Abstract
Acid-sensing ion channel (ASIC) 1a subunit is expressed in synapses of central neurons where it contributes to synaptic plasticity. However, whether these channels can conduct Ca2+ and thereby raise the cytosolic Ca2+ concentration, [Ca2+]c, and possibly alter neuronal physiology has been uncertain. We found that extracellular acidosis opened ASIC1a channels, which provided a pathway for Ca2+ entry and elevated [Ca2+]c in wild-type, but not ASIC1-/-, hippocampal neurons. Acid application also raised [Ca2+]c and evoked Ca2+ currents in heterologous cells expressing ASIC1a. Although ASIC2a is also expressed in central neurons, neither ASIC2a homomultimeric channels nor ASIC1a/2a heteromultimers showed H+-activated [Ca2+]c elevation or Ca2+ currents. Because extracellular acidosis accompanying cerebral ischemia contributes to neuronal injury, we tested the effect of acidosis on cell death measured as lactate dehydrogenase release. Eliminating ASIC1a from neurons or treating ASIC1a-expressing cells with the ASIC blocker amiloride attenuated acidosis-induced cell injury. These results indicate that ASIC1a provides a non-voltage-gated pathway for Ca2+ to enter neurons. Thus, it may provide a target for modulation of [Ca2+]c.
The mammalian CNS expresses the degenerin/epithelial Na+ (DEG/ENaC) channel acid-sensing ion channel (ASIC) 1a (1, 2). In vivo, it is found in many brain regions and in areas with high synaptic density (3-5). In cultured neurons, recombinant ASIC1a can be found on the cell body, but it also localizes to dendrites and is present at synapses (6). In central neurons, ASIC1a forms both homomultimers and heteromultimers with ASIC2a (7-10). These complexes generate cation channels that are activated by a fall in the extracellular pH. Although the extracellular pH of the CNS is tightly controlled, synaptic activity may reduce extracellular pH by releasing the acidic contents of synaptic vesicles, especially during repetitive nerve activity (11, 12). The importance of ASIC1 for normal CNS physiology was demonstrated in mice bearing disruptions of the ASIC1 gene (4, 6). Loss of ASIC1 abolished hippocampal cation currents evoked by a pH 5 stimulus and impaired hippocampal synaptic plasticity. These abnormalities were accompanied by behavioral defects in spatial learning, eye-blink conditioning, and fear conditioning.
In diseases associated with extracellular acidosis, ASIC1a may also contribute to pathophysiology. During ischemia, extracellular pH falls, and the acidosis contributes to neuronal injury (13-15). Seizures and hyperglycemia can also generate an acidic extracellular environment that contributes to neuronal injury (16, 17). Prolonged exposure to an acidic environment may also alter the number of ASIC channels in central neurons (18), perhaps altering their responsiveness to acidosis.
However, the mechanisms by which ASIC1a contributes to normal physiology and pathophysiology remain uncertain. Because of the central role that cytosolic Ca2+ concentration, [Ca2+]c, plays in normal physiology and in disease, there has been speculation that ASIC channels might directly or indirectly increase [Ca2+]c. Previous work showed that ASICs conduct predominantly Na+ although other ion permeabilities have been reported. Several studies suggested that ASIC1a had an appreciable Ca2+ permeability and might constitute a Ca2+ entry pathway (2, 19-22). Other work suggested a very low Ca2+ permeability (23, 24). In addition, because ASIC currents desensitize with time, it has been suggested that these channels are not likely to make a significant contribution to membrane depolarization or changes in [Ca2+]c during prolonged acidosis (25).
Thus, it is uncertain whether ASIC1a can directly mediate Ca2+ influx and can increase [Ca2+]c in neurons. Moreover, the presence of ASIC1a/2a heteromultimers in central neurons raises the possibility that they might provide a pathway for Ca2+ entry. It is also uncertain whether ASIC1a might contribute to neuronal toxicity in the setting of ischemia or seizures through an H+-evoked increase in [Ca2+]c. Therefore, the goal of this work was to test the hypothesis that ASIC1 channels can alter [Ca2+]c. When we found that they did, we asked whether their activation might lead to neuronal injury.
Methods
Cells and Transfection. COS-7 and Chinese hamster ovary cells were cultured on collagen-coated glass cover slips or 35-mm Petri dishes. ASIC1a has similar function in both cell types (10). For measurements of [Ca2+]c, COS-7 cells were transfected by electroporation (26) and plated on collagen-coated glass cover slips. For patch-clamp studies and measurements of lactate dehydrogenase (LDH) release, Chinese hamster ovary cells, and COS-7 cells, respectively, were transfected with TransFast Lipid Reagent (Promega) and cultured on 35-mm Petri dishes. We used human ASIC1a and ASIC2a cDNA (27) and for patch-clamp studies a vector encoding green fluorescent protein (pGreenLantern, GIBCO) at a ratio of 6:1 ASIC:GFP. Experiments were performed 2-3 days after transfection.
Cultures of hippocampal neurons from mice with a disrupted ASIC1 gene and from wild-type littermates were prepared from postnatal day 1-2 pups as described (28). Cultures were maintained at 37°C with 5% CO2 in air for 10-14 days before use in the experiments.
Fluorescence Measurements. COS-7 cells were loaded with 1 μM fura-2, AM (Molecular Probes) for 1 h, washed, and mounted in a spectrofluorometer (F-4500, Hitachi, Tokyo). The standard recording medium contained (in mM): 140 NaCl/5 KCl/1 MgCl2/2 CaCl2/5 glucose/10 Hepes/10 2-(4-morpholino)-ethanesulfonic acid (Mes), pH 7.4 (NaOH). High K+ medium substituted 30 mM KCl for 30 mM of the NaCl; Na-free medium contained 140 mM N-methyl-D-glucamine instead of Na+; and nominally Ca2+-free medium contained no added Ca2+. The chamber was continuously perfused. Measurements of [Ca2+]c were performed as described (29). Fura-2 was excited at 340 and 380 nm, and emission was measured at 510 nm. Background fluorescence was determined after each experiment by quenching fura-2 fluorescence with 10 mM MnCl2 in nominally Ca2+-free medium in the presence of 5 μM ionomycin. Background fluorescence at 340 and 380 nm was subtracted from the respective experimental readings, and the ratio of signals at 340 and 380 nm was obtained. For Ca2+ concentration, Rmax and Rmin were determined after incubation of fura-2-loaded cells in the presence of 5 μM ionomycin and either 10 mM Ca2+-containing medium for 30 min or 10 mM EGTA-containing medium for 1 h, respectively. Data were digitized at 1-s intervals. In the experiments involving amiloride, calcium green 1 (Molecular Probes) was used instead of fura-2 to avoid interference of UV light-induced amiloride fluorescence. Calcium green-1 was excited at 506 nm, and fluorescence was measured at 531 nm.
Neurons plated on 25-mm round glass cover slips were loaded with fura-2, AM similar to COS-7 cells and mounted in the recording chamber of a Nikon Eclipse TE200 microscope. Optical recordings from cell bodies were performed with an I-PentaMAX charge-coupled device camera (Princeton Instruments, Trenton, NJ). A ×40 SuperFluor oil objective, a dichroic 7200 fura-2 filter set (Chroma Technology, Brattleboro, VT) and 340 and 380 excitation filters were used for all recordings. Recording and image analysis were performed by using metaf luor software (Universal Imaging, Media, PA). Measurements in COS-7 cells and neurons were performed at 22°C. Statistical significance was evaluated by ANOVA or Student's t test.
Electrophysiology. Transfected cells were identified in epifluorescence microscopy. For whole-cell patch clamp studies, transfected Chinese hamster ovary cells were bathed with (in mM): 107.5 NaCl/5.4 KCl/2 CaCl2/1 MgCl2/20.5 tetramethyl ammonium chloride (TMACl)/10 Hepes/10 Mes/5.55 glucose, pH 7.4. Before measurements of Ca2+ currents, we measured Na+ current as described (10). To ensure that cells were expressing heteromultimeric ASIC1a/2a channels, we checked the rate of recovery from desensitization in Na+-containing solution (not shown, but see refs. 10 and 30 for examples). For Ca2+ current experiments, the 2 mM Ca2+-solution contained (in mM): 2 CaCl2/10 Hepes/10 Mes/280 D-mannitol, pH 7.4 or pH 4.5. Ca2+-free solution contained (in mM): 10 Hepes, 10 Mes and 286 D-mannitol (pH 7.4 or pH 4.5). The pipette solution contained (in mM): 140 KCl/2 MgCl2/10 Hepes/10 Mes/5 D-mannitol/10 EGTA, pH 7.25. pH was adjusted with tetramethylammonium hydroxide (TMA-OH) or HCl, respectively. Holding voltage was -70 mV. Data acquisition and analyses were performed as described (10). Data were sampled at 5 kHz and filtered at 2 KHz.
LDH Release Assay. The degree of cell injury was assessed by a quantitative measurement of released LDH (31). Twenty-four hours after exposure to acidic solution, culture medium was removed, and cells were incubated with necessary cofactors as described by the manufacturer's assay kit (Sigma Diagnostics). One percent Triton X-100 in PBS was used to extract the remaining LDH from the cultures. Absorbance measurements at 340 nM were taken every 30 s for 3 min by using a 96-well microplate reader (GENios Spectra Plus, TECAN, Grödig, Austria). The resulting slopes defined LDH units per sample. Data are expressed as a ratio of LDH released/(LDH released + LDH Triton-X extracted).
Results
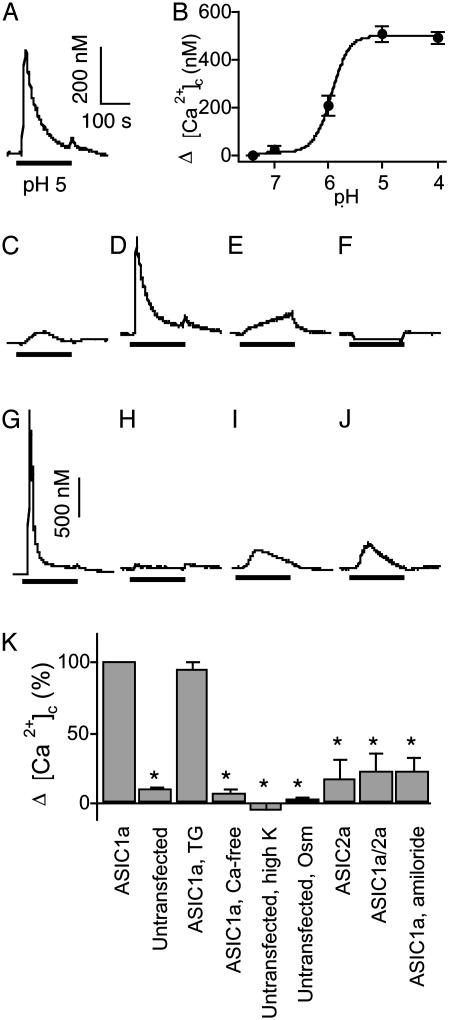
Acid-Evoked Elevations of [Ca2+]c in COS Cells Expressing ASIC1a. To learn whether ASIC1a activation could increase [Ca2+]c, we expressed this subunit in COS-7 cells and measured Ca2+-dependent changes in fura-2 fluorescence. Lowering extracellular pH from pH 7.4 to 5 evoked a rapid rise in [Ca2+]c that was not observed in untransfected cells (Fig. 1 A and C). More acidic extracellular solutions produced greater elevations of [Ca2+]c (Fig. 1B), with a half-maximal effect at approximately pH 5.9. This value is in the same range as previously reported relationships between pH and ASIC1a current activation (2, 23, 30, 32).
Fig. 1.
ASIC-mediated changes in [Ca2+]c in COS-7 cells. (A) [Ca2+]c elevation generated by a pH 5 stimulus (indicated by bar) in cells expressing ASIC1a. The pH of the control solution was 7.4. (Scale bars show change in [Ca2+]c for all panels except G, in which bar represents 1 μM.) (B) Relationship between the pH of the stimulus and the maximal [Ca2+]c increase in ASIC1a-transfected cells (n = 4). (C) Response of untransfected cells to a pH 5 stimulus. (D) Response to pH 5 stimulus in ASIC1a-transfected cells pretreated with 1 μM thapsigargin (TG). (E) Response to pH 5 in ASIC1a-transfected cells in nominally Ca2+-free medium. (F) Response to application of medium containing a high K+ concentration (35 mM) in untransfected cells, pH 7.4. (G) Response to pH 5 in Na+-free medium in ASIC1a-transfected cells. The increase in [Ca2+]c was 1.39 ± 0.37 μM (n = 6). (H) Response to 10% reduced osmolarity (pH 7.4) medium in untransfected cells. (I) Response to pH 5 stimulus in ASIC2atransfected cells. (J) Response to pH 5 stimulus in cells transfected with ASIC1a plus ASIC2a. (K) Average data from experiments shown in A, C-F, and H-J. Change in [Ca2+]c of ASIC1a-expressing cells is indicated as 100%. Data are mean ± SEM; n = 6-8. *, P < 0.05 compared with ASIC1a-transfected cells.
An elevation of [Ca2+]c could result from increased Ca2+ entry or from release of intracellular Ca2+ stores. To distinguish between these alternatives, we pretreated cells with 1 μM thapsigargin in nominally Ca2+-free medium for 10 min to empty intracellular Ca2+ stores and prevent their refilling (33). Subsequent applications of thapsigargin failed to induce Ca2+ transients, confirming its action (not shown). However, H+-evoked [Ca2+]c elevation remained the same as that in cells with intact Ca2+ stores (Fig. 1 D and K). Moreover, in the absence of extracellular Ca2+, an acid challenge failed to increase [Ca2+]c (Fig. 1 E and K). As an additional test of the involvement of ASIC1a, we applied the ASIC channel blocker amiloride (300 μM) (2) and found that it reduced the amplitude of the [Ca2+]c response (Fig. 1K).
We considered the possibility that an acid-evoked [Ca2+]c elevation might be secondary to ASIC1a-mediated Na+ influx that could depolarize the plasma membrane, thereby activating a voltage-sensitive Ca2+ influx pathway. Although we did not expect COS-7 cells to have such channels, we did two sets of studies to test this possibility. First, we applied a solution containing 35 mM K+ to depolarize untransfected COS-7 cells but found no increase in [Ca2+]c (Fig. 1 F and K). Second, we applied a pH 5 stimulus in the absence of Na+ to ASIC1atransfected cells. Instead of a reduced [Ca2+]c response, [Ca2+]c increased to an even greater extent (Fig. 1G); this result suggests that extracellular Na+ may attenuate the Ca2+ conductance of ASIC1a. We also tested the possibility that the H+ stimulus increased [Ca2+]c by enhancing Na+ influx and causing cell swelling. However, applying a solution with 10% reduced osmolarity to untransfected cells caused no change in [Ca2+]c (Fig. 1 H and K).
In addition to ASIC1a homomultimers, hippocampal neurons also express ASIC2, and some of the H+-activated current is produced by ASIC1a/2a heteromultimeric channels (3, 5, 7-10, 34). Therefore, we also tested these channels expressed in COS-7 cells. In contrast to ASIC1a, neither ASIC2a nor ASIC1a/2a heteromultimers generated a significant increase in [Ca2+]c in response to pH 5 (Fig. 1 I-K). Although it remains possible that more extreme reductions in pH could elevate [Ca2+]c, these results are consistent with the observation that presence of the ASIC2a subunit reduced Ca2+ permeability (7).
To further test whether ASIC channels conduct Ca2+, we used the whole-cell patch-clamp to measure Ca2+ current through heterologously expressed ASIC channels. With 2 mM Ca2+ as the only cation in the extracellular medium, an acid stimulus evoked an inward current (Fig. 2B). This current was not observed in Ca2+-free medium or in untransfected cells (Fig. 2 A and B). Moreover, ASIC2a and ASIC1a/2a channels yielded little if any Ca2+ current (Fig. 2 C and D). These data agree well with our measurements of [Ca2+]c, and taken together the results indicate that extracellular acidosis elevates [Ca2+]c by increasing Ca2+ influx across the plasma membrane through ASIC1a channels.
Fig. 2.
Acid-evoked currents in Chinese hamster ovary cells expressing ASIC subunits. Cells were superfused with Na+-free solution containing 2 mM Ca2+ (Left) or Ca2+-free solution (Right); both panels are from the same cell. The pH was reduced from 7.4 to 4.5 during times indicated by bars. (A) Untransfected cells. Maximal H+-activated current was -24 ± 6 pA in the presence of Ca2+ and -13 ± 5 pA in the absence of Ca2+ (n = 6). (B) Cells expressing ASIC1a. Maximal H+-activated current was -1432 ± 297 pA in the presence of Ca2+ and -43 ± 24 pA in the absence of Ca2+ (n = 6). (C) Cells expressing ASIC2a. Maximal H+-activated current was -24 ± 12 pA in the presence of Ca2+ and -35 ± 14 pA in the absence of Ca2+ (n = 5). (D) Cells expressing ASIC1a/2a. Maximal H+-activated current was -80 ± 71 pA in the presence of Ca2+ and -76 ± 35 pA in the absence of Ca2+ (n = 5).
ASIC1a-Dependent Increases in [Ca2+]c in Hippocampal Neurons. Based on the results described above, we asked whether activating ASIC1a would increase [Ca2+]c in central neurons. After 10-14 days in culture, hippocampal neurons showed spontaneous Ca2+ oscillations (Fig. 3A), likely related to spontaneous synaptic activity (35). When we applied a pH 6 solution, [Ca2+]c increased substantially, and the spontaneous oscillations disappeared. The elimination of the [Ca2+]c oscillations probably results from the known inhibitory effect of a reduced pH on Ca2+ channels (36).
Fig. 3.
[Ca2+]c response to a pH 6 stimulus in cultured mouse hippocampal neurons. (A) Representative traces from wild-type and ASIC1-/- neurons. Control solution was pH 7.4, and pH 6 application is indicated by bar. Average frequency and amplitude of the oscillations before pH 6 application were 4.1 ± 2.8 oscillations/min and 276 ± 64 nM Ca2+ for wild-type, and 4.4 ± 2.3 oscillations/min and 316 ± 71 nM Ca2+ for ASIC1-/-. These values were not statistically different. pH 6 eliminated oscillations in neurons of both genotypes but increased [Ca2+]c in ASIC1+/+ neurons only. (B) Basal [Ca2+]c in wild-type and ASIC1 null neurons. (C) Maximal change in [Ca2+]c induced by pH 6. (D) [Ca2+]c after 4 min of continuous superfusion of pH 6 solution. Data are mean ± SEM, n = 8. *, P < 0.05.
To learn whether the [Ca2+]c elevation required ASIC1a, we repeated the experiments using hippocampal neurons from ASIC1-/- mice. There were no significant differences between ASIC1-/- and wild-type neurons in basal [Ca2+]c or the frequency and amplitude of the spontaneous [Ca2+]c oscillations (Fig. 3 A and B and legend). However, in the absence of ASIC1, pH 6 solution failed to increase [Ca2+]c (Fig. 3 A and C). Moreover, after 4 min in a pH 6 solution, [Ca2+]c in wild-type neurons was elevated compared to that in ASIC1-/- neurons (Fig. 3D). As the pH fell, the amplitude of the H+-evoked [Ca2+]c elevation increased (Fig. 3E). Addition of nifedipine (2 μM) failed to prevent the acid-stimulated increase in [Ca2+]c (not shown). These results indicate that ASIC1a mediates an acidosis-induced Ca2+ influx that elevates [Ca2+]c. Although we cannot exclude some contribution of Ca2+ release from intracellular stores or entry through voltage-activated Ca2+ channels, the major component of Ca2+ entry seemed to be through ASIC1a.
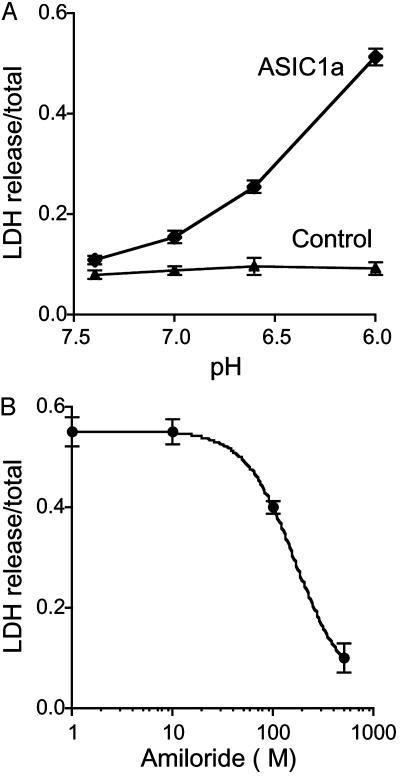
Acid-Induced Toxicity in Cells Expressing ASIC1a. Extracellular acidosis is thought to contribute to neuronal toxicity after ischemia and seizures (13, 17). Based on our finding that ASIC1a activation generates a significant elevation of [Ca2+]c in response to extracellular acidosis and the known potential for elevated [Ca2+]c to cause cell injury (37), we asked whether ASIC1a might mediate cell toxicity when pH falls. To test the potential contribution of ASIC1a, we applied acidic solutions to COS-7 cells expressing ASIC1a and then measured LDH release as an assay of cell damage. Exposure to acidic solutions increased LDH release in cells expressing ASIC1a, but not in cells expressing GFP as a control (Fig. 4A). Amiloride attenuated the toxicity, with half-maximal inhibition observed at ≈166 μM (Fig. 4B), a value within the range of amiloride concentrations (1 μM-1 mM) that inhibit ASIC currents in sensory neurons, PC12 cells, and Xenopus oocytes (2, 20).
Fig. 4.
Acidosis-induced LDH release from COS-7 cells expressing ASIC1a. (A) Effect of pH on LDH release from cells expressing ASIC1a or control cells (expressing GFP). Cells were exposed to solution of the indicated pH for 6 h, and LDH release was assessed 24 h later. (B) Effect of amiloride on LDH release from ASIC1a-transfected cells exposed to pH 6 solutions (n = 3).
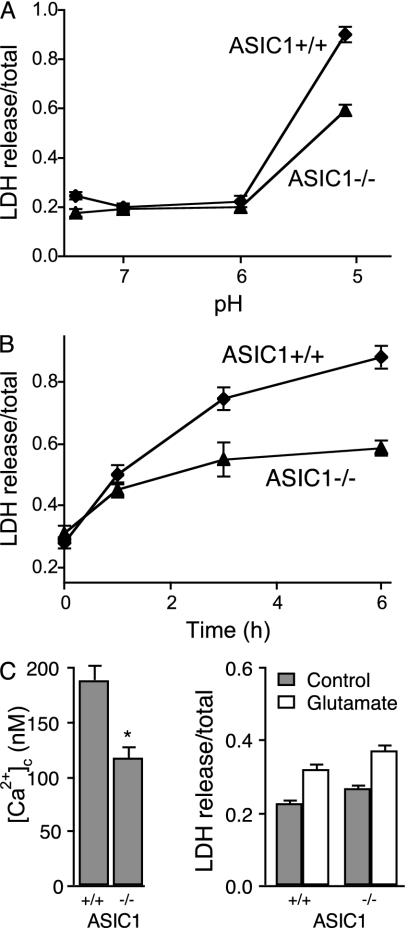
To test the hypothesis that ASIC1a activation could contribute to neuronal injury associated with extracellular acidosis, we studied hippocampal neurons from wild-type and ASIC1-/- animals. When extracellular pH was reduced to 5, wild-type neurons released more LDH than ASIC1-/- neurons; the injury depended on both the duration and extent of the acidosis (Fig. 5 A and B). When we measured [Ca2+]c 24 h after the acid stimulus, at the time the LDH measurements were performed, wild-type neurons had elevated [Ca2+]c compared to ASIC1-/- neurons (Fig. 5C). This result is consistent with an acid-induced injury and suggests the presence of either a persistent increase in Ca2+ entry or impaired Ca2+ extrusion mechanisms. In contrast to the protection from injury in the ASIC1 null neurons, glutamate addition induced a similar injury in neurons of both genotypes (Fig. 5D).
Fig. 5.
LDH release from hippocampal neurons. (A) Effect of pH on LDH release from wild-type and ASIC1a null neurons. Solutions were applied for 6 h, and LDH release measured 24 h later (n = 3). (B) Effect of duration of acid (pH 5) application on LDH release (n = 3). (C) [Ca2+]c in wild-type and ASIC1-/- neurons measured 24 h after exposure to a pH 6 solution for 6 h (n = 6). (D) Glutamate-induced LDH release in wild-type and ASIC1-/- neurons. Glutamate (100 μM) was applied for 30 min, and LDH release was measured 24 h later (n = 3). Gray bars indicate control (no treatment), and open bars indicate glutamate treatment.
Discussion
Hippocampal neurons express homomultimeric ASIC1a channels and heteromultimeric ASIC1a/2a channels (7-10). Our data indicate that activation of ASIC1a homomultimers provides a pathway for Ca2+ influx in hippocampal neurons. In contrast, ASIC1a/2a heteromultimers or ASIC2a homomultimers generate little Ca2+ influx. Thus, ASIC1 represents a non-voltage-gated pathway for Ca2+ entry into central neurons.
Previous studies have reported conflicting results about the Ca2+ permeability of ASIC1 channels. ASIC1a expressed in Xenopus oocytes has been reported to show significant Ca2+ permeability (PNa/PCa = 2.5) (2) or a very low Ca2+ permeability (PNa/PCa = 18.5 or greater) (23, 24). Studies of isolated rat sensory neurons, PC12, and HEK-293 cells expressing endogenous ASIC1a, and COS-7 cells expressing recombinant ASIC1a have reported H+-gated currents with PNa/PCa values ranging from 3.2 to 50 (19-22). The reason for these differences is not apparent. In some cases, the molecular identity of the channels was uncertain. Moreover, in some cases, permeability was measured by varying Ca2+ and Na+ concentrations in the presence of other monovalent and divalent cations; this result could limit the accuracy of the determinations, especially for a channel that may not only conduct Ca2+ but also be regulated by it (38, 39). Our studies of recombinant channels indicate that ASIC1a can carry Ca2+ current; an advantage of these studies is that Ca2+ was the only abundant extracellular cation. But more importantly, our measurements of [Ca2+]c in heterologous cells expressing recombinant subunits and in wild-type and ASIC1 null neurons indicate that ASIC1a serves as a pathway for Ca2+ entry. Moreover, in the presence of physiologic concentrations of extracellular Ca2+ and Na+, the acid-induced Ca2+ flux was sufficient to significantly raise [Ca2+]c.
Given the localization of ASIC1 at synapses (6) and the profound importance of [Ca2+]c in synaptic function and plasticity, the ability of ASIC1 to elevate [Ca2+]c at that location could have an important physiologic role. Consistent with that speculation, disruption of the ASIC1 gene in mice impaired hippocampal long-term potentiation, spatial learning, and eye-blink conditioning (4, 6). Thus, activating ASIC1 could elevate [Ca2+]c either directly by allowing Ca2+ influx or indirectly by depolarizing the plasma membrane and thereby activating voltage-gated Ca2+ channels.
The contribution of ASIC1 to physiologic [Ca2+]c alterations will depend on its ligand. Acidic extracellular solutions activate ASIC1, and it has been proposed that protons are the physiologic stimulus (2). Indeed, transient acidification of the extracellular space has been reported during stimulation of hippocampal slices and isolated hippocampal neurons (11, 12). The activity of these channels is also modulated by FMRFamide (Phe-Met-Arg-Phe-NH2)-related peptides and extracellular zinc ions (13, 32, 40), which might further enhance activity and hence Ca2+ entry. It is also possible that other ligands will regulate the activity of these channels.
It has also been proposed that ASIC1 might play a role in the pathogenesis of diseases associated with extracellular acidosis, including seizures and cerebral ischemia (18, 20, 41). Lactate and arachidonic acid, which are released by ischemia, might also enhance ASIC activity (13, 42, 43). An ASIC1-mediated increase in Ca2+ influx could contribute to Ca2+ overload, an important mechanism of cell damage and death in ischemia (37). Consistent with this hypothesis, we found that H+-evoked ASIC1a activation contributes to cell death in vitro in heterologous cells and in hippocampal neurons.
Although the data indicate that ASIC1a activity enhances acidosis-induced injury, our study leaves unanswered several questions about how this enhancement occurs. For example, during prolonged acid application, ASIC1a currents desensitize, and [Ca2+]c returns toward basal values. Thus, whereas a prolonged elevation of [Ca2+]c can be toxic (37), it is not certain that an increased [Ca2+]c is how ASIC1a activity causes injury. We also found that LDH release occurred at pH 6 in COS cells expressing ASIC1a, but at pH 5 in neurons. The reason for the difference is unknown but might relate to the amount of ASIC1a expression or to differences between neurons and COS-7 cells in Ca2+ extrusion mechanisms or susceptibility to injury. Finally, a fall in pH activated ASIC1a Ca2+ current within milliseconds, and current then desensitized within a few seconds. A similar time course has been measured for ASIC1a Na+ currents (2, 24). Yet, after acid application, the increase in [Ca2+]c occurred over the course of seconds and persisted for minutes. Whereas values of [Ca2+]c depend on Ca2+ extrusion, the difference in time course was striking. Perhaps the kinetic differences relate to experimental procedures associated with cell perfusion during whole-cell patch-clamping or to some aspect of measuring [Ca2+]c. Thus, additional studies are required to understand how ASIC1a contributes to acidosis-induced injury and to changes in [Ca2+]c.
Our results suggest that ASIC1a could be a target for agents designed to alter synaptic function or the consequences of diseases associated with central acidosis. Although amiloride is neither a potent nor a selective ASIC inhibitor, its ability to reduce acid-dependent toxicity in cells expressing ASIC1a suggest the potential value of a pharmaceutical approach to reduce neuronal damage from ischemic insults.
Acknowledgments
We thank Amin Nekoomand, Pary Weber, and Tami Nesselhauf for their assistance with cell culture. This work was supported in part by the National Heart, Blood, and Lung Institute (Grant HL14388). A.S.L. and M.K.S. are Associates, and M.J.W. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: ASIC, acid-sensing ion channel; [Ca2+]c, cytosolic Ca2+ concentration; LDH, lactate dehydrogenase.
References
- 1.Krishtal, O. (2003) Trends Neurosci. 26, 477-483. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann, R., Champigny, G., Bassilana, F., Heurteaux, C. & Lazdunski, M. (1997) Nature 386, 173-177. [DOI] [PubMed] [Google Scholar]
- 3.García-Añoveros, J., Derfler, B., Neville-Golden, J., Hyman, B. T. & Corey, D. P. (1997) Proc. Natl. Acad. Sci. USA 94, 1459-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wemmie, J. A., Askwith, C. C., Lamani, E., Cassell, M. D., Freeman, J. H. J. & Welsh, M. J. (2003) J. Neurosci. 23, 5496-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez de la Rosa, D., Krueger, S. R., Kolar, A., Shao, D., Fitzsimonds, R. M. & Canessa, C. M. (2003) J. Physiol. 546, 77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wemmie, J. A., Chen, J., Askwith, C. C., Hruska-Hageman, A. M., Price, M. P., Nolan, B. C., Yoder, P. G., Lamani, E., Hoshi, T., Freeman, J. H. J. & Welsh, M. J. (2002) Neuron 34, 463-477. [DOI] [PubMed] [Google Scholar]
- 7.Bassilana, F., Champigny, G., Waldmann, R., de Weille, J. R., Heurteaux, C. & Lazdunski, M. (1997) J. Biol. Chem. 272, 28819-28822. [DOI] [PubMed] [Google Scholar]
- 8.Varming, T. (1999) Neuropharmacology 38, 1875-1881. [DOI] [PubMed] [Google Scholar]
- 9.Baron, A., Waldmann, R. & Lazdunski, M. (2002) J. Physiol. 539, 485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askwith, C. C., Wemmie, J. A., Price, M. P., Rokhlina, T. & Welsh, M. J. (2004) J. Biol. Chem. [DOI] [PubMed]
- 11.Krishtal, O. A., Osipchuk, Y. V., Shelest, T. N. & Smirnoff, S. V. (1987) Brain Res. 436, 352-356. [DOI] [PubMed] [Google Scholar]
- 12.Miesenbock, G., De Angelis, D. A. & Rothman, J. E. (1998) Nature 394, 192-195. [DOI] [PubMed] [Google Scholar]
- 13.Allen, N. J. & Attwell, D. (2002) J. Physiol. 543, 521-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diarra, A., Sheldon, C., Brett, C. L., Baimbridge, K. G. & Church, J. (1999) Neuroscience 93, 1003-1016. [DOI] [PubMed] [Google Scholar]
- 15.Obrenovitch, T. P., Scheller, D., Matsumoto, T., Tegtmeier, F., Holler, M. & Symon, L. (1990) J. Neurophysiol. 64, 1125-1133. [DOI] [PubMed] [Google Scholar]
- 16.Deitmer, J. W. & Rose, C. R. (1996) Prog. Neurobiol. 48, 73-103. [DOI] [PubMed] [Google Scholar]
- 17.Li, P. A. & Siesjo, B. K. (1997) Acta Physiol. Scand. 161, 567-580. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, M. B., Jin, K., Minami, M., Chen, D. & Simon, R. P. (2001) J. Cereb. Blood Flow Metab. 21, 734-740. [DOI] [PubMed] [Google Scholar]
- 19.Kovalchuk Yu, N., Krishtal, O. A. & Nowycky, M. C. (1990) Neurosci. Lett. 115, 237-242. [DOI] [PubMed] [Google Scholar]
- 20.Chu, X. P., Miesch, J., Johnson, M. B., Root, L., Zhu, X., Chen, D., Simon, R. P. & Xiong, Z. (2002) J. Neurophysiol. 87, 2555-2561. [DOI] [PubMed] [Google Scholar]
- 21.Gunthorpe, M. J., Smith, G. D., Davis, J. B. & Randall, A D. (2001) Pflügers Arch. 442, 668-674. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland, S. P., Benson, C. J., Adelman, J. P. & McCleskey, E. W. (2001) Proc. Natl. Acad. Sci. USA 98, 711-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, P. & Canessa, C. M. (2002) J. Gen. Physiol. 120, 553-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassler, E. L., Ngo-Anh, T. J., Geisler, H. S., Ruppersberg, J. P. & Grunder, S. (2001) J. Biol. Chem. 276, 33782-33387. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez de la Rosa, D., Zhang, P., Shao, d., White, F. & Canessa, C. M. (2002) Proc. Natl. Acad. Sci. USA 99, 2326-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hruska-Hageman, A. M., Wemmie, J. A., Price, M. P. & Welsh, M. J. (2001) Biochem. J. 361, 443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard, S. A., Yermolaieva, O., Hruska-Hageman, A., Askwith, C. C., Price, M. P., Wemmie, J. A. & Welsh, M. J. (2003). (2003) Proc. Natl. Acad. Sci. USA 100, 2029-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mennerick, S., Que, J., Benz, A. & Zorumski, C. F. (1995) J. Neurophysiol. 73, 320-332. [DOI] [PubMed] [Google Scholar]
- 29.Grynkiewicz, G., Poenie, M. & Tsien, R. Y. (1985) J. Biol. Chem. 260, 3440-3450. [PubMed] [Google Scholar]
- 30.Benson, C. J., Xie, J., Wemmie, J. A., Price, M. P., Henss, J. M., Welsh, M. J. & Snyder, P. M. (2002) Proc. Natl. Acad. Sci. USA 99, 2338-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNamara, D. & Dingledine, R. (1990) J. Neurosci. 10, 3970-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron, A., Schaefer, L., Lingueglia, E., Champigny, G. & Lazdunski, M. (2001) J. Biol. Chem. 276, 35361-35367. [DOI] [PubMed] [Google Scholar]
- 33.Thastrup, O., Cullen, P. J., Drobak, B. K., Hanley, M. R. & Dawson, A. P. (1990) Proc. Natl. Acad. Sci. USA 87, 2466-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waldmann, R. (2001) in Hypoxia: From Genes to the Bedside, eds. Roach, R. C., Wagner, P. D. & Hackett, P. M. (Kluwer/Plenum, New York), Vol. 502, Chap. 19, pp. 293-304. [Google Scholar]
- 35.Liu, Z., Geng, L., Li, R., He, X., Zheng, J. Q. & Xie, Z. (2003) J. Neurosci. 23, 4156-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prod'hom, B., Pietrobon, D. & Hess, P. (1987) Nature 329, 243-246. [DOI] [PubMed] [Google Scholar]
- 37.Kristian, T. & Siesjo, B. K. (1998) Stroke 29, 705-718. [DOI] [PubMed] [Google Scholar]
- 38.Babini, E., Paukert, M., Geisler, H. S. & Gründer, S. (2002) J. Biol. Chem. 277, 41597-41603. [DOI] [PubMed] [Google Scholar]
- 39.de Weille, J. & Bassilana, F. (2001) Brain res. 900, 277-281. [DOI] [PubMed] [Google Scholar]
- 40.Askwith, C. C., Cheng, C., Ikuma, M., Benson, C. J., Price, M. P. & Welsh, M. J. (2000) Neuron 26, 133-141. [DOI] [PubMed] [Google Scholar]
- 41.Biagini, G., Babinski, K., Avoli, M., Marcinkiewicz, M. & Seguela, P. (2001) Neurobiol. Dis. 8, 45-58. [DOI] [PubMed] [Google Scholar]
- 42.Obrenovitch, T. P., Garofalo, O., Harris, R. J., Bordi, L., Ono, M., Momma, F., Bachelard, H. S. & Symon, L. (1988) J. Cereb. Blood Flow Metab. 8, 866-874. [DOI] [PubMed] [Google Scholar]
- 43.Immke, D. C. & McCleskey, E. W. (2001) Nat. Neurosci. 4, 869-870. [DOI] [PubMed] [Google Scholar]