Abstract
Apomorphine, a nonselective dopamine receptor agonist, facilitates penile erection and is effective in patients suffering from erectile dysfunction. The specific dopamine receptor subtype(s) responsible for its erectogenic effect is not known. Here we report that the dopamine D4 receptor plays a role in the regulation of penile function. ABT-724 is a selective dopamine D4 receptor agonist that activates human dopamine D4 receptors with an EC50 of 12.4 nM and 61% efficacy, with no effect on dopamine D1, D2, D3, or D5 receptors. ABT-724 dose-dependently facilitates penile erection when given s.c. to conscious rats, an effect that is blocked by haloperidol and clozapine but not by domperidone. A proerectile effect is observed after intracerebroventricular but not intrathecal administration, suggesting a supraspinal site of action. s.c. injections of ABT-724 increase intracavernosal pressure in awake freely moving rats. In the presence of sildenafil, a potentiation of the proerectile effect of ABT-724 is observed in conscious rats. The ability of ABT-724 to facilitate penile erection together with the favorable side-effect profile indicates that ABT-724 could be useful for the treatment of erectile dysfunction.
Penile erection is the result of a complex series of integrated neuronal and vascular events that leads to accumulation of blood in the penis to achieve rigidity. The coordination of several neural events is required to release endogenous mediators (i.e., nitric oxide) at the level of the penile smooth muscle to induce relaxation, whereas a disruption of this series of events can lead to erectile dysfunction (ED) (1, 2). Traditionally, drugs used for the treatment of ED have had a peripheral site of action, including phosphodiesterase (PDE)-5 inhibitors like sildenafil, but the recent demonstration that apomorphine can facilitate penile erection in ED patients has introduced a new approach to treatment, because apomorphine acts on central dopaminergic systems (3, 4).
Dopamine is the main catecholamine in the CNS and is involved in a variety of physiological functions, including sexual behavior, cognition, motor coordination, cardiovascular control, reward, hormonal regulation; abnormalities in dopaminergic neurotransmission have been implicated in Parkinson's disease, schizophrenia, attention-deficit disorder, depression, and drug abuse, among other disorders (5, 6). The regional distribution of the receptor subtypes, the recent generation of knock-out animals, and the availability of a large number of dopaminergic agents have aided researchers in clarifying the biological role of the dopamine receptors. Dopamine receptors in mammalian tissues have been classified as D1-like (D1 and D5) and D2-like (D2, D3, and D4) based on their binding properties and their ability to activate or inhibit forskolin-induced adenylate cyclase activity, a classification supported by the cloning of the different subtypes during the last decade (7, 8).
Within the D2-like family, the D2 receptor is highly expressed in the caudate, accumbens, cortex, hypothalamus, pituitary gland, and areas where the cell bodies that project to these regions are located, like the ventral tegmental area and the substantial nigra. The systemic administration of dopamine D2 agonists increases locomotion and modulates prolactin release but, more importantly, the blockade of D2 receptors in mesocortical and mesolimbic pathways has been associated with the therapeutic effect of antipsychotic drugs (8). D3 receptors are highly expressed in the island of Calleja, hypothalamus, and thalamus, and are an attractive target for the potential treatment of drug abuse in view of the inhibition of cocaine-seeking behavior induced by the partial D3 agonist BP897 (9). On the other hand, the D4 receptor is more abundant in the frontal cortex, hippocampus, amygdala, and hypothalamus, but pharmacological studies have failed to reveal any psychopharmacological effect in rats (10). The therapeutic effect of clozapine in psychotic patients that do not respond to classical antipsychotic agents like haloperidol prompted the hypothesis that D4 receptors may be responsible for the antipsychotic activity as clozapine is a preferential D4 antagonist (11).
Figure 1.
With regard to sexual function, a substantial body of experimental data indicates that several central neurotransmitters and neuropeptides are involved in the control of penile erection and sexual behavior, including dopamine (12, 13). The participation of dopamine is supported by studies demonstrating that several dopamine receptor agonists like apomorphine, quinpirole, quinelorane, and (-)-3-(3-hydroxyphenyl)-N-n-propylpiperidine (3-PPP) induce penile erection after systemic administration in mammals (12). The proerectile effect of apomorphine exhibits a characteristic inverted-U response and is observed in rats, rabbits, and monkeys (13-15). Apomorphine is a potent nonselective dopamine receptor agonist that activates all of the dopamine receptor subtypes (8), and it has been reported by several laboratories that the proerectile effect of apomorphine is mediated via the D2 receptor subtype; however, recent studies with selective dopamine agonists did not confirm this hypothesis (16). The dopamine D4 receptor is expressed in brain areas like the cortex and hypothalamus, areas that are known to control sexual function in mammals (17). The role of dopamine D4 receptors in penile erection was investigated in our laboratory by using ABT-724 (2-[(4-pyridin-2-ylpiperazin-1-yl)methyl]-1H-benzimidazole), a highly selective D4 agonist that is structurally different from apomorphine. To determine the potential therapeutic use of ABT-724 for the treatment of male ED, the effect on cardiovascular, CNS, and gastrointestinal function was investigated in animal models.
Methods
Binding Assay. For the D4 receptor-binding assays, membranes were prepared from stable cell lines expressing either human D4.4 or rat D4 in human embryonic kidney (HEK)293 cells. Membranes containing recombinant human D4.2 and human D4.7 receptors were obtained from Perkin-Elmer. Saturation binding assays were carried out with the D4 agonist [3H]-A-369508 (R.B.M. and J.D.B., unpublished work). The Kds for [3H]-A-369508 on human D4.2, D4.4, and D4.7 were 1.68 ± 0.28, 4.04 ± 0.69, and 1.22 ± 0.19 nM, respectively. For rat D4, Kd = 4.35 ± 0.43 nM. Competition binding assays were initiated by the addition of 250 μl of membrane (50 μg of protein final) to 200 μl of [3H]-A-369508 (88.1 Ci/mmol; final concentration in assay, 2 nM) and were incubated at room temperature for 1 h. Nonspecific binding was defined with 10 μM PD168077. Radioactivity was counted in a TopCount Microplate Scintillation Counter (Packard). The IC50 values are determined from the competition studies by using a one-site model with graphpad (GraphPad, San Diego) analysis, which calculates the Ki values using the Cheng-Prusoff equation. For the D2 receptor-binding assays, membranes were prepared from a stable cell line expressing human D2L in HEK293 cells (gift of Liliane Unger, Abbott Laboratories). Binding assays were initiated by addition 250 μl of membrane to 200 μl of [125I]-PIPAT [PIPAT, trans-7-hydroxy-2-(N-n-propyl-N-3′-iodo-2′-propenyl)] (Amersham Pharmacia Biotech) and incubated at room temperature for 1 h. Radioactivity was counted and IC50 values determined as described above. A-369508 and ABT-724 were synthesized at Abbott Laboratories.
Fluorometric Imaging Plate Reader (FLIPR) Assay. Agonist-induced intracellular Ca2+ changes were assessed by using Fluo-4 in conjunction with FLIPR (Molecular Devices). Fluo-4 was dissolved in anhydrous DMSO and diluted in Dulbecco's phosphate buffer saline containing 0.5 μM 3-isobutyl-1-methylxanthine and 0.004% ascorbic acid to a final concentration of 2 mM. After agonist addition, Ca2+ dynamics were recorded. Independent measurements of 10 μM dopamine (100%) and unloaded (0%) cells were performed on each plate to normalize values from plate to plate. Concentration-response data were analyzed by using PRISM (GraphPad), and the corresponding EC50 values were derived from single-curve fits. FLIPR studies were conducted in human, rat, and ferret D4 receptors. Cloning of the ferret D4 receptor in our laboratory revealed that the ferret D4 receptor has 74% and 67% amino acid sequence identities to the rat D4 and human D4.4 receptors, respectively.
Conscious Rat Model of Penile Erection. Male adult Wistar rats (≈300 g of body weight, obtained from Charles River Breeding Laboratories) were used as animal models to study penile erection in vivo as reported (18). All animals were housed in American Association for the Accreditation of Laboratory Animal Care-approved facilities at Abbott Laboratories in a temperature-regulated environment under a controlled 12-h light-dark cycle, with lights on at 6:00 a.m. Food and water were available ad libitum except during testing. All testing was done following procedures outlined in protocols approved by Abbott's Institutional Animal Care and Use Committee. The experiments were carried out between 9:00 a.m. and 3:00 p.m. On the day of testing, animals were allowed to adapt to a diffusely illuminated testing room with red light for 1 h before the start of the experiment. Rats were placed individually into a transparent Plexiglas cage (20 × 30 × 30 cm) immediately after the drug injection. A mirror was placed behind and under the observation cages to facilitate observation of the animals. Each rat was used only once. A penile erection was considered to occur when the following behaviors were presented: repeated pelvic thrusts immediately followed by an upright position, and an emerging engorged penis that the rat proceeded to groom.
ABT-724 (maleate salt) was freshly prepared and administered to rats via s.c. injection into the back neck area (1 ml/kg injection volume). For intracerebroventricular (ICV) or intrathecal administrations, rats were allowed at least 1 week of recovery from surgery before behavioral testing. The compounds of interest were infused ICV (left lateral ventricle) in a 5-μl volume or 10 μl intrathecally. After the experiment was completed, infusion of 0.5% fast-green dye in saline solution and subsequent dissection were carried out to confirm the diffusion of the injection solution.
Repeated dosing experiments were also conducted in the rat conscious penile erection model. For these studies, rats were dosed with either vehicle or ABT-724 (0.03 μmol/kg s.c.) once daily for 5 days. Animals were tested in the morning on day 1 and day 5 immediately after drug injection. To characterize the in vivo effect of sildenafil in combination with ABT-724 in conscious rats, sildenafil (1 μmol/kg, i.p.) was injected 30 min before ABT-724 s.c., and the rats were observed for 60 min for the occurrence of erections.
Penile erection episodes were recorded by direct observation for a period of 60 min after compound dosing, and erection incidence (%) was defined as the percent of animals exhibiting one or more erections during the observation period. Data were expressed as incidence (%) ± SE. SE was calculated by the Wald equation, and the statistical evaluation of the data was performed by the χ2 test. The number of penile erections was also counted and the data expressed as mean ± SEM. These data were analyzed by the Mann-Whitney nonparametric U test.
Intracavernosal Pressure Registration in Conscious Rats. Sixteen male Sprague-Dawley rats (350-420 g) were anesthetized with pentobarbital and ketamine given by i.p. injection. Through a midline scrotal incision, the base of the penis was exposed. Using a 25-gauge needle, a small hole was made in the tunica albuginea of the crus cavernosum on one side and a heparinized (100 international equivalents per/ml) PE50 polyethylene catheter was introduced. The catheter was secured by a 5/0 pouch-suture in the fibrous tunica and by application of adhesive skin glue around the entrance hole. The scrotal skin was sutured and the catheter tunneled s.c. to the neck where it was anchored to the skin by 3/0 sutures. Twelve hours after implantation of the catheter, the animals were placed in metabolic cages. Experiments were performed after a stabilizing period of 30 min. Continuous direct measurements of intracavernous pressure (ICP) were performed with P23 DC transducers and registered on a Grass Polygraph 7E (Grass Instruments, Quincy, MA). ABT-724 [1 μg/kg (0.0025 μmol/kg) or 10 μg/kg (0.025 μmol/kg)] or vehicle (saline) was then given by s.c. injection, and changes in ICP were registered during 60 min. Basal ICP, the total number of responses [>50 cmH2O (38 mmHg, 1 mmHg = 133 Pa)], total duration of responses (the added duration of all responses >50 cmH2O or (38 mmHg)], time to first response, and peak ICP were analyzed in each rat. When appropriate, the results are given as mean values ± SEM. One-way ANOVA was used for comparison between groups, followed by Bonferroni correction. All calculations are based on the number of individuals in the experiments.
Results and Discussion
The human D4.4 receptor was cloned and stably expressed in HEK293 cells to determine the efficacy of the dopamine receptor agonists. Mouse, rat, and human D4 receptors have been shown to couple via Gi or Go to inhibit cAMP synthesis (19-22). However, coexpressing the D4 receptor with chimeric G proteins (where the last five amino acids of Gq are replaced by the last five amino acids of Go to generate Gqo5) allows coupling to phospholipase C pathways (23). In this way, activation of the D4 receptor leads to changes in intracellular Ca2+ levels.
Dopamine activated D4 receptors stably expressed in a recombinant cell line with 2.2 nM potency (Table 1). Apomorphine and ABT-724 induced potent but partial agonist responses in comparison to dopamine with EC50s of 4.3 and 12.4 nM, respectively. In this cell line, selective D1 (SKF81297) or D2 (PNU-95666E) agonists did not increase intracellular Ca2+ levels, whereas a significant effect was observed after incubation with the D4 receptor agonists PD168077 and CP226269 (EC50 = 5.6 and 32.0 nM, respectively) (R.B.M. and J.D.B., unpublished work). The dopamine effect was blocked by the dopamine antagonists haloperidol and clozapine (Kb = 10.5 ± 1.4 and 2.5 ± 0.8 nM, respectively), indicating that its effect is mediated via dopamine receptors. The intracellular Ca2+ changes induced by ABT-724 were also blocked by haloperidol and clozapine with Kb = 1.2 ± 0.3 and 5.1 ± 1.6 nM, respectively. The rat and ferret D4 receptor were cloned and stably expressed in HEK293 cells with coexpression of the G protein chimera Gqo5. Functional studies demonstrated that dopamine increased intracellular Ca2+ levels in rat D4 cells with 2.4 ± 0.2 nM and in ferret D4 cells with 2.7 ± 0.3 nM potency. ABT-724 is a potent partial agonist at the rat D4 (EC50 = 14.3 ± 0.6 nM; 70% efficacy) and the ferret D4 receptor (EC50 = 23.2 ± 1.3 nM; 64% efficacy), consistent with its activity at the human D4 receptor.
Table 1. Potency and efficacy of dopamine receptor agonists in HEK293 cells stably expressing cloned human D4.4 receptors and the G protein chimera Gqo5.
| EC50, nM | Efficacy, % | |
|---|---|---|
| Dopamine | 2.2 ± 0.2 | 100 |
| Apomorphine | 4.3 ± 0.2 | 84.0 ± 3.3 |
| ABT-724 | 12.4 ± 1.0 | 61.0 ± 3.7 |
Dopamine-induced increases in intracellular Ca++ were measured by fluorometric imaging plate reader. Data represent the mean ± SEM EC50 (nM) and efficacy (%) to increase intracellular Ca++ levels in comparison to dopamine 10 μM (n = 4-18).
The human dopamine D4 receptor is expressed in multiple forms that vary with the ethnic origin of the population, and the D4.4 is the most abundant variant (64.3% of population) followed by the D4.7 (20.6%) and D4.2 (8.2%). Competition binding with the D4 receptor agonist [3H]-A-369508 was conducted for dopamine, apomorphine, and ABT-724 on the three most abundant variants. Table 2 shows that dopamine, apomorphine, and ABT-724 bind with nanomolar potency to the different alleles of the human D4 receptor, and that the affinity of ABT-724 is similar across the three major alleles. ABT-724 exhibits a selective biochemical profile, as indicated by a lack of binding affinity for >70 neurotransmitter/uptake/ion channels including D1, D2, D3, and D5 receptors up to a 10 μM concentration. A weak affinity to 5-HT1A receptors (Ki = 2,780 ± 642 nM) was observed. ABT-724 did not inhibit the PDE activity of PDE1, PDE5, or PDE6 at 10 μM concentrations (see supporting information, which is published on the PNAS web site).
Table 2. Radioligand-binding affinity (KI, nM) for the dopamine receptor agonists using [3H]-A-369508 and membrane preparations expressing the human D4 variants.
| D4.2 | D4.4 | D4.7 | |
|---|---|---|---|
| Dopamine | 11.6 ± 0.6 | 56.2 ± 9.1 | 9.8 ± 1.3 |
| Apomorphine | 1.1 ± 0.1 | 4.0 ± 0.5 | 1.2 ± 0.2 |
| ABT-724 | 57.5 ± 8.0 | 63.6 ± 6.6 | 46.8 ± 4.8 |
Data represent the mean ± SEM (n = 4).
Because activation of the D2 receptor with selective D2 receptor agonists like PNU-95666E can induce emetic responses (see below), detailed biochemical studies were conducted in human D2 receptors. Binding studies using [125I]-PIPAT [PIPAT, trans-7-hydroxy-2-(N-n-propyl-N-3′-iodo-2′-propenyl)] demonstrated that dopamine, PNU-95666E, and apomorphine exhibit nanomolar affinity to the D2 receptor (Table 3), whereas ABT-724 was inactive up to 10 μM. Functional activity was determined in human D2 receptors expressed in HEK293 cells with coexpression of the G protein chimera Gqo5. PNU-95666E and apomorphine were potent and efficacious D2 receptor agonists, whereas ABT-724 did not activate human D2 receptors in concentrations up to 10 μM (Table 3).
Table 3. Radioligand-binding affinity (KI, nM) for the dopamine receptor agonists using membrane preparations expressing the human D2 receptor and functional efficacy in HEK293 cells expressing a cloned human D2 receptor and the G protein chimera Gqo5.
| D2 binding
|
D2 function
|
||
|---|---|---|---|
| KI, nM | EC50, nM | Efficacy, % | |
| Dopamine | 10.0 ± 1.5 | 18.0 ± 2.0 | 100 |
| Apomorphine | 3.6 ± 0.4 | 5.8 ± 0.3 | 86.0 ± 6.3 |
| PNU-95666E | 53.8 ± 4.2 | 165.0 ± 11.5 | 92.8 ± 2.0 |
| ABT-724 | > 10,000 | > 10,000 | - |
In the binding studies, data represent the mean ± SEM (n = 4) using [125I]-PIPAT as ligand. Functional data are represented as the mean ± SEM EC50 and % efficacy (n = 4) to increase intracellular calcium levels in comparison to dopamine 10 μM (n = 4).
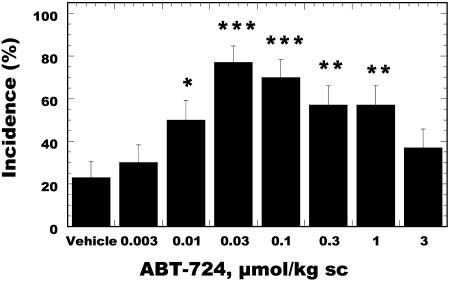
Behavioral pharmacological studies were conducted in conscious Wistar rats to determine the effect of the D4 receptor agonist on erectile function. This model was established in-house based on standard procedures used in several laboratories (24). ABT-724 induced a significant facilitation of penile erection in rats after s.c. injections, as shown in Fig. 1. A 77% maximal effect was observed at the 0.03 μmol/kg dose vs. 23% incidence in the control group. The latency to induce a penile erection in rats for ABT-724 was 18.7 min. Clozapine (10 μmol/kg, i.p.) and haloperidol (0.3 μmol/kg, i.p.), but not domperidone (10 μmol/kg, i.p.), blocked the erectogenic effect of ABT-724 in rats, indicating that the effect was mediated via central dopaminergic mechanisms, because the peripheral dopaminergic antagonist domperidone did not block the proerectile effect of ABT-724. A separate study was conducted to determine whether the proerectile effect of ABT-724 is maintained after five daily dosings. There was no tolerance to the proerectile effect of ABT-724 at 0.03 μmol/kg, because the incidence of penile erections in conscious rats was 71 ± 12% on day 1 and 78 ± 11% on day 5. The maximal concentration of ABT-724 at the 0.03 μmol/kg dose reached 5 min after drug administration was 5.0 ng/ml (17 nM), a concentration comparable to the concentration needed to activate the D4 receptor. ABT-724 rapidly crossed the blood-brain barrier in rats and reached concentrations of 4.9 ng/g of brain tissue 15 min after s.c. injection.
Fig. 1.
Proerectile effect of ABT-724 in conscious Wistar rats after s.c. administration. Penile erection was assessed during a 60-min period after the injection of ABT-724. Data are expressed as erection incidence (%) ± SE (n = 30 rats per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. the vehicle group (χ2 test).
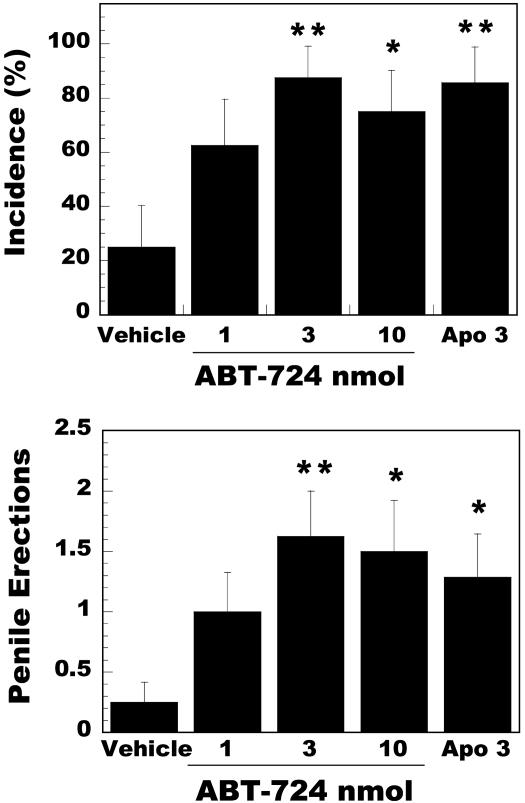
To determine the site of action of ABT-724 and to compare the effect of ABT-724 vs. apomorphine, a group of rats with chronically implanted cannulas were injected with these drugs directly into the lateral ventricle. A dose-response study was conducted with ABT-724 in comparison to the best dose of apomorphine based on previous studies (16). Fig. 2 shows that nanomolar amounts of ABT-724 significantly facilitated erectile responses in conscious rats. The incidence and number of erections during the 60-min observation period were similar between ABT-724 (3 nmol) and apomorphine (3 nmol). The onset of action was also similar between these two groups (23.1 ± 4.0 and 15.3 ± 2.6 min, respectively). On the contrary, ABT-724 had no proerectile effect when injected intrathecally at the spinal level of L4-L6 in rats at doses of 3, 10, and 30 nmol. A modest effect was observed at the 100-nmol dose (50 ± 14% incidence vs. 9 ± 7% incidence in the vehicle-injected group), a dose 30-fold higher than the dose that induced a maximal effect after ICV injections.
Fig. 2.
Proerectile effect of ICV injections of ABT-724 and apomorphine (3 nmol) via chronically implanted cannulas in conscious Wistar rats. Penile erection was assessed during a 60-min period after drug administration. Data are expressed as erection incidence (%) ± SE (Upper) and as the mean ± SEM number of erections (Lower). Eight rats were included in each group. *, P < 0.05; **, P < 0.01 vs. the vehicle group.
To investigate whether ABT-724 would affect the cavernosal smooth muscle directly, a series of experiments was conducted in rabbit corpus cavernosum smooth muscle strips precontracted with phenylephrine. ABT-724 had no effect in concentrations up to 10 μM, whereas sildenafil induced a complete relaxation with an EC50 = 44.37 ± 11.2 nM (see supporting information). Apomorphine was also inactive in this assay in concentrations up to 10 μM, consistent with previous data in the literature using rat cavernosal strips (25).
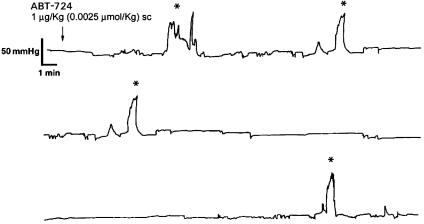
The effect of ABT-724 on ICP responses was also investigated in awake freely moving rats. A mean basal ICP of 28.6 ± 4.3 cmH2O (21.7 ± 3.3 mmHg, n = 16) was recorded at the beginning of the experiments. In control experiments, rats receiving saline exhibited 0.2 ± 0.2 (n = 4) erectile responses with a mean duration of 1.0 min and mean peak ICP (PICP) of 92 cmH2O (69.9 mmHg) (see supporting information). After s.c. administration of ABT-724, erectile responses measured as increases in ICP were observed in 11 of 12 rats. On administration of the lowest dose of ABT-724 (0.0025 μmol/kg s.c., n = 5), an average of 2.0 ± 0.6 (P < 0.05) episodes of increases in ICP was observed. A representative recording is shown in Fig. 3. The mean time to the first erectile response [time to first response (TFR)] was calculated to 15.4 ± 7.7 min. Mean PICP amounted to 139.6 ± 18.6 cmH2O (106.1 ± 14.1 mmHg), and the mean duration of the increases in ICP was 3.6 ± 1.5 min. At this dose of ABT-724, one rat did not respond with an increase in ICP. ABT-724 produced 7.2 ± 2.8 (n = 5, P < 0.05) episodes of increases in ICP at 0.025 μmol/kg, with a mean duration of 7.0 ± 2.3 min. The mean TFR was 19.3 ± 5.8 min, and PICP amounted to 134.1 ± 19.2 cmH2O (101.9 ± 14.6 mmHg). During the later third of the observation period, after administration of 0.025 μmol/kg, two of the rats exhibited clusters of increases in ICP (16 and 11 erectile responses, respectively). All rats receiving 0.025 μmol/kg ABT-724 responded with erectile responses measured as increases in ICP.
Fig. 3.
Original recording of ICP responses in an awake freely moving rat after s.c. administration of ABT-724 (0.0025 μmol/kg, s.c.). Erectile responses registered as increases in ICP are highlighted (*).
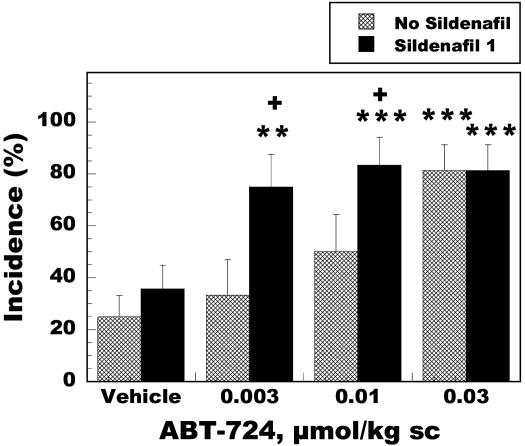
The effect of ABT-724 in combination with the PDE5 inhibitor sildenafil was also investigated in rats. Fig. 4 demonstrates that sildenafil (1 μmol/kg, i.p.) did not induce a significant effect at this dose in comparison to the vehicle group, and that ABT-724 by itself induced a maximal effect at the 0.03 μmol/kg dose. However, in the presence of sildenafil, a 10-fold potentiation of the incidence of erections was observed as ABT-724 reached a maximal effect at the 0.003 μmol/kg dose. These data suggest that the combination of a centrally acting agent with a peripherally acting agent might be beneficial for the treatment of patients suffering from ED.
Fig. 4.
Effects of ABT-724 and sildenafil (1 μmol/kg, i.p.) on penile erection in Wistar rats. Sildenafil was injected 30 min before ABT-724 dosing, and penile erection was assessed during a 60-min period. Data are expressed as erection incidence (%) ± SE (12-16 rats were included in each group). **, P < 0.01; ***, P < 0.001 vs. the vehicle group; + P < 0.05 vs. the corresponding no-sildenafil group.
Nonselective dopaminergic agents like quinpirole, 7-hydroxy-2-(di-n-propylamino)tetralin, quinelorane, and apomorphine exhibit affinity for D2, D3, and D4 receptors and are known to induce nausea/emesis. ABT-724 was evaluated in conscious male ferrets, a preclinical model to determine the emetic potential of drugs. ABT-724 was administered s.c. at 0.03, 0.3, and 3 μmol/kg. The number of emetic episodes and the presence of stereotypical behaviors that correlate with the sensation of nausea in ferrets were recorded during 90 min. ABT-724 did not cause emesis or nauseogenic behavior at any of the doses tested (Table 4), despite its ability to activate ferret D4 receptors. The highest dose tested is 100-fold greater than the maximally efficacious dose in the rat model of penile erection. The plasma levels reached in ferrets after the s.c. administration of 3 μmol/kg was 812 ± 74 ng/ml. Because the plasma level required for maximal efficacy in rats is 5 ng/ml, the apparent tolerability index for ABT-724 is >160-fold. On the contrary, apomorphine induced emesis at the 0.3 μmol/kg dose in ferrets (2.3 ± 0.4 episodes with a 10.5 ± 2.6 min latency). The selective dopamine D2 agonist PNU-95666E was identified from the literature (26, 27) and when tested in the ferret model PNU-95666E induced emesis after systemic injections of 1 and 3 μmol/kg (Table 4). The emetic effect of PNU-95666E was completely blocked by the dopaminergic antagonist haloperidol (0.1 μmol/kg, s.c.). These data indicate that the activation of dopamine D2 receptors is involved in the regulation of emetic responses, whereas the dopamine D4 receptor does not play a role in emesis.
Table 4. Effect of ABT-724 and PNU-95666E in male ferrets after s.c. injection.
| Emesis, % incidence | Emetic episodes | Latency to vomit | |
|---|---|---|---|
| Vehicle | 0 | 0 | - |
| ABT-724 0.03 μmol/kg | 0 | 0 | - |
| ABT-724 0.3 μmol/kg | 0 | 0 | - |
| ABT-724 3 μmol/kg | 0 | 0 | - |
| PNU-95666E 0.1 μmol/kg | 0 | 0 | - |
| PNU-95666E 0.3 μmol/kg | 0 | 0 | - |
| PNU-95666E 1 μmol/kg | 83.3 | 3.8 ± 0.7 | 8.1 ± 1.6 |
| PNU-95666E 3 μmol/kg | 100 | 5.2 ± 1.1 | 8.0 ± 2.6 |
Data are expressed as the percentage of ferrets exhibiting emesis, the mean ± SEM number of emetic episodes, and the mean ± SEM latency to vomit in minutes. Six ferrets were included in each group.
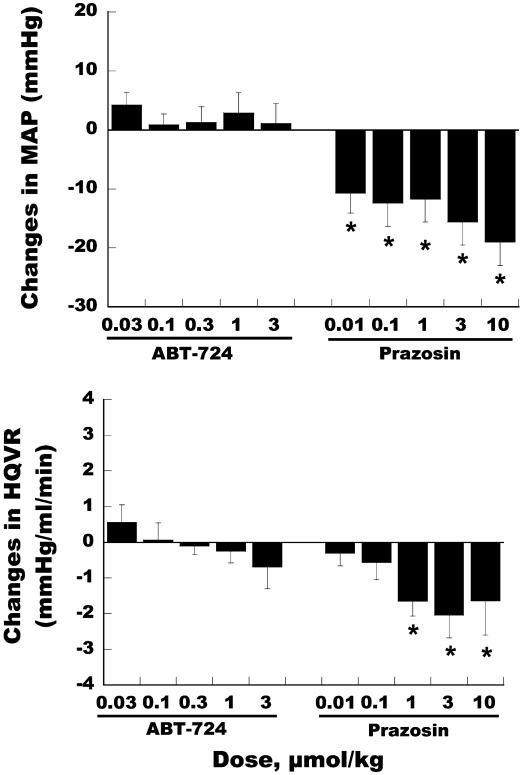
Expression of D4 receptor mRNA has been reported in peripheral tissues including the atrium and ganglia (28, 29). Although mRNA expression is limited, the effect of ABT-724 on cardiovascular and regional hemodynamic functions was assessed in rats to determine the peripheral actions of ABT-724. i.v. administration of ABT-724 in anesthetized Sprague-Dawley rats produced no significant effects on mean arterial pressure (MAP), heart rate, dP/dt, or regional blood flow to renal, mesenteric, and hindquarter beds. Fig. 5 shows that ABT-724 was devoid of any effects on MAP, whereas a significant hypotensive effect is induced in this model by the adrenergic antagonist prazosin. A plasma concentration of 277 ± 37 ng/ml was achieved at the end of the high-dose infusion (3 μmol/kg) of ABT-724, a concentration 55-fold greater than the efficacious plasma level (5 ng/ml) in the rat model of penile erection. Prazosin also significantly decreased hindquarter vascular resistance in rats, whereas ABT-724 did not affect this parameter. These data indicate that D1 and D2, but not D4, receptors can significantly affect hemodynamic parameters in mammals (30, 31).
Fig. 5.
Effects of ABT-724 and prazosin on mean arterial pressure (Upper) and hindquarter vascular resistance (Lower) in rats. Drugs were administered i.v. over a 30-min infusion for each dosing (n = 5 for ABT-724 and n = 7 for prazosin). Values collected from the last 5-min time point of infusion are presented as mean ± SEM of hemodynamic changes from drug predosing. Data were analyzed by unpaired t test. *, P < 0.05 compared to the baseline of drug predosing.
The effect of ABT-724 on CNS behaviors was investigated to rule out potential stimulatory or propsychotic effects due to D4 receptor stimulation. s.c. injections of ABT-724 (0.003-1.0 μmol/kg) did not affect locomotion of Wistar rats in the open field during a 20-min observation period. The doses tested in this study were 30-fold higher than the effective dose in rats (0.03 μmol/kg), and there was no indication of a stimulatory effect on locomotion. Amphetamine injections (1-3 μmol/kg, s.c.) significantly increased locomotion in rats. The prepulse inhibition (PPI) model in male Sprague-Dawley rats is used as a model to determine the potential antipsychotic properties of drugs. Amphetamine, a propsychotic agent in humans, disrupted the PPI response in rats (8.2 μmol/kg, s.c.), and this effect was blocked by haloperidol and clozapine. However, ABT-724 (0.03-1.0 μmol/kg, s.c.) neither blocked nor increased the PPI response. The Irwin test (32) was performed to determine the effects of ABT-724 on behavior, neurological symptoms, and rectal temperature in CD-1 mice. ABT-724 (0.1-100 μmol/kg, i.p.) did not induce observable effects up to 10 μmol/kg. The lowest dose of ABT-724 determined to cause behavioral side effects in mice was 100 μmol/kg, which produced only mild hypoactivity and ptosis (see supporting information). Methylphenidate was used as a positive control and induced clear signs of CNS stimulation, including hyperlocomotion, aggression, and convulsions. These CNS-mediated behaviors were not induced by ABT-724, indicating that activation of the D4 receptor does not lead to CNS stimulation, as observed for methylphenidate and amphetamine.
These preclinical data demonstrate that the selective D4 agonist ABT-724 can facilitate penile erection in conscious rats at doses devoid of cardiovascular, emetic, or CNS side effects. The proerectile effect of ABT-724 can be detected in a visual observation model in conscious rats and also by the increase in intracavernosal pressure responses in conscious rats.
There is extensive evidence in the literature demonstrating that pharmacological agents like apomorphine can facilitate penile erection by direct action in central areas of the nervous system (33), in contrast to sildenafil, which acts at the level of the cavernosal smooth muscle. Our data show that ABT-724 facilitates penile erection in rats after systemic and ICV administration. The effect of low doses of ABT-724 injected ICV in the rat, together with the lack of effect at the spinal level, its inability to relax corpus cavernosum strips, and lack of effect on different vascular beds provide further pharmacological support to the notion that the proerectile effect of ABT-724 is mediated by supraspinal dopaminergic mechanisms.
Although several publications have suggested that the erectogenic effect of nonselective dopamine receptor agonists like apomorphine is related to its activity at the D2 receptor, our preclinical studies did not confirm this hypothesis (16) and indicate that the D2 receptor agonist PNU-95666E induces modest nonsignificant effects on penile erection in rats, whereas it induces emesis in ferrets. Thus, activation of D2 and D4 receptors leads to a differential pharmacological profile, and the D4 receptor plays a unique role in the control of penile erection. Because apomorphine is a potent D2 agonist, the activation of D2 receptors may be responsible for the higher incidence of nausea at higher doses that limits its clinical efficacy in ED patients. The use of a selective D4 agonist like ABT-724 that does not induce nausea will allow the use of a larger dose range to determine the maximal efficacy in ED patients.
ED affects a large population of men and can be classified based on severity, etiology, and onset of the disease. Based on the severity of ED, patients can be stratified as mild, moderate, or severe, depending on their ability to achieve and maintain an erection (34). Although sildenafil is generally an efficacious agent for the treatment of ED, this drug has limited efficacy in a subpopulation of ED patients, e.g., diabetics. A PDE5 inhibitor cannot increase cGMP levels by itself unless there is release of NO that activates guanylate cyclase to increase cGMP levels (35). In this situation, PDE5 inhibitors can maintain cGMP levels by blocking the metabolism of cGMP. The reduced efficacy of PDE5 inhibitors like sildenafil in diabetic patients may be due to the impaired neuronal and endothelium-dependent mechanisms (36-38). Centrally acting agents may be able to enhance the signal that stimulates NO release from the nerve terminal, a critical process to increase cGMP levels in the smooth muscle. Either by itself or in combination with a PDE5 inhibitor, ABT-724 could achieve greater efficacy in diabetic patients.
During the past decade, the D4 receptor has received significant attention in the psychiatric field due to the therapeutic effect of clozapine in psychotic patients that do not respond to classical dopamine receptor antagonists like haloperidol. Clozapine is a preferential antagonist of the dopamine D4 receptor (11), and several potent D4 antagonists were discovered aiming to improve the therapeutic efficacy of the antipsychotic agents (19, 39). However, the D4 hypothesis has not been confirmed in the clinic as the mechanism of action of antipsychotic agents (40, 41). Other hypotheses regarding the physiological or pathophysiological role of the D4 receptor have been based on knock-out animals (42, 43) or genetic association studies suggesting a potential role in novelty-seeking behavior, alcoholism, attention deficit, and depression; however, neither clinical nor preclinical research has confirmed these hypotheses (29).
The dopamine D4 receptor is expressed in various brain areas, and in situ hybridization as well as autoradiographic studies have demonstrated that the D4 receptor is most abundant in the prefrontal cortex, hippocampus, amygdala, and hypothalamus (17). In view of the preclinical evidence on the effect of apomorphine at the level of the periventricular nucleus of the hypothalamus to facilitate penile erection (33) and the proerectile effect of the selective D4 receptor agonist ABT-724, the D4 receptor expressed in the hypothalamus merits further investigation in relation to the control of penile erection and sexual function.
Conclusion
ABT-724 is a previously undescribed and selective dopamine D4 receptor agonist that facilitates penile erection in rats at doses devoid of cardiovascular, emetic, or CNS side effects. The present preclinical studies demonstrate that dopamine D4 receptors are involved in the regulation of penile function in mammals. The availability of a highly selective dopamine D4 receptor agonist like ABT-724 will facilitate validation of this hypothesis in humans.
Supplementary Material
Acknowledgments
We thank L. Miller, M. Namovic, M. Terranova, R. Chang, J. McVey, J. Darbyshire, T. Seifert, G. Fox, P. Curzon, and B. Cox for helpful discussions and technical assistance.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY394848).
Abbreviations: ED, erectile dysfunction; PDE, phosphodiesterase; ICV, intracerebroventricular; ICP, intracavernous pressure; HEK, human embryonic kidney.
References
- 1.Andersson, K. & Wagner, G. (1995) Physiol. Rev. 75, 191-236. [DOI] [PubMed] [Google Scholar]
- 2.Moreland, R., Hsieh, G., Nakane, M. & Brioni, J. (2001) J. Pharmacol. Exp. Ther. 296, 225-234. [PubMed] [Google Scholar]
- 3.Lal, S., Laryea, E., Thavundayil, J., Nair, N., Negrete, J., Ackman, D., Blundell, P. & Gardiner, R. (1987) Prog. Neuropsychopharmacol. Biol. Psychiatry 11, 235-242. [DOI] [PubMed] [Google Scholar]
- 4.Dula, E., Keating, W., Siami, P., Edmonds, A., O'Neil, J. & Butler, S. (2000) Urology 56, 130-135. [DOI] [PubMed] [Google Scholar]
- 5.Jackson, A. & Westlind-Danielsson, A. (1994) Pharmacol. Ther. 64, 291-369. [DOI] [PubMed] [Google Scholar]
- 6.Emilien, G., Maloteaux, J., Geurts, M., Hoogenberg, K. & Cragg, S. (1999) Pharmacol. Ther. 84, 133-156. [DOI] [PubMed] [Google Scholar]
- 7.Kebabian, J. & Calne, D. (1979) Nature 277, 93-96. [DOI] [PubMed] [Google Scholar]
- 8.Missale, C., Nash, S., Robinson, S., Jaber, M. & Caron, M. (1998) Physiol. Rev. 78, 189-225. [DOI] [PubMed] [Google Scholar]
- 9.Pilla, M., Perachon, S., Sautel, F., Garrido, F., Mann, A., Wermuth, C., Schwartz, J., Everitt, B. & Sokoloff, P. (1999) Nature 400, 371-375. [DOI] [PubMed] [Google Scholar]
- 10.Clifford, J. & Waddington, J. (2000) Neuropsychopharmacology 22, 538-544. [DOI] [PubMed] [Google Scholar]
- 11.Seeman, P. & VanTol, H. (1994) Trends Pharmacol. Sci. 15, 264-266. [DOI] [PubMed] [Google Scholar]
- 12.Melis, M. & Argiolas, A. (1995) Neurosci. Biobehav. Rev. 19, 19-38. [DOI] [PubMed] [Google Scholar]
- 13.Andersson, K. (2001) Pharmacol. Rev. 53, 417-450. [PubMed] [Google Scholar]
- 14.Bitran, D. & Hull, E. (1987) Neurosci. Biobehav. Rev. 11, 365-389. [DOI] [PubMed] [Google Scholar]
- 15.Barros, H., Braz, S. & Carlini, E. (1989) Pharmacology 38, 335-340. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh, G., Hollingsworth, P., Martino, B., Chang, R., Terranova, M., O'Neill, A., Lynch, J., Moreland, R., Donnelly-Roberts, D., Kolasa, T., et al. (2004) J. Pharmacol. Exp. Ther. 308, 330-338. [DOI] [PubMed] [Google Scholar]
- 17.Primus, R., Thurkauf, A., Xu, J., Yevich, E., Mcinerney, S., Shaw, K., Tallman, J. & Gallagher, D. (1997) J. Pharmacol. Exp. Ther. 282, 1020-1027. [PubMed] [Google Scholar]
- 18.Hsieh, G., O'Neill, A., Moreland, R., Sullivan, J. & Brioni, J. (2003) Eur. J. Pharmacol. 458, 183-189. [DOI] [PubMed] [Google Scholar]
- 19.VanTol, H., Bunzow, J., Guan, H., Sunahara, R., Seeman, P., Niznik, H. & Civelli, O. (1991) Nature 350, 610-614. [DOI] [PubMed] [Google Scholar]
- 20.Cohen, A., Todd, R., Harmon, S. & O'Malley, K. (1992) Proc. Natl. Acad. Sci. USA 89, 12093-12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang, L., Todd, R., Heller, A. & O'Malley, K. (1994) J. Pharmacol. Exp. Ther. 260, 495-502. [PubMed] [Google Scholar]
- 22.Asghari, V., Sanyal, S., Buchwaldt, S., Paterson, A., Jovanovic, V. & VanTol, H. (1995) J. Neurochem. 65, 1157-1165. [DOI] [PubMed] [Google Scholar]
- 23.Milligan, G. & Rees, S. (1999) Trends Pharmacol. Sci. 20, 118-124. [DOI] [PubMed] [Google Scholar]
- 24.Heaton, J., Varrin, S. & Morales, A. (1991) J. Urol. 145, 1099-1102. [DOI] [PubMed] [Google Scholar]
- 25.Andersson, K., Gemalmaz, H., Waldeck, K., Chapman, T., Tuttle, J. & Steers, W. (1999) J. Urol. 161, 1707-1712. [PubMed] [Google Scholar]
- 26.Sethy, V., Ellerbrock, B. & Wu, H. (1997) Prog. Neuropsychopharmacol. Biol. Psychiatry 21, 873-883. [DOI] [PubMed] [Google Scholar]
- 27.Romero, A., Darlington, W. & McMillan, M. (1997) J. Org. Chem. 62, 6582-6587. [Google Scholar]
- 28.Xie, G., Jones, K., Peroutka, S. & Palmer, P. (1998) Brain Res. 785, 129-135. [DOI] [PubMed] [Google Scholar]
- 29.Oak, J., Oldenhof, J. & VanTol, H. (2000) Eur. J. Pharmacol. 405, 303-327. [DOI] [PubMed] [Google Scholar]
- 30.Ng, S. & Pang, C. (2000) Br. J. Pharmacol. 129, 853-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damase-Michel, C., Montastruc, J., Gharib, C., Geelen, G., Saint-Blanquat, G. & Tran, M. (1990) J. Pharmacol. Exp. Ther. 252, 770-777. [PubMed] [Google Scholar]
- 32.Irwin, S. (1968) Psychopharmacologia 13, 222-257. [DOI] [PubMed] [Google Scholar]
- 33.Melis, M., Argiolas, A. & Gessa, G. (1987) Brain Res. 415, 98-104. [DOI] [PubMed] [Google Scholar]
- 34.Capelleri, J., Rosen, R., Smith, M., Mishra, A. & Osterloh, I. (1999) Urology 54, 346-351. [DOI] [PubMed] [Google Scholar]
- 35.Brioni, J., Nakane, M., Hsieh, G., Moreland, R., Kolasa, T. & Sullivan, J. (2002) Int. J. Impot. Res. 14, 8-14. [DOI] [PubMed] [Google Scholar]
- 36.SaenzdeTejada, I., Goldstein, I., Azadzoi, K., Krane, R. & Cohen, R. (1989) N. Engl. J. Med. 16, 1025-1030. [DOI] [PubMed] [Google Scholar]
- 37.Hurt, K., Musicki, B., Palese, M., Crone, J., Becker, R., Moriarity, J., Snyder, S. & Burnett, A. (2002) Proc. Natl. Acad. Sci. USA 99, 4061-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang, S., Hypolite, J., Changolkar, A., Wein, A., Chacko, S. & DiSanto, M. (2003) Int. J. Impot. Res. 15, 53-62. [DOI] [PubMed] [Google Scholar]
- 39.Handley, M. (1996) Med. Res. Rev. 16, 507-526. [DOI] [PubMed] [Google Scholar]
- 40.Bristow, L., Kramer, M., Kulagowski, J., Patel, S., Ragan, C. & Seabrook, G. (1997) Trends Pharmacol. Sci. 18, 186-188. [DOI] [PubMed] [Google Scholar]
- 41.Kramer, M., Last, B. & Reines, S. (1997) Arch. Gen. Psychiatry 54, 567-572. [DOI] [PubMed] [Google Scholar]
- 42.Rubinstein, M., Phillips, T., Bunzow, J., Falzone, T., Zhang, G., Fang, Y., Larson, J., Chester, J., Saenz, C., Pugsley, T., Gershanik, O., Low, M. & Grandy, D. (1997) Cell 90, 991-1001. [DOI] [PubMed] [Google Scholar]
- 43.Ralph, R., Varty, G., Kelly, M., Wang, Y., Caron, M., Rubinstein, M., Grandy, D., Low, M. & Geyer, M. (1999) J. Neurosci. 19, 4627-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.