Abstract
Adipocyte-specific secretory factor (ADSF)/resistin is a small cysteine-rich protein secreted from adipose tissue that belongs to a gene family found in inflammatory zone (FIZZ) or found in resistin-like molecule (RELM). ADSF has been implicated in modulating adipogenesis and insulin resistance. To examine the long-term function of ADSF in adipogenesis and glucose homeostasis, we constructed an expression vector for a dominant inhibitory form of ADSF by fusing it to the human IgGγ constant region (hFc). ADSF-hFc not only homodimerizes but heterooligomerizes with ADSF/resistin and prevents ADSF/resistin inhibition of adipocyte differentiation of 3T3-L1 cells in a dominant negative manner. Transgenic mice overexpressing ADSF-hFc in adipose tissue show increased adiposity with elevated expression of adipocyte markers as well as enlarged adipocyte size. This finding clearly demonstrates in vivo the inhibitory role of ADSF in adipogenesis. ADSF-hFc transgenic mice with impaired ADSF function exhibit improved glucose tolerance and insulin sensitivity either on chow or high-fat diets. Because of the enhanced adipocyte differentiation, the ADSF-hFc transgenic mice show increased expression of leptin and adiponectin in adipose tissue. The elevated circulating levels for these adipocyte-derived hormones with decreased plasma triglyceride and free fatty acid levels may account for the improved glucose and insulin tolerance in these transgenic mice.
Keywords: ADSF-hFc, differentiation, transgenic, mice
Obesity is a prevalent health hazard in industrialized countries and is closely associated with insulin resistance and type 2 diabetes. Obesity arises from increased size of adipocytes caused by lipid accumulation and from an increased number of adipocytes arising from differentiation of adipose precursor cells to mature adipocytes under the appropriate nutritional and hormonal conditions (1). Gene expression studies during adipocyte differentiation have firmly established that peroxisome proliferator-activated receptor γ (PPARγ) and the CCAAT enhancer-binding protein (C/EBP) family of transcription factors play central roles in adipocyte differentiation (2, 3). However, various factors in cell-cell and cell-matrix communications govern expression of the adipocyte transcription factors and therefore regulate conversion of preadipocytes to adipocytes (4). Although adipose tissue serves as the major energy reservoir in higher eukaryotes, the role of adipose tissue as a secretory organ has emerged through the discovery of leptin (5). In addition to leptin, other secretory factors including adiponectin and tumor necrosis factor α, as well as preadipocyte-specific preadipocyte factor 1 (Pref-1) are known to be secreted from adipose tissue (6-8). These factors are involved in regulating a variety of physiological functions including satiety and energy metabolism, as well as adipocyte differentiation. In this regard, we have shown that Pref-1, which is secreted specifically by preadipocytes, inhibits adipocyte differentiation (8-10).
We previously identified adipocyte-specific secretory factor (ADSF) as an adipocyte-secreted factor that inhibits adipocyte differentiation of 3T3-L1 cells in vitro (11). ADSF expression in adipose tissue is tightly controlled by the nutritional and hormonal status of animals. We reported that ADSF mRNA levels were very low during fasting and increased drastically when fasted animals were refed a high-carbohydrate diet. ADSF mRNA levels were also low in streptozotocin-diabetes but increased by insulin treatment. Therefore, we hypothesized that ADSF may be an adipose sensor for the nutritional state of the animals and that inhibition of adipocyte differentiation by ADSF implicates its function as a feedback regulator of adipogenesis. At the same time, Steppan et al. (12) also identified ADSF as an adipocyte-secreted hormone that was suppressed by insulin-sensitizing PPARγ agonist thiazolidinediones, and proposed that ADSF functions for insulin resistance, thus linking obesity and diabetes. They reported that mice treated with ADSF had increased insulin resistance and therefore named this molecule resistin. In addition, ADSF was originally identified by Holcomb et al. (13) as FIZZ3 (found in inflammatory zone 3), a member of protein family FIZZ by an EST database search against FIZZ1, which is induced during lung inflammation. Therefore, ADSF belongs to a FIZZ/resistin-like molecule (RELM) family of proteins consisting of FIZZ1/RELMα, FIZZ2/RELMβ, and RELMγ (14, 15). ADSF exists as both monomer and homodimer. ADSF also forms heterooligomer with FIZZ1/RELMα or FIZZ2/RELMβ (16, 17). Interestingly, another member, FIZZ1/RELMα, has also been shown to inhibit adipocyte differentiation of 3T3-L1 preadipocytes in vitro (17). In addition, FIZZ2/RELMβ was reported to have similar action to ADSF in insulin resistance (18).
Although the effect of acute administration of ADSF on insulin sensitivity has been studied (12), there are no known reports on the effect of ADSF on adipogenesis and its consequence in glucose homeostasis in vivo. With the inhibitory effect of ADSF on adipocyte differentiation in vitro, it would be necessary to examine the long-term effect of ADSF in vivo to understand the physiological role of ADSF in adipogenesis as well as insulin resistance. Here, we generated transgenic mice overexpressing ADSF fused to the human IgGγ constant region (hFc) in adipose tissue. We found that ADSF-hFc functions in a dominant negative manner resulting in prevention of ADSF-mediated inhibition of adipocyte differentiation of 3T3-L1 cells in vitro. Transgenic mice overexpressing ADSF-hFc in adipose tissue showed increased adiposity with enhanced adipogenesis. Despite the development of obesity, these ADSF-hFc transgenic mice had improved glucose disposal and insulin sensitivity with increased plasma leptin and adiponectin levels, but with decreased plasma free fatty acids and triglyceride levels, suggesting the contribution of the long-term effect of ADSF on enhanced adipogenesis and its effect on insulin sensitivity.
Materials and Methods
Cell Culture. The 3T3-L1 cells were cultured in media containing 75% conditioned media collected from COS7 cells transiently transfected with various expression vectors and 25% DMEM with 10% FBS and were differentiated as described (11).
Construction of Plasmids and Generation of Transgenic Mice. A hemagglutinin (HA)-tagged full-length ADSF fragment was prepared by PCR amplification as described (11). ADSF-hFc fusion protein with EagI site at the 3′ end was ligated in frame to the RT-PCR product of the human Fc region sequence encoding the C-terminal 235 aa, then ligated into pCR3.1 (Invitrogen). The ADSF-hFc gene was cloned into a vector containing the 5.4-kb aP2 promoter/enhancer and used for the generation of ADSF-hFc transgenic mice. Food intake was determined every day for 7 consecutive days. Indirect calorimetry was performed in the Jackson Laboratory at the University of California, Davis.
Immunoprecipitation and Western Blot Analysis. Conditioned media were incubated with anti-hFc agarose (Sigma) overnight at 4°C, and, after washing, the precipitated proteins were resolved by 15% SDS/PAGE. Serum (10 μl) or white adipose tissue (WAT) lysates were subjected to 15% SDS/PAGE and transferred to nitrocellulose followed by immunoblot analysis with guinea pig anti-mouse ADSF antibody (Linco Research Immunoassay, St. Charles, MO) or goat anti-human IgG conjugated with peroxidase.
Northern Blot Analysis and RT-PCR. Total RNA (10 μg) prepared by using TRIzol (Invitrogen) was subjected to Northern blot analysis as described (11). Total RNA was reverse transcribed with Super-Script II (Invitrogen). Primers for RT-PCR were designed from nucleotide sequences deposited in a National Center for Biotechnology Information database.
Glucose Tolerance Tests (GTTs) and Insulin Tolerance Tests (ITTs) and Measurement of Plasma Hormone and Metabolite Levels. GTTs and ITTs and plasma triglycerides, cholesterol, free fatty acids, leptin, and adiponectin levels were analyzed as described (10). Triglyceride content in liver and muscle was determined by the modified method of Bligh and Dyer (19).
Results
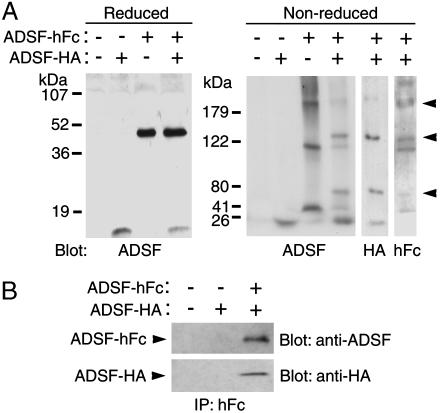
ADSF-hFc Functions as a Dominant Negative ADSF to Enhance Adipocyte Differentiation in Vitro. We used human IgGγ heavy chain Fc region to generate ADSF-hFc fusion protein to investigate the long-term physiological effect of ADSF in adipogenesis and its effect on insulin resistance. We hypothesized that hFc fusion not only enhances oligomerization and protein stability (10, 20, 21) but may also make ADSF-hFc heterodimerize with the endogenous ADSF. Because of the relatively smaller size of ADSF, fusion to a larger Fc protein may make it interfere with endogenous ADSF function. We first tested whether ADSF-hFc can form heterodimer with ADSF. Expression vectors for ADSF tagged with HA and ADSF-hFc were transfected separately or together into COS7 cells, and the conditioned media were collected and analyzed by Western blotting with antibodies against ADSF, HA, or hFc. Under reducing conditions, ADSF-HA and ADSF-hFc migrated as a monomer of 12.5 and 40 kDa, respectively (Fig. 1A). Under nonreducing conditions, ADSF-HA migrated as a dimer, whereas ADSF-hFc migrated as multiple oligomers. Furthermore, a series of novel heterooligomers consisting of ADSF-HA and ADSF-hFc were detected when these two forms were coexpressed in COS7 cells (Fig. 1A). We also found that ADSF-HA was coimmunoprecipitated with ADSF-hFc, suggesting that ADSF-hFc can bind ADSF-HA, resulting in the formation of multiple heterooligomers (Fig. 1B).
Fig. 1.
Binding of ADSF-hFc to ADSF. 3T3-L1 cells were differentiated in conditioned media from COS7 cells transfected with pcDNA3.1 control vector or ADSF expression vectors. (A) Conditioned media containing ADSF-HA, ADSF-hFc, or both ADSF-HA and ADSF-hFc were subjected to reducing (15%) or nonreducing (6%) SDS/PAGE followed by Western blot analysis with antibodies against ADSF, HA, or human Fc fragment. (B) Conditioned media containing ADSF-HA or ADSF-HA and ADSF-hFc were immunoprecipitated by using anti-hFc agarose beads followed by Western blot analysis with antibodies against ADSF and HA.
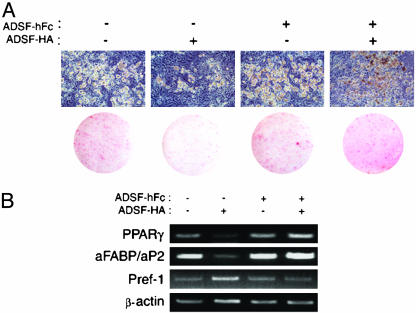
Next, to test possible dominant negative function of ADSF-hFc, we examined the effect of ADSF-hFc on adipocyte differentiation. Conditioned media harvested from COS7 cells transfected with expression vectors for ADSF-HA, ADSF-hFc, or both were used. 3T3-L1 cells were treated with dexamethasone (Dex) and 1-methylisobutylxanthine (MIX) for 2 days, and the cells were further maintained without the drugs for 7 days. Conditioned media were used during adipocyte differentiation. Judged by cell morphology and Oil red O staining, ≈70% of cells were differentiated into adipocytes when the cells were maintained in conditioned media from COS7 cells transfected with control empty vector (Fig. 2A). In agreement with our previous study (11), 3T3-L1 cells treated with conditioned media containing ADSF-HA showed inhibition of adipocyte differentiation with fibroblastic morphology and little lipid accumulation with only ≈10% of the cells differentiating into adipocytes. However, the differentiation of the cells treated with conditioned media containing ADSF-hFc was slightly higher than the cells treated with control media. Furthermore, the differentiation of the 3T3-L1 cells treated with conditioned media containing both ADSF-HA and ADSF-hFc was not attenuated. This was probably caused by the prevention of the inhibitory function of ADSF-HA in adipocyte differentiation caused by ADSF-hFc binding to ADSF-HA. We also addressed the effect of ADSF-hFc on adipocyte differentiation at the molecular level. RT-PCR analysis for an adipocyte transcription factor, PPARγ, and a late differentiation marker, aFABP/aP2, as well as a preadipocyte marker, Pref-1, was performed by using mRNA prepared from 3T3-L1 cells differentiated into adipocytes in the presence of various conditioned media. 3T3-L1 cells treated with conditioned media containing ADSF-HA exhibited lower expressions of PPARγ and adipocyte fatty acid-binding protein (aFABP)/aP2 and increased Pref-1 expression compared with those treated with control media, indicating the inhibitory effect of ADSF-HA in adipocyte differentiation. On the other hand, 3T3-L1 cells treated with conditioned media containing ADSF-hFc, or both ADSF-HA and ADSF-hFc, had similar or slightly higher expression of adipocyte markers with reduced Pref-1 expression compared with cells treated with control media (Fig. 2B). These results clearly show that ADSF-hFc functions in a dominant negative manner and prevents the inhibitory function of ADSF on adipocyte differentiation of 3T3-L1 cells in vitro.
Fig. 2.
Effect of conditioned media from COS7 cells transfected with ADSFHA, ADSF-hFc, or ADSF-HA and ADSF-hFc on 3T3-L1 adipocyte differentiation. (A) Microscopic pictures for cell morphology and Oil red O staining of 3T3-L1 cells after subjection to differentiation protocol. (B) Adipocyte markers (PPARγ and aFABP/aP2) and a preadipocyte marker (Pref-1) were analyzed by RT-PCR by using total RNA isolated from conditioned-medium-treated 3T3-L1 adipocytes. β-Actin was used as control.
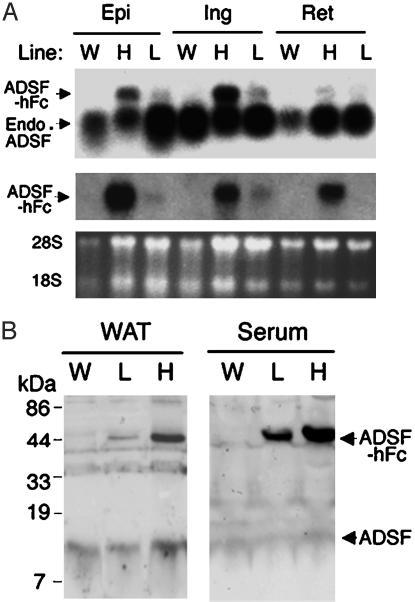
Generation of ADSF-hFc Transgenic Mice. To examine the long-term physiological significance of ADSF in adipogenesis, we generated transgenic mice expressing ADSF-hFc in adipose tissue. Two lines of ADSF-hFc transgenic mice (lines L and H for mice expressing low and high levels of transgene, respectively) were studied. Mice of both lines were viable throughout adulthood without apparent abnormality. ADSF-hFc expression in both lines was restricted to WAT, including epididymal, inguinal, and retroperitoneal fat pads (Fig. 3A), and brown adipose tissue; other tissues we examined included brain, heart, kidney, liver, lung, and skeletal muscle (data not shown). We next examined the cellular and plasma levels of ADSF-hFc fusion protein in both lines of transgenic as well as wild-type mice using anti-ADSF antibody. The endogenous ADSF protein level in WAT was similar in wild-type and transgenic mice, although line H had a slightly higher level of endogenous ADSF than wild-type mice, possibly because of the increased adipogenesis resulting from attenuated ADSF function by ADSF-hFc binding. The level of ADSF-hFc fusion protein expressed in WAT from both lines of transgenic mice was slightly higher than that of endogenous ADSF. However, circulating levels of ADSF-hFc fusion protein were greatly elevated as compared with the endogenous ADSF level, probably because of the increased protein stability of circulating ADSF-hFc fusion protein caused by the presence of hFc. Line H of ADSF-hFc transgenic mice showed 5-fold-higher circulating ADSF-hFc fusion protein levels compared with those shown in line L (Fig. 3B).
Fig. 3.
ADSF-hFc expression in transgenic mice. (A) Northern blot analysis in lines H and L of ADSF-hFc mice and wild-type (W) mice. Total RNA was extracted from the tissues of 10-week-old mice and probed with radiolabeled ADSF (Top) or hFc (Middle) cDNA probes. Epi, epididymal fat pad; Ing, inguinal fat pad; Ret, retroperitoneal fat pad; Endo, endogenous. (B) Western blot analysis for ADSF-hFc fusion protein in serum from lines H and L of ADSF-hFc and wild-type mice. The serum samples were subjected to SDS/PAGE and probed with ADSF antibody. The antibody detected both the 40 kDa of ADSDF-hFc fusion protein and the 12.5 kDa of endogenous ADSF in transgenic mice, but only the 12.5 kDa of ADSF was detected in wild-type mice. Positions of 28S and 18S rRNA are shown.
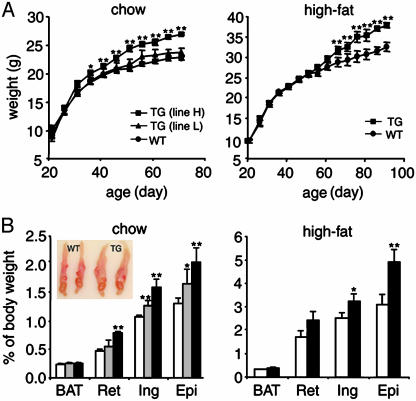
Increased Adipogenesis in ADSF-hFc Transgenic Mice. To test the function of ADSF-hFc in adipogenesis, we used ADSF-hFc transgenic and wild-type mice fed a chow or high-fat diet for 10 weeks. Regardless of the type of diet, ADSF-hFc transgenic mice developed a marked increase in body weight in comparison with wild-type mice. The difference in body weight between transgenic and wild-type mice became apparent after 30 days of age (Fig. 4A). At 10 weeks of age, line H of ADSF-hFc transgenic mice fed a chow diet had an average 20.3% increase in body weight relative to wild-type littermates. Although statistically not significant, line L also exhibited slightly increased body weight compared with wild-type littermates over the 10 weeks of study. However, we observed no significant differences in food intake (transgenic versus wild-type mice: 3.98 ± 0.04 and 3.80 ± 0.16 g/g of body weight per day), oxygen consumption (transgenic versus wild-type mice: 3016.05 ± 252.89 and 2925.63 ± 121.54 ml of O2/kg/hr), or respiratory quotient (transgenic versus wild-type mice: 0.75 ± 0.04 and 0.74 ± 0.02) between transgenic and wild-type littermates (n = 4 for each group). Body weights of those mice fed a high-fat diet were also measured. Line H of ADF-hFc transgenic mice showed ≈23% higher body weight than wild type at 10 weeks of age (Fig. 4A). We then investigated whether the increased body weight in transgenic mice was associated with the change in relative weight of individual fat pads and other organs. There was no significant difference in weights of other organs, expressed as percentage of body weight (transgenic versus wild-type mice: liver, 4.70 ± 0.07 and 4.67 ± 0.10; kidney, 1.35 ± 0.06 and 1.46 ± 0.04; lung, 0.82 ± 0.06 and 0.67 ± 0.02; heart, 0.52 ± 0.02 and 0.52 ± 0.01; thymus, 0.19 ± 0.01 and 0.21 ± 0.02, respectively). At 10 weeks of age, line L of ADSF-hFc mice had 19%, 22%, and 27% higher retroperitoneal, inguinal, and epididymal fat pad weights as expressed in percent of body weight, respectively, compared with those of wild-type littermates. Line H of ADSF-hFc transgenic mice had 68%, 48%, and 56% higher retroperitoneal, inguinal, and epididymal fat pad weights, respectively, compared with wild-type littermates (Fig. 4B). The major fat pad weights of transgenic and wild-type mice on a high-fat diet were also examined. The ADSF-hFc transgenic mice on a high-fat diet also had higher retroperitoneal, inguinal, and epididymal fat pad weights compared with wild-type littermates (39%, 29%, and 63%, respectively; Fig. 4B). We did not, however, observe any difference in the brown adipose tissue weight of transgenic and wild-type mice. A representative enlarged epididymal fat pad from transgenic mice compared with that from wild-type littermates is shown in Fig. 4B. These results indicate that the increase in fat pad weights in both transgenic lines paralleled the increase in body weights, suggesting that the change in body weight is mainly attributed to increased adipose tissue mass. Furthermore, the magnitude of increase in white fat pad weights was positively correlated with the level of transgene expression.
Fig. 4.
Growth curves and fat pad weights of ADSF-hFc transgenic and wild-type mice. (A) Body weight of male mice on a chow or high-fat diet was measured at 5-day intervals; each point represents mean ± SEM; n = 8-9 for each group. (B) Fat pad weights of 10-week-old transgenic mice of line H (filled bar), line L (hatched bar), and wild-type littermates (open bar) are presented as percentage of body weight (n = 8-14 for each group). BAT, brown adipose tissue; Ret, retroperitoneal fat pad; Ing, inguinal fat pad; Epi, epididymal fat pad. Results are mean ± SEM. Statistically significant differences between the groups are indicated: *, P < 0.05. **, P < 0.01. (Inset) Epididymal fat pads from 10-week-old mice of line H and wild-type littermates.
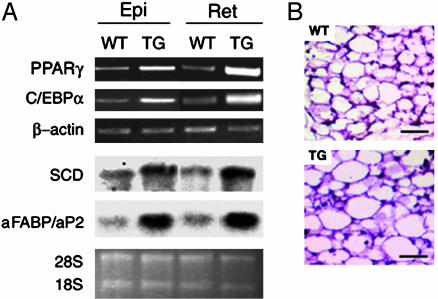
Adipose cell differentiation involves changes in the expression of genes that result in the acquisition of fat cell phenotype and adipocyte function. RT-PCR analysis of adipose tissues and epididymal and retroperitoneal fat pads from ADSF-hFc transgenic mice showed increased expression of adipocyte transcriptional factors PPARγ and CCAAT enhancer-binding protein α (C/EBPα). Expression of late markers of adipocyte differentiation, stearoyl-CoA desaturase (SCD) and aFABP/aP2 (measured by Northern blot analysis), was also increased in adipose tissues from ADSF-hFc transgenic mice (Fig. 5A). In addition, we also observed larger adipocyte cell size when we examined histological staining of representative epididymal WAT from line H of ADSF-hFc transgenic mice (Fig. 5B). Taken together, these observations suggest that the increased adiposity in ADSF-hFc transgenic mice reflects an enhanced adipogenesis as well as increased adipocyte cell size.
Fig. 5.
Adipocyte marker expression and histological analysis of adipose tissue in ADSF-hFc transgenic and wild-type mice. (A) PPARγ, C/EBPα, and β-actin mRNA levels were determined by RT-PCR using total RNA isolated from adipose tissues of 10-week-old ADSF-hFc and wild-type mice fed a chow diet. SCD and aFABP/aP2 were analyzed by Northern blot analysis using 10 μg of total RNA. Epi, epididymal fat pad; Ret, retroperitoneal fat pad. (B) Paraffin-embedded sections of epididymal WAT from 10-week-old mice were stained with hematoxylin and eosin. (Scale bar = 50 μm.) Positions of 28S and 18S rRNA are shown.
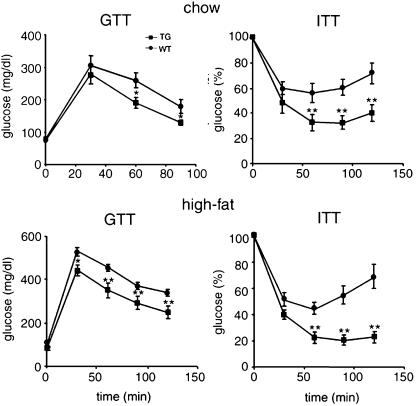
Improved Glucose Tolerance and Insulin Sensitivity in ADSF-hFc Transgenic Mice. Changes in adipose tissue mass are frequently associated with alterations in glucose homeostasis and insulin sensitivity. Furthermore, ADSF has been implicated in causing impairment in glucose tolerance and insulin action. We therefore carried out a GTT and ITT in mice fed a chow diet to determine whether insulin and glucose homeostasis were affected by ADSF-hFc expression. GTT revealed that the blood glucose levels after glucose injection were lower in ADSF-hFc transgenic mice (line H) than those in wild-type mice. This result suggests an improved glucose tolerance in ADSF-hFc mice. We next performed ITT (0.5 units/kg of body weight) in these mice. ADSF-hFc transgenic mice revealed a hypoglycemic response to insulin compared with wild-type littermates (Fig. 6). We also performed GTT and ITT in ADSF-hFc and wild-type mice fed a high-fat diet to examine the effect of diet-induced obesity on insulin action. Wild-type mice developed impaired glucose tolerance caused by the high-fat feeding, whereas ADSF-hFc transgenic mice still exhibited lower blood glucose levels during GTT. For ITT, we used a higher dosage of insulin (0.75 units/kg of body weight) to compare insulin sensitivity between ADSF-hFc transgenic and wild-type mice, because wild-type mice showed a severe insulin resistance at insulin dosage of 0.5 units/kg of body weight. We observed that ADSF-hFc transgenic mice fed a high-fat diet also had a hypoglycemic response to insulin injection compared with wild type as shown in mice on chow diet. Line L transgenic mice also showed slightly improved glucose tolerance and insulin sensitivity (data not shown). These results suggest that ADSF-hFc mice have resistance to high-fat diet-mediated glucose intolerance and insulin resistance and are protected from the development of obesity-induced insulin resistance (Fig. 6). Overall, expression of ADSF-hFc mice caused significant insulin sensitivity under both chow and high-fat diets when compared with wild-type littermates in a dose-dependent manner.
Fig. 6.
GTTs and ITTs in ADSF-hFc transgenic mice. For GTT, overnight-fasted mice that were fed either a chow or a high-fat diet were given an i.p. injection of glucose (2 mg/g of body weight). Blood samples from the tail were analyzed for glucose concentration. For ITT, mice on chow or high-fat diet were fasted for 5 h before i.p. administration of insulin, 0.5 units/kg of body weight and 0.75 units/kg of body weight, respectively. Glucose (%) represents percent of glucose concentration at 0 min. Each point represents mean ± SEM; n = 6-8 for each group. *, P < 0.05; **, P < 0.01.
Plasma and Tissue Metabolite Levels in ADSF-hFc Transgenic Mice. Next, we measured the levels of plasma metabolites because the changes in plasma triglyceride and free fatty acid levels are known to be related to diabetic status (22). Despite the development of obesity in ADSF-hFc transgenic mice, no significant differences were observed in the levels of fasted plasma glucose (transgenic versus wild-type mice: 52.30 ± 0.32 and 50.00 ± 1.05 mg/dl, respectively; n = 6-15) and cholesterol (transgenic versus wild-type mice: 77.03 ± 4.24 and 78.82 ± 7.76 mg/dl, respectively; n = 6-15) between ADSF-hFc transgenic mice and wild-type littermates. Surprisingly, the levels of plasma triglyceride (transgenic versus wild-type mice: 57.09 ± 3.91 and 69.13 ± 3.67 mg/dl, respectively; n = 6-15; P < 0.05) and free fatty acid (transgenic versus wild-type mice: 0.87 ± 0.05 and 1.08 ± 0.09 mmol/liter, respectively; n = 6-15; P < 0.05) were lower by 17% and 20% in ADSF-hFc transgenic mice compared with wild-type littermates, respectively. Although the ADSF-hFc transgenic mice had a substantial increase in adipose tissue mass with enhanced adipogenesis, they did not exhibit increased plasma triglycerides and free fatty acids, which are normally observed in other obesity mouse models. Obesity-mediated triglyceride deposition in insulin-sensitive tissues such as liver and muscle has been proposed as one of the mechanisms for insulin resistance associated with obesity as well as that of lipodystrophy syndrome (23). Therefore, we measured tissue triglyceride content of ADSF-hFc and wild-type mice and found similar levels of triglyceride deposition in the liver (transgenic versus wild-type mice: 61.01 ± 2.02 and 60.39 ± 3.95 mg/g of tissue, respectively; n = 6-15) and skeletal muscle (transgenic versus wild-type mice: 36.40 ± 3.71 and 36.12 ± 3.81 mg/g of tissue, respectively; n = 6-15). In an attempt to estimate the intramyocellular triglyceride content, we carried out lipid staining of the soleus muscle by using Oil red O but did not find significant difference in staining between ADSF-hFc transgenic and wild-type mice (data not shown). Our findings suggest that the adipose tissue from obese ADSF-hFc transgenic mice still has the ability to maintain normal tissue lipid homeostasis.
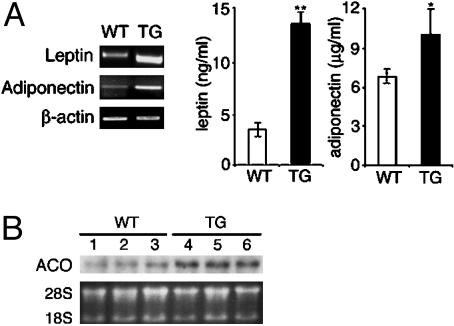
Previously known mechanisms by which adipocytes can regulate insulin action include the release of adipocyte-derived hormones that can affect insulin sensitivity, such as leptin and adiponectin. We thus measured both expression levels in adipose tissue and plasma levels for leptin and adiponectin in ADSF-hFc transgenic mice. RT-PCR showed that ADSF-hFc transgenic mice had ≈4-fold-higher leptin and adiponectin mRNA levels than wild-type littermates (Fig. 7A Left). ADSF-hFc transgenic mice also showed a 4-fold-higher plasma leptin and a 1.3-fold-higher plasma adiponectin compared with those of wild-type littermates (Fig. 7A Right). Northern blot analysis showed 3-fold-higher acyl-CoA oxidase (ACO) mRNA levels in skeletal muscle from ADSF-hFc transgenic mice as compared with wild-type littermates (Fig. 7B). These findings suggest that the increased insulin sensitivity observed in ADSF-hFc transgenic mice may have resulted from the enhanced adipogenesis and accompanying increase in leptin and adiponectin levels.
Fig. 7.
Characterization of mRNA and plasma levels of leptin and adiponectin and fatty acid metabolism in ADSF-hFc transgenic mice. (A) mRNA levels for leptin and adiponectin were measured by RT-PCR using total RNA isolated from epididymal WAT of 10-week-old mice fed a chow diet (Left). β-Actin was used as control. Plasma levels for leptin and adiponectin were measured from overnight-fasted 10-week-old mice fed a chow diet (Right). Results are mean ± SEM from 6-15 mice in each group. Statistically significant differences between the groups are indicated: *, P < 0.05; **, P < 0.01. (B) ACO mRNA levels were determined by Northern blot analysis using total RNA isolated from skeletal muscle of wild-type (lanes 1-3) and ADSF-hFc transgenic mice (lanes 4-6) fed a chow diet. WT, wild-type; TG, transgenic; ACO, acyl-CoA oxidase. Positions of 28S and 18S rRNA are shown.
Discussion
ADSF shares significant sequence homology with FIZZ1/RELMα, FIZZ2/RELMβ, and RELMγ. Although the physiological roles of FIZZ1/RELMα, FIZZ2/RELMβ, and RELMγ are unclear, previous reports have shown that ADSF is able to bind both lung and adipose tissue-specific FIZZ1/RELMα and intestine-specific FIZZ2/RELMβ. FIZZ1/RELMα has also been shown to inhibit adipocyte differentiation of 3T3-L1 preadipocytes in vitro (17). FIZZ2/RELMβ was reported to have similar action to ADSF in insulin resistance (18). These observations suggest a potential involvement of all FIZZs/RELMs in the regulation of adipogenesis as well as in insulin resistance (16, 17). Based on the fact that ADSF-hFc binds ADSF, it is plausible that ADSF-hFc would form protein complexes with not only the endogenous ADSF but also with other endogenous FIZZs/RELMs in ADSF-hFc transgenic mice. Thus, the phenotype shown in our study may have resulted from the binding of ADSF-hFc to FIZZs/RELMs members and may have provided dominant negative effects to their putative function in adipogenesis and insulin action. Therefore, our ADSF-hFc transgenic mice may have shown phenotype that may not be manifested in a single-gene loss-of-function mutation of ADSF.
Recent studies have shown conflicting results for the role of ADSF in obesity and insulin action in rodents and humans (24-26). Reports correlating expression levels for ADSF or circulating ADSF levels with genetic or diet-induced obesity in rodents were inconsistent (27-29). Furthermore, human studies attempting to demonstrate ADSF expression correlating with pathophysiology of obesity and type 2 diabetes (24-26) have been controversial at best. In this study, we demonstrate that ADSF-hFc functions in a dominant negative manner and prevents ADSF inhibition of adipocyte differentiation in vitro, possibly because of the presence of the bulky hFc protein at the C terminus of ADSF where all of the conserved cysteines are located. We also show that ADSF-hFc enhances adipogenesis in vivo. Mice overexpressing ADSF-hFc (line H) in adipose tissue showed ≈62% increase in total WAT percentage of body weight compared with wild-type littermates when fed a chow diet and ≈83% increase in total WAT percentage of body weight when fed a high-fat diet (Fig. 5A). This increase was caused by the increased adipogenesis in ADSF-hFc transgenic mice with elevated adipocyte marker expression in an ADSF-hFc gene dose-dependent manner, which was probably caused by the attenuation of inhibitory effect of the endogenous ADSF as well as possibly FIZZ1/RELMα and FIZZ2/RELMβ. The mechanism(s) by which ADSF inhibits adipocyte differentiation or signal pathway is not known. The observed prevention of ADSF function by ADSF-hFc in the present study can only be speculated to be via its capacity to heterodimerization and potential prevention of ADSF binding to its putative receptor. However, the function of ADSF as a feedback regulator to sense the nutrient status in regulating adipocyte differentiation seems to be critical. Thus, the impaired ADSF function in adipose tissue of ADSF-hFc transgenic mice would alleviate the feedback regulation mechanism of adipogenesis, resulting in markedly increased adipogenesis.
Despite the development of obesity in ADSF-hFc transgenic mice, these mice showed improved glucose tolerance and insulin sensitivity compared with normal wild-type littermates. Of note, 10-week-old wild-type littermates used in this study showed normal insulin action judged by plasma glucose and lipid metabolite levels, GTT, and ITT. The basis for further improved glucose tolerance and insulin sensitivity in ADSF-hFc transgenic mice compared with wild-type littermates, despite the accompanying obesity, is worthy of note. The ADSF-hFc transgenic mouse model is one of few known mouse models in which obesity is accompanied by increased insulin sensitivity. Previous studies of other animal models showed only the dissociation of obesity from impaired glucose disposal, showing normal insulin sensitivity in obese animals. This finding may result from the functional nature of the proteins examined: ADSF is involved in adipocyte differentiation itself, whereas the other transgenic or knockout mouse models represent changes in expression of the molecules involved in metabolism (30, 31).
Leptin and adiponectin are proposed to be associated with insulin sensitivity. The increase in plasma leptin level was suggested to prevent accumulation of triglycerides in nonadipose tissues during overnutrition (32). In addition, the treatment of animals with leptin or adiponectin reversed insulin resistance with decreased circulating levels of triglycerides and free fatty acids (33, 34). In our ADSF-hFc transgenic mice, because of the enhanced differentiation of adipocytes, not only the leptin and adiponectin mRNA levels increased in adipose tissue by ≈4-fold, but the circulating leptin and adiponectin levels also increased by 4- and 1.3-fold, respectively. These mice showed normal triglyceride deposition in liver and muscle. However, plasma triglyceride and free fatty acid levels were decreased. As observed on adiponectin administration (34) and adiponectin transgenic mice (35), we also found an increase in ACO mRNA levels in skeletal muscle of ADSF-hFc transgenic mice. We propose that increased leptin and adiponectin levels accompanying decrease in circulating triglycerides and free fatty acids may contribute to the observed improved insulin sensitivity in our ADSF-hFc transgenic mice. We cannot, however, rule out the possibility that our transgenic mice showed improved insulin sensitivity because of the acute dominant negative effect of ADSF-hFc on insulin signaling and glucose homeostasis. Steppan et al. (12) originally reported that ADSF is a target gene down-regulated by insulin-sensitizing agents (thiazolidinediones) and proposed ADSF function in insulin resistance. Recently, by using pancreatic insulin clamp technique, Rajala et al. (18) showed that ADSF causes severe hepatic insulin resistance, which may cause the activation of glucose-6-phosphatase. However, we did not observe any change in expression of glucose-6-phosphatase or phosphoenolpyruvate carboxykinase mRNA levels in liver of ADSF-hFc transgenic mice (data not shown).
We conclude that the complex phenotype of enhanced insulin sensitivity with increased adipogenesis shown in ADSF-hFc transgenic mice is likely to be caused by the chronic effects of the impairment of the inhibitory function of ADSF in adipogenesis. We previously observed that inhibition of adipogenesis by overexpression of Pref-1 causes a decrease in circulating leptin and adiponectin levels with increased serum triglyceride and free fatty acid levels. Those lean transgenic mice overexpressing Pref-1 had impaired glucose tolerance and insulin sensitivity (10). It has been well documented that adipocyte marker expression is decreased in adipose tissue of insulin-resistant obese animals and humans (36, 37). Interestingly, thiazolidinedione therapy brings about increased body weight and adiposity as well as increased insulin sensitivity in type 2 diabetic patients (38). We propose that the degree of adipocyte differentiation and thus its functional capacity as an endocrine organ is critical in overall energy homeostasis and insulin sensitivity.
Acknowledgments
We thank David A. Bernlohr (University of Minnesota) for the aP2 promoter, Kichoon Lee for helping prepare ADSF-hFc/pcDNA3.1 vector and animal handling, and members of the Sul laboratory for discussion and comments. This work was supported by National Institutes of Health Grant DK50828 (to H.S.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ADSF, adipocyte-specific secretory factor; FIZZ, found in inflammatory zone; RELM, resistin-like molecule; hFc, human IgGγ constant region; PPARγ, peroxisome proliferator-activated receptor γ; Pref-1, preadipocyte factor 1; HA, hemagglutinin; aFABP, adipocyte fatty acid-binding protein; GTT, glucose tolerance test; ITT, insulin tolerance test.
References
- 1.Klyde, B. J. & Hirsch, J. (1979) J. Lipid Res. 20, 705-715. [PubMed] [Google Scholar]
- 2.Gregoire, F. M., Smas, C. M. & Sul, H. S. (1998) Physiol. Rev. 78, 783-809. [DOI] [PubMed] [Google Scholar]
- 3.MacDougald, O. A. & Mandrup, S. (2002) Trends Endocrinol. Metab. 13, 5-11. [DOI] [PubMed] [Google Scholar]
- 4.Zhao, L., Gregoire, F. & Sul, H. S. (2000) J. Biol. Chem. 275, 16845-16850. [DOI] [PubMed] [Google Scholar]
- 5.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. & Friedman, J. M. (1994) Nature 372, 425-432. [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil, G. S., Peraldi, P., Budavari, A., Ellis, R., White, M. F. & Spiegelman, B. M. (1996) Science 271, 665-668. [DOI] [PubMed] [Google Scholar]
- 7.Maeda, K., Okubo, K., Shimomura, I., Funahashi, T., Matsuzawa, Y. & Matsubara, K. (1996) Biochem. Biophys. Res. Commun. 221, 286-289. [DOI] [PubMed] [Google Scholar]
- 8.Smas, C. M. & Sul, H. S. (1993) Cell 73, 725-734. [DOI] [PubMed] [Google Scholar]
- 9.Moon, Y. S., Smas, C. M., Lee, K., Villena, J. A., Kim, K. H., Yun, E. J. & Sul, H. S. (2002) Mol. Cell. Biol. 22, 5585-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, K., Villena, J. A., Moon, Y. S., Kim, K. H., Lee, S., Kang, C. & Sul, H. S. (2003) J. Clin. Invest. 111, 453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, K. H., Lee, K., Moon, Y. S. & Sul, H. S. (2001) J. Biol. Chem. 276, 11252-11256. [DOI] [PubMed] [Google Scholar]
- 12.Steppan, C. M., Bailey, S. T., Bhat, S., Brown, E. J., Banerjee, R. R., Wright, C. M., Patel, H. R., Ahima, R. S. & Lazar, M. A. (2001) Nature 409, 307-312. [DOI] [PubMed] [Google Scholar]
- 13.Holcomb, I. N., Kabakoff, R. C., Chan, B., Baker, T. W., Gurney, A., Henzel, W., Nelson, C., Lowman, H. B., Wright, B. D., Skelton, N. J., et al. (2000) EMBO J. 19, 4046-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steppan, C. M., Brown, E. J., Wright, C. M., Bhat, S., Banerjee, R. R., Dai, C. Y., Enders, G. H., Silberg, D. G., Wen, X., Wu, G. D. & Lazar, M. A. (2001) Proc. Natl. Acad. Sci. USA 98, 502-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerstmayer, B., Kusters, D., Gebel, S., Muller, T., Van Miert, E., Hofmann, K. & Bosio, A. (2003) Genomics 81, 588-595. [DOI] [PubMed] [Google Scholar]
- 16.Chen, J., Wang, L., Boeg, Y. S., Xia, B. & Wang, J. (2002) J. Endocrinol. 175, 499-504. [DOI] [PubMed] [Google Scholar]
- 17.Blagoev, B., Kratchmarova, I., Nielsen, M. M., Fernandez, M. M., Voldby, J., Andersen, J. S., Kristiansen, K., Pandey, A. & Mann, M. (2002) J. Biol. Chem. 277, 42011-42016. [DOI] [PubMed] [Google Scholar]
- 18.Rajala, M. W., Obici, S., Scherer, P. E. & Rossetti, L. (2003) J. Clin. Invest. 111, 225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartsook, E. W., Hershberger, T. V. & Nee, J. C. (1973) J. Nutr. 103, 167-178. [DOI] [PubMed] [Google Scholar]
- 20.Kaneta, M., Osawa, M., Sudo, K., Nakauchi, H., Farr, A. G. & Takahama, Y. (2000) J. Immunol. 164, 256-264. [DOI] [PubMed] [Google Scholar]
- 21.Ohno, N., Izawa, A., Hattori, M., Kageyama, R. & Sudo, T. (2001) Stem Cells 19, 71-79. [DOI] [PubMed] [Google Scholar]
- 22.Boden, G. (2001) Endocr. Pract. 7, 44-51. [DOI] [PubMed] [Google Scholar]
- 23.Unger, R. H. (2002) Annu. Rev. Med. 53, 319-336. [DOI] [PubMed] [Google Scholar]
- 24.Shuldiner, A. R., Yang, R. & Gong, D. W. (2001) N. Engl. J. Med. 345, 1345-1346. [DOI] [PubMed] [Google Scholar]
- 25.McTernan, C. L., McTernan, P. G., Harte, A. L., Levick, P. L., Barnett, A. H. & Kumar, S. (2002) Lancet 359, 46-47. [DOI] [PubMed] [Google Scholar]
- 26.Nagaev, I. & Smith, U. (2001) Biochem. Biophys. Res. Commun. 285, 561-564. [DOI] [PubMed] [Google Scholar]
- 27.Way, J. M., Gorgun, C. Z., Tong, Q., Uysal, K. T., Brown, K. K., Harrington, W. W., Oliver, W. R., Jr., Willson, T. M., Kliewer, S. A. & Hotamisligil, G. S. (2001) J. Biol. Chem. 276, 25651-25653. [DOI] [PubMed] [Google Scholar]
- 28.Fujita, H., Fujishima, H., Morii, T., Koshimura, J., Narita, T., Kakei, M. & Ito, S. (2002) Biochem. Biophys. Res. Commun. 298, 345-349. [DOI] [PubMed] [Google Scholar]
- 29.Fukui, Y. & Motojima, K. (2002) Diabetes Obes. Metab. 4, 342-345. [DOI] [PubMed] [Google Scholar]
- 30.Franckhauser, S., Munoz, S., Pujol, A., Casellas, A., Riu, E., Otaegui, P., Su, B. & Bosch, F. (2002) Diabetes 51, 624-630. [DOI] [PubMed] [Google Scholar]
- 31.Chen, H. C., Stone, S. J., Zhou, P., Buhman, K. K. & Farese, R. V., Jr. (2002) Diabetes 51, 3189-3195. [DOI] [PubMed] [Google Scholar]
- 32.Unger, R. H. (2003) Annu. Rev. Physiol. 65, 333-347. [DOI] [PubMed] [Google Scholar]
- 33.Chen, G., Koyama, K., Yuan, X., Lee, Y., Zhou, Y. T., O'Doherty, R., Newgard, C. B. & Unger, R. H. (1996) Proc. Natl. Acad. Sci. USA 93, 14795-14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamauchi, T., Kamon, J., Waki, H., Terauchi, Y., Kubota, N., Hara, K., Mori, Y., Ide, T., Murakami, K., Tsuboyama-Kasaoka, N., et al. (2001) Nat. Med. 7, 941-946. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi, T., Kamon, J., Waki, H., Imai, Y., Shimozawa, N., Hioki, K., Uchida, S., Ito, Y., Takakuwa, K., Matsui, J., et al. (2003) J. Biol. Chem. 278, 2461-2468. [DOI] [PubMed] [Google Scholar]
- 36.Nadler, S. T., Stoehr, J. P., Schueler, K. L., Tanimoto, G., Yandell, B. S. & Attie, A. D. (2000) Proc. Natl. Acad. Sci. USA 97, 11371-11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moraes, R. C., Blondet, A., Birkenkamp-Demtroeder, K., Tirard, J., Orntoft, T. F., Gertler, A., Durand, P., Naville, D. & Begeot, M. (2003) Endocrinology 144, 4773-4782. [DOI] [PubMed] [Google Scholar]
- 38.Larsen, T. M., Toubro, S. & Astrup, A. (2003) Int. J. Obes. Relat. Metab. Disord. 27, 147-161. [DOI] [PubMed] [Google Scholar]