Abstract
Addiction to psychostimulants, including cocaine and amphetamine, is associated with dysregulation of dopamine and serotonin (5-HT) neurotransmitter systems. Neuroadaptations in these systems vary depending on the stage of the drug taking-abstinence-relapse cycle. Consequently, the effects of potential treatments that target these systems may vary depending on whether they are given during abstinence or relapse. In this review, we discuss evidence that dopamine D3 receptors (D3Rs) and 5-HT1B receptors (5-HT1BRs) are dysregulated in response to both chronic psychostimulant use and subsequent abstinence. We then review findings from preclinical self-administration models which support targeting D3Rs and 5-HT1BRs as potential medications for psychostimulant dependence. Potential side effects of the treatments are discussed and attention is given to studies reporting positive treatment outcomes that depend on: 1) whether testing occurs during abstinence versus relapse, 2) whether escalation of drug self-administration has occurred, 3) whether the treatments are given repeatedly, and 4) whether social factors influence treatment outcomes. We conclude that D3/D2 agonists may decrease psychostimulant intake; however, side effects of D3/D2R full agonists may limit their therapeutic potential, whereas D3/D2R partial agonists likely have fewer undesirable side effects. D3-selective antagonists may not reduce psychostimulant intake during relapse, but nonetheless, may decrease motivation for seeking psychostimulants with relatively few side-effects. 5-HT1BR agonists provide a striking example of treatment outcomes that are dependent on the stage of the addiction cycle. Specifically, these agonists initially increase cocaine’s reinforcing effects during maintenance of self-administration, but after a period of abstinence they reduce psychostimulant seeking and the resumption of self-administration. In conclusion, we suggest that factors contributing to dysregulation of monoamine systems, including drug history, abstinence, and social context, should be considered when evaluating potential treatments to better model treatment effects in humans.
Keywords: addiction, cocaine, amphetamine, incentive motivation, reinstatement/extinction, self-administration
1. Introduction
Several theories of addiction postulate that chronic intake of drugs of abuse challenges homeostatic regulation of the neurotransmitter systems that are directly affected by the drugs, resulting in compensatory changes that are likely at the heart of the pathology underlying the development of dependence (Ahmed and Koob, 1998; Antelman and Caggiula, 1996; Kalivas, 2009; Koob et al., 2004; Koob et al., 1997; McEwen, 2000). Because these changes are caused by drug-induced neurotransmission outside of the physiological range associated with natural stimuli, we refer to the process causing them as dysregulation. Neurons that are directly affected by a drug may cause dysregulation of downstream neurons, which then continues to cascade throughout interconnected neurons within brain circuits.
Monoamine transporters are primary sites of action of psychostimulants (Akimoto et al., 1990; Reith et al., 1997; Zetterstrom et al., 1983). Dysregulation of circuitries involving monoamine neurotransmitters occurs with repeated psychostimulant use and may manifest as 1) changes that oppose the drug’s effects resulting in tolerance and withdrawal syndromes (Ahmed et al., 2002); 2) changes that amplify the drug’s effects leading to sensitization (Robinson and Berridge, 2000); and 3) gradual shifts in the relative activity among parallel circuits (e.g., mesolimbic versus nigrostriatal pathways) (Porrino et al., 2007). Escalation of drug intake is a hallmark sign of the development of addiction and continues to challenge homeostasis making the dysregulation progressively more pronounced and possibly distinct from that occurring with limited drug intake (e.g., Orio et al., 2010). Abstinence results in the loss of drug-induced input to these circuits, again challenging homeostasis and causing its own dysregulation. Abstinence-induced dysregulation may become more pronounced with time causing the emergence and strengthening of drug craving (Gawin and Kleber, 1986; Tran-Nguyen et al., 1998), a phenomenon known as the incubation effect (Grimm et al., 2001).
Dysregulation is dynamic with different changes occurring in the brain at different stages of the drug abuse-abstinence-relapse cycle. In this review, we discuss evidence of dysregulation involving two different monoamine receptor subtypes: dopamine D3 receptors (D3Rs) and 5-HT1B receptors (5-HT1BRs). We then discuss evidence from psychostimulant self-administration (SA) animal models that supports the development of medications that target these receptors. To aid with interpretation of this literature, we first discuss how various measures of drug SA are differentially sensitive to the incentive motivation and reinforcement processes that are involved in addiction and how these measures may inform treatment efficacy that is dependent on the stage of the abuse-abstinence-relapse cycle. We conclude that both D3Rs and 5-HT1BRs are good targets for development of medications for psychostimulant dependence and emphasize that research in this area will benefit from considering the dynamic changes in monoamine neurotransmitter systems that have been identified from basic neuroscience research.
1.1 Relevance of animal models to the chronic relapsing cycle of dependence
Drug self-administration involves incentive motivation, the process by which organisms are energized to seek drug, and reinforcement, the process by which response-contingent delivery of drug increases the probability of performing the response (Markou et al., 1993). There are procedures that can increase sensitivity to detecting effects of a manipulation on incentive motivation, such as requiring high workloads or testing under extinction condition so that drug reinforcement is not available. This review focuses on four approaches to testing potential therapeutics: 1) psychostimulant SA under low demand schedules of reinforcement, 2) SA under progressive ratio (PR) schedules of reinforcement, 3) reinstatement of extinguished drug-seeking behavior either by acute stress, drug-associated cues, or drug priming injections, and 4) resumption of drug SA after a period of abstinence. Extinction and reinstatement of drug seeking reflects incentive motivational effects of the reinstating stimulus, and when response-contingent cues are used, also reflects conditioned reinforcing effects of the cues. There are many parallels between psychostimulant-seeking behavior measured in this model and self-reports of craving (Fuchs et al., 1998; Markou et al., 1993; Stewart, 1983), supporting the predictive validity of the model as a screen for anti-craving effects of medications. Resumption of psychostimulant SA after a period of abstinence is less commonly used to screen potential treatment effects, but in our view, offers a model with strong face validity for screening anti-relapse effects. Screening treatment effects during maintenance of SA is a common approach. The use of PR schedules to assess effects of a treatment on maintenance of SA is more sensitive to motivation than low ratio or interval schedules because the work demand increases across successive reinforcers (Markou et al., 1993; Salamone and Correa, 2012). With low ratio or interval schedules, the psychostimulant dose-effect function is typically an inverted U-shaped function whereas on PR schedules it is typically linear within non-toxic dose ranges. Typically, treatment-induced shifts of the psychostimulant SA dose-effect function to the left reflect facilitation of a pharmacological action at a receptor involved in drug reinforcement, shifts to the right reflect blockade of a pharmacological action at a receptor involved in drug reinforcement, upward shifts reflect enhancement of drug reinforcement, and downward shifts reflect attenuation of drug reinforcement (Mello and Negus, 1996). The latter outcome is optimal for a potential treatment because drug intake is reduced regardless of the self-administered dose.
2. Dysregulation of dopamine D3 receptors
One interesting characteristic of D3Rs that make them particularly relevant in addiction is their localization. D3R expression is primarily restricted to structures in the mesolimbic pathway, including the nucleus accumbens shell (NAcsh), islands of Calleja, olfactory tubercle, and the ventral tegmental area (VTA) (reviewed by Heidbreder et al., 2005; Sokoloff et al., 2006). Importantly, the mesolimbic pathway is strongly implicated in drug addiction (Kalivas and Volkow, 2005; Wise, 2004). D3Rs are expressed on dopaminergic cell bodies where they may function as autoreceptors (Diaz et al., 2000). They are also expressed post-synaptically in the NAcsh on medium spiny GABAergic neurons (Ridray et al., 1998). Convergent evidence suggests that psychostimulant use up-regulates D3Rs and that this effect is related to an increase in motivation for drug. In humans, psychostimulant overdose increases D3R binding in the ventral striatum (Boileau et al., 2012; Segal et al., 1997; Staley and Mash, 1996). In rodents, an increase in D3R binding in the ventral striatum emerges during the course of abstinence from a chronic cocaine regimen (Collins et al., 2011; Conrad et al., 2010; Marcellino et al., 2007; Neisewander et al., 2004) in parallel with the time-dependent enhancement of cocaine-seeking behavior (Morgan et al., 2002a; Neisewander et al., 2000; Tran-Nguyen et al., 1998). Furthermore, chronic administration of the D3/D2R agonist 7-OH-DPAT during abstinence from cocaine SA normalizes the elevated striatal D3R levels and reduces cocaine-seeking behavior (Fuchs et al., 2002a; Neisewander et al., 2004). These findings suggest that there is a positive relationship between D3R binding and motivation for cocaine. Interestingly, the D3R up-regulation may depend on learning. Rats that have learned to associate a distinct environment with cocaine, as revealed by the expression of conditioned locomotor hyperactivity, have increased D3R levels in both the nucleus accumbens core (NAcc) and shell (NAcsh) in the ventral striatum (Le Foll et al., 2002). Such an increase is absent in rats exposed to cocaine in a familiar environment (home cage).
2.1 Limitations and complexities in evaluating potential D3R-targeted treatments
One complication in classifying D3R compounds into agonist, partial agonist and antagonist is that some compounds show functional selectivity. Functional selectivity describes the phenomenon that a ligand can have different intrinsic activities on different second messenger signaling systems that are coupled to the same receptor (Mailman, 2007; Urban et al., 2007). For example, the D3/D2R compound BP 897 acts as a partial agonist at the D3R in signaling pathways of mitogenesis (Pilla et al., 1999) but acts as an antagonist in GTPγS-dependent pathways (Gyertyan et al., 2007a). Several other D3R compounds also show functional selectivity, including aripiprazole (Shapiro et al., 2003), the D3R-selective phenylpiperazine PG 619 (Blaylock et al., 2011), and some novel phenylpiperazines (Taylor et al., 2010). Further studies are required to examine the role of functional selectivity in mediating the effects of D3R compounds. It is possible that the therapeutic effect of a functional selective D3R compound is mediated by one D3R-coupled second messenger signaling pathway, and the side effect of the same D3R compound is mediated by another D3R-coupled signaling pathway. If this is the case, then it would be greatly beneficial to develop D3R compounds with functional selectivity that targets the therapeutic effect alone (Mailman, 2007).
Due to a current lack of D3R selective agonists (Newman et al., 2005), the behavioral effect of D3R activation has mainly been examined using receptor agonists that only display moderate to low selectivity for D3 over D2 receptors as summarized in Table 1. In addition to binding affinity, evidence of functional activation of D3 and D2Rs is suggested from effects of the compounds on yawning (Collins et al., 2005). D3R agonists, including the selective agonist pramipexole, produce an inverted U-shaped dose-effect function for yawning (Collins et al., 2005; Khroyan et al., 1995). The induction of yawning is blocked by D3R antagonists, whereas the inhibition of yawning at high doses is attenuated by a D2R-preferring antagonist (Collins et al., 2005; Newman et al., 2012). This suggests that D3R agonists primarily activate D3Rs at low doses resulting in yawning, and that D2Rs are also activated at higher doses resulting in inhibition of D3R-induced yawning. There is also evidence that most D3R agonists have less D3R:D2R selectivity in vivo than in vitro (Collins et al., 2007). Such is the case for both pramipexole and PD128907, which we refer to as D3/D2R agonists despite their ~200-fold D3R selectivity in vitro.
Table 1.
Mechanism of action and specificity of D3Rdrugs.
| Compound | Mechanism of Action | Specificity (nM) | D3R:D2R selectivity (% IA*) | |
|---|---|---|---|---|
| Agonists | ||||
| Pramipexole | D3R-preferring agonist | Ki: hD2R, 790; hD3R, 4.1 | 192.7 | (Sautel et al., 1995) |
| PD128907 | D3R-preferring agonist | Ki: hD2R, 339; hD3R, 1.89 | 179.4 | (Audinot et al., 1998) |
| PF-592,379 | D3R-selective agonist | IC50: hD2R, <10000; Ki: hD3R, 215; hD4R, 4165 | <46.5 | (Collins et al., 2012a) |
| Quinelorane | D3R-preferring agonist | Ki: hD2R, 265; hD3R, 6.1 | 43.44 | (Millan et al., 1995) |
| 7-OH-DPAT | D3R-preferring agonist | Ki: hD2R, 92; hD3R, 2.2 | 41.8 | (Audinot et al., 1998) |
| WC44 | D3R-preferring agonist (antagonist in the 7-OH-DPAT-induced yawning test, unpublished). | Ki: hD2R, 56.5; hD3R, 2.4; hD4R, 804 | 23.5 | (Kumar et al., 2009) |
| Quinpirole | D3R-preferring agonist | Ki: hD2R, 911; hD3R, 43 | 21.2 | (Millan et al., 1995) |
| (−)–NPA | Mixed D2R/D3R agonist | Ki: hD2R, 2.9; hD3R, 0.36 | 8.1 | (Sautel et al., 1995) |
| Sumanirole | D2R-selective agonist | Ki: hD2R, 9.0; hD3R, 1940; hD4R, <2190 | 0.0046 | (McCall et al., 2005) |
| Antagonists | ||||
| YQA14 | D3R-selective antagonist | Ki: hD2R, 353.3; hD3R (high affinity site), 0.68 × 10−4; hD3R (low affinity site), 2.11; hD4R, <105 | 167.3 | (Song et al., 2012a) |
| PG-01037 | D3R-selective antagonist | Ki: hD2R, 93.3; hD3R, 0.7; hD4R, 375 | 133.3 | (Grundt et al., 2007) |
| SB-277011A | D3R-selective antagonist | pKi: hD2R, 5.98; hD3R, 7.95 | 93.3 | (Reavill et al., 2000) |
| NGB-2904 | D3R-preferring antagonist | Ki: hD2R, 112; hD3R, 2.0 | 56.0 | (Grundt et al., 2007) |
| WC10 | D3R-preferring antagonist | Ki: hD2R, 33.4; hD3R, 0.8; hD4R, 896 | 41.8 | (Kumar et al., 2009) |
| L-745,829 | D3R/D4R-preferring antagonist | Ki: rD2R, <1900; rD3R, 46.5; rD4R, 2.7 | 40.9 | (Caine et al., 2002) |
| S33138 | D3R-preferring antagonist | pKi: hD2LR, 7.1; hD2SR, 7.3; hD3R, 8.7; hD4R, <5 | 25.1 | (Millan et al., 2008) |
| Raclopride | Mixed D2R/D3R antagonist | Ki: hD2R, 1.1; hD3R, 1.4 | 0.79 | (Millan et al., 1995) |
| Eticlopride | Mixed D2R/D3R antagonist | Ki: hD2R, 0.07; hD3R, 0.16 | 0.44 | (Mackenzie et al., 1994) |
| Sulpiride | Mixed D2R/D3R antagonist | Ki: hD2R, 71.6; hD3R, 570 | 0.13 | (Mackenzie et al., 1994) |
| L-741,626 | D2R-preferring antagonist | Ki: rD2R, 7.1; rD3R, 155; rD4R, 596 | 0.046 | (Caine et al., 2002) |
| Partial Agonists | ||||
| RGH-237 | D3R-selective partial agonist | Ki: hD2R, <2900; hD3R, 1.6 | 1812.5 (52%) | (Gyertyan et al., 2007a) |
| WW-III-55 | D3R-selective partial agonist | Ki: hD2LR, <15000; hD3R, 19.8 | 757.6 (68%) | (Unpublished data) |
| OS-3-106 | D3R-preferring partial agonist | Ki: hD2LR, 26.0; hD3R, 0.23 | 113.0 (58%) | (Unpublished data) |
| BP 897 | D3R-preferring partial agonist | Ki: hD2R, 61; hD3R, 0.92 | 66.3 (60%, but shows functional selectivity) | (Pilla et al., 1999) (Gyertyan et al., 2007a) |
| CJB 090 | D3R-preferring partial agonist | Ki: hD2R, 24.8; hD3R, 0.4 | 62.0 (30%) | (Grundt et al., 2007) |
| WC26 | D3R-preferring partial | Ki: hD2R, 30.7; hD3R, 0.6; hD4R, 674 | 51.2 (69%) | (Kumar et al., 2009) |
| Aripiprazole | Mixed D2R/D3R partial | Ki: hD2R, 4.4; hD3R, 3.1; hD4R, <1000 | 1.42 ((47–52%) | (Burstein et al., 2005) (Tadori et al., 2008) |
| Terguride | Mixed D2R/D3R partial agonist | pKi: hD2SR, 9.1; hD2LR, 8.94; hD3R, 9.0 | 0.79 (81–77%) | (Millan et al., 2002) (Tadori et al., 2008) |
| SDZ-208-911 | Mixed D2R/D3R partial agonist | Ki: rD2R (rat striatum), 0.11; hD3R, 0.17 | 0.65 (26–16%) | (Kula et al., 1994) (Tadori et al., 2008) |
In the case of partial agonists, the % intrinsic activity relative to agonist control (%IA) is also shown in parentheses next to the selectivity ratio.
2.2 D3/D2R agonist effects on maintenance of psychostimulant SA
D3/D2R agonists, which are also referred to as D3-preferring agonists, produce opposite effects on cocaine SA depending on dose. These biphasic effects may reflect actions at D3 versus D2Rs. As detailed in Table 2, low doses of D3/D2R agonists reduce SA of low doses of cocaine on the ascending limb of the dose-effect function (Caine and Koob, 1995; Caine et al., 1999). This effect may be due to a preferential action at D3 or D2R autoreceptors which counters effects of cocaine by inhibiting dopamine release. In contrast, higher doses of D3/D2R agonists shift the cocaine dose-effect function to the left, increasing SA of low doses of cocaine and decreasing SA of high doses of cocaine (Caine and Koob, 1995; Caine et al., 1997; Caine et al., 1999; Gál and Gyertyán, 2003). Likewise, acute treatment with the D3/D2R agonist (−)–NPA at doses that cause little behavioral disruption reduces choice of a high dose of cocaine relative to food (Czoty and Nader, 2013), an effect similar to acute amphetamine treatment (Negus, 2003). Early studies suggested that D3R activation may enhance the reinforcing value of cocaine (Caine et al., 1997); however, Caine et al. (2002) later found that the D2R-preferring antagonist L-741,626, but not the D3/D4R antagonist L-745,829, reversed the quinelorane-induced reduction of intake at a high dose of cocaine. Also, D2R deletion in mice increases SA of high doses of cocaine without affecting SA of low doses of cocaine (Caine et al., 2002). Collectively, the findings suggest that D2Rs mediate the decrease in intake of high dose cocaine, whereas D2 and/or D3Rs mediate the decrease in intake of low dose cocaine, perhaps via preferential action at autoreceptors.
Table 2.
Effects of D3/D2R agonists on psychostimulant abuse-related behaviors.
| Model | Treatment (D3:D2R selectivity) | Dose of treatment | Effect (Psychostimulant dose); Species; Schedule; Further comments | Reference |
|---|---|---|---|---|
| Cocaine: SA maintenance | Pramipexole (193-fold) | 1–5 μmol/kg, SC | ↓ descending limb (0.25 mg, IV); rats; FR5 | (Caine et al., 1997) |
| PD128907 (179-fold) | 0.6 mg/kg, SC | ↑ ascending limb (0.01 mg, IV); rats; FR5 | (Caine et al., 1997; Gál and Gyertyán, 2003) | |
| 0.3–1 mg/kg, SC | ↓ descending limb (0.25–0.3 mg, IV); rats; FR5 | |||
| Quinelorane (43-fold) | 0.0032 mg/kg, IP | ↓ ascending limb (0.032 mg, IV); rats; FR5 | (Caine et al., 1999) | |
| 0.01–0.03 mg/kg, IP | ↑ ascending limb (0.01–0.03 mg, IV); rats; FR5 | (Barrett et al., 2004; Caine et al., 1999) | ||
| 0.001–0.032 mg/kg, IP | ↓ descending limb (0.1–1 mg, IV); rats; FR5 | |||
| 7-OH-DPAT (42-fold) | 0.001 mg/kg, IV | ↓ ascending limb (0.03 mg, IV); rats; FR5 | (Caine and Koob, 1995) | |
| 0.4 mg/kg, SC; 0.32–1 mg/kg, IP | ↑ ascending limb (0.01–0.03 mg, IV); rats; FR5 | (Barrett et al., 2004; Caine and Koob, 1995; Gál and Gyertyán, 2003) | ||
| 0.001–0.004 mg/kg, IV; 0.1–1.6 mg/kg, SC; 1 mg/kg, IP | ↓ descending limb (0.12–1 mg, IV); rats; FR5 | |||
| 1–4 μg/side, intra–CeA | ↓ descending limb (0.75 mg/kg, IV); rats; VR5 | (Thiel et al., 2010) | ||
| 0.004 mg/kg, IV (co-infused with cocaine reinforcers) | Ø (0.06–0.5 mg/kg, IV); rats; PR | (Caine and Koob, 1995) | ||
| WC44 (24-fold, in vivo partial agonist/antagonist) | 10 mg/kg, IP | ↓ ascending/descending limb (0–0.375 mg/kg, IV); ↑ descending limb (1.5 mg/kg, IV); rats; VI60s | (Cheung et al., 2012) | |
| Quinpirole (22-fold) | 0.003–.1 mg/kg, IM | Ø peak dose (0.18–0.3 mg/kg, IV); monkeys; 2nd order schedule | (Platt et al., 2003) | |
| (−)–NPA (8-fold) | 0.003–0.0056 mg/kg, IV, acute | ↓ high dose (0.1 mg/kg, IV); monkeys; choice vs. food | (Czoty and Nader, 2013) | |
| 0.003–0.0056 mg/kg/day, IV, after 5 daily treatments | ↑ low dose (0.003–0.03 mg/kg, IV); dominant monkeys; choice vs. food | |||
| ↑ low dose (0.003 mg/kg, IV); ↓ high dose (0.03–0.1 mg/kg, IV); subordinate monkeys; choice vs. food | ||||
| Amphetamine: SA maintenance | Quinpirole | 0.1–1 mg/kg, SC | Ø (0.12 mg/kg, IV); rats; PR | (Izzo et al., 2001) |
| Cocaine seeking, early abstinence (<48h) | 7-OH-DPAT | 0.01–1 mg/kg, SC | ↓ 1st h; rats; non-contingent cues | (Fuchs et al., 2002b) |
| 1 mg/kg, SC | ↑ 2nd h; rats; non-contingent cues | |||
| 3–10 mg/kg, IP | ↑ rats; no cues | (Self et al., 1996) | ||
| Quinpirole | 0.05 mg/kg, SC | ↓ rats; non-contingent cues | (Marinelli et al., 2003) | |
| 0.1–3 mg/kg, IP | ↑ rats; no cues | (Self et al., 1996) | ||
| Cocaine seeking, protracted abstinence | PD128907 (179-fold) | 0.01–.1 mg/kg, IV | Ø monkeys; response-contingent cues | (Khroyan et al., 2000) |
| 0.3 mg/kg, IV | ↑ monkeys; response-contingent cues | (Achat-Mendes et al., 2010a) | ||
| 7-OH-DPAT, chronic | 1 mg/kg/day for 14 days, SC | ↓ rats; non-contingent cues; 17–23 h post daily treatment | (Fuchs et al., 2002b) | |
| 7-OH-DPAT, acute | 0.1–0.3 mg/kg, IP | ↓ rats; discriminative cues | (Cervo et al., 2003a) | |
| 1, 3 mg/kg, IP | ↑ rats; discriminative cues | |||
| 4 μg/side, intra-CeA | ↓ rats; response-contingent cues | (Thiel et al., 2010) | ||
| Quinpirole | 0.01–0.05 mg/kg, SC | ↓ rats; non-contingent cues | (Marinelli et al., 2003) | |
| 0.2–5 mg/kg, SC | ↑ rats; no cues | (De Vries et al., 2002; De Vries et al., 1999) | ||
| 0.1 mg/kg, IV | ↑ monkeys; no cues | (Blaylock et al., 2011) | ||
| Sumanirole (0.005-fold) | 2–3 mg/kg, IV | ↑ monkeys; response-contingent cues | (Achat-Mendes et al., 2010a) | |
| Cocaine-primed reinstatement of cocaine seeking* | PD128907 | 0.01–0.18 mg/kg, IM | Ø (prime: maximum effective dose, IV); monkeys; cues on 2nd order schedule | (Khroyan et al., 2000) |
| 7-OH-DPAT | 0.01–0.1 mg/kg, IM | Ø (prime: maximum effective dose, IV); monkeys; cues on 2nd order schedule | (Khroyan et al., 2000) | |
| 0.3 mg/kg, IP | ↑ (prime: 0.5 mg/kg, IV); rats | (Self et al., 1996) | ||
| 1 mg/kg, SC (after < 36 days of daily treatment) | ↑ 0 but not 2 or 4 h post-7-OH-DPAT (prime: 15 mg/kg, IP); rats | (Fuchs et al., 2002b) | ||
| 4 μg/side, intra-CeA | ↓ (prime: 10 mg/kg, IP); rats | (Thiel et al., 2010) | ||
| Quinpirole | 0.01–0.05 mg/kg, SC | Ø (prime: 20 mg/kg, IP); rats; 1 day abstinence | (Marinelli et al., 2003) | |
| ↓ (prime: 20 mg/kg, IP); rats; 10 days abstinence | ||||
| 0.1 mg/kg, IV | Ø (prime: 0.1 mg/kg, IV); monkeys | (Blaylock et al., 2011) | ||
| (−)–NPA | 0.0003–0.01 mg/kg, IM | Ø (prime: maximum effective dose, IV); monkeys, cues on a 2nd order schedule | (Khroyan et al., 2000) | |
| Resumption of extinguished cocaine SA* | 7-OH-DPAT, chronic | 1 mg/kg/day for 20 days, SC | ↓ descending limb (0.25 mg/kg, IV); rats; VR5; 17–23 h post daily treatment | (Fuchs et al., 2002b) |
Table key. ↑: increased compared with control. ↓: decreased compared with control. Ø: no effect compared with control. SA: self-administration. FR: fixed ratio schedule of reinforcement. PR: progressive ratio schedule of reinforcement. VI: variable interval schedule of reinforcement. BLA: Basolateral amygdala. CeA: Central amygdala. NAc: Nucleus accumbens. dSt: Dorsal striatum.
: tests carried out after protracted abstinence (<48h).
In contrast to the above studies, we found that WC44, a compound with a 23-fold D3R:D2R selectivity that acts as a full agonist at the D3R in the adenylyl cyclase bioassay (Chu et al., 2005; Kumar et al., 2009), shifted the cocaine SA dose-effect function to the right (Cheung et al., 2012). The most likely explanation for this discrepancy is that WC44 may act as a D3R antagonist in vivo (Weber et al., 2009). Consistent with this idea, WC44 attenuates yawning induced by 7-OH-DPAT (unpublished), similar to the effects of D3R antagonists (Collins et al., 2005).
Under schedules requiring relatively high effort, D3/D2R agonists appear to be less effective in altering psychostimulant SA. Co-infusion of 7-OH-DPAT with each cocaine reinforcer fails to significantly affect PR responding (Caine and Koob, 1995). Similarly, quinpirole fails to affect amphetamine SA on a PR schedule (Izzo et al., 2001), as well as responding for cocaine under a second order schedule in monkeys (Platt et al., 2003).
2.3 D3/D2R agonist effects in relapse models
The effects of D3/D2R agonists on cocaine seeking (i.e., responses emitted in extinction) are biphasic with respect to dose and time course at higher doses (Table 2). For instance, acute treatment with low doses of D3/D2R agonists reduces cue-elicited cocaine seeking, whereas a high dose initially inhibits, but later in the session enhances, the behavior (Fuchs et al., 2002b; Marinelli et al., 2003). Similar biphasic dose effects have been observed with 7-OH-DPAT on the reinstatement of extinguished cocaine seeking by discriminative cues (Cervo et al., 2003b). High doses of D3/D2R agonists are also effective as priming injections which reinstate extinguished cocaine seeking (Blaylock et al., 2011; De Vries et al., 2002; De Vries et al., 1999; Self et al., 1996), possibly via the NAcsh (Schmidt and Pierce, 2006). The low dose effects of D3/D2R agonists likely inhibit cocaine-seeking behavior due to preferential action at D3 versus D2Rs, whereas higher doses may act upon both D3 and D2Rs, with the action at D2Rs likely increasing cocaine-seeking behavior (Cervo et al., 2003b; Fuchs et al., 2002b). In support, the D2R-selective agonist sumanirole partially primes cocaine seeking while the more D3R-selective agonist PD128907 has less consistent effects (Achat-Mendes et al., 2010b; Khroyan et al., 2000).
Time-dependent effects are also observed across days of repeated D3/D2R agonist administration. For instance, repeated treatments with high dose 7-OH-DPAT increase cocaine-primed cocaine seeking when testing occurs immediately after the treatment, have no effect when testing occurs 4 h after the treatment, and reduce cocaine seeking when testing occurs 23 h after the treatment (Fuchs et al., 2002b). These findings suggest a biphasic effect of the chronic 7-OH-DPAT treatments in rats where initially the treatment produces a transient enhancement of incentive motivation for cocaine followed by a protracted decrease in incentive motivation for cocaine (Fuchs et al., 2002a). Chronic daily 7-OH-DPAT treatments also reduce resumption of cocaine SA after a period of abstinence in rats tested 17–23 h after their daily 7-OH-DPAT treatments (Fuchs et al., 2002b). Given that the test occurred 17–23 h post-treatment, the decreases in cocaine-primed seeking and cocaine SA are likely due to neuroadaptations that occur in response to chronic 7-OH-DPAT during cocaine withdrawal rather than an acute effect of the treatment. Low dose quinpirole effects are also time-dependent with greater reductions in cocaine seeking, cocaine-primed seeking, and dopamine neuron firing observed after 10 days of withdrawal compared to 1 day of withdrawal (Marinelli et al., 2003).
There may be species differences in the effects of D3/D2R agonists on cocaine-primed cocaine seeking. In contrast to rats for instance, D3/D2R agonists and the D3/D2R agonists (−)–NPA fail to alter cocaine-primed reinstatement of cocaine seeking in monkeys (Blaylock et al., 2011; Khroyan et al., 2000). Interestingly, the effect of (−)–NPA may depend on treatment duration, cocaine dose and social hierarchy, because five daily treatments of (−)–NPA during abstinence from cocaine SA reduced preference for cocaine relative to food in a subsequent choice test in subordinate, but not in dominant, monkeys; however it increased choice of a low cocaine dose in both groups of monkeys (Czoty and Nader, 2013).
2.4 D3R-selective and D3/D2R antagonist effects on maintenance of cocaine SA
D3R antagonists may affect psychostimulant motivation rather reinforcement, whereas D2R antagonists, possibly in combination with D3R antagonists, may be needed to decrease psychostimulant reinforcement. As summarized in Table 3, several D3R-selective antagonists are relatively ineffective in modulating SA of high doses of cocaine (Caine et al., 2002; Di Ciano et al., 2003b; Gál and Gyertyán, 2003; Gyertyan et al., 2007a; Xi et al., 2005; Xi et al., 2006) or methamphetamine (Higley et al., 2011a; Higley et al., 2011b; Orio et al., 2010) under low effort, fixed ratio schedules. In contrast, the D3/D2R antagonist eticlopride and the D3/D2R functional antagonist WC44 reduce intake of low cocaine doses (ascending limb) and increase intake of high cocaine doses (descending limb) under low effort schedules of reinforcement, consistent with a rightward shift of the cocaine dose-effect function (Barrett et al., 2004; Cheung et al., 2012). The increased SA of high doses of cocaine may be mediated via D2R antagonism because D2R knockout mice exhibit a similar effect that is not reversed by eticlopride (Caine et al., 2002). Not all D3/D2R antagonists produce a rightward shift of the cocaine SA function. For instance, the D3/D2R antagonist S33138 reduces SA at a high dose of cocaine (descending limb) under FR2 schedule (Peng et al., 2009). The reason for this discrepancy is currently unclear.
Table 3.
Effects of D2/D3R antagonists on psychostimulant abuse-related behaviors.
| Model | Treatment (D3:D2R selectivity) | Dose of treatment | Effect (Psychostimulant dose); Species; Schedule; Further comments | Reference |
|---|---|---|---|---|
| Cocaine: SA maintenance on FR1 or FR2 | YQA14 (167-fold) | 6.25–25 mg/kg, IP | ↓ ascending/peak dose (0.03–0.125 mg/kg, IV); rats; FR2 | (Song et al., 2012a) |
| 25 mg/kg, IP | ↓ descending limb (0.25 mg/kg, IV); rats; FR2 | |||
| 25–50 mg/kg, IP | ↓ descending limb (1 mg/kg, IV); mice; FR1; Ø in D3R knockout mice | |||
| SB-277011A (93-fold) | 5–20 mg/kg, IP or PO | Ø descending limb (0.25 mg, IV); rats; FR1 | (Gál and Gyertyán, 2003; Gyertyan and Saghy, 2007) | |
| 0.3–30 mg/kg, IP | Ø descending limb (0.75 mg/kg, IV); rats; FR1 | (Di Ciano et al., 2003b; Xi et al., 2005) | ||
| 100 mg/kg, IP | ↓ descending limb (1 mg/kg, IV); mice; FR1; Ø in D3R knockout mice | (Song et al., 2012a) | ||
| 12.5–25 mg/kg, IP | ↓ ascending/peak dose (0.03-0.125 mg/kg, IV); rats; FR2 | |||
| ↓ descending limb (0.25 mg/kg, IV); rats; FR2 | ||||
| NGB-2904 (56-fold) | 0.1–10 mg/kg, IP | Ø descending limb (0.5 mg/kg, IV); rats; FR2 | (Xi et al., 2006) | |
| L-745,829 (41-fold) | 0.1–10 mg/kg, IP | Ø descending limb (1 mg/kg, IV); rats; FR1 | (Caine et al., 2002) | |
| 5.6 mg/kg, IP | Ø ascending/descending limb (0.032–3.2 mg/kg, IV); rats; FR1 | |||
| S33138 (25-fold) | 2.5 mg/kg, PO | ↑ descending limb (0.5 mg/kg, IV); rats; FR2 | (Peng et al., 2009) | |
| 5 mg/kg, PO | ↓ descending limb (0.5 mg/kg, IV); rats; FR2 | |||
| Eticlopride (0.44-fold) | 0.1–0.18 mg/kg, IP | ↑ descending limb (1 mg/kg, IV); mice; FR1; Ø in D2R knockout mice | (Caine et al., 2002) | |
| Methamphetamine: SA maintenance on FR1 or FR2 | PG-01037 (133-fold) | 3–30 mg/kg, IP | Ø descending limb (0.05 mg/kg, IV); rats; FR2 | (Higley et al., 2011b) |
| 8–32 mg/kg, SC | Ø descending limb (0.05 mg/kg, IV); rats; FR1; long and short access | (Orio et al., 2010) | ||
| SB-277011A | 6–24 mg/kg, IP | Ø descending limb (0.05 mg/kg, IV); rats; FR2 | (Higley et al., 2011a) | |
| Cocaine: SA maintenance on other schedules | YQA14 | 1.04–12.5 mg/kg, IP | ↓ (0.5 mg/kg, IV); rats; PR | (Song et al., 2012a) |
| 50 mg/kg, IP | ↓ (1 mg/kg, IV); mice; PR; Ø in D3R knockout mice | |||
| PG-01037 | 30 mg/kg, IM | Ø (0.03–0.3 mg/kg, IV); monkeys; 2nd order schedule | (Achat-Mendes et al., 2010a) | |
| 100 mg/kg, IM | Ø (0.1 mg/kg, IV); monkeys; 2nd order schedule | |||
| SB-277011A | 24 mg/kg, IP | ↓ peak/descending limb (0.125–0.25 mg/kg/inf, IV); rats; FR10 | (Xi et al., 2005) | |
| 12–24 mg/kg, IP | ↓ (0.5 mg/kg, IV); rats; PR | |||
| 50 mg/kg, IP | ↓ (1 mg/kg, IV); mice; PR; Ø in D3R knockout mice | (Song et al., 2012b) | ||
| 20–30 mg/kg, IP | ↓ descending limb (0.75 mg/kg, IV); rats; 2nd order schedule | (Di Ciano et al., 2003b) | ||
| NGB-2904 | 1–5 mg/kg, IP | ↓ (0.5 mg/kg, IV); rats; PR | (Xi et al., 2006) | |
| 1–5.6 mg/kg, IV | Ø (1 mg/kg, IV); monkeys; 2nd order schedule | (Martelle et al., 2007) | ||
| WC10 (42-fold) | 10 mg/kg, IP | ↓ ascending/descending limb (0–0.375 mg/kg, IV); rats; VI60s | (Cheung et al., 2012) | |
| Eticlopride | 0.1–0.32 mg/kg, IP | ↓ ascending limb (0.1–0.32 mg, IV); rats; FR5 | (Barrett et al., 2004) | |
| 0.1 mg/kg, IP | ↑ descending limb (1 mg, IV); rats; FR5 | |||
| Methamphetamine: SA maintenance on other schedules | PG-01037 | 10–30 mg/kg, IP | ↓ (0.05 mg/kg, IV); rats; PR | (Higley et al., 2011b) |
| 32 mg/kg, IP | ↓ (0.05 mg/kg, IV); rats; PR; long but not short access | (Orio et al., 2010) | ||
| SB-277011A | 12–24 mg/kg, IP | ↓ (0.05 mg/kg, IV); rats; PR | (Higley et al., 2011a) | |
| Cocaine seeking, early abstinence (<48h) | SB-277011A | 10–30 mg/kg, IP | ↓ before 1st reinforcer; rats; 2nd order schedule | (Di Ciano et al., 2003b) |
| 4 μg/side, intra-BLA but not dSt or NAc shell | ↓ before 1st reinforcer; rats; 2nd order schedule | (Di Ciano, 2008) | ||
| Cocaine seeking, protracted abstinence | SB-277011A | 5–20 mg/kg, IP or PO | ↓ rats; response-contingent cues | (Gyertyan and Saghy, 2007; Xi et al., 2005) |
| 10–30 mg/kg, IP | ↓ rats; discriminative cues | (Cervo et al., 2007b) | ||
| NGB-2904 | 5 mg/kg, IP | ↓ rats; response-contingent cues | (Gilbert et al., 2005) | |
| Raclopride (0.79-fold) | 1 mg/kg, SC | ↓ rats; discriminative cues | (Cervo et al., 2003a) | |
| Methamphetamine seeking* | PG-01037 | 10–30 mg/kg, IP | ↓ rats; response-contingent cues | (Higley et al., 2011b) |
| Cocaine-primed reinstatement of cocaine seeking* | PG-01037 | 30 mg/kg, IM | ↓ (prime: 0.3–1 mg/kg, IV); monkeys; cues on a 2nd order schedule | (Achat-Mendes et al., 2010a) |
| SB-277011A | 6–12 mg/kg, IP | ↓ (prime: 1 mg/kg, IV); rats | (Vorel et al., 2002) | |
| NGB-2904 | 1–5 mg/kg, IP | ↓ (prime: 10 mg/kg, IP); rats | (Xi et al., 2006) | |
| S33138 | 0.625–2.5 mg/kg, PO | ↓ (prime: 10 mg/kg, IP); rats | (Peng et al., 2009) | |
| Sulpiride (0.13-fold) | 2 μg/side, intra-NAc shell but not core | ↓ (prime: 10 mg/kg, IP); rats | (Anderson et al., 2006) | |
| Methamphetamine-primed reinstatement of methamphetamine seeking* | SB-277011A | 12–24 mg/kg, IP | ↓ (prime: 1 mg/kg, IP); rats | (Higley et al., 2011a) |
| Stress-primed reinstatement of cocaine seeking* | SB-277011A | 12 mg/kg, IP | ↓ rats; footshock stress-prime | (Xi et al., 2004) |
| 1.5 μg/side, intra-NAc but not dSt | ↓ rats; footshock stress-prime |
Table key. ↑: increased compared with control. ↓: decreased compared with control. Ø: no effect compared with control. SA: self-administration. FR: fixed ratio schedule of reinforcement. PR: progressive ratio schedule of reinforcement. VI: variable interval schedule of reinforcement. BLA: Basolateral amygdala. CeA: Central amygdala. NAc: Nucleus accumbens. dSt: Dorsal striatum.
: tests carried out after protracted abstinence (<48h).
In contrast to the lack of effect of D3R-selective antagonists on psychostimulant intake under low demand reinforcement schedules, these antagonists decrease intake under higher demand schedules. This pattern suggests that D3R-selective antagonists affect motivation for cocaine. For example, under FR10, PR, or second order schedules of reinforcement, D3R-selective antagonists reduce responding for cocaine and methamphetamine (Di Ciano et al., 2003a; Higley et al., 2011a; Higley et al., 2011b; Song et al., 2012a; Xi et al., 2005; Xi et al., 2006). Furthermore, Nader et al. (1999) reported a correlation between the reduction in the number of responses emitted per cocaine reinforcer on an interval schedule (i.e., effort measure) and selectivity for D3 versus D2R binding of antagonists. We have also found that the D3/D2R functional antagonist WC44 increases the latency to respond for cocaine but not for sucrose, which may reflect a selective decrease in motivation for cocaine (Cheung et al., 2012). However, there may be species differences in D3R antagonist effects because both NGB-2904 and PG-01037 are ineffective in monkeys responding under high demand, second order schedules (Achat-Mendes et al., 2010b; Martelle et al., 2007). In rats, dysregulation of D3Rs may underlie D3R antagonist reduction of methamphetamine intake on PR since this effect is only observed in rats given extended daily access and not those given limited access (Orio et al., 2010). The former exposure regimen presumably produces a more severe challenge to homeostasis.
The lack of D3R-selective antagonist effects on cocaine SA under low effort schedules may be due to the use of high cocaine doses. Testing against a full cocaine dose-effect function, Song et al. (2012a) found that SB-277011A and the novel D3R-selective antagonist YQA14 reduce SA of low and moderate doses of cocaine (ascending and descending limb) under a low demand schedule. These effects are absent in D3R knockout mice. However, D3R knockout mice still acquire cocaine SA (Caine et al., 2012; Song et al., 2012a; Song et al., 2012b) and exhibit enhanced cocaine-conditioned place preference (Kong et al., 2011), suggesting cocaine reward is unaffected.
Interestingly, cocaine self-administration alters adenosine A2A receptors (A2ARs), in addition to D3Rs, in the NAc (Marcellino et al., 2007) and there is evidence that dopamine in the NAc modulates effort via an interaction with A2ARs (Salamone and Correa, 2009; Salamone et al., 2009). These findings have suggested the possibility that D3R antagonism lowers the motivation to engage in effortful responses for cocaine via A2AR-D3R heteromers (Fuxe et al., 2010).
2.5 D3R-selective and D3/D2R antagonist effects in relapse models
There is substantial evidence that both D3/D2R and D3R-selective antagonists are effective in reducing psychostimulant seeking. For example, SB-277011A attenuates cocaine seeking under second order schedules (Di Ciano et al., 2003b; Gal and Gyertyan, 2006), in part via receptors in the basolateral amygdala (Di Ciano, 2008). D3R-selective and D3/D2R antagonists also attenuate cue reinstatement of extinguished cocaine seeking behavior (Cervo et al., 2003b; Cervo et al., 2007a; Gilbert et al., 2005; Higley et al., 2011b), as well as cocaine-primed reinstatement (Achat-Mendes et al., 2010b; Peng et al., 2009; Vorel et al., 2002; Xi et al., 2006). The latter is mediated in part via D2/D3Rs in the NAcc (Anderson et al., 2006). Similarly, methamphetamine-primed reinstatement of methamphetamine seeking is blocked by SB-277011A (Higley et al., 2011a). In addition, SB-277011A given either systemically or by infusion into the NAc, but not the dorsal striatum (dStr), attenuates footshock stress-induced reinstatement of cocaine seeking (Xi et al., 2004). Collectively, these findings suggest that D3Rs play a pivotal role in motivation to seek psychostimulants regardless of the trigger for the motivation.
2.6 D3R-selective and D3/D2R partial agonist effects on maintenance of cocaine SA
D3R partial agonists have some degree of intrinsic activity that is less than that of the endogenous neurotransmitter dopamine. This property allows for dual functional effects (Pulvirenti and Koob, 2002): 1) they can prevent the full effects of high synaptic levels of dopamine, such as those observed during exposure to psychostimulants or to psychostimulant-associated cues (Schultz et al., 1997; Volkow et al., 2006); 2) their partial intrinsic activity at D3Rs when endogenous dopamine release is low, such as during abstinence (Volkow et al., 1997), may allow for signaling levels that restore some degree of tonic stimulation of the receptors. The latter effect may help to relieve cocaine withdrawal symptoms including craving.
The dual action of D3/D2R partial agonists is likely responsible for the mixed effects that they have on psychostimulant SA (Table 4). For example, the D3/D2R partial agonist terguride increases SA of cocaine (Pulvirenti et al., 1998) and amphetamine (Izzo et al., 2001) on the descending limb of the SA dose-effect curve, similar to effects of D3/D2R antagonists (Caine et al., 2002). In contrast, the D3/D2R partial agonist aripiprazole appears to shift the cocaine dose-effect function downward in mice, reducing responding over a wide range of cocaine doses (Sørensen et al., 2008); however, this effect is not observed in rats (Feltenstein et al., 2007). Aripiprazole delivered via osmostic minipumps (0.56 mg/kg/h for 2 h) is effective in decreasing choice of cocaine over food in rats, an effect that dissipates after 5 days of continuous aripiprazole delivery and daily cocaine SA (Thomsen et al., 2008). The D3/D2R compound BP 897, which has functional selectivity and may act as a partial agonist (Pilla et al., 1999) or a full antagonist (Gyertyan et al., 2007a) at D3Rs, has no effect on cocaine SA (Pilla et al., 1999) except at a relatively high dose which increases cocaine infusions (Gal and Gyertyan, 2006).
Table 4.
Effects of D2/3R partial agonists on psychostimulant abuse-related behaviors.
| Model | Treatment (D3:D2R selectivity) | Dose of treatment | Effect (Psychostimulant dose); Species; Schedule; Further comments | Reference |
|---|---|---|---|---|
| Cocaine: SA maintenance on FR1 or FR2 | RGH-237 (1813-fold) | 10–30 mg/kg, PO | Ø descending limb (0.25 mg, IV); rats; FR1 | (Gyertyan et al., 2007a) |
| BP 897 (66-fold; functional selective) | 0.05–1 mg/kg, IP | Ø descending limb (0.25 mg, IV); rats; FR1 | (Pilla et al., 1999) | |
| 1 mg/kg, IP | ↑ descending limb (0.25 mg, IV); rats; FR1 | (Gál and Gyertyán, 2003) | ||
| Aripiprazole (1.4-fold) | 0.4 m/kg, PO | ↓ ascending/descending limb (0.003–1 mg, IV); mice; FR1 | (Sørensen et al., 2008) | |
| 0.5–2.5 mg/kg, IP | Ø descending limb (0.5 mg/kg, IV); rats; FR1 | (Feltenstein et al., 2007) | ||
| Terguride (0.79) | 0.4 mg/kg, IP | ↑ descending limb (0.125–0.5 mg, IV); rats; FR1 | (Pulvirenti et al., 1998) | |
| Amphetamine: SA maintenance | Terguride | 0.1–.4 mg/kg, IP | ↑ (0.12 mg, IV); rats; FR1 | (Izzo et al., 2001) |
| Methamphetamine: SA maintenance | CJB 090 (62-fold) | 10 mg/kg, IV | ↓ descending limb (0.05 mg, IV); rats; FR1; long but not short access | (Orio et al., 2010) |
| Cocaine: SA maintenance on other schedules | WW-III-55 (758-fold) | 3–10 mg/kg, IP | Ø descending limb (0.375 mg, IV); rats; VI60s | Unpublished |
| 5.6 mg/kg, IP | ↓ (0.375 mg/kg, IV); rats; PR | |||
| OS-3-106 (113-fold) | 10 mg/kg, IP | ↓ descending limb (0.375 mg, IV); rats; VI60s | Unpublished | |
| CJB 090 | 3 mg/kg, IV | ↓ ascending limb/peak dose (0.1 mg/kg, IV); monkeys; 2nd order schedule | (Martelle et al., 2007) | |
| 17.8 mg/kg, IM | Ø ascending limb/peak dose (0.03–0.3 mg/kg, IV); monkeys; 2nd order schedule | (Achat-Mendes et al., 2009) | ||
| WC26 (51-fold) | 10 mg/kg, IP | ↓ descending limb (0.375 mg, IV); rats; VI60s | (Cheung et al., 2012) | |
| Terguride | 0.4 mg/kg, IP | ↓ (0.25 mg/kg, IV); rats; PR | (Pulvirenti et al., 1998) | |
| 0.1 mg/kg, IM | ↓ peak dose (0.18–0.3 mg/kg, IV); monkeys; 2nd order schedule | (Platt et al., 2003) | ||
| Amphetamine: SA maintenance on other schedules | Terguride | 0.2–0.4 mg/kg, IP | ↓ (0.12 mg/kg, IV); rats; PR | (Izzo et al., 2001) |
| Methamphetamine: SA maintenance on other schedules | CJB 090 | 5 mg/kg, IV | ↓ (0.05 mg, IV); rats; PR; long but not short access | (Orio et al., 2010) |
| 10 mg/kg, IV | ↓ (0.05 mg, IV); rats; PR; both short and long access | |||
| Cocaine: SA, Acute vs. Chronic treatment | Aripiprazole, acute | 0.56 mg/kg/h, SC via osmosis pump for 2 h | ↑ peak dose (0.18 mg/kg, IV); rats; choice vs. food | (Thomsen et al., 2008) |
| 0.056–0.1 mg/kg, IV, acute | ↑ low dose (0.03–0.01 mg/kg, IV); monkeys; choice vs. food | (Czoty and Nader, 2013) | ||
| Aripiprazole, chronic | 0.32–1 mg/kg/h, SC via osmosis pump for 5 days, with cocaine SA each day | Ø ascending/descending limb (0.18 mg/kg, IV); rats; choice vs. food | (Thomsen et al., 2008) | |
| 0.01–0.1 mg/kg/day, IV, for 5 days, with no cocaine SA days 2–4 | ↓ high dose (0.03–0.1 mg/kg, IV); dominant monkeys; choice vs. food | (Czoty and Nader, 2013) | ||
| ↓ low dose (0.003–0.01 mg/kg, IV); subordinate monkeys; choice vs. food | ||||
| 0.01-0.1 mg/kg/day, IV, for 5 days, with cocaine SA each day | Ø low/high dose (0.003–0.1 mg/kg, IV); dominant monkeys; choice vs. food | |||
| Cocaine seeking, early abstinence (<48h) | BP 897 | 0.5-1 mg/kg, IP | ↓ before 1st reinforcer; rats; 2nd order schedule | (Pilla et al., 1999) |
| CJB 090 | 3 mg/kg, IV | ↓ before 1st reinforcer; rats; 2nd order schedule | (Martelle et al., 2007) | |
| Cocaine seeking, protracted abstinence | RGH-237 | 10–30 mg/kg, PO | ↓ rats; response-contingent cues | (Gyertyan et al., 2007a) |
| BP 897 | 1-3 mg/kg, IP | ↓ rats; response-contingent cues | (Gilbert et al., 2005; Gyertyan et al., 2007b) | |
| 1 mg/kg, IP | ↓ rats; discriminative cues | (Cervo et al., 2003a) | ||
| Aripiprazole | 1–15 mg/kg, IP | ↓ rats; response-contingent cues | (Feltenstein et al., 2007) | |
| 0.25–1 mg/kg, IP, 3 daily treatments | ↓ rats; response-contingent cues or no cues; but only if acute treatment also given pre-test | (Feltenstein et al., 2009) | ||
| 1 mg/kg, IP, 7 daily treatments | Ø; rats; response-contingent cues or no cues; no acute treatment given pre-test | |||
| Cocaine-primed reinstatement of cocaine seeking* | CJB 090 | 17.8 mg/kg, IM | Ø (prime: 0.1–1 mg/kg, IV); monkeys; cues on a 2nd order schedule | (Achat-Mendes et al., 2009) |
| Aripiprazole | 0.25 – 15 mg/kg, IP | ↓ (prime: 10 mg/kg, IP); rats | (Feltenstein et al., 2007) | |
| 0.25–1 mg/kg, IP, 3 daily treatments | ↓ (prime: 10 mg/kg, IP); rats; but only if acute treatment also given pre-test | (Feltenstein et al., 2009) | ||
| 1 mg/kg, IP, 7 daily treatments | Ø (prime: 10 mg/kg, IP); rats; no acute treatment given pretest | |||
| Terguride | 0.1 mg/kg, IM | Ø (prime: maximum effective dose, IV); monkeys; cues on a 2nd order schedule | (Khroyan et al., 2000) | |
| SDZ-208-911 (0.65-fold) | 0.1 mg/kg, IM | ↓ (prime: maximum effective dose, IV); monkeys; cues on a 2nd order schedule | (Khroyan et al., 2000) |
In the case of partial agonists, the % intrinsic activity relative to agonist control (%IA) is also shown in parentheses next to the selectivity ratio.
The amount of exposure to psychostimulant SA prior to testing is a factor that may contribute to treatment effects. Cocaine SA regimens dynamically alter extracellular dopamine concentrations during and after the sessions (Kiyatkin and Stein, 1994; Parsons et al., 1995; Wise et al., 1995), which in turn affects whether D3R partial agonists produce a dopamine-like or dopamine antagonist-like effect. For instance, Orio et al. (2010) reported that the D3/D2R partial agonist CJB 090 has no effect on methamphetamine SA in rats that have intermittent access (1 h/session, 3 sessions/week), but normalizes escalated methamphetamine SA in rats that have extended access (6 h/session, 7 sessions/week). These findings suggest that neuroadaptations in response to extended methamphetamine intake alter the effects of D3R partial agonist challenge.
D3/D2R partial agonists with some intrinsic activity at D2Rs may be more effective in reducing cocaine SA than highly selective D3R partial agonists (Joyce and Millan, 2005). Our recent findings with OS-3-106, a D3/D2R partial agonist, and WW-III-55, a selective D3R partial agonist, are consistent with this idea. We found that only OS-3-106 reduced cocaine infusions on a low demand VI60 s schedule. OS-3-106 also selectively increased the latency to the first response in the session only when the reinforcer was cocaine and not when the reinforcer was sucrose (unpublished observations). These results are similar to our findings with WC26, a 51-fold selective D3R partial agonist (Cheung et al., 2012). In contrast, the highly selective D3R partial agonist WW-III-55 failed to affect cocaine or sucrose reinforcement rates or response latencies (unpublished observation). Similarly, Gyertyan et al. (2007a) reported that the most selective D3R partial agonist RGH-237 currently available (<1800 D3R:D2R selectivity) also fails to affect cocaine SA, suggesting that some co-occupancy of D2Rs may be required.
Similar to D3R antagonists, D3R partial agonists more consistently decrease psychostimulant SA under high-demand schedules of reinforcement than under low demand schedules. For example, terguride reduces breakpoints for both cocaine (Pulvirenti et al., 1998) and amphetamine (Izzo et al., 2001) under PR schedules. Moreover, CJB 090 reduces the breakpoint for methamphetamine, and this effect is more potent in rats given extended access during training relative to rats given intermittent access (Orio et al., 2010). We have also found that WW-III-55 reduces breakpoint for cocaine (unpublished observations). Furthermore, responding for cocaine under a second order schedule is reduced by terguride and by CJB 090 (Martelle et al., 2007; Platt et al., 2003; although see Achat-Mendes, 2009).
2.7 D3R-selective and D3/D2R partial agonist effects in relapse models
D3R-selective partial agonists reduce psychostimulant seeking, similar to D3R antagonists. For example, BP 897, CJB 090 and RGH-237 attenuate cocaine seeking after one-day of protracted abstinence (Gyertyan et al., 2007a; Martelle et al., 2007; Pilla et al., 1999). The D3/D2R partial agonists WC26 and OS-3-106, but not the highly selective D3R partial agonist WW-III-55, also increase the latency to respond for cocaine (Cheung et al., 2012; unpublished observation), consistent with reduced motivation to seek cocaine. In agreement with these results, cue-primed reinstatement (Cervo et al., 2003b; Feltenstein et al., 2007; Feltenstein et al., 2009; Gilbert et al., 2005) and cocaine-primed reinstatement of cocaine seeking is attenuated by SDZ-208-911 (Khroyan et al., 2000), although CJB 090 is ineffective (Achat-Mendes et al., 2009).
Treatment administered during abstinence from SA provides additional promising results regarding anti-relapse effects of D3R partial agonists. Czoty and Nader (2013) reported that five daily aripiprazole treatments during abstinence, but not during maintenance of cocaine SA, decreases preference for high doses of cocaine in dominant monkeys, while preference for low doses of cocaine was increased in subordinate monkeys. These findings suggest that D3R partial agonists may reduce motivation to self-administer cocaine in dominant monkeys, but only after a period of abstinence.
The increased efficacy of D3R partial agonists with chronic treatment during abstinence suggests that these drugs may normalize abstinence-induced changes that underlie withdrawal symptoms and the incubation effect. Withdrawal from psychostimulants causes anhedonia, which is reflected in rats as a lower breakpoint on a PR schedule of food reinforcement (Barr and Phillips, 1999). Acute treatments with terguride or aripiprazole reverse the effect of amphetamine withdrawal on reducing breakpoint for food (Orsini et al., 2001; Schwabe and Koch, 2007). In contrast, methamphetamine withdrawal induces a more severe reduction in breakpoint for food that is not reversed by acute terguride, but is reversed after 5 daily treatments with terguride (Hoefer et al., 2006). This suggests that methamphetamine induces a more severe withdrawal that results in greater dopaminergic dysregulation, which requires more exposure to D3R partial agonists to normalize. Furthermore, the effect of chronic terguride persisted for 4 days after the cessation of treatment. Therefore D3R partial agonists may be useful in countering dopaminergic dysfunction associated with psychostimulant withdrawal, similar to that observed with 7-OH-DPAT (Fuchs et al., 2002b; Neisewander et al., 2004)
2.8 Potential side effects of D3R compounds
Currently available D3R agonists have potential side effects that may limit their usefulness. For instance, D3/D2R agonists are self-administered when substituted for cocaine (Caine et al., 1999; Caine et al., 2002; Freeman et al., 2012; Koffarnus et al., 2012). This may be a D2R-mediated effect since D2R knock-out mice do not self-administer quinelorane when substituted for cocaine, unlike wild-type controls (Caine et al., 2002). Furthermore, the more selective D3R agonist PF-592,379 does not support SA when substituted for cocaine (Collins et al., 2012a). However, the D2R-selective agonist sumanirole does not support SA when substituted for cocaine either (Koffarnus et al., 2012), suggesting that activation of both D2 and D3Rs may be required. SA of D3/D2R agonists when substituted for cocaine may be driven in part by the reinforcing or incentive motivational effects of drug-conditioned stimuli. For instance, intake of D3/D2R agonists is higher during substitution tests when cocaine-conditioned stimuli (CS+) are presented response-contingently compared to when the CS+ is omitted (Collins et al., 2012b; Collins and Woods, 2009). This effect is antagonized by a D3/D2R antagonist and to a lesser degree by a D3R-selective antagonist. Importantly, D3/D2R agonists do not sustain responding for a CS+ paired with food (Collins and Woods, 2009; Dias et al., 2004), suggesting selective enhancement of the incentive salience and/or conditioned reinforcing effects of cocaine-conditioned stimuli. Another potential side effect of D3/D2R agonists is a general decrease in motivation. D3/D2R agonists reduce locomotion (Chang et al., 2011; Cheung et al., 2012; Fuchs et al., 2002b; Li et al., 2010) and responding for food (Caine and Koob, 1995; Cheung et al., 2012; Czoty and Nader, 2013; Platt et al., 2003) at doses that reduce responding for cocaine.
D3R partial agonists have a more suitable side-effect profile than high efficacy agonists. Despite some intrinsic activity at D3Rs, D3R partial agonists do not support SA when substituted for cocaine (Pilla et al., 1999; Pulvirenti et al., 1998; Sørensen et al., 2008) nor do they prime the reinstatement of cocaine-seeking behavior (Khroyan et al., 2000). However, these drugs have mixed effects on locomotion, motivation, and alternative reinforcers. For instance, aripiprazole (Feltenstein et al., 2007), WC26 (Cheung et al., 2012), OS-3-106 and WW-III-55 (unpublished observations) reduce locomotion, whereas terguride, CJB 090 and RGH-237 have no effect at doses that reduce responding for psychostimulants (Achat-Mendes et al., 2009; Gyertyan et al., 2007a; Platt et al., 2003). Terguride, CJB 090, WC26, OS-3-106 and WW-III-55 reduce responding for food and sucrose (Achat-Mendes et al., 2009; Cheung et al., 2012; unpublished observations; Martelle et al., 2007; Platt et al., 2003; Pulvirenti et al., 1998), although terguride produces the opposite effect (i.e., increase responding for food) during amphetamine withdrawal (Orsini et al., 2001). Other D3R partial agonists, including BP 897, RGH-237, or low doses of aripiprazole fail to alter responding for food or under extinction of food or water reinforcement (den Boon et al., 2012; Feltenstein et al., 2007; Gal and Gyertyan, 2006; Gyertyan et al., 2007a). The reduction in responding for natural rewards by some D3R partial agonists may reflect reduced dopaminergic signaling caused by activation of D3R autoreceptors.
D3R-selective antagonists have the fewest side effects of all of the D3R drug classes. They do not substitute for cocaine in SA tests (Xi et al., 2005; Xi et al., 2006). They have little effects on locomotion or operant behavior associated with other reinforcers and the effects that they do have on these measures are at high doses that are dissociated from doses that selectively decrease motivation for cocaine (Di Ciano et al., 2003b; Gal and Gyertyan, 2006; Gilbert et al., 2005; Song et al., 2012a; Thanos et al., 2008; Vorel et al., 2002; Xi et al., 2005; Xi et al., 2006). In contrast to these highly selective D3R antagonists, the less selective functional antagonists WC10 and WC44 reduce both responding for sucrose and locomotor activity at doses that reduce cocaine intake, although WC44 does selectively increase response latency for cocaine but not sucrose (Cheung et al., 2012). Similarly, the D3/D2R antagonist S33138 impairs rotorod performance at a dose that reduces cocaine intake (Peng et al., 2009). The relatively low distribution of D3Rs in the dStr may account for the improved side effect profile of D3R-selective antagonists compared to nonselective antagonists (Millan et al., 2004).
Because any therapeutic medication is likely to be given repeatedly, it is important to also consider potential side effects that can arise from chronic treatment. There is evidence that chronic treatment with the D3/D2R agonist pramipexole, which is used to treat early Parkinson’s disease, can lead to pathological behaviors such as gambling and hypersexuality (Ahlskog, 2011; Dagher and Robbins, 2009; Fenu et al., 2009). These symptoms may reflect pathological obsession and/or compulsion resulting from a dysregulated reward system (Ahlskog, 2011). In contrast, motoric side effects are more prominently associated with non-selective D3/2R antagonists such as haloperidol. While acute treatments can cause catalepsy (Wadenberg et al., 2000), chronic treatments can lead to extrapyramidal symptoms such as tardive dyskinesia (Dayalu and Chou, 2008; Kinon and Lieberman, 1996). D2R blockade likely plays a role in these motoric side effects (Ossowska, 2002). Clinical evidence suggests that chronic treatments with the non-selective D2/3R partial agonist aripiprazole can also cause extrapyramidal symptoms, although not as frequently as non-selective D3/2R antagonists (De Fazio et al., 2010; Miller et al., 2007; Peña et al., 2010). Finally, it is unclear what side effects are associated with chronic treatment with selective D3R antagonists. However, because the acute side effects of selective D3R antagonists appear relatively mild, the chronic side effects of these selective D3R antagonists are likely more mild than other types of dopaminergic compounds.
2.9 Conclusions and future considerations for D3R-targeted medications development
Currently, findings with selective D3R antagonists suggest that these drugs effectively reduce motivation for psychostimulants while producing minimal side effects. Impact on motivation is evident from reduced seeking behavior and drug intake on high demand PR schedules of reinforcement. In humans, motivation for psychostimulants is often measured as self-reports of craving. The stimuli that elicit craving in humans (i.e., drug cues, stress, or drug priming) (Childress et al., 1988a; Jaffe et al., 1989; Sinha et al., 1999) also reinstate psychostimulant seeking (Shaham et al., 2003; Stewart, 1983). These similarities suggest that the two measures tap into similar processes and that the D3R antagonist reduction of seeking predicts that the D3R antagonists will have anti-craving effects in humans. Cocaine craving is thought to be a major factor contributing to relapse in cocaine-dependent individuals, although these phenomena are not completely related as craving can occur without relapse and relapse can occur without craving (Childress et al., 1988b; Tiffany and Carter, 1998; Washton, 1988; Weiss and Griffin, 1995). Although research is needed to better understand the relationship between craving and relapse, it seems likely that some individuals would benefit from anti-craving medication used as an adjunct to other therapies aimed at preventing relapse. A potential limitation of D3R antagonists is that they are likely ineffective in reducing psychostimulant intake if a lapse occurs since they fail to alter psychostimulant SA under low demand schedules of reinforcement.
Although less selective D3/D2R antagonists and partial agonists will likely produce some unwanted side effects, these drugs may be more effective against relapse given that they reduce drug intake under high and low demand schedules of reinforcement and in some cases, regardless of dose of psychostimulant available. These findings suggest that D3/D2R antagonists and partial agonists may modulate the reinforcing effects of cocaine. The reinforcing effects of psychostimulants are unlikely to be mediated solely by D3 or D2Rs because cocaine SA is not abolished in D3R- (Caine et al., 2012; Song et al., 2012b) or D2R-knockout mice (Caine et al., 2002). The development of highly D2R-selective compounds may help identify the precise role that D2 and D3Rs play in cocaine reward (Luedtke et al., 2012). It is possible that reinforcing effects of psychostimulants are most effectively reduced by the co-occupancy of D2 and D3Rs. In vivo PET imaging using D2R- and D3R-selective imaging agents may aid the development of compounds with “threshold” D3R:D2R occupancy levels that can inhibit cocaine SA while minimizing the potential side effects (Mach et al., 2011; Tu et al., 2011).
Further research characterizing the link between regulatory changes in D3Rs during abstinence and efficacy of D3R-selective and D3/D2R drugs is needed. Given the dynamic changes in D3Rs during abstinence, a multi-stage therapeutic strategy may be needed that would utilize different compounds depending on whether one is in early or protracted abstinence. For instance, early during abstinence when relapse rate is high (Willett and Singer, 1993), moderately selective D3R:D2R partial agonists might be useful in suppressing craving and attenuating the reinforcing effects of psychostimulants should relapse occur. Furthermore, D2/D3R partial agonists may normalize elevated D3R levels in the ventral striatum (Fuchs et al., 2002b; Neisewander et al., 2004) as well as reduce psychostimulant withdrawal symptoms (Hoefer et al., 2006; Orsini et al., 2001; Schwabe and Koch, 2007). At a later stage of recovery, a transition to a highly selective D3R partial agonist or antagonist may be effective in attenuating seeking behavior while minimizing both extrapyramidal side effects associated with D2R antagonism (Millan et al., 2004) and “impulse control disorder” such as pathological gambling associated with D2/D3R activation (Ahlskog, 2011; Dagher and Robbins, 2009; Fenu et al., 2009).
Another issue that requires attention in preclinical research is social influences on drug effects (Bardo et al., 2013; Neisewander et al., 2012). As mentioned above, D3R compounds such as the agonist (−)–NPA and the antagonists NGB-2904 and PG-01037 reduce cocaine-related behavior in rats but not in monkeys (Achat-Mendes et al., 2010; Blaylock et al. 2011; Khroyan et al., 2000; Martelle et al. 2007), and although the reason for the discrepancy is unclear, social context may play a role. For instance, the efficacy of D3R compounds in reducing cocaine intake depends on social hierarchy, cocaine dose and abstinence from cocaine use (Czoty and Nader, 2013; Thomsen et al., 2008). Social hierarchy in monkeys changes D2/D3R availability in the striatum, which may play a role in these effects (Morgan et al., 2002b; Nader et al., 2012). Also, as reviewed above, 5 daily treatments of aripiprazole during abstinence, but not during daily cocaine SA, decrease preference for high doses of cocaine in dominant monkeys. In contrast, this treatment increases preference for low doses of cocaine in subordinate monkeys. Because research in rats has been conducted using isolate housing whereas monkeys have some degree of social housing, this difference may contribute to discrepancies across studies using these different species. The dependency on various factors likely contributes to the mixed effects of aripiprazole in other preclinical (Bergman, 2008) and clinical studies (Haney et al., 2011; Lile et al., 2008; Meini et al., 2011; Stoops et al., 2007; Tiihonen et al., 2007; Vorspan et al., 2008). These discrepancies suggest that D3R-targeted medications alone may not be sufficient to blunt cocaine’s reinforcing effect in all individuals, especially those who resume frequent use. It may be necessary to target other monoamine systems that are dysregulated in addition to dopamine, such as 5-HT systems in which 5-HT1BRs appear to be dysregulated similar to D3Rs.
3. Dysregulation of 5-HT1BRs
Cocaine inhibition of 5-HT transporters increases synaptic 5-HT above normal, resulting in dysregulation, including changes in 5-HT1BRs. Genetic studies implicate a link between 5-HT1BRs and substance abuse (Cao et al., 2013; Drago et al., 2010; Hasegawa et al., 2002; Huang et al., 2003; Proudnikov et al., 2006; Sun et al., 2002); but see (Cigler et al., 2001; Proudnikov et al., 2006), suggesting that the dysregulation of 5-HT1BRs may be a critical part of substance abuse pathology. 5-HT1BRs are terminal autoreceptors on 5-HT neurons (Boschert et al., 1994; Doucet et al., 1995; Voigt et al., 1991), as well as heteroreceptors on medium spiny GABAergic neurons originating in striatum and projecting to the ventral pallidum, ventral tegmental area (VTA), and substantia nigra (Bruinvels et al., 1994; Ghavami et al., 1999; Riad et al., 2000; Sari et al., 1997; Sari et al., 1999). These receptors likely regulate both dopamine and 5-HT since both transmitters are reduced in the striatum of 5-HT1BR knockout mice (Ase et al., 2000). Repeated cocaine administration followed by brief abstinence (1–5 days) increases 5-HT1BR mRNA and 5-HT1BR binding within regions of the mesolimbic and nigrostriatal pathways (Hoplight et al., 2007; Przegalinski et al., 2003). In contrast, following 14 days of abstinence from cocaine SA, 5-HT1BR mRNA is reduced throughout the striatum compared to controls without a history of abstinence (Neumaier et al., 2009). Functional sensitivity to the 5-HT1B/1AR agonist RU24969 also varies depending on abstinence, with cocaine-experienced rats exhibiting hypolocomotion when tested during maintenance of SA and hyperlocomotion when tested after prolonged abstinence. Both effects are more robust in rats given a 12-h binge for the last SA session than those given 3-h access (O’Dell et al., 2006). These effects are likely mediated via heteroreceptors on VTA GABA neurons that tonically inhibit mesolimbic dopamine neurons because the agonist also decreases VTA GABA and enhances NAc dopamine responses to cocaine (O’Dell et al., 2006; O’Dell and Parsons, 2004). Collectively, the findings suggest that cocaine versus protracted abstinence may alter 5-HT1BR systems in opposite directions.
3.1 5-HT1BR agonist effects on maintenance of psychostimulant SA
In one of the first studies to implicate 5-HT1BRs in psychostimulant reinforcement, Parsons et al. (1996) showed that the non-selective 5-HT1A/1BR partial agonist CGS-12066B shifts the dose-effect function for SA of the dopamine transport inhibitor GBR-12909 to the left under low demand schedules of reinforcement, consistent with enhancing the reinforcing value of GBR-12909. As summarized in Table 5, Parsons et al. (1998) subsequently showed that the more selective 5-HT1BR agonists RU24969, CP94253 and CP93129 produce a similar effect on cocaine SA, and that the effect of CP94253 is reversed by the 5-HT1B/1DR antagonist GR127935. These findings have been replicated by two other laboratories (Pentkowski et al., 2009; Przegalinski et al., 2007). Furthermore, we found that viral enhancement of 5-HT1BR expression in terminals of medial NAcsh neurons shifts the dose– effect function for cocaine SA upward and to the left under a low demand reinforcement schedule (Pentkowski et al., 2012a). Recently, Miszkiel et al. (2012) found that the CP94253 also shifts the descending limb of the amphetamine SA dose-effect function to the left, and this effect is blocked by a 5-HT1BR antagonist. Interestingly, administration of 5-HT1BR antagonists alone has minimal if any effect on cocaine SA, suggesting that tonic stimulation of these receptors by endogenous 5-HT does not modulate the reinforcing effects of cocaine. Similar to the effects observed under low demand schedules of reinforcement, 5-HT1BR agonists dose-dependently increase cocaine intake and breakpoints on PR schedules of reinforcement and these effects are reversed by a 5-HT1B/1DR antagonist (Parsons et al., 1998). Elevated 5-HT1BR expression in terminals of the medial NAcsh also increases cocaine intake and breakpoints on a PR schedule (Pentkowski et al., 2012a). Collectively, the findings provide strong support for 5-HT1BR-mediated enhancement of the reinforcing effects of psychostimulants during maintenance (Parsons et al., 1998; Pentkowski et al., 2009; Przegalinski et al., 2007).
Table 5.
Effects of increased 5-HT1BR stimulation on psychostimulant abuse-related behaviors.
| Model | Treatment (selectivity) | Dose of treatment | Effect (Psychostimulant dose); Species; Schedule; Further comments | Reference |
|---|---|---|---|---|
| Cocaine: SA maintenance | RU24968:5-HT1B/1AR agonist (7-fold; Macor et al., 1990) | 1 mg/kg, SC 1–3 mg/kg, SC 0.3-3 mg/kg, SC |
↑ ascending limb (0.03 mg, IV); rats; FR5 ↓ descending limb (0.125 mg, IV); rats; FR5 ↑ (0.125 mg, IV); rats; PR |
(Parsons et al., 1998) |
| CP94253: 5-HT1B/1D/1AR agonist (24-fold; Koe et al., 1992) | 1 mg/kg, SC 1–3 mg/kg, SC 1–3 mg/kg, SC |
↑ ascending limb (0.03 mg, IV); rats; FR5 ↓ descending limb (0.125 mg, IV); rats; FR5 ↑ (0.125 mg, IV); rats; PR+ |
(Parsons et al., 1998) | |
| 5.6 mg/kg, SC | ↓ descending limb (0.1875–0.75 mg/kg, IV); rats; FR5 | (Pentkowski et al., 2009) | ||
| 5.6 mg/kg, SC | ↓ descending limb (0.75); rats; FR5+ | (Pentkowski et al., 2012b) | ||
| 5–7.5 mg/kg, IP | ↓ descending limb (0.125–0.5 mg, IV); rats; FR5+ | (Przegalinski et al., 2007) | ||
| CP93129: 5-HT1BR agonist: (146-fold; Macor et al., 1990) | 10 μg, ICV 3–10 μg, ICV |
↑ ascending limb (0.03 mg, IV); rats; FR5 ↓ descending limb (0.125 mg, IV); rats; FR5 |
(Parsons et al., 1998) | |
| Viral mediated 5-HT1BR gene transfer (elevated 5-HT1BR expression) | ~200,000 infective units infused into the NAc shell | ↑ ascending limb (0.032–0.1 mg/kg, IV), Ø descending limb (0.32–1 mg/kg, IV); rats; FR5 ↑ (0.75 mg/kg, IV); rats; PR |
(Pentkowski et al., 2012a) | |
| Amphetamine: SA maintenance | CP94253 | 2.5–5 mg/kg, IP | ↓ descending limb (0.06–0.12 mg/kg, IV); rats; FR5+ | (Miszkiel et al., 2012) |
| Cocaine: SA protracted abstinence | CP94253 | 5.6 mg/kg, SC | ↓ ascending/descending limbs (0.075–0.75 mg/kg, IV); rats; FR5+ ↓ (0.375 mg/kg, IV); rats; PR+ |
(Pentkowski et al., 2012b) |
| Viral mediated 5-HT1BR gene transfer | ~200,000 infective units infused into the NAc shell | ↓ (0.75 mg/kg); rats; PR | (Pentkowski et al., 2012a) | |
| Cocaine seeking, early abstinence (<48hr) | CP94253 | 5.6 mg/kg, SC | Ø (1 day), ↓ (5 days); rats; response-contingent cues; FR1 | (Pentkowski et al., 2012b) |
| Cocaine seeking, protracted abstinence | CP94253 | 3–10 mg/kg, SC | Ø rats; no cues | (Pentkowski et al., 2009) |
| RU24968 | 1–3 mg/kg, SC | ↓ rats; response-contingent cues; FR1+ | (Acosta et al., 2005) | |
| CP94253 | 5.6–10 mg/kg, SC | ↓ rats; response-contingent cues; FR1 | (Pentkowski et al., 2009) | |
| 5 mg/kg, IP | ↓ rats; response-contingent cues; FR1 | (Przegalinski et al., 2008) | ||
| Viral mediated 5-HT1BR gene transfer | ~200,000 infective units infused into the NAc shell | ↓ rats; response-contingent cues; FR1; with history of extinction or abstinence only | (Pentkowski et al., 2012a) | |
| Cocaine-primed reinstatement of cocaine seeking* | CP94253 | 5.6–10 mg/kg, SC 10 mg/kg, SC |
↓ (prime: 10 mg/kg, IP); rats ↓ (prime: 2.5 mg/kg, IP); rats |
(Pentkowski et al., 2009) |
| 5 mg/kg, IP 2.5 mg/kg, IP |
↓ (prime: 10 mg/kg, IP); rats ↑ (prime: 2.5 mg/kg, IP); rats |
(Przegalinski et al., 2008) | ||
| RU24968 | 1–3 mg/kg, SC | ↓ (prime: 10 mg/kg, IP); rats+ | (Acosta et al., 2005) | |
| Viral mediated 5-HT1BR gene transfer | ~200,000 infective units infused into the NAc shell | ↓ (prime: 10 mg/kg, IP); rats | (Pentkowski et al., 2012a) |
Key:↑: increased compared with control. ↓: decreased compared with control. Ø: no effect compared with control. SA: self-administration. FR: fixed ratio schedule of reinforcement. PR: progressive ratio schedule of reinforcement. NAc: Nucleus accumbens.
tests carried out after protracted abstinence (<48h).
agonist effect blocked by a 5-HT1BR antagonist
3.2 5-HT1BR agonist effects in relapse models
Targeting 5-HT1BRs for medications development for psychostimulant addiction initially seemed counter intuitive given the above findings that 5-HT1BR agonists enhance the reinforcing value of cocaine and amphetamine during maintenance of SA while antagonists have no effect. However, in apparent contrast to these effects, we found that RU24969 (Acosta et al., 2005) and CP94253 (Pentkowski et al., 2009) dose-dependently decrease cue- and cocaine-primed reinstatement of extinguished cocaine-seeking behavior. The RU24969-induced decreases in reinstatement of cocaine seeking are reversed by the antagonist GR127935, suggesting that they are 5-HT1BR-mediated. Since 5-HT1BR agonists enhance cocaine reinforcement, we initially thought that the effects of the agonists on cocaine-primed reinstatement (10 mg/kg, IP) resulted from a cocaine-like satiation effect (Acosta et al., 2005). Subsequently, however, we found that CP94253 (10 mg/kg, SC) reduces reinstatement by a lower cocaine priming dose (2.5 mg/kg, IP), which we had predicted would enhance seeking similar to a higher dose of cocaine (Pentkowski et al., 2009). These findings suggest that 5-HT1BR agonists attenuate motivation for cocaine. Consistent with this interpretation, CP94253 pretreatment or substitution for cocaine during extinction of cocaine SA during maintenance reduces responding relative to saline pretreatment or saline substitution, respectively (Parsons et al., 1998; Pentkowski et al., 2009). We suggest the CP94253-induced decrease in responding under this extinction condition also reflects a decrease in motivation, similar to the decrease in seeking during reinstatement tests. In contrast to our findings, Przegalinski et al. (2008) found that a low dose of CP94253 (2.5 mg/kg, IP) enhances reinstatement of cocaine seeking by a low cocaine priming dose (2.5 mg/kg, IP). This discrepancy may be due to differences in test doses of CP94253, training doses of cocaine, and/or rat strains used in these studies. Importantly, both laboratories found that higher doses of CP94253 suppress cocaine-primed reinstatement of cocaine seeking. We also showed that CP94253 fails to reinstate cocaine-seeking behavior when administered as a priming injection, suggesting that 5-HT1BR stimulation alone does not elicit incentive motivation for cocaine (Pentkowski et al., 2012b). Furthermore, we found that viral mediated 5-HT1BR-gene transfer (i.e., increased 5-HT1BR expression) attenuates cue and cocaine-primed reinstatement of cocaine seeking (Pentkowski et al., 2012a) similar to the effects of the 5-HT1BR agonists. More recently we found that CP94253 attenuates cue and cocaine-primed reinstatement of cocaine seeking in rats tested after 5 days, but not after only 1 day, of forced abstinence (Pentkowski et al., 2012b). Collectively, the findings suggest that 5-HT1BR stimulation decreases motivation for cocaine and that this effect becomes stronger during the course of abstinence.
We hypothesized that the seemingly paradoxical effect of 5-HT1BR agonists in enhancing cocaine reinforcement while decreasing incentive motivation for cocaine may be due to an abstinence-induced switch in the functional consequences of 5-HT1BR stimulation. To test this hypothesis we examined the effects of viral increases in 5-HT1BR expression on resumption of cocaine SA after 21 days of forced abstinence. In striking contrast to the increase in cocaine reinforcement observed during maintenance, elevated 5-HT1BR expression not only decreased cue- and cocaine-primed reinstatement, but also cocaine infusions and breakpoints on an exponential PR schedule of reinforcement (see Figure 1 adapted in part from Pentkowski et al., 2012a). We subsequently found that CP94253 pretreatment given prior to a test for resumption of cocaine SA after 21 days of abstinence attenuates cocaine intake and breakpoints on a PR schedule of reinforcement (Figure 1). These attenuating effects of CP94253 on PR responding are independent of extinction as cocaine intake was reduced in rats with or without prior extinction training. The attenuating effects of CP94253 are independent of the SA reinforcement schedule since reductions in cocaine intake are observed after 21 days of abstinence on a PR schedule, as well as on a FR5 schedule of reinforcement with either a low (0.075 mg/kg, IV) or high (0.75 mg/kg, IV) cocaine dose available (unpublished observation). The decrease in cocaine SA at both the low cocaine dose, which is on the ascending limb of the dose-effect function, and the high dose of cocaine, which is on the descending limb of the dose-effect function, suggests that CP94253 flattens the cocaine dose-effect function when given during protracted abstinence. Collectively, these findings suggest that 5-HT1BR agonists may counter the dysregulation of homeostatic control over motivation/reinforcement that occurs with chronic psychostimulant exposure and abstinence. These exciting findings suggest that 5-HT1BR agonists may offer a new treatment strategy for psychostimulant addiction that would not only decrease the incentive motivational effects of psychostimulant-conditioned cues, but also reduce cocaine intake if a lapse occurs.
Figure 1.
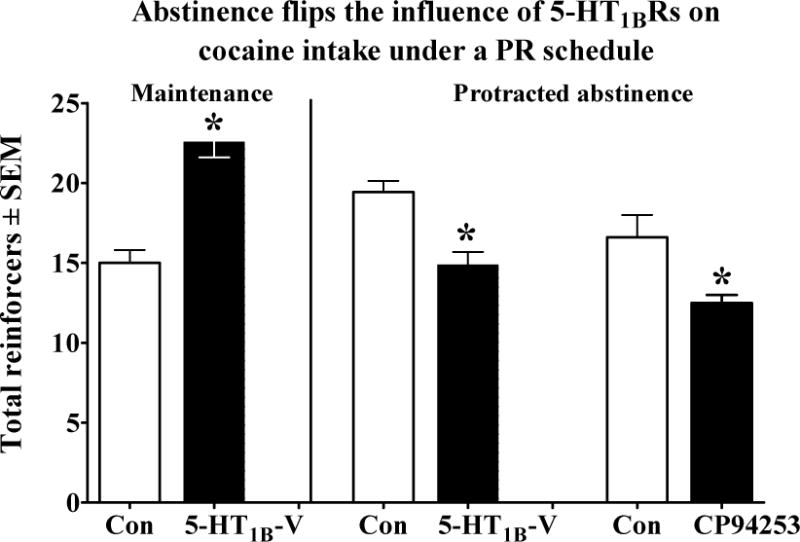
Abstinence-dependent changes in 5-HT1BR manipulations on cocaine SA under a PR schedule of reinforcement in male rats. Viral vectors containing GFP (Con) or 5-HT1BR+ GFP (5-HT1B-V) transcripts were infused into the NAc either during maintenance (left) or after 21 days of protracted abstinence (right). Rats were tested for the effects of the virus 4 days later on an exponential PR schedule of cocaine reinforcement (0.75 mg/kg, IV). The 5-HT1BR agonist CP94253 (5.6 mg/kg) or saline (Con) was administered on abstinence day 21 and the test for responding on the exponential PR schedule of reinforcement began 15 min later. *different from Con, p<0.05. The effects of viral over-expression of 5-HT1BRs has been published previously (Pentkowski et al., 2012).
3.3 Potential side effects of 5-HT1BR agonists
Currently available 5-HT1BR agonists have several potential side effects, including anxiety, stimulant effects, and general decreases in motivation. At doses that affect cocaine SA, CP94253 increases anxiety-like behavior in the elevated plus maze (EPM) in both drug naïve rats (Lin and Parsons, 2002) and in rats that have undergone prolonged abstinence from a cocaine SA regimen (Pentkowski et al., 2009). In contrast, others report anxiolytic-like effects in the EPM and Vogel conflict-drinking test following administration of similar doses of CP94253 (Tatarczynska et al., 2004). Furthermore, CP94253 dose-dependently reduces the expression of conditioned freezing (McDevitt et al., 2011), suggesting that 5-HT1BR agonists attenuate conditioned anxiety. Clinical research investigating the effects of the triptan class of 5-HT1R agonists on anxiety has yielded mixed result with some studies reporting no effects of acute administration of sumatriptan, a 5-HT1A/1B/1DR agonist (Pian et al., 1998), or zolmitriptan, a 5-HT1B/1D/1FR agonist (Boshuisen and den Boer, 2000) in patients with obsessive-compulsive disorder (OCD). However, others have reported symptom exacerbation in OCD patients using the same acute doses (Stern et al., 1998) and following chronic administration (Koran et al., 2001), suggesting that 5-HT1BR agonists may be counter indicated for patients with OCD.
Several reports suggest that 5-HT1BR agonists increase spontaneous locomotion in rats (Acosta et al., 2005; Bendotti and Samanin, 1987; Chaouloff et al., 1999; Green et al., 1984; Koe et al., 1992; O’Neill and Parameswaran, 1997; Oberlander et al., 1987) and mice (Chaouloff et al., 1999; Cheetham and Heal, 1993; Goodwin and Green, 1985). In contrast, others have found that acute administration of CP94253 either in rats with a history of cocaine SA (Pentkowski et al., 2009) or in drug naïve rats (Halford and Blundell, 1996; Przegalinski et al., 2007) fails to alter locomotion at doses that alter psychostimulant SA. Furthermore, 5-HT1BR gene transfer into the NAc shell also fails to alter spontaneous locomotion (Neumaier et al., 2002; Pentkowski et al., 2012a). There is some evidence that RU24969-induced hyperlocomotion involves 5-HT1ARs (Kalkman, 1995);1997 #15102}, suggesting that the use of selective 5-HT1BR agonists may circumvent motor side effects.
5-HT1BR agonists may produce general satiation. Indeed, acute administration of 5-HT1BR agonists decrease sucrose (10% solution) intake (Lee and Simansky, 1997) and reinstatement of sucrose seeking in free-feeding rats (Acosta et al., 2005), as well as food intake in food-restricted and non-restricted rodents (Bendotti and Samanin, 1987; Halford and Blundell, 1996; Koe et al., 1992; Lee et al., 2002; Nonogaki et al., 2007). In contrast, CP94253 failed to alter reinforcement rates for sucrose or sweetened milk at doses that significantly altered cocaine-seeking behavior (Pentkowski et al., 2009; Przegalinski et al., 2008). Furthermore, Koe et al. (1992) found that CP94253 effects on food intake dissipated with repeated administration. Collectively, these data suggest that the potential for a general disruption in motivation by 5-HT1BR agonists would be transient.
Given that 5-HT1BR agonists enhance the reinforcing effects of cocaine, it is conceivable that these agonists may have some abuse potential. However, SA was not maintained when either RU24969 or CP94253 were substituted for cocaine (Parsons et al., 1998), nor does CP94253 produce conditioned place preference when given alone (Cervo et al., 2002). These findings suggest that 5-HT1BR agonists themselves likely have limited or no abuse potential. However, the circumstances under which these agonists can enhance cocaine’s reinforcing effects need to be examined more thoroughly as discussed below.
There are case reports of the triptan class of 5-HT1BR agonists causing the 5-HT syndrome, a potentially serious side effect. In 2006 the Food and Drug Administration (FDA) issued a warning regarding the combined use of triptans with selective serotonin reuptake inhibitors (SSRIs) or selective norepinephrine reuptake inhibitors (SNRIs; see www.fda.gov/cder/drug/InfoSheets/HCP/triptansHCP.htm). The FDA warning applies specifically to triptans, which are not selective for 5-HT1BRs. 5-HT syndrome is characterized by hallucinations, loss of coordination, restlessness, overactive reflexes, tachycardia, blood pressure fluctuations, elevated body temperature, nausea, vomiting, and diarrhea, with symptom severity ranging from mild to fatal (Evans and Sebastian, 2007). Since this warning, several reports have questioned the validity of the FDA’s warning based on: 1) triptans mechanism of action (Gillman, 2010) (i.e., triptans target 5-HT1Rs while 5-HT2ARs are implicated in 5-HT syndrome), 2) the inclusion of only 29 subjects in the FDA report (Evans, 2007), 3) and the lack of appropriate diagnostic criteria for subjects included in the report (Evans, 2007). Despite the FDA warning, the combined use of triptans with SSRIs or SNRIs is currently widespread (Sclar et al., 2012) without any indication of serious side effects (Rolan, 2012). Collectively, there are no contraindications for the monotherapeutic use of triptans or selective 5-HT1BR agonists, however, patients also taking SSRIs or SNRIs should be carefully monitored until thorough toxicity studies have been conducted.
3.4 Conclusions and future considerations for 5-HT1BR-targeted medications development
Preclinical research with 5-HT1BR agonists suggests that these drugs may be effective anti-relapse medications in cocaine-experienced individuals who have undergone a period of abstinence (i.e., detoxification). Based on the effects of the agonists on cocaine seeking, we suggest that they may decrease craving elicited by drug-associated cues or a lapse of drug use (Acosta et al., 2005; Pentkowski et al., 2009; Przegalinski et al., 2008). More importantly, based on the effects of the agonists on SA after a period of abstinence, we suggest that they may decrease cocaine intake if a lapse occurs (Miszkiel et al., 2012; Parsons et al., 1998; Pentkowski et al., 2009; Pentkowski et al., 2012b; Przegalinski et al., 2007). There are important questions that need to be address to test the above hypotheses. An important question is whether or not the abstinence switch to an inhibition of cocaine intake is limited to high demand schedules of reinforcement similar to effects of selective D3R antagonists or whether a decrease in intake is also observed under low demand schedules of reinforcement. More importantly, the question of how stable the inhibition of cocaine intake is once it occurs during abstinence is critical to address. For example, it is important to rule out the possibility that the effects of the5-HT1BR agonists may switch back to a facilitatory effect on cocaine intake after a longer abstinence period, repeated agonist treatment, or repeated relapse episodes.
Sole use of cocaine or amphetamine is infrequent compared to poly-drug use among psychostimulant abusers. Poly-drug use presents a major challenge in developing treatments for drug dependence (Preti, 2007; Rowan-Szal et al., 2000). In particular, cocaine- and amphetamine-dependent patients often exhibit co-morbidity for alcohol and opiate abuse (Leri et al., 2003; Miller et al., 1993; Prinzleve et al., 2004), as well as mood and anxiety disorders (NIDA Research Report Series: Comorbidity). Interestingly, polymorphisms of 5-HT1BRs have been linked to substance abuse not only for cocaine, but also opiates and alcohol (Cao et al., 2011; Huang et al., 2003; Lee et al., 2009; Proudnikov et al., 2006; Sun et al., 2002). Neuroimaging studies in humans have also revealed that alcohol dependence is associated with increased levels of ventral striatal 5-HT1BRs (Hu et al., 2010), effects similar to those detected following cocaine administration in rats (Hoplight et al., 2007; Neumaier et al., 2009). Furthermore, administration of systemic 5-HT1BR agonists have been shown to decrease ethanol (Tomkins and O’Neill, 2000; Wilson et al., 2000), amphetamine (Miszkiel et al., 2012) and cocaine (Collins and Woods, 2009; Parsons et al., 1998; Pentkowski et al., 2009; Pentkowski et al., 2012b; Przegalinski et al., 2007) intake, particularly on the descending limb of the SA dose-effect function, as well as cue- and cocaine-primed reinstatement of extinguished drug-seeking behavior (Acosta et al., 2005; Pentkowski et al., 2009; Przegalinski et al., 2008). Collectively these data indicate that 5-HT1BRs are involved in modulating drug intake across a range of abused substances, including alcohol (see Sari, 2013 for a detailed review), amphetamine and cocaine; however, further research investigating the effects of 5-HT1BRs on poly-drug intake and the influence of these receptors on the incentive motivational effects of stimuli that elicit craving for alcohol and opiates is needed.
4. Summary and conclusions
This review of preclinical research strongly suggests that D3Rs and 5-HT1BRs are dysregulated by cocaine SA and cocaine abstinence, implicating both receptors in the pathology underlying psychostimulant abuse. Taking these dynamics into consideration, we draw the important conclusion that potential treatments need to be evaluated at various time points, including maintenance and early and late abstinence. Many treatment-seeking individuals go through detoxification, voluntary periods of abstinence or forced abstinence (i.e., incarceration or treatment programs) prior to starting treatment. Although abstinence is employed in most preclinical studies examining treatment effects on psychostimulant seeking (i.e., extinction/reinstatement), it is rarely employed in studies examining self-administration because these studies focus mostly on the maintenance of self-administration. Future preclinical studies should examine resumption of SA after a period of abstinence given the clinical relevance of this time point and the preclinical evidence, as reviewed in this paper, that abstinence with (Fuchs et al., 2002; Czoty and Nader, 2012) or without concomitant chronic treatments (Pentkowski et al., 2012b) strongly influences treatment outcomes. Furthermore, we suggest that this latter approach will be particularly useful for examining efficacy of treatment for preventing relapse and decreasing drug intake should relapse occur.
Elucidating the neural mechanisms of dysregulation within the D3R and 5-HT1BR systems is a crucial step in bridging basic understanding of the pathologies of drug addiction and the development of effective medications. Specifically, treatments aimed at reversing the dysregulation may be provide useful tools in reducing relapse. In support, we have found a relationship between effects of 7-OH-DPAT in attenuating the resumption of cocaine SA and its effects in reversing abstinence-induced up-regulation of D3Rs (Fuchs et al., 2002; Neisewander et al., 2004). The findings that both 5-HT1BR mRNA expression and the effectiveness of 5-HT1BR agonists are increased after a period of abstinence (Neumaier et al., 2009; Pentkowski et al., 2012b) also suggests that targeting dysregulated systems may be beneficial. It is possible that treatment strategy should vary depending on the stage of the drug taking-abstinence-relapse cycle during which treatment is sought; understanding mechanisms of dysregulation may help to inform such strategies.
Another important issue is the potential side effect of treatments. Although D3R full agonists can reverse D3R dysregulation, they also have abuse potential (Collins et al., 2012b) and may impair “impulse control” (Ahlskog, 2011; Dagher and Robbins, 2009; Fenu et al., 2009). We reviewed findings that suggest that D3R partial agonists are promising candidates for capturing D3R agonists’ therapeutic effects with a more suitable side-effect profile. Preclinical evidence for the ability of D3R partial agonists to reverse dysregulation include their ability to attenuate anhedonia induced by psychostimulant abstinence (Hoefer et al., 2006; Schwabe and Koch, 2007) and to decrease cocaine choice after chronic treatment during abstinence in monkeys (Czoty and Nader, 2013). However, whether D3R partial agonists can mimic D3R full agonists in reversing abstinence-induced up-regulation of D3R expression remains to be established. Similarly, whether abstinence-induced changes in 5-HT1BR expression can be reversed by 5-HT1BR agonists has not yet been examined.
This review also raises other important questions. For example, do treatment effects vary depending on the amount and severity of drug exposure (Hoefer et al., 2006; O’Dell et al., 2006; Orio et al., 2010; Orsini et al., 2001)? Do treatment effects depend on the number of episodes of abstinence/resumption, and will the beneficial effects of a treatment persist with repeated administration? For instance, the beneficial effects of aripiprazole seem to dissipate with repeated treatment (Thomsen et al., 2008), although it may be due to an interaction with cocaine SA (Czoty and Nader, 2013), whereas terguride becomes effective with repeated treatment (Hoefer et al., 2006). The acute effectiveness of D3R and 5-HT1BR compounds may depend crucially on how dysregulated their respective systems are when treatment begin, while their chronic effectiveness may depend on neuroadaptations in response to treatments. Further research is required to address these questions.
As mentioned in this review, highly selective D3R antagonists in general appear to have the fewest side effects amongst the D3R compounds. However, they also seem to be less effective in reducing psychostimulant-related behavior, suggesting that some co-occupancy of D2Rs may have therapeutic benefits at the cost of incurring some side effects. Basic research in developing highly selective D2R and D3R compounds will allow researchers to better isolate how each receptor subtype contribute separately to therapeutic and side effects, and how treatment effectiveness is increased by their interaction (Wang et al., 2010). This may inform improvement of extant D3/D2R compounds by identifying the binding ratios and relative intrinsic activities at D3 versus D2Rs needed to optimize therapeutic benefit and reduce side effects.
In the absence of a single “silver bullet” pharmacological treatment that comprehensively nullifies psychostimulant addiction, evidence provided in this review suggests that the use of different therapeutic compounds at different stages of the addiction cycle may provide the greatest therapeutic efficacy. For instance, one way to balance therapeutic efficacy against side effect may be to shift from chronic treatment with less selective D3/D2 partial agonists during abstinence, which may attenuate anhedonia and reduce resumption should it occur, to D3-selective antagonists once an individual has achieved prolonged abstinence, which may attenuate incentive motivational effects of environmental stimuli with minimal side effects. 5-HT1BR agonists may also complement this treatment as evidence suggests that they reduce both drug seeking and resumption of self-administration after a period of abstinence. This approach, in addition to limiting the severity of side effects, may allow for the restoration of multiple neuropharmacological systems that have become dysregulated during psychostimulant abuse, and may thus lead to reduced relapse rate even when patients are no longer under active treatments.
Dopamine D3 and 5-HT1B receptors are dysregulated by psychostimulants and abstinence.
Data suggest that these receptors are novel targets for treating psychostimulant dependence.
Drug history, abstinence and social context should be considered in medication development.
Non-standard abbreviations
- D3Rs
dopamine D3 receptors
- 5-HT1BRs
5-HT1B receptors
- SA
self-administration
- PR
progressive ratio
- FR
fixed ratio
- VI
variable interval
- NAc
nucleus accumbens
- NAcsh
nucleus accumbens shell
- NAcc
nucleus accumbens core
- A2ARs
adenosine A2A receptors
- EPM
elevated plus maze
- OCD
obsessive-compulsive disorder
- VTA
ventral tegmental area
- BLA
basolateral amygdala
- CeA
central amygdala
- dSt
dorsal striatum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. J Pharmacol Exp Ther. 2010a;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Grundt P, Cao JJ, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 Receptor Mechanisms in the Abuse-Related Behavioral Effects of Cocaine: Studies with Preferential Antagonists in Squirrel Monkeys. Journal of Pharmacology and Experimental Therapeutics. 2010b;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Platt DM, Newman AH, Spealman RD. The dopamine D3 receptor partial agonist CJB 090 inhibits the discriminative stimulus but not the reinforcing or priming effects of cocaine in squirrel monkeys. Psychopharmacology. 2009;206:73–84. doi: 10.1007/s00213-009-1581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Boynton FA, Kirschner KF, Neisewander JL. Stimulation of 5-HT1B receptors decreases cocaine- and sucrose-seeking behavior. Pharmacol Biochem Behav. 2005;80:297–307. doi: 10.1016/j.pbb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE. Pathological behaviors provoked by dopamine agonist therapy of Parkinson’s disease. Physiology & Behavior. 2011;104:168–172. doi: 10.1016/j.physbeh.2011.04.055. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Akimoto K, Hamamura T, Kazahaya Y, Akiyama K, Otsuki S. Enhanced Extracellular Dopamine Level May Be the Fundamental Neuropharmacological Basis of Cross-Behavioral Sensitization between Methamphetamine and Cocaine – an Invivo Dialysis Study in Freely Moving Rats. Brain Research. 1990;507:344–346. doi: 10.1016/0006-8993(90)90295-m. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 Dopamine Receptor Antagonist Sulpiride into the Shell, but not the Core, of the Nucleus Accumbens Attenuates Cocaine Priming-Induced Reinstatement of Drug Seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR. Oscillation follows drug sensitization: implications. Critical Reviews in Neurobiology. 1996;10:101–117. doi: 10.1615/critrevneurobiol.v10.i1.50. [DOI] [PubMed] [Google Scholar]
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT(1A) or 5-HT(1B) receptor knockout mice. Journal of Neurochemistry. 2000;75:2415–2426. doi: 10.1046/j.1471-4159.2000.0752415.x. [DOI] [PubMed] [Google Scholar]
- Audinot V, Newman-Tancredi A, Gobert A, Rivet JM, Brocco M, Lejeune F, Gluck L, Desposte I, Bervoets K, Dekeyne A, Millan MJ. A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103,691 and U 99194. Journal of Pharmacology and Experimental Therapeutics. 1998;287:187–197. [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Psychosocial influences on the neurobehavioral pharmacology of abused drugs. Pharmacological Reviews. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology. 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47:256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bendotti C, Samanin R. The role of putative 5-HT1A and 5-HT1B receptors in the control of feeding in rats. Life Sci. 1987;41:635–642. doi: 10.1016/0024-3205(87)90418-8. [DOI] [PubMed] [Google Scholar]
- Bergman J. Medications for Stimulant Abuse: Agonist-Based Strategies and Preclinical Evaluation of the Mixed-Action D2 Partial Agonist Aripiprazole (Abilify ®) Experimental and Clinical Psychopharmacology. 2008;16:475–483. doi: 10.1037/a0014398. [DOI] [PubMed] [Google Scholar]
- Blaylock BL, Gould RW, Banala A, Grundt P, Luedtke RR, Newman AH, Nader MA. Influence of Cocaine History on the Behavioral Effects of Dopamine D-3 Receptor-Selective Compounds in Monkeys. Neuropsychopharmacology. 2011;36:1104–1113. doi: 10.1038/npp.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong JC, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Higher Binding of the Dopamine D3 Receptor-Preferring Ligand [C-11] -(+)-Propyl-Hexahydro-Naphtho-Oxazin in Methamphetamine Polydrug Users: A Positron Emission Tomography Study. Journal of Neuroscience. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Boshuisen ML, den Boer JA. Zolmitriptan (a 5-HT1B/1D receptor agonist with central action) does not increase symptoms in obsessive compulsive disorder. Psychopharmacology (Berl) 2000;152:74–79. doi: 10.1007/s002130000529. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, Nash NR, Olsson R, Davis RE, Hacksell U, Weiner DM, Brann MR. Intrinsic efficacy of antipsychotics at human D2, D3, and D4 dopamine receptors: identification of the clozapine metabolite N-desmethylclozapine as a D2/D3 partial agonist. Journal of Pharmacology and Experimental Therapeutics. 2005;315:1278–1287. doi: 10.1124/jpet.105.092155. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behav Pharmacol. 1995;6:333–347. [PubMed] [Google Scholar]
- Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport. 1997;8:2373–2377. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self- administer cocaine. Journal of Pharmacology and Experimental Therapeutics. 1999;291:353–360. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: Studies with D2 receptor mutant mice and novel D2 receptor antagonists. Journal of Neuroscience. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, Newman AH, Butler P, Xu M. Cocaine Self-Administration in Dopamine D3 Receptor Knockout Mice. Experimental and Clinical Psychopharmacology. 2012;20:352–363. doi: 10.1037/a0029135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Larocque E, Li D. Associations of the 5-hydroxytryptamine (serotonin) Receptor 1B gene (HTR1B) with alcohol, cocaine, and heroin abuse. Am J Med Genet B Neuropsychiatr Genet. 2013 doi: 10.1002/ajmg.b.32128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JX, Hu J, Ye XM, Xia Y, Haile CA, Kosten TR, Zhang XY. Association between the 5-HTR1B gene polymorphisms and alcohol dependence in a Han Chinese population. Brain Research. 2011;1376:1–9. doi: 10.1016/j.brainres.2010.12.039. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003a;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: Involvement receptors of D-3 and D-2 dopamine receptors. Neuropsychopharmacology. 2003b;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychopharmacol. 2007a;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D(3) receptors attenuates cocaine-seeking behaviour in the rat. International Journal of Neuropsychopharmacology. 2007b;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Cervo L, Rozio M, Ekalle-Soppo CB, Carnovali F, Santangelo E, Samanin R. Stimulation of serotonin1B receptors induces conditioned place aversion and facilitates cocaine place conditioning in rats. Psychopharmacology (Berl) 2002;163:142–150. doi: 10.1007/s00213-002-1145-8. [DOI] [PubMed] [Google Scholar]
- Chang WL, Breier MR, Yang A, Swerdlow NR. Disparate effects of pramipexole on locomotor activity and sensorimotor gating in Sprague-Dawley rats. Pharmacology Biochemistry and Behavior. 2011;99:634–638. doi: 10.1016/j.pbb.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F, Courvoisier H, Moisan MP, Mormede P. GR 127935 reduces basal locomotor activity and prevents RU 24969-, but not D-amphetamine-induced hyperlocomotion, in the Wistar-Kyoto hyperactive (WKHA) rat. Psychopharmacology (Berl) 1999;141:326–331. doi: 10.1007/s002130050841. [DOI] [PubMed] [Google Scholar]
- Cheetham SC, Heal DJ. Evidence that RU 24969-induced locomotor activity in C57/B1/6 mice is specifically mediated by the 5-HT1B receptor. Br J Pharmacol. 1993;110:1621–1629. doi: 10.1111/j.1476-5381.1993.tb14010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung THC, Nolan BC, Hammerslag LR, Weber SM, Durbin JP, Peartree NA, Mach RH, Luedtke RR, Neisewander JL. Phenylpiperazine derivatives with selectivity for dopamine D3 receptors modulate cocaine self-administration in rats. Neuropharmacology. 2012;63:1346–1359. doi: 10.1016/j.neuropharm.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O’Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988a;81:74–80. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr. 1988b;84:25–43. [PubMed] [Google Scholar]
- Chu WH, Tu Z, McElveen E, Xu JB, Taylor M, Luedtke RR, Mach RH. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D-3 receptor ligands. Bioorganic and Medicinal Chemistry. 2005;13:77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Cigler T, LaForge KS, McHugh PF, Kapadia SU, Leal SM, Kreek MJ. Novel and previously reported single-nucleotide polymorphisms in the human 5-HT(1B) receptor gene: no association with cocaine or alcohol abuse or dependence. Am J Med Genet. 2001;105:489–497. doi: 10.1002/ajmg.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Butler P, Wayman C, Ratcliffe S, Gupta P, Oberhofer G, Caine SB. Lack of abuse potential in a highly selective dopamine D-3 agonist, PF-592,379, in drug self-administration and drug discrimination in rats. Behavioural Pharmacology. 2012a;23:280–291. doi: 10.1097/FBP.0b013e3283536d21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Cunningham AR, Chen JY, Wang SM, Newman AH, Woods JH. Effects of pramipexole on the reinforcing effectiveness of stimuli that were previously paired with cocaine reinforcement in rats. Psychopharmacology. 2012b;219:123–135. doi: 10.1007/s00213-011-2382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen JY, Wang SM, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Truong YNT, Levant B, Chen JY, Wang SM, Woods JH. Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D3 and D2 receptor sensitivity. Psychopharmacology. 2011;215:609–620. doi: 10.1007/s00213-010-2154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao JJ, Woods JH. Dopamine agonist-induced yawning in rats: A dopamine D3 receptor-mediated behavior. Journal of Pharmacology and Experimental Therapeutics. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Influence of conditioned reinforcement on the response-maintaining effects of quinpirole in rats. Behavioural Pharmacology. 2009;20:492–504. doi: 10.1097/FBP.0b013e328330ad9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Ford K, Marinelli M, Wolf ME. Dopamine receptor expression and distribution dynamically change in the rat nucleus accumbens after withdrawal from cocaine self-administration. Neuroscience. 2010;169:182–194. doi: 10.1016/j.neuroscience.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Effects of dopamine D2/D3 receptor ligands on food-cocaine choice in socially housed male cynomolgus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2013;344:329–338. doi: 10.1124/jpet.112.201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Robbins TW. Personality, Addiction, Dopamine: Insights from Parkinson’s Disease. Neuron. 2009;61:502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Dayalu P, Chou KL. Antipsychotic-induced extrapyramidal symptoms and their management. Expert Opinion on Pharmacotherapy. 2008;9:1451–1462. doi: 10.1517/14656566.9.9.1451. [DOI] [PubMed] [Google Scholar]
- De Fazio P, Girardi P, Maina G, Mauri MC, Mauri M, Monteleone P, Perini GI, Perugi G, Rossi A. Aripiprazole in acute mania and long-term treatment of bipolar disorder: a critical review by an Italian working group. Clinical Drug Investigation. 2010;30:827–841. doi: 10.2165/11584270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer ANM, Binnekade R, Raaso H, Vanderschuren L. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer ANM, Binnekade R, Vanderschuren L. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology. 1999;143:254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- den Boon FS, Body S, Hampson CL, Bradshaw CM, Szabadi E, de Bruin N. Effects of amisulpride and aripiprazole on progressive-ratio schedule performance: comparison with clozapine and haloperidol. Journal of Psychopharmacology. 2012;26:1231–1243. doi: 10.1177/0269881111421974. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behavioral Neuroscience. 2008;122:129–139. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003a;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D-3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003b;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Dias C, Lachize S, Boilet V, Huitelec E, Cador M. Differential effects of dopaminergic agents on locomotor sensitisation and on the reinstatement of cocaine-seeking and food-seeking behaviour. Psychopharmacology. 2004;175:414–427. doi: 10.1007/s00213-004-1839-1. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. Journal of Neuroscience. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet E, Pohl M, Fattaccini CM, Adrien J, Mestikawy SE, Hamon M. In situ hybridization evidence for the synthesis of 5-HT1B receptor in serotoninergic neurons of anterior raphe nuclei in the rat brain. Synapse. 1995;19:18–28. doi: 10.1002/syn.890190104. [DOI] [PubMed] [Google Scholar]
- Drago A, Alboni S, Brunello N, De Ronchi D, Serretti A. HTR1B as a risk profile maker in psychiatric disorders: a review through motivation and memory. Eur J Clin Pharmacol. 2010;66:5–27. doi: 10.1007/s00228-009-0724-6. [DOI] [PubMed] [Google Scholar]
- Evans CE, Sebastian J. Serotonin syndrome. Emergency medicine journal: EMJ. 2007;24:e20. doi: 10.1136/emj.2006.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and Triptans: an analysis of the 29 case reports. MedGenMed: Medscape general medicine. 2007;9:48. [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Altar CA, See RE. Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biological Psychiatry. 2007;61:582–590. doi: 10.1016/j.biopsych.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Do PH, See RE. Repeated aripiprazole administration attenuates cocaine seeking in a rat model of relapse. Psychopharmacology. 2009;207:401–411. doi: 10.1007/s00213-009-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenu S, Wardas J, Morelli M. Impulse control disorders and dopamine dysregulation syndrome associated with dopamine agonist therapy in Parkinson’s disease. Behavioural Pharmacology. 2009;20:363–379. doi: 10.1097/FBP.0b013e32833109a0. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Heal DJ, McCreary AC, Woolverton WL. Assessment of ropinirole as a reinforcer in rhesus monkeys. Drug and Alcohol Dependence. 2012;125:173–177. doi: 10.1016/j.drugalcdep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LT, Weber SM, Khroyan TV, Neisewander JL. Effects of 7-OH-DPAT on cocaine-seeking behavior and on re-establishment of cocaine self-administration. Pharmacol Biochem Behav. 2002a;72:623–632. doi: 10.1016/s0091-3057(02)00731-1. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LTL, Weber SM, Khroyan TV, Neisewander JL. Effects of 7-OH-DPAT on cocaine-seeking behavior and on re-establishment of cocaine self-administration. Pharmacology Biochemistry and Behavior. 2002b;72:623–632. doi: 10.1016/s0091-3057(02)00731-1. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Borroto-Escuela DO, Guescini M, Fernandez-Duenas V, Tanganelli S, Rivera A, Ciruela F, Agnati LF. Adenosine-Dopamine Interactions in the Pathophysiology and Treatment of CNS Disorders. Cns Neuroscience & Therapeutics. 2010;16:e18–e42. doi: 10.1111/j.1755-5949.2009.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gál K, Gyertyán I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Research Bulletin. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Ghavami A, Stark KL, Jareb M, Ramboz S, Segu L, Hen R. Differential addressing of 5-HT1A and 5-HT1B receptors in epithelial cells and neurons. J Cell Sci. 1999;112(Pt 6):967–976. doi: 10.1242/jcs.112.6.967. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman PK. Triptans, serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50:264–272. doi: 10.1111/j.1526-4610.2009.01575.x. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Green AR. A behavioural and biochemical study in mice and rats of putative selective agonists and antagonists for 5-HT1 and 5-HT2 receptors. Br J Pharmacol. 1985;84:743–753. doi: 10.1111/j.1476-5381.1985.tb16157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Guy AP, Gardner CR. The behavioural effects of RU 24969, a suggested 5-HT1 receptor agonist in rodents and the effect on the behaviour of treatment with antidepressants. Neuropharmacology. 1984;23:655–661. doi: 10.1016/0028-3908(84)90147-3. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation – Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao JJ, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: Potential substance abuse therapeutic agents. Journal of Medicinal Chemistry. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Gyertyan I, Kiss B, Gal K, Laszlovszky I, Horvath A, Gemesi LI, Saghy K, Pasztor G, Zajer M, Kapas M, Csongor EA, Domany G, Tihanyi K, Szombathelyi Z. Effects of RGH-237 [N-{4-[4-(3-aminocarbonyl-phenyl)-piperazin-1-yl]-butyl}-4-bromobenzami de], an orally active, selective dopamine D-3 receptor partial agonist in animal models of cocaine abuse. Journal of Pharmacology and Experimental Therapeutics. 2007a;320:1268–1278. doi: 10.1124/jpet.106.107920. [DOI] [PubMed] [Google Scholar]
- Gyertyan I, Kiss B, Gal K, Laszlovszky I, Horvath A, Gemesi LI, Saghy K, Pasztor G, Zajer M, Kapas M, Csongor EA, Domany G, Tihanyi K, Szombathelyi Z. Effects of RGH-237 [N-{4-[4-(3-aminocarbonyl-phenyl)-piperazin-1-yl]-butyl}-4-bromobenzamide ], an orally active, selective dopamine D(3) receptor partial agonist in animal models of cocaine abuse. J Pharmacol Exp Ther. 2007b;320:1268–1278. doi: 10.1124/jpet.106.107920. [DOI] [PubMed] [Google Scholar]
- Gyertyan I, Saghy K. The selective dopamine D3 receptor antagonists, SB 277011-A and S 33084 block haloperidol-induced catalepsy in rats. Eur J Pharmacol. 2007;572:171–174. doi: 10.1016/j.ejphar.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Halford JC, Blundell JE. The 5-HT1B receptor agonist CP-94,253 reduces food intake and preserves the behavioural satiety sequence. Physiol Behav. 1996;60:933–939. doi: 10.1016/0031-9384(96)00073-x. [DOI] [PubMed] [Google Scholar]
- Haney M, Rubin E, Foltin RW. Aripiprazole maintenance increases smoked cocaine self-administration in humans. Psychopharmacology. 2011;216:379–387. doi: 10.1007/s00213-011-2231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Higuchi S, Matsushita S, Miyaoka H. Association of a polymorphism of the serotonin 1B receptor gene and alcohol dependence with inactive aldehyde dehydrogenase-2. J Neural Transm. 2002;109:513–521. doi: 10.1007/s007020200042. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR. The role of central dopamine D-3 receptors in drug addiction: a review of pharmacological evidence. Brain Research Reviews. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Kiefer SW, Li X, Gaal J, Xi ZX, Gardner EL. Dopamine D3 receptor antagonist SB-277011A inhibits methamphetamine self-administration and methamphetamine-induced reinstatement of drug-seeking in rats. European Journal of Pharmacology. 2011a;659:187–192. doi: 10.1016/j.ejphar.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacol. 2011b;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer ME, Voskanian SJ, Koob GF, Pulvirenti L. Effects of terguride, ropinirole, and acetyl-L-carnitine on methamphetamine withdrawal in the rat. Pharmacol Biochem Behav. 2006;83:403–409. doi: 10.1016/j.pbb.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Hoplight BJ, Vincow ES, Neumaier JF. Cocaine increases 5-HT1B mRNA in rat nucleus accumbens shell neurons. Neuropharmacology. 2007;52:444–449. doi: 10.1016/j.neuropharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Hu J, Henry S, Gallezot JD, Ropchan J, Neumaier JF, Potenza MN, Sinha R, Krystal JH, Huang Y, Ding YS, Carson RE, Neumeister A. Serotonin 1B receptor imaging in alcohol dependence. Biol Psychiatry. 2010;67:800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Oquendo MA, Friedman JM, Greenhill LL, Brodsky B, Malone KM, Khait V, Mann JJ. Substance abuse disorder and major depression are associated with the human 5-HT1B receptor gene (HTR1B) G861C polymorphism. Neuropsychopharmacology. 2003;28:163–169. doi: 10.1038/sj.npp.1300000. [DOI] [PubMed] [Google Scholar]
- Izzo E, Orsini C, Koob GF, Pulvirenti L. A dopamine partial agonist and antagonist block amphetamine self-administration in a progressive ratio schedule. Pharmacol Biochem Behav. 2001;68:701–708. doi: 10.1016/s0091-3057(01)00472-5. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalkman HO. Ru-24969-Induced Locomotion in Rats Is Mediated by 5ht(1a) Receptors. Naunyn-Schmiedebergs Archives of Pharmacology. 1995;352:583–584. doi: 10.1007/BF00169395. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Baker DA, Neisewander JL. Dose-dependent effects of the D-3-preferring agonist 7-OH-DPAT on motor behaviors and place conditioning. Psychopharmacology. 1995;122:351–357. doi: 10.1007/BF02246265. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1-and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Kinon BJ, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: A critical analysis. Psychopharmacology. 1996;124:2–34. doi: 10.1007/BF02245602. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Biphasic changes in mesolimbic dopamine signal during cocaine self-administration. Neuroreport. 1994;5:1005–1008. doi: 10.1097/00001756-199404000-00038. [DOI] [PubMed] [Google Scholar]
- Koe BK, Nielsen JA, Macor JE, Heym J. Biochemical and Behavioral-Studies of the 5-Ht(1b) Receptor Agonist, Cp-94,253. Drug Development Research. 1992;26:241–250. [Google Scholar]
- Koffarnus MN, Collins GT, Rice KC, Chen JY, Woods JH, Winger G. Self-administration of agonists selective for dopamine D-2, D-3, and D-4 receptors by rhesus monkeys. Behavioural Pharmacology. 2012;23:331–338. doi: 10.1097/FBP.0b013e3283564dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong H, Kuang W, Li S, Xu M. Activation of dopamine D3 receptors inhibits reward-related learning induced by cocaine. Neuroscience. 2011;176:152–161. doi: 10.1016/j.neuroscience.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Koran LM, Pallanti S, Quercioli L. Sumatriptan, 5-HT(1D) receptors and obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2001;11:169–172. doi: 10.1016/s0924-977x(01)00082-7. [DOI] [PubMed] [Google Scholar]
- Kula NS, Baldessarini RJ, Kebabian JW, Neumeyer JL. S-(+)-aporphines are not selective for human D 3 dopamine receptors. Cellular and Molecular Neurobiology. 1994;14:185–191. doi: 10.1007/BF02090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Riddle LR, Griffin SA, Chu WH, Vangveravong S, Neisewander J, Mach RH, Luedtke RR. Evaluation of D2 and D3 dopamine receptor selective compounds on L-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology. 2009;56:956–969. doi: 10.1016/j.neuropharm.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. European Journal of Neuroscience. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Lee MD, Kennett GA, Dourish CT, Clifton PG. 5-HT1B receptors modulate components of satiety in the rat: behavioural and pharmacological analyses of the selective serotonin1B agonist CP-94,253. Psychopharmacology (Berl) 2002;164:49–60. doi: 10.1007/s00213-002-1162-7. [DOI] [PubMed] [Google Scholar]
- Lee MD, Simansky KJ. CP-94,253: A selective serotonin(1B) (5-HT1B) agonist that promotes satiety. Psychopharmacology. 1997;131:264–270. doi: 10.1007/s002130050292. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lin WW, Huang SY, Kuo PH, Wang CL, Wu PL, Chen SL, Wu JY, Ko HC, Lu RB. The relationship between serotonin receptor 1B polymorphisms A-161T and alcohol dependence. Alcohol Clin Exp Res. 2009;33:1589–1595. doi: 10.1111/j.1530-0277.2009.00990.x. [DOI] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Li SM, Collins GT, Paul NM, Grundt P, Newman AH, Xu M, Grandy DK, Woods JH, Katz JL. Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behavioural Pharmacology. 2010;21:171–181. doi: 10.1097/FBP.0b013e32833a5c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Hays LR, Rush CR. The Safety, Tolerability, and Subject-Rated Effects of Acute Intranasal Cocaine Administration During Aripiprazole Maintenance II: Increased Aripipirazole Dose and Maintenance Period. American Journal of Drug and Alcohol Abuse. 2008;34:721–729. doi: 10.1080/00952990802308262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Parsons LH. Anxiogenic-like effect of serotonin(1B) receptor stimulation in the rat elevated plus-maze. Pharmacol Biochem Behav. 2002;71:581–587. doi: 10.1016/s0091-3057(01)00712-2. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Mishra Y, Wang Q, Griffin SA, Bell-Horner C, Taylor M, Vangveravong S, Dillon GH, Huang RQ, Reichert DE, Mach RH. Comparison of the Binding and Functional Properties of Two Structurally Different D2 Dopamine Receptor Subtype Selective Compounds. Acs Chemical Neuroscience. 2012;3:1050–1062. doi: 10.1021/cn300142q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach RH, Tu ZD, Xu JB, Li SH, Jones LA, Taylor M, Luedtke RR, Derdeyn CP, Perlmutter JS, Mintun MA. Endogenous Dopamine (DA) Competes With the Binding of a Radiolabeled D-3 Receptor Partial Agonist In Vivo: A Positron Emission Tomography Study. Synapse. 2011;65:724–732. doi: 10.1002/syn.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie RG, Vanleeuwen D, Pugsley TA, Shih YH, Demattos S, Tang L, Todd RD, Omalley KL. Characterization of the human dopamine D3 receptor expressed in transfected cell lines. European Journal of Pharmacology-Molecular Pharmacology Section. 1994;266:79–85. doi: 10.1016/0922-4106(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Macor JE, Burkhart CA, Heym JH, Ives JL, Lebel LA, Newman ME, Nielsen JA, Ryan K, Schulz DW, Torgersen LK, Koe BK. 3-(1,2,5,6-Tetrahydropyrid-4-Yl)Pyrrolo[3,2-B]Pyrid-5-One – a Potent and Selective Serotonin (5-Ht1b) Agonist and Rotationally Restricted Phenolic Analog of 5-Methoxy-3-(1,2,5,6-Tetrahydropyrid-4-Yl)Indole. Journal of Medicinal Chemistry. 1990;33:2087–2093. doi: 10.1021/jm00170a007. [DOI] [PubMed] [Google Scholar]
- Mailman RB. GPCR functional selectivity has therapeutic impact. Trends in Pharmacological Sciences. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Roberts DCS, Navarro G, Filip M, Agnati L, Lluis C, Franco R, Fuxe K. Increase in A(2A) receptors in the nucleus accumbens after extended cocaine self-administration and its disappearance after cocaine withdrawal. Brain Research. 2007;1143:208–220. doi: 10.1016/j.brainres.2007.01.079. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology. 2003;168:84–98. doi: 10.1007/s00213-003-1491-1. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D-3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxa mide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benza mide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- McCall RB, Lookingland KJ, Bedard PJ, Huff RM. Sumanirole, a highly dopamine D2-selective receptor agonist: In vitro and in vivo pharmacological characterization and efficacy in animal models of Parkinson’s disease. Journal of Pharmacology and Experimental Therapeutics. 2005;314:1248–1256. doi: 10.1124/jpet.105.084202. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Hiroi R, Mackenzie SM, Robin NC, Cohn A, Kim JJ, Neumaier JF. Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol Psychiatry. 2011;69:780–787. doi: 10.1016/j.biopsych.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Meini M, Moncini M, Cecconi D, Cellesi V, Biasci L, Simoni G, Ameglio M, Pellegrini M, Forgione RN, Rucci P. Safety, Tolerability, and Self-Rated Effects of Aripiprazole and Ropinirole Treatment for Cocaine Dependence: A Pilot Study. American Journal on Addictions. 2011;20:179–180. doi: 10.1111/j.1521-0391.2010.00106.x. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. Journal of Pharmacology and Experimental Therapeutics. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Mannoury la Cour C, Novi F, Maggio R, Audinot V, Newman-Tancredi A, Cussac D, Pasteau V, Boutin JA, Dubuffet T, Lavielle G. S33138 [N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrr ol-2(3H)-yl)-ethyl]phenylacetamide], a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent: I. Receptor-binding profile and functional actions at G-protein-coupled receptors. Journal of Pharmacology and Experimental Therapeutics. 2008;324:587–599. doi: 10.1124/jpet.107.126706. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Peglion JL, Vian J, Rivet JM, Brocco M, Gobert A, Newmantancredi A, Dacquet C, Bervoets K, Girardon S, Jacques V, Chaput C, Audinot V. Functional correlates of dopamine D3 receptor activation in the rat in vivo and their modulation by the selective antagonist, (+)-S 14297: 1. Activation of postsynaptic D3 receptors mediates hypothermia, whereas blockade of D2 receptors elicits prolactin secretion and catalepsy. Journal of Pharmacology and Experimental Therapeutics. 1995;275:885–898. [PubMed] [Google Scholar]
- Millan MJ, Seguin L, Gobert A, Cussac D, Brocco M. The role of dopamine D3 compared with D2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats. Psychopharmacology. 2004;174:341–357. doi: 10.1007/s00213-003-1770-x. [DOI] [PubMed] [Google Scholar]
- Miller DD, Eudicone JM, Pikalov A, Kim E. Comparative assessment of the incidence and severity of tardive dyskinesia in patients receiving aripiprazole or haloperidol for the treatment of schizophrenia: A post hoc analysis. Journal of Clinical Psychiatry. 2007;68:1901–1906. doi: 10.4088/jcp.v68n1210. [DOI] [PubMed] [Google Scholar]
- Miller NS, Summers GL, Gold MS. Cocaine dependence: alcohol and other drug dependence and withdrawal characteristics. J Addict Dis. 1993;12:25–35. doi: 10.1300/J069v12n01_03. [DOI] [PubMed] [Google Scholar]
- Miszkiel J, Adamczyk P, Filip M, Przegalinski E. The effect of serotonin 5HT(1B) receptor ligands on amphetamine self-administration in rats. Eur J Pharmacol. 2012;677:111–115. doi: 10.1016/j.ejphar.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Morgan D, Brebner K, Lynch WJ, Roberts DCS. Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behavioural Pharmacology. 2002a;13:389–396. doi: 10.1097/00008877-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nature Neuroscience. 2002b;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nader MA, Green KL, Luedtke RR, Mach RH. The effects of benzamide analogues on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1999;147:143–152. doi: 10.1007/s002130051154. [DOI] [PubMed] [Google Scholar]
- Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, Blaylock BL, Kaplan JR, Garg PK, Davies HM, Morton D, Garg S, Reboussin BA. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biological Psychiatry. 2012;72:414–421. doi: 10.1016/j.biopsych.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: Effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LTL, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. Journal of Neuroscience. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology. 2012;224:33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, McDevitt RA, Polis IY, Parsons LH. Acquisition of and withdrawal from cocaine self-administration regulates 5-HT mRNA expression in rat striatum. Journal of Neurochemistry. 2009;111:217–227. doi: 10.1111/j.1471-4159.2009.06313.x. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA., Jr Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P. Medication discovery for addiction: Translating the dopamine D3 receptor hypothesis. Biochemical Pharmacology. 2012;84:882–890. doi: 10.1016/j.bcp.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. Journal of Medicinal Chemistry. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Nozue K, Takahashi Y, Yamashita N, Hiraoka S, Kumano H, Kuboki T, Oka Y. Fluvoxamine, a selective serotonin reuptake inhibitor, and 5-HT2C receptor inactivation induce appetite-suppressing effects in mice via 5-HT1B receptors. Int J Neuropsychopharmacol. 2007;10:675–681. doi: 10.1017/S1461145706007206. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Manzardo AM, Polis I, Stouffer DG, Parsons LH. Biphasic alterations in serotonin-1B (5-HT1B) receptor function during abstinence from extended cocaine self-administration. Journal of Neurochemistry. 2006;99:1363–1376. doi: 10.1111/j.1471-4159.2006.04163.x. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- O’Neill MF, Parameswaran T. RU24969-induced behavioural syndrome requires activation of both 5HT1A and 5HT1B receptors. Psychopharmacology (Berl) 1997;132:255–260. doi: 10.1007/s002130050343. [DOI] [PubMed] [Google Scholar]
- Oberlander C, Demassey Y, Verdu A, Van de Velde D, Bardelay C. Tolerance to the serotonin 5-HT1 agonist RU 24969 and effects on dopaminergic behaviour. European Journal of Pharmacology. 1987;139:205–214. doi: 10.1016/0014-2999(87)90253-6. [DOI] [PubMed] [Google Scholar]
- Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addiction Biology. 2010;15:312–323. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini C, Koob GF, Pulvirenti L. Dopamine partial agonist reverses amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:789–792. doi: 10.1016/S0893-133X(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Ossowska K. Neuronal basis of neuroleptic-induced extrapyramidal side effects. Polish Journal of Pharmacology. 2002;54:299–312. [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Serotonin Dysfunction in the Nucleus-Accumbens of Rats during Withdrawal after Unlimited Access to Intravenous Cocaine. Journal of Pharmacology and Experimental Therapeutics. 1995;274:1182–1191. [PubMed] [Google Scholar]
- Parsons LH, Weiss F, Koob GF. Serotonin1b receptor stimulation enhances dopamine-mediated reinforcement. Psychopharmacology (Berl) 1996;128:150–160. doi: 10.1007/s002130050120. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Weiss F, Koob GF. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18:10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña MS, Yaltho TC, Jankovic J. Tardive Dyskinesia and Other Movement Disorders Secondary to Aripiprazole. Movement Disorders. 2010;26:147–152. doi: 10.1002/mds.23402. [DOI] [PubMed] [Google Scholar]
- Peng XQ, Ashby CR, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaer E, Munoz C, Gardner EL, Xi ZX. The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharmacology. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Acosta JI, Browning JR, Hamilton EC, Neisewander JL. Stimulation of 5-HT(1B) receptors enhances cocaine reinforcement yet reduces cocaine-seeking behavior. Addict Biol. 2009;14:419–430. doi: 10.1111/j.1369-1600.2009.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Cheung TH, Toy WA, Adams MD, Neumaier JF, Neisewander JL. Protracted Withdrawal from Cocaine Self-Administration Flips the Switch on 5-HT(1B) Receptor Modulation of Cocaine Abuse-Related Behaviors. Biol Psychiatry. 2012a doi: 10.1016/j.biopsych.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Harder B, Brunwasser S, Yanamandra K, Bastle RM, Der-Ghazarian T, Adams MD, Alba J, Neisewander JL. The effects of 5-HT1B receptors on motivation for cocaine vary depending on the length of abstinence. Society for Neuroscience 2012b [Google Scholar]
- Pian KL, Westenberg HG, van Megen HJ, den Boer JA. Sumatriptan (5-HT1D receptor agonist) does not exacerbate symptoms in obsessive compulsive disorder. Psychopharmacology (Berl) 1998;140:365–370. doi: 10.1007/s002130050777. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rodefer JS, Rowlett JK, Spealman RD. Suppression of cocaine- and food-maintained behavior by the D2-like receptor partial agonist terguride in squirrel monkeys. Psychopharmacology. 2003;166:298–305. doi: 10.1007/s00213-002-1347-0. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJR. The effects of cocaine: A shifting target over the course of addiction. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- Prinzleve M, Haasen C, Zurhold H, Matali JL, Bruguera E, Gerevich J, Bacskai E, Ryder N, Butler S, Manning V, Gossop M, Pezous AM, Verster A, Camposeragna A, Andersson P, Olsson B, Primorac A, Fischer G, Guttinger F, Rehm J, Krausz M. Cocaine use in Europe – a multi-centre study: patterns of use in different groups. Eur Addict Res. 2004;10:147–155. doi: 10.1159/000079835. [DOI] [PubMed] [Google Scholar]
- Proudnikov D, LaForge KS, Hofflich H, Levenstien M, Gordon D, Barral S, Ott J, Kreek MJ. Association analysis of polymorphisms in serotonin 1B receptor (HTR1B) gene with heroin addiction: a comparison of molecular and statistically estimated haplotypes. Pharmacogenet Genomics. 2006;16:25–36. doi: 10.1097/01.fpc.0000182782.87932.d6. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Czepiel K, Nowak E, Dlaboga D, Filip M. Withdrawal from chronic cocaine up-regulates 5-HT1B receptors in the rat brain. Neurosci Lett. 2003;351:169–172. doi: 10.1016/j.neulet.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Golda A, Filip M. Effects of serotonin (5-HT)(1B) receptor ligands on cocaine-seeking behavior in rats. Pharmacol Rep. 2008;60:798–810. [PubMed] [Google Scholar]
- Przegalinski E, Golda A, Frankowska M, Zaniewska M, Filip M. Effects of serotonin 5-HT1B receptor ligands on the cocaine- and food-maintained self-administration in rats. Eur J Pharmacol. 2007;559:165–172. doi: 10.1016/j.ejphar.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Balducci C, Piercy M, Koob GF. Characterization of the effects of the partial dopamine agonist terguride on cocaine self-administration in the rat. J Pharmacol Exp Ther. 1998;286:1231–1238. [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. Being partial to psychostimulant addiction therapy. Trends Pharmacol Sci. 2002;23:151–153. doi: 10.1016/s0165-6147(00)01991-x. [DOI] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DNC, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AKK, Hagan JJ. Pharmacological actions of a novel, high-affinity, and selective human dopamine D-3 receptor antagonist, SB-277011-A. Journal of Pharmacology and Experimental Therapeutics. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology (Berl) 1997;134:309–317. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Ridray S, Griffon N, Mignon V, Souil E, Carboni S, Diaz J, Schwartz JC, Sokoloff P. Coexpression of dopamine D-1 and D-3 receptors in islands of Calleja and shell of nucleus accumbens of the rat: opposite and synergistic functional interactions. European Journal of Neuroscience. 1998;10:1676–1686. doi: 10.1046/j.1460-9568.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rolan PE. Drug interactions with triptans: which are clinically significant? CNS drugs. 2012;26:949–957. doi: 10.1007/s40263-012-0002-5. [DOI] [PubMed] [Google Scholar]
- Rowan-Szal GA, Chatham LR, Simpson DD. Importance of identifying cocaine and alcohol dependent methadone clients. Am J Addict. 2000;9:38–50. doi: 10.1080/10550490050172218. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Dopamine/adenosine interactions involved in effort-related aspects of food motivation. Appetite. 2009;53:422–425. doi: 10.1016/j.appet.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Frontiers in Behavioral Neuroscience. 2009;3:12. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Lefevre K, Bancila M, Quignon M, Miquel MC, Langlois X, Hamon M, Verge D. Light and electron microscopic immunocytochemical visualization of 5-HT1B receptors in the rat brain. Brain Research. 1997;760:281–286. doi: 10.1016/s0006-8993(97)00400-9. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Verge D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Sautel F, Griffon N, Levesque D, Pilon C, Schwartz JC, Sokoloff P. A functional test identifies dopamine agonists selective for D3 versus D2 receptors. Neuroreport. 1995;6:329–332. doi: 10.1097/00001756-199501000-00026. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking bahavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Koch M. Effects of aripiprazole on operant responding for a natural reward after psychostimulant withdrawal in rats. Psychopharmacology. 2007;191:759–765. doi: 10.1007/s00213-006-0520-2. [DOI] [PubMed] [Google Scholar]
- Sclar DA, Robison LM, Castillo LV, Schmidt JM, Bowen KA, Oganov AM, Skaer TL, Kogut SJ. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52:198–203. doi: 10.1111/j.1526-4610.2011.02067.x. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: A therapeutic target for the treatment of neuropsychiatric disorders. CNS & Neurological Disorders-Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaal J, Xi ZX, Gardner EL. YQA14: a novel dopamine D-3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D-3 receptor-knockout mice. Addiction Biology. 2012a;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D-3 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2012b;109:17675–17680. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen G, Sager TN, Petersen JH, Brennum LT, Thøgersen P, Bengtsen CH, Thomsen M, Wörtwein G, Fink-Jensen A, Woldbye DPD. Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. Psychopharmacology. 2008;199:37–46. doi: 10.1007/s00213-008-1069-z. [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. Journal of Neuroscience. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern L, Zohar J, Cohen R, Sasson Y. Treatment of severe, drug resistant obsessive compulsive disorder with the 5HT1D agonist sumatriptan. Eur Neuropsychopharmacol. 1998;8:325–328. doi: 10.1016/s0924-977x(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Stewart J. Conditioned and unconditioned drug effects in relapse to opiate and stimulant drug self-adminstration. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:591–597. doi: 10.1016/0278-5846(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Lofwall MR, Rush CR. The safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance. American Journal of Drug and Alcohol Abuse. 2007;33:769–776. doi: 10.1080/00952990701651556. [DOI] [PubMed] [Google Scholar]
- Sun HF, Chang YT, Fann CS, Chang CJ, Chen YH, Hsu YP, Yu WY, Cheng AT. Association study of novel human serotonin 5-HT(1B) polymorphisms with alcohol dependence in Taiwanese Han. Biol Psychiatry. 2002;51:896–901. doi: 10.1016/s0006-3223(01)01366-x. [DOI] [PubMed] [Google Scholar]
- Tadori Y, Forbes RA, McQuade RD, Kikuchi T. Characterization of aripiprazole partial agonist activity at human dopamine D-3 receptors. European Journal of Pharmacology. 2008;597:27–33. doi: 10.1016/j.ejphar.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Tatarczynska E, Klodzinska A, Stachowicz K, Chojnacka-Wojcik E. Effects of a selective 5-HT1B receptor agonist and antagonists in animal models of anxiety and depression. Behav Pharmacol. 2004;15:523–534. doi: 10.1097/00008877-200412000-00001. [DOI] [PubMed] [Google Scholar]
- Taylor D, Lincoln AJ, Foster SL. Impaired behavior regulation under conditions of concurrent variable schedules of reinforcement in children with ADHD. Journal of Attention Disorders. 2010;13:358–368. doi: 10.1177/1087054708329974. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Ho CW, Wang GJ, Newman AH, Heidbreder CA, Ashby CR, Jr, Gardner EL, Volkow ND. The effects of two highly selective dopamine D3 receptor antagonists (SB-277011A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacol Biochem Behav. 2008;89:499–507. doi: 10.1016/j.pbb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Wenzel JM, Pentkowski NS, Hobbs RJ, Alleweireldt AT, Neisewander JL. Stimulation of dopamine D2/D3 but not D1 receptors in the central amygdala decreases cocaine-seeking behavior. Behav Brain Res. 2010;214:386–394. doi: 10.1016/j.bbr.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye DPD, Wortwein G, Sager TN, Holm R, Pepe LM, Caine SB. Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology. 2008;201:43–53. doi: 10.1007/s00213-008-1245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Fohr J, Tuomola P, Kuikanmaki O, Vorma H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. American Journal of Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, O’Neill MF. Effect of 5-HT(1B) receptor ligands on self-administration of ethanol in an operant procedure in rats. Pharmacol Biochem Behav. 2000;66:129–136. doi: 10.1016/s0091-3057(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Tu ZD, Li SH, Cui JQ, Xu JB, Taylor M, Ho D, Luedtke RR, Mach RH. Synthesis and Pharmacological Evaluation of Fluorine-Containing D-3 Dopamine Receptor Ligands. Journal of Medicinal Chemistry. 2011;54:1555–1564. doi: 10.1021/jm101323b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. Journal of Pharmacology and Experimental Therapeutics. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Voigt MM, Laurie DJ, Seeburg PH, Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. The EMBO journal. 1991;10:4017–4023. doi: 10.1002/j.1460-2075.1991.tb04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma YM, Wong C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Asbhy CR, Paul M, Liu XH, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D-3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward. Journal of Neuroscience. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorspan F, Bellais L, Keijzer L, Lepine JP. An open-label study of aripiprazole in nonschizophrenic crack-dependent patients. Journal of Clinical Psychopharmacology. 2008;28:570–572. doi: 10.1097/JCP.0b013e3181858311. [DOI] [PubMed] [Google Scholar]
- Wadenberg MLG, Kapur S, Soliman A, Jones C, Vaccarino F. Dopamine D2 receptor occupancy predicts catalepsy and the suppression of conditioned avoidance response behavior in rats. Psychopharmacology. 2000;150:422–429. doi: 10.1007/s002130000466. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mach RH, Luedtke RR, Reichert DE. Subtype Selectivity of Dopamine Receptor Ligands: Insights from Structure and Ligand-Based Methods. Journal of Chemical Information and Modeling. 2010;50:1970–1985. doi: 10.1021/ci1002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washton AM. Preventing relapse to cocaine. J Clin Psychiatry. 1988;49(Suppl):34–38. [PubMed] [Google Scholar]
- Weber M, Chang WL, Durbin JP, Park PE, Luedtke RR, Mach RH, Swerdlow NR. Using prepulse inhibition to detect functional D3 receptor antagonism: Effects of WC10 and WC44. Pharmacology Biochemistry and Behavior. 2009;93:141–147. doi: 10.1016/j.pbb.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML. Craving in hospitalized cocaine abusers as a predictor of outcome. American Journal of Drug and Alcohol Abuse. 1995;21:289–301. doi: 10.3109/00952999509002698. [DOI] [PubMed] [Google Scholar]
- Willett JB, Singer JD. Investigating onset, cessation, relapse, and recovery: why you should, and how you can, use discrete-time survival analysis to examine event occurrence. Journal of Consulting and Clinical Psychology. 1993;61:952–965. doi: 10.1037//0022-006x.61.6.952. [DOI] [PubMed] [Google Scholar]
- Wilson AW, Costall B, Neill JC. Manipulation of operant responding for an ethanolpaired conditioned stimulus in the rat by pharmacological alteration of the serotonergic system. J Psychopharmacol. 2000;14:340–346. doi: 10.1177/026988110001400402. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D-3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D-3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. European Journal of Neuroscience. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Zetterstrom T, Sharp T, Marsden CA, Ungerstedt U. Invivo Measurement of Dopamine and Its Metabolites by Intracerebral Dialysis – Changes after D-Amphetamine. Journal of Neurochemistry. 1983;41:1769–1773. doi: 10.1111/j.1471-4159.1983.tb00893.x. [DOI] [PubMed] [Google Scholar]


