Abstract
The effects of ingesting the common foodborne mycotoxin deoxynivalenol (DON) on body weight and composition were characterized in the high fat (HF) diet-induced obese mouse, a model of human obesity. Female B6C3F1 mice were initially fed HF diets containing 45% kcal (HF45) or 60% kcal (HF60) as fat for 94 d to induce obesity. Half of each group was either continued on unamended HF diets or fed HF diets containing 10 mg/kg DON (DON-HF45 or DON-HF60) for another 54 d. Additional control mice were fed a low fat (LF) diet containing 10% kcal as fat for the entire 148 d period. DON induced rapid decreases in body weights and fat mass, which stabilized to those of the LF control within 11 d. These effects corresponded closely to a robust transient decrease in food consumption. While lean body mass did not decline in DON-fed groups, further increases were suppressed. DON exposure reduced plasma insulin, leptin, insulin-like growth factor 1 and insulin-like growth factor acid labile subunit as well as increased hypothalamic mRNA level of the orexigenic agouti-related protein. Overall, DON-mediated effects on body weight, fat mass, food intake and hormonal levels were consistent with a state of chronic energy restriction.
Keywords: deoxynivalenol, diet-induced obesity, food intake, hormone, trichothecene
1 Introduction
Deoxynivalenol (DON), a trichothecene mycotoxin produced by the phytopathogenic, fungus Fusarium, commonly contaminates grains such as wheat, barley and corn worldwide and is relatively recalcitrant to food processing [1]. DON intake by humans has been estimated to be 2.4,1.6, 1.4, 1.2 and 0.8 μg/kg bw/d in the Middle East, Far East, Europe, Latin America and Africa, respectively [2]. Because of its frequent occurrence in grain-based foods, it is critical to understand how DON and related trichothecenes impact humans. Exposure of monogastric species such as pigs to low or moderate DON concentrations in feed causes impaired weight gain whereas high DON doses cause malaise, diarrhea and emesis [3].
Suppression of weight gain has in mice been used as the metric for establishing the tolerable daily intake of this toxin [2], however, the underlying mechanisms remain poorly understood. Many investigations have suggested that DON impairs body weight increase and induces body weight loss by suppressing food intake [4, 5]. For example, the consumption of DON at 0.25 to 1.0 mg/kg bw and at 1.0 mg/kg bw by female and male rats, respectively, for 9 weeks caused both decreased feed consumption and body weight gain [4]. Likewise, DON consumption (0.35 and 1.3 mg/kg bw) caused reductions in food consumption and body weight after 1 wk in mice [5].
DON’s anorexic effects are possibly mediated via the brain. The hypothalamus controls food intake by balancing the levels of anorexic and orexigenic peptides such as pro-opiomelanocortin (POMC), α-melanocyte stimulating hormone (α-MSH), neuropeptide Y (NPY) and agouti-related peptide (AgRP) [6]. Notably, there is a strong inverse relationship between food consumption and brain levels of serotonin (5-hydroxytryptamine, 5-HT), an important upstream regulator of aforementioned peptides [7]. Since DON is detectable in brain following oral administration [8], the toxin has the potential to interfere with normal appetite regulation. In support of this contention, exposure to DON at 2.5 mg/kg- bw significantly increases serotonin levels in cerebellum and hypothalamus in rats at 24 hr after administration [9, 10]. Relatedly, acute DON administration (0.25 mg/kg bw) enhances the level of serotonin in hypothalamus of swine [11]. Therefore, altered serotonin in the brain could mediate DON-induced feed refusal and subsequent body weight effects.
Two other hormones that may impact DON-induced weight changes are insulin-like growth factor 1 (IGF-1) and insulin-like growth factor acid labile subunit (IGFALS). Prior studies in our laboratory demonstrated that DON exposure reduced expression of IGF-1 and IGFALS mRNA and protein [12, 13]. Notably, the latter protein prolongs half-life of circulating IGF-1 by forming ternary complex with IGF-1 and insulin-like growth factor-binding protein (IGFBP)-3/IGFBP-5 [14].
Given the current worldwide epidemic of human obesity, it is essential to understand how foodborne toxins such as DON might affect obese individuals. This is further important because DON and its derivatives have been proposed as potential pharmacotherapeutics for treating human obesity [15]. We have recently reported that DON suppresses body weight increase in mice fed high fat (HF)-diets as well as causes reduction in body weights in HF-induced obese mice [16]. These effects were linked to decreased terminal periuterine fat suggesting that there was a concurrent reduction in adiposity. Several critical questions remain regarding the effects of DON on body weight loss in obese adult animals, particularly as related to mechanisms and whether these effects are linked to loss of lean body mass as occurs in cachexia.
The purpose of this study was to relate DON-induced body weight loss in HF-induced obese mice to food intake, fat mass, lean mass and obesity-related hormones. DON-mediated body weight effects were found to correspond to transiently reduced food intake and to decreased body fat mass but not to reduction of basal lean weight. Further consistent with a state of chronic energy restriction, DON exposure decreased plasma concentrations of insulin, leptin, IGF-1 and IGFALS in obese mice as well as increased the hypothalamic mRNA level of the orexigenic peptide agouti-related protein (AgRP).
2 Materials and methods
2.1 Experimental design
Mice were maintained according to National Institute of Health guidelines as overseen by the All University Committee on Animal Use and Care at Michigan State University (Approval No. 01/08-007-00). Pathogen-free female adult B6C3F1 mice (11-wk-old, Charles River Laboratories, Portage, MI) were housed in polycarbonate cages under controlled temperature (21-24°C), humidity (40-55%) and lighting (12 h light:dark cycle). Boxes were covered with filter bonnets and mice were allowed free access to food and water.
Diets employed are summarized in Table 1. Mice (n=40) were fed low fat (LF) containing 10% kcal in fast diet (LF, D12450B) (Research Diet Inc., New Brunswick, NJ) during a 1 week acclimatization period. The remaining mice were fed HF diets containing either 45 kcal% as fat (HF45, Research Diet D12451) (n=16) or 60 kcal% as fat (HF60, Research Diet D12492) (n=16) to induce obesity. Following induction of the obese state at 94 d, each of these HF groups was subdivided into 2 groups (n=8) and fed either unamended HF diets (HF45 or HF60) or HF diets amended with 10 mg/kg DON (DON-HF45 or DON-HF60) until 147 d. This dose was selected based on its capacity to evoke body weight loss in our previous study [16]. In addition to these groups, another control group of mice was maintained on LF (n=8) for experiment duration (147 d).
Table 1.
Composition of LF (10% kcal from fat [LF]) and HF Diets (45% kcal from fat [HF45] and 60% kcal from fat [HF-60]
| Component | LF | HF45 | DON-HF45 | HF60 | DON-HF60 |
|---|---|---|---|---|---|
| casein, 80 mesh, g | 200 | 200 | 200 | 200 | 200 |
| L-cystine, g | 3 | 3 | 3 | 3 | 3 |
| corn starch, g | 315 | 72.8 | 72.8 | 0 | 0 |
| maltodextrin 10, g | 35 | 100 | 100 | 125 | 125 |
| sucrose, g | 350 | 172.8 | 172.8 | 68.8 | 68.8 |
| cellulose, BW200, g | 50 | 50 | 50 | 50 | 50 |
| soybean oila, g | 25 | 25 | 25 | 25 | 25 |
| lardb, g | 20 | 177.5 | 177.5 | 245 | 245 |
| Mineral Mix S10026, g | 10 | 10 | 10 | 10 | 10 |
| dicalcium phosphate, g | 13 | 13 | 13 | 13 | 13 |
| calcium carbonate, g | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| potassium citrate, 1 H2O, g | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 |
| Vitamin Mix V10001, g | 10 | 10 | 10 | 10 | 10 |
| choline bitartrate, g | 2 | 2 | 2 | 2 | 2 |
| deoxynivalenol, g | - | - | 0.012 | - | 0.013 |
| Total, g | 1055.0 | 858.1 | 858.1 | 773.8 | 773.8 |
Fatty acid composition of soybean oil: C16, 10.4%; C18, 3.8%; C18:1, 24.3%; C18:2, 53.5%; C18:3, 7.8%.
Fatty acid composition of lard: C10. 0.1%; C12, 0.2%; C14, 1.3%; C16:1, 2.7%; C18, 13.5%; C18:1, 41.2%; C18:2, 10.2%; C18:3, 1.0%; C20:1, 1.0% (USDA Nutrient Database, NDB no. 04002).
Food intake, body weight, fat mass and lean mass of the mice were monitored and blood was collected from the saphenous vein at intervals throughout the experiment. At the end of the feeding period, blood was collected from anesthetized mice that had been fasted for 4 h to achieve a non-fed state and the hypothalamus was removed following euthanasia. Plasma was frozen at -80°C for glucose and hormone analysis. The hypothalamus was stored in RNAlater® (Ambion, Inc., Austin, TX) at -20°C prior to RNA extraction.
2.2 Body composition
Body fat mass and lean mass were quantified in live mice by magnetic resonance imaging using an EchoMRI-100™ (Echo Medical Systems, LLC., Houston, TX). The body fat percentage was calculated using the following formula: body fat percentage (%) = body fat mass (g)/body weight (g) X 100.
2.3 Plasma glucose and hormones
Commercial assays were used to analyze plasma for glucose, insulin, leptin, and IGF-1 according to manufacturers’ instructions. Glucose Assay Kit was obtained from BioVision, Inc., (Mountain View, CA). Mouse/Rat IGF-1 Quantikine ELISA Kit was purchased from R&D Systems, Inc., (Minneapolis, MN). Mouse/Rat Leptin ELISA Kit was obtained from BioVendor Laboratory Medicine, Inc. (Candler, NC). Ultrasensitive Mouse Insulin ELISA kit was provided by Mercodia, Inc. (Winston Salem, NC). Plasma IGFALS level was measured according to a previously described method [12].
2.4 Quantitative real-time PCR
Total RNA from hypothalamus was extracted with TRI Reagent® (Sigma-Aldrich, St. Louis, MO), treated with DNaseI and then purified using an RNeasy Mini kit (Qiagen, Hilden, Germany). Real-time PCR was performed on an ABI PRISM 7900 HT Sequence Detection System with Taqman One-Step Real-Time PCR Master Mix and Assays-on-Demand primer/probe gene expression products according to the manufacturer’s instructions (Applied Biosystems, Foster City, NY). The mRNA levels of pro-opiomelanocortin (POMC) and agouti-related protein (AgRP) were expressed as the relative value to that of β2-microglobulin because its mRNA expression is not affected by DON exposure [17]. PCR amplifications were performed in duplicate wells in the same 384-well plate under the following conditions: 2 min at 50°C, 10 min at 95°C followed by 45 cycles of 30 s at 95°C and 1 min at 59°C.
2.5 Statistics
The data were analyzed using the Student-Newman-Keuls test following one-way analysis of variance using SigmaStat v 3.1 (Jandel Scientific, San Rafael, CA). The data were expressed the means ± standard error of the mean (SEM). All results with p<0.05 were evaluated as being statistically significant.
3 Results
3.1 Effects of DON on body weight in HF-induced obese mice
Body weights of mice fed HF45 and HF60 were significantly higher than that for mice fed LF by 37 d (Fig. 1). Upon onset of DON feeding (94 d), body weights of the LF, HF45 and HF60 were 33.8 ± 1.2 g, 41.4 ± 1.2 g and 42.2± 1.6 g, respectively. Following initiation of DON feeding, mean body weights of the DON-HF45 and DON-HF60-fed mice decreased dramatically as compared to those fed unamended HF diets. After reaching body weights of LF at 105 d, weights of the DON-fed mice stabilized for the remainder of the study. There was no difference in the rates of the initial body weight increase or DON-induced weight loss between HF45- and HF60-fed mice. At termination (147 d), body weights of mice on HF45 and HF60 were 133 and 134 %, respectively, of that for mice on LF diet, whereas body weights of mice on DON-HF45 and DON-HF60 were 94 and 97 %, respectively.
Figure 1.
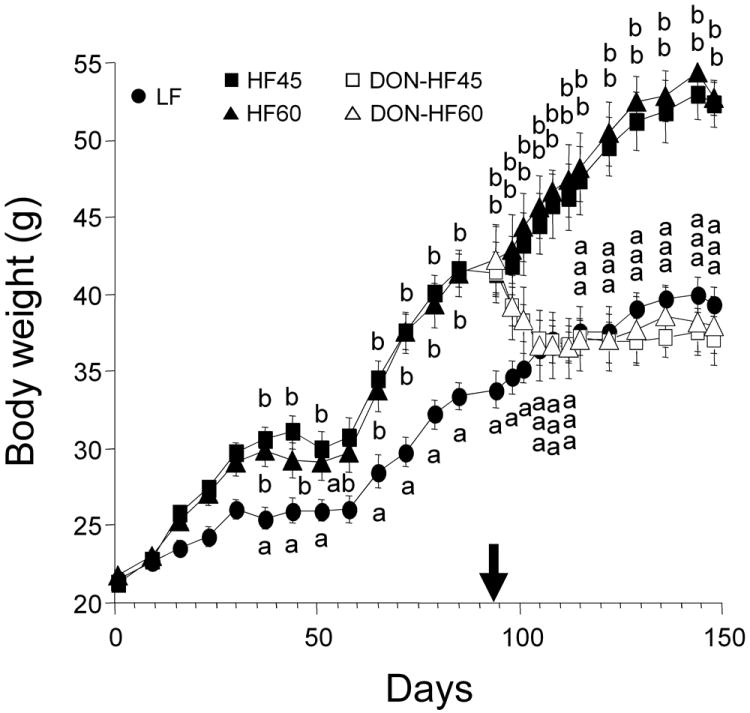
DON induces body weight decrease in HF-induced obese mice. Mice were fed LF, HF45 and HF60 to induce obesity for 94 d. After inducing obesity, the mice were divided into 5 groups and fed LF, unamended (HF45 and HF60) and HF diets with 10 mg/kg of DON (DON-HF45 and DON-HF60) from 94 d to 147 d. The arrow indicates the time at which DON feeding was initiated. Data are expressed as mean ± SEM (n=8). Data at a given time without the same letter differ significantly (p < 0.05).
3.2 Effects of DON on body composition in HF-induced mice
Body fat mass (Fig. 2A) and body fat percentage (Fig. 2B) of the DON-HF45 and DON-HF60 groups steadily declined to those observed in the LF group after 108 d and were significantly less than the those of the HF45 and HF60 groups, respectively. Body fat mass losses of 11.4 g and 12.2 g were observed for the DON-HF45 and DON-HF60 groups, respectively. In contrast, lean weights of DON-HF45- and DON-HF60-fed mice over the duration of toxin exposure did not differ significantly from those observed just prior to initiation of DON feeding (Fig. 3). Lean mass did not increase over the starting basal levels for DON-HF45 and DON-HF60 groups whereas it did for LF, HF45 and HF60 groups.
Figure 2.
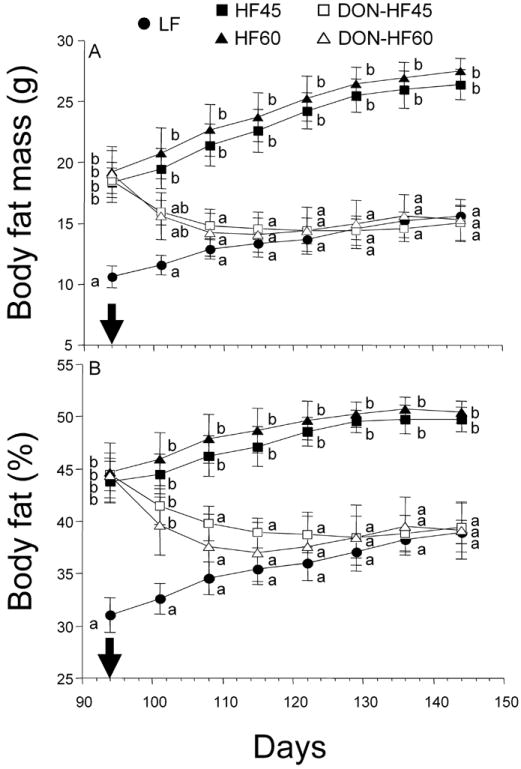
DON induces body fat decrease in HF-induced obese mice. Panels represent body fat mass (A) and body fat percentage (B). Arrows indicate the day at which the HF diets were changed to HF diets containing DON. Data are expressed as mean ± SEM (n=8). Data at a specified time without the same letter differ significantly (p < 0.05).
Figure 3.
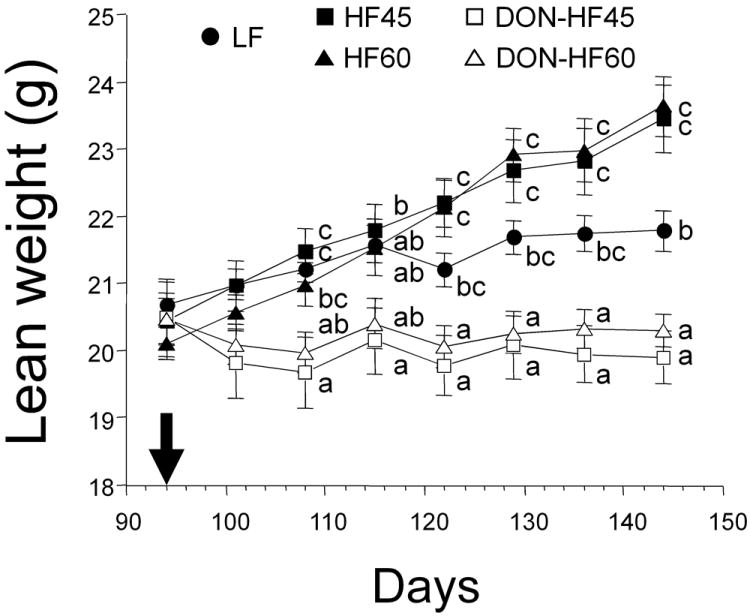
DON prevents lean mass increase in HF-induced obese mice. The arrow indicates the time at which DON feeding was initiated. Data are expressed as mean ± SEM (n=8). Data at a given time without the same letter differ significantly (p < 0.05).
3.3 Effects of DON on food intake in HF-induced obese mice
Food intake by the DON-HF45 and DON-HF60 groups was decreased dramatically at 98 d and 101 d, as compared to the HF45 and HF60 groups, respectively (Fig. 4). By 108 d, mean food intake of DON-HF45-fed mice had largely recovered; however, significant differences from HF45-fed mice were still evident at 122 d and 136 d. Food intake in the DON-HF-60 group partially recovered at 108 d but remained significantly lower than the HF60 group until experiment termination.
Figure 4.
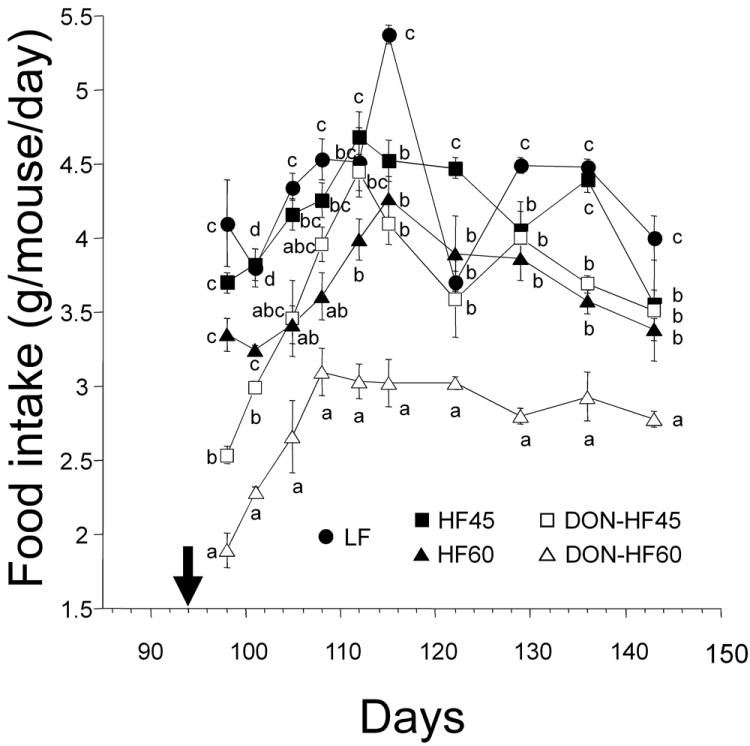
Effects of DON on food intake by HF-induced obese mice. Food intakes were measured after initiation of DON (indicated by arrow). Data at the specified time without the same letter differ significantly (p < 0.05).
3.4 Effects of DON on plasma glucose, insulin and leptin in HF-induced obese mice
Plasma glucose, insulin and leptin concentrations at the final day were measured upon experiment termination. Although no differences were observed in glucose levels among LF-, HF- and DON-treated groups (Fig. 5A), terminal plasma insulin concentrations in the DON-HF45 and DON-HF60 groups were significantly reduced as compared to their corresponding unamended HF controls (Fig. 5B). Furthermore, the insulin level in the DON-fed groups was significantly lower than that of the LF-fed group. Finally, plasma leptin was significantly decreased in the DON-fed groups as compared to their corresponding HF controls (Fig. 5C). Analyses of pooled plasma samples suggested that insulin reduction occurred relatively rapid after the onset of DON feeding (95 d) while leptin reduction occurred later (109 d) during the toxin feeding regimen (see supplemental information).
Figure 5.
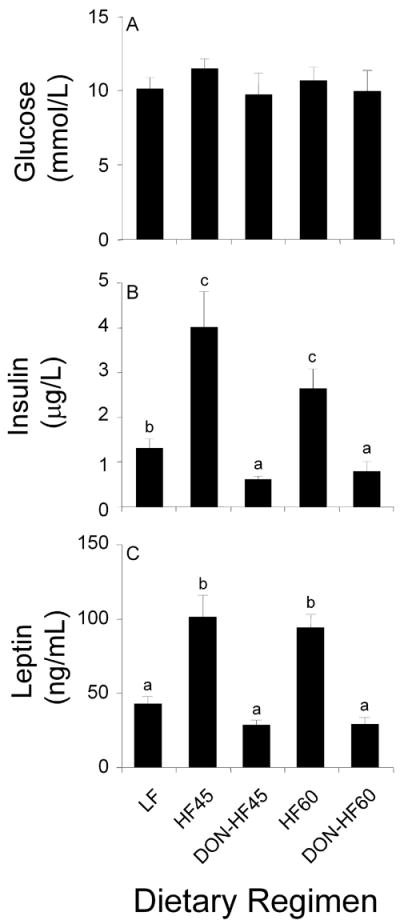
Effect of DON on glucose, insulin and leptin of HF-induced obese mice. Data are expressed as mean ± SEM (n=8). Bars without the same letter differ significantly (p < 0.05).
3.5 Effects of DON on IGF1 and IGFALS in HF-induced obese mice
Plasma IGF-1 concentrations in DON-HF45- and DON-HF60-fed mice were significantly lower than HF45- and HF60-fed mice, respectively, and identical to LF-fed mice (Fig. 6A). Similarly, plasma IGFALS in the DON-HF45 group was significantly lower than that of HF45 and DON-HF60-fed mice exhibited a similar trend (Fig. 6B). Plasma concentrations IGFALS in both DON-fed groups were significantly lower than that of LF.
Figure 6.
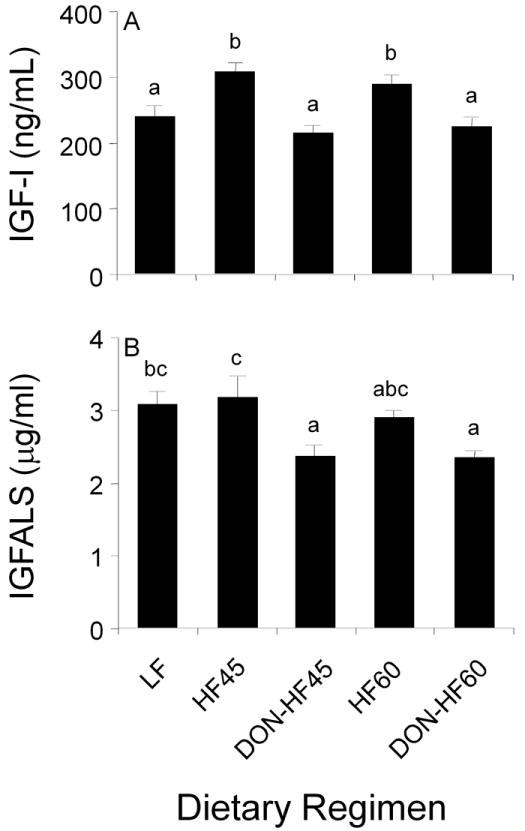
Effect of DON on plasma IGF-1 and IGFALS in HF-induced obese mice at experiment termination. Data are expressed as mean ± SEM (n=8). Bars without the same letter differ significantly (p < 0.05).
3.6 Effect of DON on hypothalamic mRNA levels of appetite-related hormones in HF-induced obese mice
DON feeding did not affect hypothalamic mRNA expression of the anorexic peptide POMC at experiment termination (Fig. 7A). In contrast, the DON-HF45 group showed a significant increase in the mRNA level of the orexigenic peptide AgRP and a similar trend was observed for DON-HF60 group (Fig. 7B).
Figure 7.
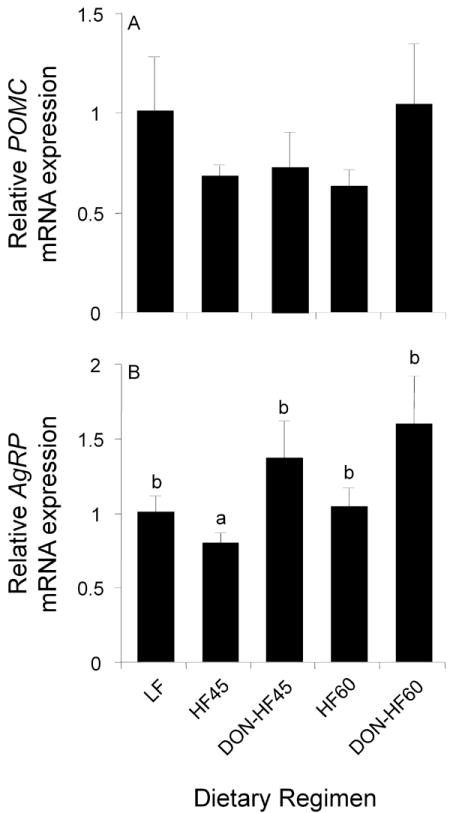
Effects of DON on hypothalamic POMC and AgRP levels in HF-induced obese mice. The mRNA level of POMC and AgRP in the hypothalamus was measured by real-time PCR at experiment termination. mRNA levels of POMC and AgRP were expressed as the relative value to that of β2-microglobulin. Data are expressed as means ± SEM (n=8). Bars without the same letter differ significantly (p<0.05).
4 Discussion
Relatively little is known how foodborne toxins might impact obese individuals. Recently, we have demonstrated that DON induces profound body weight loss in the obese adult mouse model [16], but the relationship between this effect and the regulation of body fat and lean body mass is still not fully understood. The results presented herein indicate that DON-mediated attenuation of body weight in HF-induced obese mice corresponded closely with reductions in body fat mass and body fat percentage. While DON did not affect basal lean mass, it inhibited further increases in lean mass as were observed for control LF and HF groups. Thus, DON-mediated reduction in body weight of the HF-induced obese mice at the dose employed here is likely attributable to both a decrease in body fat mass and impairment of lean mass increase, but not to loss of lean mass from muscle wasting. Concurrent with body weight loss, DON induced dramatic decreases in food intake during the first several days of exposure with marked restoration of feeding being observed within 2 wk of initiating DON exposure. Reduced food intake likely evoked a state of chronic energy restriction that contributed to both observed reductions in body weight and fat mass as well as impairment of lean weight increase. Putative mechanisms for these aforementioned effects based on this study and prior investigations are summarized in Fig. 8.
Figure 8.
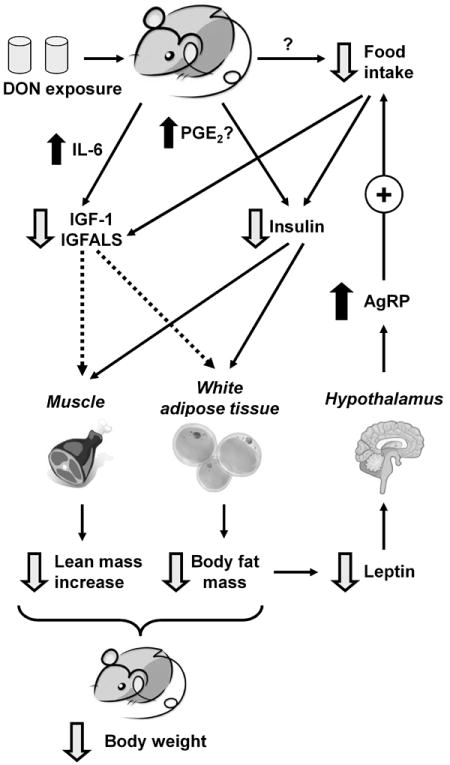
Putative mechanisms for DON-induced weight loss in HF-induced obese mice. DON induces reduced food intake, possibly via serotonin-mediated modulation of appetite in the brain or other mechanism. Reduced calorie intake can result in decreased plasma insulin and IGF-1/IGFALS. DON might also downregulate insulin and IGF-1/IGFALS by upregulating COX-2/PGE2 and IL-6, respectively. Downregulation of these hormones can contribute to decreased body fat mass and prevent further increase in lean mass, ultimately decreasing body weights of HF-induced obese mice. Another downstream effect is downregulation of leptin, which can increase expression of the orexigenic peptide AgRP and facilitate partial recovery of food intake.
Since insulin suppresses hormone-sensitive lipase activity and consequently inhibits lipolysis in adipose tissues [18], it might be speculated that DON-induced insulin reduction contributed to increased fat degradation observed here as well as subsequent the loss of body fat mass (Fig. 8) with corresponding increases in plasma free fatty acids (see supplementary data). It is known that diminished calorie intake reduces blood glucose and concurrently decreases insulin secretion from pancreatic β-cells [19], however, effects on the terminal plasma glucose level were not observed here. An alternative mechanism for reduced insulin might involve the modulation of prostaglandin E2 (PGE2) which inhibits glucose-induced insulin secretion [20]. Intriguingly, DON induces COX-2 expression in the mouse [21], and furthermore, the toxin enhances production of the COX-2 metabolite PGE2 in murine macrophages [22]. Another possibility for DON-mediated suppression of insulin might be via direct toxic effects to the pancreas, the insulin-producing organ. Szkudelska et al. [23] reported that DON increased serum insulin level in rats after a single subcutaneous injection of DON for 3 days indicating that the compound affects pancreas function. However, in vivo studies suggest DON does not selectively affect the protein synthesis in the pancreas [24] or absolute pancreatic weight [25, 26] suggesting that the toxin might not target this organ. Further investigation of how DON modulates insulin is thus warranted.
It was notable that reduced IGF-1 and IGFALS occurred in conjunction with DON-induced body weight reduction. Both reduction of these two proteins and body weight decreases are similarly observed in normal mice challenged acutely with DON or fed standard lab diet amended with DON [12]. Because IGFALS plays an important role in increasing the half-life of circulating IGF-1 [14], its decrease in DON-fed mice might also be associated with reduced IGF-1 stability. A proposed mechanism for the DON-induced IGF-1/IGFALS suppression involves the induction of IL-6 and other proinflammatory cytokines, which downregulate responsivity of the growth hormone receptor via suppressor of cytokines (SOCS)- and signal transducers and activator of transcription (STAT)-related mechanisms [12, 13]. IGF-1 and its binding partners are also very sensitive to nutritional alterations particularly with regard to total energy and protein intake [27-29]. For example, when normal human subjects are fasted for 3 d, serum IGF-1 level is decreased by 42% [30]. Caloric deprivation to 40% and protein deprivation for 30 d lowers serum levels of IGF-1 and IGFBP-3 in young rats [29]. Relatedly, caloric restriction induces significant reductions in serum IGFALS in rats [31] and human patients with anorexia nervosa [32].
Since IGF-1 increases lipogenesis and inhibits lipolysis [18, 28, 33], its reduction in plasma might contribute, in part, to the loss of body weight and fat mass in the DON-fed mice (Fig. 8). In addition, IGF-1 also stimulates the synthesis of protein in skeletal muscle [27]. IGF-1 has a net anabolic effect by increasing protein synthesis and it improves body composition favorably by increasing lean body weight and decreasing fat mass [33]. Therefore, it is possible that the reduced food intake in conjunction with decreased plasma IGF-1 and insulin led to the impairment of lean weight increases noted in this study.
Oral administration of DON results in accumulation of the toxin in the brain [8], raising the possibility that this toxin could act directly on the brain and modulate appetite. Although DON is known to increase the brain concentrations of serotonin, a molecule that can modulate appetite [9-11], similar information is unavailable on the toxin’s effects on the hypothalamic level of appetite-related hormones. To address this question, we measured two appetite-related transcripts in the hypothalamus focusing on POMC and AgRP because serotonin is located in the upstream of melanocortin pathway. Serotonin inhibits the activity of AgRP neurons and their downstream inhibition of POMC cells via activation of 5-HT1B receptors [7]. Serotonin also activates POMC neurons via activation of 5-HT2c receptors. This leads to reciprocal increases in α-MSH release, which is produced by processing of POMC, and decreases in AgRP release at the MC4 receptor in target sites, and finally serotonin reduces food intake via activating of MC4 receptor.
Chronic energy restriction causes weight loss coupled with upregulation of the hypothalamic mRNA level of AgRP and another orexigenic peptide, neuropeptide Y (NPY), but does not alter mRNA levels of anorexic peptides POMC and cocaine-and amphetamine-regulated transcript (CART) [34]. Thus, reduced calorie intake could effectively increase orexigenic peptide mRNA levels without increasing anorexic peptide mRNA expression in hypothalamus. In the present study, chronic DON exposure similarly increased hypothalamic AgRP mRNA level, without changing expression of POMC. Thus, the increase in the hypothalamic level of only orexigenic peptide mRNA at experiment termination might simply reflect a state of energy restriction after chronic exposure to DON.
Our results also demonstrate that DON exposure decreased plasma leptin, a hormone that is produced by adipocytes and is involved in appetite control and energy metabolism via its hypothalamic receptor [35, 36]. Leptin level positively correlates with differences in body fat [35]. Indeed, the reduced body fat mass after DON intake paralleled decreases in plasma leptin levels. This hormone also positively regulates POMC and CART to activate satiety and to inhibit the NPY/AgRP pathway [37]. Interestingly, food intake in DON-fed mice was partially restored within the first few weeks of initiating toxin exposure. It is therefore plausible that DON’s initial robust anorexic effects might be dampened by subsequent decreases in leptin and increase in AgRP mRNA levels. Nevertheless, since mean food intake per mouse of both DON-fed groups did not fully recover to that of the corresponding HF diet groups, these compensatory mechanisms were not totally sufficient to overcome DON’s anorexic effects.
No mice succumbed to DON toxicity in this study. Consistent with this observation, DON exposure here (1 mg/kg bw per mouse) is considerably lower than its reported oral LD50s which range from 46 to 78 mg/kg b.w. (Pestka) Further consistent with our findings is the report that the survival rates normal B6C3F1 mice over two years were 72%, 68%, 86% and 84% for 0, 1, 5, 10 mg/kg DON-fed males and 72%, 84%, 74% and 74% for 0, 1, 5, 10 mg/kg DON-fed females.
The present study provides further insight into feasibility of and mechanistic targets for novel DON-based anti-obesity agents. While DON’s capacity to decrease body weight and fat mass in this obesity model are consistent a possible therapeutic application [15], the toxin has considerable potential for adverse side effects, most notably emesis in susceptible animal species [2]. Nevertheless, understanding how DON modulates food intake could lead to identification of unique targets and novel pharmacotherapeutic agents for obesity. In support of this contention, several structurally modified DON derivatives have been developed that reportedly evoke reduction of food intake without concurrent toxicity [15]. There is need to for comparative studies with other structurally unrelated toxins to determine whether DON’s effects are truly specific or represent nonspecific inanition classically observed in toxicity studies.
Taken together, this investigation has demonstrated for the first time that DON-induced body weight loss in HF-induced obese mice corresponded to decreases in food intake and body fat mass as well as impaired lean weight increases - effects that were highly consistent with concomitant reductions in plasma insulin, IGF-1 and IGFALS (Fig. 8). These hormonal changes and subsequent compensatory effects involving leptin and AgRP are consistent with chronic calorie restriction that was likely evoked by DON-induced anorexia. Further clarification of the mechansistic roles for these hormones will require kinetic analyses in conjunction with the use of pair-fed controls.
Supplementary Material
Acknowledgments
Dr. Kobayashi-Hattori was supported by a fellowship from Tokyo University of Agriculture. Financial support for this work was obtained from the Michigan Agricultural Experiment Station, Public Health Service Grant ES 3358 (J.J.P.) from the National Institute for Environmental Health Sciences, and the U.S. Department of Agriculture (USDA), under a cooperative project with the U.S. Wheat and Barley Scab Initiative. Any findings, opinions, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of USDA. We thank Mary Rosner for technical assistance.
Abbreviations
- AgRP
agouti-related protein
- CART
cocaine-and amphetamine-regulated transcript
- DON
deoxynivalenol
- ELISA
enzyme-linked immunosorbent assay
- GH
growth hormone
- HF
high fat
- 5-HT
5-hydroxytryptamine
- IGF-1
insulin-like growth factor 1
- IGFALS
insulin-like growth factor acid labile subunit
- IGFBP
insulin-like growth factor-binding protein
- LF
low fat
- MC4
melanocortin 4
- α-MSH
alpha-melanocyte stimulating hormone
- NPY
neuropeptide Y
- PBS
phosphate-buffered saline
- PBST
PBS containing 0.05% Tween-20
- PCR
polymerase chain reaction
- POMC
pro-opiomelanocortin
- SEM
standard error of the mean
Footnotes
The authors verify that there are no financial of personal conflicts of interest related to this work.
References
- 1.Pestka JJ, Smolinski AT. Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health B Crit Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- 2.Canady RA, Coker RD, Rgan SK, Krska R, et al. Safety evaluation of certain mycotoxins in food. Fifty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Vol. 2001. International Programme on Chemical Safety - World Health Organization; Geneva: pp. 420–555. [Google Scholar]
- 3.Pestka JJ. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol. 2010;84:663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- 4.Arnold DL, Karpinski KF, McGuire PF, Nera EA, et al. A short-term feeding study with deoxynivalenol (vomitoxin) using rats. Fundam Appl Toxicol. 1986;6:691–696. doi: 10.1016/0272-0590(86)90182-x. [DOI] [PubMed] [Google Scholar]
- 5.Robbana-Barnat S, Loridon-Rosa B, Cohen H, Lafarge-Frayssinet C, et al. Protein synthesis inhibition and cardiac lesions associated with deoxynivalenol ingestion in mice. Food Additives and Contaminants. 1987;4:49–56. doi: 10.1080/02652038709373614. [DOI] [PubMed] [Google Scholar]
- 6.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 7.Heisler LK, Jobst EE, Sutton GM, Zhou L, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Pestka JJ, Islam Z, Amuzie CJ. Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicology Letters. 2008;178:83–87. doi: 10.1016/j.toxlet.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick DW, Boyd KE, Watts BM. Comparison of the trichothecenes deoxynivalenol and T-2 toxin for their effects on brain biogenic monoamines in the rat. Toxicology Letters. 1988;40:241–245. doi: 10.1016/0378-4274(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick DW, Boyd KE, Wilson LM, Wilson JR. Effect of the trichothecene deoxynivalenol on brain biogenic monoamines concentrations in rats and chickens. J Environ Sci Health B. 1988;23:159–170. doi: 10.1080/03601238809372594. [DOI] [PubMed] [Google Scholar]
- 11.Prelusky DB, Yeung JM, Thompson BK, Trenholm HL. Effect of deoxynivalenol on neurotransmitters in discrete regions of swine brain. Arch Environ Contam Toxicol. 1992;22:36–40. doi: 10.1007/BF00213300. [DOI] [PubMed] [Google Scholar]
- 12.Amuzie CJ, Pestka JJ. Suppression of insulin-like growth factor acid-labile subunit expression--a novel mechanism for deoxynivalenol-induced growth retardation. Toxicol Sci. 2010;113:412–421. doi: 10.1093/toxsci/kfp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amuzie CJ, Shinozuka J, Pestka JJ. Induction of suppressors of cytokine signaling by the trichothecene deoxynivalenol in the mouse. Toxicol Sci. 2009;111:277–287. doi: 10.1093/toxsci/kfp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol. 2001;170:63–70. doi: 10.1677/joe.0.1700063. [DOI] [PubMed] [Google Scholar]
- 15.Krantis A, Durst T. Novel multi-ring organic compounds for regulating gut motility and food intake. 2004/0102514. United States Patent Application. 2004:1–54.
- 16.Amuzie CJ, F BM, Ulrich AM, Pestka JJ. Effects of deoxynivalenol consumption on body weight and adiposity in the diet-induced obese mouse. Journal of Toxicology and Environmental health. 2011 doi: 10.1080/15287394.2011.539119. in press. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, Pestka JJ. Mechanisms for suppression of interleukin-6 expression in peritoneal macrophages from docosahexaenoic acid-fed mice. Journal of Nutritional Biochemistry. 2009;20:358–368. doi: 10.1016/j.jnutbio.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells T. Ghrelin - Defender of fat. Prog Lipid Res. 2009;48:257–274. doi: 10.1016/j.plipres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 20.Robertson RP. Eicosanoids as pluripotential modulators of pancreatic islet function. Diabetes. 1988;37:367–370. doi: 10.2337/diab.37.4.367. [DOI] [PubMed] [Google Scholar]
- 21.Moon Y, Pestka JJ. Cyclooxygenase-2 mediates interleukin-6 upregulation by vomitoxin (deoxynivalenol) in vitro and in vivo. Toxicol Appl Pharmacol. 2003;187:80–88. doi: 10.1016/s0041-008x(02)00033-9. [DOI] [PubMed] [Google Scholar]
- 22.Moon Y, Pestka JJ. Vomitoxin-induced cyclooxygenase-2 gene expression in macrophages mediated by activation of ERK and p38 but not JNK mitogen-activated protein kinases. Toxicological Sciences. 2002;69:373–382. doi: 10.1093/toxsci/69.2.373. [DOI] [PubMed] [Google Scholar]
- 23.Szkudelska K, Szkudelski T, Nogowski L. Short-time deoxynivalenol treatment induces metabolic disturbances in the rat. Toxicology Letters. 2002;136:25–31. doi: 10.1016/s0378-4274(02)00281-3. [DOI] [PubMed] [Google Scholar]
- 24.Danicke S, Goyarts T, Doll S, Grove N, et al. Effects of the Fusarium toxin deoxynivalenol on tissue protein synthesis in pigs. Toxicology Letters. 2006;165:297–311. doi: 10.1016/j.toxlet.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Awad WA, Bohm J, Razzazi-Fazeli E, Ghareeb K, Zentek J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult Sci. 2006;85:974–979. doi: 10.1093/ps/85.6.974. [DOI] [PubMed] [Google Scholar]
- 26.Awad WA, Bohm J, Razzazi-Fazeli E, Zentek J. Effects of feeding deoxynivalenol contaminated wheat on growth performance, organ weights and histological parameters of the intestine of broiler chickens. J Anim Physiol Anim Nutr (Berl) 2006;90:32–37. doi: 10.1111/j.1439-0396.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 27.Clemmons DR, Underwood LE. Nutritional regulation of IGF-I and IGF binding proteins. Annu Rev Nutr. 1991;11:393–412. doi: 10.1146/annurev.nu.11.070191.002141. [DOI] [PubMed] [Google Scholar]
- 28.Wabitsch M, Hauner H, Heinze E, Teller WM. The role of growth hormone/insulin-like growth factors in adipocyte differentiation. Metabolism. 1995;44:45–49. doi: 10.1016/0026-0495(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 29.Oster MH, Fielder PJ, Levin N, Cronin MJ. Adaptation of the growth hormone and insulin-like growth factor-I axis to chronic and severe calorie or protein malnutrition. J Clin Invest. 1995;95:2258–2265. doi: 10.1172/JCI117916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merimee TJ, Zapf J, Froesch ER. Insulin-like growth factors in the fed and fasted states. J Clin Endocrinol Metab. 1982;55:999–1002. doi: 10.1210/jcem-55-5-999. [DOI] [PubMed] [Google Scholar]
- 31.Oster MH, Levin N, Fielder PJ, Robinson IC, et al. Developmental differences in the IGF-I system response to severe and chronic calorie malnutrition. Am J Physiol. 1996;270:E646–653. doi: 10.1152/ajpendo.1996.270.4.E646. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda I, Hotta M, Hizuka N, Takano K, et al. Decreased serum levels of acid-labile subunit in patients with anorexia nervosa. J Clin Endocrinol Metab. 1999;84:2034–2036. doi: 10.1210/jcem.84.6.5737. [DOI] [PubMed] [Google Scholar]
- 33.Bluher S, Kratzsch J, Kiess W. Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best Pract Res Clin Endocrinol Metab. 2005;19:577–587. doi: 10.1016/j.beem.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, Deng C, Huang XF. Obese reversal by a chronic energy restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice. Behav Brain Res. 2009;205:50–56. doi: 10.1016/j.bbr.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 36.Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83–89. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- 37.Konturek PC, Konturek JW, Czesnikiewicz-Guzik M, Brzozowski T, et al. Neuro-hormonal control of food intake: basic mechanisms and clinical implications. J Physiol Pharmacol. 2005;56(Suppl 6):5–25. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


