Abstract
Background
Bedside ultrasonography in the diagnosis of pneumothorax has been well described in emergency and trauma medicine literature. Its role in detection of iatrogenic pneumothoraces has not been studied. We describe the performance of bedside ultrasonography in detection of procedure related pneumothoraces and highlight some limitations.
Methods
185 patients underwent thoracentesis (n=60), transbronchial biopsy (n=48), CT-guided lung biopsy (n=76), and CT-guided cryoablation of a lung mass (n=1). Bedside transthoracic ultrasound examination and post-procedure chest radiograph were performed in all patients. Patients in whom pleural surface was not well imaged with ultrasound were said to have a limited exam. Chest x-ray was the standard for diagnosing pneumothorax.
Results
Chest x-ray detected pneumothorax in 8/185 patients (4.0%). Ultrasound diagnosed pneumothorax in seven of these patients. Sensitivity, specificity and diagnostic accuracy were 88%, 97% and 97%, respectively. Limited quality ultrasound examinations due to pre-existing lung disease was seen in 43/185 patients. The positive and negative likelihood ratios for patients with adequate scans were 55 and 0.17, respectively. Likelihood ratio for patients with limited quality scan was 1.08.
Conclusions
Bedside chest ultrasonography, in the presence of good quality scan, is a valuable tool in the evaluation of post procedure pneumothorax. Patients with preexisting lung disease in whom the quality of ultrasound examination is limited should be studied with a chest x-ray.
Correspondence information: Abraham Sanders, MD Department of Pulmonary and Critical Care Medicine New York Presbyterian Hospital/Weill Cornell Medical Center 1305 York Avenue New York, NY 10021 abs2001@med.cornell.edu
INTRODUCTION
Iatrogenic pneumothorax is a complication of various procedures involving bronchoscopic and percutaneous lung biopsies. Rates of iatrogenic pneumothorax depend on the type of procedure, operator experience, and presence of underlying lung disease. For CT guided transthoracic needle biopsy, the observed rate of pneumothorax is 15-20%1 and up to 50% if aerated lung is traversed during needle penetration. Ultrasound guided thoracentesis and fluoroscopic transbronchial needle biopsies have reported rates of pneumothorax of 2-4%.2-4
Thoracic ultrasonography has been used in the diagnosis of pneumothorax. Early studies in trauma patients have shown ultrasound to be more sensitive and specific than portable antero-posterior (AP) chest x-ray.5-7 Many emergency rooms perform thoracic ultrasound together with the Focused Assessment and Sonography for Trauma (FAST) exam before ordering an initial screening chest x-ray (CXR).8,9 Ultrasonography has also been used to diagnose pneumothorax in critically ill patients.10
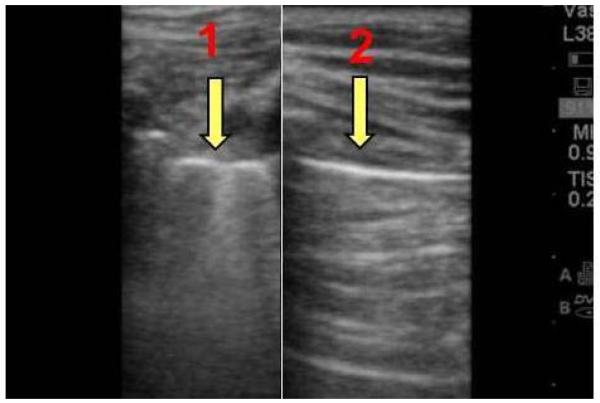
Imaging aerated lungs produces a series of artifacts, many of which have been previously defined by Lichtenstein et al.11 Vertical artifacts, known as comet tails or “B lines”, are due to a difference in acoustic impedance between two structures, such as visceral pleural and aerated lung. They appear as vertical lines that originate from the pleural surface and extend to the edge of the screen. Reverberation artifacts, known as “A lines”, are seen in the presence of air within pleural space, and appear as equidistantly spaced horizontal lines below the pleura (Fig. 1). Lung sliding is the back and forth movement of a pleural line that occurs during a respiratory movement. The presence of lung sliding and/or comet tails reliably excludes a pneumothorax at the site of examination.11 Absence of lung sliding is seen in pneumothorax but may also be present in other conditions such as pleural adhesions and bullous emphysema.11 Therefore, when used in isolation, absence of lung sliding cannot definitively confirm a pneumothorax. “Lung point”, or a transition point between aerated lung and pneumothorax, confirms the diagnosis of pneumothorax at the examination site.
Figure 1.
Ultrasound image comparing normally aerated lung with pneumothrax
Experience with ultrasonography in the identification of iatrogenic pneumothorax is limited12,13. This study was conducted to determine the diagnostic accuracy, advantages as well as limitations of ultrasound in the evaluation of iatrogenic pneumothorax in patients undergoing thoracentesis, CT guided transthoracic lung biopsy, and transbronchial lung biopsy.
MATERIALS AND METHODS
Study Design
185 patients were enrolled in an observational cross-sectional study between August 2011 and March 2012. Institutional Board Review approval (protocol # 1107011827) was obtained prior to the study commencement. Patients were 18 years or older and scheduled to undergo a thoracentesis, CT guided needle lung biopsy or transbronchial lung biopsy. Patients were excluded if they developed an intraoperative pneumothorax requiring manual aspiration or chest tube drainage prior to ultrasound examination, declined to provide informed consent, to undergo an ultrasound examination or chest x-ray, or were pregnant. After informed consent, every participant underwent a pre-procedure thoracic ultrasound examination with a 7.5 MHz linear vascular transducer (Sonosite M Turbo, Bothell, WA.) Most were supine during the examination, others were semi-upright, sitting or standing. The pleural surface was identified as a hyperechoic line between two rib shadows. Images were acquired in a longitudinal scanning plane with the transducer indicator in a cephalad position along the mid-clavicular, anterior-axillary, mid-axillary, and posterior-axillary lines. Each line was scanned for 10 seconds, allowing a complete examination of the thorax to be performed in 40 seconds. The scan was deemed adequate if the pleural surface with lung sliding was imaged in at least 3 consecutive intercostal spaces along each line of scanning. Ultrasound scans that did not meet this criteria were deemed limited. All ultrasound examinations were performed and interpreted by the clinical investigator at the patient’s bedside and then saved as a video file for later review by an attending radiologist. The clinical investigator was a second year pulmonary fellow who underwent a 2 hour introductory thoracic ultrasound course at the start of his fellowship and routinely used ultrasound for central line placement. The co-interpreter was a general board certified radiologist with no specialty training in thoracic ultrasonography. Both investigators were blinded to the chest x-ray and computed tomography results. All patients received a post procedure ultrasound examination. Pneumothorax was excluded if either the lung sliding or comet tail artifacts were visualized in each intercostal space examined. The following criteria were used to establish the diagnosis of pneumothorax on ultrasound: loss of both lung sliding and comet tails, with or without the appearance of A-lines or identification of a lung point. Neither the clinical investigator nor the radiologist co-interpreting the ultrasound was present during the procedures. Patient’s health care providers were not allowed to communicate any clinically relevant information to the investigator and the latter was not permitted to interview or examine the patient. The results of a positive post procedure ultrasound scan for a pneumothorax were communicated to patient’s healthcare providers. Each patient underwent a post procedure anterior-posterior (AP) chest radiograph that was ordered emergently. Chest x-ray was the standard for diagnosis of pneumothorax. Both chest x-ray and ultrasound were performed within 60 minutes of completion of the procedure. All chest radiographs were uploaded into the computer database system in a digital format and interpreted by board certified radiologists who were unaware of the ongoing study.
Description of Procedures
CT-guided Transthoracic Needle Biopsy
Most CT guided lung biopsies biopsies were performed using local anesthetic. An outer 19-gauge cannula (Cardinal Health Temno Biopsy System, Dublin, OH) was advanced into the lesion under CT guidance. Through this outer cannula, fine needle aspirates using a 22-gauge needle (Cardinal Health BD Wescott and Cardinal Health BD Chiba, Dublin, OH) were obtained, followed by a limited CT of the biopsy site. Patients recovered in a position dependent to the biopsy tract for two hours. Post procedure chest x-ray was obtained during the recovery period.
Cryoablation
Cryoablation was performed under general anesthesia. A 17gauge cryoprobe (Endocare Cryocare CS, Irvine,CA.) was advanced and positioned within the lesion under CT guidance. Two cycles or freeze and thaw, lasting 8 and 7 minutes, respectively, were performed utilizing manufacturer recommendations. A post procedure portable chest radiograph was also performed during the recovery.
Transbronchial Lung Biopsy
Transbronchial biopsies were performed using an Olympus flexible bronchoscope (Olympus Videobronchoscope Evis Exera II BF Type Q180, Melville, NY, USA) under moderate sedation. All procedures were performed by pulmonary fellows under direct supervision of a pulmonary attending. Transbronchial biopsy forceps (Boston Scientific Radial Edge, Global Park Heredia, CR) were advanced to the target lesion under fluoroscopic guidance. A chest x-ray was obtained at the conclusion of the procedure.
Thoracentesis
Thoracentesis was performed by medical housestaff and/or pulmonary fellows, without direct pulmonary attending supervision. Ultrasound was used in all cases to identify the pleural effusion and nearby anatomical structures. Thoracentesis needle (Arrow-Clarke Pleura-Seal Thoracentesis Kit, Reading PA) was advanced into the pleural space and pleural effusion was drained using a manual syringe pump method. A chest x-ray was obtained at the conclusion of the procedure.
Statistical Methods
A sample size of 160 produced a two-sided 95% confidence interval with a width equal to 0.100 when the sample specificity was 0.90. The sample size calculation was computed using a Wilson Score confidence interval with continuity correction. Basic descriptive statistics were reported. Cohen kappa statistics were computed with corresponding p-values to evaluate the inter-observer agreement of the readers. The sensitivity, specificity and diagnosis accuracy of ultrasound readings were calculated with 95% exact binomial confidence intervals (Collett 1999). Likelihood ratios were also computed. All data was analyzed with a statistical software package.14
RESULTS
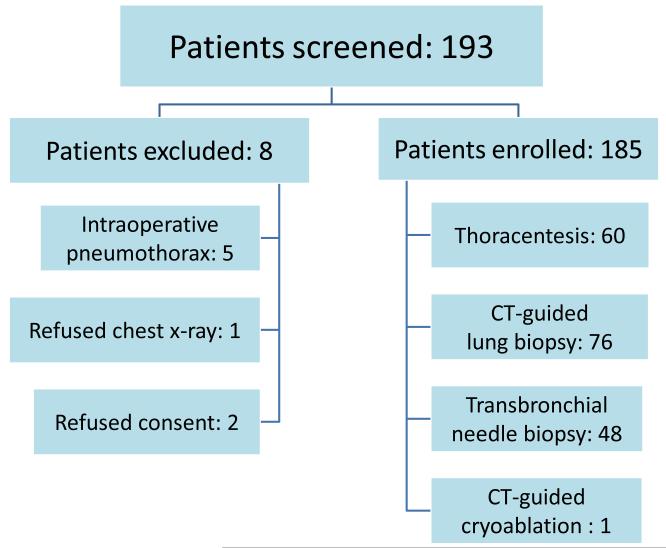
193 patients were screened and 185 were enrolled into the study. There were 91 men and 94 women. The average age was 67 (range 23-92). Reasons for exclusions and performed procedures are summarized in Figure 2. The average time between ordering a chest film and obtaining an image for interpretation was 49 minutes. Four percent of patients (8/185) were diagnosed with a pneumothorax based on a chest x-ray. Seven pneumothoraces occurred in CT-guided lung biopsy group (Table 1). Ultrasound correctly identified 7/8 pneumothoraces when interpreted by clinical investigator and 6/8 pneumothoraces when interpreted by radiologist. The diagnostic performance of ultrasound for each operator is summarized in Table 2. The clinical investigator and the radiologist had an excellent agreement in the interpretation of ultrasound scans (k=0.8± 0.07, p value≤0.001). Reduced lung sliding was a common reason for limited quality ultrasound scans and a frequent source of discordance between chest x-ray and ultrasound. (Table 3). One patient with false positive ultrasound scan had an occult pneumothorax confirmed with chest CT and another patient developed a large pneumothorax requiring drainage after an initial chest x-ray was negative. Patients with a false negative ultrasound scan had clinically insignificant pneumothoraces at the time of ultrasound examination, although on repeat imaging one patient developed an enlarging pneumothorax that required chest tube placement. Limited quality ultrasound examinations were present in 43/185 (23%) of patients (Table 4.) Common causes for limited quality scans were reduced lung sliding from underlying lung disease such as bullous emphysema, and previous lung surgeries and radiation treatments resulting in pleural adhesions. Ultrasound showed high diagnostic accuracy in a setting of an adequate scan (Table 5.) Ultrasound offered no diagnostic value in patients with limited quality examination. Chest x-ray excluded a pneumothorax in all patients with false positive ultrasound scans. No invasive procedures were performed solely on the basis of ultrasound examination.
Table 1.
Prevalence of Pneumothoraces, Diagnosed on Chest X-ray, Among Various Procedure Groups
| Procedure Group | Sample Size (n) | Prevalence of pneumothorax – no. (%) |
|---|---|---|
|
CT-guided
transthoracic lung biopsy1,2 |
77 | 7 (9%) |
|
Transbronchial
needle lung biopsy3 |
48 | 1 (2%) |
| Thoracentesis | 60 | 0 (0%) |
| Total | 185 | 8 (4%) |
Includes one patient with CT-guided cryoablation.
Four patients developed intra-operative pneumothorax requiring chest tube drainage and were excluded from the study.
One patient developed intra-operative pneumothorax requiring chest tube drainage and was excluded from the study
Table 2.
Diagnostic Performance of Ultrasound in Detection of Pneumothorax
| Operator | Sensitivity, % [95% CI] | Specificity, % [95% CI] | Diagnostic Accuracy % [95% CI] |
|---|---|---|---|
| Clinical Investigator | 88 [0.35-0.90] |
97 [0.93-0.98] |
97 [0.93-0.98] |
| Radiologist | 75 [0.35-0.90] |
97 [0.93-0.98] |
96 [0.93-0.98] |
Table 3.
Etiologies of Disagreements Between Chest X-ray and Ultrasound
| Disagreement | Clinician | Radiologist | Ultrasound Limitation |
Etiology / Comments1 |
|
|---|---|---|---|---|---|
| US | CXR | ||||
| Positive | Negative | X | X | Reduced lung sliding | Bullous emphysema |
| Positive | Negative | X | X | None | Timing of scan2 |
| Positive | Negative3 | X | X | Reduced lung sliding | Pleural adhesions |
| Positive | Negative | X | Reduced lung sliding | Pleural adhesions | |
| Positive | Negative | X | None | None | |
| Positive | Negative | X | Reduced lung sliding | Pleural adhesions | |
| Positive | Negative | X | None | None | |
| Negative | Positive | X | X | None | None |
| Negative | Positive | X | None | None | |
Etiologies for reduced lung sliding inferred based on patient’s medical history.
Pneumothorax was seen on ultrasound obtained 30 minutes after the initial negative chest x-ray. A significant pneumothorax requiring chest tube drainage was confirmed on a repeat chest x-ray.
Occult pneumothorax confirmed on chest computed tomography.
US = ultrasound. CXR = chest x-ray
Table 4.
Etiology of Limited Quality Ultrasound Examinations
| Etiology | N |
|---|---|
| Reduced lung sliding1 | 18 |
| Pleural effusions | 13 |
| Obesity | 3 |
| Pacemaker | 3 |
| Tunneled catheter | 3 |
| Breast implants | 3 |
| Total | 43 |
One patient had bullous emphysema, the remaining patients most likely had pleural adhesions based on their medical history.
Table 5.
Diagnostic Performance of Ultrasound When Performed by Clinical Investigator in Detection of Pneumothorax in Patients with Adequate and Limited Sonographic Examinations
| Chest X-ray | ||||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | Likelihood ratio | 95% Confidence Interval |
||
|
Adequate Limited
Ultrasound |
Adequate
Positive |
5 | 2 | 142 | 55 | (0.45-0.85) |
|
Adequate
Negative |
1 | 134 | 0.17 | (0.36-0.52) | ||
| Limited | 2 | 41 | 43 | 1.08 | (0.85-0.90) | |
| Total | 8 | 177 | 185 | |||
DISCUSSION
Most studies performed in emergency medicine and trauma patients report sensitivity and specificity of ultrasound in detection of pneumothorax to be above 90%10. Our study differs from those published in several important ways. Many previous studies used chest CT as the standard for diagnosing pneumothorax 7,10,15. Given the cost and exposure to ionizing radiation, we chose to compare ultrasound with a chest x-ray. In contrast to earlier studies reporting on patients with traumatic pneumothorax, patients in our study had significant underlying lung disease, as evidenced by the nature of performed procedures and a high prevalence rate of limited quality ultrasound scans.10 As shown in table 4, the most common cause of limited scans was reduced lung sliding from pleural adhesions due to prior thoracic surgeries and radiation therapies. The use of ultrasound for diagnosis of pneumothorax in such patient population may be non-diagnostic and obtaining a baseline pre-procedure scan may identify patients in whom chest x-ray should be the preferred imaging modality. Unlike earlier studies in which ultrasound examinations were performed by emergency medicine physicians and radiologists, all ultrasound scans in our study were performed by a pulmonary fellow.7,10 Finally, in our study the entire hemithorax was examined with ultrasound. This is in contrast to previous studies where a more limited ultrasound examination was performed7. It is unclear whether a comprehensive examination is necessary, although we hypothesize that it may improve diagnostic accuracy by detecting occult pneumothoraces. To our knowledge there have been no studies comparing various scanning techniques on the diagnostic accuracy of ultrasound, and since we did not detect any pneumothoraces in the posterior axillary line, we suggest that it is not necessary to routinely include this region of the thorax in the ultrasonographic examination.
Since loss of lung sliding following a procedure suggests procedure-related pneumothorax, performing a pre-procedure scan allows the clinician to diagnose pneumothorax with greater confidence. Pre-procedure scan can also identify patients in whom ultrasound may be of limited diagnostic value due to reduced lung sliding. Although previously suggested by Koenig et al16, to our knowledge, we are the first to systematically include pre-procedure ultrasound examination in the evaluation of pneumothorax.
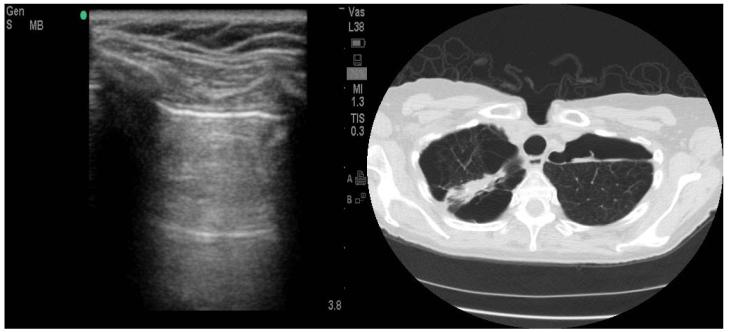
Out of 5 patients falsely diagnosed with pneumothorax by the clinician, two had reduced lung sliding due to pleural adhesions from prior lung surgeries and one had bullous emphysema. (Fig.3). These disease states have been previously demonstrated to reduce lung sliding and result in false positive ultrasound scans11. Chest CT was available in 3 patients with false positive ultrasound scan and confirmed an occult pneumothorax in one. Since CT was not performed every time there was discrepancy between chest x-ray and ultrasound, the true prevalence of occult pneumothoraces is unknown.
Figure 3.
Bullous Emphysema Resulting in False Positive Ultrasound Scan
Operator experience is an important determinant of the diagnostic accuracy of the ultrasound. In our study, all scans were acquired by a second year pulmonary fellow who underwent a two hour thoracic ultrasound course. Studies show that intensive care unit residents with no prior ultrasound experience can reliably perform thoracic ultrasonography for detection of pneumothorax after 2 hours of training.17
Although neither the radiologist nor the clinical investigator were permitted to receive any clinical information, it is conceivable that by being present at the bedside and physically seeing the patient, the investigator was clinically biased. While low prevalence rate of pneumothoraces makes direct comparison between the radiologist and the clinical investigator difficult, we hypothesize that including clinical suspicion in the evaluation of pneumothorax may improve the diagnostic accuracy of ultrasound.
We noted several advantages that ultrasound offers over chest x-ray. The first is that ultrasound exam can be performed more quickly than a chest x-ray. Chest x-rays were available for review an average 48 minutes following the procedure. A second advantage is the lack of ionizing radiation. One disadvantage of ultrasound is lack of accuracy in the evaluation of pneumothorax size.18,19 At this time we recommend following pneumothorax size with serial chest x-rays. A second disadvantage is the need for a trained clinician. A third disadvantage is the cost of purchasing and maintaining the equipment. However, many institutions are already using ultrasound for thoracenthesis and central line placement, and will bear no additional cost by including an ultrasound in the evaluation of post procedure pneumothorax.
This study has several limitations. First, the diagnostic accuracy seen in this study reflects the skill of a single clinical investigator. Studies in which ultrasound exams are performed by several clinicians are needed to determine the inter-operator variability and reproducibility of this data. A second limitation is the low prevalence rate of pneumothoraces.
Given low incidence of pneumothoraces following transbronchial biopsy and thoracentesis, several authors recommend against performing routine post procedure chest x-ray in the absence of clinical symptoms. 20,21 Such recommendations have not been extended for patients undergoing CT-guided needle lung biopsy, where the incidence of pneumothorax is higher. None of our study patients who developed post procedure pneumothorax had clinical symptoms at the time of diagnosis. We therefore recommend that every patient having undergone a lung biopsy or a thoracentesis be evaluated for post procedure pneumothorax using an ultrasound as the first imaging modality. In the presence of good quality ultrasound scan obtaining a chest film becomes unnecessary. Patients with reduced quality ultrasound examinations should be studied with a chest x-ray. Performing a pre-procedure ultrasound scan can identify patients in whom ultrasound may not be diagnostic. We believe that this approach will greatly reduce the number of unnecessary chest films.
CONLCUSION
Bedside ultrasound is a valuable imaging tool that can reliably exclude post procedure pneumothorax provided that pleural surface is adequately imaged and lung sliding is present on a pre-procedure scan. Patients in whom ultrasound examination is limited should be studied with a chest x-ray.
ACKNOWLEDGEMENTS
This research was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-RR024996) Sandra M. Hurtado Rúa, PhD Division of Biostatistics and Epidemiology Department of Public Health Weill Medical College of Cornell University --------Contributed to the design and statistical analysis of data.
ABBREVIATION LIST
- US
ultrasound
- CXR
chest x-ray
- CT
computed tomography
- FAST
focused assessment and sonography for trauma
- AP
antero-posterior
- BMI
body mass index
- mAs
milliampere second
- kVp
peak kilovoltage
- PACS
Picture Archiving and Communication System
Footnotes
COI Disclosure – Above authors have no conflicts of interest to disclose
Study approved by Institutional Review of Board Committee from August 22, 2011-August 7, 2012. Protocol number 1107011827
Author contributions: Dr Sanders had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Shostak: contributed to study design, data collection, analyses, and manuscript writing.
Dr Brylka: contributed to study design, data collection, analyses, and manuscript writing.
Dr Krepp: contributed to study design, data collection, analyses, and manuscript writing.
Dr Pua: contributed to study design, data collection, analyses, and manuscript writing.
Dr Sanders: contributed to study design, data collection, analyses, and manuscript writing.
Other contributors: Ronald G. Crystal, MD Bruce Webster Professor of Internal Medicine Professor of Genetic Medicine Chairman of the Department of Genetic Medicine Department of Pulmonary and Critical Care Medicine Weill Cornell Medical Center --------Contributed to the design, interpretation of data and manuscript writing.
David E. Ost, MD, MPH, FACP Associate Professor Department of Pulmonary Medicine Division of Internal Medicine University of Texas MD Anderson Cancer Center --------Contributed to the design, interpretation of data and manuscript writing.
Financial/nonfinancial disclosures: none
REFERENCES
- 1.Cox J, et al. Transthoracic Needle Aspiration Biopsy: Variables That Affect Risk of Pneumothorax. Radiology. 1999;212:165–168. doi: 10.1148/radiology.212.1.r99jl33165. [DOI] [PubMed] [Google Scholar]
- 2.Duncan D, et al. Reducing Iatrogenic Risk in Thoracentesis. Establishing Best Practice Via Experiential Training in a Zero-Risk Environment. Chest. 2009;135(5):1315–1320. doi: 10.1378/chest.08-1227. [DOI] [PubMed] [Google Scholar]
- 3.Barnes B, et al. Sonographically Guided Thoracentesis and Rate of Pneumothorax. Journal of Clinical Ultrasound. 2005;33:442–446. doi: 10.1002/jcu.20163. [DOI] [PubMed] [Google Scholar]
- 4.Zink R, et al. Pneumothorax After Transbronchial Biopsy or Fine Needle Aspiration: Can it be Predicted? A Prospective Study of 94 Cases. Journal of Bronchology. 2007;14(3):162–164. [Google Scholar]
- 5.Soldati G, et al. Occult Traumatic Pneumothorax: Diagnostic Accuracy of Lung Ultrasonography in the Emergency Department. Chest. 2008;133(1):204–11. doi: 10.1378/chest.07-1595. [DOI] [PubMed] [Google Scholar]
- 6.Nagarsheth K, Kurek S. Ultrasound Detection of Pneumothorax Compared with Chest x-ray and Computed Tomography Scan. Am Surg. 2011;77(4):480–4. [PubMed] [Google Scholar]
- 7.Rowan S, et al. Traumatic Pneumothorax Detection with Thoracic US: Correlation with Chest Radiography and CT – Initial Experience. Radiology. 2002;225:210–214. doi: 10.1148/radiol.2251011102. [DOI] [PubMed] [Google Scholar]
- 8.Chan S. Emergency Bedside Ultrasound to Detect Pneumothorax. Acad Emerg Med. 2003;(10):91–95. doi: 10.1111/j.1553-2712.2003.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 9.Wilkerson B, et al. Sensitivity of Bedside Ultrasound and Supine Anteroposterior Chest Radiographs for the Identification of Pneumothorax After Blunt Trauma. Acad Emerg Med. 2010;17:11–17. doi: 10.1111/j.1553-2712.2009.00628.x. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein DA, et al. A Bedside Ultrasound Sign Ruling Out Pneumothorax in the Critically Ill. Chest. 1995;108(5):1345–8. doi: 10.1378/chest.108.5.1345. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein DA, et al. The Comet-ail Artifact: An Ultrasound Sign of Ruling Out Pneumothorax. Intensive Care Med. 1999;25:383–388. doi: 10.1007/s001340050862. [DOI] [PubMed] [Google Scholar]
- 12.Reiβig A, Kroegel C. Europena. Accuracy of Transthoracic Sonography in Excluding Post Interventional Pneumothorax and Hydropneumothorax. Comparison to Chest Radiography. Journal of Radiology. 2005;53:463–470. doi: 10.1016/j.ejrad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Sartori, et al. Accuracy of Transthoracic Sonography in Detection of Pneumothorax After Sonographically Guided Lung Biopsy: Prospective Comparison with Chest Radiography. AJR. 2007;188:37–41. doi: 10.2214/AJR.05.1716. [DOI] [PubMed] [Google Scholar]
- 14.A Language and Environment for Statistical Computing. R Development Core Team; 2011. http://www.R-project.org. [Google Scholar]
- 15.Slater, et al. COPD Can Mimic the Appearance of Pneumothorax on Thoracic Ultrasound. Chest. 2006;129:545–550. doi: 10.1378/chest.129.3.545. [DOI] [PubMed] [Google Scholar]
- 16.Koenig, et al. Thoracic Ultrasonography for the Pulmonary Specialist. Chest. 2011;140(5):1333–1341. doi: 10.1378/chest.11-0348. [DOI] [PubMed] [Google Scholar]
- 17.Galbois, et al. Pleural Ultrasound Compared With Chest Radiographic Detection of Pneumothorax Resolution After Drainage. Chest. 2010;138:648–655. doi: 10.1378/chest.09-2224. [DOI] [PubMed] [Google Scholar]
- 18.Kim, et al. Approximation of the Size of Pneumothorax by Ultrasound for Patients with a Possibility of Pneumothorax. J Korean Soc Emerg Med. 2006;17(5):493–499. [Google Scholar]
- 19.Sistrom, et al. Detection and Estimation of the Volume of Pneumothorax Using Real-time Sonography: Efficacy Determined by Receiver Operating Characteristic Analysis. AJR. 1996;166(2):317–321. doi: 10.2214/ajr.166.2.8553938. [DOI] [PubMed] [Google Scholar]
- 20.Izbicki, et al. Is Routine Chest Radiography After Transbronchial Biopsy Necessary? - A Prospective Study of 350 Cases. Chest. 2006;129(6):1561–1564. doi: 10.1378/chest.129.6.1561. [DOI] [PubMed] [Google Scholar]
- 21.Petersen W, Zimmerman R. Limited Utility of Chest Radiograph After Thoracentesis. Chest. 2000;117(4):1038–1042. doi: 10.1378/chest.117.4.1038. [DOI] [PubMed] [Google Scholar]