Abstract
Background
Annual chlamydia screening is recommended for all sexually active women aged <25 years. Substantial limitations exist in ascertaining chlamydia trends. Reported case rates have increased likely due to increased screening and improved test technology. Other data suggest that prevalence has decreased.
Methods
Data from the Infertility Prevention Project (IPP), a national chlamydia screening program, were used to assess trends in chlamydia positivity from 2004 to 2008 among women aged 15 to 24 years who were tested in family planning clinics reporting data to IPP. Using the clinic as the unit of analysis, a correlated, longitudinal data analysis with a random intercept was conducted among clinics reporting ≥3 years of data during the analysis time-frame. Sensitivity analyses were performed to address the impact of various clinic participation levels in addition to the assessment of various correlation structures.
Results
Over 5 million chlamydia tests were reported to IPP family planning clinics from 2004 to 2008. A majority of tests were conducted among white women (clinic-specific mean: 63.2%, inter-quartile range: 37.6%–91.5%); the clinic-specific mean percent of tests conducted among black women was 17.9% (interquartile range: 0.8%–25.7%). Overall chlamydia positivity from 2004 to 2008 was 7.0%. The odds ratio associated with a single year change (1.00; 95% confidence interval: 0.99, 1.00) suggested that chlamydia positivity did not change from 2004 to 2008, after controlling for clinic-specific population factors (age, race, test usage, and geography).
Conclusions
Findings support previous analyses suggesting that chlamydia prevalence is not increasing despite apparent increasing rates based on case reports.
Chlamydia trachomatis infection, a sexually transmitted disease associated with serious adverse outcomes among women, including pelvic inflammatory disease, ectopic pregnancy, tubal-factor infertility, and chronic pelvic pain, is the most commonly reported nationally notifiable disease in the United States.1,2 Over 1.2 million cases were reported to the Centers for Disease Control and Prevention from state and local health departments in 2008.3 However, an estimated 2.8 million chlamydia cases occur annually, suggesting that under-detection of cases is substantial.4 Chlamydia screening recommendations were first made in 1993 and expanded in 2001.5,6 Currently, the US Preventive Services Task Force recommends that all sexually active women under the age of 25 years be screened annually for chlamydia.7 Given the national effort to prevent chlamydia and its complications, efforts to monitor trends in infections are critical.
Data sources available to assess chlamydia disease burden and temporal trends on a national scale are limited. Trends in US chlamydia case report data, collected routinely from state and local health departments, show case rates increasing over the last 2 decades.3 However, increasing trends are likely due to better case detection through improvements in test technology and more widespread screening.8 Contrary to national case report data, a recent analysis of data from the National Health and Nutrition Examination Survey (NHANES) suggested that chlamydia prevalence from 1999 to 2006 was stable or decreasing among a nationally representative sample of men and women aged 14 to 39 years.9 While a valuable data source, NHANES only allows for analyses at the national level, not smaller geographic areas, and is costly to reproduce at the local level. Moreover, a limited sample size restricts the ability to track chlamydia trends over time in subgroups.
There are additional data sources that supplement case report data and NHANES and allow for assessment of national chlamydia trends. The National Job Training Program (NJTP) is a program serving young, socioeconomically disadvantaged men and women aged 16 to 24 years. All participants are screened for chlamydia at program entrance. In this high-risk population, chlamydia prevalence declined from 2003 to 2007.10 In addition, data from young women screened for chlamydia are available through the Infertility Prevention Project (IPP). IPP is a national program targeting young, sexually active women for chlamydia and gonorrhea screening to prevent sequelae leading to infertility.
Previous analyses of data reported through IPP have focused on using the individual test-based data to ascertain positivity trends.3,11 These analyses have generally suggested an increase in chlamydia positivity over time among young women attending family planning clinics. However, there are substantial limitations when using this approach, primarily, the lack of covariate availability and subsequent inability to adequately assess and control for confounding. Analyzing the proportion of positive tests at the clinic level, rather than the individual encounter level, may help minimize some of these limitations. In such an analysis, the clinic itself may be considered a proxy for possible confounders, such as screening practices, demographic and behavioral population characteristics, and healthcare access. Treating the clinic as a confounder in individual test-based analyses is not possible due to the large number of participating clinics; regression models fail when adding clinic as a covariate (i.e., a large number of parameters are required). Thus, analyzing data at the clinic level may improve in some aspects of individual test-based analyses. Developing reproducible methodology to better use IPP family planning data is an important step in assessing chlamydia prevalence trends in the United States overall and at state and local levels.
The objective of this analysis was to describe trends in chlamydia positivity from 2004 to 2008 among women aged 15 to 24 years who were tested at publicly funded family planning clinics participating in IPP. Given the limitations of using individual test result data reported through IPP, we applied an analytic approach utilizing clinic-level data to determine if a linear trend existed in positivity.
MATERIALS AND METHODS
Data Source and Study Population
Administered primarily through family planning clinics, data on chlamydia test results have been reported through IPP since 1997. Data are routinely collected from facilities participating in IPP and reported to the Centers for Disease Control and Prevention on a quarterly basis. IPP data are test-based (i.e., each observation is 1 test conducted, and an individual may have multiple tests), with no personal or unique identifiers included. Available variables in IPP data include demographics (age, sex, race/ethnicity, and geography) and information specific to the test performed (technology, specimen type, and test results). Variables describing the facility conducting the test (state, region) and the type of facility (family planning, prenatal, etc.) are also available from a facility reference file. Information found on the facility reference file is combined with the test-based data using a unique facility identifier variable.
The study population consisted of family planning clinics reporting data to IPP from 2004 to 2008. Sensitivity analyses were performed using various levels of clinic participation (clinics reporting ≥3, ≥4, or 5 years of data). Since findings were consistent, regardless of levels of participation, family planning clinics reporting ≥3 years of data to IPP from 2004 to 2008 were included to use the maximum available data. Family planning clinics were defined as either a stand-alone family planning clinic, or as a designated component of an integrated clinic, in which family planning visits may be distinguished. Only individual test results from women aged 15 to 24 years were included. In order to contribute data for a single calendar year, a clinic must have reported at least 25 total tests (positive and negative) conducted among women aged 15 to 24 years during that year. Exclusion of data from low-volume clinics was intended to reduce the influence of outlier chlamydia positivity values and increase analytic stability.
Analysis
A correlated, longitudinal analysis was conducted to assess trends over a 5-year time span. The unit of analysis was the individual clinic performing chlamydia tests (clinic-based analysis), as opposed to an analysis based only on individual test results that would assume independence of test results within and across clinics.
The outcome of interest was chlamydia positivity within a clinic, defined as the proportion of positive tests out of all positive and negative tests reported by that clinic (events/trials model). This approach allowed for the incorporation of clinic size (denominator) into the modeled outcome. Chlamydia positivity has been found to be a reasonable approximation of prevalence.12 To assess for trends, the primary independent exposure of interest was defined as calendar year, from 2004 to 2008. In order to determine if year should be treated as ordinal or categorical, a linearity assessment was performed using 2 methods. First, estimated logit plots were created, which suggested a linear pattern. Linearity was then assessed using a generalized estimating equation model, to account for the correlated data. Similar to the logit plot results, modeling showed a general linear relationship between estimates associated with dummy variables representing year as categorical; therefore, year was treated as ordinal for the purposes of this analysis.
Four covariates were considered. The proportion of tests conducted using nucleic acid amplification test (NAAT) technology performed by a given clinic was likely important because these tests demonstrate substantially increased sensitivity over prior-generation tests.13 Two demographic covariates were also considered as follows: the proportion of tests occurring among young women aged 15 to 19 years, the group generally considered to be at the highest risk for chlamydial infection, and the proportion of tests occurring among black women, a group disproportionally affected by chlamydia.3 The fourth covariate identified the region (Fig. 1) where the clinic was geographically located. All 2-way interaction terms (product of year with each of the 4 covariates) were assessed, in order to detect differences in trends over time in subgroups.
Figure 1.
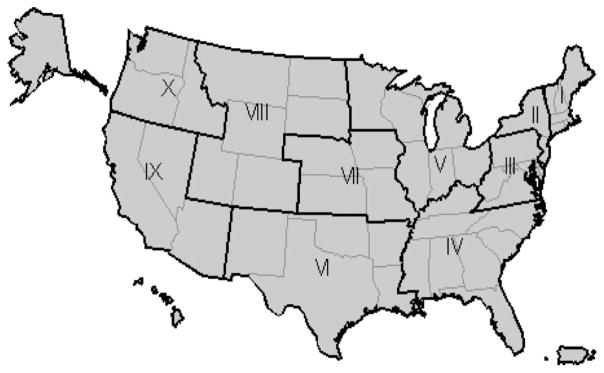
US Department of Health and Human Services Regions.
Using SAS (version 9.2, GLIMMIX procedure), a random effects regression model with an events/trials approach for the outcome (chlamydia positivity, logit [probability {total positive chlamydia tests/total positive and negative chlamydia tests in each clinic for each analysis year}]) was applied to assess linear trends from 2004 to 2008 (Supplemental Digital Content, http://links.lww.com/OLQ/A24). The exposure of interest (year) and other explanatory variables were fixed, but the intercept was treated as random, given the substantial variation in the underlying chlamydia positivity across clinics (e.g., in 2004, median: 5.8%, range: 0.0%–29.5%). A Wald test was conducted to test for the significance of the random intercept. A random slope was also considered. After reviewing respective logit plots and confirming appropriateness, the 3 proportional covariates (age, race, test technology) were treated as continuous in the model.
Before assessing correlation structures, all covariates and interaction terms were entered into an initial model to assess for covariate collinearity. Using a logistic model that accounted for correlated data but assumed a fixed effect rather than a random effect for the intercept (SAS version 9.2, GENMOD procedure), condition indices (>30) were evaluated first, followed by variance decomposition proportions (>0.5), both produced using the inverse of the information matrix (collingenmodv9c.sas macro, Emory University, Atlanta, GA, modified).
A variety of possible correlation structures were considered. The default G-matrix for this analysis was defined by σ02, a scalar parameter given to the variance component associated with the random intercept. There was no obvious R-matrix correlation structure that described the correlations between 2 different outcomes (i.e., observed proportions) within the same clinic that could be identified on the basis of clinical or biologic rationale. However, a σ12I5 R correlation matrix may be appropriate if clinic populations are similar over time. This matrix, with an empirical option and a random intercept, produces an overall covariance structure that is approximately exchangeable (compound symmetric). Unstructured, autoregressive, and toeplitz structures were also tested. Results were compared by evaluating coefficient estimates and standard errors to identify if results were consistent or discrepant when using different correlation structures. In addition, results of type III tests of fixed effects (F-statistics and associated significance tests) were obtained and compared.
To assess for confounding and precision, all possible model combinations involving subsets of the 4 covariates were considered by comparing the odds ratio (OR) for year to the corresponding OR from the full analytic model containing all 4 covariates. Sixteen possible models, including the full model, were considered. If the OR estimate was within 10% of the OR estimate for the full model, the model was determined to be eligible for further consideration by assessing precision, based on the 95% confidence intervals (CI) around the OR point estimate. The final model was selected based upon precision and appropriateness of including covariates based on demonstrated prior associations with the outcome (chlamydia positivity).
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
From 2004 to 2008, more than 5 million tests conducted among young women aged 15 to 24 years were reported to IPP. Approximately 1 million tests were reported annually. Of the 4253 clinics reporting data to IPP for at least 1 year during the analysis time frame, 58.2% (2475) reported ≥3 years of data, representing 88% of all tests reported. This group of clinics was located in 49 states. About 30% of clinics were located in region IV (Fig. 1).
The clinic-specific mean percent of tests conducted among black women aged 15 to 24 years was 17.9% (Table 1). Slightly less than half of the population tested was comprised of young women aged 15 to 19 years. Mean use of NAAT technology increased over time, from 69.7% in 2004 to 87.6% in 2008; however, median usage was consistently 100.0%. When stratified by year, race/ethnicity and age did not vary substantially. The mean proportion of tests conducted among women aged 15 to 19 years ranged from 44.2% to 46.2% (median: 43.9%– 46.3%); the mean proportion of tests conducted among black women ranged from 17.2% to 19.0% (median: 4.8%–5.9%). The overall mean clinic-specific chlamydia positivity from 2004 to 2008 was 7.0%. In 2004, positivity was 6.9%, remaining fairly stable through 2008, when positivity was 7.2%. When compared to clinics reporting only 1 or 2 years of data to IPP not included in the analysis, clinics reporting ≥3 years of data had a slightly lower mean proportion of women tested who were black (17.9% vs. 20.4%); the distribution of age and NAAT usage was nearly identical. The overall mean positivity among clinics not included in the analysis was 7.5%.
TABLE 1.
Clinic-specific Population Characteristics of Family Planning Clinics Reporting ≥3 Years of Data to the Infertility Prevention Project From Chlamydia Tests Conducted Among Women Aged 15 to 24 Years, 2004–2008
| Clinic Percent or Count*
|
||
|---|---|---|
| Mean | Median (Interquartile Range) | |
| Race/ethnicity† | ||
| Hispanic | 11.7 | 2.6 (0.4–11.8) |
| Non-Hispanic black | 17.9 | 5.4 (0.8–25.7) |
| Non-Hispanic white | 63.2 | 73.2 (37.6–91.5) |
| Other | 3.3 | 1.0 (0–2.8) |
| Unknown/missing | 3.9 | 0.2 (0–1.9) |
| Age group† | ||
| 15–19 y | 45.4 | 45.3 (38.2–52.1) |
| 20–24 y | 54.5 | 54.7 (47.9–61.8) |
| NAAT† usage | ||
| 2004 | 69.7 | 100.0 (2.9–100.0) |
| 2005 | 77.5 | 100.0 (99.5–100.0) |
| 2006 | 83.0 | 100.0 (100.0–100.0) |
| 2007 | 86.1 | 100.0 (97.9–100.0) |
| 2008 | 87.6 | 100.0 (100.0–100.0) |
| No. tests | 396 | 250 (123–512) |
Mean and median of all clinic values.
Proportions.
NAAT indicates nucleic acid amplification test.
Correlated Analysis of Continuously Reporting Clinics
Only the σ12I5 R-matrix model converged consistently. The random intercept was statistically significant and retained in the model. Inclusion of the random slope did not alter the findings, and the random slope was not significant (data not shown). All 4 product terms (involving year with each covariate) were sequentially dropped because of collinearity; consequently, the resulting model did not address interaction.
After adjusting for all covariates in the no interaction model, the estimated effect of year, the independent predictor of interest, was null (OR: 1.00; CI: 0.99, 1.00; P = 0.69), suggesting that chlamydia positivity did not change from 2004 to 2008 (Table 2). OR values for the continuous variables for the proportion of tests conducted among 15- to 19-year-old women (OR: 1.00; CI: 1.00, 1.00; P < 0.001), among black women (OR: 1.02; CI: 1.01, 1.02; P < 0.001), and using NAAT technology (OR: 1.00; CI: 1.00, 1.00; P < 0.001) were extremely close to 1 (with narrow CIs). The estimated effect of region varied.
TABLE 2.
Clinic-based Model Output Assessing Chlamydia Positivity in Family Planning Clinics Reporting Data to the Infertility Prevention Project From Chlamydia Tests Conducted Among Women Aged 15 to 24 Years, 2004–2008
| Variable | Odds Ratio (95% CI) | |
|---|---|---|
| Full model | Year | 1.00 (0.99, 1.00) |
| Proportion 15–19 | 1.00 (1.00, 1.00) | |
| Proportion black | 1.02 (1.01, 1.02) | |
| Proportion NAAT | 1.00 (1.00, 1.00) | |
| Region I | 0.73 (0.67, 0.79) | |
| Region II | 0.81 (0.72, 0.90) | |
| Region III | 0.65 (0.61, 0.69) | |
| Region IV | 0.90 (0.85, 0.96) | |
| Region V | 0.97 (0.92, 1.03) | |
| Region VI | 1.14 (1.07, 1.22) | |
| Region VII | 0.93 (0.87, 0.99) | |
| Region VIII | 1.16 (1.05, 1.27) | |
| Region IX | 1.19 (1.08, 1.30) | |
| Region X | Ref | |
| Reduced model* | Year | 1.02 (1.01, 1.03) |
No covariates included.
CI indicates confidence interval; NAAT, nucleic acid amplification test.
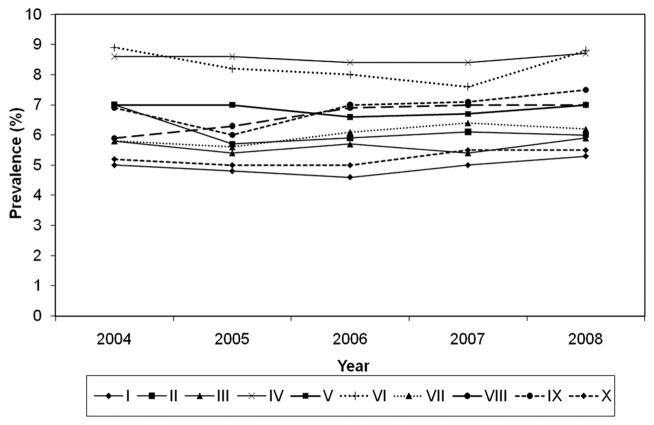
When examining crude chlamydia positivity by region, the mean clinic-specific positivity appeared to fluctuate (Fig. 2). However, when trends were modeled for each region, adjusting for race, test technology, and age, some minor variation in trends was noted, but no substantial changes in chlamydia positivity from 2004 to 2008 were seen. In regions I, II, IX, and X, no changes in positivity were evident. In regions IV (OR: 0.95; CI: 0.95, 0.96), V (OR: 0.98; CI: 0.97, 1.00), and VI (OR: 0.97; CI: 0.95, 0.99), a decrease in chlamydia positivity was detected, although the magnitude of change was negligible. Similarly, in regions VII (OR: 1.04; CI: 1.02, 1.05) and VIII (OR: 1.04; CI: 1.01, 1.07), increase in chlamydia positivity was detected, but changes were effectively null. The model for region III did not converge due to data limitations.
Figure 2.
Mean clinic-specific chlamydia positivity among women aged 15 to 24 years who attended family planning clinics reporting data to the Infertility Prevention Project, by region, 2004–2008.
DISCUSSION
Our analysis suggests that chlamydia positivity did not change among the population of women aged 15 to 24 years who were screened in family planning clinics reporting data to IPP from 2004 to 2008. When combined with other findings from earlier prevalence studies examining chlamydia trends, such as recently conducted analyses using data from NHANES and the NJTP, this analysis adds more evidence that chlamydia prevalence is not increasing.9,10
This conclusion runs counter to common misinterpretations using case report data, which is often incorrectly cited as evidence that the burden of chlamydia is increasing. From 2004 to 2008, reported chlamydia case rates increased more than 20% among women aged 15 to 24 years.3 During this same time period, NAAT usage increased. In 2004, 64.4% of all chlamydia tests conducted in surveyed public health laboratories were NAATs; by 2007, 81.6% of all tests performed utilized NAAT technology.14,15 Expanded use of more sensitive test technology likely resulted in increased case detection, as has been previously reported.16 Concurrently, chlamydia screening coverage increased. Among sexually active women aged 16 to 20 years with commercial health insurance who were seeking health care, coverage increased from 32.6% in 2004 to 40.1% in 2008.17 Similarly, coverage among young women in Medicaid managed care increased from 45.9% to 52.7%. Both of these factors, increasing NAAT usage and increasing coverage, affect case report trends, but not actual disease burden.
Findings presented in this analysis differ from other analyses of IPP data. IPP data from women aged 15 to 24 years who were tested for chlamydia in family planning clinics suggested an upward trend in median state-specific positivity, which increased from 6.3% in 2004 to 7.4% in 20083,18; no adjustments for clinic or other possible confounders were made. This possible increase, similar to chlamydia case report trends, is at least partially explained by greater NAAT usage; for instance, when NAAT usage among women screened in NJTP increased from about 20% to 88%, positivity increased from 9.1% to 13.9% in the absence of any other measured population changes.10 When overall chlamydia crude positivity was stratified by a region, slight increases were noted in most regions (Prevention, November 20103). Conversely, findings reported in this article showed that chlamydia prevalence changed little by a region. In this analysis, test technology, represented in the model as the proportion of tests performed in a clinic that utilized NAATs, was treated as a confounder. However, because different test technologies have varying sensitivity and specificity values, even within different types of NAATs, measurement error is a concern. Adjusting for test technology in the model presented in this analysis does not adequately address this type of error; rather, including test technology represents broader clinic practices.
Analyzing IPP data at the clinic level may be preferable to individual test-based analyses. Few covariates are available in IPP data. However, clinic populations are likely similar over time, and use of a correlated analysis approach allows for clinics to act as a proxy for unmeasured confounding. Inclusion of a random intercept accounted for the natural heterogeneity among clinics on the prediction of chlamydia positivity trends. Because of the initial uncertainty in identifying a single appropriate correlation matrix, several were tested. While only the σ12I5 R-matrix model consistently converged, this correlation matrix approximates a compound symmetric structure, supporting the original posit of a compound symmetric matrix being a possible matrix, if clinic populations maintain some consistency over time. Failure of models using other R-matrix structures should not have adverse implications. Overall, this analysis may better characterize national trends in chlamydia prevalence and positivity than unadjusted test-based analyses commonly reported.
As a result of sensitivity analyses assessing the importance of clinic participation in examining trends, IPP data were maximized by including all clinics reporting at least 3 years of data. This important finding allowed for inclusion of 58% of all clinics reporting any data from 2004 to 2008, and 88% of all tests reported. If analyses had revealed that stability and estimates were compromised by allowing incompletely participating clinic data, only 42% of available clinics would have been included in the analysis (those clinics reporting 5 years of data, 2004–2008). Programmatic and funding decisions frequently affect annual clinic participation. Thus, a more inclusive approach should allow for maximum data usage, enhancing IPP data utility, and allowing for broader generalizability within the IPP family planning clinic population. In addition, the developed approach is easily reproducible for future surveillance usage.
Monitoring trends in chlamydia prevalence is a critical component to assessing the impact of prevention efforts. While prevention strategies may be having some effect, more remains to be done. Despite steady improvements in screening eligible women seeking healthcare, chlamydia trends remain stable. The burden of disease is substantial; this analysis shows regional variation in positivity of 5% to 9% among young women aged 15 to 24 years tested in family planning clinics analyzed. In addition, other reports have demonstrated substantial racial/ethnic disparities.3,9,19
This analysis has several limitations. Although use of data summarized at the clinic level likely minimized the influence of some unmeasured confounders, only a limited number of covariates were available. In particular, sexual behaviors were not measured, and thus could not be accounted for in the analysis. Changes in clinic characteristics, such as screening policies, or uncaptured differences in clinic population, such as minor changes within a demographic stratum that resulted in a lower-risk population being screened may be missed using the modeling approach presented. In addition, as with all analyses of national IPP data, lack of personal identifiers meant that some individuals may have been tested more than once, thus contributing more than 1 test result to the analysis. Another limitation was the inability to assess for effect measure modification. Due to collinearity between the product terms and the component covariates, all 4 interaction terms were removed from the model in the early assessment stages. This did not demonstrate the absence of interaction between year and the covariates, but rather interactions could not be properly assessed because of collinearity. However, when the model was run using a stratified approach (women aged 15–19 years, women aged 20–24 years, black women, white women, Hispanic women), findings were not meaningfully different (data not shown).
IPP primarily serves socio-economically disadvantaged young women; however, clinics participating in IPP have diverse populations and screen a substantial population of young women of various races and ages. Moreover, the IPP mean clinic-specific chlamydia positivity of 7.0% reported here is similar to an estimate of chlamydia prevalence among a nationally representative (NHANES) sample of sexually active 14- to 19-years old women (7.1%).20 Although the ages represented are not identical, this suggests that the IPP family planning clinic population in this analysis may be somewhat similar to the general population of sexually active young women in the United States. However, women tested in family planning clinics likely represent a heterogeneous risk group, seeking healthcare for a variety of reasons; other clinic types in IPP, such as prenatal clinics where women are less impacted by healthcare seeking behaviors, may better approximate the general population.
In addition to some of the aforementioned strengths of the modeling approach, this analysis revealed consistent results using well-defined methodology, regardless of clinic participation level. Utilization of clinic-level summary data likely accounted for some unmeasured confounding and allowed for trend assessment in this population. Use of a correlated analysis approach with a random intercept included likely minimized the impact of varying clinic populations and policies. Moreover, the analytic approach applied in this study is easily reproducible; a correlated analysis with a random intercept addresses the study question using the best available data and moves beyond limitations of current IPP analyses. Other approaches, such as utilizing a multilevel model that included variables at the individual level as well as at the clinic level, may have also been appropriate if more extensive individual test data were available, such as sexual behavior data.
In summary, findings suggest that, in US family planning clinics reporting chlamydia tests to IPP, chlamydia positivity did not change substantially from 2004 to 2008 among 15- to 24-years old women. These findings are consistent with other national prevalence analyses, suggesting that chlamydia prevalence in the United States is not increasing, despite increases in chlamydia case reports.
Supplementary Material
Acknowledgments
The authors thank and acknowledge Dr. Stuart Berman and Dr. James Buehler for providing valuable insights in the early conceptualization of this analysis, and Robert Nelson for graphical assistance.
Footnotes
Supplemental digital content is available for this article. A direct URL citation appears in the printed text, and a link to the digital file is provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
The findings and conclusions in this report have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed to represent any agency determination or policy.
References
- 1.Centers for Disease Control and Prevention. Summary of notifiable diseases—United States, 2008. Morb Mortal Wkly Rep. 2008;57:1–94. [Google Scholar]
- 2.Stamm WE. Chlamydia trachomatis infections of the adult. In: Holmes KK, Sparling PF, Stamm WE, et al., editors. Sexually Transmitted Diseases. 4. New York, NY: McGraw-Hill; 2008. pp. 575–593. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2008. Atlanta, GA: US Department of Health and Human Services; 2009. [Google Scholar]
- 4.Weinstock H, Berman S, Cates W. Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Recommendations for the prevention and management of Chlamydia trachomatis infections, 1993. Morb Mortal Wkly Rep. 1993;42:1–38. [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force. Screening for chlamydial infection: Recommendations and rationale. Am J Prev Med. 2001;20:90–94. [Google Scholar]
- 7.US Preventive Services Task Force. Screening for chlamydial infection: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;147:128–134. doi: 10.7326/0003-4819-147-2-200707170-00172. [DOI] [PubMed] [Google Scholar]
- 8.Miller WC. Epidemiology of chlamydial infection: Are we losing ground? Sex Transm Infect. 2008;84:82– 86. doi: 10.1136/sti.2007.028662. [DOI] [PubMed] [Google Scholar]
- 9.Datta SD, Sternberg M, Satterwhite C, et al. Trends in Chlamydia trachomatis prevalence in the US, 1999–2006: Results from the National Health and Nutrition Examination Survey (NHANES). Poster presented at: 48th Annual ICAAC/IDSA 46th Annual Meeting; October 25–28, 2008; Washington, DC. [Google Scholar]
- 10.Satterwhite CL, Tian LH, Braxton J, et al. Chlamydia prevalence among women and men entering the National Job Training Program: United States, 2003–2007. Sex Transm Dis. 2010;37:63–67. doi: 10.1097/OLQ.0b013e3181bc097a. [DOI] [PubMed] [Google Scholar]
- 11.Fine D, Dicker L, Mosure D, et al. Increasing chlamydia positivity in women screened in family planning clinics: Do we know why? Sex Transm Dis. 2008;35:47–52. doi: 10.1097/OLQ.0b013e31813e0c26. [DOI] [PubMed] [Google Scholar]
- 12.Dicker LW, Mosure DJ, Levine WC. Chlamydia positivity versus prevalence. What’s the difference? Sex Transm Dis. 1998;25:251–253. doi: 10.1097/00007435-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Black C, Marrazzo J, Johnson R, et al. Head-to-head multicenter comparison of DNA probe and nucleic acid amplification tests for Chlamydia trachomatis infection in women performed with an improved reference standard. J Clin Microbiol. 2002;40:3757–3763. doi: 10.1128/JCM.40.10.3757-3763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dicker L, Mosure D, Steece R, et al. Testing for sexually transmitted diseases in US Public health laboratories in 2004. Sex Transm Dis. 2007;34:41– 46. doi: 10.1097/01.olq.0000222708.70594.8e. [DOI] [PubMed] [Google Scholar]
- 15.Yee E, Satterwhite CL, Braxton J, et al. Current STD laboratory testing and volume in the United States among public health laboratories, 2007. Poster presented at: 18th ISSTDR (International Society for STD Research); June 28–July 1, 2009; London, England. [Google Scholar]
- 16.Dicker LW, Mosure DJ, Levine WC, et al. Impact of switching laboratory tests on reported trends in Chlamydia trachomatis infections. Am J Epidemiol. 2000;151:430– 435. doi: 10.1093/oxfordjournals.aje.a010223. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Quality Assurance. The state of health-care quality. Washington, DC: National Committee for Quality Assurance; 2009. [Google Scholar]
- 18.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2004. Atlanta, GA: U. S. Department of Health and Human Services; 2005. [Google Scholar]
- 19.Datta SD, Sternberg M, Johnson RE, et al. Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann Intern Med. 2007;147:89–96. doi: 10.7326/0003-4819-147-2-200707170-00007. [DOI] [PubMed] [Google Scholar]
- 20.Forhan S, Gottlieb S, Sternberg M, et al. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics. 2009;124:1505–1512. doi: 10.1542/peds.2009-0674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.