Abstract
BACKGROUND
The cause of acute uncomplicated cystitis is determined on the basis of cultures of voided midstream urine, but few data guide the interpretation of such results, especially when gram-positive bacteria grow.
METHODS
Women from 18 to 49 years of age with symptoms of cystitis provided specimens of midstream urine, after which we collected urine by means of a urethral catheter for culture (catheter urine). We compared microbial species and colony counts in the paired specimens. The primary outcome was a comparison of positive predictive values and negative predictive values of organisms grown in midstream urine, with the presence or absence of the organism in catheter urine used as the reference.
RESULTS
The analysis of 236 episodes of cystitis in 226 women yielded 202 paired specimens of midstream urine and catheter urine that could be evaluated. Cultures were positive for uropathogens in 142 catheter specimens (70%), 4 of which had more than one uropathogen, and in 157 midstream specimens (78%). The presence of Escherichia coli in midstream urine was highly predictive of bladder bacteriuria even at very low counts, with a positive predictive value of 102 colony-forming units (CFU) per milliliter of 93% (Spearman’s r = 0.944). In contrast, in midstream urine, enterococci (in 10% of cultures) and group B streptococci (in 12% of cultures) were not predictive of bladder bacteriuria at any colony count (Spearman’s r = 0.322 for enterococci and 0.272 for group B streptococci). Among 41 episodes in which enterococcus, group B streptococci, or both were found in midstream urine, E. coli grew from catheter urine cultures in 61%.
CONCLUSIONS
Cultures of voided midstream urine in healthy premenopausal women with acute uncomplicated cystitis accurately showed evidence of bladder E. coli but not of enterococci or group B streptococci, which are often isolated with E. coli but appear to rarely cause cystitis by themselves. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases.)
Urinary tract infection is a bacterial infection that is encountered frequently in outpatient settings in the United States, accounting for 8.6 million visits in 2007.1 Half of all women report having had at least one urinary tract infection by 32 years of age.2 The hallmark of such infections is the presence of bladder bacteriuria. Although a urine specimen collected by suprapubic aspiration or catheter is the reference for determining the microbial cause of infection, neither type of specimen is routinely obtained because of inconvenience, discomfort, and the potential for adverse events. Thus, when an examination of urine is indicated in women with symptoms suggestive of cystitis to assist with diagnosis or to determine antimicrobial susceptibility, the customary urinary specimen collected for culture is the midstream portion of voided urine. However, the interpretation of such cultures in women is complicated by the potential for contamination of voided specimens by periurethral microorganisms, since it is not possible to distinguish whether bacteriuria originates from the bladder or the periurethra. As a result, colony-count thresholds have been established to help interpret colony counts.
Early studies showed the value of quantitative urine culture in discriminating between true urinary tract infection and contaminated urine specimens.3–11 Bacterial counts of 105 colony-forming units (CFU) per milliliter or higher in midstream urine cultures were predictive of bladder bacteriuria in asymptomatic women and women with pyelonephritis, whereas lower counts were more likely to be associated with contamination.5–9 However, later studies showed that women with symptoms of cystitis often had lower colony counts.12–14 In a 1982 study involving women with cystitis, proven bladder bacteriuria had a significant correlation with urinary coliform-colony counts as low as 102 CFU per milliliter.15 To our knowledge, in the three decades since this seminal finding, no confirmatory studies of gram-negative rods have been published, nor has a study addressed the positive predictive value for true bladder bacteriuria of colony counts of other organisms causing cystitis, such as enterococcus or group B streptococcus. To address this issue, we performed detailed microbiologic analyses of paired specimens of midstream urine and urine collected by means of a urethral catheter (catheter urine) obtained from women with episodes of cystitis; these analyses included all organisms that are considered to be causative in acute uncomplicated cystitis.
METHODS
STUDY POPULATION
We conducted the study at the Hall Health Primary Care Center, an outpatient clinic at the University of Washington, Seattle, and at the Clinical Research Unit at the University of Miami, Miami. Women were eligible if they were between the ages of 18 and 49 years, were in good general health, and had had typical symptoms of cystitis (dysuria and urinary frequency or urgency) for 7 days or less. Women were not eligible if they had a temperature of 38°C or higher or tenderness in the costovertebral angle, diabetes mellitus, known anatomical abnormalities of the urinary tract, exposure to a systemic antimicrobial agent in the previous 2 weeks, or a diagnosis of urinary tract infection in the previous month or if they were pregnant or not using reliable contraception.
The ethics committees at the University of Washington and the University of Miami approved the study, and all participants provided written informed consent. All the authors vouch for the integrity and completeness of the data and analysis presented and for adherence to the study protocol.
STUDY PROCEDURES
Flyers, newspaper ads, and discussions with local clinicians were the main methods of recruitment, and participants were enrolled as soon as possible after contacting study personnel. At the study visit, a medical history was taken, and all participants underwent a physical examination and were interviewed with the use of a standardized questionnaire. Participants were instructed to obtain a clean-catch midstream urine specimen after spreading the labia with one hand and then wiping the urethra with two premoistened 2% castile soap towelettes. After the participant provided the midstream specimen, a 2% xylocaine gel was applied locally to minimize the pain of catheter insertion. Shortly afterward, the participant was placed in the lithotomy position, and a lubricated 8-French urethral catheter was inserted into the bladder to collect a first-flow urine specimen through the catheter. Urine samples for culture were refrigerated and transported to the laboratory within 24 hours after collection. Women were treated with standard antimicrobial agents and asked to call or return to the clinic if their symptoms did not resolve.
LABORATORY PROCEDURES
Methods for collecting urine specimens and isolating, identifying, and quantifying uropathogens have been described previously.15,16 In catheter urine cultures, all microorganisms were fully identified and quantified to 10 CFU per milliliter. In midstream urine cultures, all uropathogens (gram-negative rods, enterococci, group B streptococci, and Staphylococcus saprophyticus) were identified and quantified to 10 CFU per milliliter. Organisms that are not usually considered to be uropathogens (e.g., lactobacilli, diphtheroids, coagulase-negative staphylococci, Gardnerella vaginalis, and alpha-hemolytic streptococci) were grouped together as mixed gram-positive flora, quantified as a group, and not further identified. S. aureus in midstream urine was also considered to be a component of mixed gram-positive flora. However, all organisms that were found in pure culture of midstream urine were identified and quantified to 10 CFU per milliliter. There were no changes in laboratory methods during the course of the study. A hemocytometer was used to quantify leukocytes in urine specimens. Pyuria was defined as 8 or more leukocytes per cubic millimeter.15
OUTCOME MEASURES
The primary study outcome was a comparison of positive predictive values and negative predictive values of organisms grown in midstream urine, with the presence or absence of the organism in urine obtained by means of a catheter used as the reference and with an emphasis on the predictive values for enterococcus and group B streptococci. Secondary study outcomes were a determination of the prevalence of organisms that are not usually considered to be uropathogens (e.g., lactobacilli) in bladder urine, the frequency of polymicrobic cystitis, and the presence of pyuria. The treatment outcome was not evaluated as a study outcome.
The primary unit of analysis was the cystitis episode. The analyses were limited to the episodes in which paired specimens of both midstream urine and catheter urine were obtained and organisms could be identified and quantified.
STATISTICAL ANALYSIS
We constructed two-way tables to compare the presence and quantity of organisms in the specimens of midstream urine with those in the specimens of catheter urine. We calculated the sensitivity, specificity, positive predictive value, and negative predictive value to assess the accuracy of the findings provided by the midstream urine culture at log10 quantities 1 to 5 in relation to the findings provided by the catheter urine culture, using the presence of an organism in the catheter urine as the reference.
Plots were generated to illustrate the relationship between colony counts in midstream urine cultures and those in catheter urine cultures, according to the specific organism or group. We then computed Spearman’s correlation coefficients to measure the extent of the association between the two specimen sites.
RESULTS
STUDY PARTICIPANTS
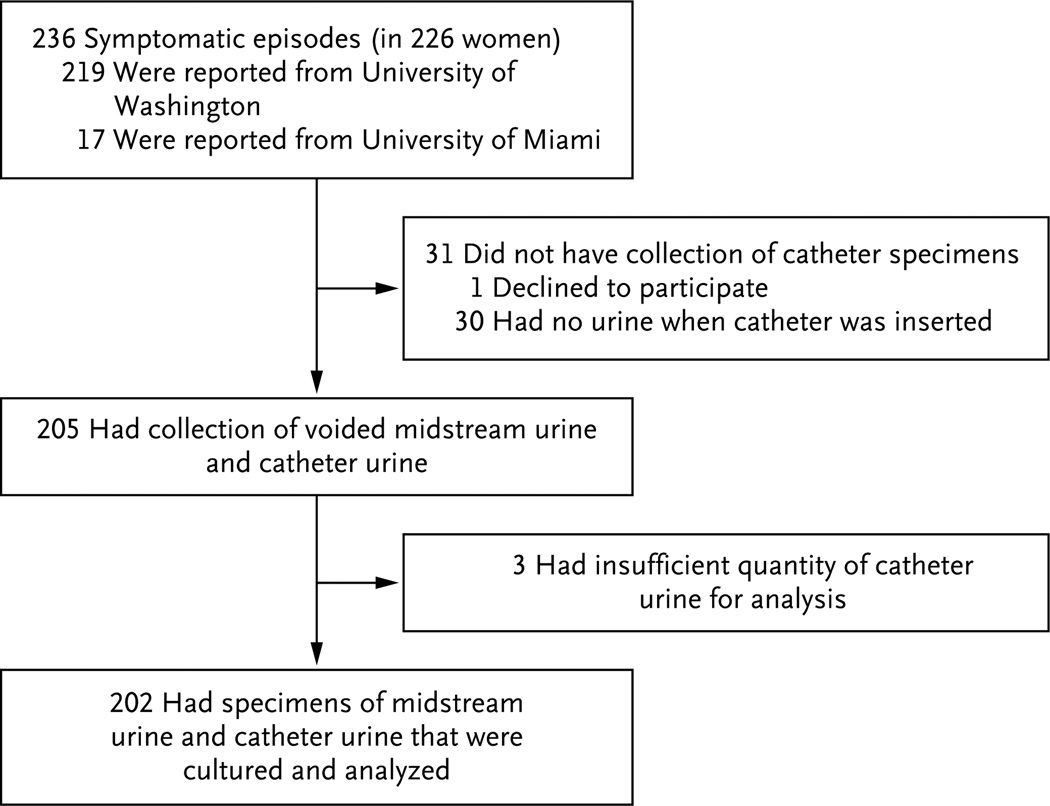
Enrollment of participants took place from 2002 through 2012. A total of 236 episodes of acute cystitis were evaluated with the use of a specimen of voided midstream urine. Nine women had more than 1 episode, for a total of 19 episodes among these women. Paired specimens of midstream urine and catheter urine were available for 202 episodes (Fig. 1).
Figure 1. Enrollment and Outcomes.
The 236 symptomatic episodes occurred in 226 women; 8 women had 2 episodes each, and 1 woman had 3 episodes.
The characteristics of the women at presentation are shown in Table 1. The median age was 22 years, and 73% were white. In almost all the episodes, women had two or more symptoms, most of which were moderate to severe, with a median duration of 2 days. Pyuria was present in 88% of midstream urine specimens.
Table 1.
Characteristics of Participants at the Time of an Episode of Cystitis.*
| Characteristic | Value |
|---|---|
| Total no. of episodes | 202 |
| Median age (range) — yr | 22 (18–49) |
| Race or ethnic group — no./total no. (%)† | |
| White | 147/200 (73) |
| Black | 6/200 (3) |
| Asian or Pacific Islander | 25/200 (12) |
| Hispanic | 8/200 (4) |
| Other | 14/200 (7) |
| No. of sex partners in the past month — no. (%) | |
| 0 | 14(7) |
| ≥1 | 188 (93) |
| Symptoms at clinic visit for cystitis episode — no. (%) | |
| Dysuria or urinary frequency or urgency | 202 (100) |
| Dysuria | 199 (99) |
| Urinary frequency | 197 (98) |
| Urinary urgency | 193 (96) |
| ≥1 Symptoms rated as moderate or severe — no./total no. (%) | 170/176 (97) |
| Median duration of urinary symptoms before presentation (range) — days‡ | 2 (<1–14) |
| Pyuria in midstream urine— no./total no. (%) | |
| Any episode | 160/182 (88) |
| Episode with any bladder bacteriuria | 122/126 (97) |
| Episode with Escherichia coli bladder bacteriuria | 106/109 (97) |
All participants with symptoms of cystitis provided specimens of midstream urine, after which paired specimens of catheter urine for culture were collected.
Race or ethnic group was self-reported.
One woman had mild symptoms of urinary tract infection for 14 days before enrollment. All the other participants had a symptom duration of 1 week or less; 60% had symptoms for 1 to 2 days.
COMPARISON OF PAIRED SPECIMENS
All Organisms
At least one organism grew in 149 cultures (74%) of catheter urine and 200 cultures (99%) of voided midstream urine. Uropathogens (gram-negative rods, enterococci, group B streptococci, or S. saprophyticus) grew in 142 cultures (70%) of catheter urine and in 157 cultures (78%) of midstream urine — with growth in 1 culture of catheter urine only (0.5%), 16 cultures of midstream urine only (8%), and 141 cultures of both catheter urine and midstream urine (70%) (Table 2); more than one uropathogen grew in 4 cultures (2%) of catheter urine and in 35 cultures (17%) of midstream urine. Midstream urine yielded multiple organisms of any species in 173 cultures (86%). Among the 53 cultures without growth in catheter urine, 13 (25%) had uropathogens in the paired midstream urine. The distribution of organisms that were cultured in midstream urine was similar over the course of the study period.
Table 2.
Isolation of Uropathogens from 202 Paired Specimens of Voided Midstream Urine and Catheter Urine.*
| Organism | Catheter Urine Only |
Midstream Urine Only |
Both Catheter and Midstream Urine |
Neither Catheter nor Midstream Urine |
|---|---|---|---|---|
| number of cultures (percent) | ||||
| Any uropathogen | 1(<1) | 16(8) | 141 (70) | 44 (22) |
| Gram-negative uropathogen | 1(<1) | 11(5) | 133 (66) | 57 (28) |
| Escherichia coli | 1(<1) | 11(5) | 120 (59) | 70 (35) |
| Non–Escherichia coli | 0 | 2(1) | 14(7) | 186 (92) |
| Klebsiella pneumoniae | 0 | 0 | 10(5) | 192 (95) |
| Enterobacter aerogenes | 0 | 1(<1) | 2(1) | 199 (99) |
| Proteus mirabilis | 0 | 0 | 1(<1) | 201 (>99) |
| Citrobacter diversus | 0 | 0 | 1(<1) | 201 (>99) |
| Pseudomonas species | 0 | 1(<1) | 0 | 201 (>99) |
| Gram-positive uropathogen | 0 | 37 (18) | 10(5) | 155 (77) |
| Enterococci | 0 | 18(9) | 2(1) | 182 (90) |
| Group B streptococci | 0 | 23 (11) | 2(1) | 177 (88) |
| Staphylococcus saprophyticus | 0 | 0 | 6(3) | 196 (97) |
A positive culture was defined as a culture with more than 0 colony-forming units per milliliter.
Gram-Negative Uropathogens
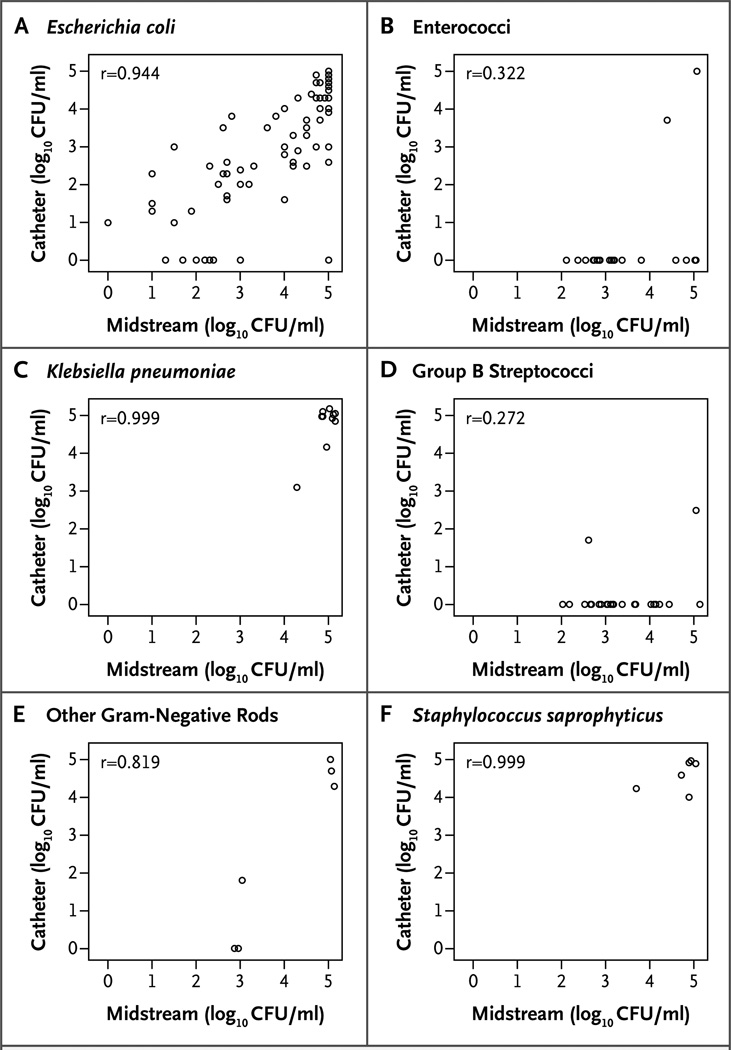
Gram-negative rods grew in the cultures of midstream urine or catheter urine in 145 episodes of cystitis (72%). Escherichia coli grew in the cultures in 132 episodes (65%). In 131 episodes, E. coli grew in midstream urine cultures, and in 120 of these episodes (92%), E. coli was also found in catheter urine cultures (Table 2 and Fig. 2A). Among 114 episodes in which E. coli grew along with any other organism as part of mixed flora in midstream urine cultures, 104 episodes (91%) showed E. coli growth in catheter urine cultures. Colony counts in midstream urine and catheter urine were strongly correlated (Spearman’s r = 0.944). The colony-count agreement was also strong between midstream urine and catheter urine for the 10 episodes in which Klebsiella pneumoniae was detected (Spearman’s r = 0.999) (Fig. 2C).
Figure 2. Correlation between Counts of Colony-Forming Units (CFUs) in Catheter Urine Cultures and in Midstream Urine Cultures.
Shown are plots indicating the correlation between bacterial counts in cultures of urine collected by means of a urethral catheter and those in cultures of voided midstream urine in women with symptoms of acute uncomplicated cystitis. Spearman’s correlation coefficients, which are shown in the upper left corners of the panels, are based on all observations. The left-hand column of plots (Panels A, C, and E) show gram-negative organisms, and the right-hand column (Panels B, D, and F) show gram-positive organisms.
Gram-Positive Uropathogens
Enterococci were isolated from cultures of midstream urine in 20 episodes (10%) but from cultures of catheter urine in only 2 episodes (Spearman’s r = 0.322) (Table 2 and Fig. 2B). In one of the latter episodes, both the midstream urine culture and the catheter urine culture grew more than 105 CFU per milliliter of enterococci; in the other episode, 104 CFU per milliliter of enterococci grew in midstream urine and 103 CFU per milliliter in catheter urine; no other uropathogen was isolated. Among the 18 episodes in which enterococci were detected only in midstream urine, 4 cultures had at least 104 CFU per milliliter; E. coli was also isolated from 16 midstream urine cultures and 11 catheter urine cultures.
Group B streptococci were isolated from mid-stream urine cultures in 25 episodes (12%) but from catheter urine cultures in only 2 episodes (Spearman’s r = 0.272) (Table 2 and Fig. 2D). E. coli was concurrently isolated from midstream urine cultures in the latter 2 episodes and from catheter urine cultures in 1 episode. Among the 23 episodes in which group B streptococci were detected in midstream urine cultures only, 6 cultures had at least 104 CFU per milliliter; E. coli was concurrently isolated from 18 midstream urine cultures and 16 catheter urine cultures.
There were 41 episodes in which enterococcus, group B streptococci, or both were found in midstream urine. E. coli grew from catheter urine cultures in 25 (61%) of these episodes.
In contrast, for S. saprophyticus (6 episodes), colony counts were strongly correlated between midstream urine and catheter urine cultures (Spearman’s r=0.999) (Fig. 2F).
PERFORMANCE CHARACTERISTICS OF MIDSTREAM URINE CULTURE
Sensitivity, specificity, positive predictive values, and negative predictive values for E. coli, enterococci, and group B streptococci in midstream urine cultures are shown in Table 3. The positive predictive values for E. coli in midstream urine cultures were 93% for growth of at least 102 CFU per milliliter and 99% for growth of at least 104 CFU per milliliter, but for enterococci and group B streptococci, positive predictive values were low for all colony counts: 10% for enterococcus colony counts of at least 102 CFU per milliliter and 33% for counts of at least 104 CFU per milliliter; for group B streptococci, the positive predictive values were 8% and 14%, respectively.
Table 3.
Sensitivity, Specificity, and Positive and Negative Predictive Values in 202 Specimens of Voided Midstream Urine.*
| Organism | Sensitivity | Specificity | Positive Predictive Value |
Negative Predictive Value |
|---|---|---|---|---|
| number/total number (percent) | ||||
| E. coli (CFU/ml) | ||||
| ≥10 | 120/121 (99) | 70/81 (86) | 120/131(92) | 70/71 (99) |
| ≥102 | 114/121 (94) | 72/81 (89) | 114/123 (93) | 72/79 (91) |
| ≥103 | 106/121 (88) | 77/81 (95) | 106/110(96) | 77/92 (84) |
| ≥104 | 98/121 (81) | 80/81 (99) | 98/99 (99) | 80/103 (78) |
| ≥105 | 72/121 (60) | 80/81 (99) | 72/73 (99) | 80/129 (62) |
| Enterococci (CFU/ml) | ||||
| ≥102 | 2/2 (100) | 182/200 (91) | 2/20 (10) | 182/182 (100) |
| ≥103 | 2/2 (100) | 187/200 (94) | 2/15 (13) | 187/187 (100) |
| ≥104 | 2/2 (100) | 196/200 (98) | 2/6 (33) | 196/196 (100) |
| ≥105 | 1/2 (50) | 197/200 (98) | 1/4 (25) | 197/198 (99) |
| Group B streptococci (CFU/ml) | ||||
| ≥102 | 2/2 (100) | 177/200 (88) | 2/25 (8) | 177/177 (100) |
| ≥103 | 1/2 (50) | 183/200 (92) | 1/18 (6) | 183/184 (99) |
| ≥104 | 1/2 (50) | 194/200 (97) | 1/7 (14) | 194/195 (99) |
| ≥105 | 1/2 (50) | 199/200 (>99) | 1/2 (50) | 199/200 (>99) |
The presence or absence of any quantity of each organism in catheter urine was the reference. CFU denotes colony-forming units.
CATHETER URINE CULTURES YIELDING ORGANISMS NOT USUALLY CONSIDERED UROPATHOGENS
Seven cultures yielded lactobacilli (≤104 CFU per milliliter), with pyuria only in the four cultures in which gram-negative rods were concurrently isolated. Three cultures yielded G. vaginalis (all 105 CFU per milliliter), with pyuria only in the two cultures in which gram-negative rods were concurrently isolated. S. simulans (105 CFU per milliliter) was identified in pure growth in one culture with pyuria.
Although the study protocol did not call for the identification of S. aureus in midstream urine cultures when the bacteria were present with mixed gram-positive flora, S. aureus was identified in five midstream urine cultures. In one episode, the colony count for S. aureus was at least 105 CFU per milliliter in both midstream urine and catheter urine. In the other four episodes, all of which had 103 CFU per milliliter or less of S. aureus and other flora in the midstream urine culture, S. aureus did not grow in catheter urine cultures.
DISCUSSION
Millions of episodes of cystitis occur in the United States annually, and midstream urine culture is often obtained to confirm the diagnosis or to provide data regarding antimicrobial susceptibility. However, documentation of the usefulness of midstream urine cultures in the clinical evaluation of women with symptoms of cystitis is sparse, especially when cultures do not yield E. coli, the most common cause of cystitis. Our study provides additional information for interpreting midstream urine cultures in healthy premenopausal women with cystitis.
In our study, colony counts of E. coli as low as 10 to 102 CFU per milliliter in midstream urine were sensitive and specific for the presence of E. coli in catheter urine in symptomatic women, a finding that has been reported in previous studies.15 However, similar data are lacking for enterococci and group B streptococci, organisms that are often considered to be minority species causing acute uncomplicated cystitis.17–23 In our study, both enterococci and group B streptococci were frequently isolated from midstream urine but rarely from paired specimens of catheter urine. The positive predictive values for enterococci and group B streptococci in midstream urine cultures were very low, even at high colony counts, in contrast to the high positive predictive values for E. coli (Table 3). These data suggest that enterococci and group B streptococci only rarely cause acute uncomplicated cystitis. Since K. pneumoniae and S. saprophyticus were found only in high quantities in paired specimens of midstream urine and catheter urine, the predictive values of lower quantities of these organisms in midstream urine are not known.
Organisms that are usually considered to be contaminants, such as lactobacilli, grew from catheter urine cultures in 11 episodes (5%), but in only 1 episode was there growth (a coagulase-negative staphylococcus) with pyuria without the presence of gram-negative rods, suggesting that these bacteria rarely cause cystitis.
In 53 of the 202 episodes that were evaluated (26%), there was no organism growth in catheter specimens (sterile cultures). Although infections with Chlamydia trachomatis or Neisseria gonorrhoeae cause dysuria and pyuria in some women,15,24,25 we rarely detected these pathogens in a previous study of this population,26 and thus we test for them only if there is clinical suspicion on the basis of exposure or infection history. With the use of culture-independent gene sequencing of 16S ribosomal RNA (rRNA), bacteria that cannot be cultivated are commonly found in bladder urine collected by catheter or suprapubic aspirates from asymptomatic women,27 but this technique has not been used to evaluate women with cystitis. It is possible that some of the women with sterile catheter urine cultures had urethritis, a syndrome previously described in symptomatic women with E. coli cultured from the urethra or from midstream urine but with sterile catheter urine cultures.15,28 In this regard, 25% of episodes with sterile catheter urine cultures had growth of a uropathogen in paired specimens of midstream urine. It is also possible that noninfectious conditions may underlie symptoms in some women.
The strengths of our study are the number of well-characterized participants with the clinical presentation of cystitis and the detailed microbiologic analysis of specimens of midstream urine and catheter urine with quantification of organisms to 10 CFU per milliliter. Although urethral catheterization may be contaminated by urethral organisms carried into the bladder by the catheter, previous studies have found no significant differences between colony counts in bladder urine that was collected by suprapubic aspiration and those in bladder urine that was collected by urethral catheterization.15,27 We believe that our study population is representative of the relatively homogeneous population of healthy premenopausal women with cystitis and that our microbiologic findings are generalizable to this population. Our findings with respect to E. coli support observations from an earlier study that compared midstream urine cultures with catheter urine cultures.15
The lack of association between midstream urine cultures and catheter urine cultures that was observed with enterococci and group B streptococci confirms beliefs held by some practitioners in the biomedical community that these organisms are infrequent colonizers of the bladder. It is not possible, however, to draw definitive conclusions about enterococci and group B streptococci with the relatively small number of cultures yielding these organisms in this study. The growth in midstream urine culture of K pneumoniae and S. saprophyticus, which are universally considered to be uropathogens, was strongly associated with growth in catheter urine cultures, even though both species were found in few episodes.
National surveys have shown that urine cultures are obtained in 23 to 77% of female outpatients with symptoms of cystitis.29,30 However, urine cultures in healthy women with symptoms of cystitis are not usually warranted because of the delay in obtaining results, expense and inconvenience to the patient, and minimal clinical value in women without concurrent vaginal symptoms.31–33 Several short-course antimicrobial regimens have been recommended for empirical treatment.34 A culture of midstream urine may be appropriate, however, in women with an unclear diagnosis or to examine the resistance profile of strains.32
For episodes of cystitis in which it is decided that a culture might be helpful, the findings of this study should provide instructive information with respect to the interpretation of results. First, some commercial clinical laboratories routinely report growth only for counts of 104 CFU per milliliter or higher, so cultures yielding low E. coli colony counts may be falsely reported as negative. Second, enterococci and group B streptococci are commonly found in midstream urine cultures obtained from women with cystitis,17–23 often in counts of 104 CFU per milliliter or higher, but appear to rarely cause cystitis. E. coli is commonly found in the catheter urine specimens from women with enterococci or group B streptococci in the midstream urine and is the likely cause of many of these episodes. It is possible that predictive values of enterococci and group B streptococci are higher in counts of 104 CFU per milliliter or higher without the concurrent isolation of E. coli, but we found this pattern in only 2 of 202 episodes (both with enterococci). Third, E. coli in mixed flora in midstream urine predicts bladder bacteriuria in 91% of episodes and should not be considered a contaminant.
In conclusion, the data from our study reinforce the view that cultures of midstream urine generally are not indicated in the treatment of healthy premenopausal women with presumptive cystitis. Misinterpretation of such cultures may result in the undertreatment of low-quantity or mixed E. coli infections or inappropriate treatment if enterococcus or group B streptococcus is reported by the laboratory. If treatment of suspected cystitis is to be delayed pending the results of midstream urine culture, one should consider asking the laboratory to quantify E. coli to 102 CFU per milliliter to improve sensitivity15 (Table 3). For epidemiologic and research studies of healthy premenopausal women with cystitis, the use of 103 CFU per milliliter as a compromise threshold for a positive culture growing E. coli may be considered, given feasibility and cost constraints in the microbiology laboratory. On the basis of our data, similar recommendations cannot be made for other gram-negative rods or S. saprophyticus. In order to determine the generalizability of our findings, studies comparing midstream urine and catheter urine cultures will need to be performed in other types of patients with urinary tract infection.
Acknowledgments
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK088830, P01DK053369, and ORWH SCOR P50 DK064540).
We thank Dr. Walter E. Stamm (deceased) for his involvement in discussions leading to the conception and conduct of this study; at the University of Washington: the patients, Medical Director D.C. Dugdale, and the staff at Hall Health Primary Care Center, along with Niki Deshaw, M.A., and Ellen Cassen, A.R.N.P., for the enrollment and follow-up of participants, and Sheila Manuguid, B.S., for laboratory assistance; at the University of Miami: the patients, Wisvline Labrousse, Ph.D., A.R.N.P., for enrollment and follow-up of participants, and Nadege Atis for laboratory assistance.
Footnotes
Presented in part at the 47th Annual Meeting of the Infectious Diseases Society of America, Philadelphia, October 29–November 1, 2009.
Dr. Hooton reports receiving consulting fees from Merck and Pinnacle Pharmaceuticals and having an equity interest in Fimbrion Therapeutics. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 2011;169:1–38. [PubMed] [Google Scholar]
- 2.Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17:227–41. doi: 10.1016/s0891-5520(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 3.Marple CD. The frequency and character of urinary tract infections in an unselected group of women. Ann Intern Med. 1941;14:2220–39. [Google Scholar]
- 4.Barr RH, Rantz LA. The incidence of unsuspected urinary tract infection in a selected group of ambulatory women. Calif Med. 1948;68:437–40. [PMC free article] [PubMed] [Google Scholar]
- 5.Kass EH. Asymptomatic infections of the urinary tract. Trans Assoc Am Physicians. 1956;69:56–64. [PubMed] [Google Scholar]
- 6.Kass EH. The role of asymptomatic bacteriuria in the pathogenesis of pyelonephritis. In: Quinn EL, Kass EH, editors. Biology of pyelonephritis. Boston: Little, Brown; 1960. pp. 399–412. [Google Scholar]
- 7.Kass EH. Bacteriuria and pyelonephritis of pregnancy. Arch Intern Med. 1960;105:194–8. doi: 10.1001/archinte.1960.00270140016003. [DOI] [PubMed] [Google Scholar]
- 8.Kass EH. Pyelonephritis and bacteriuria: a major problem in preventive medicine. Ann Intern Med. 1962;56:46–53. doi: 10.7326/0003-4819-56-1-46. [DOI] [PubMed] [Google Scholar]
- 9.Norden CW, Kass EH. Bacteriuria of pregnancy — a critical appraisal. Annu Rev Med. 1968;19:431–70. doi: 10.1146/annurev.me.19.020168.002243. [DOI] [PubMed] [Google Scholar]
- 10.Sanford JP, Favour CB, Mao FH, Harrison JH. Evaluation of the positive urine culture: an approach to the differentiation of significant bacteria from contaminants. Am J Med. 1956;20:88–93. doi: 10.1016/0002-9343(56)90175-9. [DOI] [PubMed] [Google Scholar]
- 11.Monzon OT, Ory EM, Dobson HL, Carter E, Yow EM. A comparison of bacterial counts of the urine obtained by needle aspiration of the bladder, catheterization and midstream-voided methods. N Engl J Med. 1958;259:764–7. doi: 10.1056/NEJM195810162591603. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher DJ, Montgomerie JZ, North JD. Acute infections of the urinary tract and the urethral syndrome in general practice. Br Med J. 1965;1:622–6. doi: 10.1136/bmj.1.5435.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabeck CE. Studies in urinary tract infections. I. The diagnosis of bacteriuria in women. Acta Med Scand. 1969;186:35–8. [PubMed] [Google Scholar]
- 14.Stamey TA, Timothy M, Millar M, Mihara G. Recurrent urinary infections in adult women: the role of introital entero-bacteria. Calif Med. 1971;115:1–19. [PMC free article] [PubMed] [Google Scholar]
- 15.Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463–8. doi: 10.1056/NEJM198208193070802. [DOI] [PubMed] [Google Scholar]
- 16.Murray PR, Baron EJ, Pfaller MA, et al. Manual of clinical microbiology. 7th ed. Washington, DC: American Society for Microbiology; 1999. [Google Scholar]
- 17.Naber KG, Schito G, Botto H, Palou J, Mazzei T. Surveillance study in Europe and Brazil on clinical aspects and Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC): implications for empiric therapy. Eur Urol. 2008;54:1164–75. doi: 10.1016/j.eururo.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto T, Hamasuna R, Ishikawa K, et al. Sensitivities of major causative organisms isolated from patients with acute uncomplicated cystitis against various antibacterial agents: results of subanalysis based on the presence of menopause. J Infect Chemother. 2012;18:597–607. doi: 10.1007/s10156-012-0419-2. [DOI] [PubMed] [Google Scholar]
- 19.Kahlmeter G. Prevalence and antimicrobial susceptibility of pathogens in uncomplicated cystitis in Europe: the ECO.SENS study. Int J Antimicrob Agents. 2003;22(Suppl 2):49–52. doi: 10.1016/s0924-8579(03)00229-2. [DOI] [PubMed] [Google Scholar]
- 20.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(Suppl 1A):14S–19S. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- 21.Hooton TM, Roberts PL, Stapleton AE. Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial. JAMA. 2012;307:583–9. doi: 10.1001/jama.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooton TM, Scholes D, Gupta K, Stapleton AE, Roberts PL, Stamm WE. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA. 2005;293:949–55. doi: 10.1001/jama.293.8.949. [DOI] [PubMed] [Google Scholar]
- 23.Cai T, Mazzoli S, Nesi G, Boddi V, Mondaini N, Bartoletti R. 14-Day prulifloxacin treatment of acute uncomplicated cystitis in women with recurrent urinary tract infections: a prospective, open-label, pilot trial with 6-month follow-up. J Chemother. 2009;21:535–41. doi: 10.1179/joc.2009.21.5.535. [DOI] [PubMed] [Google Scholar]
- 24.Stamm WE, Wagner KF, Amsel R, et al. Causes of the acute urethral syndrome in women. N Engl J Med. 1980;303:409–15. doi: 10.1056/NEJM198008213030801. [DOI] [PubMed] [Google Scholar]
- 25.Curran JW. Gonorrhea and the urethral syndrome. Sex Transm Dis. 1977;4:119–21. doi: 10.1097/00007435-197707000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Gupta K, Hooton TM, Roberts PL, Stamm WE. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9–16. doi: 10.7326/0003-4819-135-1-200107030-00004. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50:1376–83. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fihn SD, Johnson C, Stamm WE. Escherichia coli urethritis in women with symptoms of acute urinary tract infection. J Infect Dis. 1988;157:196–9. doi: 10.1093/infdis/157.1.196. [DOI] [PubMed] [Google Scholar]
- 29.Kallen AJH, Welch HG, Sirovich BE. Current antibiotic therapy for isolated urinary tract infections in women. Arch Intern Med. 2006;166:635–9. doi: 10.1001/archinte.166.6.635. [DOI] [PubMed] [Google Scholar]
- 30.McIsaac WJ, Prakash P, Ross S. The management of acute uncomplicated cystitis in adult women by family physicians in Canada. Can J Infect Dis Med Microbiol. 2008;19:287–93. doi: 10.1155/2008/404939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287:2701–10. doi: 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 32.Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028–37. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 33.Little P, Moore MV, Turner S, et al. Effectiveness of five different approaches in management of urinary tract infection: randomised controlled trial. BMJ. 2010;340:cl99. doi: 10.1136/bmj.c199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):el03–el20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]