Abstract
Background
Nail psoriasis occurs in up to 50% of patients affected by psoriasis, with a significant impact on quality of life that leads to a real clinical need for new therapeutic options.
Aim
To confirm whether the strengthening and hardening properties of the hydroxypropyl-chitosan (HPCH) nail lacquer could improve the structure of the nail plates on psoriatic nails.
Materials and methods
A randomized, double-blind, placebo controlled, parallel-group trial was carried out to evaluate the efficacy and tolerability of a hydrosoluble nail lacquer containing HPCH, Equisetum arvense, and methylsulfonylmethane on nail psoriasis. The test product or a placebo was applied once daily for 24 weeks to all fingernails. Efficacy assessments were performed on the target fingernail by means of the modified Nail Psoriasis Severity Index score. A cut-off score of 4 was considered to define the clinical cure rate (ie, Cure ≤4, Failure >4).
Results
After 24 weeks, the clinical cure rate showed the statistically significant superiority of the HPCH nail lacquer compared to placebo in both the intention-to-treat (Fisher’s exact test, P=0.0445) and the per protocol population (Fisher’s exact test, P=0.0437). This superiority was already present after 16 weeks of treatment. Moreover, the analysis of the modified Nail Psoriasis Severity Index-50 showed a statistically significant clinical improvement after 12 weeks of treatment in comparison to the results obtained after 8 weeks (Fisher’s exact test, P<0.05).
Conclusion
The trial showed that HPCH nail lacquer could be a new, valid, effective, and safe option for decreasing the signs of nail dystrophy in psoriatic patients.
Keywords: nail psoriasis, psoriatic onychodystrophy
Introduction
Psoriasis is a chronic skin disease that causes distress and morbidity, affecting approximately 2% of the population with an emotional, social, and physical impact.1,2
Nail involvement has long been recognized as a common manifestation of psoriasis, occurring in up to 50% of patients, with an estimated lifetime incidence of 80%–90%.3 Its impact on quality of life is well known, since nail dystrophies are often highly visible, causing embarrassment for the patient and considerable disability in many cases.4,5 The most common clinical sign in nail psoriasis is pitting, which occurs in almost 70% of patients, followed by onycholysis.6 Other nail manifestations associated with psoriasis are splinter hemorrhages, leukonychia, red spots in the lunula, nail plate crumbling, salmon patches, and hyperkeratosis.1 Before starting an appropriate therapy for nail psoriasis, the severity of nail changes and the extent of skin and/or joint involvement should be considered. If a patient has extensive skin psoriasis accompanied with nail changes, systemic therapy will be appropriate, while for a patient who has predominant nail psoriasis with mild or no skin involvement, topical treatments would be an ideal initiating option for therapy.7
The choice of the topical formulation to be used depends on the presence of nail bed and/or nail matrix disease. Many products have been used in the topical treatment of nail psoriasis; corticosteroids and vitamin D analogs are still the most frequently used topical therapies found in literature.8 However, none of them have been fully satisfactory, thus determining a real clinical need for new therapeutic options.
A product currently on the market, consisting of a hydro-soluble nail lacquer and containing hydroxypropyl-chitosan (HPCH), horsetail extract (Equisetum arvense), and methylsulfonylmethane, as its main ingredients, has shown its effectiveness in the management of nail splitting and nail brittleness.9 In a previous, preliminary study, we reported the effectiveness of this nail lacquer on signs of dystrophy in psoriatic nails,10 where the HPCH nail lacquer was able to improve clinical signs such as pitting, leukonychia, and onycholysis (ie, the most common manifestations of nail psoriasis).
The present placebo-controlled study was designed to confirm whether the strengthening and hardening properties of this product could improve the structure of the nail plates on psoriatic nails.
Materials and methods
The study was a randomized, single-center, double-blind, placebo controlled, parallel-group trial to evaluate the effects and tolerability of an HPCH nail lacquer on nail psoriasis (the lacquer Genadur®, also known as Ecocel®, Sililevo®, Betalfatrus®, Psoriatec®, and Kitolac®; Polichem SA, Lugano, Switzerland; it is also known as VERALAC® distributed by Valeo Pharma Inc., Kirkland, QC, Canada). It was approved by the independent Ethics Committee of the “Azienda Policlinico Umberto I”, Rome, Italy, and was performed at the Department of Dermatology of the Sapienza University, Rome, Italy.
A total of 87 patients of both sexes and aged 16–80 years were enrolled after providing written informed consent. Patients affected by mild to moderate nail psoriasis of the nail matrix and/or of the nail bed in at least one fingernail, with a clinical diagnosis of psoriasis at least 6 months previously, were randomly allocated in a 1:1 ratio to one of the study groups corresponding to the HPCH nail lacquer or to placebo (which was the vehicle of the HPCH nail lacquer). The main exclusion criteria were the use of any systemic treatment for the cutaneous lesions in the previous 3 months, any other topical product (drug or cosmetic) on any nail in the 4 weeks before the baseline visit, and a positive mycology test. The patients were treated once daily for 24 weeks on all fingernails in the evening at bedtime. The nails were not washed for 6 hours after application.
The clinical features of nail psoriasis were evaluated every 4 weeks using the modified Nail Psoriasis Severity Index (mNAPSI) on the target nail defined at baseline. According to the mNAPSI definition,11 the target nail is divided into four quadrants, and to each quadrant, a score between 0 and 3 (0= none, 1= mild, 2= moderate, 3= severe) is given for each parameter of the nail matrix (presence or absence of pitting, leukonychia, red spots on the lunula, and plate crumbling) or the nail bed disease (salmon patch, onycholysis, hyperkeratosis, and splinter hemorrhages). The total score, resulting from the sum of all the partial scores, may vary between 0 (no signs) and 96, derived from 4 (quadrants) ×8 (parameters) ×3 (severe disease). The primary end point was the clinical cure rate, which was defined as an mNAPSI score ≤4 at the end of treatment. Any patients having an mNAPSI score >4 were considered as a failure. A further clinical end point was the proportion of patients who achieved a 50% reduction in the score (mNAPSI-50) compared to baseline value (ie, an improvement ≥50% of the baseline mNAPSI score).
The acceptability of the product was assessed by the patient at the end of the study according to a five-point scale: 1= very good; 2= good; 3= fair; 4= poor; 5= very poor.
Data were processed by means of the program SAS for Windows, release 9.2 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were performed, including the mean, standard deviation, standard error, minimum value, median, and maximum value for continuous variables. According to the Last Observation Carried Forward approach,12 the last observed value of the mNAPSI score was used as an end point value in the event that patients prematurely withdrew from the study (for any reason). The significance level was declared as a P-value <0.05 (two-tailed test).
The superiority of the test product versus placebo was tested for efficacy parameters in the intention-to-treat (ITT) population by means of Fisher’s exact test. The same model was applied on the per protocol (PP) population as a supportive efficacy analysis for the aforementioned parameters.
The difference in patients’ judgments on treatment acceptability was analyzed by means of the chi-square test.
Results
A total of 87 patients were randomized: 43 in the HPCH nail lacquer group and 44 in the placebo group. Eighty-one out of 87 patients were considered evaluable for the ITT efficacy analysis since six patients (three in each group) had no postbaseline assessments. The two groups were similar with respect to their demographic characteristics (Table 1).
Table 1.
Demographic and baseline characteristics of the two groups
| HPCH nail lacquer N=43 | Placebo N=44 | |
|---|---|---|
| Sex, N (%) | ||
| Males | 25 (58.1) | 26 (59.1) |
| Females | 18 (41.9) | 18 (40.9) |
| Age, years | ||
| Mean ± SD | 54.1±15.4 | 52.7±16.9 |
| Median | 56 | 55.8 |
| Range | 24.02–80.7 | 16.5–81.7 |
| Race, N (%) | ||
| Caucasian | 43 (100) | 43 (97.7) |
| Asian | 0 (0) | 1 (2.3) |
| Working activities, N (%) | ||
| Worker | 23 (53.5) | 25 (56.8) |
| Housewife | 7 (16.3) | 5 (11.4) |
| Retired | 9 (20.9) | 11 (25) |
| Student | 3 (7) | 2 (4.5) |
| Unemployed | 1 (2.3) | 1 (2.3) |
| Psoriasis diagnosis, N (%) | ||
| ≤1 year | 5 (11.6) | 6 (13.6) |
| >1 and ≤3 | 10 (23.25) | 8 (18.2) |
| >3 and ≤5 | 18 (41.9) | 17 (38.6) |
| >5 | 10 (23.25) | 13 (29.6) |
| mNAPSI baseline (ITT population) | ||
| Mean ± SD | 13±6.64 | 14.32±8.10 |
| Median | 12 | 14 |
| Range | 2–32 | 4–42 |
| mNAPSI baseline (PP population) | ||
| Mean ± SD | 12.68±5.88 | 15.75±8.45 |
| Median | 12 | 14.5 |
| Range | 6–30 | 4–42 |
Abbreviations: HPCH, hydroxypropyl-chitosan; N, number; SD, standard deviation; mNAPSI, modified Nail Psoriasis Severity Index; ITT, intention-to-treat; PP, per protocol.
The clinical cure rate, which was defined as the primary end point and evaluated every 4 weeks, showed that after 24 weeks (end of treatment) there was a statistically significant superiority of the HPCH nail lacquer compared to placebo in both the ITT (Fisher’s exact test, P=0.0445) and PP populations (Fisher’s exact test, P=0.0437). To be precise, in the ITT dataset, 55% (22/40) of patients treated with the HPCH nail lacquer versus 31.7% (13/41) of the placebo patients were cured after 24 weeks (Figure 1). The superiority of the test product over placebo was already evident after 16 weeks in 35.0% (14/40) of the HPCH nail lacquer treated patients versus 14.6% (6/41) of the placebo patients (Fisher’s exact test, P=0.0415; ITT population).
Figure 1.
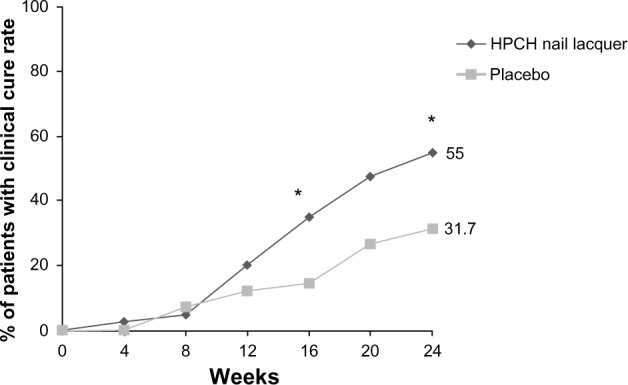
Proportion of patients with a clinical cure from baseline to week 24 at each measured time point (ITT population).
Notes: HPCH nail lacquer versus placebo; *P>0.05 (Fisher’s exact test).
Abbreviations: HPCH, hydroxypropyl-chitosan; ITT, intention-to-treat.
The difference was higher when the PP dataset, including all patients who completed the trial according to the protocol, was considered: 67.7% (21/31) of patients treated with the HPCH nail lacquer and 40.6% (13/32) of patients randomized to the placebo had become cured at the end of the treatment period (Figure 2).
Figure 2.
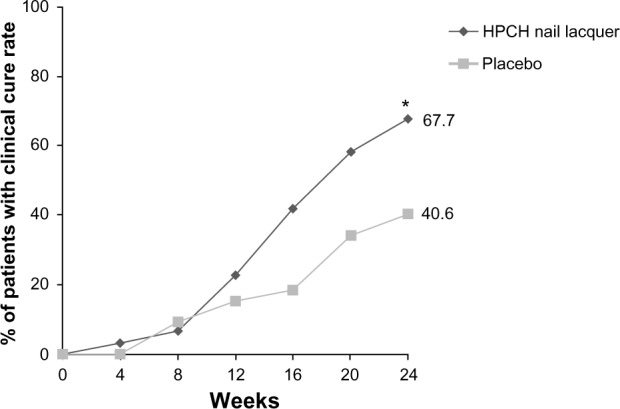
Proportion of patients with a clinical cure from baseline to week 24 at each measured time point (PP population).
Notes: HPCH nail lacquer versus placebo; *P<0.05 (Fisher’s exact test).
Abbreviations: HPCH, hydroxypropyl-chitosan; PP, per protocol.
The analysis of mNAPSI-50 indicates a trend of superiority of HPCH nail lacquer compared to placebo (Figure 3) throughout the 24-week treatment course. Moreover, the clinical improvement observed in the test product group had already been confirmed after 12 weeks of treatment, in fact the mNAPSI-50 score was statistically superior in comparison to the results obtained after 8 weeks of treatment (Fisher’s exact test, P<0.05).
Figure 3.
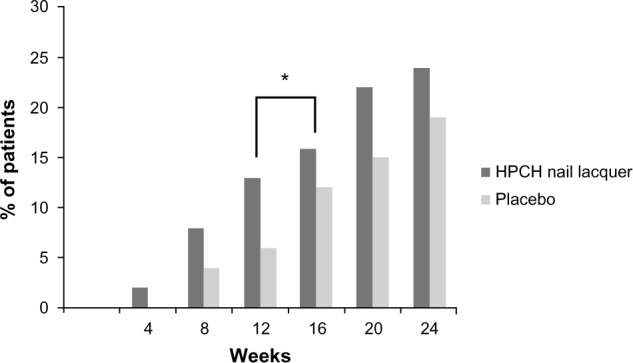
Proportion of patients with improvement in mNAPSI of ≥50% from baseline to week 24 at each measured time point (ITT population).
Notes: HPCH nail lacquer week 16 versus week 12; *P<0.05 (Fisher’s exact test).
Abbreviations: HPCH, hydroxypropyl-chitosan; mNAPSI, modified Nail Psoriasis Severity Index; ITT, intention-to-treat.
The improvement of clinical signs of nail psoriasis at the end of treatment is documented in Figures 4 and 5.
Figure 4.
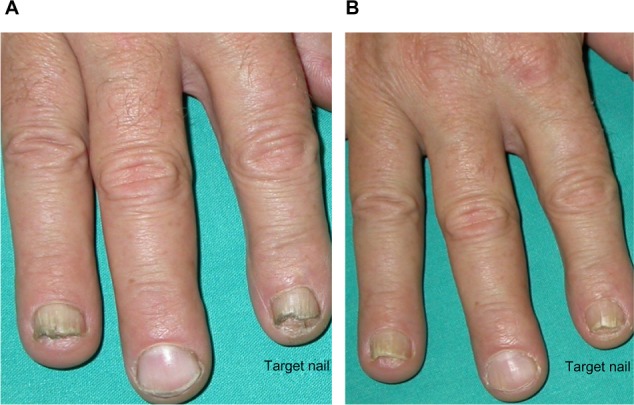
Improvement of onycholysis, leukonychia, and hyperkeratosis compared to baseline.
Notes: (A) Pretreatment (baseline mNAPSI =14); (B) posttreatment (mNAPSI =4).
Abbreviation: mNAPSI, modified Nail Psoriasis Severity Index.
Figure 5.
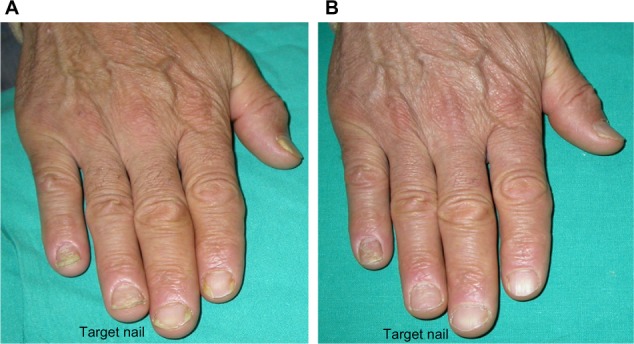
Onycholysis and leukonychia completely resolved, and improvement of nail plate crumbling compared to baseline.
Notes: (A) Pretreatment (baseline mNAPSI =12); (B) posttreatment (mNAPSI =2).
Abbreviation: mNAPSI, modified Nail Psoriasis Severity Index.
The level of acceptability of the products by the patients was also evaluated in the ITT population. A statistically significant difference was observed between treatments, in favor of the HPCH nail lacquer (Figure 6) at the end of the treatment period (chi-square test, P=0.0265). In detail, the level of acceptability was assessed as “good” or “very good” by 96.8% of patients in the HPCH nail lacquer group (30/31) compared to 78.1% (25/32) of placebo-treated subjects. Compliance was excellent in all patients.
Figure 6.
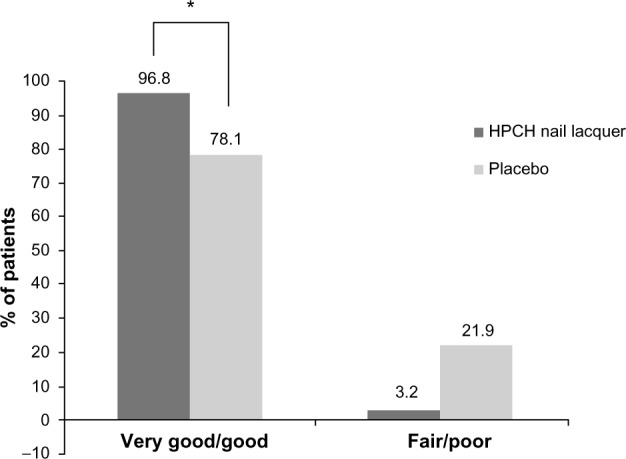
Proportion of patients who judged the treatment positively/negatively at week 24 (PP population).
Note: HPCH nail lacquer versus placebo; *P<0.05 (chi-square test).
Abbreviations: HPCH, hydroxypropyl-chitosan; PP, per protocol.
A case report of a patient who had not been cured at the end of the treatment period is described as follows. The patient, encouraged by the result obtained after 6 months of treatment, decided to continue the product application, and after 27 months, the nail psoriasis was cured without using any systemic or other topical nail treatments (Figure 7).
Figure 7.
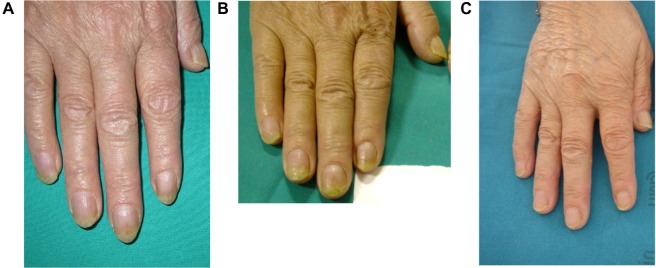
Nail plate crumbling, hyperkeratosis, and splinter hemorrhage improved after 24 weeks and resolved after 27 months compared to baseline.
Notes: (A) Baseline; (B) improvement after 24 weeks; (C) resolved after 27 months.
No local adverse reactions were reported in either treatment group. Eight patients reported nonserious adverse events, six in the placebo group and two in the HPCH nail lacquer group. No adverse events were assessed by the investigator as being related to the study treatment.
Discussion
Psoriasis of the nail is associated with disability, as the nails are weak and often painful in this condition; this has remained an unsatisfied medical need over the last 2 decades.4,13 The choice of treatment for nail psoriasis depends on the clinical features of the disease, as well as on individual factors for each patient. If the associated skin psoriasis is not systemically treated, or if nail psoriasis is the sole manifestation of the disease, topical therapies are the appropriate first choice. The current treatment options for nail psoriasis are poorly efficacious, and patients need long-term treatments in order to obtain any clinical benefit.5,14 The situation has not greatly changed after the introduction of biologics in the management of patients with psoriasis.13 Biologic agents have demonstrated the best outcome compared with other available therapeutic methods; however, the relatively higher social costs and greater risks of adverse effects remain a serious concern. Hence, systemic treatment should be reserved for a minority of patients with severe skin psoriasis and/or joint involvement.7,13
At the moment, topical treatment options are limited or absent due to the great difficulty experienced in delivering the drug to the site of action, and also due to the fact that the commercially available products are usually in the form of creams or gels, which are unsuitable to remain in contact with the nails for a sufficient period of time, unless they are applied by means of complicated occlusive or semiocclusive medications.15–17 Topical therapy for nail psoriasis would be very welcome if nail lacquers become available that demonstrate activity in this condition.
We previously reported the ability of this HPCH nail lacquer to decrease signs of dystrophy in psoriatic nails,10 although that study was neither randomized nor blind, and it had only a preliminary value. The present randomized, placebo-controlled, double-blind trial could be considered as one of the most powerful clinical studies performed to date, and the results confirm the effectiveness of HPCH nail lacquer applied for 24 weeks in nail psoriasis, with the test product proving to be statistically superior to placebo. Currently, no other powered study on the management of nail psoriasis that compares a topical medical device to placebo has been published.18 The Nail Psoriasis Severity Index (NAPSI) has been developed as an objective and reproducible tool to standardize the treatment outcome in nail psoriasis treatment. Specifically, the current NAPSI system, which uses a 32-point scale for the target nail without taking into consideration disease severity, may lack the sensitivity to reflect meaningful clinical improvement.19
In 2005, the mNAPSI, with scores ranging between 0–96 for the target nail, and using a qualitative gradation of severity for each parameter, (from 0–3) in each quadrant, was proposed by Parrish et al.11 This alternative evaluation index was developed in order to increase the sensitivity of the NAPSI to reflect the response to therapy of the disease.
Nevertheless, the simple decrease of the aforementioned scores may not reflect the real clinical outcome and the complete or almost complete resolution of the signs and symptoms of nail psoriasis. For this purpose, in our investigation, we proposed an mNAPSI cut-off score of 4 based on the clinical observation that a value ≤4 does not cause disability and shows a robust clearing of the psoriatic nail lesion. This value is consistent with the published literature.19 In fact, Rigopoulos et al20 have already defined that a value between 3–4 indicates an almost complete resolution of psoriatic nail involvement.
Moreover, the proportion of patients achieving an mNAPSI-50 was calculated. This evaluation is the current benchmark used in most clinical trials of psoriasis.21 It could be hypothesized that a 50% reduction in the mNAPSI score represents a meaningful change in the clinical outcome.
Although the clinical benefit of the treatment was already evident after 12 weeks, the 24-week treatment course is the minimum period required to demonstrate evidence of the treatment’s meaningful effects. No differences in terms of the safety profile between the product and the placebo were found during the study.
In addition, the clinical setting suggests that the longer the treatment period is, the greater the efficacy of the HPCH nail lacquer. However, considering that a treatment lasting longer than 6 months for the resolution of nail psoriasis is justified by the high safety and tolerability profile of the product, further experiments should be designed in order to confirm the reported results.
Conclusion
In conclusion, considering the poor clinical outcome of the available systemic and topical treatments for nail psoriasis, HPCH nail lacquer is a valid, effective, and safe option that can be used to decrease the signs of nail dystrophy in psoriatic patients.
Footnotes
Disclosure
The clinical study was entirely supported by Polichem SA, which is the holder of all the rights related to the study. MC and RP are employees of Polichem SA. The other authors report no conflicts of interest in this work.
References
- 1.Jiaravuthisan MM, Sasseville D, Vender RB, Murphy F, Muhn CY. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol. 2007;57(1):1–27. doi: 10.1016/j.jaad.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 2.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–284. [PubMed] [Google Scholar]
- 3.Van Laborde S, Scher RK. Developments in the treatment of nail psoriasis, melanonychia striata, and onychomycosis. A review of the literature. Dermatol Clin. 2000;18(1):37–46. doi: 10.1016/s0733-8635(05)70145-5. [DOI] [PubMed] [Google Scholar]
- 4.de Jong EM, Seegers BA, Gulinck MK, Boezeman JB, van de Kerkhof PC. Psoriasis of the nails associated with disability in a large number of patients: results of a recent interview with 1,728 patients. Dermatology. 1996;193(4):300–303. doi: 10.1159/000246274. [DOI] [PubMed] [Google Scholar]
- 5.Gupta AK, Cooper EA. Psoriatic nail disease: quality of life and treatment. J Cutan Med Surg. 2009;13(Suppl 2):S102–S106. doi: 10.2310/7750.2009.00027. [DOI] [PubMed] [Google Scholar]
- 6.Tham SN, Lim JJ, Tay SH, et al. Clinical observations on nail changes in psoriasis. Ann Acad Med Singapore. 1988;17(4):482–485. [PubMed] [Google Scholar]
- 7.Tan ES, Chong WS, Tey HL. Nail psoriasis: a review. Am J Clin Dermatol. 2012;13(6):375–388. doi: 10.2165/11597000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura RC, Abreu Ld, Duque-Estrada B, Tamler C, Leverone AP. Comparison of nail lacquer clobetasol efficacy at 0.05%, 1% and 8% in nail psoriasis treatment: prospective, controlled and randomized pilot study. An Bras Dermatol. 2012;87(2):203–211. doi: 10.1590/s0365-05962012000200003. [DOI] [PubMed] [Google Scholar]
- 9.Sparavigna A, Setaro M, Genet M, Frisenda L. Equisetum arvense in a new transungual technology improves nail structure and appearance. Journal of Plastic Dermatology. 2006;2(1):31–38. [Google Scholar]
- 10.Cantoresi F, Sorgi P, Arcese A, et al. Improvement of psoriatic onychodystrophy by a water-soluble nail lacquer. J Eur Acad Dermatol Venereol. 2009;23(7):832–834. doi: 10.1111/j.1468-3083.2009.03209.x. [DOI] [PubMed] [Google Scholar]
- 11.Parrish CA, Sobera JO, Elewski BE. Modification of the Nail Psoriasis Severity Index. J Am Acad Dermatol. 2005;53(4):745–746. doi: 10.1016/j.jaad.2004.11.044. author reply 746. [DOI] [PubMed] [Google Scholar]
- 12.Desouza CM, Legedza ATR, Sankoh AJ. An overview of practical approaches for handling missing data in clinical trials. J Biopharm Stat. 2009;19:1055–1073. doi: 10.1080/10543400903242795. [DOI] [PubMed] [Google Scholar]
- 13.Lawry M. Biological therapy and nail psoriasis. Dermatol Ther. 2007;20(1):60–67. doi: 10.1111/j.1529-8019.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 14.De Simone C, Maiorino A, Tassone F, D’Agostino M, Caldarola G. Tacrolimus 0.1% ointment in nail psoriasis: a randomized controlled open-label study. J Eur Acad Dermatol Venereol. 2013;27(8):1003–1006. doi: 10.1111/j.1468-3083.2012.04642.x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer-Levancini C, Sánchez-Regaña M, Llambí F, Collgros H, Expósito-Serrano V, Umbert-Millet P. Nail psoriasis: treatment with tazarotene 0.1% hydrophilic ointment. Actas Dermosifiliogr. 2012;103(8):725–728. doi: 10.1016/j.ad.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez Regaña M, Márquez Balbás G, Umbert Millet P. Nail psoriasis: a combined treatment with 8% clobetasol nail lacquer and tacalcitol ointment. J Eur Acad Dermatol Venereol. 2008;22(8):963–969. doi: 10.1111/j.1468-3083.2008.02679.x. [DOI] [PubMed] [Google Scholar]
- 17.Rigopoulos D, Gregoriou S, Katsambas A. Treatment of psoriatic nails with tazarotene cream 0.1% vs clobetasol propionate 0.05% cream: a double-blind study. Acta Derm Venereol. 2007;87(2):167–168. doi: 10.2340/00015555-0195. [DOI] [PubMed] [Google Scholar]
- 18.de Vries AC, Bogaards NA, Hooft L, et al. Interventions for nail psoriasis. Cochrane Database Syst Rev. 2013;1:CD007633. doi: 10.1002/14651858.CD007633.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49(2):206–212. doi: 10.1067/s0190-9622(03)00910-1. [DOI] [PubMed] [Google Scholar]
- 20.Rigopoulos D, Gregoriou S, Stratigos A, et al. Evaluation of the efficacy and safety of infliximab on psoriatic nails: an unblinded, nonrandomized, open-label study. Br J Dermatol. 2008;159(2):453–456. doi: 10.1111/j.1365-2133.2008.08686.x. [DOI] [PubMed] [Google Scholar]
- 21.Fabroni C, Gori A, Troiano M, Prignano F, Lotti T. Infliximab efficacy in nail psoriasis. A retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25(5):549–553. doi: 10.1111/j.1468-3083.2010.03826.x. [DOI] [PubMed] [Google Scholar]


