Abstract
The continuation paradigm is often used to investigate the behavioral and neural mechanisms of timing. Typically, a movement rate is established by pacing with a metronome. Then, the metronome is turned off and the subject continues at the established rate. Performance during continuation is assumed to be based on internal timing mechanisms. Here, we investigated the degree to which the neural activity underlying time representation depends on the initial pacing context, that is, whether pacing was established by moving in-phase (the usual procedure) or anti-phase (syncopation) with an auditory metronome. Functional MRI was measured from 14 subjects during four conditions: synchronized pacing, synchronized continuation, syncopated pacing, and syncopated continuation. In general, movements were timed consistently for all four conditions. However, a much broader network of activation was engaged during syncopation compared with synchronization, including increased activation in supplementary motor area, left premotor area, right thalamus, bilateral inferior frontal gyrus, and cerebellum. No differences were found when comparing continuation with the preceding pacing phase except for decreased activity in auditory-related regions due to the absence of the metronome. These results demonstrate that the cortical and subcortical areas recruited to support a simple motor timing task depend crucially on the method used to establish the temporal reference. Thus, the neural mechanisms underlying time and timing are highly flexible, reflecting the context in which the timing is established.
The ability of humans to accurately maintain temporal information after the removal of external environmental cues is often exploited to investigate the neural basis of internal timing mechanisms (1). An illustrative approach first used by Stevens (2) and popularized by Wing and Kristofferson (3) is the continuation paradigm. The task consists of two stages. In the first stage (termed pacing), movements are made to coincide with an external periodic stimulus or metronome. In the second so-called continuation stage, the metronome is removed and the subject is required to maintain movement at the rate previously established during pacing. During continuation it is assumed that timing is based on internal mechanisms that use a representation of the required interval developed during pacing. When applied to patient populations, this approach has been central in identifying cerebellum and basal ganglia as putative structures mediating these temporal processes during continuation (4-6). More recent functional imaging studies employing this paradigm have identified broader networks of cortical and subcortical structures underlying timing (7, 8). Although these latter studies demonstrate that pacing and continuation, in addition to imposing similar task demands, activate substantially overlapping networks, the degree to which the neural areas supporting continuation are influenced by the network of neural activity generated during pacing has not been investigated. Here, we explore the relationship between how the time representation is established during the pacing stage and its later neural expression when pacing information is removed. We explicitly inquire into whether changing the way in which a movement rate is established during pacing modulates the functional network activated during subsequent continuation.
This question may be crucial because, although synchronization is the preferred method of coordination used during pacing, it represents only one coordination pattern that can be adopted to establish rhythmic movements. For instance, both synchronization and syncopation (moving between beats) are easily performed at low movement rates and can therefore be used effectively to establish pacing. However, within the framework of coordination dynamics (9), it is known that synchronization is a more stable form of coordination (10-12) that imposes fewer demands on neural resources than alternative timing relationships such as syncopation (13-15). Even at low movement rates, syncopation compared with synchronization produces additional activity across a broad range of cortical and subcortical areas, including supplementary motor area (SMA), basal ganglia, cerebellum, and lateral premotor areas (refs. 14 and 16; see also ref. 17), structures implicated either directly in the processing of temporal information (18) or indirectly in nontemporal processes, such as attention and working-memory (19), that may support timing. Although increased or additional activity within this functional network may reflect an increase in timing demands imposed by the syncopation pattern, it is not clear whether the network supporting the subsequent continuation phase (when the metronome is removed and coordination constraints no longer exist) are influenced by this prior context.
To address this question, we used functional MRI to investigate paced finger flexion to an auditory metronome by using a modified continuation paradigm. During pacing subjects were directed to coordinate by using either a synchronized (on the beat) or syncopated (off the beat) coordination pattern with an auditory metronome presented at 1.25 Hz. When the metronome was removed, subjects were instructed to continue moving at the established rate. Based on existing work (14), we expected syncopated pacing to result in greater activity within a characteristic cortical-subcortical network. If the areas supporting continuation are independent of the method for establishing pacing (i.e., reflect only the timing demands imposed during continuation), no differences in activity should be observed when comparing continuation after synchronized pacing and continuation after syncopated pacing. An alternative hypothesis is that the method of pacing influences the activity observed during continuation. This hypothesis predicts that, during the continuation phase when no metronome is present, neural activity reflects not only the task demands of the current behavior, but also the preceding mode of pacing. As a result, differences in neural activity between the two continuation conditions should mirror initial differences between synchronization and syncopation even though behavioral performance may be, to all intents and purposes, identical.
Methods
Fourteen neurologically normal volunteers (12 male, 2 female; mean age 28.5 yr, ranging from 23 to 37) gave informed consent to participate in the study. All subjects reported being strongly right handed. Procedures were carried out in accordance with the guidelines set out by the Internal Review Board at Florida Atlantic University and the human subject guidelines of the National Institutes of Health. During pacing, subjects coordinated finger-thumb opposition movements with a series of tones presented at a constant rate of 1.25 Hz (pacing conditions). The tones were then discontinued, and subjects were required to continue moving at the same rate in the absence of pacing (continuation conditions). During pacing, finger movements were either synchronized with the stimulus such that the point of peak flexion coincided with each tone, or syncopated with the stimulus such that each movement occurred directly in between consecutive tones. Regardless of the coordination pattern during pacing, subjects were instructed to maintain the movement rate as accurately as possible during continuation. A single 1-s tone was presented at the end of the continuation stage signaling the subject to rest until the start of the next pacing interval. Auditory stimuli (1,000 Hz sine tones; 60-ms duration) were presented binaurally to the subject through headphones. Behavioral responses were recorded as a change in pressure in a small air-filled pillow placed between the index finger and thumb of the right hand.
MRI. Changes in neural activity were characterized as changes in local blood oxygenation [blood oxygen level-dependent (BOLD) effect] by using echo planar imaging on a 1.5-Tesla GE Signa Scanner (General Electric). Echo-planar images were acquired by using a single shot, gradient-echo, echo planar pulse sequence [echo time (TE)/repetition time (TR)/flip angle (FA)/field of view (FOV) = 60 ms/3 s/90°/24 cm, 64 × 64 matrix). Twenty axial 5-mm-thick slices spaced 2.5 mm provide coverage of the entire brain. High-resolution anatomical spoiled gradient recall (SPGR) images (TE/TR/FA/FOV = in-phase/325 ms/90°/24 cm; 5-mm thickness, 2.5-mm spacing, number of excitations (NEX) = 2) were collected at the same slice locations as the functional images. These images were used to coregister the functional scans onto anatomical 3D SPGR axial images (TE/TR/FA/FOV = 5 ms/34 ms/45°/24 or 26 cm; resolution = 256 × 256; thickness = 2 mm).
A modified block design was used in which a single block comprised a rest period (9 images; 27 s) followed by pacing (7 images; 21 s) and continuation (7 images; 21 s) conditions, respectively. A total of four blocks were completed for both syncopation and synchronization. Blocks were grouped such that all syncopation trials were presented together and all synchronization trials were presented together. The order of grouped blocks was randomized such that half the subjects started with syncopation and the other half with synchronization.
Behavioral Analysis. The time of each behavioral response was defined as the point of maximum compression of the air pillow (i.e., peak flexion of the index finger and thumb). The time of each response was corrected by 30 ms to account for the temporal delay of the pneumatic device as determined by multiplying the length of the tube by the speed of sound in air. Two relative measures of performance were calculated. Inter-response interval (IRI) was defined as the time between consecutive behavioral responses, and relative timing (phase) was defined as the time between each behavioral response and the preceding stimulus onset, divided by the stimulus period (20). In addition to motor timing, simple kinematic analysis was performed by averaging individual response profiles for syncopation and synchronization conditions, respectively. Finally, according to the two-process model of Wing and Kristofferson (3), variance and lag-one autocovariance measures were computed on the response intervals observed during continuation to detect relative changes in variability within putative central clock and motor mechanisms.
Neuroimaging Analysis. Unless otherwise stated, all analyses were performed by using AFNI (21, 22). Preprocessing included motion detection and correction followed by spatial smoothing by convolution with a Gaussian kernel [full-width half-maximum (FWHM) 4 mm] and temporal filtering below 0.1 Hz. Multiple regression was used to determine the relative contribution of pacing and continuation model functions to the observed time series of each voxel. Model time series consisted of vectors comprised of ones when a stimulus was present (pacing or continuation), and zeros otherwise, convolved with a hemodynamic response function. The resulting fit coefficient for each regressor of interest was divided by the average offset of each voxel to give a measure of percent signal change. SPM99 was used to coregister functional images to 3D anatomical images that were later transformed into the coordinate space of Talairach and Tournoux (23) before being subjecting to further statistical evaluation.
To characterize the BOLD activity elicited by each experimental condition, group t tests were performed comparing the mean of activity for all subjects to rest (no activation). Voxels were considered task related if they, exceeding a statistical threshold of P ≤ 0.0005, were members of a spatially continuous cluster across an area of at least 500 mm3 (corrected to P ≤ 0.01). Significant differences between experimental conditions were assessed by a 2-way ANOVA with factors of mode (synchronize, syncopate) and task (pace, continue). The resulting statistical maps were thresholded at P ≤ 0.005 and clustered with a minimum volume of 632 mm3 (corrected P < 0.05). A more stringent threshold was selected for the more robust comparisons (activation vs. rest) to provide a focused view of the distinct cortical and subcortical regions involved in the performance of the individual conditions.
Results
Performance. During pacing, subjects successfully produced both the synchronized and syncopated patterns. The average relative phase across subject was 17.7 ± 27.9° (mean ± SD) for synchronized pacing and 187.9 ± 50.7° for syncopated pacing. Both patterns are performed with similar stability as indexed by the SD of the relative phase. No significant difference (P > 0.05) was found in the variability of the relative phase between paced synchronization (29.8 ± 4.9°) and syncopation (39.6 ± 17.9°).
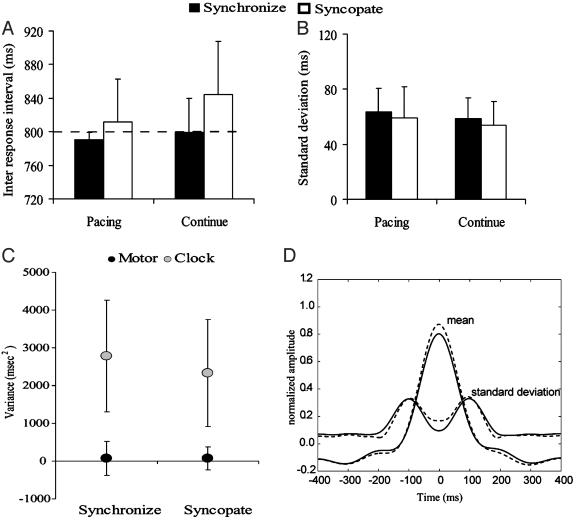
During all conditions, subjects were able to produce an average interval close to the required 800 ms (Fig. 1A). Synchronization (filled bars) was performed slightly faster than the metronome with a mean IRI of 791 ± 8.6 ms and 799 ± 50.3 ms for the pacing and continuation phases, respectively. During syncopation (open bars), the average response rate was slightly slower with mean IRIs of 812 ± 40.5 ms for pacing and 844 ± 65.3 ms for continuation. A two-way ANOVA performed using factors of mode (synchronize and syncopate) and task (pacing and continuation) showed a main effect for mode (F1,26 = 6.25, P ≤ 0.016), but no effect of task. All conditions were performed with similar stability, as reflected in the mean SD (Fig. 1B). SDs across conditions ranged from 54 ± 16.97 ms to 63.5 ± 17.18 ms. A two-way ANOVA showed no significant difference between conditions.
Fig. 1.
Means (A) and SDs (B) of the IRIs for both synchronization (filled) and syncopation (open) conditions. The dashed line in A marks the required response interval. (C) Covariance analysis separating the response variability into a clock component (shaded) and a motor component (filled). (D) Mean movement profile averaged across synchronization (solid lines) and syncopation (dotted lines) conditions shown together with the SD. For A, B, and C, error bars are at 1 SD.
A paired, two-tailed t test comparing the mean clock variance (±SD, Fig. 1C) during continuation after synchronization (2,782 ms2, left side) and continuation after syncopation (2,333 ms2, left side) conditions revealed no statistical differences (P = 0.12). Similarly, no differences were observed when comparing the motor variance during continuation (synchronization, 70 ms2; syncopation, 68 ms2; P = 0.99). These data indicate that, according to the hierarchical timing model proposed by Wing and Kristofferson (3), the use of alternative coordination patterns during pacing does not affect variability in the underlying timing process, or in the independent motor delay.
Fig. 1D shows the normalized movement profiles averaged within an 800-ms window centered on the point of peak flexion. Movement trajectories and amplitude are virtually identical for synchronized (solid lines) and syncopated (dotted lines) responses. Only a slight nonsignificant increase in amplitude is observed for syncopation, indicating that subjects performed very similar movement trajectories for both coordination conditions. Taken together, these behavioral results support two conclusions. First, subjects were able to perform equally well in both pacing conditions, whether synchronizing or syncopating. Second, regardless of the coordination pattern used during pacing, reproduction of the required interval during continuation was performed basically similar on all behavioral measures.
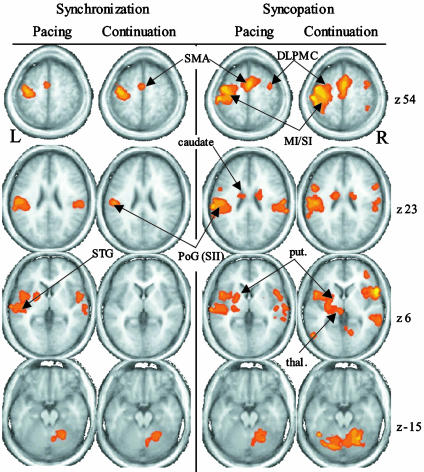
Neuroimaging. Fig. 2 shows the average parametric maps from each of the four conditions overlaid on selected slices of an average anatomical scan. As expected, syncopated pacing (column 3) activates a broader network of areas than synchronized pacing (column 1). These differences carry over into continuation (columns 2 and 4) such that the two continuation conditions activate substantially different neural networks.
Fig. 2.
Average parametric maps from the four experimental conditions overlaid on selected slices of an average anatomical scan. Data from each condition are organized into columns. M1/S1, primary sensorimotor cortex; thal, thalamus; put, putamen.
Synchronized Pacing. Regions of significant activation for synchronized pacing (Fig. 2, column 1) were identified in contralateral precentral gyrus (PcG), corresponding to primary sensori-motor cortex (M1/S1) and extending anteriorly into the ventrolateral premotor region of the middle frontal gyrus (MFG). In addition, a single activation within the medial wall of the MFG was identified (corresponding to SMA). Bilateral activity occurred in more inferior temporal regions, with clusters centered in the precentral gyrus (PcG) extending from the inferior aspects of the PcG [corresponding to secondary somatosensory cortex (SII)] to the superior temporal gyrus (STG). Subcortical activity was observed in the contralateral putamen and in the lateral declive of the ipsilateral cerebellum.
Synchronized Continuation. Synchronized continuation showed a very similar activity pattern to synchronized pacing, with clusters located within contralateral M1, SMA, and ventrolateral premotor region (column 2). In addition, a small cluster was present in the inferior lateral portion of the post central gyrus (PoG), a region in the vicinity of SII. A final cluster of activity was observed within the ipsilateral declive. Neither the putamen nor the STG was active, the latter likely related to the absence of the auditory stimulus.
Syncopated Pacing. Syncopated pacing resulted in a broader network of activity than synchronized pacing. In addition to those regions active during synchronization, syncopated pacing (Fig. 2, column 3) resulted in activity bilaterally in dorsolateral premotor cortex (DLPMC). In addition, MFG activation extended bilaterally into both the left and right SMA and inferiorly into the cingulate. Activations centered in bilateral PoG extended into STG, the planum temporale, and the insula, encompassing large portions of both primary auditory cortex (A1) and SII. In addition to subcortical activity in the left putamen, thalamus, and right declive, significant BOLD activity was also observed more inferiorly in bilateral caudate.
Syncopated Continuation. The activations observed during syncopated continuation (Fig. 2, column 4) paralleled those described for syncopated pacing. Moreover, the number and extent of active regions was much greater for syncopated than synchronized continuation. Activation was found in bilateral DLPMC, SMA, cingulate, and also in the inferior parietal lobe [Brodmann's area (BA) 40]. A single large contralateral cluster centered on the left post central gyrus BA3 encompassed M1, S1, and also extended inferolaterally to include the SII region of the PoG but did not extend beyond the lateral fissure into auditory regions. A similar smaller cluster extended laterally and inferiorly into the SII region on the ipsilateral side. Bilateral middle temporal gyrus (MTG, BA22), superior frontal gyrus, and insula were also significantly activated during syncopated continuation. Subcortical activity occurred in bilateral caudate and putamen, left thalamus, and bilaterally in the cerebellum.
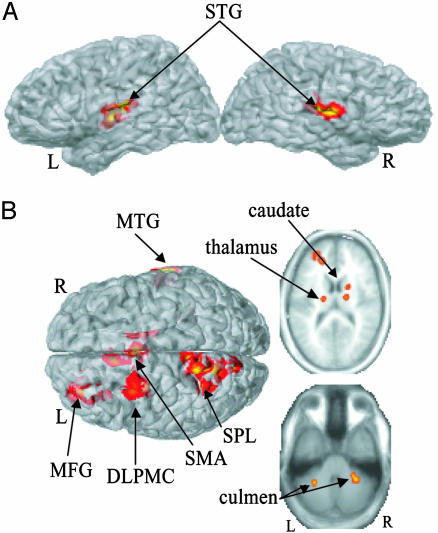
Statistical Comparisons. Main effects from the two-way ANOVA are displayed in Fig. 3. Areas demonstrating a significant main effect for task (Fig. 3A, pacing > continuation) were identified exclusively in bilateral superior temporal gyrus (for details, see table 1). These effects resulted from greater activity during pacing than during continuation and reflect a decrease in activity in primary auditory cortex in the absence of the metronome. Areas demonstrating a main effect of mode (syncopation > synchronization) are shown in Fig. 3B. Significant cortical activity (Table 1) was found in the medial (SMA) and right lateral (DLPMC and left prefrontal cortex, BA10) portions of the MFG, left superior parietal lobe, and the right MTG. Subcortical clusters were identified in the right caudate extending into the thalamus, the left ventral posterior lateral nucleus of the thalamus, as well as bilaterally in the culmen of the cerebellum. There were no voxels showing a significant mode × task interaction, indicating that the observed differences between synchronization and syncopation occurred during both pacing and continuation.
Fig. 3.
Results from a two-way ANOVA. (A) A main effect for task (pacing vs. continuation) was observed in bilateral STG. (B) A main effect for mode (syncopate vs. synchronize) occurred in several regions, including SMA, left superior parietal lobe (SPL), left DLPMC, left MFG, right MTG, bilateral thalamus, right caudate, and bilateral culmen.
Table 1. Brain regions showing significant effects.
| Side | x | y | z | Vol, cm3 | BA | Region |
|---|---|---|---|---|---|---|
| Pacing > continuation | ||||||
| L | −47 | −21 | 11 | 6.99 | 41 | STG |
| R | 52 | −20 | 15 | 5.05 | 40 | STG |
| Synchronization > syncopation | ||||||
| B | 0 | 3 | 46 | 5.67 | 32 | SMA |
| L | −24 | −3 | 53 | 3.28 | 6 | MFG |
| L | −12 | −55 | 61 | 7.74 | 7 | SPL |
| L | −28 | 40 | 23 | 5.85 | 10 | MFG |
| R | 62 | −22 | −6 | 2.19 | 21 | MTG |
| R | 16 | −3 | 20 | 2.26 | - | Caudate |
| L | −19 | −19 | 7 | 2.16 | - | VPL |
| L | −14 | −37 | −17 | 2.24 | - | Culmen |
| R | 28 | −43 | −28 | 1.88 | - | Culmen |
| L | −30 | −50 | −33 | 2.08 | - | Tonsil |
L, left; R, right; B, bilateral; SPL, superior parietal lobe; VPL, ventro posterior lateral thalamus.
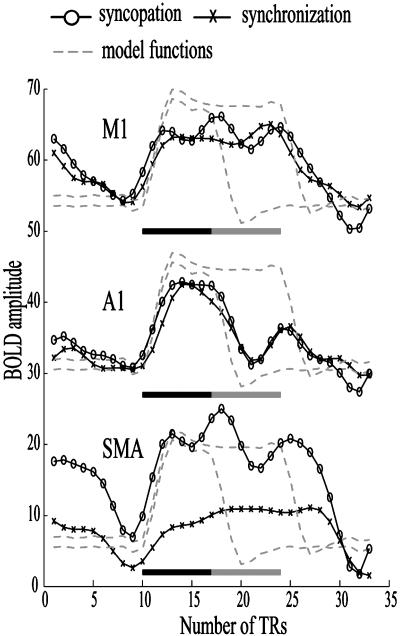
A possible explanation of greater BOLD activity during continuation after syncopation is that of “carry-over” of the hemodynamic response generated during the preceding pacing phase. To evaluate this methodological issue, we investigated the hemodynamic response to the different task conditions within key brain regions. Representative mean functional time series averaged across voxels and presentation blocks within three functionally defined regions of interest (ROI) are illustrated in Fig. 4. Also plotted are two model functions (dashed lines) derived by convolving a hemodynamic response function with boxcars representing the pacing phase alone (early return to baseline) and the pacing and continuation phases together (later return to baseline). As expected, BOLD signal increases in contralateral primary motor area (M1) remain elevated across pacing (filled bars) and continuation (shaded bars) blocks for both the syncopation (open circles) and synchronization (Xs) conditions. This sustained activity reflects the continuous nature of the movement. Within primary auditory cortex (A1), a decrease in BOLD amplitude is associated with the end of the pacing phase for both syncopation and synchronization. The mean time series corresponds well to the function that explicitly models BOLD increases only during pacing and is consistent with the ANOVA result showing significant difference in BOLD signal amplitude between continuation and pacing within STG. The final time series is from the SMA where, according to the statistical analysis, BOLD signal amplitude was unaffected by the transition from pacing to continuation but was sensitive to the coordination pattern (differences between syncopation and synchronization). It is clear from this trace that activity during continuation is not a result of a slow return to baseline after pacing (see A1) but signifies persistent activation that does not begin to decline until the end of the continuation phase.
Fig. 4.
BOLD time series from a representative subject are averaged across presentation blocks and voxels within three regions of interest (ROI). Functionally defined ROI were identified in contralateral precentral gyrus (M1), STG (A1), and in the medial aspect of the MFG (SMA). Dashed lines show theoretical bold responses to the pacing phase alone (filled bar) or pacing and continuation together (shaded bar). Mean signals are shown for both syncopation (open circles) and synchronization (X). BOLD amplitude is shown on the vertical axis and time in terms of scan repetitions [repetition time (TR) = 3 s] relative to the onset of the rest period is shown on the horizontal axis.
Discussion
This research provides insight into the neural basis of coordination and timing by exploiting two well established paradigms, the syncopation-synchronization paradigm of coordination dynamics and the continuation paradigm of classical motor program theory. Results demonstrate how neural areas recruited in the performance of a simple timing behavior not only reflect the temporal and motor demands of the task at hand, but also seem to be strongly influenced by the way in which the required temporal interval is established. During continuation, the pattern of hemodynamic activity generated in the production of paced finger flexion was considerably different when the pacing was established by using the syncopated pattern compared with the synchronized one. On the other hand, when comparing the two continuation conditions with their respective pacing counterparts, the only common difference was a relative decrease in activity within auditory processing areas. This finding leads to the interesting consequence that two related but different timing networks were invoked to support a simple self-paced motor task.
The general pattern of activity supporting continuation after synchronization reported here bears similarity to the results of Jäncke et al. (8), who also observed no statistical difference between auditory synchronization and continuation. Several differences are noted, however, when comparing the current results with those of Rao et al. (7). During synchronized pacing, in addition to those areas of activation reported by Rao et al. (7), we observed additional activity in SMA, putamen, and insula whereas the STG activity reported by Rao et al. (7) was modest in comparison with that reported here. Furthermore, whereas we show no significant difference between synchronization and continuation, Rao et al. (7) indicate that continuation results in additional activity in SMA, thalamus, putamen, and inferior frontal gyrus. However, care must be taken in interpreting the Rao et al. (7) results because a statistical comparison between pacing and continuation conditions was not performed. Although the source of differences between the current results and those of Rao et al. (7) is not immediately clear, differences in analysis and experimental parameters (such as movement rate) may certainly contribute.
The location of regions showing significantly greater activity during syncopated pacing compared with synchronized pacing are in agreement with those reported recently by Mayville et al. (14). Increased activity within subsystems associated with timing (basal ganglia and cerebellum) (4-6, 18) motor planning and preparation (SMA and dorsal-premotor) (24), and working memory and attention (prefrontal cortex, superior parietal lobe, and MTG) (19) have been postulated to reflect increases in cognitive demand for performance of the off-the-beat coordination pattern. What is most interesting, however, is that these differences in neural activity are still observed during continuation when no metronome is present and the specific cognitive constraints expressed during pacing no longer exist.
Differentiation in BOLD activity between syncopation and synchronization and the subsequent differences between continuation conditions cannot be explained by the presence of differences in behavioral parameters. Although there is evidence that the BOLD signal may be modulated by movement parameters such as rate (25) and force (26), the similarity of the movement profiles for the two coordination modes indicates that movement parameters did not significantly influence the present results. Similarly, differences in BOLD amplitude were not related to overall performance of the task, as indicated by the mean and standard deviation of the IRI. These performance data further suggest that both syncopation and synchronization were adequate in generating a representation of the required movement interval.
Increases in clock variance (and in some cases motor variance) have been demonstrated to occur in patient populations suffering from cerebellar lesions (6), frontal lobe lesions (27), and Parkinson's disease (4, 5), and in normal participants when simultaneously performing a secondary task (28). However, although BOLD increases within basal ganglia, cerebellum, and frontal areas, as seen during syncopated continuation, may be considered as representing greater demand within similar subsystems as those affected in patient populations, no concomitant increase in clock variance was observed. Such a result suggests that recruitment of a broader cortical-subcortical network during syncopated continuation compensates for increased temporal demand, allowing for stable performance of interval production.
Differential expression of neural activity during synchronization and syncopation may reflect the functioning of distinct processing networks such as those postulated recently by Lewis and Miall (29) for automatic and cognitively controlled timing. All experimental conditions in the present study activated a network compatible with the automatic, motor-related timing network (M1, SMA, basal ganglia, and cerebellum). However, the additional activity during syncopation tasks in prefrontal, dorsolateral premotor, and parietal areas is compatible with increased participation of memory and attention processes, presumably reflecting increased cognitive control (29). Moreover, this interpretation is consistent with behavioral findings showing that anti-phase relationships impose greater cognitive and attentional demands than in-phase patterns (17, 30). Within the current context, this finding not only suggests that different timing networks are required for the performance of the different pacing patterns, but that once established, the same timing networks continue to operate during continuation.
Our results show that a more restricted timing network than the one used after syncopation can clearly meet the motor and temporal demands of continuation. This finding leads to the question of why the more extended network recruited for syncopation persists during continuation. Although other mediating factors may contribute, one distinct possibility is that these BOLD patterns reflect differential representation of temporal information during the two pacing phases. Most popular process models of interval timing propose the existence of specific mechanisms for representing and storing temporal intervals (31-33). However, there has been little consideration of the specific neural form this memory might take. Based on the present results, we propose that the pattern of activity within and across the various subsystems involved in pacing may comprise specific sensory-motor and timing information required for accurate performance during continuation. The global pattern of neural activity that underlies temporal information and processing may thus be defined not only by activity within functionally specific timing regions, but also by the connectivity or communication between regions. Thus, the same timing network recruited to meet the constraints of the syncopated coordination pattern must also be used during subsequent continuation, or as long as the temporal information continues to be referenced. The overriding consequence of this proposed mechanism is that substantially different networks become recruited for processing of the same temporal task.
A primary goal of studies employing the continuation paradigm is to add to our understanding of the neural structures and processes underlying timing (1). Imperative to this understanding is an appreciation for the degree to which the networks revealed by using this paradigm are dependent on the experimental context. Although it is recognized that pacing and continuation involve partially overlapping task demands and engage similar neural systems (7, 8), the specific role that the pacing plays in determining brain mechanisms mediating continuation has not previously been considered. Results presented here strongly suggest that neural activity underlying continuation does not generalize across all timing contexts but is strongly influenced by the prior pacing context. It seems that, with respect to the distributed neural networks engaged, both time (in the form of time interval information) and timing (in the form of a sensorimotor relationship) play an integral role in the venerable continuation paradigm.
Acknowledgments
We are grateful to a reviewer for proposing the carry-over hypothesis and encouraging us to test it. This work was supported by National Institute of Mental Health Grants MH42900 and MH01386.
Abbreviations: SMA, supplementary motor area; STG, superior temporal gyrus; MFG, middle frontal gyrus; MTG, middle temporal gyrus; BA, Brodmann's area; BOLD, blood oxygen level-dependent; IRI, inter-response interval; SII, secondary somatosensory cortex; PoG, post central gyrus; DLPMC, dorsolateral premotor cortex.
References
- 1.Wing, A. M. (2002) Brain Cognit. 48, 7-30. [DOI] [PubMed] [Google Scholar]
- 2.Stevens, L. T. (1886) Mind 11, 393-404. [Google Scholar]
- 3.Wing, A. & Kristofferson, A. (1973) Percept. Psychophys., 14, 5-12. [Google Scholar]
- 4.Harrington, D. L. & Haaland, K. Y. (1998) in Timing of Behavior: Neural, Psychological and Computational Perspectives, eds. Rosenbaum, D. A & Collyer, D. E. (MIT Press, Cambridge, MA), pp. 35-61.
- 5.Harrington, D. L., Haaland, K. Y. & Knight, R. T. (1998) J. Neurosci. 18, 1085-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivry, R. B. & Keele, W. (1989) J. Cognit. Neurosci. 1, 136-153. [DOI] [PubMed] [Google Scholar]
- 7.Rao, S. M., Harrington, D. L., Haaland, K. Y., Bobholz, J. A., Cox, R. W. & Binder, J. R. (1997) J. Neurosci. 17, 5528-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jäncke, L., Loose, R., Lutz, K., Specht, K. & Shah, N. J. (2000) Brain Res. Cognit. Brain Res. 10, 51-66. [DOI] [PubMed] [Google Scholar]
- 9.Jirsa, V. K. & Kelso, J. A. S. eds. (2004) Coordination Dynamics: Issues and Trends, Springer Series in Understanding Complex Systems (Spinger, Heidelberg).
- 10.Kelso, J. A. S., Bressler, S. L., Buchanan, S., deGuzman, G. C., Ding, M., Fuchs, A. & Holroyd, T. (1992) Phys. Lett. A 169, 134-144. [Google Scholar]
- 11.Kelso, J. A. S., DelColle, J. D. & Schöner, G. (1990) in Attention and Performance XIII, ed. Jeannerod, M. (Erlbaum, Hillsdale, NJ), pp. 139-169.
- 12.Kelso, J. A. S., Fink, P. W., DeLaplain, C. R. & Carson, R. G. (2001) Proc. R. Soc. London B Biol. Sci. 268, 1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayville, J. M., Fuchs, A., Ding, M., Cheyne, D., Deecke, L. & Kelso, J. A. S. (2001) Hum. Brain Mapp. 14, 65-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayville, J. M., Jantzen, K. J., Fuchs, A., Steinberg, F. L. & Kelso, J. A. S. (2002) Hum. Brain Mapp. 17, 214-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelso, J. A. S., Fuchs, A., Lancaster, R., Holroyd, T., Cheyne, D. & Weinberg, H. (1998) Nature 392, 814-818. [DOI] [PubMed] [Google Scholar]
- 16.Jantzen, K. J., Fuchs, A., Mayville, J. M., Deecke, L. & Kelso, J. A. S. (2001) J. Clin. Neurophysiol. 112, 1685-1697. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Lindenberg, A., Ziemann, U., Hajak, G., Cohen, L. & Berman, K. F. (2002) Proc. Natl. Acad. Sci. USA, 99, 10948-10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diedrichsen, J., Ivry, R. & Pressing, J. (2003) in Functional and Neural Mechanisms of Interval Timing, ed. Meck, W. H., (CRC, Boca Raton, FL), pp. 457-484.
- 19.Smith, E. E. & Jonides, J. (1998) Proc. Natl. Acad. Sci. USA 95, 12061-12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanone, P. & Kelso, J. A. S. (1992) J. Exp. Psychol. Hum. Percept. Perform. 18, 403-421. [DOI] [PubMed] [Google Scholar]
- 21.Cox, R. W. (1996) Comput. Biomed. Res. 29, 162-173. [DOI] [PubMed] [Google Scholar]
- 22.Cox, R. W. & Hyde, J. S. (1997) NMR Biomed. 10, 171-178. [DOI] [PubMed] [Google Scholar]
- 23.Talairach, J. & Tournoux, P. (1988) Co-planar Stereotaxic Atlas of the Brain (Thieme, New York).
- 24.Larsson, J., Gulyás, B. & Roland, P. E. (1996) NeuroReport, 7, 463-468. [DOI] [PubMed] [Google Scholar]
- 25.Jäncke, L., Specht, K., Mirzazade, S., Loose, R., Himmelbach, M., Lutz, K. & Shah, N. J. (1998) Neurosci. Lett. 252, 37-40. [DOI] [PubMed] [Google Scholar]
- 26.Thickbroom, G. W., Phillips, B. A., Morris, I., Byrnes, M. L. & Mastaglia, F. L., (1998) Exp. Brain Res. 121, 59-64. [DOI] [PubMed] [Google Scholar]
- 27.Mangels, J. A., Ivry, R. & Shimuzu, N. (1998) Cognit. Brain Res. 7, 15-39. [DOI] [PubMed] [Google Scholar]
- 28.Seargent, V., Hellige, J. B. & Cherry, B. (1993) Brain Cognit. 23, 243-262. [DOI] [PubMed] [Google Scholar]
- 29.Lewis, P. & Miall, C. (2003) in Functional and Neural Mechanisms of Interval Timing, ed. Meck., W. (CRC, Boca Raton, FL), pp. 515-532.
- 30.Monno, A., Temprado, J. J., Zanone, P. G. & Laurent, M. (2002) Acta Psychol. 110, 187-211. [DOI] [PubMed] [Google Scholar]
- 31.Creelman, C. D. (1962) J. Acoust. Soc. Am. 34, 582-593. [Google Scholar]
- 32.Treisman, M. (1963) Psychol. Monogr. 77, 1-31. [DOI] [PubMed] [Google Scholar]
- 33.Church, R. M. (2003) in Functional and Neural Mechanisms of Interval Timing, ed. Meck., W. (CRC, Boca Raton, FL), pp. 3-22.