Summary
COPII coated vesicles bud from an ER domain termed the transitional ER (tER), but the mechanism that clusters COPII vesicles at tER sites is unknown. tER sites are closely associated with early Golgi or pre-Golgi structures, suggesting that the clustering of nascent COPII vesicles could be achieved by tethering to adjacent membranes. This model challenges the prevailing view that COPII vesicles are clustered by a scaffolding protein at the ER surface. Although Sec16 was proposed to serve as such a scaffolding protein, recent data suggest that rather than organizing COPII into higher-order structures, Sec16 acts at the level of individual COPII vesicles to regulate COPII turnover. A plausible synthesis is that tER sites are created by tethering to Golgi membranes and are regulated by Sec16. Meanwhile, the COPII vesicles that bud from tER sites are thought to nucleate new Golgi cisternae. Thus, an integrated self-organization process may generate tER-Golgi units.
Keywords: self-organization, transitional ER, ER exit sites, Golgi, COPII
Introduction
The transitional ER (tER), also known as ER exit sites, is a ribosome-free ER domain surrounded by the ribosome-studded rough ER [1–3] (Fig. 1). All eukaryotes studied to date have tER sites, which range in number from one or two in some protists to several hundred in a cultured mammalian cell [4, 5]. COPII (coat protein complex II) coated vesicles bud from tER sites to deliver secretory cargo to the Golgi [6]. COPII assembly is initiated when the guanine nucleotide exchange factor Sec12 recruits the small GTPase Sar1 to the ER membrane. Sar1-GTP then recruits the cargo-binding Sec23/Sec24 complex, which in turn recruits the coat-forming Sec13/Sec31 complex. A COPII vesicle is about 60 nm in diameter whereas a typical tER site is about 400 nm in diameter. Thus, a tER site is a functionally, structurally, and biochemically specialized ER domain that generates multiple COPII vesicles.
Figure 1.
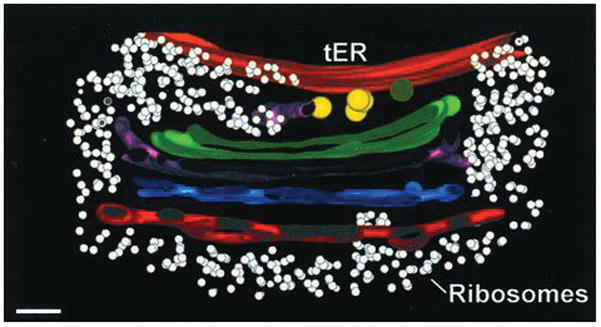
Cross-section through a tomographic reconstruction of a tER-Golgi unit in P. pastoris. The ribosome-free tER site (orange) is continous with the ribosome-excluding Golgi matrix, which extends to the trans-Golgi network (red). COPII vesicles (yellow) are found at the interface between the tER site and the early Golgi (green). Bar, 100 nm. Adapted with permission from Ref. 2.
Although COPII vesicle formation is reasonably well understood, the biogenesis of tER sites is still mysterious. These two processes are clearly related. However, there is a key difference: a COPII vesicle is a transient carrier, whereas a tER site is a long-lived domain. tER dynamics have been characterized in the budding yeast Pichia pastoris, which contains several tER sites per cell [7, 8]. A tER site forms de novo, grows to a steady-state size, and persists indefinitely. If two tER sites collide, they fuse into a larger domain that gradually shrinks back to the steady-state size. Similar dynamics are seen with mammalian tER sites [5, 9]. Therefore, tER sites have a mechanism for preserving their size and identity in the face of rapid turnover of COPII components. This mechanism is probably based on self-organization.
Self-organization of membrane compartments
The concept of self-organization is well established in cell biology [10]. Indeed, one might argue that for cells, self-organization is the only option because no external organizer is present. But there is another way to build a cellular structure: the organization problem could have been solved long ago in evolutionary time, with modern cells simply copying an ancient template. The most obvious example of template-driven organization is DNA replication. George Palade extended this idea to membrane compartments by postulating that “membranes act as their own templates… They recognize and incorporate like components, grow by expansion in two dimensions, and eventually divide into two sets of descendant membranes, one for each daughter cell” [11]. This paradigm is clearly valid for compartments such as the ER and mitochondria [12]. As a result, the organelle biogenesis field was slow to embrace self-organization.
The current view is that self-organization plays an important role in the secretory and endocytic pathways. There is strong evidence that early Golgi cisternae mature into late Golgi cisternae, and that early endosomes mature into late endosomes [13, 14]. When an early compartment matures out of existence, it is apparently replenished by a self-organization process in which vesicles fuse with one another to generate a new compartment [15]. Self-organization can also generate domains within a given membrane. In the best-studied example, Rab GTPases and phosphoinositides and their effectors operate in feedback loops to create transient membrane domains [16]. However, unlike Rab domains, tER sites are stable and depend on Sar1 activation, suggesting that a novel self-organization mechanism is at work.
Does Sec16 organize COPII at tER sites?
The known properties of COPII explain how a single vesicle forms, but do not explain how multiple nascent COPII vesicles cluster to form a tER site. One way to cluster COPII would be to place a COPII-organizing scaffold at the ER surface. A candidate for such a scaffold component is Sec16, a large peripheral ER membrane protein that is concentrated at tER sites [17, 18]. Sec16 has been shown to associate with all four COPII coat proteins, and also with Sec12 and perhaps Sar1 [18–22]. In mammalian and Drosophila cells, depletion of Sec16 disrupts tER sites, whereas depletion of COPII still allows Sec16 to form punctate structures [19, 21, 23]. A punctate Sec16 distribution persists during mammalian mitosis even when most of the COPII dissociates from tER sites [24]. When Sec16 was ectopically localized to endosomes in Drosophila cells, COPII proteins were found in the Sec16-containing structures [21]. These data are consistent with the view that Sec16 defines tER sites upstream of COPII recruitment [6, 19, 21].
What might be the molecular basis for a COPII organizing activity of Sec16? Sec16 can oligomerize [21, 23, 25, 26], suggesting that Sec16 might crosslink patches of COPII into higher-order structures [17]. Another perspective came from a crystallographic analysis [25] of the central conserved domain (CCD), which is the most conserved region of Sec16 [17, 23]. The CCD forms an ancestral coatomer element 1 (ACE1) structure, prompting speculation that this part of Sec16 nucleates COPII coat assembly [25]. Crosslinking and/or nucleating activities of Sec16 could promote clustering of COPII at tER sites (Fig. 2A). Moreover, a conserved C-terminal region of Sec16 recruits Sec12 to tER sites in P. pastoris and possibly also in mammalian cells [22], so Sec16 could be viewed as a scaffold for localizing Sec12. Yet despite these tantalizing results, we still lack a clear mechanistic model for how Sec16 might constrain COPII assembly to tER sites.
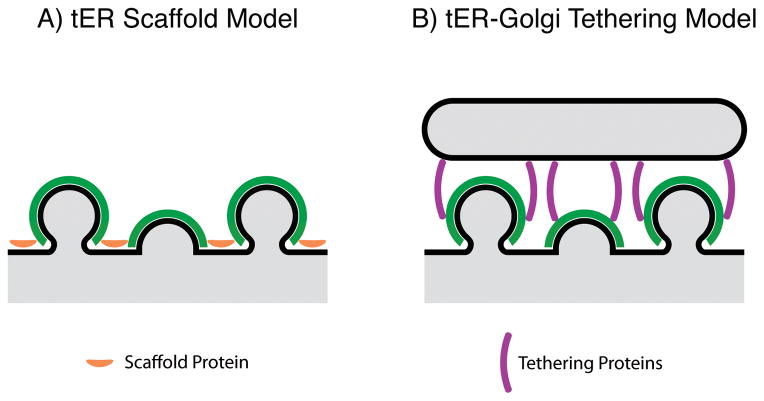
Figure 2.
Two possible mechanisms for clustering COPII vesicles at tER sites.
A: According to the tER scaffold model, a protein such as Sec16 resides at the ER surface, where it recruits and/or crosslinks COPII components. B: According to the tER-Golgi tethering model, proteins such as p115/Uso1 link COPII components to adjacent early Golgi or pre-Golgi membranes.
Sec16 is a regulator of COPII turnover and tER dynamics
Recent biochemical studies revealed that Sec16 can slow Sar1 GTPase activity, suggesting that Sec16 acts as a negative regulator of COPII turnover [26, 27]. In vivo support for this idea comes from our finding that displacement of P. pastoris Sec16 to the cytosol accelerates COPII turnover, causing tER sites to form and shrink at a faster rate [28]. These effects are reversed by expressing a dominant inhibitory mutant of Sar1. It seems likely that Sec16 suppresses Sar1 GTPase activity in order to stabilize the assembling COPII coat and promote efficient vesicle formation [6, 28].
Thus, Sec16 has been proposed to have two distinct functions—organizing COPII assembly, and regulating COPII turnover—but our analysis of P. pastoris Sec16 leads to a simpler interpretation. Deletion of the CCD from P. pastoris Sec16 has very little effect on cell growth, Sec16 localization, or tER structure, so the CCD is not actually required to nucleate COPII assembly. In P. pastoris, the major COPII-binding region of Sec16 is also the region that mediates tER localization, and displacement of COPII from tER sites also displaces Sec16 [28]. The implication is that instead of Sec16 recruiting COPII to tER sites, COPII assembles at tER sites and then recruits Sec16 as a regulator.
This model explains why the association of P. pastoris Sec16 with tER sites is saturable [17], and why displacement of P. pastoris Sec16 to the cytosol does not abolish tER sites [28]. We postulate that loss of Sec16 changes the appearance of tER sites by accelerating COPII turnover. Thus, when P. pastoris Sec16 is inactivated, tER sites become smaller and more numerous due to their abnormally fast dynamics. According to this view, P. pastoris Sec16 has a single major function in regulating COPII turnover, and there is no compelling reason to think that this protein has an additional role in organizing COPII.
Can this revised conclusion be extended to metazoan Sec16 proteins? The jury is still out. Protein domain analysis suggests that the tER localization mechanism of P. pastoris Sec16 [28] is similar to that of mammalian and Drosophila Sec16 [19, 21, 23], consistent with the idea that COPII recruits Sec16 to tER sites in metazoans as well. This effect may only become apparent with methods that remove virtually all of the COPII from tER sites [28]. Alternatively, if metazoan Sec16 binds to the ER and establishes tER sites upstream of COPII, the molecular basis of this pathway will need to be demonstrated. For now, the conservative interpretation is that Sec16 serves only to regulate COPII turnover and tER dynamics.
tER sites are associated with early Golgi or pre-Golgi structures
If Sec16 is not the glue that keeps COPII proteins at tER sites, then what other component could perform this function? An attractive candidate is early Golgi membranes. In many cell types, tER sites are closely associated with Golgi stacks [3]. For example, in P. pastoris, tER sites form, fuse, and move in conjunction with Golgi stacks, because both tER sites and Golgi stacks are embedded in a continuous ribosome-excluding matrix [2, 7] (Fig. 1). The secretory system of P. pastoris therefore consists of integrated tER-Golgi units. Similarly, in plant cells, mobile Golgi stacks are tethered to the ER, with COPII proteins concentrated at the ER-Golgi interface [29, 30]. Mammalian cells are superficially different because many of the tER sites are distant from the juxtanuclear Golgi, but mammalian tER sites are stably associated with pre-Golgi membranes of the ER-Golgi intermediate compartment (ERGIC) [5, 31]. Even in Saccharomyces cerevisiae, which has a fragmented secretory system consisting of small tER sites and non-stacked Golgi cisternae, many of the tER sites are next to early Golgi cisternae [32, 33]. These observations suggest that the association of tER sites with early Golgi or pre-Golgi membranes may be universal.
Early Golgi or pre-Golgi membranes could act as a framework for organizing tER sites (Fig. 2B). In this scenario, early Golgi or pre-Golgi membranes are tethered to nascent and completed COPII vesicles, thereby constraining the COPII components to the tER site and the immediately adjacent region of cytoplasm. This concept fits with evidence that COPII vesicles are restricted to the tER-Golgi interface [2, 30, 34] and can associate with early Golgi membranes prior to uncoating [35]. It seems likely that COPII vesicles do not diffuse freely, but instead become attached to their targets as they form [36]. A typical tER site in a mammalian or P. pastoris cell contains about 4–15 budding or completed COPII vesicles [2, 32, 36], providing the opportunity for multiple tethering interactions. Tethering presumably occurs with assembled COPII coats and not with free COPII subunits, in a process that may be regulated by phosphorylation of COPII coat proteins [37]. In addition, tethers may bind to other COPII vesicle components such as Rabs and SNAREs [38].
The model shown in Fig. 2B can explain the observation that in mammalian cells, a single ERGIC element can associate with multiple tER sites from distantly connected ER regions [36]. Apparently, tethering of the ERGIC element to nascent COPII vesicles generates not only two-dimensional tER sites, but also a three-dimensional “export complex”.
Various components could participate in tethering to COPII vesicles [35]. One such component is a conserved coiled-coil protein known as p115 in mammalian cells or Uso1 in yeast. p115/Uso1 is potentially long enough to link Golgi membranes to nascent COPII vesicles. For example, the coiled-coil portion of Uso1 is ~150 nm in length [39], and the distance between the ER surface and the first Golgi cisterna in P. pastoris is ~100 nm (Fig. 1). CASP (Coy1 in yeast) is also a conserved coiled-coil protein that may participate in tethering at the ER-Golgi interface [40, 41]. Another component is the multi-subunit TRAPP complex, which binds to Golgi membranes and also to the COPII coat protein Sec23. In some cells, the tER-Golgi interface includes other putative tethers such as the yeast GRASP protein Grh1 [32] and the animal cell protein TFG-1 [42].
The tER-Golgi tethering model predicts that if the tER-Golgi association were lost, tER organization would be disrupted. Such a result was observed after RNAi-mediated depletion of p115 in Drosophila S2 cells [43]. During the course of p115 depletion, tER sites progressively lost their association with Golgi membranes, and at the same time, tER sites broke down into smaller structures. If this finding can be extended to other cell types, it will provide evidence that clustering of COPII at tER sites requires linkage to early Golgi or pre-Golgi membranes.
Integrated self-organization of associated compartments
Association between two compartments is a well-known way to generate membrane domains. Examples include the nucleus-vacuole junction as well as contact sites between the ER and other organelles [44]. Membrane domains can also be established by cell-cell interactions, as exemplified by neural and immunological synapses [45]. However, in those cases the apposing compartments have an independent existence, whereas tER and Golgi compartments are more intimately related because tER sites appear to nucleate the formation of Golgi cisternae [15]. Golgi cisternae may return the favor by helping to define tER sites. Thus, self-organization is proposed to occur at the level of an integrated unit consisting of a tER site and its associated early Golgi or pre-Golgi membranes.
The details of such a self-organization process are speculative, but a reasonable scenario can be envisioned. As COPII vesicles bud from the ER, they tether and fuse with one another to generate pre-Golgi membranes [15]. The pre-Golgi membranes remain temporarily linked to the ER, thereby clustering COPII components to form a tER site. This tER site continues to nucleate new pre-Golgi membranes, which in turn continue to tether and cluster COPII, leading to a dynamic but long-lived tER-Golgi unit.
Higher levels of organization can be achieved by linking tER-Golgi units to other cellular structures. In vertebrate cells, microtubule-dependent transport generates a ribbon of interconnected Golgi stacks. In many other organisms, individual tER-Golgi units show distinctive positioning [3]. Centrins appear to play a role in tER-Golgi positioning in Trypanosoma brucei [46]. It seems likely that different cell types use a variety of mechanisms to position tER-Golgi units.
Testing the model
A central prediction of the new model is that a tER site will be stable only if it is associated with early Golgi or pre-Golgi membranes. To falsify this model, one could seek to identify conditions in which tER sites exist stably when the Golgi is absent. Novel approaches would be needed because previous treatments with Golgi-perturbing drugs have been inconclusive. For example, brefeldin A disrupts the Golgi without substantially perturbing tER sites, but the tER sites in those cells remain associated with ERGIC-like membranes [47]. Moreover, when the mammalian Golgi ribbon is fragmented by depolymerizing microtubules with nocodazole, the individual Golgi stacks are closely associated with tER sites [5]. Thus, tER sites have not been shown to exist on their own.
As described above, a promising strategy is to characterize and disrupt the matrix that links tER sites to early Golgi or pre-Golgi membranes. The expectation is that loss of this matrix will eliminate stable tER sites. Another possible strategy builds on the observation that in P. pastoris, about half of the tER sites are on the nuclear envelope, which is part of the ER [8]. Purified nuclei from P. pastoris might therefore be a good experimental system to study the requirements for tER site assembly.
Conclusions
Cell biologists typically take a reductionist approach when trying to understand the properties of a specific cellular compartment. This perspective led to the assumption that clustering of COPII at tER sites must be due to an upstream organizer. Sec16 emerged as a candidate for such an organizer, but reevaluation of the evidence suggests that the main role of Sec16 is actually to regulate COPII turnover and tER dynamics. tER sites may not in fact be stand-alone membrane domains. Instead, they may be part of integrated self-organization units that include early Golgi or pre-Golgi membranes. Tests of this model will help us to understand how the cell creates the compartments of the secretory pathway.
Acknowledgments
Thanks to members of the Glick lab for helpful discussion. This work was supported by NIH grant R01 GM061156.
Abbreviations
- CCD
central conserved domain
- COPII
coat protein complex II
- ERGIC
ER-Golgi intermediate compartment
- tER
transitional ER
References
- 1.Bannykh SI, Rowe T, Balch WE. The organization of endoplasmic reticulum export complexes. J Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mogelsvang S, Gomez-Ospina N, Soderholm J, Glick BS, Staehelin LA. Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris. Mol Biol Cell. 2003;14:2277–2291. doi: 10.1091/mbc.E02-10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whaley WG. The Golgi Apparatus. Vienna: Springer-Verlag; 1975. p. 190. [Google Scholar]
- 4.Becker B, Melkonian M. The secretory pathway of protists: spatial and functional organization and evolution. Microbiol Rev. 1996;60:697–721. doi: 10.1128/mr.60.4.697-721.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond AT, Glick BS. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol Biol Cell. 2000;11:3013–3030. doi: 10.1091/mbc.11.9.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlowe CK, Miller EA. Secretory protein biogenesis and traffic in the early secretory pathway. Genetics. 2013;193:383–410. doi: 10.1534/genetics.112.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bevis BJ, Hammond AT, Reinke CA, Glick BS. De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat Cell Biol. 2002;4:750–756. doi: 10.1038/ncb852. [DOI] [PubMed] [Google Scholar]
- 8.Rossanese OW, Soderholm J, Bevis BJ, Sears IB, O’Connor J, Williamson EK, Glick BS. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J Cell Biol. 1999;145:69–81. doi: 10.1083/jcb.145.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephens DJ. De novo formation, fusion and fission of mammalian COPII-coated endoplasmic reticulum exit sites. EMBO Rep. 2003;4:210–217. doi: 10.1038/sj.embor.embor736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misteli T. The concept of self-organization in cellular architecture. J Cell Biol. 2001;155:181–185. doi: 10.1083/jcb.200108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palade GE. Membrane biogenesis: an overview. Methods Enzymol. 1983;96:xxix–lv. doi: 10.1016/s0076-6879(83)96004-4. [DOI] [PubMed] [Google Scholar]
- 12.Warren G, Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- 13.Glick BS, Nakano A. Membrane traffic within the Golgi stack. Annu Rev Cell Dev Biol. 2009;25:113–132. doi: 10.1146/annurev.cellbio.24.110707.175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glick BS. Can the Golgi form de novo? Nat Rev Mol Cell Biol. 2002;3:615–619. doi: 10.1038/nrm877. [DOI] [PubMed] [Google Scholar]
- 16.Jean S, Kiger AA. Coordination between RAB GTPase and phosphoinositide regulation and functions. Nat Rev Mol Cell Biol. 2012;13:463–470. doi: 10.1038/nrm3379. [DOI] [PubMed] [Google Scholar]
- 17.Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS. Sec16 is a determinant of transitional ER organization. Curr Biol. 2005;15:1439–1447. doi: 10.1016/j.cub.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 18.Shaywitz DA, Espenshade PJ, Gimeno RE, Kaiser CA. COPII subunit interactions in the assembly of the vesicle coat. J Biol Chem. 1997;272:25413–25416. doi: 10.1074/jbc.272.41.25413. [DOI] [PubMed] [Google Scholar]
- 19.Hughes H, Budnik A, Schmidt K, Palmer KJ, Mantell J, Noakes C, Johnson A, Carter DA, Verkade P, Watson P, Stephens DJ. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci. 2009;122:2924–2934. doi: 10.1242/jcs.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iinuma T, Shiga A, Nakamoto K, O’Brien MB, Aridor M, Arimitsu N, Tagaya M, Tani K. Mammalian Sec16/p250 plays a role in membrane traffic from the endoplasmic reticulum. J Biol Chem. 2007;282:17632–17639. doi: 10.1074/jbc.M611237200. [DOI] [PubMed] [Google Scholar]
- 21.Ivan V, de Voer G, Xanthakis D, Spoorendonk KM, Kondylis V, Rabouille C. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol Biol Cell. 2008;19:4352–4365. doi: 10.1091/mbc.E08-03-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montegna EA, Bhave M, Liu Y, Bhattacharyya D, Glick BS. Sec12 binds to Sec16 at transitional ER sites. PLoS ONE. 2012;7:e31156. doi: 10.1371/journal.pone.0031156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharyya D, Glick BS. Two mammalian Sec16 homologs have nonredundant functions in ER export and transitional ER organization. Mol Biol Cell. 2007;18:839–849. doi: 10.1091/mbc.E06-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes H, Stephens DJ. Sec16A defines the site for vesicle budding from the endoplasmic reticulum on exit from mitosis. J Cell Sci. 2010;123:4032–4038. doi: 10.1242/jcs.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittle JRR, Schwartz TU. Structure of the Sec13-Sec16 edge element, a template for assembly of the COPII vesicle coat. J Cell Biol. 2010;190:347–361. doi: 10.1083/jcb.201003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yorimitsu T, Sato K. Insights into structural and regulatory roles of Sec16 in COPII vesicle formation at ER exit sites. Mol Biol Cell. 2012 doi: 10.1091/mbc.E12-05-0356. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kung LF, Pagant S, Futai E, D’Arcangelo JG, Buchanan R, Dittmar JC, Reid RJ, Rothstein R, Hamamoto S, Snapp EL, Schekman R, Miller EA. Sec24p and Sec16p cooperate to regulate the GTP cycle of the COPII coat. EMBO J. 2011;31:1014–1027. doi: 10.1038/emboj.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharucha N, Liu Y, Papanikou E, McMahon C, Esaki M, Jeffrey PD, Hughson FM, Glick BS. Sec16 influences transitional ER sites by regulating rather than organizing COPII. Mol Biol Cell. 2013 doi: 10.1091/mbc.E13-04-0185. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.daSilva LL, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F. Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell. 2004;16:1753–1771. doi: 10.1105/tpc.022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staehelin LA, Kang BH. Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol. 2008;147:1454–1468. doi: 10.1104/pp.108.120618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- 32.Levi SK, Bhattacharyya D, Strack RL, Austin JRI, Glick BS. The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic. 2010;11:1168–1179. doi: 10.1111/j.1600-0854.2010.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto M, Kurokawa K, Matsuura-Tokita K, Saito C, Hirata R, Nakano A. High-curvature domains of the ER are important for the organization of ER exit sites in Saccharomyces cerevisiae. J Cell Sci. 2012;125:3412–3420. doi: 10.1242/jcs.100065. [DOI] [PubMed] [Google Scholar]
- 34.Zeuschner D, Geerts WJ, van Donselaar E, Humbel BM, Slot JW, Koster AJ, Klumperman J. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat Cell Biol. 2006;8:377–383. doi: 10.1038/ncb1371. [DOI] [PubMed] [Google Scholar]
- 35.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Bannykh SI, Balch WE. Membrane dynamics at the endoplasmic reticulum-Golgi interface. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhandari D, Zhang J, Menon S, Lord C, Chen S, Helm JR, Thorsen K, Corbett KD, Hay JC, Ferro-Novick S. Sit4p/PP6 regulates ER-to-Golgi traffic by controlling the dephosphorylation of COPII coat subunits. Mol Biol Cell. 2013;24:2727–2738. doi: 10.1091/mbc.E13-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bröcker C, Engelbrecht-Vandré S, Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Curr Biol. 2010;20:R943–R952. doi: 10.1016/j.cub.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Yamakawa H, Seog DH, Yoda K, Yamasaki M, Wakabayashi T. Uso1 protein is a dimer with two globular heads and a long coiled-coil tail. J Struct Biol. 1996;116:356–365. doi: 10.1006/jsbi.1996.0053. [DOI] [PubMed] [Google Scholar]
- 40.Gillingham AK, Pfeifer AC, Munro S. CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a Golgi membrane protein related to giantin. Mol Biol Cell. 2002;13:3761–3774. doi: 10.1091/mbc.E02-06-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osterrieder A. Tales of tethers and tentacles: golgins in plants. J Microsc. 2012;247:68–77. doi: 10.1111/j.1365-2818.2012.03620.x. [DOI] [PubMed] [Google Scholar]
- 42.Witte K, Schuh AL, Hegermann J, Sarkeshik A, Mayers JR, Schwarze K, Yates JR, 3rd, Elmer S, Audhya A. TGF-1 function in protein secretion and oncogenesis. Nat Cell Biol. 2011;13:550–558. doi: 10.1038/ncb2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondylis V, Rabouille C. A novel role for dp115 in the organization of tER sites in Drosophila. J Cell Biol. 2003;162:185–198. doi: 10.1083/jcb.200301136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elbaz Y, Schuldiner M. Staying in touch: the molecular era of organelle contact sites. Trends Biochem Sci. 2011;36:616–623. doi: 10.1016/j.tibs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Dustin ML, Colman DR. Neural and immunological synaptic relations. Science. 2002;298:785–789. doi: 10.1126/science.1076386. [DOI] [PubMed] [Google Scholar]
- 46.He CY, Pypaert M, Warren G. Golgi duplication in Trypanosoma brucei requires Centrin2. Science. 2005;310:1196–1198. doi: 10.1126/science.1119969. [DOI] [PubMed] [Google Scholar]
- 47.Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol. 2001;155:557–570. doi: 10.1083/jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]