Abstract
Minichromosome complex maintenance component 7 (MCM7) is a critical component of DNA replication licensing. Amplification and overexpression of MCM7 leads to high rate of prostate cancer metastasis. Recent studies indicate that MCM7 genome encodes a putative “super-oncogene” cluster including MCM7 oncogene and a miRNA cluster that knocks down the expression of several critical tumor suppressor genes. In this study, we constructed a vector that constitutively expresses siRNA specific for MCM7. Introduction of this vector into prostate cancer cell lines PC3 or Du145 decreases the expression of MCM7 by 80%. The vector inhibits DNA synthesis and generates growth arrest of these cancer cells. SCID mice were xenografted PC3 or Du145 tumors, and subsequently treated with this vector through tail vein injection with polyethylenimine (PEI). The animals had dramatically smaller tumor volume, less metastasis and better survival rate in comparison with the controls. As a result, intervention of MCM7 expression using siRNA approach may hold the promise for treating androgen refractory prostate cancer.
Keywords: MCM7, siRNA knock-down, prostate, cancer, expression
Introduction
MCM7 is a critical DNA replication licensing factor in both yeast and xenopus oocytes (Blow & Hodgson, 2002). Its role in mammalian cells, however, also involves in chromosome unwinding besides being a component of DNA replication licensing complex (You et al., 1999). To ensure that DNA replication is initiated only once per cell division cycle, the synthesis of the MCM complex is temporally regulated. For example, MCM7 expression is shut down during S, G2 and early M phase to prevent re-initiation of DNA synthesis. Our previous study, along with others, indicated that MCM7 is amplified and overexpressed in prostate cancers that relapse (Levesque et al., 2007; Ren et al., 2006). Continuous expression of MCM7 in the prostate cancer cell line Du145 resulted in a higher level of DNA synthesis, cell proliferation and tumor invasiveness. A transgenic mouse model with a knock-in MCM7 expression under the control of a keratin promoter generated a strain of mouse prone to the development of multiple malignancies (Honeycutt et al., 2006). Over-expression of MCM7 was also identified in a number of human malignancies, including endometrial carcinoma (Li et al., 2005), melanoma (Gambichler et al., 2009), esophageal adenocarcinoma (Kan et al., 2009), colorectal adenocarcinoma (Nishihara et al., 2008), oral squamous cell carcinoma (Feng et al., 2008), glioblastoma (Facoetti et al., 2006), and thyroid cancer (Kebebew et al., 2006). Recent studies indicate that miRNA cluster miR-106b-25/miR-17-92 encoded in the intron 13 of MCM7 plays a critical role in suppressing the expression of tumor suppressor genes such as p21 (Ivanovska et al., 2008) and BIM (Ventura et al., 2008), and undercut TGFβ anti-proliferative signaling (Petrocca et al., 2008). Thus, amplification and overexpression of MCM7 gene will have dramatic tumorigenic effects. It appears that high level of MCM7 expression is one of the determining factors for abnormal cell growth and tumorigenesis. MCM7 also interacts directly with androgen receptor (AR) (Shi et al., 2008). Excessive activation of AR, however, leads to decrease of MCM7 expression, inhibition of DNA synthesis and growth arrest in both LNCaP and PC3 cells with forced expression of wild-type AR. Mutant AR that does not interact with MCM7 has no such effect. In this study, we constructed a pENTR-siMCM7 vector expressing siRNA specific for MCM7. Introduction of this vector into PC3 and Du145 cells suppressed tumor cell growth and DNA synthesis. Delivery of this vector through Polyethylenimine (PEI) into mice xenografted with PC3 or Du145 tumors dramatically reduced the tumor volumes, incidences of tumor metastasis and mortalities of these mice.
Materials and Methods
Cells, Culture Conditions, and Antibodies
PC3 and Du145 were purchased from American Type Cell Culture (Manassas, VA). PC3 cells were cultured with F12K medium supplemented with 10% fetal bovine serum (InVitrogen, Carlsbad, CA). Du145 cells were cultured with modified Eagle medium supplemented with 10% fetal bovine serum (InVitrogen).
Immunoblot detection of MCM7
To examine the MCM7 expression, the cells were washed with PBS and lysed by RIPA buffer (50 mM Tris-HCl, pH 7.4, 1%NP-40: 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 microgram/ml each of Aprotinin, leupeptin, pepstatin, 1 mM Na3VO4). The lysates were sonicated and centrifuged to remove the insoluble materials. The proteins were resolved in SDS-PAGE in 8.5% polyacrylamide gel, and blotted onto PVDF membrane. The membrane was blocked with 5% powdered skim milk in Tris-tween 20 buffer, pH 7.4 for 1 hour at room temperature, followed by 2 hours of incubation of primary anti-MCM7 antibodies (Santa Cruz biotechnology, Santa Cruz, CA). The membrane was then washed three times with Tris-Tween 20 buffer and incubated with horseradish peroxidase-conjugated secondary antibody specific for mouse for 1 hour at room temperature. The MCM7 expression was detected with the ECL system (Amersham Life Science) according to the manufacturer's protocols.
Construction of small interfering RNA Vector for MCM7
To construct the small interfering RNA (siRNA) vectors for MCM7, and a scrambled control sequence, oligonucleotides corresponding to the following region of MCM7 mRNA (5’- caccgcacggccctcggcagcgattcgaaaatcgctgccgagggccgtgc -3’/ 5’-aaaagcacggccctcggcagcgattttcgaatcgctgccgagggccgtgc -3’), or scrambled siRNA (5’-caccgtaatgtattggaacgcatattttgatatccgaatatgcgttccaatacatta-3’/5’-aaaataatgtattggaacgcatattcggatatcaaaatatgcgttccaatacatta-3’) were annealed and ligated into a pENTR™/U6 vector. The ligated products were transfected into E. coli and plated on kanamycin plates (50 μg/mL). Six colonies per transfection were picked and sequenced for the presence of inserts. The DNA from the selected clones, was then transfected into cultured PC3 or Du145 cells using Lipofectamine 2000 transfection kit (Invitrogen).
For transient siRNA analysis, 125 pmol siRNA specific for MCM7 regions (α1: 5’-caccgcacggccctcggcagcgattcgaaaatcgctgccgagggccgtgc-3’ / 5’-aaaagcacggccctcggcagcgattttcgaatcgctgccgagggccgtgc-3’; α2: 5’-caccgcaaggccctagcagcaacattcgaaaatgttgctgctagggccttg-3’ /5’-aaaacaaggccctagcagcaacattttcgaatgttgctgctagggccttgc-3’; α3: 5’-caccgtccaggagatgaagatgcattcgaaaatgcatcttcatctcctgga-3’ /5’-aaaatccaggagatgaagatgcattttcgaatgcatcttcatctcctggac-3’) or scramble (uaauguauuggaacgcauauu/uaugcguuccaaua cauua) were transfected into PC3 cells using the Lipofectamine 2000 transfection kit (Invitrogen). Immunoblots were performed 24 or 48 hours after transfection.
MTT assay
Three thousand LNCaP or PC3 cells were seeded per well in 96-well plate and incubated (37°C, 5% CO2) overnight. pENTR-siMCM7 or pENTR-siScramble was transfected into these cells for 96 hours. One hundred microliters of 1.2 M MTT/medium solution were added to each well and incubated at 37°C for 4 hours to allow the MTT to be metabolized. After removal of medium, cells were lysed with 200 μl of DMSO and placed on a rotating table for 5 minutes to thoroughly mix the formazan into the solvent. The formazan concentration was quantified at 595 nm in a spectrophotometer.
Bromo-Uridine labeling analysis
To label LNCaP or PC3 cells transfected with pENTR-siMCM7 or pENTR-siScramble, 10 μl of BrdU solution (1 mM BrdU in 1× PBS) was added directly to each ml of tissue culture media. The treated cells were then incubated for 3 hours at 37°C. Cells were then resuspended with 100 μl of BD Cytofix/Cytoperm Buffer per sample (BD-Pharmagen) and incubated for 30 minutes at room temperature. The cells were then pelleted and washed with 1 ml of 1× BD Perm/Wash Buffer. The cells were then incubated with Cytoperm Plus Buffer for 10 minutes on ice. The permeation procedure was repeated twice. The cells were resuspended with 100 μl of diluted DNase (diluted to 300 μg/ml in DPBS) per tube, (ie, 30 μg of DNase to each tube), and incubated for 1 hour at 37°C. The cells were washed with 1× BD Perm/Wash Buffer, and incubated with 50 μl of BD Perm/Wash Buffer containing diluted fluorescent anti-BrdU and propidium iodide for 20 minutes at room temperature. The incubation was washed with 1 ml of 1× BD Perm/Wash Buffer. The results of the stains were analyzed in LSC-II flowcytometer.
Tumor growth and spontaneous metastasis
Severe combined immunodeficient (SCID) mice (Taconic, Germantown, NY) were subcutaneously implanted at the abdominal flank with ~1 × 107 viable PC3 or Du145 cells, suspended in 0.2 ml of Hanks’ balanced salt solution. The animals were observed daily. Body weight, tumor size, and other special findings, including lymph-node enlargement, were recorded weekly. After two weeks of xenografting, fifty micrograms of pENTR-siMCM7 or pENTR-siScr were resuspended in 200 μl/mouse therapeutic injection cocktail containing 20 mM HEPES, 5% glucose and 6.75 mM polyethylenimine in final concentration. The therapeutic cocktails were then injected into tail vein of each mouse twice a week for one week. After 6 weeks of xenografting or when the mice became moribund, they were sacrificed, and autopsies were performed. Serial sections of lung, brain, liver, kidneys, vertebra and lymph nodes were collected. These tissues were formalin-fixed and paraffin-embedded. The sections were stained with H&E and subject to histology examination. All animal procedures were approved by the University of Pittsburgh IACUC.
Results and discussion
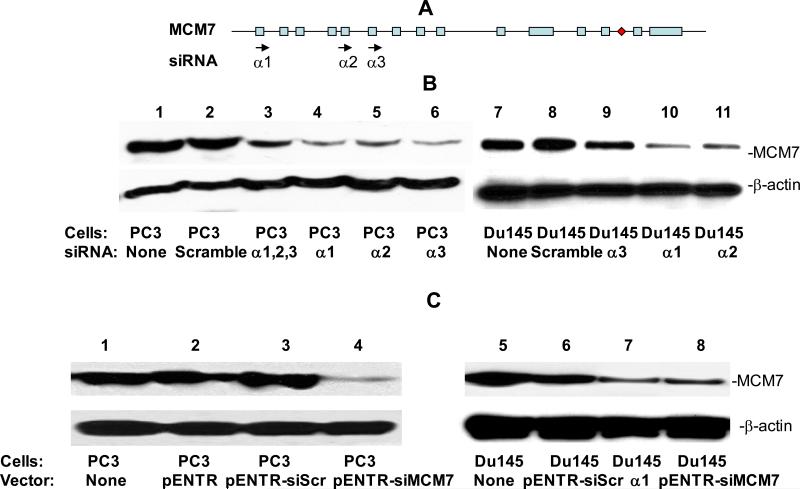
Overexpression and/or amplification of MCM7 had been shown to occur in prostate cancer cell lines and primary cancer samples (Padmanabhan et al., 2004; Ren et al., 2006). To investigate whether reduction of MCM7 expression in cancer cells impedes tumor growth and invasiveness, we design 3 sets of siRNA duplexes corresponding to the coding regions of MCM7 mRNA. These siRNA were then transfected individually or in combination into PC3 cells which overexpress MCM7 mRNA. As shown in figure 1A and B, all three different siRNA effectively decreased the MCM7 protein levels by 80% or more in either PC-3 or Du145 cells. However, combination of these 3 siRNA did not appear to increase the effectiveness in suppressing the expression of MCM7 in PC3 cells. Subsequently, we ligated the oligonucleotide duplex corresponding to the α1 region into pENTR™ to allow the constitutive expression of the siRNA specific for MCM7 through U6 promoter. Similar down-regulation of MCM7 expression was found (figure 1C).
Figure 1. siRNAs specific for MCM7 inhibit MCM7 expression in PC3 cells.
(A) Diagram of MCM7 genome and siRNAs corresponding to the regions of MCM7. Exons are indicated by box. miRNA-106b-25 is indicated by a diamond. Introns are indicated by line. (B) Immunoblot analysis of MCM7 expression inhibition by siRNA specific for MCM7. PC3 (lanes 1-6) or Du145 (lanes 7-11) cells were transfected with siScramble (lanes 2 and 8), siMCM7α1 (lanes 4 and 10), siMCM7α2 (lanes 5 and 11) and siMCM7α3 (lanes 6 and 9) or combination of all three siMCM7 (lane 3). Immunoblot analysis were subsequently performed on the protein extracts from these cells using antibody specific for MCM7 (upper panel) or β-actin (lower panel). (C) Immunoblot analysis of MCM7 expression inhibition by pENTR-siMCM7. PC3 (lanes 1-4) or Du145 cells (lanes 5-8) were transfected with pENTR (lanes 2), pENTR-siScramble (lanes 3 and 6), pENTR-siMCM7 (lanes 4 and 8), and siMCM7α1 (lane 7). Immunoblot analysis were subsequently performed on the protein extracts from these cells using antibody specific for MCM7 (upper panel) or β-actin (lower panel).
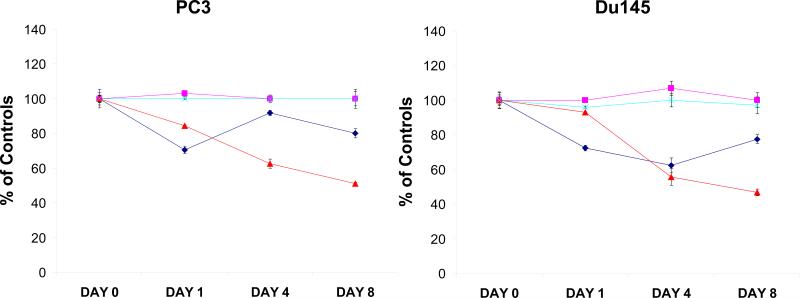
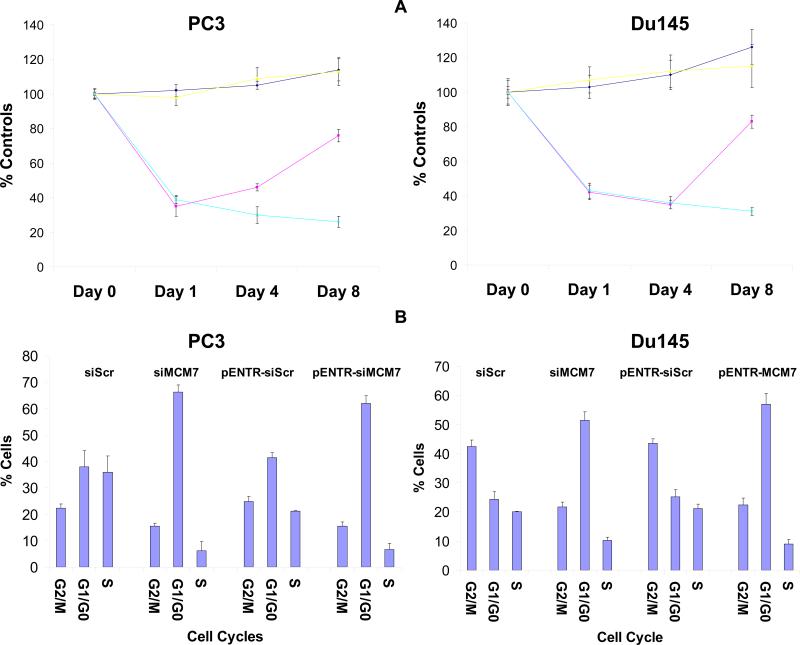
To examine whether down-regulation of MCM7 inhibits tumor cell growth, PC3 and Du145 cells transfected with pENTR-siMCM7 or siRNA specific for MCM7 (α1) or their corresponding scramble controls were synchronized with serum-free medium for 24 hours, and were then allowed to grow. As shown in figure 2, down-regulation of MCM7 using siRNA decreased cell growth for 1 day after treatment (30% inhibition, p=0.001), but growth appeared to recover partially (20% inhibition) after 8 days of incubation, suggesting the transient nature of MCM7 expression suppression. In contrast, cells transfected with pENTR-siMCM7 showed a more dramatic decrease of cell growth (49% inhibition at 8th day treatment, p<0.001) for longer period of time, reflecting constitutive expression of MCM7 siRNA. Subsequently, the pENTR-siMCM7 or its scramble control transfected PC3 or Du145 cells were examined for cell cycle alterations. Both PC3 and Du145 treated with pENTR-siMCM7 were synchronized by serum starvation, stimulated with 10% fetal bovine serum containing media and pulse labeled with BrdU for 3 hours. As shown in figure 3A, cells transfected with pENTR-siMCM7 inhibited DNA synthesis by 61% (p=0.002) and 57% (p<0.001) in day 1 in PC3 and Du145 cells, respectively. pENTR-siMCM7 produced long lasting inhibition of BrdU incorporation (74% inhibition on day 8 for PC3, p=0.002), while the effect of siMCM7 appeared to phase out after 8 days of transfection (25% inhibition for PC3, p<0.001). Knocking down of MCM7 by either pENTR-siMCM7 or siMCM7 siRNA generated a significant reduction of both PC3 and Du145 cells in S phase: For pENTR-siMCM7, it is 5.8 fold reduction for PC3 (p=0.005) and 2 fold for Du145 cells (p=0.03). For siMCM7, the reduction is 3.2 fold for PC3 and 2.4 fold for Du145 cells. This was accompanied with accumulation of cells in G1/G0 phase 2 days after the treatment (figure 3B), i.e. 1.8 (p<0.001) and 2.1 fold (p<0.001) increase for PC3 and Du145 cells, respectively, when treated with pENTR-siMCM7, and 1.5 (p=0.001) and 2.3 fold (p<0.001) increase for PC3 and Du145 cells, respectively, when they were treated with siMCM7, supporting the knockdown of MCM7 generating an entry block to DNA replication.
Figure 2. siRNA specific for MCM7 inhibits prostate cancer cell growth.
PC3 (left) or Du145 (right) cells transfected with siRNA for scramble ( ), siMCM7α1 (
), siMCM7α1 ( ), pENTR-siScramble (
), pENTR-siScramble ( ) or pENTR-siMCM7 (
) or pENTR-siMCM7 ( ), were cultured for the indicated time. MTT assays were performed to examine the cell growths. Standard deviations were indicated.
), were cultured for the indicated time. MTT assays were performed to examine the cell growths. Standard deviations were indicated.
Figure 3. siRNA specific for MCM7 inhibits prostate cancer cell DNA synthesis.
(A) Inhibition of Bromo-deoxy-uridine incorporation into DNA by siRNA specific for MCM7. PC3 (left) or Du145 cells (right) transfected with siRNA for scramble ( ), siMCM7α1 (
), siMCM7α1 ( ), pENTR-siScramble (
), pENTR-siScramble ( ) or pENTR-siMCM7 (
) or pENTR-siMCM7 ( ), were cultured for the indicated time. The cells were then pulsed labeled with BrdU for 3 hours, and the BrdU incorporations were quantified. (B) Cell cycle arrest induced by siRNA specific for MCM7. PC3 or Du145 cells transfected with the indicated siRNAs or vectors for 24 hours. The cells were then synchronized by serum starvation. These cells were later serum stimulated and pulsed labeled with BrdU for 3 hours and stained with DAPI. FACS analyses were then performed on these cells to examine cell cycle distribution.
), were cultured for the indicated time. The cells were then pulsed labeled with BrdU for 3 hours, and the BrdU incorporations were quantified. (B) Cell cycle arrest induced by siRNA specific for MCM7. PC3 or Du145 cells transfected with the indicated siRNAs or vectors for 24 hours. The cells were then synchronized by serum starvation. These cells were later serum stimulated and pulsed labeled with BrdU for 3 hours and stained with DAPI. FACS analyses were then performed on these cells to examine cell cycle distribution.
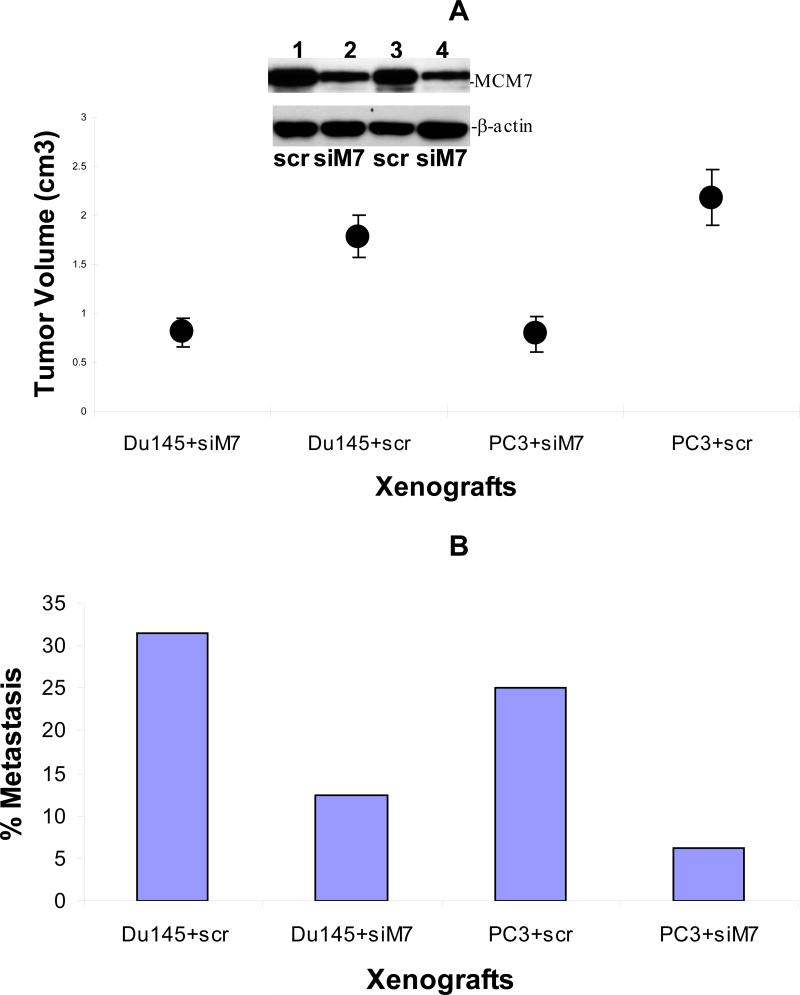
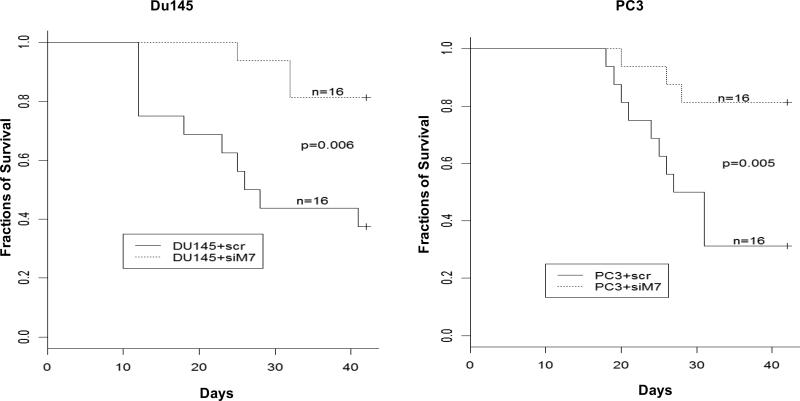
To investigate whether knocking down of MCM7 is a viable approach to inhibit tumor growth in vivo, we xenografted PC3 and Du145 cells subcutaneously onto SCID mice. Subsequently, we delivered pENTR-siMCM7 or its control pENTR-siScramble to the xenografted PC3 or Du145 tumors in SCID mice through tail veins using polyethyleneimime (PEI) as carrier. We found an average 2.2 -fold reduction in Du145 tumor volumes when mice were treated with pENTR-siMCM7, in comparison with pENTR-siScramble (p=0.0007), while an average of 2.8 fold decrease in PC3 tumor volumes was found for PC3 tumors (p=0.0002, figure 4A). Treatment of pENTR-siMCM7 also decreased the metastasis rate of Du145 tumors from 31.5% (5/16) to 12.5% (2/16) and of PC3 tumors from 25% (4/16) to 6.25% (1/16, figure 4B). The mortality associated with xenografted prostate cancer was also significantly reduced when mice were treated with pENTR-siMCM7 (figure 5): Only 3 of 16 mice xenograted with PC3 tumors died within 42 days versus 11 of 16 for the controls (p=0.005), and with Du145 tumors, it was 3/16 versus 10/16 (p=0.006). As a result, down-regulation of MCM7 appears effective in inhibiting prostate cancer growth in vivo.
Figure 4. pENTR-siMCM7 inhibits growth and metastasis of xenografted PC3 and Du145 tumors.
(A) Average of tumor volumes within 6 weeks of xenografting of Du145 or PC3 tumors treated with pENTR-siMCM7 (siM7) or pENTR-siScramble (scr). Inset: Western blot of xenografted tumors from mice treated with siM7 or scr. Lanes 1 and 2 represent protein extracts from PC3 tumors while lanes 3 and 4 from Du145. (B) Rates of metastasis of xenografted PC3 or Du145 tumors treated with pENTR-siMCM7 (siM7) or pENTR-siScramble (scr).
Figure 5. pENTR-siMCM7 reduces mortality of xenografted PC3 and Du145 tumors.
Kaplan-Meier analysis of mortality of mice xenografted with PC3 (right) or Du145 (left) tumors, and treated with pENTR-siMCM7 (siM7) or pENTR-siScramble (scr).
MCM7 is a critical component of DNA replication licensing complex. Amplification and overexpression of MCM7 have been identified in several types of human malignancies (Brake et al., 2003; Honeycutt et al., 2006; Padmanabhan et al., 2004; Ren et al., 2006). Recent study indicates that MCM7 encodes a cluster of miRNA inhibiting the translation of several tumor suppressor genes. Based on these findings, the genome sequence of MCM7 and the MCM7 transcript contain both oncogene and anti-tumor suppressor gene activities, representing a “super-oncogene” cluster. It is possible that MCM7 amplification and overexpression play an initiator role in developing multiple human malignancies. A recent study indicates that the expressions of MCM7 and its embedded miRNA-106b-25 are not always correlated with each other, raising the possibility of another yet to be discovered mechanism in controlling the expressions of MCM7 and miRNA-106b-25 in prostate cancer cells (Sikand et al., 2009). Our recent study also suggests that MCM7 serves as a down-stream signaling molecule for androgen receptor, and mediates testosterone induced cell growth. This raises the possibility to intervene at the expression level of MCM7 in prostate cancer cells to control their growth rate and metastatic behavior. Because of the wide spread involvements of MCM7 “super-oncogene” cluster in human malignancies, and potency of the oncogene, targeting MCM7 transcript could be an efficient approach in treating a variety of human cancers. Our study indicates that systemic (blood) delivery of small interference RNA expression vector to xenografted tumors through PEI appears effective since immunoblot analysis (figure 4) indicates a significant down-regulation of MCM7 protein expression in xenografted tumors of mice treated with pENTR-siMCM7 in comparison with the scramble control. In addition, PCR analysis using primers specific to pENTR vector sequence showed the presence of vector sequences in the xenografted tumor cells (data not shown). Our study demonstrated that down-regulation of MCM7 through small interference RNA inhibits tumor cell growth in vitro, and reduces tumor volume, the rate of metastasis and animal mortality in vivo. The siRNA designed in this study is specific for human. This gene targeted therapy does not have effect on MCM7 expression of mouse host cells. Nevertheless, this study lays down the foundation for treatment of prostate cancer through manipulation of MCM7 expression.
The current treatment of relapse of prostate cancers, particularly those being androgen ablation insensitive, is still problematic. Our previous analysis indicates that up to 80% of prostate cancers with MCM7 amplification and/or overexpression relapse within 5 years of surgical removal of the cancer. In contrast, 90% of prostate cancers without overexpression or amplification of MCM7 survive cancer free for at least 5 years (Ren et al., 2006). Analysis of these cases indicates that 50% of the relapse cases with MCM7 amplification appear refractory to androgen ablation therapy. The amplification or overexpression MCM7 not only serves as an autonomous signal for DNA synthesis and cell growth, and bypass the inhibitory signal from androgen inhibition, but also increases the expression of its embedded miR-106b cluster (Ambs et al., 2008) which in turn inhibits several critical tumor suppressor genes. As a result, intervention of MCM7 expression may hold the promise for the treatment of aggressive prostate cancer.
Acknowledgement
This work was supported by grants from National Cancer Institute (RO1 CA098249 to JHL), Department of Defense (W81 XWH-09-1-0376 to JHL), and American Cancer Society (RSG-08-137-01-CNE to YPY).
References
- Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, Yfantis HG, Stephens RM, Croce CM. Cancer Res. 2008;68:6162–70. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Hodgson B. Trends Cell Biol. 2002;12:72–8. doi: 10.1016/s0962-8924(01)02203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake T, Connor JP, Petereit DG, Lambert PF. Cancer Res. 2003;63:8173–80. [PubMed] [Google Scholar]
- Facoetti A, Ranza E, Benericetti E, Ceroni M, Tedeschi F, Nano R. Anticancer Res. 2006;26:1071–5. [PubMed] [Google Scholar]
- Feng CJ, Li HJ, Li JN, Lu YJ, Liao GQ. Anticancer Res. 2008;28:3763–9. [PubMed] [Google Scholar]
- Gambichler T, Shtern M, Rotterdam S, Bechara FG, Stucker M, Altmeyer P, Kreuter A. J Am Acad Dermatol. 2009;60:808–13. doi: 10.1016/j.jaad.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Honeycutt KA, Chen Z, Koster MI, Miers M, Nuchtern J, Hicks J, Roop DR, Shohet JM. Oncogene. 2006;25:4027–32. doi: 10.1038/sj.onc.1209435. [DOI] [PubMed] [Google Scholar]
- Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson AL, Linsley PS, Cleary MA. Mol Cell Biol. 2008;28:2167–74. doi: 10.1128/MCB.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan T, Sato F, Ito T, Matsumura N, David S, Cheng Y, Agarwal R, Paun BC, Jin Z, Olaru AV, Selaru FM, Hamilton JP, Yang J, Abraham JM, Mori Y, Meltzer SJ. Gastroenterology. 2009;136:1689–700. doi: 10.1053/j.gastro.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebebew E, Peng M, Reiff E, Duh QY, Clark OH, McMillan A. World J Surg. 2006;30:767–74. doi: 10.1007/s00268-005-0308-2. [DOI] [PubMed] [Google Scholar]
- Levesque MH, El-Alfy M, Berger L, Labrie F, Labrie C. Urology. 2007;69:196–201. doi: 10.1016/j.urology.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Li SS, Xue WC, Khoo US, Ngan HY, Chan KY, Tam IY, Chiu PM, Ip PP, Tam KF, Cheung AN. Histopathology. 2005;46:307–13. doi: 10.1111/j.1365-2559.2005.02069.x. [DOI] [PubMed] [Google Scholar]
- Nishihara K, Shomori K, Fujioka S, Tokuyasu N, Inaba A, Osaki M, Ogawa T, Ito H. Int J Oncol. 2008;33:245–51. [PubMed] [Google Scholar]
- Padmanabhan V, Callas P, Philips G, Trainer TD, Beatty BG. J Clin Pathol. 2004;57:1057–62. doi: 10.1136/jcp.2004.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, Cavazzini L, Volinia S, Alder H, Ruco LP, Baldassarre G, Croce CM, Vecchione A. Cancer Cell. 2008;13:272–86. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Ren B, Yu G, Tseng GC, Cieply K, Gavel T, Nelson J, Michalopoulos G, Yu YP, Luo JH. Oncogene. 2006;25:1090–8. doi: 10.1038/sj.onc.1209134. [DOI] [PubMed] [Google Scholar]
- Shi YK, Yu YP, Zhu ZH, Han YC, Ren B, Nelson JB, Luo JH. Am J Pathol. 2008;173:1758–1767. doi: 10.2353/ajpath.2008.080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand K, Slane SD, Shukla GC. Cancer Cell Int. 2009;9:21. doi: 10.1186/1475-2867-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Cell. 2008;132:875–86. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z, Komamura Y, Ishimi Y. Mol Cell Biol. 1999;19:8003–15. doi: 10.1128/mcb.19.12.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]