Blood clot formation resulting in the complete occlusion of a blood vessel (thrombosis) often leads to serious life threatening events such as stroke and heart attack. Because the composition of a thrombus changes as it matures, new imaging methods that are capable of distinguishing new clot from old clot may yield important diagnostic and prognostic information.[1] To address this need we have developed an activatable MR probe that is responsive to a key biochemical process associated with recently formed clots.
New thrombi differ from mature thrombi in a variety of ways. They contain a host of enzymatic activities including those responsible for the clotting cascade; they contain various of types leukocytes; and they also contain activated platelets, which play an important role in mediating both thrombus formation and the wound healing process.[1-2] When platelets become activated, they aggregate to form a hemostatic plug at the clot site. This aggregation process is facilitated by integrins located on the surface of activated platelets that can bind fibrinogen with high affinity only after being triggered by the enzymatic activity of protein disulfide isomerase (PDI).[3]
PDI is a protein-folding chaperone that typically resides in the lumen of the endoplasmic reticulum.[4] However some extracellular sources have also been reported including on the exterior surface of platelets.[5] Studies have shown that upon platelet activation, surface-associated PDI undergoes a shift in the oxidation state of active-site cysteine residues favoring the enzymatically-active reduced form.[6] The fact that PDI activity is present on the surface of activated platelets could make it a useful biomarker of nascent blood clots. Therefore we have designed an MR imaging probe that takes advantage of this unique chemistry.
The native concentration of enzymes like PDI is often too low for detection by MR probes using direct targeting. However there are numerous examples of activatable MR probes that sense enzymatic activity.[7] These probes undergo modification by the enzyme that changes the relaxivity of the probe: this can be due to a change in hydration state,[8] or a change in rotational correlation time of the molecule.[9] A challenge remains in keeping the probe localized to the site of activation long enough for imaging. For instance Miserus et al. addressed this issue by exploiting the enzymatic activity of the transglutaminase FXIIIa, which covalently attached a Gd-based MR probe directly to fibrin.[10]
Here we describe a PDI activatable probe for detection of fresh blood clots that is retained in the clot through non-covalent binding to fibrin upon activation. This technology builds on the properties of the previously reported fibrin-imaging probe EP-2104R (Scheme 1), which binds selectively to fibrin with high affinity (106 – 107 M−1) providing strong signal enhancement to the region with its four attached gadolinium chelates.[11] EP-2104R was shown to provide persistent clot enhancement in animal models and in human clinical trials.[12] However it cannot distinguish new clots from older more stable clots. Here, we prepared compound 1, a non-binding pro-form of EP-2104R, which can be activated by PDI.
Scheme 1.
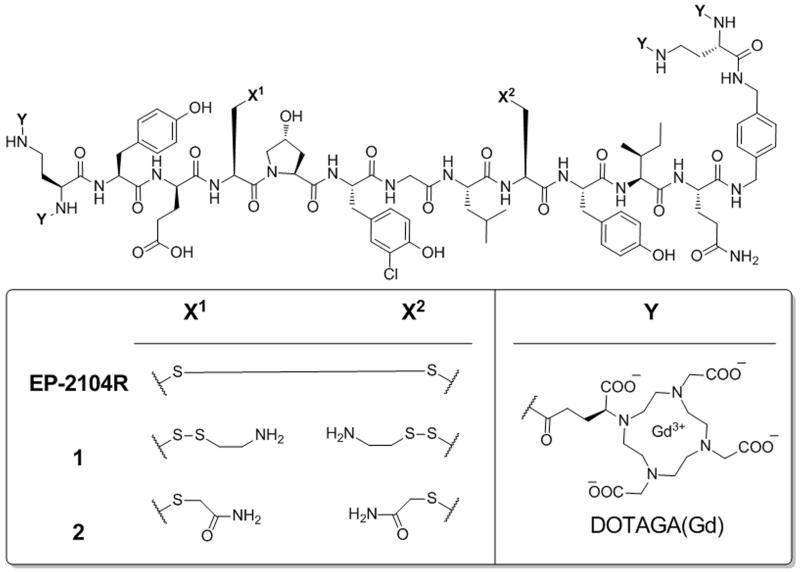
Structures of the fibrin imaging probe EP-2104R and the mixed disulfide 1, which can be converted to EP-2104R through the enzymatic activity of PDI. Compound 2 is a non-binding analogue to 1 that is not a substrate for PDI and serves as a negative control.
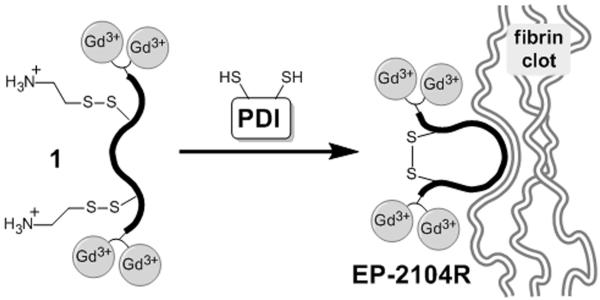
EP-2104R contains a key disulfide bond between cysteine residues that is required for binding. The presence of this bond helps to preorganize many of the dihedral angles of the peptide macrocycle and gives rigidity to the overall structure. Previous studies in our lab have demonstrated that any modifications to EP-2104R that prevent disulfide formation between these two cysteine residues blocks binding to fibrin.[13] We decided to exploit this feature by appending cystamine groups to both of the cysteine side chains to prevent cyclization until the probe encounters PDI (Figure 1). In this way we expect the probe to be converted by platelet PDI to EP-2104R, which will then be selectively retained in the clot by binding to fibrin. Older clots that no longer possess high PDI activity will not show retention of the probe.
Figure 1.
Mechanism of probe activation and retention at the clotting site. PDI activity expressed on the surface of activated platelets in newly formed thrombi catalyzes the conversion of compound 1 to EP-2104R, which then binds neighboring fibrin with high affinity.
Activatable probe 1 was prepared from EP-2104R by reduction of the disulfide bond with TCEP, followed by reoxidation to the mixed disulfide in the presence of concentrated cystamine solution. Pure 1 was isolated in 46% overall yield after HPLC purification (see SI for more details).
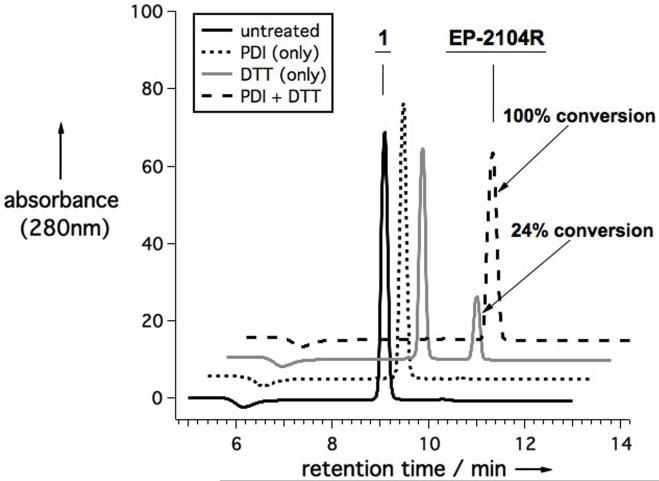
To confirm that PDI does catalyze the cyclization reaction that converts 1 to EP-2104R, we monitored the reaction by HPLC (Figure 2). Since PDI must remain in its reduced form to be active, a thiol cofactor, in this case dithiothreitol (DTT), is required. We observed quantitative conversion of 1 to EP-2104R in under 15 minutes at room temperature, while only 24% conversion was observed in presence of DTT alone, and no conversion was observed when PDI was added without DTT.
Figure 2.
Series of HPLC traces showing the extent of conversion of compound 1 (40 μM) to EP-2104R after 15 minutes under four different reaction conditions: untreated (no enzyme or DTT added); with PDI (4 mg/L); with DTT (40 μM); and with both PDI (4 mg/L) and DTT (40 μM).
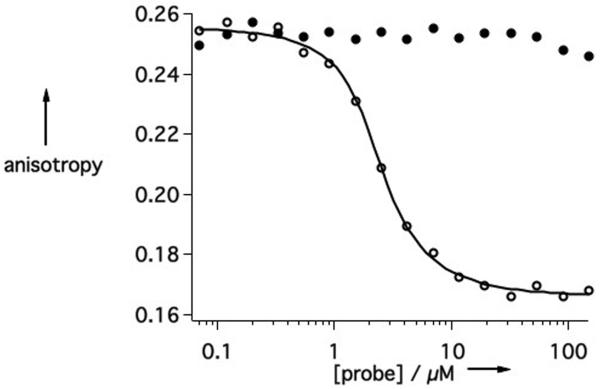
Next, the affinity of 1 for fibrin was investigated for comparison to EP-2104R. This was done using a displacement assay where the fluorescence polarization of a fibrin binding peptide (TRITC-Tn6) is measured in the presence of a competitor.[13b, 14] Here we use a soluble fragment of fibrin termed DD(E). EP-2104R can displace the fluorescent peptide resulting in a large decrease in fluorescence anisotropy (Figure 3a), allowing us to calculate a Kd value of 0.16 μM for EP-2104R. However, 1 shows virtually no competitive displacement of the TRITC-Tn6 probe across the same concentration range indicating that the dissociation constant for 1 must be at least 1000-fold greater than that of EP-2104R.
Figure 3.
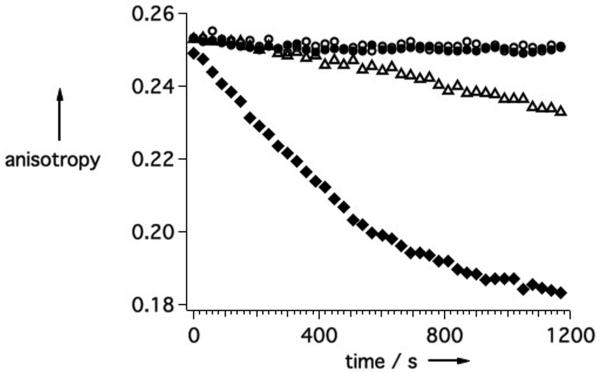
Binding of probes to DD(E) (2 μM) is detected by measuring the decrease in fluorescence anisotropy that occurs upon competitive displacement of the TRITC-Tn6 probe (0.1 μM). A) EP-2104R (○) exhibits a strong dose-dependent response whereas 1 (●) does not. B) The conversion of 1 (10 μM) to EP-2104R is followed in real-time by observing the displacement of TRITC-Tn6 from DD(E). The reaction was run under four different reaction conditions: untreated (○); 1 mg/L PDI (△); 10 μM DTT (●); and 10 μM DTT plus 1 mg/L PDI (◆).
The cyclization reaction can be followed in real-time using the same displacement assay. As 1 undergoes conversion to EP-2104R, this results in binding to DD(E) and displacement of the fluorescent peptide. The reaction was run under a series of conditions as shown in Figure 3b. Conversion of 1 proceeds rapidly when both PDI and DTT are present simultaneously compared to when just DTT alone is added. This indicates that the probe is in fact recognized as a substrate by PDI. As expected, no conversion of the probe was observed in the presence of PDI alone. Similar behaviour was observed when the reaction was followed by relaxometry (supporting info).
The relaxivities of EP-2104R, 1, and 2 are similar in the absence of DD(E). However, the relaxivity of EP-2104R is increased by 70% in the presence of excess DD(E) (Table 1) exhibiting a value similar to its relaxivity reported in fibrin.[11] This increase is due to the longer rotational correlation time of the protein bound probe. By contrast the relaxivities of 1 and 2 show no increase in the presence of DD(E).
Table 1.
Per molecule relaxivities (mM−1s−1) of probes measured in the presence and absence of DD(E) at 60 MHz, 37 °C, TBS.
| Compound |
r1 without DD(E)[b] |
r1 with DD(E)[c] |
% increase with DD(E) |
|---|---|---|---|
| EP-2104R | 38.2±0.9 | 65.8±3.4 | 72% |
| 1 | 38.6±0.7 | 40.8±1.4 | 6% |
| 2 | 35.1±1.1 | 35.0±1.1 | 0% |
[Probe] range 20 μM – 100 μM.
[Probe] range 5 μM – 25 μM and [DD(E)] = 30.5 μM.
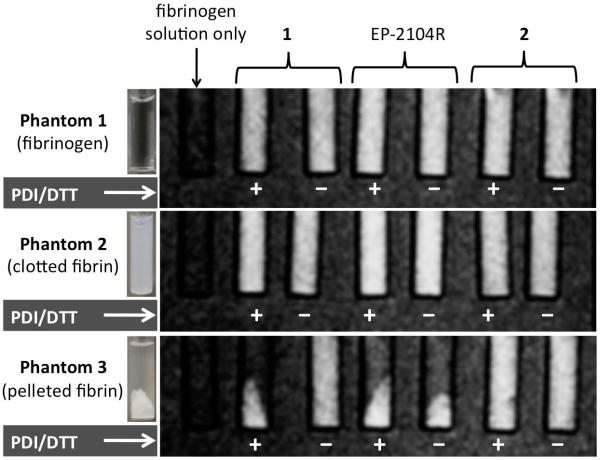
Three sets of phantoms were prepared and imaged at 1.5T in order to illustrate how the PDI catalyzed activation of the mixed disulfide probe 1 leads to fibrin binding (Figure 4). Each phantom contained a series of glass inserts immersed in water. The first insert located on the far left of each phantom contained a solution of human fibrinogen (12.4 μM) and served as a signal reference for the other inserts. The remaining inserts contained solutions of either 1, EP-2104R, or 2 displayed from left to right. For each compound, a single stock solution was divided into two separate fractions. One fraction was treated for 30 minutes with a combination of both PDI and DTT while the other was not. These solutions were then mixed with human fibrinogen to give a final fibrinogen concentration of 12.4 μM. The final probe concentration for each insert was 10 μM.
Figure 4.
T1-weighted MR images of phantoms 1-3 obtained at 1.5T. Solutions of 1, EP-2104R, and 2 treated with both PDI and DTT (+) are paired with solutions that were not treated (−). The probe-containing solutions of phantoms 2 and 3 were treated with thrombin and CaCl2 to clot the fibrinogen. The inserts of phantom 3 were centrifuged to pellet the clots.
Phantom 2 contained the same solutions as phantom 1, except these solutions were treated with thrombin and CaCl2, which converts the soluble fibrinogen into a homogeneous insoluble fibrin clot as shown in Figure 4. The solutions of phantom 3 were the same as phantom 2 except that the inserts were then centrifuged at high speed to pellet the clotted fibrin leaving a clear supernatant layer above.
In both phantoms 1 and 2 the signal enhancement from each probe was distributed uniformly throughout each of the inserts. By contrast phantom 3 clearly showed that when compound 1 was treated with both PDI and DTT, the signal enhancement co-localized with the fibrin pellet just as EP-2104R did independent of the treatment conditions. Likewise when 1 was not treated with PDI and DTT then the distribution of signal enhancement remained uniform throughout the sample as was seen for compound 2, which can neither bind fibrin nor be activated by PDI.
The binding of EP-2104R to fibrin was also marked by a measurable increase in signal enhancement due to the change in relaxivity that is associated with a decreased rotational correlation time. We compared the signal intensity ratio (SIR) between a given sample and the fibrinogen reference sample. The SIRs for each of the inserts of phantom 1 were then used to calculate the percent change in signal intensity ratio (%Δ SIR) observed for the corresponding inserts of phantoms 2 and 3 (supporting information Tables S4 and S5). When treated with PDI and DTT, compound 1 in phantom 2 exhibited a 12% increase in SIR compared to only 3% for the untreated sample. This difference was consistent with the values measured for the positive and negative fibrin binding probes, EP-2104R and 2.
The present form of 1 offers a number of properties that make it an attractive candidate for use in the identification and imaging of nascent blood clots. It is a suitable substrate for an enzyme that is uniquely expressed on the surface of activated platelets and its enzyme activated product binds fibrin selectively with an affinity 1000-fold greater than the pro-form. This activation and retention mechanism provides a high degree of specificity since it depends on two distinct biomarkers of the associated pathology. The four attached gadolinum chelates also provide high relaxivity, which is enhanced 70% further upon binding to the target. Therefore, the efficacy this probe will most likely be dictated by the amount of PDI activity that is present and accessible to the probe in newly forming clots, which in turn will depend on the number of activated platelets present. In the absence of PDI (old clot), very little of the probe will be retained at the clot site making detection difficult. A potential limitation for the current probe design is the observed background activation that occurs slowly over time in the presence of free thiols like reduced glutathione and cysteine, which are known to be present in the blood at low micromolar concentrations.[15] This could result in collateral accumulation of the probe at older clot sites where activated platelets are no longer present thereby yielding a false positive response. However this process may be slow compared to the rate of systemic elimination of the probe from the body. Future work is required to optimize the form of the mixed disulfide for rapid reaction with PDI but slow reaction with endogenous thiols.
Supplementary Material
Acknowledgments
The National Institutes of Health are acknowledged for funding to PC (HL109448)) and GSL (CA009502) and instrumentation grants (RR023385, EB015896). Chris Farrar is thanked for help with the MRI experiment.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.((Please delete if not appropriate))
References
- [1].Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, Montalescot G, Weisel JW. J. Am. Coll. Cardiol. 2011;57:1359. doi: 10.1016/j.jacc.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Front. Biosci. 2008;13:3532. doi: 10.2741/2947. [DOI] [PubMed] [Google Scholar]
- [3].Essex DW. Antioxid. Redox Signaling. 2004;6:736. doi: 10.1089/1523086041361622. [DOI] [PubMed] [Google Scholar]
- [4].Wilkinson B, Gilbet HF. Biochim. Biophys. Acta. 2004;1699:35. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- [5].a) Essex DW, Chen K, Swiatkowska M. Blood. 1995;86:2168. [PubMed] [Google Scholar]; b) Turano C, Coppari S, Altieri F, Ferraro A. J. Cell. Physiol. 2002;193:154. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- [6].Burgess JK, Hotchkiss KA, Suter C, Dudman NP, Szollosi J, Chesterman CN, Chong BH, Hogg PJ. J. Biol. Chem. 2000;275:9758. doi: 10.1074/jbc.275.13.9758. [DOI] [PubMed] [Google Scholar]
- [7].a) Major JL, Meade TJ. Acc. Chem. Res. 2009;42:893. doi: 10.1021/ar800245h. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yoo B, Pagel MD. Front. Biosci. 2008;13:1733. doi: 10.2741/2796. [DOI] [PubMed] [Google Scholar]
- [8].Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. Nat. Biotechnol. 2000;18:321. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- [9].Nivorozhkin AL, Kolodziej AF, Caravan P, Greenfield MT, Lauffer RB, McMurry TJ. Angew. Chem., Int. Ed. Engl. 2001;40:2903. [PubMed] [Google Scholar]
- [10].Miserus RJ, Herias MV, Prinzen L, Lobbes MB, Van Suylen RJ, Dirksen A, Hackeng TM, Heemskerk JW, van Engelshoven JM, Daemen MJ, van Zandvoort MA, Heeneman S, Kooi ME. JACC Cardiovasc. Imaging. 2009;2:987. doi: 10.1016/j.jcmg.2009.03.015. [DOI] [PubMed] [Google Scholar]
- [11].Overoye-Chan K, Koerner S, Looby RJ, Kolodziej AF, Zech SG, Deng Q, Chasse JM, McMurry TJ, Caravan P. J. Am. Chem. Soc. 2008;130:6025. doi: 10.1021/ja800834y. [DOI] [PubMed] [Google Scholar]
- [12].a) Uppal R, Ay I, Dai G, Kim YR, Sorensen AG, Caravan P. Stroke. 2010;41:1271. doi: 10.1161/STROKEAHA.109.575662. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Vymazal J, Spuentrup E, Cardenas-Molina G, Wiethoff AJ, Hartmann MG, Caravan P, Parsons EC., Jr. Invest. Radiol. 2009;44:697. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- [13].a) Uppal R, Medarova Z, Farrar CT, Dai G, Moore A, Caravan P. Invest. Radiol. 2012;47:553. doi: 10.1097/RLI.0b013e31825dddfb. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kolodziej AF, Nair SA, Graham P, McMurry TJ, Ladner RC, Wescott C, Sexton DJ, Caravan P. Bioconjugate Chem. 2012;23:548. doi: 10.1021/bc200613e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ciesienski KL, Yang Y, Ay I, Chonde DB, Loving GS, Rietz TA, Catana C, Caravan P. Mol. Pharmaceutics. 2013;10:1100. doi: 10.1021/mp300610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Banerjee R. J. Biol. Chem. 2012;287:4397. doi: 10.1074/jbc.R111.287995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.