Abstract
In aging humans and rodents, inter-individual differences in cognitive function have been ascribed to variations in long-term glucocorticoid exposure. 11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1) regenerates the active glucocorticoid cortisol from circulating inert cortisone, thus amplifying intracellular glucocorticoid levels in some tissues. We show that 11β-HSD1, but not 11β-HSD2, mRNA is expressed in the human hippocampus, frontal cortex, and cerebellum. In two randomized, double-blind, placebo-controlled crossover studies, administration of the 11β-HSD inhibitor carbenoxolone (100 mg three times per day) improved verbal fluency (P < 0.01) after 4 weeks in 10 healthy elderly men (aged 55-75 y) and improved verbal memory (P < 0.01) after 6 weeks in 12 patients with type 2 diabetes (52-70 y). Although carbenoxolone has been reported to enhance hepatic insulin sensitivity in short-term studies, there were no changes in glycemic control or serum lipid profile, nor was plasma cortisol altered. 11β-HSD1 inhibition may be a new approach to prevent/ameliorate cognitive decline.
Mild cognitive impairment (MCI) is a common feature of aging that may herald dementia but is, at present, untreatable (1). MCI is associated with a poor quality of life and loss of independence in older people and results in huge socioeconomic costs (2). Preventing and treating age-related cognitive decline is a research priority (3). In aged animals and humans, inter-individual differences in cognitive function have been ascribed to variations in long-term glucocorticoid exposure (4, 5). The hippocampus plays a central role in the formation of long-lasting memories, highly expresses receptors for glucocorticoids in rodents (6) and humans (7), and is particularly sensitive to the deleterious actions of chronic glucocorticoid excess (8, 9). Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis with resultant chronically increased exposure of the hippocampus to elevated glucocorticoid levels has been hypothesized to contribute to the decline in cognitive function with aging, through the detrimental effects of glucocorticoids on hippocampal neurons (10, 11). In human populations, including those with Cushing's disease, Alzheimer's disease, depression, and normal aging, higher cortisol levels have been associated with poorer memory and hippocampal shrinkage/neuronal loss (4, 12, 13). In rats, manipulations that keep glucocorticoid levels low through life, such as neonatal handling or adrenalectomy with low-dose glucocorticoid replacement, prevent the emergence of learning deficits with age (14, 15). However, in humans, chronic suppression of plasma cortisol is unsafe whereas long-term use of glucocorticoid receptor antagonists merely induces compensatory increases in corticotrophin (ACTH)-dependent steroid production (16). Recent research has highlighted the importance of metabolism of glucocorticoids within target tissues, which may offer a route for tissue-selective manipulation of glucocorticoid exposure (17).
Tissue glucocorticoid concentrations are determined not only by plasma steroid levels, but also by intracellular 11β-hydroxysteroid dehydrogenases (11β-HSDs), which interconvert active glucocorticoids (cortisol in humans, corticosterone in rats and mice) and inert 11-keto forms (cortisone and 11-dehydrocorticosterone, respectively) within specific target cells (18). The type 2 isozyme (11β-HSD2) is a potent NAD-dependent dehydrogenase that catalyzes the rapid inactivation of glucocorticoids. In the periphery, 11β-HSD2 is confined to aldosterone-selective target tissues such as the distal nephron, colon, and salivary gland, where it acts to prevent illicit occupation by glucocorticoids of intrinsically nonselective mineralocorticoid receptors (19, 20). In contrast, the type 1 isozyme (11β-HSD1) is expressed in key glucocorticoid receptor target tissues such as liver (21, 22) and adipose tissue (23). Here, 11β-HSD1 acts in the reverse (reductase) direction in intact cells (24-26) and organs (27), thus regenerating active glucocorticoids (17). Inhibition of 11β-HSD1 has been proposed to selectively lower intracellular cortisol in these tissues, an action advocated as a potential therapy for obesity, type 2 diabetes, and dyslipidemia (17, 28-30).
11β-HSDs are also expressed in the adult rat brain (31-33) where 11β-HSD1 is abundant, but 11β-HSD2 is largely absent (34). In intact rat hippocampal cells in primary culture, which express solely the 11β-HSD1 isozyme, the reaction catalyzed is reduction. Such reactivation of glucocorticoids by 11β-HSD1 allows intrinsically inert 11-keto steroids to mimic active glucocorticoids in potentiating kainate-induced neurotoxicity (35). Carbenoxolone, which inhibits both isozymes of 11β-HSD in vitro and in vivo (36), prevents regeneration of glucocorticoids by 11β-HSD1 and thereby protects primary cultures of hippocampal cells from glucocorticoid-mediated exacerbation of excitatory amino acid neurotoxicity (35). Regeneration of glucocorticoids by 11β-HSD1 within neurons seems important in vivo because 11β-HSD1 knockout mice are protected from glucocorticoid-associated hippocampal dysfunction with aging (37), despite having modestly elevated plasma glucocorticoid levels.
However, any relevance of this system to human cognitive aging is unknown. Here, we examine expression of 11β-HSDs in key cognitive regions of the adult human brain and determine the effects of carbenoxolone [which gains access to the brain (38)] on cognitive function in healthy elderly men and on cognitive function and metabolic control in middle-aged subjects with type 2 diabetes. Neither group had dementia or a diagnosed cognitive disorder, but only the expected age-related cognitive changes.
Methods
11β-HSD Isozyme mRNAs in Human Brain. Postmortem sections (n = 4 per region) of human hippocampus, frontal cortex, and cerebellum were obtained with ethical approval and relatives' consent from the Edinburgh Brain Bank. The subjects were two women and two men (mean 77 y, range 67-86 y) who died of lymphangitis carcinomatosa, lung carcinoma, esophageal adenocarcinoma, and cardiac failure and had no antemortem or postmortem evidence of CNS disorders. Brain sections were taken and processed, broadly as described (7). 11β-HSD1 mRNA was assayed by in situ hybridization histochemistry in frozen sections by using [35S]UTP-labeled antisense cRNA probes transcribed in vitro from a 900-bp HindIII-SstI fragment of ph11β-HSD1 (39) subcloned in pGEM3. 11β-HSD2 mRNA was similarly estimated by using an XbaI-linearized pCRIICtBb, which has the 531-bp 11β-HSD2 insert (corresponds to the 3′ end of the 11β-HSD2 coding region, i.e., bases +654 to +1184) (20). Sections were postfixed in 4% paraformaldehyde, acetylated (0.25% acetic anhydride in 0.1 M triethanolamine, pH 8.0), washed in PBS, dehydrated through graded alcohols, and air-dried. Hybridization with the cRNA probe was carried out as described (40). Slides were dehydrated, dipped in photographic emulsion (NTB-2, Kodak) and exposed at 4°C for 6 weeks before developing and counterstaining with 1% pyronine. Control sections were hybridized with identically labeled sense RNA probes.
11β-HSD Bioactivity in Human Brain. 11β-HSD activity was assayed in homogenates of human CNS subregions, taken within 4 h of death from three subjects aged 64-71 y without CNS disorders (causes of death: acute myocardial infarction, cardiac failure, and pancreatic cancer). The assays were performed essentially as described (41). Homogenates were assayed for protein content by the Bradford method. Protein homogenate (500 μg/ml), within the linear part of the relationship between substrate concentration and reaction product formation, was added to 100 nM [3H]cortisol with 400 μM NADP and incubated at 37°C for 1, 6, and 24 h. Assays were in the dehydrogenase direction, which is more stable in tissue homogenates. The reaction was terminated by addition of ethyl acetate, steroids separated by TLC with visualization by PhosphorImager (Molecular Dynamics) and quantification by scintillation counting with results expressed as fmol product per mg of protein per min.
11β-HSD Inhibition in Humans. Healthy elderly men. Ten healthy men [65.5 ± 5.5 (SD) y, range 55-75] were recruited by local advertisements. Exclusion criteria were any ongoing medical condition, including those that affected cognitive function; history of glucocorticoid therapy in the previous 6 months; any regular medication; history of depression; abnormal renal, liver, and thyroid function tests on screening; or alcohol intake >21 units/week. All subjects gave written informed consent to the study, which was approved by the local ethics of medical research committee.
The subjects participated in a randomized, double blinded, placebo-controlled crossover study comparing the 11β-HSD inhibitor carbenoxolone (100 mg, orally, three times per day for 4 weeks) with placebo. During each phase, subjects also received amiloride (10 mg/day) to prevent renal mineralocorticoid excess (36). The two phases were separated by an 8-week washout period. Participants were asked to look out for potential adverse effects of carbenoxolone, including weight gain and pedal edema, and blood pressure and plasma electrolytes were monitored weekly. Compliance was assessed by pill counting and detecting carbenoxolone in plasma by high performance liquid chromatography. To avoid order effects, half the participants received placebo in the first phase and half received carbenoxolone first. At the end of each phase, participants had venous blood samples taken (≈9:00 a.m.) for electrolytes and cortisol and underwent a battery of tests of different domains of cognition and mood (42). The tests took 3 h to complete and were punctuated with rest periods.
Cognitive and affective functions were assessed as described (43). Briefly, nonverbal reasoning was evaluated with Raven's Standard Progressive Matrices by using the number correct in 20 min (44). Verbal fluency, thought to assess executive function and semantic memory, was assessed with the Controlled Word Association Test (42). Verbal memory was evaluated with a test of list-learning, the Rey Auditory-Verbal Learning Test (AVLT) (42), and paragraph recall, with the Logical Memory (immediate and 30-min-delayed) subtest of the Wechsler Memory Scale-Revised (45). Visuospatial memory was evaluated with the Visual Reproduction (immediate and 30-min-delayed) subtest of the Wechsler Memory Scale-Revised (45). Attention and processing speed were evaluated with the Digit-Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised (46). Prior general cognitive ability was assessed with the National Adult Reading Test (47). Mood was assessed with the University of Wales Institute of Science and Technology (UWIST)-Mood Adjective Checklist (MACL) (48) and the Hospital Anxiety and Depression Scale (HADS) (49).
Type 2 diabetes study. Because the subjects in the healthy elderly study showed little cognitive impairment at baseline and to dissect whether the effects on cognition reflected primary actions on the CNS or merely the reported improvements in metabolic function (increased insulin sensitivity) seen in normal subjects with carbenoxolone (28), we repeated the study in middle-aged subjects with type 2 diabetes, known as a group to show a selective deficit in verbal memory unassociated with dementia (51). Twelve subjects [nine male; three female, 60 ± 4.9 (SD) y, range 52-70 y] with stable type 2 diabetes were enrolled by means of the local diabetes clinic. All were treated with diet and oral hypoglycemic agents alone. None had blood pressure >150/90 mmHg or was taking antihypertensives. Exclusion criteria were any other ongoing medical condition, including those that affected cognitive function; history of glucocorticoid therapy in the previous 6 months; history of depression; abnormal renal, liver, and thyroid function tests on screening; or alcohol intake >21 units per week. All subjects gave written informed consent to the study, which was approved by the local ethics of medical research committee.
The diabetic subjects participated in a similar randomized, double blinded, placebo-controlled crossover study comparing the 11β-HSD inhibitor carbenoxolone (100 mg, orally, three times per day for 6 weeks) with placebo. During each phase, subjects also received amiloride (10 mg/day) to prevent renal mineralocorticoid excess. The two phases were separated by a 12-week washout period. Fasting morning (≈9:00 a.m.) blood samples were taken for assay of electrolytes, cortisol, glycosylated hemoglobin (HbA1c), and lipids before the study and at the end of each treatment phase, along with cognitive function testing as in the healthy elderly study.
Blood samples were analyzed by routine autoanalyzer for electrolytes, HbA1c, and cholesterol, and plasma cortisol was estimated by RIA, as described (30, 50).
Statistical Analysis. Nonparametric tests were used to detect differences in cognitive variables between the two treatments: Wilcoxon matched pair test for two-group comparisons with exact, two-tailed significance levels; Friedman's for ANOVA. Plasma variables and blood pressures were compared by Student's t tests. Results are means ± SD.
Results
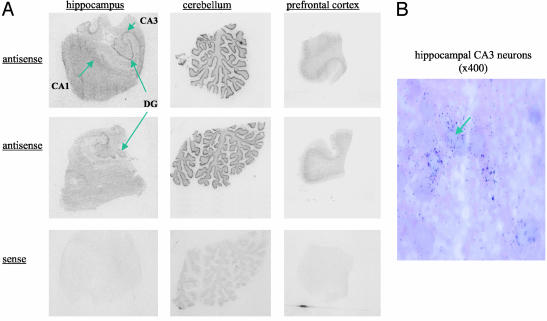
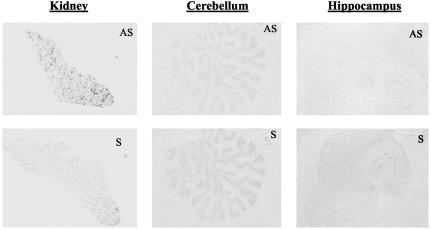
11β-HSD mRNAs and Enzyme Activity in the Human CNS. By using in situ hybridization, 11β-HSD1 mRNA was detected in hippocampus, prefrontal cortex, and cerebellum (Fig. 1). In the hippocampus, the highest expression was seen in cornu ammonis, particularly CA3, and in the dentate gyrus. High 11β-HSD1 mRNA expression was also detected in the granule cell layer of the cerebellum and throughout the prefrontal cortex. No signal was detected with “sense” controls. On microscopy, the distribution of 11β-HSD1 was neuronal in all regions examined (Fig. 1). In contrast, 11β-HSD2 mRNA was not detected in hippocampus, frontal cortex, or cerebellum (Fig. 2). Hippocampal and cerebellar homogenates all showed 11β-HSD activity (cerebellum: 39 ± 12 fmol cortisone per mg of protein per min; hippocampus: 10.4 ± 2.6 fmol cortisone per mg of protein per min).
Fig. 1.
11β-HSD1 mRNA in the human CNS. (A) In situ hybridization histochemistry showing expression of 11β-HSD1 mRNA, detected with antisense cRNA probes, in hippocampus, cerebellum, and frontal cortex from two representative human postmortem brains. Note the absence of signal with sense control probes applied to sequential sections of the same (upper) blocks. (B) Microscopic autoradiography of 11β-HSD1 mRNA expression in human hippocampus. An arrow marks pyramidal neuronal cell bodies in the CA3 subfield. Note silver grain accumulation over cell bodies indicating expression of 11β-HSD1 mRNA.
Fig. 2.
11β-HSD2 mRNA in human kidney (positive control) and CNS. 11β-HSD2 mRNA by in situ hybridization. Specific (AS) signal is seen dotted around the renal cortex, but none in cerebellum or hippocampus compared with sense (S) control.
Carbenoxolone and Cognitive Function. Healthy elderly men. There were no adverse events, and blood pressure and plasma sodium, potassium, and cortisol did not differ between study phases (table 1). Carbenoxolone levels were 8.69 ± 4.1 μg/ml during active treatment and undetectable during placebo administration.
Table 1. Effects of carbenoxolone or placebo on plasma electrolytes, cortisol, and blood pressure in two distinct, randomized, double-blind, placebo-controlled crossover studies in healthy elderly subjects and patients with stable type 2 diabetes.
| Healthy elderly subjects
|
Patients with type 2 diabetes
|
|||||
|---|---|---|---|---|---|---|
| Placebo with amiloride mean (SD) | Carbenoxolone with amiloride mean (SD) | P | Placebo with amiloride mean (SD) | Carbenoxolone with amiloride mean (SD) | P | |
| Plasma Na (mmol/liter) | 141 (2) | 142 (2) | 0.054 | 139 (3) | 139 (3) | 0.46 |
| Plasma K (mmol/liter) | 4.37 (0.34) | 4.34 (0.37) | 0.82 | 4.44 (0.41) | 4.58 (0.32) | 0.03* |
| 9:00 a.m. plasma cortisol (μg/dl) | 15.3 (9.2) | 15.6 (8.2) | 0.94 | 20.7 (7.3) | 22.7 (7.3) | 0.51 |
| Systolic blood pressure (mmHg) | 123 (14) | 123 (14) | 0.84 | 133 (14) | 133 (14) | 1.00 |
| Diastolic blood pressure (mmHg) | 70 (9) | 71 (8) | 0.12 | 78 (6) | 78 (6) | 1.00 |
, P < 0.05 compared with placebo.
Carbenoxolone significantly improved verbal fluency scores (P = 0.006) but did not improve performance in tests of visual and verbal memory (Table 2). There were no significant differences in the tests of nonverbal reasoning (Raven's matrices), attention, and processing speed (Digit-Symbol Substitution Test), or in tests of verbal or visuospatial memory (Table 2). There were no significant changes in the mood indicators, Hospital Anxiety and Depression Scale (HADS) and UWIST-Mood Adjective Checklist (MACL) scores, although there was a trend for carbenoxolone to reduce scores on anxiety subsets of both these scales (HADS-anxiety score P = 0.07; UWIST MACL-tense arousal score P = 0.08).
Table 2. Influence of carbenoxolone on cognitive function in healthy elderly men and patients with type 2 diabetes.
| Healthy elderly subjects
|
Patients with type 2 diabetes
|
||||||
|---|---|---|---|---|---|---|---|
| Cognitive domain | Neuropsychological measures | Placebo with amiloride mean (SD) | Carbenoxolone with amiloride mean (SD) | P | Placebo with amiloride mean (SD) | Carbenoxolone with amiloride mean (SD) | P |
| Executive function | Verbal fluency | 40.6 (12.4) | 44.2 (10.6) | 0.006 | 42.2 (8.4) | 42.7 (6.4) | 0.48 |
| Memory | |||||||
| Visual | WM visual reproduction | 59.2 (18.2) | 66.2 (8.4) | 0.28 | 59.9 (13.3) | 60.2 (7.9) | 0.94 |
| Verbal | WM logical memory | 47.0 (19.2) | 53.2 (18.1) | 0.13 | 49.7 (13.8) | 49.7 (17.7) | 0.78 |
| Rey AVLT | 51.5 (10.5) | 53.9 (8.5) | 0.47 | 55.2 (8.0) | 58.8 (5.2) | 0.005 | |
| Nonverbal reasoning | RSPM | 45.1 (5.9) | 46.3 (8.5) | 0.45 | 44.0 (6.6) | 45.4 (8.0) | 0.17 |
| Processing speed | DSST | 50.7 (7.8) | 51.9 (8.5) | 0.70 | 50.3 (6.7) | 50.6 (5.5) | 0.78 |
WM, Wechsler Memory Scale-revised; AVLT, Rey Auditory Verbal Learning Test; RSPM, Ravens Standard Progressive Matrices; DSST, Digit Symbol Substitution test. Values in bold indicate a significant difference compared with placebo.
Patients with type 2 diabetes. The healthy elderly subjects examined in the first study had very well preserved memory function for age. To examine a population with more frequent memory defects, we repeated the study in patients with type 2 diabetes mellitus who in general show an increased incidence of impairments in verbal memory (51). The diabetic patients were metabolically stable (being treated for diabetes for 2-11 y) and had modestly elevated HbA1c levels (mean 7.5 ± 0.6%; range 6.5-8.6) while taking dietary therapy and/or oral hypoglycemic agents (three, diet alone; five, metformin; two, sulfonylurea; two, sulfonylurea plus metformin). Again, carbenoxolone plus amiloride caused no adverse events, and blood pressure, plasma sodium, and cortisol did not differ between study phases (table 1). Plasma potassium was very slightly but significantly elevated in the carbenoxolone phase (table 1), confirming the efficacy of prevention of apparent mineralocorticoid excess by amiloride. Carbenoxolone for 6 weeks had no effects on HbA1c (placebo 7.5 ± 0.6%; carbenoxolone 7.5 ± 0.5%), or fasting serum cholesterol (placebo 5.34 ± 0.79 mmol/liter; carbenoxolone 5.24 ± 0.67 mmol/liter) or high-density lipoprotein (HDL) cholesterol (placebo 1.27 ± 0.33 mmol/liter; carbenoxolone 1.28 ± 0.32 mmol/liter) levels.
Carbenoxolone significantly improved scores on the Auditory-Verbal Learning Test (P < 0.01; Table 2), which assesses verbal memory. In this group, verbal fluency scores were unaltered. As in the healthy elderly men, there was no significant difference in the Raven's matrices and Digit-Symbol Substitution Test (DSST) scores between study phases. Again, there was a trend for lower anxiety with carbenoxolone (P = 0.14).
Discussion
The distribution of 11β-HSD1 mRNA in human brain mirrors the findings in rodents (31-33, 52), with high expression in specific neuronal subregions of the hippocampus, frontal cortex, and cerebellum. The highest signal, as in rodent brain, came from cerebellum (52). In contrast, 11β-HSD2 mRNA was not detected in these regions of the human CNS, again paralleling data in the adult rat brain, where 11β-HSD2 is confined to a few discrete mid- and hind-brain nuclei (34, 53) that may subserve central control of blood pressure and salt appetite (54), phenomena sensitive to aldosterone but not glucocorticoids. In the rat brain, 11β-HSD1 mRNA, protein, and enzyme activity parallel each other fairly closely (52). 11β-HSD activity was detected in both human cerebellum and hippocampus, with higher activity in cerebellar homogenates, suggesting that this may also be the case in humans. Although the function of 11β-HSD1, and indeed of glucocorticoids, in the cerebellum is unclear, this structure is known to be involved in cognition in humans (55, 56). The hippocampus and frontal cortex are more clearly related to cognition and here glucocorticoids play well defined roles modulating learning and memory (57). Thus, 11β-HSD1 mRNA and activity are expressed in key human CNS regions associated with cognitive processes. But does it have a function?
Carbenoxolone, a widely used 11β-HSD inhibitor, effectively inhibits both 11β-HSD2 and 11β-HSD1 in the periphery in humans (28, 36), as well as 11β-HSD1 in the rodent hippocampus in vitro and in vivo (35, 38). Carbenoxolone also inhibits other dehydrogenases and interferes with gap junction formation, but such actions require concentrations (10-5 to 10-4 M) several orders of magnitude higher than needed to inhibit 11β-HSD (typically 10-9 M in vitro) and are unlikely to have been achieved in this in vivo study (30). Amiloride was used to block the potential consequences of renal 11β-HSD2 inhibition [mineralocorticoid excess, hypertension, and hypokalemia (36)], which might perhaps have adversely influenced cognition (54). Amiloride therapy seemed successful as judged by the lack of substantial changes in blood pressure and electrolytes in both studies. Short-term carbenoxolone administration (1 week) has also been reported to enhance hepatic insulin sensitivity in healthy volunteers (28) and patients with type 2 diabetes (30), effects mediated by inhibition of 11β-HSD1 in liver and adipose tissue. Although variations of long-term glycemic control, as assessed for example by HbA1c levels, associate with cognitive performance in cross-sectional studies of both diabetic (51) and even nondiabetic elderly subjects (58, 59), in the current study in patients with diabetes, carbenoxolone did not alter HbA1c. 11β-HSD1 null mice have modestly elevated plasma glucocorticoid levels perhaps due to altered feedback control (60). However, carbenoxolone did not alter plasma cortisol, at least during the morning, in either study. Against this background, improvements in cognitive function with carbenoxolone cannot readily be attributed to effects outside the CNS or to gross changes in circulating glucocorticoid levels. Within the CNS, 11β-HSD1 is by far the most abundant isozyme expressed so that effects of carbenoxolone are unlikely to reflect inhibition of 11β-HSD2.
Carbenoxolone produced significant improvement in verbal fluency, which is variously described as a test of executive function and of semantic memory (61), in healthy unmedicated elderly men and in verbal memory in patients with type 2 diabetes. Among the determinants of verbal fluency scores are verbal long-term memory, auditory attention, and word knowledge (62). The neural substrates of verbal fluency include the medial temporal (63) and frontal lobes (64), loci where 11β-HSD1 is expressed. The diabetic patients were younger than the healthy elderly subjects and overall showed better performance. Patients with type 2 diabetes are prone to a selective deficit in verbal memory unassociated with dementia (51). In the diabetic group tested here, carbenoxolone specifically improved word list recall, which was found to be the most sensitive indicator of mild cognitive impairment in a large investigation of older people with memory complaints (65); the current results suggest that this domain may be particularly amenable to anti-glucocorticoid therapy.
The human CNS, including the hippocampus, highly expresses corticosteroid receptors (7), and glucocorticoids alter cognitive performance in humans, effects amplified by age (66-69). Here, we found carbenoxolone-induced cognitive improvements in groups of healthy and diabetic older people. The improvements were not on the same tests, but both tests involved aspects of verbal and semantic memory. However, each study was small and thus prone to type II errors, and both studies showed improvements in aspects of verbal function. Although our data clearly require extension to larger and more varied samples, it is tempting to suggest that carbenoxolone directly acts on CNS cells, reducing intraneuronal glucocorticoid levels and improving cognitive functions as in 11β-HSD1 knockout mice (37). The effect magnitude of up to 0.5 SD seems to be a worthwhile, clinically significant attainment. 11β-HSD1 may therefore afford a mechanistically tractable new therapeutic target to prevent or ameliorate age-associated cognitive dysfunction in healthy elderly subjects and in patients with type 2 diabetes. Whether or not carbenoxolone has beneficial effects in subjects with more florid cognitive dysfunction remains untested. The reexploitation of an old drug, carbenoxolone (with amiloride), may merit further evaluation until more selective inhibitors for brain 11β-HSD1 are developed.
Acknowledgments
We thank Prof. Jeanne Bell and the Edinburgh Brain Bank for postmortem samples. The 11β-HSD1 and 11β-HSD2 cDNA plasmids used in this study were kindly supplied by Drs. Perin White and Roger Brown, respectively. This work was supported by a Wellcome Trust Program Grant (to J.R.S. and B.R.W.) and grants from Catalyst Biomedica (to B.R.W. and J.R.S.) and the Scottish Hospitals Endowments Research Trust (to A.M.J.M.). B.R.W. is a British Heart Foundation Senior Research Fellow. J.L.W.Y. is the Alzheimer's Research Trust Carter Research Fellow. I.J.D. holds a Royal Society-Wolfson Research Merit Award.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 11βHSD, 11β-hydroxysteroid dehydrogenase; HbA1c, glycosylated hemoglobin.
See Commentary on page 6329.
References
- 1.Ritchie, K. & Touchon, J. (2000) Lancet 355, 225-228. [DOI] [PubMed] [Google Scholar]
- 2.Fillit, H., Butler, R., O'Connell, A., Albert, M., Birren, J., Cotman, C., Greenough, W., Gold, P., Kramer, A., Kuller, L., Perls, T., Sahagan, B. & Tully, T. (2002) Mayo Clinic Proc. 77, 681-696. [DOI] [PubMed] [Google Scholar]
- 3.National-Research-Council (2000) The Aging Mind: Opportunities in Cognitive Research (Natl. Acad. Press, Washington, DC). [PubMed]
- 4.Lupien, S. J., deLeon, M., deSanti, S., Convit, A., Tarshish, C., Thakur, M., McEwen, B. S., Hauger, R. L. & Meaney, M. J. (1998) Nat. Neurosci. 1, 69-73. [DOI] [PubMed] [Google Scholar]
- 5.Seckl, J. R. & Olsson, T. (1995) J. Endocrinol. 145, 201-211. [DOI] [PubMed] [Google Scholar]
- 6.Reul, J. M. H. M. & de Kloet, E. R. (1985) Endocrinology 117, 2505-2511. [DOI] [PubMed] [Google Scholar]
- 7.Seckl, J. R., Dickson, K. L., Yates, C. & Fink, G. (1991) Brain Res. 561, 332-337. [DOI] [PubMed] [Google Scholar]
- 8.Landfield, P. W., Waymire, J. & Lynch, G. (1978) Science 202, 1098-1102. [DOI] [PubMed] [Google Scholar]
- 9.Meaney, M. J., O'Donnell, D., Rowe, W., Tannenbaum, B., Steverman, A., Walker, M., Nair, N. P. V. & Lupien, S. (1995) Exp. Gerontol. 30, 229-251. [DOI] [PubMed] [Google Scholar]
- 10.McEwen, B. S. & Sapolsky, R. M. (1995) Curr. Opin. Neurobiol. 5, 205-216. [DOI] [PubMed] [Google Scholar]
- 11.McEwen, B. S. (1999) Front. Neuroendocrinol. 20, 49-70. [DOI] [PubMed] [Google Scholar]
- 12.Newcomer, J. W., Craft, S., Hershey, T., Askin, K. & Bardgett, M. E. (1994) J. Neurosci. 14, 2047-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Leon, M. J., McRae, T., Tsai, J. R., George, A. E., Marcus, D. L., Freedman, M., Wolf, A. P. & McEwen, B. S. (1988) Lancet ii, 391-392. [DOI] [PubMed] [Google Scholar]
- 14.Landfield, P. W., Baskin, R. K. & Pitler, T. A. (1981) Science 214, 581-584. [DOI] [PubMed] [Google Scholar]
- 15.Meaney, M. J., Aitken, D. H., van Berkel, C., Bhatnagar, S. & Sapolsky, R. M. (1988) Science 239, 766-768. [DOI] [PubMed] [Google Scholar]
- 16.Lamberts, S. W. J., Koper, J. W. & Dejong, F. H. (1991) J. Clin. Endocrinol. Metab. 73, 187-191. [DOI] [PubMed] [Google Scholar]
- 17.Seckl, J. R. & Walker, B. R. (2001) Endocrinology 142, 1371-1376. [DOI] [PubMed] [Google Scholar]
- 18.White, P. C., Mune, T. & Agarwal, A. K. (1997) Endocr. Rev. 18, 135-156. [DOI] [PubMed] [Google Scholar]
- 19.Albiston, A. L., Obeyesekere, V. R., Smith, R. E. & Krozowski, Z. S. (1994) Mol. Cell. Endocrinol. 105, R11-R17. [DOI] [PubMed] [Google Scholar]
- 20.Brown, R. W., Kotolevtsev, Y., Leckie, C., Lindsay, R. S., Lyons, V., Murad, P., Mullins, J. J., Chapman, K. E., Edwards, C. R. W. & Seckl, J. R. (1996) Biochem. J. 313, 1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakshmi, V. & Monder, C. (1988) Endocrinology 123, 2390-2398. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal, A. K., Monder, C., Eckstein, B. & White, P. C. (1989) J. Biol. Chem. 264, 18939-18943. [PubMed] [Google Scholar]
- 23.Bujalska, I., Kumar, S. & Stewart, P. M. (1997) Lancet 349, 1210-1213. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson, P. M., Chapman, K. E., Edwards, C. R. W. & Seckl, J. R. (1995) Endocrinology 136, 4754-4761. [DOI] [PubMed] [Google Scholar]
- 25.Hundertmark, S., Buhler, H., Ragosch, V., Dinkelborg, L., Arabin, B. & Weitzel, H. K. (1995) Endocrinology 136, 2573-2578. [DOI] [PubMed] [Google Scholar]
- 26.Low, S. C., Chapman, K. E., Edwards, C. R. W. & Seckl, J. R. (1994) J. Mol. Endocrinol. 13, 167-174. [DOI] [PubMed] [Google Scholar]
- 27.Jamieson, P. M., Chapman, K. E., Walker, B. R. & Seckl, J. R. (2000) J. Endocrinol. 165, 685-692. [DOI] [PubMed] [Google Scholar]
- 28.Walker, B. R., Connacher, A. A., Lindsay, R. M., Webb, D. J. & Edwards, C. R. W. (1995) J. Clin. Endocrinol. Metab. 80, 3155-3159. [DOI] [PubMed] [Google Scholar]
- 29.Alberts, P., Engblom, L., Edling, N., Forsgren, M., Klingstrom, G., Larsson, C., Ronquist-Nii, Y., Ohman, B. & Abrahmsen, L. (2002) Diabetologia 45, 1528-1532. [DOI] [PubMed] [Google Scholar]
- 30.Andrews, R. C., Rooyackers, O. & Walker, B. R. (2003) J. Clin. Endocrinol. Metab. 88, 285-291. [DOI] [PubMed] [Google Scholar]
- 31.Moisan, M.-P., Seckl, J. R. & Edwards, C. R. W. (1990) Endocrinology 127, 1450-1455. [DOI] [PubMed] [Google Scholar]
- 32.Lakshmi, V., Sakai, R. R., McEwen, B. S. & Monder, C. (1991) Endocrinology 128, 1741-1748. [DOI] [PubMed] [Google Scholar]
- 33.Sakai, R. R., Lakshmi, V., Monder, C. & McEwen, B. S. (1992) J. Neuroendocrinol. 4, 101-106. [DOI] [PubMed] [Google Scholar]
- 34.Roland, B. L., Li, K. X. Z. & Funder, J. W. (1995) Endocrinology 136, 4697-4700. [DOI] [PubMed] [Google Scholar]
- 35.Rajan, V., Edwards, C. R. W. & Seckl, J. R. (1996) J. Neuroscience 16, 65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart, P. M., Wallace, A. M., Atherden, S. M., Shearing, C. H. & Edwards, C. R. W. (1990) Clin. Sci. 78, 49-54. [DOI] [PubMed] [Google Scholar]
- 37.Yau, J. L. W., Noble, J., Kenyon, C. J., Hibberd, C., Kotelevtsev, Y., Mullins, J. J. & Seckl, J. R. (2001) Proc. Natl. Acad. Sci. USA 98, 4716-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jellinck, P. H., Monder, C., McEwen, B. S. & Sakai, R. R. (1993) J. Steroid Biochem. Mol. Biol. 46, 209-213. [DOI] [PubMed] [Google Scholar]
- 39.Tannin, G. M., Agarwal, A. K., Monder, C., New, M. I. & White, P. C. (1991) J. Biol. Chem. 266, 16653-16658. [PubMed] [Google Scholar]
- 40.Yau, J. L. W., Olsson, T., Noble, J. & Seckl, J. R. (1999) Mol. Brain Res. 70, 282-287. [DOI] [PubMed] [Google Scholar]
- 41.Brown, R. W., Chapman, K. E., Edwards, C. R. W. & Seckl, J. R. (1993) Endocrinology 132, 2614-2621. [DOI] [PubMed] [Google Scholar]
- 42.Lezak, M. (1995) Neuropsychological Assessment (Oxford Univ. Press, Oxford).
- 43.MacLullich, A. M. J., Ferguson, K. J., Deary, I. J., Seckl, J. R., Starr, J. M. & Wardlaw, J. M. (2002) Neurology 59, 169-174. [DOI] [PubMed] [Google Scholar]
- 44.Raven, J., Court, J. & Raven, J. (1977) Manual for Raven's Progressive Matrices and Vocabulary Scales (H. K. Lewis, London).
- 45.Wechsler, D. (1987) Wechsler Memory Scale-Revised (WMS-R) (Psychol. Corp., New York).
- 46.Wechsler, D. (1981) Manual of the Wechsler Adult Intelligence Scale-Revised (Psychol. Corp., New York).
- 47.Nelson, H. & Willison, J. (1991) NART Test Manual (Part II) (NFER-Nelson, New York).
- 48.Matthews, G. (1990) Br. J. Psychol. 81, 17-42. [Google Scholar]
- 49.Zigmond, A. & Snaith, R. (1983) Acta Psychiatr. Scand. 67, 361-370. [DOI] [PubMed] [Google Scholar]
- 50.Andrews, R. C., Herlihy, O., Livingstone, D. E. W., Andrew, R. & Walker, B. R. (2002) J. Clin. Endocrinol. Metab. 87, 5587-5593. [DOI] [PubMed] [Google Scholar]
- 51.Strachan, M. W. J., Deary, I. J., Ewing, F. M. E. & Frier, B. M. (1997) Diabetes Care 20, 438-445. [DOI] [PubMed] [Google Scholar]
- 52.Moisan, M.-P., Seckl, J. R., Brett, L. P., Monder, C., Agarwal, A. K., White, P. C. & Edwards, C. R. W. (1990) J. Neuroendocrinol. 2, 853-858. [DOI] [PubMed] [Google Scholar]
- 53.Robson, A. C., Leckie, C., Seckl, J. R. & Holmes, M. C. (1998) Mol. Brain Res. 61, 1-10. [DOI] [PubMed] [Google Scholar]
- 54.Seckl, J. R. (1997) Front. Neuroendocrinol. 18, 49-99. [DOI] [PubMed] [Google Scholar]
- 55.Malm, J., Kristensen, B., Karlsson, T., Carlberg, B., Fagerlund, M. & Olsson, T. (1998) Neurology 51, 433-440. [DOI] [PubMed] [Google Scholar]
- 56.Iacoboni, M. (2001) Nat. Neurosci. 4, 555-556. [DOI] [PubMed] [Google Scholar]
- 57.McEwen, B. S., de Leon, M. J., Lupien, S. J. & Meaney, M. J. (1999) Trends Endocrinol. Metab. 10, 92-96. [DOI] [PubMed] [Google Scholar]
- 58.Convit, A., Wolf, O. T., Tarshish, C. & de Leon, M. J. (2003) Proc. Natl. Acad. Sci USA 100, 2019-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacLullich, A., Deary, I., Starr, J., Walker, B. & Seckl, J. (2003) J. Am. Geriatr. Soc., in press. [DOI] [PubMed]
- 60.Harris, H. J., Kotelevtsev, Y., Mullins, J. J., Seckl, J. R. & Holmes, M. C. (2001) Endocrinology 142, 114-120. [DOI] [PubMed] [Google Scholar]
- 61.Wilson, R., Beckett, L., Barnes, L., Schneider, J., Bach, J., Evans, A. & Bennett, D. (2002) Psychol. Aging 17, 179-193. [PubMed] [Google Scholar]
- 62.Ruff, R., Light, R., Parker, S. & Levin, H. (1997) Brain Lang. 57, 394-405. [DOI] [PubMed] [Google Scholar]
- 63.Pihlajamaki, M., Tanila, H., Hanninen, T., Kononen, M., Laakso, M., Partanen, K., Soininen, H. & Aronen, H. (2000) Ann. Neurol. 47, 470-476. [PubMed] [Google Scholar]
- 64.Grafman, J. & Litvan, I. (1999) Lancet 354, 1921-1923. [DOI] [PubMed] [Google Scholar]
- 65.Lines, C., McCarroll, K., Lipton, R. & Block, G. (2003) Neurology 60, 261-266. [DOI] [PubMed] [Google Scholar]
- 66.Lupien, S. J., Nair, N. P. V., Briere, S., Maheu, F., Tu, M. T., Lemay, M., McEwen, B. S. & Meaney, M. J. (1999) Rev. Neurosci. 10, 117-139. [DOI] [PubMed] [Google Scholar]
- 67.De Leon, M. J. & Rusinek Mc, R. H. (1997) J. Clin. Endocrinol. Metab. 82, 3251-3259. [DOI] [PubMed] [Google Scholar]
- 68.Starkman, M. N., Giordani, B., Berent, S., Schork, M. A. & Schteingart, D. E. (2001) Psychosom. Med. 63, 985-993. [DOI] [PubMed] [Google Scholar]
- 69.Wolf, O. T., Convit, A., McHugh, P. F., Kandil, E., Thorn, E. L., De Santi, S., McEwen, B. S. & de Leon, M. J. (2001) Behav. Neurosci. 115, 1002-1011. [DOI] [PubMed] [Google Scholar]