Abstract
Anatomical cancer extent is an important predictor of prognosis and determines treatment choices. In non-small-cell lung cancer (NSCLC) the tumour–node–metastasis (TNM) classification developed by Pierre Denoix replaced in 1968 the Veterans Administration Lung cancer Group (VALG) classification, which was still in use for small-cell lung cancer (SCLC). Clifton Mountain suggested several improvements based on a database of mostly surgically treated United States (US) patients from a limited number of centres. This database was pivotal for a uniform reporting of lung cancer extent by the American Joint Committee of Cancer (AJCC) and the International Union against Cancer (IUCC), but it suffered increasingly from obsolete diagnostic and staging procedures and did not reflect new treatment modalities. Moreover, its findings were not externally validated in large Japanese and European databases, resulting in persisting controversies which could not be solved with the available database. The use of different mediastinal lymph-node maps in Japan, the (US) and Europe facilitated neither the exchange nor the comparison of treatment results.
Peter Goldstraw, a United Kingdom (UK) thoracic surgeon, started the process of updating the sixth version in 1996 and brought it to a good end 10 years later. His goals were to improve the TNM system in lung cancer by addressing the ongoing controversies, to validate the modifications and additional descriptors, to validate the TNM for use in staging SCLC and carcinoid tumours, to propose a new uniform lymph-node map and to investigate the prognostic value of non-anatomical factors. A staging committee was formed within the International Association for the Study of Lung Cancer (IASLC) – which supervised the collection of the retrospective data from >100,000 patients with lung cancer – treated throughout the world between 1990 and 2000, analyse them with the help of solid statistics and validate externally with the Surveillance, Epidemiology and End Results (SEER) database.
The ten modifications and the mediastinal lymph-node map – which were proposed in 2007 and adopted by the AJCC and IUCC in their respective seventh revision of the TNM system – were implemented as of 2010 and were rapidly adopted by the thoracic oncology community and cancer registries. As expected, not all controversies could be fully addressed, and the need for a prospective data set containing more granular information was felt early on. This data set of 25,000 consecutive incident cases will form the base for the eighth revision in 2017 and is currently being collected. Other threats are the role of stage migration and the increasing number of biological factors interfering with disease extent for prognostication. The latter issue will be addressed by the creation of a prognostic index, including several prognostic factors, of which stage will be one.
For the time being, the seventh TNM classification is considered the gold standard for the description of disease extent, initial treatment allocation and the reporting of treatment results. The uniform use of the TNM descriptors and the lymph-node map by all involved in lung cancer care is to be considered a process indicator of quality.
1. Introduction
1.1. Background
Prognostication of outcome is of all ages and a distinguishing feature of mankind. Similarly, linking features of a tumour to its natural history has been reported since pharaonic times. Surgical resection often being the only modality available at presentation in those days, anatomical tumour extent was from the early days associated with outcome and became a pivotal driver in treatment allocation and evaluation. It was the seminal work of the French surgeon Pierre Denoix in the 1940s and 1950s that led to the creation of the committee on Clinical Stage Classification and Applied Statistics within the Union for International Cancer Control (UICC), and the development of the tumour–node–metastasis (TNM) classification which is still the current gold standard for the anatomical staging of most solid malignant neoplasms.
In the first edition of the UICC manual, lung cancer was classified with ‘other sites’, although several publications had already addressed the relationship between anatomical extent and outcome [1–4]. The United States (US) surgeon Clifton Mountain progressively introduced new denominators and substages based on the analysis of a mostly surgical database from US institutions [5–10]. Although some of his data were externally validated in other cancer registry series, it became increasingly clear by 1996, when the sixth edition of the lung cancer TNM classification appeared, that a further refinement had become necessary, that the revision procedure had several limitations and that there was a growing need for uniformity in the nomenclature used to describe nodal stations [11–13]. Globally, two nodal maps were in use: the Mountain/Dressler [14] used in North America and parts of Europe, and the Japanese Naruke map [15] used in Asia and other parts of Europe.
The International Association for the Study of Lung Cancer (IASLC) undertook the ambitious International Staging Project in which an international database was assembled, consisting of more than 67,000 cases of lung cancer, treated between 1990 and 2000 by all modalities of care and collected retrospectively from 46 data sources from more than 19 countries around the world [16]. The size of this database allowed validation, both internal and external, of the revisions to descriptors and stages to a degree unprecedented in the history of TNM. The IASLC staging project has delivered a seventh edition of the TNM classification for lung cancer that aligns stage with prognosis more closely than before [17]. It was enacted on January 1, 2010, and all of its proposed revisions were subsequently accepted by the UICC [4] and the American Joint Committee on Cancer (AJCC) [18,19].
1.2. The seventh edition of the TNM classification of lung cancer
The major modifications are listed in Table 1 [20,21]. Tumour size has been given added importance [22]. New T size cut-points were originally identified in the node-negative, pathologically staged patients having undergone complete resection, but were also shown to be valid in the clinically staged patient cases: 2 cm separating T1a and T1b, 5 cm dividing T2a from T2b and size >7 cm becoming a T3 descriptor for the first time. If these larger tumours are node-negative, they move to stage IIA if T2b N0 M0 and to stage IIB if T3 N0 M0; previously these were all considered stage IB [23].
Table 1.
Ten modifications in the tumour–node–metastasis (TNM) of lung cancer in the seventh UICC classification [17–20].
| Summary of change | Details of new definition | References | |
|---|---|---|---|
| 1 | Subclassification of T1/2 according to largest tumour diameter | ⩽2 cm: T1a; 2.1–3.0 cm: T1b; 3.1–5.0 cm: T2a; 5.1–7.0 cm: T2b; >7 cm: T3 | [22] |
| 2 | Reclassification of synchronous additional tumour nodules (ATNs) | See Table 2 | [22] |
| 3 | New borders for mediastinal lymph-node stations | [28] | |
| 4 | Reclassification of malignant pleural/pericardial effusion | M1a | [26] |
| 5 | Subclassification of M1 | Limited to thorax: M1a; extrathoracic spread: M1b | [26] |
| 6 | Use of TNM in SCLC and carcinoid tumours | [35–37] | |
| 7 | Appropriate (sub)stage regrouping (Fig. 1) | T2bN0 becomes stage IIA instead of IB; T2aN1 becomes IIA instead of IIB; T4N0/1 becomes IIIA instead of IIIB | [23] |
| 8 | Elimination of Mx descriptor | The clinical assessment of metastasis can be based on physical examination alone (cM0/1); pM0 should be restricted to autopsy cases. Else, the pathologist should refer to cM | [19] |
| 9 | Optional descriptor for pleural (Pl) invasion | Tumour growth under internal elastic layer: Pl0 – through elastic layer but not abutting pleural surface: Pl1, T upgrading to at least T2a – abutting pleural surface: Pl2; T upgrading to at least T2a – in parietal pleura: Pl3; T upgrading to at least T3 – cannot be assessed: PlX | [38] |
| 10 | Optional descriptor for perineural (Pn) invasion | Pn0: no perineural invasion, Pn1: perineural invasion, PnX: perineural invasion cannot be assessed | [19] |
When additional tumour nodules are found synchronously with a known lung cancer, the distinction between lung metastases and multiple primary tumours has relied on clinical and morphological criteria [24]. The distinction is easy when tumours are of different cell types, and there is little debate if tumours are of the same cell type but associated with separate areas of carcinoma in situ, although in most cases this is confirmed only after resection. The other criteria are more problematic: the tumours should be distinct and separate, should lie in different segments, lobes or lungs, and should not be associated with any nodal involvement in an area of common lymphatic drainage. Although 20 years later there was a suggestion to modify these criteria by the addition of DNA ploidy [25], the criteria have otherwise remained unchanged despite the enormous advances in imaging, histopathology, immunohistochemistry, mutational analysis and biopsy techniques since that time. In the latest edition, the distinction between synchronous primary tumours of similar histological appearance and metastases has been clarified, and the pathologist has been given a central role in this process, allowing the distinction to be made on biopsy specimens before a decision is taken on the most appropriate treatment. Multiple tumours of similar histological appearance may now be considered to be synchronous primary tumours if in the opinion of the pathologist – on the basis of features such as differences in morphology, immunohistochemistry and/or molecular studies or, in the case of squamous cancers, on the basis of association with carcinoma in situ – they represent different subtypes of the same histopathological cell type. Such cases should also have no evidence of mediastinal nodal metastases or of nodal metastases within a common nodal drainage. Clearly, if the management of any particular patient is dependent on this distinction, the biopsy of more than one lesion may be necessary. In other situations, or where this is considered impractical, one may fall back on a generic principle of giving the patient the benefit of the doubt and assigning the lower T category and/or stage. Multiple synchronous primary tumours should be staged separately. These may be recorded separately, or if a single TNM category is required, the highest T category and stage of disease should be assigned and the multiplicity of the lesions categorised as (m), or the number of tumours should be indicated in parentheses, for example: T2(m) or T2(5). If the lesions are concluded to be metastases, then the appropriate T or M category will be dependent on the site of the nodules (Table 2). If in the same lobe as the primary they are now classified as T3 and, when associated with node negativity, are stage IIB. When associated with N1 or N2 disease they are now classified as stage IIIA, not IIIB. Tumours associated with additional nodules in other ipsilateral lobe(s) have been reclassified as T4 rather than M1. When associated with N0 or N1, these patient cases should be designated as stage IIIA, and with N2 or N3 as stage IIIB. Tumours associated with additional nodules in the contralateral lung remain M1 but have been reclassified as M1a.
Table 2.
The fate over time of multiple synchronous primary tumours.
| UICC 5, recommendation [8] | UICC 6 [11–13] | UICC 7 [17–20,22] | |
|---|---|---|---|
| Same lobe as primary tumour | Tn +1 | T4; at least stage IIIB | T3N0: stage IIB; 3N1/2: stage IIIA; T3N3: stage IIIB |
| Same lung, other lobe | T4: at least stage IIIB | M1: stage IV | T4N0/1: stage IIIA; T4N2/3:stage IIIB |
| Other lung | M1: stage IV | M1: stage IV | M1a: stage IV |
The T4 descriptor remained unchanged, but when associated with N0 or N1 disease, it was down-staged to stage IIIA, not IIIB. Tumours associated with malignant pleural/pericardial effusion or pleural/pericardial nodules have been reclassified as M1a rather than T4 [26]. These data reflect the algorithm previously developed to treat patients with so-called wet IIIB disease with systemic therapy. Tumours associated with distant metastases have been reclassified as M1b.
Analysis of the IASLC database allowed validation of the existing N categories, which were adopted without change [27]. Both existing lymph-node maps were unified in the IASLC nodal map [28], and the precise anatomical definitions of each nodal station are now recognised by the UICC and AJCC as the recommended means of describing regional lymph-node involvement for lung cancer. An important modification to both previous maps is the observation that the anatomical and oncological midlines in the superior mediastinum no longer coincide. The oncological midline deviates to the left lateral border of the trachea at the thoracic inlet and returns to the midline at the carina. Thus, all nodes in the superior mediastinum that lie anterior to the trachea are grouped with right upper paratracheal station 2 and right lower paratracheal station 4. Involvement of these nodes by a right-sided tumour will now be classified as N2-disease, whereas for a left-sided tumour, this will become N3-disease. This is in keeping with the observations of some Japanese colleagues [29]. In addition, the concept of nodal zones has been introduced, amalgamating adjacent nodal stations into larger anatomic units. An exploratory analysis of the IASLC database studied survival after complete resection in relation to the extent of node involvement using the zonal concept. Three groups were identified, with significant differences in survival. Those with single-zone N1 disease had the best survival, at 48% over 5 years. Patients with multizone N2 disease had the worst survival, at 20% over 5 years. The third group, with intermediate survival, consisted of patients with multizone N1 (35% 5-year survival) and those with single-zone N2-disease (34% 5-year survival). This refinement is presently under investigation, in order to make the nodal map of greater use to oncologists and radiologists, who are frequently tasked with classifying more bulky nodal disease that might transgress the boundaries of individual nodal stations.
As many as 40% of the reports on lung cancer resection specimens contains no information on mediastinal node involvement [30]. It is known that the greater the number of lymph nodes removed at thoracotomy, the higher the survival rate [31,32], even if all nodes are shown to be negative, presumably by increasing the certainty of the N0 classification [33]. The development of an internationally agreed nodal classification has allowed the reintroduction of minimum requirements for nodal assessment at surgery and subsequent pathological evaluation. In the latest edition of TNM, there is now an expanded definition of complete resection (R0), which recommends that at least six lymph nodes/nodal stations be removed/sampled and confirmed on histology to be free of disease to confer pN0 status [34]. Three of these nodes/stations should be mediastinal, including the subcarinal nodes (station 7) and three from N1 nodes/stations. It is hoped that the setting of this basic standard will improve nodal assessment and thereby the outcomes of pulmonary resection for lung cancer.
The above mentioned modifications in T and N have led to a migration of cases in between pre-existing (sub)stages (Fig. 1): T2bN0 becomes stage IIA instead of IB; T2a N1 becomes IIA instead of IIB; T4N0/1 becomes IIIA instead of IIIB.
Fig. 1.
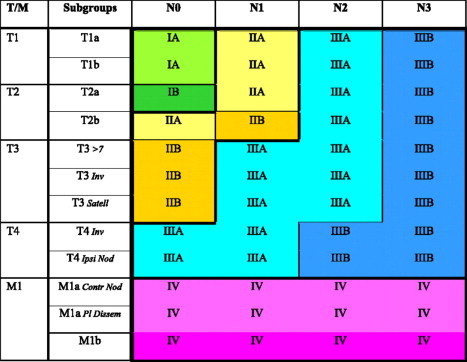
Stage groups according to tumour–node–metastasis (TNM) descriptor and subgroups. Reprinted with permission from Detterbeck et al [20]. >7: diameter >7 cm; Inv: invasion; Satell: satellite nodule in same lobe; Ipsi Nod: nodule in ipsilateral lung; Contr Nod: nodule in contralateral lung; Pl dissem: pleural or pericardial dissemination.
Small-cell lung cancer has always been excluded from the TNM classification. However, the seventh edition is the first to show that TNM has greater utility than the limited versus extensive stage split commonly used in clinically staged patients as well as those treated surgically, especially as a stratification factor in clinical trials of earlier-stage disease [35,36]. Although the TNM classification was already used in both the typical and atypical variants of carcinoid tumours, the seventh edition is the first to validate this practice [37].
There has never been an internationally agreed definition of visceral pleural invasion (VPI). This created difficulties for the IASLC staging project when attempting to define the interrelationship between VPI and other prognostic factors such as tumour size. An internationally agreed definition was therefore developed, in which VPI is defined as ‘invasion beyond the elastic layer including invasion to the visceral pleural surface’ [38]. In addition, a comment was added recommending the use of elastic stains when this feature is not clear on routine histology. With these refinements, VPI was carried forward into the seventh edition without change, and the IASLC proposed an optional more detailed classification of pleural invasion, adapting the P category developed by the Japan Lung Cancer Society to create a PL classification [39,40]. The impact of visceral pleural invasion on survival according to size was reported according to the seventh UICC classification and confirmed its proposed Pl descriptor [41].
Other changes, generic to the seventh edition of UICC, are the elimination of Mx and the introduction of a descriptor of perineural invasion Pn.
1.3. Implications of the 7th edition
Several authors have addressed the magnitude of the impact of the modifications on stage grouping. Van Meerbeeck et al estimated that in the IASLC data set of 15,952 resected patients, the change of p-TNM staging classification from UICC 6 to 7 results in the net migration of 23% of resected cases: stage pIB (–1326), stage pIIA (+2017), stage pIIB (±730), stage pIIIA (+701), stage pIIIB (–745) and stage pIV (+83) (Fig. 2) [42]. The magnitude of up- and down-migration is similar. Substage migration resulted in an increase in 5-year survival of 4% in p-IB and a decrease of 10% in p-IIIB. This stage migration should be accounted for when comparing outcome across surgical series using different TNM classifications. In a Norwegian cancer registry series from 2001–2005, the concordance index was 0.68 for both editions, indicating no overall difference in their predictive accuracy [43]. In the seventh edition, 211 (29%) stage IB patients migrated to stage II and 161 (48%) patients migrated from stage IIB to IIA. Stage migrations could change the treatment for up to 326 (17.3%) of the patients in this series [43].
Fig. 2.
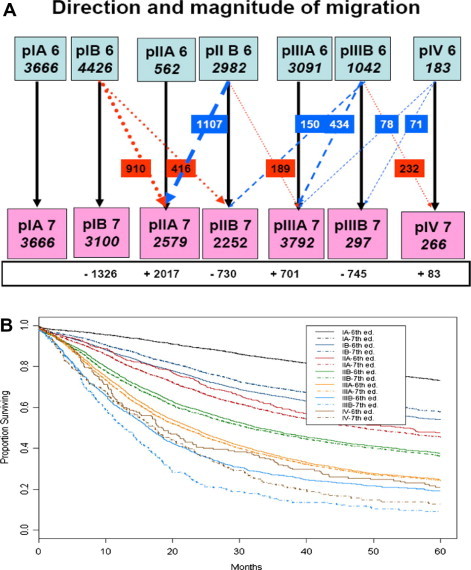
(A) Stage migration from sixth to seventh tumour–node–metastasis (TNM) classification in resected cases. (B) Impact of stage migration on overall survival. Reproduced with permission from Van Meerbeeck et al [42].
1.4. Strengths
Lung cancer stage definitions have never been subjected to such an intense validation process [44]. Internal validity was addressed by visually assessing the consistency of Kaplan–Meier curves across database types and geographic regions. External validity was addressed by assessing the similarity of curves generated using the population-based Surveillance Epidemiology and End Results (SEER) cancer registry data to those generated using the project database. Cox proportional hazards regression was used to calculate hazard ratios between the proposed stage groupings with adjustment for cell type, sex, age and region. Validation checks were robust, demonstrating that the suggested staging changes were internally and externally stable. Several series coming from cancer registries and surgical series have confirmed some or all of the proposed modifications, adding supplementary external validation to the classification [45,46].
1.5. Weaknesses
Taken together, the proposed changes are limited in number and most are either intuitive or reflect modifications that were already suggested in the analysis of cancer registries or surgical series. As some of these series were included in the IASLC database, these modifications are self-fulfilling.
With respect to the proposed boundaries for lymph-node stations, they represent a clear improvement for surgeons, but are not unambiguous for radiologists and echo-endoscopists. A recent ultrasonographic lymph-node map based on the anatomical boundaries of the seventh UICC classification might well resolve this issue [47]. The abovementioned reshaping of mediastinal lymph-node borders will result in an increase in so-called ‘minimal N2’, limited to a single station. The magnitude of this phenomenon has not yet been reported. Furthermore, stage IIIA, which used to be heterogeneous in the sixth classification, becomes a cocktail of six different T/N combinations.
Even a database of 67,000+ patients was not able to validate all the descriptors that had accrued within previous editions of TNM. One should remember, however, that many of these data were not originally defined for the purpose of evaluating the staging system, but with some other scientific questions in mind. Their prognostic role was not always confirmed by multivariate analysis. Among the T descriptors that need further study are:
-
(1)
The best way to assess tumour size clinically: measuring a single diameter, measuring the greatest diameter or measuring two or three dimensions. Computed tomography (CT) screening has accelerated the development of volumetric software, adding to the debate as to how best to determine tumour size, and whether or not volume is preferable over size [48].
-
(2)
The non-size-based descriptors of T2/3 as hilar atelectasis, obstructive pneumonitis and the cytology-negative paramalignant pleural effusion (not considered as a T-modifying condition) [49]. It is hoped that the use of [18]fluorodeoxyglucose–positron emission tomography (FDG-PET) scanning may help unravel the inflammatory and neoplastic elements.
-
(3)
The split of which invasion to adjacent structures is assigned to T3 or to T4 could not be answered because there were too few patient cases in which the precise descriptor justifying the T3 or T4 category was recorded, and even fewer in which all of the other descriptors were known to be absent.
-
(4)
The extent of dissemination was only partly addressed by the introduction of M1a. The recently increasing interest in the outcome of oligometastatic disease could not be translated into a separate descriptor for this entity.
Other details and areas in which ambiguities and difficulties exist have been reviewed [50].
1.6. Threats
Although the data are definitely more recent than in previous TNM editions, they still are from the past century and do not reflect present-day staging and treatment paradigms, wherein FDG-PET and endoscopic ultrasound are now standard staging techniques for the M and N descriptors. It has been shown in several series that the introduction of both techniques has significantly improved the accuracy of clinical staging and upstaged the stage distribution at diagnosis, hence changing treatment algorithms and improved stage-specific outcomes [51]. This process of improved stage-specific survival has been described previously at the occasion of a previous revision of the lung cancer TNM [52]. This so-called Will Rogers phenomenon has been observed in stage III patients, where the outcome was significantly ‘improved’ by the occurrence of PET staging [53–55].
Whereas pathological staging has a prognostic significance, clinical staging is meant to help the clinician in treatment allocation. The interaction between better staging techniques and improved treatment strategies does not allow the expectation that revisions in staging classification will necessarily translate into a better overall outcome, unless over decades. It is controversial whether treatment should necessarily follow a stage change, as stage should not be considered a ‘cook book’ for treatment allocation. The issue is particularly critical for the ‘down-staged’ additional tumour nodules, single zone N2 and T4N0 cases as described before. The analysis of the IASLC database was heavily influenced by surgical cases and pathological staging and cannot necessarily be extrapolated to clinical staging, which has been reported to be inaccurate [56]. Besides, individual patients with more advanced stages but an inherent indolent biological behaviour of their tumour have been reported to profit from more ‘aggressive’ surgical or radiotherapeutic approaches, whilst others with more limited stage will be given symptomatic care for reasons such as poor performance or comorbidity. pT2pN0 tumours with a diameter of 5.5 cm were previously considered pIB and hence not candidates for adjuvant chemotherapy. The same case would now be considered pT2bpN0 and staged pIIA and would be offered adjuvant treatment. We should remember that the data supporting adjuvant chemotherapy after complete resection were generated from trials using the sixth edition of the TNM classification, and that offering adjuvant chemotherapy to ‘reclassified’ stage pIB, pII and pIIIA completely resected cases is therefore not evidence-based [57,58]. A recent pooled analysis of patient cases from two multicentre trials using the size cut-points of the seventh edition of the TNM classification was unable to identify subgroups of patients who did or did not derive significant benefit from adjuvant chemotherapy after complete resection based on tumour size [59]. Prospective data from large adjuvant chemotherapy trials are necessary before clinical guidelines regarding management of surgically resected node-negative non-small-cell lung cancer (NSCLC) can be updated to reflect the changes introduced.
1.7. Opportunities
The aforementioned weaknesses and threats offer some challenging opportunities for further research. All unproven descriptors and hypotheses are carried forward for close evaluation in a 25,000-patient prospective database being collected for the future TNM- revision foreseen for 2016 [60].
The issue of T/pT classification of ground-glass opacities (GGOs) deserves special attention as these are increasingly found at CT-scan screening, with or without a solid component. By histological definition, in-situ adenocarcinoma is defined only for GGOs with a diameter of <3 cm [61]. GGOs are considered non-invasive adenocarcinoma in situ, whilst only the associated solid component is presumed to be invasive. Those ⩾3 cm are no longer ‘adenocarcinoma in situ’ and relate to a T2 description. However, their biological behaviour is less aggressive, suggesting that the measurement of the GGO in the evaluation of tumour size be discarded, analogously to the situation in breast cancer, where the tumour is coded for the invasive component only.
The concept that overall burden of lymph nodal disease (nN) might be more important than the anatomical location of the involved nodes comes from similar observations in oesophageal cancer. In a large retrospective series of resected Japanese patients, it was observed that the number of lymph nodes involved was a more precise prognostic determinant than their location and could be considered for the nodal stage classification as is done in other organs [62]. However, it is difficult to assess the exact number of the metastatic lymph nodes pre-operatively, and a uniform definition for pre- and post-operative assessment is preferable to avoid confusion. Besides, fragmentation of lymph nodes produced during the operation might result in an overestimation of the survival risk.
Pleural lavage cytology (PLC) is performed by some surgeons as the initial step after performing thoracotomy. In patients without overt effusion or pleural dissemination, a recent meta-analysis confirmed that PLC shown to be positive for cancer cells has an adverse and independent prognostic impact after complete resection [63]. When PLC is positive, the resection should be classified as R1(cy+). Sophisticated immunohistochemical and genetic techniques permit the detection of very small tumour deposits. A micrometastasis as defined by the UICC and AJCC usually is detected by routine haematoxylin and eosin staining, and typically mitoses and invasion are seen [64]. Such micrometastases in nodes or distant sites are counted as positive and denoted by the symbol (mi): for example, cN1(mi) or pN2(mi). However, the prognostic impact was not evaluated in the IASLC staging analysis. Isolated tumour cells (ITCs) are small clumps of tumour cells typically without mitoses or vascular or lymphatic invasion. ITCs within nodes (or distant sites) are not counted in the stage classification and should be coded as N0 (or M0), regardless of node level harbouring the ITC, for example, pN0(i 1) or pN0(mol 1). The prognostic value of ITCs has been inconsistent. The same applies for circulating tumour cells [65].
A staging classification describes the anatomical extent of a tumour, disregarding its biological behaviour. Stage is hence only one of several prognostic factors to be accounted for in prognostication, together with one or several biological markers which have been repeatedly linked with the outcome regardless of the treatment established [66]. Logistic regression techniques will allow the construction of a composite prognostic index in which different independent predictive factors are weighted and available for use in a nomogram or electronic outcome calculator [67,68].
Quality of health care is of increasing concern and interest. Indicators can help to describe the structural environment, the quality of the staging process and its outcome. The TNM descriptors and denominators lend themselves well as indicators of staging. Examples are the extent of intra-operative lymph-node sampling as an indicator of quality of the resection, clinicopathological correlation of resected tumours as a measure of staging accuracy and outcome according to stage. It is expected that these and others will be increasingly used to peer review medical practice.
2. Conclusion
The publication of the seventh edition of the lung cancer TNM classification has been variously applauded as a ‘seismic shift in staging’ by some or dubbed a laudable effort in ‘lumping, splitting and sorting’ by others [69,70]. It is considered a quantum leap forward in patient care, being based on an unprecedented large international database and involving extensive analysis and validation. Inevitably, the system is also more complex and far from perfect; with more refined data comes greater ability to discern granular details. This necessitates more layers of classification, manifested by additional new descriptors. As with any complex system, rules that seem clear in one context can seem awkward or conflicting in another. Implementation brings ambiguities to light. A clear knowledge of the details and difficulties should help to promote appropriate application and realisation of the full benefits of the new stage classification system.
There is much work to be done to answer these questions before the next revision scheduled for 2016. The IASLC is determined that over future revisions these shortcomings will be addressed as prospective data are accrued. The seventh edition of TNM in its present form remains, however, a surrogate for the anatomical extent of a tumour and a sequel to previous revisions. Unless we succeed in the prequel of building a composite prognostic index, including an increasing number of factors and inclusive biomarkers, ‘we might consider the TNM method for lung cancer staging to be similar to ‘brownstone’ remnants of historical interest and accept biological markers of disease extent and behaviour as the skyscrapers of our future’[71].
Conflict of interest statement
None declared.
References
- 1.1st ed. International Union Against Cancer (UICC); Geneva, Switzerland: 1968. TNM classification of malignant tumours. [Google Scholar]
- 2.Salzer G. Vorschlag einer Einteilung des bronchuskarzinoms nach pathologisch-anatomisch-klinischen Gesichtspunkten. Wien Med Wochenschr. 1951;5(6):102–103. [PubMed] [Google Scholar]
- 3.Nohl H.C. Three year follow up of classified cases of bronchogenic carcinoma after resection. Thorax. 1960;15:11–16. [PubMed] [Google Scholar]
- 4.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep. 1973;4:31–42. [PubMed] [Google Scholar]
- 5.Mountain C.F., Carr D.T., Anderson W.A. A system for the clinical staging of lung cancer. Am J Roentgenol Radium Ther Nucl Med. 1974;120:130–138. doi: 10.2214/ajr.120.1.130. [DOI] [PubMed] [Google Scholar]
- 6.2st ed. International Union Against Cancer (UICC); Geneva, Switzerland: 1975. TNM classification of malignant tumours. [Google Scholar]
- 7.American Joint Committee on Cancer Staging and End Results Reporting: AJC Cancer Staging Manual. 1st ed. Philadelphia, PA: Lippincott-Raven; 1977.
- 8.Mountain C.F. A new international staging system for lung cancer. Chest. 1986;89(Suppl. 4):225S–232S. doi: 10.1378/chest.89.4_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 9.Hermanek P., Sobin L.H., editors. TNM classification of malignant tumours. 4th ed. Springer Verlag; Berlin, Germany: 1987. [Google Scholar]
- 10.Beahrs O.H., Henson D.E., Hutter R.O., editors. Manual for Staging of Cancer. 3th ed. JB Lippincott; Philadelphia, PA: 1988. [Google Scholar]
- 11.Mountain C.F. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 12.Sobin L.H., Wittekind C., editors. UICC TNM Classification of Malignant Tumours. 6th ed. Wiley-Liss; New York, NY: 2002. [Google Scholar]
- 13.Greene F.L., Page D.L., Fleming I.D., editors. AJCC Cancer Staging Manual. 6th ed. Springer; New York, NY: 2002. [Google Scholar]
- 14.Mountain C.F., Dresler C.M. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 15.Naruke T., Suemasu K., Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg. 1978;76:832–839. [PubMed] [Google Scholar]
- 16.Goldstraw P., Crowley J. The IASLC international staging project on lung cancer. J Thorac Oncol. 2006;1:281–286. [Google Scholar]
- 17.Goldstraw P. 1st ed. EditorialRx Press; Orange Park, FL: 2009. IASLC staging handbook in thoracic oncology. [Google Scholar]
- 18.Edge S.B., Byrd D.R., Compton C.C., editors. AJCC cancer staging handbook. 7th ed. Springer; New York, NY: 2009. [Google Scholar]
- 19.Sobin L.H., Gospodarowicz M.K., Wittekind C., editors. UICC TNM Classification of Malignant Tumours. 7th ed. Wiley-Liss; New York, NY: 2009. [Google Scholar]
- 20.Detterbeck F., Boffa D.J., Tanoue L.T. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 21.Goldstraw P. New staging system: how does it affect our practice? J Clin Oncol. 2013;31:984–991. doi: 10.1200/JCO.2012.42.7922. [DOI] [PubMed] [Google Scholar]
- 22.Rami-Porta R., Ball D., Crowley J. The IASLC lung cancer staging project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 23.Goldstraw P., Crowley J., Chansky K. The IASLC lung cancer staging project: proposals for revision of the stage groupings in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 24.Martini N., Melamed M.R. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–612. [PubMed] [Google Scholar]
- 25.Antakli T., Schaefer R.F., Rutherford J.E. Second primary lung cancer. Ann Thorac Surg. 1995;59:863–866. doi: 10.1016/0003-4975(95)00067-u. [DOI] [PubMed] [Google Scholar]
- 26.Postmus P.E., Brambilla E., Chansky K. The IASLC lung cancer staging project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:686–693. doi: 10.1097/JTO.0b013e31811f4703. [DOI] [PubMed] [Google Scholar]
- 27.Rusch V.R., Crowley J., Giroux D.J. The IASLC Lung Cancer Staging Project: proposals for revision of the N descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:603–612. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- 28.Rusch V., Asamura H., Watanabe H. The IASLC lung cancer staging project: a proposal for a new international node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe Y. TNM classification for lung cancer. Ann Thorac Cardiovasc Surg. 2003;9:343–350. [PubMed] [Google Scholar]
- 30.Osarogiagbon R.U., Allen J.W., Farooq A. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol. 2012;7:390–396. doi: 10.1097/JTO.0b013e31823e5e2d. [DOI] [PubMed] [Google Scholar]
- 31.Wright G., Manser R.L., Byrnes G. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised trials. Thorax. 2006;61:597–603. doi: 10.1136/thx.2005.051995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe Si, Asamura H. Lymph node dissection for lung cancer: significance, strategy and technique. J Thorac Oncol. 2009;4:652–657. doi: 10.1097/JTO.0b013e31819cce50. [DOI] [PubMed] [Google Scholar]
- 33.Gajra A., Newman N., Gamble G.P. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21:1029–1034. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Rami-Porta R., Wittekind C. Goldstraw P for the international association for the study of lung cancer (IASLC) staging committee. Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49:25–33. doi: 10.1016/j.lungcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd F.A., Crowley J., Van Houtte P. The IASLC lung cancer staging project: proposals regarding the clinical staging of small-cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:1067–1077. doi: 10.1097/JTO.0b013e31815bdc0d. [DOI] [PubMed] [Google Scholar]
- 36.Vallières E., Shepherd F.A., Crowley J. The IASLC lung cancer staging project: proposals regarding the relevance of TNM in the pathological staging of small-cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:1049–1059. doi: 10.1097/JTO.0b013e3181b27799. [DOI] [PubMed] [Google Scholar]
- 37.Travis W.D., Giroux D.J., Chansky K. The IASLC lung cancer staging project: proposals for the inclusion of carcinoid tumours in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2008;3:1213–1223. doi: 10.1097/JTO.0b013e31818b06e3. [DOI] [PubMed] [Google Scholar]
- 38.Travis W.D., Brambilla E., Rami-Porta R. Visceral pleural invasion: pathologic criteria and use of elastic stains—proposals for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol. 2008;3:1384–1390. doi: 10.1097/JTO.0b013e31818e0d9f. [DOI] [PubMed] [Google Scholar]
- 39.Kato H., editor. Japan lung cancer society classification of lung cancer, English. 1st ed. Kanehara; Tokyo, Japan: 2000. [Google Scholar]
- 40.Hammar SP. In: Dail DH, Hammar SP, editors. Pulmonary pathology, common tumours. 2nd ed. Springer-Verlag: New York, NY; 1994.
- 41.Kudo Y., Saji H., Shimada Y. Impact of visceral pleural invasion on the survival of patients with non-small cell lung cancer. Lung cancer. 2012;78:153–160. doi: 10.1016/j.lungcan.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Van Meerbeeck J.P., Chansky K., Goldstraw P. The impact of stage migration on survival after resection in the UICC 7 TNM-classification of lung cancer. J Clin Oncol. 2010;28:15s. (suppl; abstract 7022) [Google Scholar]
- 43.Strand T.E., Rostad H., Wentzel-Larsen T., von Plessen C. A population-based evaluation of the seventh edition of the TNM system for lung cancer. ERJ. 2010;36:401–407. doi: 10.1183/09031936.00171809. [DOI] [PubMed] [Google Scholar]
- 44.Groome P.A., Bolejack V., Crowley J.J. The IASLC lung cancer staging project: validation of the proposals for revision of the T, N and M descriptors and consequent stage groupings in the forthcoming (seventh) TNM classification for lung cancer. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 45.Ou S.H., Zell J.A. Validation study of the proposed IASLC staging revisions of the T4 and M non-small cell lung cancer descriptors using data from 23,583 patients in the California Cancer Registry. J Thorac Oncol. 2008;3:216–227. doi: 10.1097/JTO.0b013e318164545d. [DOI] [PubMed] [Google Scholar]
- 46.Sawabata N., Miyaoka E., Asamura H. For the japanese joint committee for lung cancer registration. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2004;2011(6):1229–1235. doi: 10.1097/JTO.0b013e318219aae2. [DOI] [PubMed] [Google Scholar]
- 47.Tournoy K.G., Annema J.T., Krasnik M., Herth F.J., van Meerbeeck J.P. Endoscopic and endobronchial ultrasonography according to the proposed lymph node map definition in the seventh edition of the tumour, node, metastasis classification for lung cancer. J Thorac Oncol. 2009;4:1576–1584. doi: 10.1097/JTO.0b013e3181c1274f. [DOI] [PubMed] [Google Scholar]
- 48.Aberle D.R., Adams A.M., Berg C.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ou S.H., Zell J.A., Ziogas A., Anton-Culver H. Prognostic significance of the non-size-based AJCC T2 descriptors: visceral pleura invasion, hilar atelectasis, or obstructive pneumonitis in stage IB non-small cell lung cancer is dependent on tumour size. Chest. 2008;133:662–669. doi: 10.1378/chest.07-1306. [DOI] [PubMed] [Google Scholar]
- 50.Detterbeck F.C., Boffa D.J., Tanoue L.T., Wilson L.D. Details and difficulties regarding the new lung cancer staging system. Chest. 2010;137:1172–1180. doi: 10.1378/chest.09-2626. [DOI] [PubMed] [Google Scholar]
- 51.De Wever W., Ceyssens S., Mortelmans L. Additional value of PET-CT in the staging of lung cancer: comparison with CT alone, PET alone and visual correlation of PET and CT. Eur Radiol. 2007;17:23–32. doi: 10.1007/s00330-006-0284-4. [DOI] [PubMed] [Google Scholar]
- 52.Feinstein A.R., Sosin D.M., Wells C.K. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 53.Morgensztern D., Goodgame B., Baggstrom M.Q., Gao F., Govindan R. The effect of FDG-PET on the stage distribution of non-small cell lung cancer. J Thorac Oncol. 2008;3:135–139. doi: 10.1097/JTO.0b013e3181622c2c. [DOI] [PubMed] [Google Scholar]
- 54.Chee K.G., Nguyen D.V., Brown M. Positron emission tomography and improved survival in patients with lung cancer: the Will Rogers phenomenon revisited. Arch Intern Med. 2008;168:1541–1549. doi: 10.1001/archinte.168.14.1541. [DOI] [PubMed] [Google Scholar]
- 55.Gregory D.L., Hicks R.J., Hogg A. Effect of PET/CT on management of patients with non-small cell lung cancer: results of a prospective study with 5-year survival data. J Nucl Med. 2012;53:1007–1015. doi: 10.2967/jnumed.111.099713. [DOI] [PubMed] [Google Scholar]
- 56.Van Meerbeeck J.P., Nicholson M., Gilligan D. Adjuvant or neoadjuvant chemotherapy in early-stage non-small cell lung cancer (NSCLC): how would staging affect the patients treated? J Clin Oncol. 2008;26 (suppl; abstract 7509) [Google Scholar]
- 57.Pignon J.P., Tribodet H., Scagliotti G.V. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3352–3359. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 58.Strauss G.M., Herndon J.E., 2nd, Maddaus M.A. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and the North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuffe S., Bourredjem A., Graziano S. A pooled exploratory analysis of the effect of tumour size and KRAS mutations on survival benefit from adjuvant platinum-based chemotherapy in node-negative non-small cell lung cancer. J Thorac Oncol. 2012;7:963–972. doi: 10.1097/JTO.0b013e31824fe9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giroux D.J., Rami-Porta R., Chansky K. The IASLC lung cancer staging project: data elements for the prospective project. J Thorac Oncol. 2009;4:679–683. doi: 10.1097/JTO.0b013e3181a52370. [DOI] [PubMed] [Google Scholar]
- 61.Travis W.D., Brambilla E., Noguchi M. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asamura H. Clinical application of new (7th TNM staging system for lung cancer. J Thor, Oncol. 2012;7S5:S442. [Google Scholar]
- 63.Lim E., Clough R., Goldstraw P. Impact of positive pleural lavage cytology on survival in patients undergoing lung resection for non-small cell lung cancer: an international individual patient data meta-analysis. J Thorac Cardiovasc Surg. 2010;139:1441–1446. doi: 10.1016/j.jtcvs.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 64.Hermanek P., Hutter R.V.P., Sobin L.H., Wittekind C. Classification of isolated tumour cells and micrometastases. Cancer. 1999;86:2668–2673. [PubMed] [Google Scholar]
- 65.Hiltermann T.J., Pore M.M., van den Berg A. Circulating tumour cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol. 2012;23:2937–2942. doi: 10.1093/annonc/mds138. [DOI] [PubMed] [Google Scholar]
- 66.Sculier J.P., Chansky K., Crowley J.J., Van Meerbeeck J., Goldstraw P. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol. 2008;3:457–466. doi: 10.1097/JTO.0b013e31816de2b8. [DOI] [PubMed] [Google Scholar]
- 67.Kim AW, Johnson KM, Detterbeck FC. <http://www.stagingLungCancer.org> [accessed 29.04.2013].
- 68.Anonymous. <http://www.predictcancer.org> [accessed 29.04.2013].
- 69.Silvestri G.A. A seismic shift in staging. J Thorac Oncol. 2007;2:682–683. doi: 10.1097/JTO.0b013e318145af89. [DOI] [PubMed] [Google Scholar]
- 70.Detterbeck F. Lumping, splitting and sorting. J Thorac Oncol. 2007;2:581–582. doi: 10.1097/JTO.0b013e31807a2fae. [DOI] [PubMed] [Google Scholar]
- 71.Colice G. Lung cancer staging and the home insurance building constructed in 1884–1885. Chest. 2009;136:6–8. doi: 10.1378/chest.09-0797. [DOI] [PubMed] [Google Scholar]


