Abstract
Background
Both cardiopulmonary bypass (CPB) and red blood cell (RBC) storage are associated with detrimental changes in RBC structure and function that may adversely affect tissue oxygen delivery. We tested the hypothesis that in cardiac surgery patients, RBC deformability and aggregation are minimally affected by CPB with autologous salvaged blood alone, but are negatively affected by the addition of stored allogeneic blood.
Methods
In this prospective cohort study, 32 patients undergoing cardiac surgery with CPB were divided into 3 groups by transfusion status: autologous salvaged RBCs alone (Auto; n=12), autologous salvaged RBCs + minimal (<5 units) stored allogeneic RBCs (Auto+Allo min; n=10), and autologous salvaged RBCs + moderate (≥5 units) stored allogeneic RBCs (Auto+Allo mod; n=10). Ektacytometry was used to measure RBC elongation index (deformability) and critical shear stress (aggregation) before, during, and for 3 days after surgery.
Results
In the Auto group, RBC elongation index did not change significantly from the preoperative baseline. In the Auto+Allo min group, mean elongation index decreased from 32.31 ± 0.02 (baseline) to 30.47 ± 0.02 (nadir on postoperative day 1) (P = 0.003, representing a 6% change). In the Auto+Allo mod group, mean elongation index decreased from 32.7 ± 0.02 (baseline) to 28.14 ± 0.01 (nadir on postoperative day 1) (P = 0.0001, representing a 14% change). Deformability then dose-dependently recovered toward baseline over the first 3 postoperative days. Changes in aggregation were unrelated to transfusion (no difference among groups). For the 3 groups combined, mean critical shear stress decreased from 359 ± 174 mPa to 170 ± 141 mPa (P = 0.01, representing a 54% change), with the nadir at the end of surgery, and returned to baseline by postoperative day 1.
Conclusions
In cardiac surgery patients, transfusion with stored allogeneic RBCs, but not autologous salvaged RBCs, is associated with a decrease in RBC cell membrane deformability that is dose-dependent and may persist beyond 3 postoperative days. These findings suggest that autologous salvaged RBCs may be of higher quality than stored RBCs, since the latter are subject to the so-called “storage lesions.”
Introduction
Compared to other surgical patients, those undergoing cardiac surgery have a relatively high likelihood of receiving allogeneic blood transfusions.1–3 Although transfusion can be lifesaving in some clinical scenarios, most observational studies have shown a clear relationship between transfusion and adverse outcomes.4–7 In prospective clinical trials that have compared liberal to restrictive transfusion strategies, the findings have revealed either no benefit8–12 or increased morbidity and mortality13 associated with increased use of transfusions.
Of primary concern are red blood cell (RBC) quality and the detrimental biochemical and structural changes that occur during RBC storage, which have been termed storage lesions.14,15 Although the current “shelf life” of stored blood is 42 days, there is evidence that after some duration of RBC storage, perhaps even 15–21 days, the capacity for tissue oxygen delivery is diminished.16–18 In fact, studies have described adverse outcomes,19,20 including increased mortality,21,22 in patients who receive blood that has been stored for longer durations. Another concern is the potential adverse effects of cardiopulmonary bypass (CPB) and the resulting injury to the RBC cell membrane.23–25 Both the storage of blood26–28 and CPB29,30 have been shown to “stiffen” the cell membrane, resulting in a loss of deformability that may impair the ability of cells to traverse the small capillary vascular beds.31 In addition, storage of RBCs is associated with increased cellular aggregation,15,32 which may further limit blood flow and thus oxygen delivery.
Previous studies that have assessed changes in RBC structure and function after CPB may be confounded because the effect of CPB is difficult to differentiate from the effect of stored RBC transfusions. Some published reports fail to mention whether blood was transfused at all,23,25,29 and to our knowledge, the “dose response” of stored, transfused RBCs on the resulting quality of circulating RBCs has not been described.
One measure of RBC quality is cell membrane deformability, or the ability for the cell to elongate when exposed to shear stress while being forced through a thin channel (microfluidic slit flow ektacytometry).33,34 Using this method we have previously shown that both fresh RBCs drawn directly from un-transfused patients, as well allogeneic RBC stored for shorter duration (<21 days), have a greater elongation index (average ≈ 0.33), compared to RBCs stored for ≥21 days (average ≈ 0.28).26 RBC cell membrane elasticity and the ability to change shape is necessary for the cells to pass through small capillaries which can be smaller in diameter (5–10 microns) than the RBCs themselves (≈6–8 micons).35,36 Impaired RBC cell membrane deformability is associated with “entrapment and blockage” in the microcirculation, as well as reduced regional blood flow and reduced oxygen delivery.31,37
In the current prospective observational cohort study, we assessed RBC deformability and aggregation before and after CPB in patients who received autologous salvaged blood, with or without stored allogeneic blood. The primary hypothesis being tested was that CPB with autologous salvaged blood alone has minimal effects on circulating RBCs, whereas the addition of transfused stored allogeneic blood is associated with dose-dependent adverse changes in RBC deformability and aggregation.
Methods
Patient and Anesthetic Management
After receiving IRB approval (The Johns Hopkins Medical Institutions) and written informed consent, we enrolled 32 patients undergoing cardiac surgery with or without concomitant ascending aortic repair between July 2012 and July 2013. Inclusion criteria were the planned use of CPB and autologous blood salvage. Exclusion criteria included sickle cell disease or other known RBC disorders, and the refusal of blood transfusion.
Intraoperative care was standardized, and all patients received general anesthesia with a combination of midazolam, fentanyl, and isoflurane with vecuronium given for muscle relaxation. Ventilatory goals were to maintain normocapnia during surgery. Heparin (350 IU•kg−1) was used for anticoagulation, which was monitored by the kaolin activated clotting time and maintained at a level of >480 seconds. The extracorporeal circuit consisted of roller pumps (Terumo, Tokyo, Japan), a hollow fiber membrane oxygenator (Sorin, Milan, Italy), and a standard arterial line filter (Sorin). The priming consisted of 1,600 mL of lactated Ringer’s solution and a retrograde autologous prime of 700 mL. Pump flow was adjusted to 2.2 L•m−2•min−1. Nasopharyngeal temperature during CPB was maintained at 32°C, followed by rewarming to a urinary bladder temperature ≥35°C. After the termination of CPB, heparin was neutralized by protamine in a 1:1 ratio.
Autologous blood salvage (Brat-2, Sorin Group, Milan, Italy) was used during CPB to recover shed blood from the surgical field, and after CPB to process blood from the extracoporeal circuit. Blood was suctioned (150 mmHg vacuum level) into a reservoir through a 120-μm filter. Cell processing occurred using a 125-mL Baylor bowl and the standard fill rate, wash rate, and wash volume recommended by the manufacturer. Intraoperatively, both allogeneic and autologous salvaged RBC units were administered to maintain hemoglobin concentration above 7 g/dL during CPB, and above 8 g/dL after CPB. Postoperatively, hemoglobin concentration was maintained above 8 g/dL. Autologous salvaged RBCs, if available, were always given before stored allogeneic RBCs. Duration of allogeneic RBC storage was not controlled and was based on the usual practice at our institution.
All patients had radial artery catheters that were used for obtaining blood samples during and after surgery. Blood samples were drawn from the patient into heparinized syringes before incision, during CPB, upon separation from CPB, at completion of surgery, and in the morning of postoperative days (POD) 1, 2, and 3. After the arterial catheter was removed (most often on POD 2), venous samples were obtained by phlebotomy. All blood samples were stored on ice and taken promptly (within 1 hour) to the laboratory where the measurements were performed.
Ektacytometry
RBC deformability and aggregation were measured with a microfluidic slit-flow ektacytometer (Rheo Meditech, Seoul, South Korea). The principle behind this measurement has been described in detail.33 For deformability measurements, RBCs were suspended (final hematocrit ~ 0.5%) in a highly viscous polyvinylpyrrolidone solution (viscosity ~30 cP) with slow mixing and then loaded onto a sample reservoir of a microfluidic chip. During operation, a vacuum-generating mechanism allowed the sample to flow toward the waste reservoir through the micro-channel at a range of shear stresses (0.5 to 20 Pa), while the elliptical diffraction pattern of the flowing cells was generated by a laser beam (wavelength = 635 nm from a 1.5 mW laser diode) focused onto the micro-channel. Deformability is expressed as the elongation index, which was defined as (L − W)/(L + W), where L and W are the major and minor axes of the ellipse, respectively, at various shear stress values. The elongation index values used for analysis were those measured at the shear stress level of 3 Pa, as suggested by Baskurt.38 Higher elongation index values represent increased RBC cell membrane deformability.
RBC aggregation was measured with the same instrument but with a different cuvette and undiluted blood. The critical shear stress (measured in mPa) required to disperse RBCs was used to assess aggregation. Higher shear stress values represent greater RBC aggregation.
Data Analysis
We performed a sample size calculation based on previously collected RBC deformability data using the mean change in deformability attributed to stored allogeneic blood transfusion.26 In order to detect a change in the elongation index of 0.2 ± 0.15 with an alpha of 0.05 and power of 0.8, the estimated sample size was 10 patients per study group.
For the primary analysis we designated 3 groups of patients: autologous salvaged RBCs only (Auto group), autologous salvaged RBCs + minimal (<5 units) stored allogeneic RBC transfusion (Auto+Allo min group), and autologous salvaged RBCs + moderate (≥5 units) stored allogeneic RBC transfusion (Auto+Allo mod group). As in our previous study,26 the a priori definition of minimal and moderate transfusion was <5 units and ≥5 units, respectively (≈1/2 total blood volume), administered over the course of the 3-day study period. A secondary analysis was also performed with patients analyzed as 2 groups: patients who received autologous salvaged RBCs alone (Auto, n=12) and those who received autologous salvaged RBCs and any amount of stored allogeneic RBCs (Auto+Allo any, n=20).
Differences in the mean measured values within groups and between groups were analyzed by two-way repeated measures ANOVA. The Tukey-Kramer HSD post hoc test was used to adjust for multiple comparisons of means and Tukey-corrected P values are given. The statistical tests were predicated on the assumption of normal residuals and equal variance and covariance among groups. Dichotomous variables were analyzed by the Chi squared test. To assess the independent effects of CPB duration and quantity of blood transfused upon change in RBC deformability, a multiple linear regression was performed with maximum posttransfusion change in elongation index as the dependent variable. CPB duration (minutes) and stored allogeneic blood (units) were the two (and only) independent variables in this regression. All data are presented as mean ± SD, and P < 0.05 was used to define significance.
Results
Intraoperatively, all 32 patients received autologous salvaged RBCs. Twelve of these patients received autologous salvaged RBCs alone (Auto group). In addition to salvaged RBCs, 10 patients received 1–4 units of stored allogeneic RBCs (Auto+Allo min group), and 10 received 5–12 units of stored allogeneic RBCs (Auto+Allo mod group). The characteristics of these 3 groups of patients, including preoperative comorbid conditions, are shown in Table 1. There were minimal differences among groups except for less peripheral vascular disease in the Auto+Allo min group. Table 2 shows the surgical and transfusion data, comparing the 3 groups. Patients who had revision sternotomy received larger amounts of stored allogeneic RBCs. The number of stored allogeneic RBC units transfused was significantly different among the 3 groups. Duration of CPB was significantly longer in the Auto+Allo mod group compared to the other 2 groups. Over the 3 PODs, only 1 patient who did not require intraoperative stored allogeneic blood received any postoperative stored allogeneic blood, and this patient (who received 3 postoperative units) was included in the Auto+Allo min group. The average duration of storage for the allogeneic blood was 24 ± 6 days, with a median of 25 days (range, 8–34 days). Forty-nine autologous salvaged RBC units and 86 stored allogeneic RBC units were transfused.
Table 1.
Patient Characteristics
| Variable | Auto group (n=12) | Auto+Allo min group (n=10) | Auto+Allo mod group (n=10) | P Value |
|---|---|---|---|---|
| Age (yrs) | 61 ± 11 | 66 ± 10 | 65 ± 19 | 0.63 |
| Sex | ||||
| Male | 10/12 (83%) | 7/10 (70%) | 5/10 (50%) | 0.43 |
| Female | 2/12 (17%) | 3/10 (30%) | 5/10 (50%) | |
| Diabetes | 4/12 (33%) | 3/10 (30%) | 3/10 (30%) | 0.97 |
| Hypertension | 10/12 (83%) | 5/10 (50%) | 6/10 (60%) | 0.43 |
| Obesity (BMI>30) | 2/12 (17%) | 1/10 (10%) | 1/10 (10%) | 0.37 |
| Renal insufficiency | 2/12 (17%) | 5/10 (50%) | 2/10 (20%) | 0.10 |
| Congestive heart failure | 4/12 (33%) | 6/10 (60%) | 4/10 (40%) | 0.30 |
| Peripheral vascular disease | 4/12 (33%) | 1/10 (10%) | 4/10 (40%) | 0.04 |
| Chronic obstructive pulmonary disease | 3/12 (25%) | 2/10 (20%) | 2/10 (20%) | 0.85 |
Auto = autologous salvaged RBCs only.
Auto+Allo min = autologous salvaged RBCs + minimal (<5 units) stored allogeneic RBCs.
Auto+Allo mod = autologous salvaged RBCs + moderate (≥5 units) stored allogeneic RBCs.
BMI = Body mass index.
RBC = Red blood cell.
Table 2.
Surgical and Transfusion Data
| Variable | Auto group (n=12) | Auto+Allo min group (n=10) | Auto+Allo mod group (n=10) |
|---|---|---|---|
| Type of surgery | |||
| CABG or valve | 93% | 90% | 50% |
| Vascular/aortic | 17% | 10% | 50% |
| Revision-sternotomy | 8% | 40% a | 80% b |
| Blood salvage (mL) | 527 ± 165 | 703 ± 286 | 635 ± 291 |
| Intraoperative allogeneic RBC units | 0 | 2 ± 1 a | 7 ± 3 b |
| Postoperative allogeneic RBC units | 0 | 1 ± 1 a | 3 ± 3 b |
| All allogeneic RBC units | 0 | 3 ± 1 a | 10 ± 4 b |
| CPB duration (min) mean±SD (range) | 103±46 (51–181) | 114±31 (79–180) | 226±87 (103–324)b |
Auto = autologous salvaged RBCs only.
Auto+Allo min = autologous salvaged RBCs + minimal (<5 units) stored allogeneic RBCs.
Auto+Allo mod = autologous salvaged RBCs + moderate (≥5 units) stored allogeneic RBCs.
CABG = coronary artery bypass grafting; CPB = cardiopulmonary bypass.
RBC = Red blood cell.
P < 0.05 vs. Auto group.
P < 0.05 vs. Auto group and Auto+Allo min group.
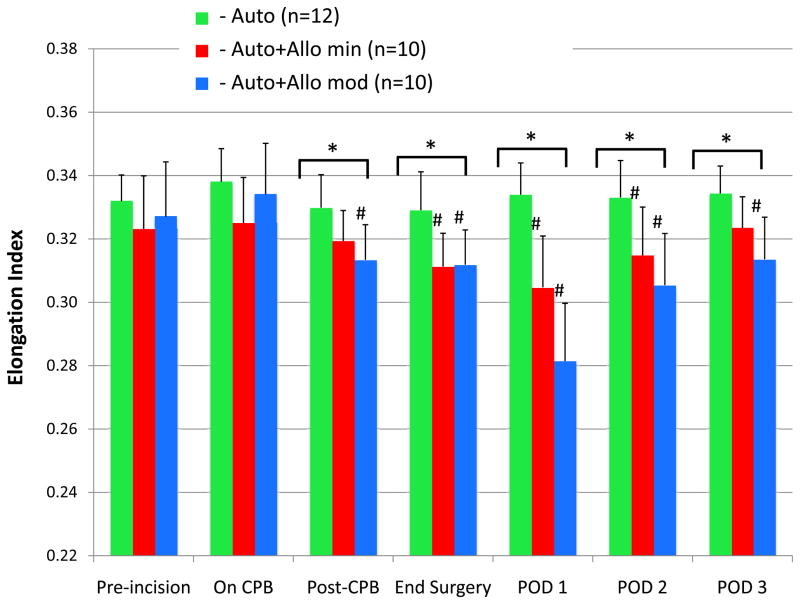
The assumptions that were made in performing the statistical analyses were satisfied after plotting histograms of the residuals, and comparison of variances and covariances to the means for the different groups. Figure 1 illustrates the 3-group comparison, which shows the changes in the RBC elongation index (deformability). Significant between-group differences were apparent at the end of CPB that persisted for the duration of the study and were still present on POD 3. There were also significant within-group changes in elongation index from baseline that persisted until POD 2 in the Auto+Allo min group, and for the duration of the study in the Auto+Allo mod group. Mean elongation index decreased in the Auto+Allo min group from the preincision baseline value of 32.31 ± 0.02 to a nadir of 30.47 ± 0.02 on POD 1 (P = 0.003), which is a 6% change. Mean elongation index in the Auto+Allo mod group decreased from 32.7 ± 0.02 (preincision baseline), to a nadir of 28.14 ± 0.01 on POD 1 (P < 0.0001), which is a 14% change. The Tukey-corrected P values revealed significant differences between the Auto and Auto+Allo min groups: after CPB (P=0.03), end surgery (P=0.008), POD 1 (P=0.003), and POD 2 (P=0.03). Likewise, corrected P values revealed significant differences between the Auto and Auto+Allo mod groups: after CPB (P=0.004), end surgery (P=0.006), POD 1 (P<0.0001), and POD 2 (P=0.01), and POD 3 (P=0.02). These findings illustrate a dose-response effect for decreased RBC deformability after transfusion with stored allogeneic blood.
Figure 1.
Red blood cell (RBC) deformability, represented as the elongation index measured by ektacytometry, is compared among the 3 groups. In patients who received autologous salvaged RBCs only, elongation index did not change from baseline over the 3-day study period. For patients who received autologous salvaged RBCs and <5 units stored allogeneic RBCs, the mean elongation index decreased by 6%, reached a nadir on postoperative day (POD) 1, and remained significantly lower than baseline until POD 2. For those who received autologous salvaged RBCs and ≥5 units stored allogeneic RBCs, the mean elongation index decreased by 14%, reached a nadir on POD 1, and remained significantly lower than baseline for all 3 postoperative days. The findings illustrate a “dose-response” for decreasing RBC deformability with increased number of stored allogeneic RBC transfusions.
Auto = autologous salvaged RBCs only.
Auto+Allo min = autologous salvaged RBCs + minimal (<5 units) stored allogeneic RBCs.
Auto+Allo mod = autologous salvaged RBCs + moderate (≥5 units) stored allogeneic RBCs.
CPB = cardiopulmonary bypass.
*P < 0.05 for between-group differences; #P < 0.05 for within-group difference from pre-incision baseline.
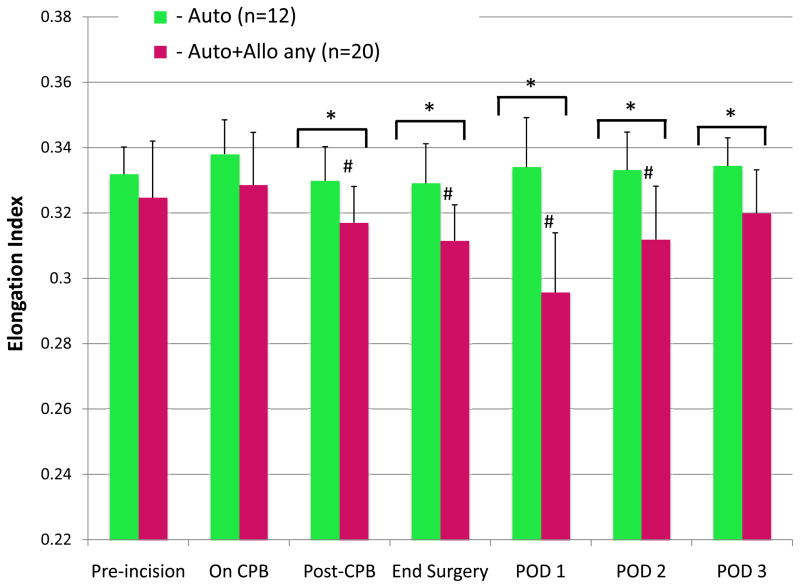
Elongation index was significantly lower in patients who received any stored allogeneic blood (Auto+Allo any) than in those who did not (Auto) beginning at the end of CPB (Fig. 2). This difference remained significant for the duration of the study. Mean elongation index decreased in the the Auto+Allo group from a preincision baseline value of 32.46 ± 0.02 to a nadir of 29.56 ± 0.01 on POD 1 (P = 0.003), which is a 9% change. The Tukey-corrected P values revealed significant differences between the Auto and Auto+Allo any groups: after CPB (P=0.04), end surgery (P=0.01), POD 1 (P=0.003), and POD 2 (P=0.02) and POD 3 (P=0.03). The Auto group exhibited no significant changes from the preincision baseline value at any time during the study.
Figure 2.
Red blood cell (RBC) deformability, represented as the elongation index measured by ektacytometry, is compared between 2 groups. In patients who received autologous salvaged RBCs only, elongation index did not change from baseline over the 3-day study period. For patients who received autologous salvaged RBCs and any amount of stored allogeneic RBCs, the mean elongation index decreased by 9% (P = 0.001), reached a nadir on postoperative day (POD) 1, and remained significantly lower than baseline until POD 3.
Auto = autologous salvaged RBCs only.
Auto+Allo any = autologous salvaged RBCs and any amount of stored allogeneic RBCs.
CPB = cardiopulmonary bypass.
*P < 0.05 for between-group differences; #P < 0.05 for within-group difference from pre-incision baseline.
Since longer duration of CPB was necessary in the patients who received the largest amount of stored allogeneic transfused blood, a multiple linear regression was used to assess both these variables as independent predictors of changes in deformability. The results of this analysis showed that the total number of units of stored allogeneic blood transfused was a significant independent predictor of maximum posttransfusion change in RBC deformability (P=0.004), but CPB duration was not (P=0.52).
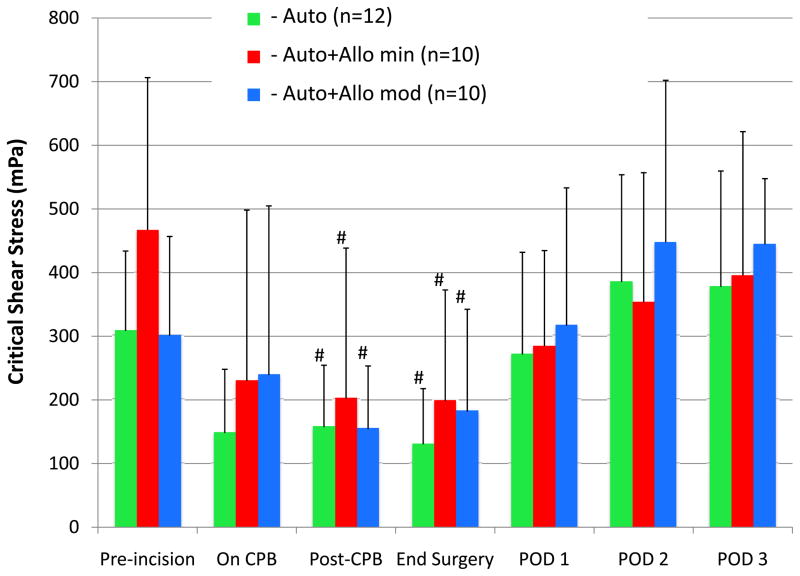
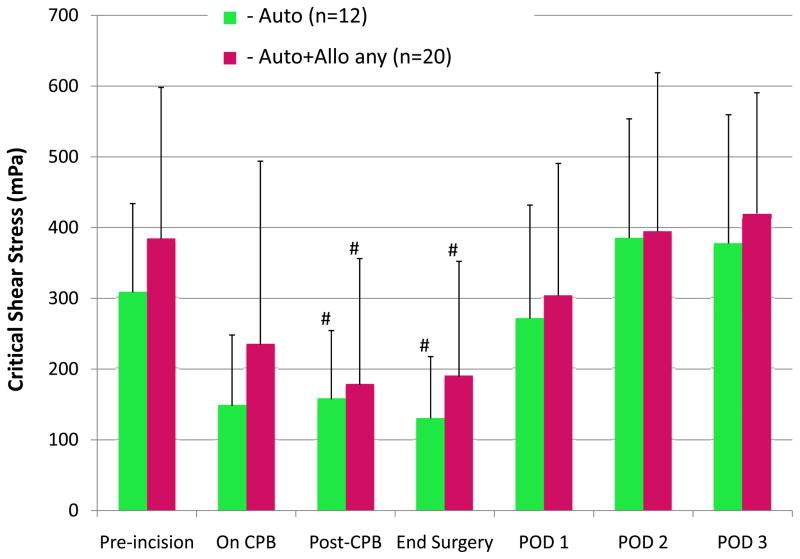
Figure 3 shows the changes in RBC critical shear stress (aggregation) over time for the 3 groups. No differences were observed among groups, but with all patients in the three groups combined, aggregation decreased to a nadir at the end of surgery and returned to baseline by POD 1. The mean critical shear stress value for all patients combined decreased from a preincision baseline of 359 ± 174 mPa to a nadir of 170 ± 141 mPa at the end of surgery (P = 0.01, Tukey-corrected), which is a 54% change. Figure 4 shows a similar comparison of changes in RBC critical shear stress between the Auto and Auto+Allo any groups. Again, there were no between-group differences at any time point, but values for both groups combined were significantly decreased at the end of CPB Tukey-corrected P = 0.01), and returned to baseline by POD 1.
Figure 3.
Red blood cell (RBC) aggregation, represented as the critical shear stress measured by ektacytometry, is compared in the 3 groups. Critical shear stress did not differ among the transfusion groups, but the changes within groups were significant. For all groups, critical shear stress decreased significantly by the end of cardiopulmonary bypass (CPB) and returned to baseline levels by postoperative day (POD) 1. These findings indicate a transient, reversible decrease in RBC aggregation associated with CPB that is unrelated to transfusion.
Auto = autologous salvaged RBCs only.
Auto+Allo min = autologous salvaged RBCs + minimal (<5 units) stored allogeneic RBCs.
Auto+Allo mod = autologous salvaged RBCs + moderate (≥5 units) stored allogeneic RBCs.
#P < 0.05 for within-group difference from pre-incision baseline.
Figure 4.
Red blood cell (RBC) aggregation, represented as the critical shear stress measured by ektacytometry, is compared in 2 groups. Critical shear stress did not differ between transfusion groups, but the changes within groups were significant. For both groups, critical shear stress decreased significantly by the end of cardiopulmonary bypass (CPB) and returned to baseline levels by postoperative day (POD) 1. These findings indicate a transient, reversible decrease in RBC aggregation associated with CPB that is unrelated to transfusion.
Auto = autologous salvaged RBCs only.
Auto+Allo any = autologous salvaged RBCs and any amount of stored allogeneic RBCs.
#P < 0.05 for within-group difference from pre-incision baseline.
Discussion
In this study, CPB combined with autologous salvaged RBC transfusion was not associated with changes in RBC deformability. In contrast, stored allogeneic RBC transfusion was associated with a dose-dependent loss of RBC deformability. The reversal of these changes was also dose-dependent, with return to baseline by POD 3 when the patients received <5 units of stored blood, but incomplete reversal by POD 3 after ≥5 units of transfused blood. The decrease in RBC aggregation associated with CPB was unrelated to transfusion. It returned to baseline by POD 1 and never increased above baseline measurements.
These findings suggest that fresh autologous salvaged RBCs may be of higher quality than stored allogeneic RBCs in regard to cell membrane deformability, an important determinant of blood flow in the microcirculation and thus of tissue oxygen delivery.31,35,39,40 The possibility that deformability of stored RBCs is impaired in a dose-dependent fashion may in part explain previous findings that the incidence of adverse outcomes is higher in patients who receive larger amounts of stored blood.4,11,41 If the laboratory-measured changes in our study do have clinical relevance, our findings would support the use of fresh autologous salvaged blood when possible, rather than stored allogeneic blood, especially when storage duration is prolonged.
Abundant evidence suggests that stored allogeneic transfused RBCs are functionally impaired relative to fresh circulating endogenous RBCs.16,26,32,42,43 Evidence also indicates that CPB adversely affects RBC structure and function,23–25 and one study has shown functional changes in salvaged autologous RBCs tested in vitro, but not in blood sampled from patients after salvaged blood transfusion.44 In our study, we found that neither salvaged RBCs nor CPB, when studied in combination, had a detrimental effect on RBC deformability. It is of note that earlier studies showing a 50–60% loss of deformability with CPB were performed with older technology, such as bubble oxygenators, which have been implicated in RBC damage and loss of deformability.23,29 In the current study, all patients had CPB with membrane oxygenators, which have been shown to preserve RBC function without causing loss of deformability.30 One particular limitation of previous research into the effects of CPB is lack of information on whether the patients received allogeneic transfusion.23,25,29 Thus, one cannot exclude the possibility that transfusion of stored RBCs confounds the effects of CPB on RBC deformability.
Although the storage lesion with allogeneic blood has been well described, it is unclear whether the various detrimental changes that RBCs undergo during storage are reversible after transfusion. The loss of 2,3-diphosphoglycerate, for example, is thought to return to baseline levels by 2 or 3 days after transfusion.45 Although nitric oxide is rapidly depleted during RBC storage,42 it is controversial whether repletion occurs after transfusion, and how quickly the levels return to baseline.14,46 Some data suggest that the decrease in RBC cell membrane deformability is irreversible,26 but the evidence is insufficient to clearly answer the question. Our findings suggest that the effect of allogeneic blood transfusion is dose dependent, and that when large amounts of stored RBCs are administered, the loss of deformability may persist for as long as 3 days, although the data did show a trend toward return to baseline. Of course it remains to be determined whether statistically significant changes in ektacytometry measurements reflect a clinically significant loss in overall RBC function.
In contrast to RBC deformability, little is known about the effects of CPB on RBC aggregation. CPB has been associated with a transient decrease in RBC aggregation, as shown in a study that compared patients who had undergone cardiac surgery either “on-pump” or “off-pump.” 25 Although this particular study comprehensively measured changes in RBC morphology and aggregation (by two different methods), the authors provided no information on whether the patients were transfused. However, the findings with regard to changes in aggregation were very similar to ours, with a transient decrease during CPB and return to baseline levels within 48 hours. Other investigators have shown that RBC aggregation in processed autologous salvaged blood, both before and after transfusion, is associated with no changes in aggregation.44 The changes that we measured were a transient decrease in aggregation that returned to baseline values by POD 1. We found no evidence of increased aggregation above baseline levels at any time point, an event that has been implicated in reduced blood flow in the microcirculation.47
Potential limitations in this study include the following. Because no patients had CPB without RBC transfusion, we are unable to comment about the effects of CPB alone on RBC characteristics. As autologous blood salvage has become routine practice for most cardiac surgical procedures, the 3 groups in our study likely represent the most common clinical scenarios, making our study design clinically relevant. A second limitation is that no patients received stored allogeneic RBCs in the absence of autologous salvaged RBCs. This scenario would also be uncommon, so again, the findings we report are most clinically relevant when considering routine practice. Another limitation is the potential confounding effect of longer-duration CPB in the Auto+Allo mod group. Hence, we cannot definitively state that the adverse changes in RBC deformability were attributable to increased transfusion. If CPB is harmful to RBC function, it is possible that confounding occurred, but we can conclude that the combination of prolonged CPB and increased stored blood transfusion appear to be detrimental. Although not definitive, the results of our multivariate analysis suggest that duration of CPB has less effect on RBC cell membrane deformability, compared to the effect of stored allogeneic blood transfusion. Lastly, our findings might have been different had we transfused allogeneic RBCs with shorter storage durations. The 24-day average duration of storage for blood administered in our study would be considered “older” blood according criteria used in the RECESS48 and ABLE49 studies, two ongoing clinical trials designed to compare outcomes in patients who receive RBCs with shorter or longer duration of storage.
In summary, for patients undergoing cardiac surgery with CPB, transfusion of salvaged autologous RBCs alone had no demonstrable adverse effect on RBC deformability or aggregation. When stored allogeneic RBCs were transfused, RBCs exhibited dose-dependent detrimental changes in deformability and a dose-dependent reversal of these changes. We also observed a transient decrease in RBC aggregation after CPB that was unrelated to transfusion. Our findings may have clinical implications for some patients, becauses transfusion of stored blood may not achieve the desired effect, namely improved oxygen delivery and improved outcome. In addition, the detrimental changes in RBC quality associated with storage may also explain the failure to show benefit in clinical trials that use stored allogeneic blood with a liberal transfusion strategy.
Acknowledgments
Funding: Support was provided from institutional and/or departmental sources and the National Institutes of Health R01 HL105296-03 (to D.E.B.), R01 HL092259-01 (to C.W.H.), and the New York Community Trust (to S.M.F.).
The authors wish to thank the Johns Hopkins cardiac surgeons and the clinicians in the CVSICU (Cardiovascular Surgery Intensive Care Unit) for allowing the patients to participate in this study. They also greatly appreciate the efforts of Elizabeth Dackiw R.N. (Research Nurse Coordinator), and Claire Levine (editorial assistance).
Footnotes
Reprints will not be available from the authors.
DISCLOSURES:
Name: Osman N. Salaria, MD
Contribution: Study design, data collection, data analysis, manuscript preparation.
Attestation: Osman Salaria approved the final manuscript. Osman Salaria also attests to the integrity of the original data and the analysis reported in this manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Viachaslau M. Barodka, MD
Contribution: Study design, data collection, data analysis, manuscript preparation.
Attestation: Viachaslau M. Barodka approved the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Charles W. Hogue, MD
Contribution: Study design, manuscript preparation.
Attestation: Charles W. Hogue approved the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Dan E. Berkowitz, MD
Contribution: Study design, manuscript preparation.
Attestation: Dan E. Berkowitz approved the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Paul M. Ness, MD
Contribution: Manuscript preparation.
Attestation: Paul M. Ness approved the final manuscript.
Conflicts of Interest: Paul M. Ness has consulted for TerumoBCT (Lakewood, Colorado) and Fenwal Labs (Lake Zurich, Illinois), both companies involved with blood storage.
Name: Jack O. Wasey, MD
Contribution: Data collection and analysis.
Attestation: Jack O. Wasey approved the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Steven M. Frank, MD
Contribution: Study design, conduct of the study, data collection, data analysis, manuscript preparation.
Attestation: Steven M. Frank approved the final manuscript. Steven M. Frank also attests to the integrity of the original data and the analysis reported in this manuscript. Steven M. Frank is the archival author.
Conflicts of Interest: Steven M. Frank has consulted for Haemonetics Corp. (Braintree, Massachusetts), a company involved with blood salvage equipment.
Contributor Information
Osman N. Salaria, Department of Anesthesiology/Critical Care Medicine, The Johns Hopkins Medical Institutions, Baltimore, Maryland.
Viachaslau M. Barodka, Department of Anesthesiology/Critical Care Medicine, The Johns Hopkins Medical Institutions, Baltimore, Maryland.
Charles W. Hogue, Department of Anesthesiology/Critical Care Medicine, The Johns Hopkins Medical Institutions, Baltimore, Maryland.
Dan E. Berkowitz, Department of Anesthesiology/Critical Care Medicine and Biomedical Engineering, The Johns Hopkins Medical Institutions, Baltimore, Maryland.
Paul M. Ness, Department of Pathology, The Johns Hopkins Medical Institutions, Baltimore, Maryland.
Jack O. Wasey, Department of Anesthesiology/Critical Care Medicine, The Johns Hopkins Medical Institutions, Baltimore, Maryland.
Steven M. Frank, Department of Anesthesiology/Critical Care Medicine, The Johns Hopkins Medical Institutions, Baltimore, Maryland.
References
- 1.Bennett-Guerrero E, Zhao Y, O’Brien SM, Ferguson TB, Jr, Peterson ED, Gammie JS, Song HK. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–75. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 2.Frank SM, Savage WJ, Rothschild JA, Rivers RJ, Ness PM, Paul SL, Ulatowski JA. Variability in blood and blood component utilization as assessed by an anesthesia information management system. Anesthesiology. 2012;117:99–106. doi: 10.1097/ALN.0b013e318255e550. [DOI] [PubMed] [Google Scholar]
- 3.Frank SM, Resar LM, Rothschild JA, Dackiw EA, Savage WJ, Ness PM. A novel method of data analysis for utilization of red blood cell transfusion. Transfusion. 2013 doi: 10.1111/trf.12227. [DOI] [PubMed] [Google Scholar]
- 4.Koch CG, Li L, Duncan AI, Mihaljevic T, Loop FD, Starr NJ, Blackstone EH. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg. 2006;81:1650–7. doi: 10.1016/j.athoracsur.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, Salloum R, Meredith UW, Osler TM. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283–92. doi: 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- 6.Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–6. doi: 10.1016/s0003-4975(02)03766-9. [DOI] [PubMed] [Google Scholar]
- 7.Kuduvalli M, Oo AY, Newall N, Grayson AD, Jackson M, Desmond MJ, Fabri BM, Rashid A. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur J Card Thor Surg. 2005;27:592–8. doi: 10.1016/j.ejcts.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 9.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacroix J, Hebert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, Joffe A, Biarent D, Meert K, Peters MJ. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 11.Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leao WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NA, Auler JO., Jr Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 12.Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, Radovancevic B, McAllister HA, Jr, Cooley DA. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39:1070–7. doi: 10.1046/j.1537-2995.1999.39101070.x. [DOI] [PubMed] [Google Scholar]
- 13.Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, Guarner-Argente C, Santalo M, Muniz E, Guarner C. Transfusion strategies for acute upper gastrointestinal bleeding. New Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 14.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol. 2009;16:515–23. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 15.Tinmouth A, Chin-Yee I. The clinical consequences of the red cell storage lesion. Transfus Med Rev. 2001;15:91–107. doi: 10.1053/tmrv.2001.22613. [DOI] [PubMed] [Google Scholar]
- 16.Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–9. [PubMed] [Google Scholar]
- 17.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfusion and Apheresis Science. 2010;43:69–78. doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raat NJ, Verhoeven AJ, Mik EG, Gouwerok CW, Verhaar R, Goedhart PT, de Korte D, Ince C. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model. Crit Care Med. 2005;33:39–45. doi: 10.1097/01.ccm.0000150655.75519.02. [DOI] [PubMed] [Google Scholar]
- 19.Gauvin F, Spinella PC, Lacroix J, Choker G, Ducruet T, Karam O, Hebert PC, Hutchison JS, Hume HA, Tucci M. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion. 2010;50:1902–13. doi: 10.1111/j.1537-2995.2010.02661.x. [DOI] [PubMed] [Google Scholar]
- 20.Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg JA, McGwin G, Jr, Vandromme MJ, Marques MB, Melton SM, Reiff DA, Kerby JD, Rue LW., 3rd Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69:1427–31. doi: 10.1097/TA.0b013e3181fa0019. discussion 31–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekestrom S, Koul BL, Sonnenfeld T. Decreased red cell deformability following open-heart surgery. Scand J Thorac Cardiovasc Surg. 1983;17:41–4. doi: 10.3109/14017438309102376. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama T, Yamaguchi H, Allers M, Roberts D. Evaluation of red cell damage during cardiopulmonary bypass. Scand J Thorac Cardiovasc Surg. 1985;19:263–5. doi: 10.3109/14017438509102729. [DOI] [PubMed] [Google Scholar]
- 25.Papp J, Toth A, Sandor B, Kiss R, Rabai M, Kenyeres P, Juricskay I, Kesmarky G, Szabados S, Toth K. The influence of on-pump and off-pump coronary artery bypass grafting on hemorheological parameters. Clin Hemorheol Microcirc. 2011;49:331–46. doi: 10.3233/CH-2011-1484. [DOI] [PubMed] [Google Scholar]
- 26.Frank SM, Abazyan B, Ono M, Hogue CW, Cohen DB, Berkowitz DE, Ness PM, Barodka VM. Decreased erythrocyte deformability after transfusion and the effects of erythrocyte storage duration. Anesth Analg. 2013;116:975–81. doi: 10.1213/ANE.0b013e31828843e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berezina TL, Zaets SB, Morgan C, Spillert CR, Kamiyama M, Spolarics Z, Deitch EA, Machiedo GW. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 28.Laczko J, Feo CJ, Phillips W. Discocyte--echinocyte reversibility in blood stored in CPD over a period of 56 days. Transfusion. 1979;19:379–88. doi: 10.1046/j.1537-2995.1979.19479250174.x. [DOI] [PubMed] [Google Scholar]
- 29.Hirayama T, Yamaguchi H, Allers M, Roberts D, William-Olsson G. Changes in red cell deformability associated with anaesthesia and cardiopulmonary bypass in open-heart surgery. Scand J Thorac Cardiovasc Surg. 1985;19:257–62. doi: 10.3109/14017438509102728. [DOI] [PubMed] [Google Scholar]
- 30.Hakoshima A, Goto H, Abe K, Benson KT, Moran JF, Arakawa K. Alteration of red cell deformability during extracorporeal bypass: membrane v bubble oxygenator. J Cardiothorac Anesth. 1989;3:189–92. doi: 10.1016/s0888-6296(89)92690-2. [DOI] [PubMed] [Google Scholar]
- 31.Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44:1626–34. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 32.Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48:136–46. doi: 10.1111/j.1537-2995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 33.Shin S, Ku Y, Park MS, Suh JS. Slit-flow ektacytometry: laser diffraction in a slit rheometer. Cytometry B Clin Cytom. 2005;65:6–13. doi: 10.1002/cyto.b.20048. [DOI] [PubMed] [Google Scholar]
- 34.Kwan JM, Guo Q, Kyluik-Price DL, Ma H, Scott MD. Microfluidic analysis of cellular deformability of normal and oxidatively damaged red blood cells. Am J Hematol. 2013;88:682–9. doi: 10.1002/ajh.23476. [DOI] [PubMed] [Google Scholar]
- 35.Chien S. Red cell deformability and its relevance to blood flow. Annu Rev Physiol. 1987;49:177–92. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- 36.Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemostasis. 2003;29:435–50. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 37.Cabrales P. Effects of erythrocyte flexibility on microvascular perfusion and oxygenation during acute anemia. Am J Physiol; Heart Circ Physiol. 2007;293:H1206–15. doi: 10.1152/ajpheart.00109.2007. [DOI] [PubMed] [Google Scholar]
- 38.Baskurt OK. Deformability of red blood cells from different species studied by resistive pulse shape analysis technique. Biorheology. 1996;33:169–79. doi: 10.1016/0006-355X(96)00014-5. [DOI] [PubMed] [Google Scholar]
- 39.Simchon S, Jan KM, Chien S. Influence of reduced red cell deformability on regional blood flow. Am J Physiol. 1987;253:H898–903. doi: 10.1152/ajpheart.1987.253.4.H898. [DOI] [PubMed] [Google Scholar]
- 40.Lipowsky HH, Cram LE, Justice W, Eppihimer MJ. Effect of erythrocyte deformability on in vivo red cell transit time and hematocrit and their correlation with in vitro filterability. Microvasc Res. 1993;46:43–64. doi: 10.1006/mvre.1993.1034. [DOI] [PubMed] [Google Scholar]
- 41.Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 42.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Nat Acad Sci. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion. 1999;39:277–81. doi: 10.1046/j.1537-2995.1999.39399219284.x. [DOI] [PubMed] [Google Scholar]
- 44.Gu YJ, Vermeijden WJ, de Vries AJ, Hagenaars JA, Graaff R, van Oeveren W. Influence of mechanical cell salvage on red blood cell aggregation, deformability, and 2,3-diphosphoglycerate in patients undergoing cardiac surgery with cardiopulmonary bypass. The Ann Thorac Surg. 2008;86:1570–5. doi: 10.1016/j.athoracsur.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 45.Heaton A, Keegan T, Holme S. In vivo regeneration of red cell 2,3-diphosphoglycerate following transfusion of DPG-depleted AS-1, AS-3 and CPDA-1 red cells. Br J Haematol. 1989;71:131–6. doi: 10.1111/j.1365-2141.1989.tb06286.x. [DOI] [PubMed] [Google Scholar]
- 46.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med. 2004;36:707–17. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 47.Vicaut E. Opposite effects of red blood cell aggregation on resistance to blood flow. J Cardiovasc Surg (Torino) 1995;36:361–8. [PubMed] [Google Scholar]
- 48.RECESS Study http://clinicaltrials.gov/ct2/show/NCT00991341.
- 49.Lacroix J, Hebert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, Cook D, Marshall JC, McIntyre L, Turgeon AF. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Trans Med Rev. 2011;25:197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]