Abstract
It is now well recognized that the atherosclerotic plaques responsible for thrombus formation are not necessarily those that impinge most on the lumen of the vessel. Nevertheless, clinical investigations for atherosclerosis still focus on quantifying the degree of stenosis caused by plaques. Many of the features associated with a high-risk plaque, including a thin fibrous cap, large necrotic core, macrophage infiltration, neovascularization, and intraplaque hemorrhage, can now be probed by novel imaging techniques. Each technique has its own strengths and drawbacks. In this article, we review the various imaging modalities used for the evaluation and quantification of atherosclerosis.
Keywords: vulnerable plaque, CT, MRI, PET, ultrasound
INTRODUCTION
Atherosclerosis-related diseases are projected to cost more than $500 billion in the United States in 2010 (1). The majority of life-threatening consequences of atherosclerosis, including myocardial infarction (MI) and stroke, result from acute thrombus formation on the surface of a plaque. The newly formed thrombus may completely occlude the lumen in situ, or embolize to occlude a distal narrower lumen, and in so doing cause infarction to the territory supplied by the artery (2). Most clinical investigations for atherosclerosis provide a readout on the degree of stenosis caused by the plaque. However, it is now well recognized that the plaque that is at risk of thrombus formation is not necessarily the plaque that impinges most on the lumen of the vessel. This dissociation between degree of stenosis and clinical outcome was initially revealed by angiography (3) and further illustrated by randomized controlled trials with HMG-CoA reductase inhibitors (statins), which markedly reduce acute cardiovascular events (4) and yet only modestly reduce stenosis (5–8). It is now well recognized that statins significantly alter the composition of plaques without necessarily impacting upon their size or the stenosis they produce. These changes in composition are thought to be partly responsible for the benefits they convey, and therefore numerous imaging techniques are currently being developed to provide information on both the composition and function of the plaques. Plaque rupture accounts for the majority of acute thrombus formation and is defined as a deep injury to the fibrous cap with consequent exposure of the underlying lipid-rich core to the arterial blood (9).
The prerupture lesion that most closely resembles a ruptured plaque is the thin-cap fibroatheroma (TCFA), characterized by a large necrotic core underneath a thin, fibrous cap (of thickness <65 μm). TCFAs contain an abundance of macrophages and show signs of neovascularization with intraplaque hemorrhage (2). All of these features can now be probed by novel imaging techniques, each with its own strengths and drawbacks. Some of these techniques, such as ultrasound imaging of carotid intima-media thickness and intravascular ultra-sound of the coronary arteries, are already in clinical use. Others, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), have found application in research in the analysis of the compositional features of carotid artery plaque, and have potential clinical utility. In this article, we review the various imaging modalities used for the evaluation and quantification of atherosclerosis.
ULTRASOUND
Carotid Intima-Media Thickness
Measurement of carotid intima-media thickness (CIMT) was the first imaging modality to be used as an endpoint in clinical trials of atherosclerosis drugs, and to date CIMT measurement is the modality that has been used most often to test drugs under evaluation as treatments for atherosclerosis (10–13).
The biological rationale for measuring CIMT is that pathological intimal thickening is one of the earliest stages of atherosclerosis. Although B mode ultrasound cannot clearly distinguish the intima-media interface, it can identify the boundaries at the intima-lumen and the adventia-media. CIMT is a one-dimensional measurement between these two boundaries and has been attractive because it is noninvasive and perceived to be relatively inexpensive.
Increased cardiovascular and total mortality risk is associated with increased CIMT (14), and CIMT has been shown to predict cardiovascular events not only in patients with coronary artery disease (15) but also in those with type 2 diabetes (16) and in asymptomatic patients (17). Furthermore, clinical trials with statins have consistently shown that a reduction in CIMT is associated with a reduction in cardiovascular events (11–13). However, a major shortcoming for use in clinical trials is that the population variability in the measurement necessitates large sample sizes that also ultimately increase cost (18).
Despite the widespread use of CIMT, critics point to major flaws in its application and argue that the feature of intimal thickening is only loosely connected to the atherosclerotic process (19). The intima-media layers thicken with both age and hypertension, even in the absence of atherosclerosis (20). Furthermore, measurements of CIMT (even on autopsy) correlate poorly with coronary and carotid disease (21), and although CIMT does correlate with coronary risk, the majority of the predictive power is lost once adjustments are made for age, sex, and blood pressure (22). Furthermore, although there is no consensus on the optimal anatomical location for a CIMT measurement, it is usually made at the common carotid artery, which is technically easier and more reproducible than measuring the bifurcation or internal carotid (23, 24). However, atherosclerosis typically occurs at branching points in the arteries where laminar flow becomes turbulent and hemodynamic shear stress is low (25). Measuring CIMT proximally to the bifurcation is likely to provide only limited information about the atherosclerotic process occurring more distally under different hemodynamic conditions. Finally, CIMT is unable to provide any information on plaque composition or function.
Therefore, while CIMT measurement occupies an important historical position as the first imaging modality to be widely used in atherosclerosis drug trials, this method has already begun to be superseded by imaging modalities that provide more information about the biology of the plaque itself.
Contrast-Enhanced Ultrasound
Contrast-enhanced ultrasound (CEUS) has attracted interest because it may potentially enable the carotid ultrasound examination to provide information about plaque composition, in addition to the structural information gathered from the standard ultrasound examination. There are several ultrasound contrast agents (microbubbles) commercially available, which act as blood pool agents. They consist of a lipid or albumin shell filled with an inert gas. To date, CEUS has chiefly been used to identify microvasculature within the carotid plaque, one feature that is associated with plaque rupture. However, a preliminary report suggests that microbubbles may be used to quantify plaque inflammation, another important feature of the vulnerable plaque (26).
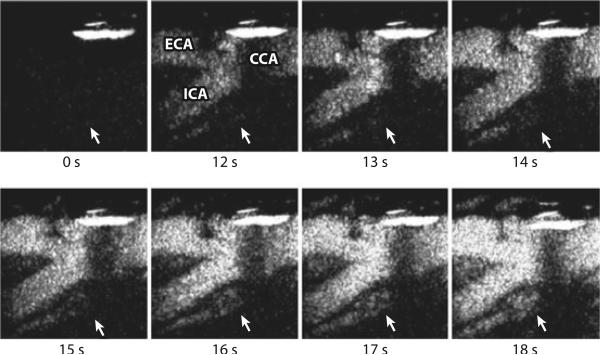
Hitherto, studies have chiefly concentrated on comparing CEUS signal from the plaque with histological indices of plaque neovascularization and found that CEUS is able to detect plaque vascularity (27, 28) (Figure 1). An association between CEUS plaque signal and cardiovascular events has also been reported (29). However, these studies used a subjective visual assessment to ascribe a discrete score to the imaging findings, because signal quantification remains a significant challenge. One recent study used automated image analysis to generate time–signal intensity curves following contrast administration, and CEUS plaque signal was shown to be greater in symptomatic patients than in asymptomatic patients (30).
Figure 1.
Contrast-enhanced ultrasound of the carotid plaque. Time 0 s shows the total subtracted image (black) in the nonlinear imaging mode at the carotid bifurcation before the arrival of the contrast agent. From time 12 s, the enhancement of the carotid lumen (white) is evident. Note the subsequent enhancement of the plaque neovascularization from time 14 s (white arrow). CCA, common carotid artery; ECA, external carotid artery; ICA, internal carotid artery. Reproduced with permission from Prof. Ed Leen, Imperial College London.
CEUS may also provide information on plaque inflammation. Microbubbles are retained in inflamed tissue through integrinand complement-based adherence to damaged endothelium and/or monocytes, which are themselves attached to the endothelium (31, 32). By performing the plaque imaging when most circulating microbubbles have been cleared (“late phase”), CEUS is able to detect retained microbubbles and distinguish between symptomatic and asymptomatic patients (26). Microbubbles can also be conjugated to ligands that target specific molecules, such as vascular cell adhesion molecule 1 (VCAM-1) (33) or αVβ3 and α5β1 integrins expressed by the endothelium of neovessels (34), although generating these agents to GMP (good manufacturing practice) standards remains challenging.
If it can be convincingly demonstrated that commercially available microbubbles provide information on plaque vascularity or inflammation, it is conceivable that CEUS could be translated into clinical practice, where it may have a role in monitoring therapy or selecting patients for surgical procedures. For this to happen, robust methods of automated signal quantification in three dimensions need to be developed and the reproducibility of the technique needs to be demonstrated.
Intravascular Ultrasound
A major limitation of conventional coronary x-ray arteriography is that it depicts the silhouette of the plaque and is therefore unable to detect positively remodeled lesions, which do not significantly impinge on the lumen (35). Intravascular ultrasound (IVUS) addresses this limitation. It is a catheter-based technique in which a miniature high-frequency transducer is transluminally placed into an affected coronary artery. Ultrasonic reflections are acquired as the transducer is automatically retracted at a rate of 0.5–1 mm/s. The atheroma cross-sectional area (CSA) is calculated by subtracting the lumen CSA from the external elastic membrane CSA. IVUS is more sensitive than conventional angiography in detecting the presence of atherosclerosis, and more accurate in measuring its extent (36). Furthermore, by distinguishing echogenic from echolucent plaques, IVUS can provide additional information on plaque composition (37) and can even detect the presence of lipid pools with high specificity, although limited sensitivity (38).
In the REVERSAL trial, 502 patients were treated for 18 months with high-dose atorvastatin (80 mg) or maintenance-dose pravastatin (40 mg). IVUS evaluation demonstrated a progression of atheroma volume in the pravastatin group, but no progression in the atorvastatin group (39). Regression of coronary plaque has been demonstrated in ASTEROID, an IVUS study evaluating 24 months’ intensive treatment with rosuvastatin (40 mg) (40), and a randomized controlled trial of only 5 weeks’ treatment with recombinant ApoA-I Milano demonstrated quite rapid plaque regression (41). The cholesterol ester transfer protein (CETP) inhibitor, torcetrapib, showed no regression of plaque established by both IVUS-derived percent atheroma volume (42) and CIMT (43) despite potent effects on low-density lipoprotein (LDL) reduction and high-density lipoprotein (HDL) elevation. The results emerged after development of the drug had been terminated due to an outcome trial showing an increase in all-cause mortality. This example suggests how imaging might provide early and instructive surrogate readouts in phase II, which could help inform decisions on which compounds to advance into clinical trials.
These studies were based on conventional grayscale IVUS, which is limited in its ability to distinguish specific plaque components such as fibrous and necrotic tissue (44). However, plaque characterization can be achieved with spectral analysis of the radiofrequency signal, known as virtual-histology IVUS (VH-IVUS). VH-IVUS can accurately detect the presence of fibrous, fibrolipidic, calcified, and calcified-necrotic regions from plaques (45). Furthermore, the PROSPECT study, by classifying lesion types depending on a combination of conventional grayscale and VH-IVUS, found that patients with highest-risk plaques had a substantial increase in cardiovascular events within three years (hazard ratio 3.8) compared with those of lowest risk (hazard ratio 0.2). VH-IVUS has already been used to provide endpoints for a drug in development. Darapladib is a reversible lipoprotein-associated phospholipase A2 inhibitor that has entered a phase III clinical outcome trial supported by the results of a VH-IVUS study, which demonstrated a reduction in the growth of the necrotic core (but not overall plaque) compared with placebo (46).
A major limitation for IVUS is its invasiveness, limiting its use to patients who are undergoing x-ray angiography for clinical indications. Furthermore, the longitudinal measurements that are required for clinical trials are restricted to nonstenotic lesions, as flow-limiting stenoses are likely to be treated with balloons or stents. Despite these practical drawbacks, conventional IVUS remains the best available imaging technique for quantifying coronary plaque burden for drug trials, and the increasing use of VH-IVUS shows promise in allowing the determination of plaque composition.
MAGNETIC RESONANCE IMAGING
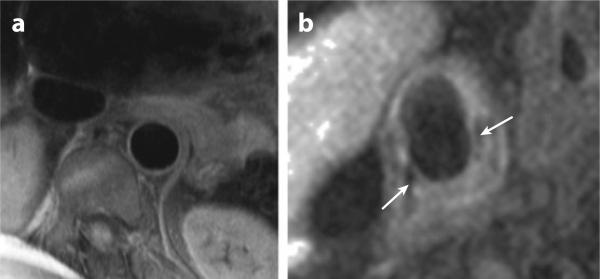
Magnetic resonance imaging (MRI) of atherosclerosis can provide information on both plaque volume and composition in multiple arterial territories (47) (Figure 2). To date, the majority of research exploring the ability of MRI to show plaque composition has centered on imaging of the carotid artery and the aorta (48–51). These studies have revealed that, compared with other imaging modalities, MRI appears to have the greatest potential for plaque characterization, through the use of multiple contrast weightings (T1, T2, and proton-density weighting). Specifically, it has been shown that MRI of the carotid artery can differentiate four main plaque components: fibrous cap (and its integrity), lipid-rich/necrotic core, intraplaque hemorrhage, and calcification (49). More recent work has shown a correlation between clinical symptoms and features of vulnerable plaque, such as cap rupture and intraplaque hemorrhage (52), raising the possibility that MRI of the carotid arteries may have a potential clinical role in the management of symptomatic carotid artery disease.
Figure 2.
Magnetic resonance imaging of the aorta and carotid artery. (a) The descending thoracic aorta adjacent to the lumbar spine. The vessel wall is clearly demarcated and can be seen to be normal. (b) Complicated right carotid plaque with a necrotic lipid core and a thin fibrous cap (white arrows). Images courtesy of Dr. Robin Choudhury.
In addition to its applications in these specific territories, the noninvasive nature of MRI allows serial scanning to assess the overall vascular response to both conventional and experimental pharmacotherapy. Corti et al. were the first to demonstrate a reduction in carotid and aortic atherosclerosis using serial MRI after 12 months of statin treatment (7). Subsequently, it was demonstrated that more intensive lipid lowering, to LDL <100 mg/dL, was associated with a larger decrease in plaque size, also over 12 months (6). MRI has provided further insights into how atherosclerotic plaque responds to cholesterol-altering medications. For example, Lee et al. used MRI to demonstrate, in the carotid arteries and aorta, reduction in the plaque index (normalized vessel wall area) as early as three months after statin initiation. In the same patients, early changes in atherosclerosis (within three months) were significantly correlated with later change at 12 months (53). More recently, MRI has been used to investigate the effects of novel treatment strategies, including the use of niacin to increase levels of HDL (8).
Conventional gadolinium-based contrast agents have been used to enhance visualization of the fibrous cap of atherosclerotic plaque, and use of dynamic contrast enhancement can give an indication of plaque vascularity and inflammation (54–56). However, the field has been advanced considerably by the development of a range of targeted molecular and cellular contrast agents, which in the future may allow identification of the molecular constituents of individual plaques. Several molecular imaging agents, including liposomes, nanoparticles, lipoproteins, and quantum dots, are under development and have shown encouraging results in early animal testing (57). Iron oxide conjugates have also shown promise as contrast agents, as their paramagnetic properties cause signal dropout on MRI; Tang et al. used ultrasmall particles of iron oxide (USPIO) to show a reduction in carotid plaque morphology in response to intensive cholesterol lowering with statin treatment (58), although this agent has now been withdrawn. McAteer et al. have also constructed larger microparticles of iron oxide (MPIO, 4.5 μm diameter) targeting endothelial P-selectin and VCAM-1 adhesion molecules, which allow binding to the arterial wall in the early stages of plaque development (59). In addition, more naturally occurring nanoparticles, such as lipoproteins, have been adapted for use as effective molecular imaging probes. Frias et al. extracted ApoA-I from human plasma and reconstituted it with a gadolinium chelate. When injected into ApoE knockout mice, a significant accumulation of this reconstituted HDL particle was seen in the aorta (60). Vascular MRI continues to evolve in terms of sequence development, hardware evolution, and contrast-agent production.
COMPUTED TOMOGRAPHY
Two forms of computed tomography (CT) scanner have been used to assess atherosclerosis: electron-beam CT and multiple-row detector CT (MDCT). The former uses stationary tungsten rings to generate x-ray images at 3-mm slice thickness from which a coronary artery calcium score is calculated to assess cardiovascular risk. In contrast, the latter uses a continuously rotating x-ray source to obtain 0.5–0.75-mm slices during a single patient breathhold. Intravenous contrast is used to produce coronary angiographic (CTA) pictures which are also capable of giving information on atherosclerotic plaque in the coronary arterial wall.
Calcium is the plaque characteristic most readily identified by CT, and the addition of coronary artery calcium scoring to current clinical risk-scoring systems has been shown to provide predictive information (61). Furthermore, the amount of coronary calcium detected has been shown to correlate with the amount of coronary atherosclerosis present by histology (62). This, coupled with the lack of a systematic method for quantification of disease using CTA, has meant that coronary artery calcium scoring has been the predominant method of risk assessment using CT so far. However, the imperative to image the vessel wall, including lipid-rich but calcium-poor stenotic plaques, has led many to argue that CTA will eventually prevail as the standard method of CT coronary artery assessment (63). In younger patients, CTA is capable of detecting both calcium- and lipid-rich plaques; in elderly patients, it can often exclude the presence of a significant stenosis even if the calcium burden is high (63).
In addition to the detection of calcium in the arterial wall, CT is capable of differentiating predominantly lipid-rich from predominantly fibrous plaque. However, a large degree of overlap exists in the degree of attenuation caused by these types of lesions, which decreases the specificity of CT (64), particularly given the highly heterogeneous nature of most plaques. Also, regardless of plaque composition, accurate estimation of the degree of stenosis can be difficult if a high signal from calcium-rich plaque obscures the arterial lumen. These issues and the particular biology of late-stage calcific plaques mean that CT may not be suitable for assessing drug interventions. A recent meta-analysis of ten trials that have used CT to assess changes in coronary plaque in response to a variety of therapies concluded that one-year change in coronary artery calcification was not a suitable surrogate endpoint for treatment trials of patients with existing coronary artery disease or kidney disease (65). However, the majority of research to date has been performed on 64-slice CT scanners; ongoing technical developments and the introduction of 256- and 512-slice scanners are likely to increase the applicability of CT in the future. In addition, the development of novel molecular imaging probes, either for use in CT or combined CT/SPECT (single photon emission computed tomography) examinations, is likely to further enhance the plaque-imaging capabilities of CT. For example, the CT contrast agent N1177 (66) and gold HDL nanoparticles (67) are capable of detecting intraplaque macrophages.
Two major challenges remain regarding the future clinical use of CT for atherosclerosis imaging. First, the risk from the radiation exposure required for an MDCT scan of the coronary arteries is estimated to be high; for example, the lifetime cancer risk conferred by a standard cardiac scan in a 20-year-old female has been calculated to be 1 in 143 (68). However, recent technical advances, including the use of volume scanning (as opposed to helical scanning) to reduce the effective radiation dose by 90% (69), have led to substantially decreased radiation doses for the average examination. Second, even if radiation exposure can be minimized, the optimal investigative use of serial MDCT plaque imaging in the clinical arena remains to be clarified. Although plaque progression has been shown in the clinical setting (70), it is not yet clear how this relates to patient prognosis. On the contrary, a negative CT coronary angiogram has been shown to have a high negative predictive value for coronary artery disease; therefore, many centers currently use CT prior to invasive coronary angiography for patients at low or intermediate risk of coronary artery disease (71).
POSITRON EMISSION TOMOGRAPHY
Nuclear imaging techniques involve the intravenous administration of radiolabeled ligands that accumulate preferentially in tissues rich in their target. Radionuclide decay is detected externally to produce the signal, which can be measured as standardized uptake values or tissue-to-background ratios. Time-activity curves can also be generated and converted to physiological parameters with kinetic models. Although these techniques offer an unparalleled sensitivity to quantify cellular parameters such as receptor density, their anatomical resolution is poor (4–5 mm for PET and 10–15 mm for SPECT), and thus they require coregistration with either CT or MRI to enable accurate placement of regions of interest. The use of ionizing radiation, cost of studies, and short half-lives of the tracers (often requiring proximity to a cyclotron) are further disadvantages.
SPECT ligands designed to probe various processes of atherosclerosis progression and rupture, including chemotaxis (72), angiogenesis (73), lipoprotein accumulation (74), proteolysis (75), and thrombogenicity (76), have been manufactured and studied in animals (77). A few PET tracers, including 18fluorodeoxyglucose (FDG), translocator protein (TSPO) ligands, and choline ligands, have been studied in humans.
18Fluorodeoxyglucose
FDG is a glucose analogue that is taken up by cells at the same rate as glucose and phosphorylated by the same hexokinase, which prevents its egress back across the cell membrane. Once inside the cell, the phosphorylated FDG is halted in its progression through the glycolysis pathway. FDG accumulation is therefore interpreted as a measure of cellular metabolic activity. The rationale for using FDG to identify the vulnerable plaque is that metabolic activity within the plaque represents macrophage activity, and thus a plaque rich in macrophages will have high FDG uptake.
FDG-PET was first used for the purpose of studying atherosclerosis in a patient with recent carotid-territory transient ischemic attack who was scheduled to undergo carotid endarterectomy. FDG uptake in the symptomatic carotid artery was significantly higher than in the contralateral asymptomatic artery, and autoradiography of excised plaques confirmed accumulation of deoxyglucose in macrophage-rich areas (78). In studies of similar design, the PET signal from the carotid plaque has been found to correlate with macrophage staining from the corresponding histological section (79) and gene expression of markers of atherosclerotic plaque vulnerability (80). Of note, FDG uptake appeared to add value for the detection of inflammation, since the signal did not correlate with plaque area, plaque thickness, or smooth muscle cell staining (79).
The FDG signal can also be altered by therapeutic interventions. A cohort of cancer patients in whom a baseline FDG scan had shown arterial uptake were treated with simvastatsin or placebo. Simvastastin produced a reduction in FDG uptake relative to placebo at 12 weeks (81). Although signal changes were relatively modest, the cohort studied was a population of cancer patients with less original uptake than might be expected from a population of atherosclerosis patients. Lifestyle modification, by inducing favorable effects on blood pressure and lipid profile, has also been shown to reduce arterial FDG uptake over 18 months (82).
FDG-PET demonstrates impressive repeatability for quantification of plaque uptake in the carotid and aorta. For examinations repeated over a two-week period, inter- and intraobserver agreement is high across all territories (except the aortic arch) with intraclass correlation coefficients ranging from 0.90 to 0.98 (83).
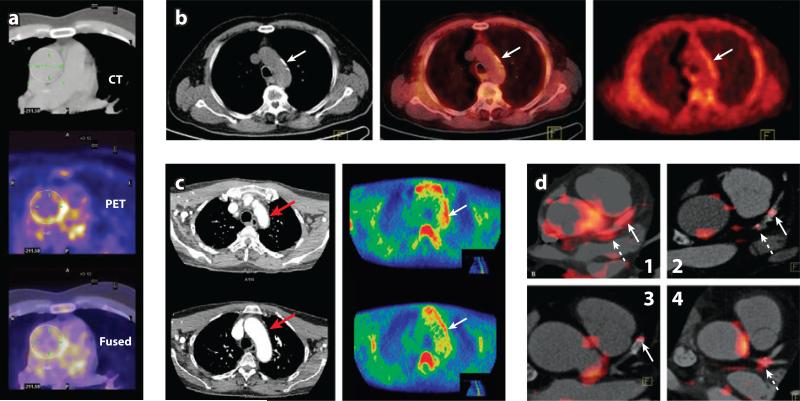
Measuring FDG uptake in coronary arteries is limited by uptake into the myocardium, but uptake can be reduced by preparation with a low-carbohydrate and high-fat diet prior to an FDG scan. In a recent study, such preparation produced adequate suppression of myocardial uptake in two thirds of patients, and inadequate suppression was associated with self-reported dietary nonadherence. Although a trend was observed in the correlation between FDG signal in the vessel and the presence of coronary artery disease on angiography, significance was not achieved (84). However, this was a retrospective study in which the imaging was performed for the investigation of cancer, without cardiac or respiratory gating. This would have impacted greatly on spatial and temporal resolution. A recent prospective study reported promising results, demonstrating that the FDG signal in the left main coronary artery is greater in patients with recent acute coronary syndrome than in those with stable disease (Figure 3) (85).
Figure 3.
(a) [18F]FDG PET/CT image (CT/PET/Fused) of aorta. Image courtesy of Prof. Fayad. (b) [11C]-choline PET/CT images of the aortic arch: CT (left), PET (middle), and fused (right). Tracer uptake coincides with calcification (arrows indicate calcification site). (c) [11C]PK11195 CT angiogram (left), PET image (right). Patient with active giant cell arteritis with increased tracer uptake in the lateral aspect of the aortic arch (arrow). The CT angiography demonstrates corresponding thickening of the aortic wall (arrow). (d ) Coregistered [18F]FDG PET/CT images show FDG uptake within specified coronary vascular locations (85). Arrows show stent locations. Hatched arrows show lesions within the left main coronary artery (LM). (d1) Increased uptake in both LM and stented culprit lesions in a subject presenting with acute coronary syndrome (ACS). (d2) More modest uptake in a coronary lesion that was recently stented for a stable coronary syndrome (some FDG uptake is also noted in a mixed plaque within the LM). (d3) Modest FDG uptake within a lesion stented several months before imaging. (d4) The FDG uptake at the trifurcation of the LM in a subject presenting with ACS.
A question yet to be addressed is whether high FDG-PET signal is predictive of future cardiovascular events. A retrospective study of 932 patients who underwent whole-body FDGPET for cancer showed that increased FDG up-take in major arteries was a strong predictor of subsequent vascular events (86). These results require confirmation in a prospective study. However, because the annual rate of cardiovascular events is low, even in patients with known atherosclerotic disease, such studies must necessarily be large.
Other Tracers
The 18-kDa translocator protein (TSPO), previously known as the peripheral benzodiazepine receptor (PBR), is expressed in large numbers within microglia and macrophages (87). For this reason, the TSPO has been used successfully as a target for the PET radioligand, [11C]PK11195, in order to study diseases that involve the recruitment of macrophages, including multiple sclerosis (88). The TSPO is therefore a promising imaging target for atherosclerosis, as it offers the opportunity to quantify intraplaque macrophage expression with greater specificity than FDG, although myocardial binding may complicate quantification as the TSPO is expressed in the heart (89).
Ex vivo autoradiography with human carotid plaques has shown that TSPO binding colocalizes with macrophage expression, and receptor density is sufficiently great to be quantified in a PET study (90). [11C]PK11195 has already been used to detect vascular inflammation within the arterial wall in patients with systemic inflammatory disorders (Figure 3) (91), and the first clinical PET study with [11C]PK11195 in atherosclerosis is under way. A plethora of new tracers targeting the TSPO, with vastly improved signal-to-noise ratio and higher affinity for the TSPO compared to PK11195, are now available for use in human studies (92), although heterogeneity in affinity among patients may complicate interpretation of signal (93, 94).
18F-labeled fluorocholine (FCH) is a tracer already in clinical use to image prostate cancer on the basis that activity of the choline-specific transporter increases in proliferating cells. Following import, FCH is phosphorylated and incorporated into the cell membrane (95). Like tumor cells, activated macrophages show enhanced FCH uptake (95), and in a mouse model, FCH has shown greater sensitivity in detecting plaques than FDG (84% versus 64%) (96). A major advantage offered by FCH imaging over both TSPO and FDG is the lack of FCH uptake in the myocardium (97). Two retrospective clinical studies in cohorts of old male patients undergoing PET/CT with FCH for prostate cancer have shown that signal from the aorta and common carotid is frequently observed (Figure 3) (98, 99). As with FDG, the signal tends not to colocalize with areas of vessel calcification (100), implying that the tracer is more likely to detect active plaques. To date, a prospective study of FCH to image atherosclerotic plaques has not yet been published.
FUTURE HORIZONS
The conventional imaging techniques of MRI, CT, and ultrasound provide detailed information on structure. However, functional changes normally precede structural changes in both development and resolution of pathology. By attaching molecular probes that target specific molecules or metabolic processes to contrast-generating materials, MRI, CT, and ultrasound can visualize fundamental cellular processes, examples of which are discussed above. In the field of atherosclerosis, lipoprotein-based contrast agents are generating considerable interest. Lipoproteins are an attractive family for imaging atherosclerosis because they are endogenous (and therefore nonimmunogenic and biodegradable), they are fundamental to the pathology of atherosclerosis, and their composition can be easily altered. Hitherto, LDL has been most exploited as an imaging agent (101), but HDL offers various advantages. These include its smaller size (allowing improved penetration into the plaque) and the fact that it is beneficial in atherosclerosis whereas LDL is deleterious. HDL imaging is therefore a very promising approach, and reports of success have been published (60, 102).
CONCLUSION
The past 15 years have witnessed great strides forward in the field of imaging atherosclerosis. Far from merely detecting stenoses, conventional imaging techniques are now routinely able to characterize plaque composition, and many of these techniques are already being employed as endpoints in trials of novel therapeutics. Pure anatomical imaging is already being enhanced with the use of molecular probes to provide functional and cellular information, and this area of imaging is likely to be the most rapidly advancing in the coming years. The improvement of the techniques reviewed herein, which will allow a wide variety of molecular pathways to be probed in greater detail, will provide invaluable tools enabling researchers to investigate disease processes, clinicians to risk-stratify patients, and pharmaceutical companies to make go/no-go decisions on candidate molecules.
CIMT: carotid intima-media thickness
CEUS: contrast-enhanced ultrasound
IVUS: intravascular ultrasound
MDCT: multiple-row detector computed tomography
SPECT: single photon emission computed tomography
SUMMARY POINTS.
MRI provides information, without ionizing radiation, on plaque volume and composition in multiple arterial territories. The use of contrast agents can add incremental information on function, making MRI a powerful tool, particularly to test novel therapies.
Although multiple-row detector CT, using ionizing radiation, may be able to differentiate lipid-rich from fibrous plaque, some overlap exists in the degree of attenuation caused by these lesions, thereby limiting CT to identification of plaque calcium. Because plaque calcium does not rapidly change, CT has less use in drug trials than MRI, CIMT, and PET, but it is currently employed to assist clinical decision making in patients of low to medium risk.
PET offers unparalleled sensitivity to quantify cellular parameters, but its anatomical resolution is poor and is limited by its use of radiation. Though potentially very useful in early-phase drug trials, it is unclear if PET will be in widespread use as a clinical technique of investigation.
Imaging coronary atherosclerosis is challenging because of motion and the small diameter of the vessels. IVUS and CT can both provide information on coronary plaque, and encouraging progress is being made in coronary MRI and PET.
By generating contrast agents that target specific molecules or metabolic processes, we can improve the ability of MRI, CT, and ultrasound to visualize fundamental biological processes at the molecular, cellular, and functional levels.
Footnotes
DISCLOSURE STATEMENT
R.P.C. holds equity in Oxford Contrast, Molecular Diagnostics. His research is funded by the Wellcome Trust and British Heart Foundation. D.R.J.O.'s fellowship is partly funded by GlaxoSmithKline.
LITERATURE CITED
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Hellings WE, Peeters W, Moll FL, et al. From vulnerable plaque to vulnerable patient: the search for biomarkers of plaque destabilization. Trends Cardiovasc. Med. 2007;17(5):162–71. doi: 10.1016/j.tcm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Hackett D, Davies G, Maseri A. Pre-existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur. Heart J. 1988;9(12):1317–23. doi: 10.1093/oxfordjournals.eurheartj.a062449. [DOI] [PubMed] [Google Scholar]
- 4.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–89. [PubMed] [Google Scholar]
- 5.Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92(8):2333–42. doi: 10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 6.Corti R, Fuster V, Fayad ZA, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years’ follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation. 2002;106(23):2884–87. doi: 10.1161/01.cir.0000041255.88750.f0. [DOI] [PubMed] [Google Scholar]
- 7.Corti R, Fayad ZA, Fuster V, et al. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation. 2001;104(3):249–52. doi: 10.1161/01.cir.104.3.249. [DOI] [PubMed] [Google Scholar]
- 8.Lee JM, Robson MD, Yu LM, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J. Am. Coll. Cardiol. 2009;54(19):1787–94. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Schaar JA, Muller JE, Falk E, et al. Eur. Heart J. 12. Vol. 25. Santorini, Greece: Jun 17 18, 2004. 2003. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque; pp. 1077–82. [DOI] [PubMed] [Google Scholar]
- 10.Thoenes M, Oguchi A, Nagamia S, et al. The effects of extended-release niacin on carotid intimal media thickness, endothelial function and inflammatory markers in patients with the metabolic syndrome. Int. J. Clin. Pract. 2007;61(11):1942–48. doi: 10.1111/j.1742-1241.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 11.de GE, Jukema JW, van Boven AJ, et al. Effect of pravastatin on progression and regression of coronary atherosclerosis and vessel wall changes in carotid and femoral arteries: a report from the Regression Growth Evaluation Statin Study. Am. J. Cardiol. 1995;76(9):40C–46C. doi: 10.1016/s0002-9149(99)80469-x. [DOI] [PubMed] [Google Scholar]
- 12.Crouse JR, III, Byington RP, Bond MG, et al. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II). Am. J. Cardiol. 1995;75(7):455–59. doi: 10.1016/s0002-9149(99)80580-3. [DOI] [PubMed] [Google Scholar]
- 13.Crouse JR, III, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297(12):1344–53. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 14.Simons PC, Algra A, Bots ML, et al. Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of ARTerial disease). Circulation. 1999;100(9):951–57. doi: 10.1161/01.cir.100.9.951. [DOI] [PubMed] [Google Scholar]
- 15.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann. Intern. Med. 1998;128(4):262–69. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 16.Yamasaki Y, Kodama M, Nishizawa H, et al. Carotid intima-media thickness in Japanese type 2 diabetic subjects: predictors of progression and relationship with incident coronary heart disease. Diabetes Care. 2000;23(9):1310–15. doi: 10.2337/diacare.23.9.1310. [DOI] [PubMed] [Google Scholar]
- 17.Bots ML, Hoes AW, Koudstaal PJ, et al. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96(5):1432–37. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 18.Bots ML, Evans GW, Riley WA, et al. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke. 2003;34(12):2985–94. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 19.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler. Thromb. Vasc. Biol. 2010;30(2):177–81. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 20.Beere PA, Glagov S, Zarins CK. Experimental atherosclerosis at the carotid bifurcation of the cynomolgus monkey. Localization, compensatory enlargement, and the sparing effect of lowered heart rate. Arterioscler. Thromb. 1992;12(11):1245–53. doi: 10.1161/01.atv.12.11.1245. [DOI] [PubMed] [Google Scholar]
- 21.Young W, Gofman JW, Tandy R, et al. The quantitation of atherosclerosis. II. Quantitative aspects of the relationship of blood pressure and atherosclerosis. Am. J. Cardiol. 1960;6:294–99. doi: 10.1016/0002-9149(60)90318-0. [DOI] [PubMed] [Google Scholar]
- 22.O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 23.Crouse JR, III, Craven TE, Hagaman AP, et al. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation. 1995;92(5):1141–47. doi: 10.1161/01.cir.92.5.1141. [DOI] [PubMed] [Google Scholar]
- 24.Howard G, Sharrett AR, Heiss G, et al. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC Investigators. Stroke. 1993;24(9):1297–304. doi: 10.1161/01.str.24.9.1297. [DOI] [PubMed] [Google Scholar]
- 25.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282(21):2035–42. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 26.Owen DR, Shalhoub J, Miller S, et al. Inflammation within carotid atherosclerotic plaque: assessment with late-phase contrast-enhanced US. Radiology. 2010;255(2):638–44. doi: 10.1148/radiol.10091365. [DOI] [PubMed] [Google Scholar]
- 27.Shah F, Balan P, Weinberg M, et al. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc. Med. 2007;12(4):291–97. doi: 10.1177/1358863X07083363. [DOI] [PubMed] [Google Scholar]
- 28.Coli S, Magnoni M, Sangiorgi G, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J. Am. Coll. Cardiol. 2008;52(3):223–30. doi: 10.1016/j.jacc.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 29.Staub D, Patel MB, Tibrewala A, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke. 2010;41(1):41–47. doi: 10.1161/STROKEAHA.109.560342. [DOI] [PubMed] [Google Scholar]
- 30.Xiong L, Deng YB, Zhu Y, et al. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology. 2009;251(2):583–89. doi: 10.1148/radiol.2512081829. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsui JM, Xie F, Cano M, et al. Detection of retained microbubbles in carotid arteries with real-time low mechanical index imaging in the setting of endothelial dysfunction. J. Am. Coll. Cardiol. 2004;44(5):1036–46. doi: 10.1016/j.jacc.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 32.Lindner JR, Coggins MP, Kaul S, et al. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation. 2000;101(6):668–75. doi: 10.1161/01.cir.101.6.668. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116(3):276–84. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 34.Leong-Poi H, Christiansen J, Heppner P, et al. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005;111(24):3248–54. doi: 10.1161/CIRCULATIONAHA.104.481515. [DOI] [PubMed] [Google Scholar]
- 35.Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987;316(22):1371–75. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 36.Tobis JM, Mallery J, Mahon D, et al. Intravascular ultrasound imaging of human coronary arteries in vivo. Analysis of tissue characterizations with comparison to in vitro histological specimens. Circulation. 1991;83(3):913–26. doi: 10.1161/01.cir.83.3.913. [DOI] [PubMed] [Google Scholar]
- 37.Yamagishi M, Terashima M, Awano K, et al. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J. Am. Coll. Cardiol. 2000;35(1):106–11. doi: 10.1016/s0735-1097(99)00533-1. [DOI] [PubMed] [Google Scholar]
- 38.Prati F, Arbustini E, Labellarte A, et al. Correlation between high frequency intravascular ultra-sound and histomorphology in human coronary arteries. Heart. 2001;85(5):567–70. doi: 10.1136/heart.85.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291(9):1071–80. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 40.Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–65. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 41.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290(17):2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 42.Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 2007;356(13):1304–16. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 43.Vergeer M, Bots ML, van Leuven SI, et al. Cholesteryl ester transfer protein inhibitor torcetrapib and off-target toxicity: a pooled analysis of the rating atherosclerotic disease change by imaging with a new CETP inhibitor (RADIANCE) trials. Circulation. 2008;118(24):2515–22. doi: 10.1161/CIRCULATIONAHA.108.772665. [DOI] [PubMed] [Google Scholar]
- 44.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2001;37(5):1478–92. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 45.Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J. Am. Coll. Cardiol. 2006;47(12):2405–12. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 46.Serruys PW, Garcia-Garcia HM, Buszman P, et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118(11):1172–82. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 47.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451(7181):953–57. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 48.Yuan C, Mitsumori LM, Beach KW, et al. Carotid atherosclerotic plaque: noninvasive MR characterization and identification of vulnerable lesions. Radiology. 2001;221(2):285–99. doi: 10.1148/radiol.2212001612. [DOI] [PubMed] [Google Scholar]
- 49.Cai JM, Hatsukami TS, Ferguson MS, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106(11):1368–73. doi: 10.1161/01.cir.0000028591.44554.f9. [DOI] [PubMed] [Google Scholar]
- 50.Fayad ZA, Nahar T, Fallon JT, et al. In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography. Circulation. 2000;101(21):2503–9. doi: 10.1161/01.cir.101.21.2503. [DOI] [PubMed] [Google Scholar]
- 51.Chan SK, Jaffer FA, Botnar RM, et al. Scan reproducibility of magnetic resonance imaging assessment of aortic atherosclerosis burden. J. Cardiovasc. Magn. Reson. 2001;3(4):331–38. doi: 10.1081/jcmr-100108587. [DOI] [PubMed] [Google Scholar]
- 52.Yuan C, Zhang SX, Polissar NL, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation. 2002;105(2):181–85. doi: 10.1161/hc0202.102121. [DOI] [PubMed] [Google Scholar]
- 53.Lee JM, Wiesmann F, Shirodaria C, et al. Early changes in arterial structure and function following statin initiation: quantification by magnetic resonance imaging. Atherosclerosis. 2008;197(2):951–58. doi: 10.1016/j.atherosclerosis.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan C, Kerwin WS, Ferguson MS, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J. Magn. Reson. Imaging. 2002;15(1):62–67. doi: 10.1002/jmri.10030. [DOI] [PubMed] [Google Scholar]
- 55.Kerwin W, Hooker A, Spilker M, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation. 2003;107(6):851–56. doi: 10.1161/01.cir.0000048145.52309.31. [DOI] [PubMed] [Google Scholar]
- 56.Kerwin WS, O'Brien KD, Ferguson MS, et al. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006;241(2):459–68. doi: 10.1148/radiol.2412051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Briley-Saebo KC, Mulder WJ, Mani V, et al. Magnetic resonance imaging of vulnerable atherosclerotic plaques: current imaging strategies and molecular imaging probes. J. Magn. Reson. Imaging. 2007;26(3):460–79. doi: 10.1002/jmri.20989. [DOI] [PubMed] [Google Scholar]
- 58.Tang TY, Howarth SP, Miller SR, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 2009;53(22):2039–50. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 59.McAteer MA, Schneider JE, Ali ZA, et al. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler. Thromb. Vasc. Biol. 2008;28(1):77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frias JC, Williams KJ, Fisher EA, et al. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J. Am. Chem. Soc. 2004;126(50):16316–17. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 61.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 62.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J. Am. Coll. Cardiol. 1998;31(1):126–33. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 63.Schuijf JD, van der Wall EE, Bax JJ. Lesions without calcium: lessons from CT angiography. Heart. 2009;95(13):1038–40. doi: 10.1136/hrt.2008.164582. [DOI] [PubMed] [Google Scholar]
- 64.Ferencik M, Chan RC, Achenbach S, et al. Arterial wall imaging: evaluation with 16-section multidetector CT in blood vessel phantoms and ex vivo coronary arteries. Radiology. 2006;240(3):708–16. doi: 10.1148/radiol.2403051204. [DOI] [PubMed] [Google Scholar]
- 65.McCullough PA, Chinnaiyan KM. Annual progression of coronary calcification in trials of preventive therapies: a systematic review. Arch. Intern. Med. 2009;169(22):2064–70. doi: 10.1001/archinternmed.2009.382. [DOI] [PubMed] [Google Scholar]
- 66.Hyafil F, Cornily JC, Feig JE, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 2007;13(5):636–41. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 67.Cormode DP, Frias JC, Ma Y, et al. HDL as a contrast agent for medical imaging. Clin. Lipidol. 2009;4(4):493–500. doi: 10.2217/clp.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317–23. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 69.Einstein AJ, Elliston CD, Arai AE, et al. Radiation dose from single-heartbeat coronary CT angiography performed with a 320-detector row volume scanner. Radiology. 2010;254(3):698–706. doi: 10.1148/radiol.09090779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehman SJ, Schlett CL, Bamberg F, et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc. Imaging. 2009;2(11):1262–70. doi: 10.1016/j.jcmg.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meijboom WB, Mollet NR, Van Mieghem CA, et al. 64-Slice CT coronary angiography in patients with non-ST elevation acute coronary syndrome. Heart. 2007;93(11):1386–92. doi: 10.1136/hrt.2006.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohtsuki K, Hayase M, Akashi K, et al. Detection of monocyte chemoattractant protein-1 receptor expression in experimental atherosclerotic lesions: an autoradiographic study. Circulation. 2001;104(2):203–8. doi: 10.1161/01.cir.104.2.203. [DOI] [PubMed] [Google Scholar]
- 73.Haubner R. αvβ3-integrin imaging: a new approach to characterise angiogenesis? Eur. J. Nucl. Med. Mol. Imaging. 2006;33(Suppl. 1):54–63. doi: 10.1007/s00259-006-0136-0. [DOI] [PubMed] [Google Scholar]
- 74.Virgolini I, Rauscha F, Lupattelli G, et al. Autologous low-density lipoprotein labelling allows characterization of human atherosclerotic lesions in vivo as to presence of foam cells and endothelial coverage. Eur. J. Nucl. Med. 1991;18(12):948–51. doi: 10.1007/BF00180413. [DOI] [PubMed] [Google Scholar]
- 75.Choudhary S, Higgins CL, Chen IY, et al. Quantitation and localization of matrix metalloproteinases and their inhibitors in human carotid endarterectomy tissues. Arterioscler. Thromb. Vasc. Biol. 2006;26(10):2351–58. doi: 10.1161/01.ATV.0000239461.87113.0b. [DOI] [PubMed] [Google Scholar]
- 76.Greco C, Di LM, Ciavolella M, et al. Immunodetection of human atherosclerotic plaque with 125I-labeled monoclonal antifibrin antibodies. Atherosclerosis. 1993;100(2):133–39. doi: 10.1016/0021-9150(93)90199-5. [DOI] [PubMed] [Google Scholar]
- 77.Langer HF, Haubner R, Pichler BJ, et al. Radionuclide imaging: a molecular key to the atherosclerotic plaque. J. Am. Coll. Cardiol. 2008;52(1):1–12. doi: 10.1016/j.jacc.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–11. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 79.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J. Am. Coll. Cardiol. 2006;48(9):1818–24. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 80.Pedersen SF, Graebe M, Fisker Hag AM, et al. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl. Med. Commun. 2010;31(5):423–29. doi: 10.1097/MNM.0b013e32833767e0. [DOI] [PubMed] [Google Scholar]
- 81.Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J. Am. Coll. Cardiol. 2006;48(9):1825–31. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 82.Lee SJ, On YK, Lee EJ, et al. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J. Nucl. Med. 2008;49:1277–82. doi: 10.2967/jnumed.108.052233. [DOI] [PubMed] [Google Scholar]
- 83.Rudd JH, Myers KS, Bansilal S, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J. Nucl. Med. 2008;49(6):871–78. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 84.Wykrzykowska J, Lehman S, Williams G, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J. Nucl. Med. 2009;50(4):563–68. doi: 10.2967/jnumed.108.055616. [DOI] [PubMed] [Google Scholar]
- 85.Rogers IS, Nasir K, Figueroa AL, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc. Imaging. 2010;3(4):388–97. doi: 10.1016/j.jcmg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J. Nucl. Med. 2009;50(10):1611–20. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 87.Papadopoulos V, Baraldi M, Guilarte TR, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006;27(8):402–9. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 88.Banati RB, Newcombe J, Gunn RN, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123(Pt. 11):2321–37. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- 89.Imaizumi M, Briard E, Zoghbi SS, et al. Brain and whole-body imaging in nonhuman primates of [11C]PBR28, a promising PET radioligand for peripheral benzodiazepine receptors. Neuroimage. 2008;39(3):1289–98. doi: 10.1016/j.neuroimage.2007.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fujimura Y, Hwang PM, Trout IH, et al. Increased peripheral benzodiazepine receptors in arterial plaque of patients with atherosclerosis: an autoradiographic study with [(3)H]PK 11195. Atherosclerosis. 2008;201:108–11. doi: 10.1016/j.atherosclerosis.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 91.Pugliese F, Gaemperli O, Kinderlerer AR, et al. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J. Am. Coll. Cardiol. 2010;56:653–61. doi: 10.1016/j.jacc.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 92.Chauveau F, Boutin H, Van Camp N, et al. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur. J. Nucl. Med. Mol. Imaging. 2008;35(12):2304–19. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- 93.Owen DR, Howell OW, Tang SP, et al. Two binding sites for [(3)H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J. Cereb. Blood Flow Metab. 2010;30:1608–18. doi: 10.1038/jcbfm.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Owen DRJ, Gunn RN, Rabiner EA, et al. Mixed affinity binding in humans with 18 kDa translocator protein (TSPO) ligands. J. Nucl. Med. 2010 doi: 10.2967/jnumed.110.079459. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boggs KP, Rock CO, Jackowski S. Lysophosphatidylcholine and 1-O-octadecyl-2-O-methyl-racglycero-3-phosphocholine inhibit the CDP-choline pathway of phosphatidylcholine synthesis at the CTP:phosphocholine cytidylyltransferase step. J. Biol. Chem. 1995;270(13):7757–64. doi: 10.1074/jbc.270.13.7757. [DOI] [PubMed] [Google Scholar]
- 96.Matter CM, Wyss MT, Meier P, et al. 18F-choline images murine atherosclerotic plaques ex vivo. Arterioscler. Thromb. Vasc. Biol. 2006;26(3):584–89. doi: 10.1161/01.ATV.0000200106.34016.18. [DOI] [PubMed] [Google Scholar]
- 97.Roivainen A, Yli-Kerttula T. Whole-body distribution of (11)C-choline and uptake in knee synovitis. Eur. J. Nucl. Med. Mol. Imaging. 2006;33(11):1372–73. doi: 10.1007/s00259-006-0184-5. [DOI] [PubMed] [Google Scholar]
- 98.Kato K, Schober O, Ikeda M, et al. Evaluation and comparison of 11C-choline uptake and calcification in aortic and common carotid arterial walls with combined PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2009;36(10):1622–28. doi: 10.1007/s00259-009-1152-7. [DOI] [PubMed] [Google Scholar]
- 99.Bucerius J, Schmaljohann J, Bohm I, et al. Feasibility of 18F-fluoromethylcholine PET/CT for imaging of vessel wall alterations in humans—first results. Eur. J. Nucl. Med. Mol. Imaging. 2008;35(4):815–20. doi: 10.1007/s00259-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 100.Ben Haim S, Kupzov E, Tamir A, et al. Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J. Nucl. Med. 2004;45(11):1816–21. [PubMed] [Google Scholar]
- 101.Skajaa T, Cormode DP, Falk E, et al. High-density lipoprotein-based contrast agents for multi-modal imaging of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30(2):169–76. doi: 10.1161/ATVBAHA.108.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cormode DP, Briley-Saebo KC, Mulder WJ, et al. An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small. 2008;4(9):1437–44. doi: 10.1002/smll.200701285. [DOI] [PubMed] [Google Scholar]