Summary
During vertebrate development the gonad has two possible fates, the testis or the ovary. The choice between these fates is made by a variety of sex-determining mechanisms, from the sex-determining gene on the Y chromosome (Sry) in mammals, to nongenetic temperature-dependent systems in many reptiles. Despite the differences in the mechanisms at the top of the sex-determining cascade, the resulting morphology and many genes involved in early testis and ovarian development are common to most vertebrates, leading to the hypothesis that the underlying processes of sex determination are conserved. In this study, we examined the early steps of gonad development in the red-eared slider turtle (Trachemys scripta), a species that uses the temperature of egg incubation to determine sex. A dramatic increase in cell proliferation was observed in the male gonad during the earliest stages of sex determination. Using the localization of Wilms’ Tumor suppressor 1 (WT1), we determined that this proliferation increase occurred in a population that contained pre-Sertoli cells. The proliferation of pre-Sertoli cells has been documented during sex determination in both mice and alligators, suggesting that proliferation of this cell type has an important role in vertebrate testis organogenesis and the determination of male fate.
Keywords: sex determination, gonad development, testis, Sertoli cell, turtle, WT1, cell proliferation, T. scripta
INTRODUCTION
During sex determination in vertebrates, the rudimentary gonad is induced to form one of two different organs, the testis or the ovary. Initially, the gonad is formed through the same mechanisms in both sexes and the early testis and early ovary are morphologically indistinguishable. During this period, the gonad is considered to be bipotential, poised in a delicate balance between male and female developmental pathways. The morphology, cell types, function, and many of the genes that are necessary for gonad development are well conserved in most, if not all, vertebrates. However, the factor that directs the choice between male and female pathways is not conserved. In fact, vertebrates exhibit a surprising diversity of sex-determining mechanisms. In mammals, the expression of one gene on the Y chromosome (Sry) induces a cascade of genes and events in the XY gonad that initiates testis development. In the absence of Sry expression (in XX gonads), an ovary forms (Lovell-Badge and Robertson, 1990; Page et al., 1990; Koopman et al., 1991). Thus, Sry is considered to be the dominant factor in mammalian sex determination: a master switch that diverts the development of the bipotential gonad to the testis pathway.
Outside of eutherian mammals and marsupials, no homolog of Sry has been identified (reviewed in Spotila et al., 1994). In chickens, males have two Z chromosomes, while females have one Z and one W chromosome. Not only is the heterogametic sex reversed in chickens, but the Z and W chromosomes do not share significant homology to the X and Y chromosomes in mammals. Instead, chickens appear to use the relative dosage of factors from both the Z and W to determine sex (reviewed in Smith and Sinclair, 2001). Although the sex-determining factors differ, both mammals and birds use genetic mechanisms to determine sex. However, many vertebrates do not rely on genetic determinates. In crocodilians and many species of fish, lizards, and turtles, the sex of the embryo is determined by environmental cues such as the temperature of egg incubation.
Despite the fact that the master switch of sex determination is not conserved among vertebrates, the morphology and function of vertebrate testes and ovaries are remarkably similar. Homologs of genes known to be part of the sex-determining pathway in mammals, such as Wt1, Sox9, Mis, Sf1, and Dmrt, have been found in the developing gonads of various birds, turtles, fish, and alligators, indicating that the underlying forces behind sex determination in these systems may not be very different (Kent et al., 1995; Spotila and Hall, 1998; Fleming et al., 1999; Moreno-Mendoza et al., 1999; Kettlewell et al., 2000; Smith and Sinclair, 2001; Western and Sinclair, 2001). Comparison of early gonad development in vertebrates has turned up many similarities. For example, an early size increase of the male gonad over the female gonad has been observed not only in all mammalian species so far examined, but also in vertebrates that are not known to possess Sry, such as chickens, alligators, and turtles (reviewed in Mittwoch, 1986). The conservation of this male-specific size increase during the earliest stages of gonad development in a diverse array of species suggested that this size increase was somehow important in sex determination. In fact, before the discovery of Sry it was hypothesized that differential growth rates determined the sex of the gonad, such that gonads with more rapid growth rates became testes, while gonads which failed to reach a certain size by a given stage became ovaries (Mittwoch, 1969, 1989; Hunt and Mittwoch, 1987). Recently, we have shown that one of the earliest functions of the mammalian master switch (Sry) is the induction of cell proliferation, leading to the early size increase of the XY gonad.
Early proliferation in the mouse gonad occurs in a population that contains the precursors of a male-specific cell type called the Sertoli cell (Schmahl et al., 2000). Sertoli cells have critical roles in early testis development. Not only are they support cells for germ cells and essential components of testis cords, but there is also evidence that Sertoli cells express Sry and direct the morphogenesis of the entire testis (Burgoyne and Palmer, 1993; Albrecht and Eicher, 2001). In alligators, an early proliferation in Sertoli cell precursors is one of the first morphological differences between the sexes (Smith and Joss, 1994), indicating that proliferation in this critical cell type may be a conserved mechanism in vertebrate testis organogenesis.
In this study, we investigated the early mechanisms of sex determination in the red-eared slider turtle (Trachemys scripta). T. scripta are not known to possess Sry, or any other genetic differences between the sexes. Instead, this species uses temperature-dependent sex determination. When T. scripta eggs are incubated at 26°C, the embryos become male. Incubation at a higher temperature (31°C) produces female embryos (Bull and Vogt, 1981). Using a marker of cell proliferation and the immunolocalization of WT1, we examined the development of early gonads of T. scripta to determine if some of the early steps of sex determination are conserved between genetic and temperature-dependent sex determination.
RESULTS
Size Increase of Male Gonads Over Female Gonads Precedes Any Other Morphological Differences Between the Sexes
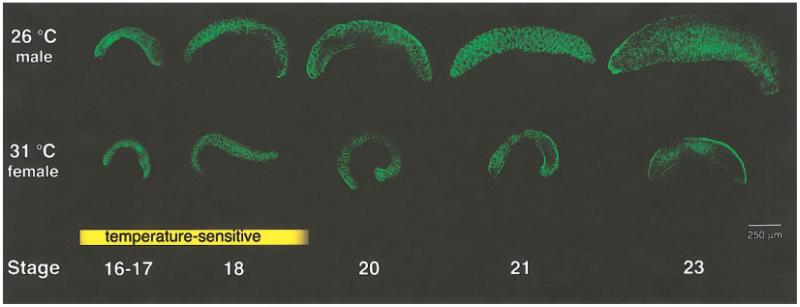
In T. scripta, the gonad forms on the ventromedial surface of the mesonephric kidney at stage 14. During its early development the gonad is morphologically identical in embryos incubated at either the male- (26°C) or female- (31°C) producing temperatures and is considered bipotential. In the turtle, this means that the gonad is not committed to either the testis or ovarian fate and switching the temperature of egg incubation during this period will change the sex of the gonad (Wibbels et al., 1991). Thus, the bipotential stage of development in T. scripta is also called the temperature-sensitive period. At roughly stage 20 in the male and stage 19 in the female, the gonad becomes committed to the testis or ovarian pathway. After these stages, changing the temperature has no effect on the sex of the embryo. Previously, no morphological differences between the sexes were observed before stages 18-19 in the turtle, when sex-specific basement membranes form around presumptive testis or ovarian structures (Wibbels et al., 1991). However, when the size of turtle gonads were compared at different stages of development (Fig. 1), it was apparent that embryos incubated at the male temperature developed larger, thicker gonads than those incubated at the female temperature. This size difference between the sexes was obvious as early as stages 16-17, during the period when the sex of the gonad is still sensitive to temperature and is not yet determined. The size difference between the testis and the ovary was maintained at least through stage 23 (Fig. 1). By hatching (stage 26), male and female gonads are closer in size, due to increased growth of the ovary at later stages (Wibbels et al., 1991).
FIG. 1.
Differential growth of male and female gonads. The gonads of both sexes increased in size during development. However, using an antibody against WT1 to detect many of the somatic cells of the gonad (green), it was observed that gonads from embryos incubated at the male-producing temperature (26°C) were larger than gonads from embryos incubated at the female-producing temperature (31°C). This size difference was observed from the bipotential, temperature-sensitive period (yellow box), through the time when the gonads have formed distinct testis or ovarian structures (stages 20–23).
Localization of WT1 in the Early Turtle Gonad
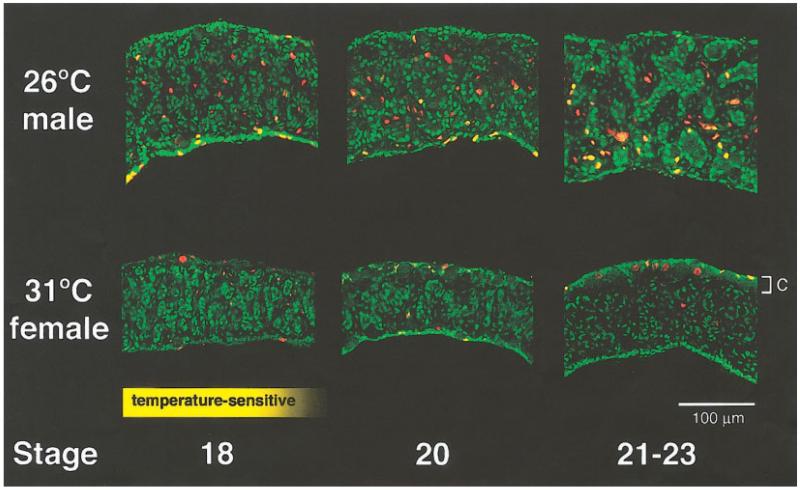
To examine the cell types and structures during the development of T. scripta gonads, we used an antibody against WT1, a DNA-binding protein expressed in the early gonads of many species (Pelletier et al., 1991; Kent et al., 1995). During the temperature-sensitive period, WT1 was observed in the nuclei of somatic cells in the epithelial layer that surrounds the gonad and in cells in the underlying medullary sex cords (Fig. 2, green). These structures have been previously described in turtles by light microscopy and are found in both males and females during the temperature-sensitive period (Wibbels et al., 1991). WT1 was also observed at a lower level in a few somatic cells in the medulla of the gonad, between the primitive sex cords.
FIG. 2.
Localization of WT1 during gonad development. WT1 (green) was observed within the nuclei of cells in the sex cords and in the surface epithelium during the period when the sex of the gonad is still sensitive to temperature (yellow box). After sex determination, the sex cords of the male increased in size and diameter, resulting in clearly defined, widely spaced testis cords by stages 21–23. BrdU labeling detects many dividing cells in the medulla of the male gonad at all stages (red, or yellow where double-labeled with WT1). In the female, the sex cords degenerated by stages 21–23. At this time a distinct cortex (c) was observed, containing dividing WT1-positive somatic cells and germ cells (identified by their large spherical nuclei).
Using WT1 localization, differences between the internal structures of gonads incubated at 26°C or 31°C could be observed as early as stage 18. At this stage the sex cords in gonads at the male-producing temperature become more defined and begin to separate, while the sex cords become less distinct at the female-producing temperature (Figs. 2, 3). After sex is determined (stage 19-20) the sex cords of the testis continue to separate and widen to enclose germ cells as they become testis cords. By stage 21, strongly WT1-positive cells are specific to the surface epithelium and the testis cords. Judging by their close association with germ cells and the location of their nuclei in the basal layer of testis cords, these WT1-positive cells in the cords are Sertoli cells.
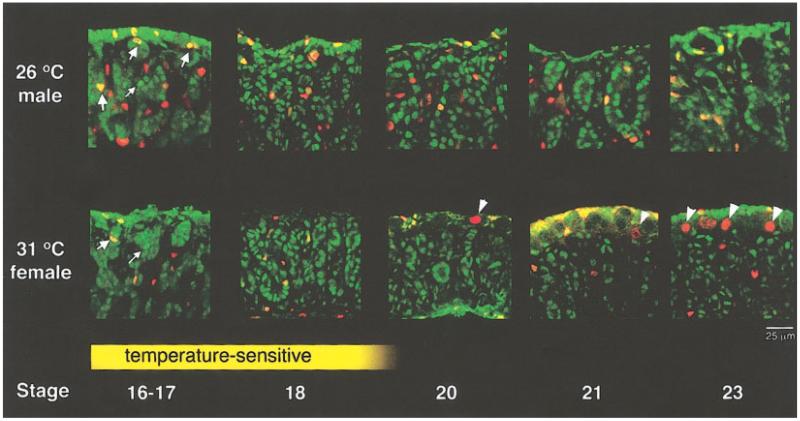
FIG. 3.
Proliferating cell types. During the temperature-sensitive period of sex determination (yellow box), more dividing cells (red and yellow) were observed in the male gonad than in the female gonad. Some cells in the primitive sex cords were more strongly labeled with WT1 (bright green, examples indicated by small arrows). These cells were observed at both the male and female temperatures, but many more were dividing in the male (bright yellow, large arrows). After sex determination, a short burst of proliferation was observed in WT1-positive cells in the female epithelium (stages 20–21). This burst of somatic proliferation was accompanied by an increase in germ cell proliferation in the cortex of the female gonad (arrowheads).
During ovarian development, localization of WT1 clearly showed the degeneration of the sex cords, leaving weakly WT1-positive cells scattered within the medulla. WT1 was transiently downregulated in the surface epithelium of the ovary between stages 18 and 20. During this period germ cells at the surface of the gonad began to proliferate and contribute to the thickening of the cortex in the ovary (Figs. 2, 3, arrowheads). By stages 20–21, WT1 is again strongly localized to somatic cells in the female surface epithelium. These WT1-positive cells are on top of and extending between germ cells in the cortex, in arrangements consistent with epithelial and prefollicle cells.
Before Sex Determination Male-Specific Proliferation Increased in a Population That Contained Pre-Sertoli Cells
The size increase of the male gonad over the female was observed long before any differences in internal morphology. To investigate the mechanism of this size increase, we used 5′-bromo-2′-deoxy-uridine (BrdU) to label dividing cells in the early gonad (the red label in Figs. 2, 3, 4). Dividing cells were observed at both temperatures at all stages. However, more dividing cells were observed at the male-producing temperature, concentrated in the cords and interstium of the medulla (Figs. 2, 3). During early gonad development (stages 16-17), some cells within the sex cords labeled more strongly with WT1 than others. These strongly WT1-positive cells were initially observed at both temperatures, but colocalization with BrdU showed that more of these cells were dividing at the male temperature. As the gonads developed, the level of WT1 decreased in the sex cords of the female gonad and these strongly labeled cells were no longer visible in the medulla by the end of the temperature-sensitive stage. In the male, the level of WT1 increased in the cells of sex cords until nearly all of the somatic cells in these structures expressed a high level of WT1, a process coincident with their differentiation into Sertoli cells.
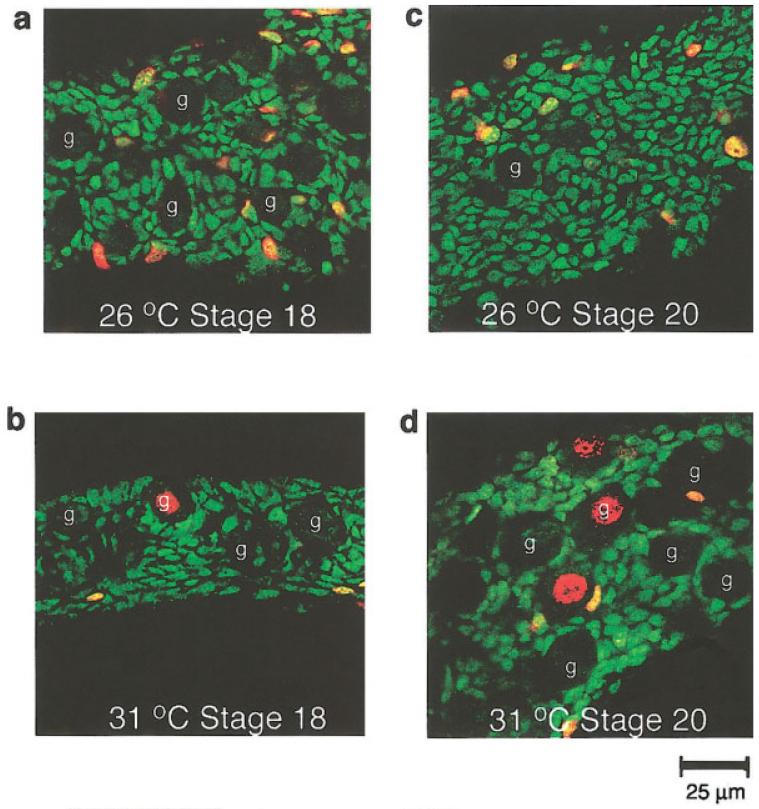
FIG. 4.
Development of the gonad cortex. Confocal sections were taken through just the upper surface layers (cortex) of the gonad. During the temperature-sensitive period, germ cells could be identified in the cortex of the gonad at both male (a) and female (b) temperatures by their large size, spherical nuclei, and lack of WT1 (examples indicated by g). At later stages, germ cells all but disappeared from the cortex of the male (c), presumably due to sequestering inside testis cords. In the female, an increase in germ cell proliferation was observed in the cortex as development progressed (d).
Between the cords, a large number of proliferating cells were observed that did not express WT1 (Fig. 3). Based on their location outside of the cords and their lack of WT1 expression, these cells are unlikely to be pre-Sertoli cells. Proliferation in these cells may contribute to the expansion of the interstitium and the separation of cords observed in this study and in Wibbels et al. (1991).
After Sex Determination, Proliferation Was Observed in Sex-Specific Patterns
Proliferation at the male temperature remained high as development progressed and presumably drove the size increase and the growth of testis cords at this temperature. At the female-producing temperature, the initial pattern of proliferation during the temperature-sensitive period was similar to the male, but fewer dividing cells were observed. After sex determination the pattern of proliferation in the female changed drastically. By stage 20, few dividing cells were observed in the medulla, while an increase in proliferation was observed in both somatic and germ cells at the surface of the ovary, in the developing cortex. The somatic proliferation in this area occurred in epithelial and presumptive pre-follicle cells strongly expressing WT1, possibly contributing to the eventual formation of primary follicles in this region.
Germ Cell Proliferation Exhibits Sex-Specific Differences
Germ cells, identified by their large size, spherical nuclei, and the absence of WT1, were found at the surface of the gonad during the temperature-sensitive period and showed low levels of proliferation in both sexes. After the temperature-sensitive period, germ cells ceased dividing in the male and by stage 20 the majority of the germ cells had moved into the gonad to populate the testis cords (Fig. 4). In the female, germ cells stayed at the surface of the gonad. Germ cell proliferation increased in this area, beginning at roughly stage 20 and lasting at least until hatching (Figs. 3, 4, and data not shown).
DISCUSSION
WT1 Expression Pattern Suggests a Conserved Role in Gonad Development and Sex Determination
WT1 is a zinc-finger DNA-binding protein that is essential for gonad development and sex determination in humans and mice (Hastie, 1992; Kriedberg et al., 1993; Vidal and Schedl, 2000; Hammes et al., 2001). In mammals, WT1 is localized to somatic cells in the gonad primordium of both sexes. After sex determination, WT1 becomes specific to Sertoli cells in the testis, follicle cells in the ovary, and cells in the surface epithelium of both sexes (Pelletier et al., 1991; Mundlos et al., 1993). Using RNA expression studies, WT1 has been observed in the gonads of nonmammalian species, such as chickens (Kent et al., 1995), Xenopus (Semba et al., 1996), and alligators (Western et al., 2000). In T. Scripta, Northern blots of the urogenital ridge (Spotila et al., 1998) suggested that WT1 may have a role in gonad development in turtles.
Using an antibody against WT1, we determined that WT1 was found within the nuclei of many cells in the gonads of both sexes, including the cells of the sex cords, the surface epithelium, and some cells in the interstitium. As in mammals, strong WT1 labeling becomes specific to particular cell types after sex determination, including the cells in the surface epithelium and the support cell lineages of both sexes, i.e., Sertoli cells in the testis and pre-follicle cells in the ovary. In mammals, a specific isoform of WT1 is essential for normal levels of expression of the male sex-determining gene (Sry) and the development of the testis pathway (Klamt et al., 1998; Hammes et al., 2001). Interestingly, the region that gives rise to this isoform is conserved in all vertebrate WT1 sequences yet examined and WT1 RNA with and without this region is expressed in the turtle urogenital ridge during the temperature-sensitive period (Spotila and Hall, 1998). The expression of this critical WT1 isoform during turtle sex determination, combined with the conserved localization pattern, suggests that WT1 has a conserved role in vertebrate sex determination.
Increase in Gonad Size and Cell Proliferation Is Conserved During Sex Determination in Vertebrates
Administration or inhibition of steroid hormones can override the temperature control of sex, indicating that hormones can play a role in sex determination. Some of the molecular components controlled by temperature or hormone action have been tentatively identified, such as Sf1 and Dmrt1. The expression of these two genes is affected by temperature in T. scripta as early as stages 15-17 (Kettlewell et al., 2000; Crews et al., 2001). However, using light microscopy (Wibbels et al., 1991) or WT1 localization, changes in internal gonad morphology are not apparent until the end of the temperature-sensitive period (stage 18). At this stage, shifting the temperature changes the sex of less than 50% of the gonads (Wibbels et al., 1991), indicating that more than half of the gonads are already committed to the testis or ovarian pathways by the time the first distinct male or female structures have formed. To investigate the cellular mechanisms that might be initiating the commitment to one pathway or the other, we looked for temperature-specific differences earlier in development. In this study, we observed an increase in the size of gonads at the male-producing temperature over the size of gonads at the female temperature. This size increase was detected by stages 16-17, before other morphological differences between the sexes and before temperature shift experiments show commitment to either pathway.
Although gonad size and growth rates vary according to species and temperatures, a size increase of the male gonad over the female gonad at the equivalent developmental stage has been observed during the development of other turtles, such as the sea-turtles Dermochelys coriacea (Rimblot et al., 1985) and Lepidochelys olivacea (Merchant-Larios et al., 1997), and the fresh-water turtle Emys orbicularis (Pieau et al., 1998). In E. orbicularis, the ovary undergoes a post-sex determination growth increase and becomes larger than the testis by hatching. However, as in T. scripta, the size increase of the gonad at the male temperature in E. orbicularis is observed during the earliest stages of the temperature-sensitive period, before the sex of the gonad is established, suggesting that events contributing to this size increase may be necessary not only for the development of testis structure, but also for the choice of the testis fate in turtles.
An early size increase of the male gonad has been observed in many vertebrates (reviewed in Mittwoch, 1986). In mice, a male-specific size increase is one of the earliest effects of Sry expression and male sex determination and is linked to early proliferation at the surface of the XY gonad (Schmahl et al., 2000). In T. scripta, an increase in proliferation was observed in the surface epithelium, the sex cords, and in the interstitium of gonads from embryos incubated at the male temperature. During the temperature-sensitive period, gonads at the male temperature had more proliferating cells than gonads at the female temperature, but the location of dividing cells appeared to be the same in both sexes (Fig. 5). After the temperature-sensitive period, dramatic sex-specific differences in the localization of proliferating cells were observed. In the developing testis, dividing cells were observed in the testis cords, consistent with the expansion of these structures after sex determination. In the ovary, a lower amount of somatic cell proliferation was observed at most stages. However, in both E. orbicularis and T. scripta the female gonad increased in size after the temperature-sensitive period. In T. scripta, this female-specific size increase was probably due to the expansion of the cortex, driven by an increase in somatic and germ cell proliferation in this region. Germ cells in T. scripta continue to proliferate in the female cortex at least until hatching. In mammals, germ cells in the ovary arrest in meiosis shortly after sex determination. The longer period of germ cell proliferation in the turtle may be related to the huge demand for oocytes in egg-laying vertebrates. In the testis of T. scripta, germ cell proliferation ceased as these cells migrated into the testis cords, similar to the mitotic arrest of male germ cells observed after cord formation in mammals.
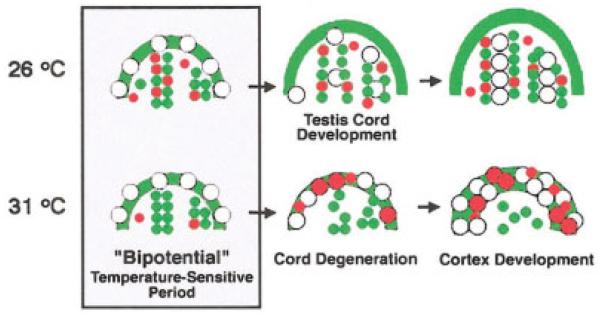
FIG. 5.
Timeline of gonad development in T. scripta. During early gonad development, the sex of the gonad is sensitive to the temperature of egg incubation and the gonad is considered bipotential. During this period gonads from embryos incubated at the male (26°C) and female (31°C) temperatures were morphologically similar, with WT1-positive cells making up the sex cords (green circles) and the somatic component of the cortex (green arch). Germ cells are also observed in the cortex at this stage (large white cells). The first differences between the male and female gonads were observed during the temperature-sensitive period as an increase in proliferation of cells within the medulla of the male gonad (red). After sex determination, the sex cords in the medulla of the male enclose germ cells and become testis cords. In the female, the sex cords degenerate and the cortex thickens, due to an increase in somatic and germ cell proliferation (red).
It is not unexpected that the rate of cell proliferation may be affected by temperature. However, in turtle gonads the higher rate of proliferation and larger organ size was observed at the lower, male-determining temperature. Incubation of clutches of E. orbicularis eggs at a pivotal temperature (28.5°C) gives rise to both male and female offspring (Pieau and Dorizzi, 1981). In these 28.5°C clutches the gonads of future males and females enter the temperature-sensitive period at the same size. Despite the fact that the eggs are incubated at the same temperature, by the end of sex determination the male gonads are larger than female gonads (Pieau et al., 1998), indicating that the size increase of the gonad is dependent on (or part of) the sex-determining mechanism and it is not a general effect of temperature on cell proliferation.
Although the proliferation increase at the male temperature contributes to the size increase of the male gonad, the influence of other cellular events should not be overlooked. In both mice and alligators, hyperplasia of pre-Sertoli cells is observed during the early stages of sex determination (Magre and Jost, 1980; Smith and Joss, 1993). Cell migration from adjacent tissue is specific to male gonads during sex determination in mice and is necessary for events in testis development (Martineau et al., 1997). Our study does not rule out the contribution of these or other cellular events to the size increase of the male gonad, but it does strongly suggest that one of the forces of the size increase in T. scripta is an increase in cell proliferation at the male temperature.
Proliferation of Sertoli Precursors May Have a Role in Vertebrate Sex Determination
The American alligator (Alligator mississippiensis) also uses temperature-dependent sex determination. However, in A. mississippiensis incubation at either high or low temperatures (35°C or 30°C) gives rise to females, while incubation at mid-temperatures (around 33°C) gives rise to males. Despite the different temperature profile, gonad development in the alligator is very similar to the turtle (Smith and Joss, 1993). The fine resolution of electron microscopy also allowed Smith and Joss (1993) to identify pre-Sertoli cells within the sex cords of the alligator gonad and showed that the first difference between the sexes of the alligator is the proliferation and differentiation of pre-Sertoli cells. In the alligator, it is theorized that the determination of the sex of the gonad is dependent on Sertoli cell proliferation, such that if the number of pre-Sertoli cells increases over a particular threshold, these cells begin to organize testis structures and recruit other cells to the male fate. If the number of Sertoli cells stays below this threshold, ovary formation (possibly driven by a germ cell-mediated signal) will take over (Smith and Joss, 1994). This hypothesis would also explain the equal sex ratio of clutches at pivotal temperatures, where slight variations in pre-Sertoli proliferation would determine which gonads exceed the threshold number necessary for testis determination. Alternatively, a commitment to the male pathway may be reflected in a coincident upregulation of proliferation in pre-Sertoli cells, as is the case in mammals.
At first glance, the pattern of proliferation and location of pre-Sertoli cells observed in the medulla of T. scripta and A. mississippiensis appears to be opposite that observed in mice, where many proliferating pre-Sertoli cells are located in the surface epithelium. However, in mice pre-Sertoli cells quickly migrate from the surface into the medulla of the gonad, where they undergo the first signs of differentiation (Karl and Capel, 1998). Thus, the early testis of the turtle and the mouse might not be so different, as both show the first differentiating Sertoli cells in the medulla, and it remains possible that studies in T. scripta and A. mississippiensis were not able to resolve undifferentiated pre-Sertoli cells located at the surface of the gonad. In fact, the connection of sex cords with the surface epithelium has lead to the hypothesis that the sex cords in reptiles originate from this surface layer. Alternatively, this position of pre-Sertoli cells may indicate a mechanistic difference between reptiles and mammals, with pre-Sertoli cells in reptiles already located in the primitive sex cords at undifferentiated stages.
Sertoli cells appear to have an essential role in early sex determination in many vertebrates. In mammals, Sertoli cell precursors express the male sex-determining gene Sry, which appears to act through this cell type to divert the gonad from the ovarian to the testis pathway (Burgoyne and Palmer, 1993; Albrecht and Eicher, 2001). In mice, one of the first differences between the sexes is an increase in proliferation of Sertoli cells (Schmahl et al., 2000). Reductions of the number of Sertoli cells in mice and humans often leads to the failure of testis formation, as seen in XX/Sry mice with Sry on a preferentially deactivated X chromosome (Fechner et al., 1994; Kusz et al., 1999) and in XX-XY chimeras (Palmer and Burgoyne, 1991), indicating that a threshold number of Sertoli cells may be necessary for testis development in mammals as well. Such commonalties between species which use disparate mechanisms of sex determination suggests that proliferation of pre-Sertoli cells is an essential process that must be initiated to direct the bipotential gonad to the male pathway, regardless of the master switch used to determine sex in the organism.
MATERIALS AND METHODS
Dissection, Staging, and BrdU Labeling
Shipments of freshly laid eggs of red-eared slider turtles were obtained commercially from Kleibert Turtle Farms (Hammond, LA). Viability was established with candling, then the eggs were incubated at either 26°C or 31°C (temperatures which produce only males or females, respectively; Bull and Vogt, 1981). The progress of development was monitored by dissection of 1-2 eggs at regular intervals. Embryos were staged according to criteria established by Yntema (1968). Gonads were removed from 4-6 embryos at specific stages of gonad development (stages 16-24). BrdU is an analog of thymidine and incorporates into the DNA of dividing cells. BrdU labeling was done in vitro by culturing dissected gonads at the male or female temperature for 1 h in culture media consisting of 10% FCS (Gibco, Grand Island, NY), 50 μg/ml ampicillin, and 5% CO2 in DMEM (without phenol red) containing 10 μm BrdU. These samples were then rinsed in PBS and fixed in 4% paraformaldehyde in PBS at 4°C overnight.
BrdU Detection and Immunohistochemistry
BrdU was detected using procedures modified from Schutte et al. (1987) and Carayon and Boyd (1992). Samples were rinsed three times with PBS, then rinsed once in 0.01N HCL for 3 min. Samples were incubated in 1 mg/ml pepsin (Sigma, St. Louis, MO; P-6887) in 0.01N HCL for 1 h at 37°C and rinsed thoroughly three times in reaction buffer (0.1M Tris pH 7.5, 50 mM NaCl, 10 mM MgCl2), about 3 min per wash. Samples were incubated for 1 h at 37°C in 100 U/ml DNAse I (Sigma DN-25) in reaction buffer. Samples were incubated for 1 h at room temperature in 10% goat serum, 0.1% Triton X-100 in PBS, incubated overnight in primary antibodies diluted in 1% goat serum, 0.01% Triton X-100 in PBS at 4°C, rinsed three times in PBS, and incubated in secondary antibodies diluted 1:500 in 1% goat serum, 0.01% Triton X-100 in PBS for 2 h at room temperature. Proliferating cells were identified using an antibody against BrdU diluted 1:100 (mouse monoclonal IgG antibody from Boehringer Mannheim, Germany; clone BMC 9318). Many somatic cells were identified with a rabbit polyclonal antibody against WT1 from Santa Cruz Biotechnology (C-19), used at 1:100 dilutions. Whole samples were mounted for confocal imaging as described in Karl and Capel (1998) and images were collected using a Zeiss LSM 410 confocal microscope. All gonads were examined at low magnification (10×) to verify that there were no abnormally advanced or delayed samples. At early stages, high magnification (40-100×) confocal sectioning was preformed for one of the pair of gonads from each embryo. At later stages (after the temperature-sensitive stages) the gonads from embryos at the same temperature and stage were pooled, examined under low magnification, and 4-6 representatives were selected for detailed confocal sectioning.
ACKNOWLEDGMENTS
We wish to thank Jordan Batchvarov for his technical assistance with this project, and other members of the laboratory for helpful discussions. This work was supported by grants from NSF, NIH, and the Labor Foundation.
LITERATURE CITED
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Bull JJ, Vogt RC. Temperature-sensitive periods of sex determination in Emydid turtles. J Exp Zool. 1981;218:435–440. doi: 10.1002/jez.1402180315. [DOI] [PubMed] [Google Scholar]
- Burgoyne P, Palmer S. Cellular basis of sex determination and sex reversal in mammals. In: Hillier SG, editor. Gonadal development and function. Raven Press; New York: 1993. pp. 17–29. [Google Scholar]
- Carayon P, Boyd A. Identification of DNA-replicating lymphocyte subsets using a new method to label the bromo-deoxyuridine incorporated into the DNA. J Immunol Methods. 1992;147:225–230. doi: 10.1016/s0022-1759(12)80012-3. [DOI] [PubMed] [Google Scholar]
- Crews D, Fleming A, Willingham E, Baldwin R, Skipper J. Role of steroidogenic factor 1 and aromatase in temperature-dependent sex determination in the red-eared slider turtle. J Exp Zool. 2001;290:597–606. doi: 10.1002/jez.1110. [DOI] [PubMed] [Google Scholar]
- Fechner PY, Rosenberg C, Stetten G, Cargile CB, Pearson PL, Smith KD, Migeon CJ, Berkovitz GD. Nonrandom inactivation of the Y-bearing X chromosome in a 46,XX individual: evidence for the etiology of 46,XX true hermaphroditism. Cytogenet Cell Genet. 1994;66:22–26. doi: 10.1159/000133656. [DOI] [PubMed] [Google Scholar]
- Fleming A, Wibbels T, Skipper JK, Crews D. Developmental expression of steroidogenic factor 1 in a turtle with temperature-dependent sex determination. Gen Comp Endocrinol. 1999;116:336–346. doi: 10.1006/gcen.1999.7360. [DOI] [PubMed] [Google Scholar]
- Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, Gubler MC, Schedl A. Two splice variants of the Wilms’ tumor 1 gene have distinct functions during sex determination and nephron formation. Cell. 2001;106:319–329. doi: 10.1016/s0092-8674(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Hastie ND. Dominant negative mutations in the Wilms tumour (WT1) gene causes Denys-Drash syndrome — proof that a tumour-suppressor gene plays a crucial role in normal genitourinary development. Hum Mol Genet. 1992;1:293–295. doi: 10.1093/hmg/1.5.293. [DOI] [PubMed] [Google Scholar]
- Hunt SE, Mittwoch U. Y-chromosomal and other factors in the development of testis size in mice. Genet Res. 1987;50:205–211. doi: 10.1017/s0016672300023715. [DOI] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Kent J, Coriat AM, Sharpe PT, Hastie ND, van Heyningen V. The evolution of WT1 sequence and expression pattern in the vertebrates. Oncogene. 1995;11:1781–1792. [PubMed] [Google Scholar]
- Kettlewell JR, Raymond CS, Zarkower D. Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. genesis. 2000;26:174–178. [PubMed] [Google Scholar]
- Klamt B, Koziell A, Poulat F, Wieacker P, Scambler P, Berta P, Gessler M. Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/−KTS splice isoforms. Hum Mol Genet. 1998;7:709–714. doi: 10.1093/hmg/7.4.709. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Kriedberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. Wt-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Kusz K, Kotecki M, Wojda A, Szarras-Czapnik M, Latos-Bielenska A, Warenik-Szymankiewicz A, Ruszczynska-Wolska A, Jaruzelska J. Incomplete masculinisation of XX subjects carrying the SRY gene on an inactive X chromosome. J Med Genet. 1999;36:452–456. [PMC free article] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the murine primary testis determining gene, Tdy. Development. 1990;109:635–646. doi: 10.1242/dev.109.3.635. [DOI] [PubMed] [Google Scholar]
- Magre S, Jost A. The initial phases of testicular organogenesis in the rat. An electron microscopy study. Arch Anat Microsc Morphol Exp. 1980;69:297–318. [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Ruiz-Ramirez S, Moreno-Mendoza N, Marmolejo-Valencia A. Correlation among thermosensitive period, estradiol response, and gonad differentiation in the sea turtle Lepidochelys olivacea. Gen Comp Endocrinol. 1997;107:373–385. doi: 10.1006/gcen.1997.6946. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Do genes determine sex? Nature. 1969;221:446–448. doi: 10.1038/221446a0. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Males, females and hermaphrodites. Ann Hum Genet. 1986;50:103–121. doi: 10.1111/j.1469-1809.1986.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Sex differentiation in mammals and tempo of growth: probabilities vs. switches. J Theor Biol. 1989;137:445–455. doi: 10.1016/s0022-5193(89)80039-6. [DOI] [PubMed] [Google Scholar]
- Moreno-Mendoza N, Harley VR, Merchant-Larios H. Differential expression of SOX9 in gonads of the sea turtle Lepidochelys olivacea at male- or female-promoting temperatures. J Exp Zool. 1999;284:705–710. doi: 10.1002/(sici)1097-010x(19991101)284:6<705::aid-jez12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B. Unclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development. 1993;119:1329–1341. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- Page DC, Fisher E, McGillivray B, Brown LG. Additional deletion in sex-determining region of human Y chromosome resolves paradox of X,t(Y,22) female. Nature. 1990;346:279–281. doi: 10.1038/346279a0. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS. In situ analysis of fetal, prepuberal and adult XX-XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development. 1991;112:265–268. doi: 10.1242/dev.112.1.265. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Schalling M, Buckler AJ, Rogers A, Haber DA, Housman D. Expression of the Wilms’ tumour gene WT1 in the murine urogenital system. Genes Dev. 1991;5:1345–1356. doi: 10.1101/gad.5.8.1345. [DOI] [PubMed] [Google Scholar]
- Pieau C, Dorizzi M. Determination of temperature sensitive stages for sexual differentiation of the gonads in embryos of the turtle, Emys orbicularis. J Morphol. 1981;170:373–382. doi: 10.1002/jmor.1051700308. [DOI] [PubMed] [Google Scholar]
- Pieau C, Dorizzi M, Richard-Mercier N, Desvages G. Sexual differentiation of gonads as a function of temperature in the turtle Emys orbicularis: endocrine function, intersexuality and growth. J Exp Zool. 1998;281:400–408. doi: 10.1002/(sici)1097-010x(19980801)281:5<400::aid-jez5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rimblot F, Fretey J, Mrosovsky N, Lescure J, Pieau C. Sexual differentiation as a function of the incubation temperature of eggs in the sea-turtle Dermochelys coriacea (Vandelli, 1761) Amphibia-Reptilia. 1985;6:83–92. [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- Schutte B, Reynders MMJ, Bosman FT, Blijham GH. Effect of tissue fixation on anti-bromodeoxyuridine immunohistochemistry. J Histochem Cytochem. 1987;35:1343–1345. doi: 10.1177/35.11.3116075. [DOI] [PubMed] [Google Scholar]
- Semba K, Saito-Ueno R, Takayama G, Kondo M. cDNA cloning and its pronephros-specific expression of the Wilms’ tumor suppressor gene, WT1, from Xenopus laevis. Gene. 1996;175:167–172. doi: 10.1016/0378-1119(96)00143-6. [DOI] [PubMed] [Google Scholar]
- Smith CA, Joss JM. Gonadal sex differentiation in Alligator mississippiensis, a species with temperature-dependent determination. Cell Tissue Res. 1993;273:149–163. [Google Scholar]
- Smith CA, Joss JM. Sertoli cell differentiation and gonadogenesis in Alligator mississippiensis. J Exp Zool. 1994;270:57–70. [Google Scholar]
- Smith CA, Sinclair AH. Sex determination in the chicken embryo. J Exp Zool. 2001;290:691–699. doi: 10.1002/jez.1119. [DOI] [PubMed] [Google Scholar]
- Spotila LD, Hall SH. Expression of a new RNA-splice isoform of WT1 in developing kidney-gonadal complexes of the turtle, Trachemys scripta. Comp Biochem Physiol Part B. 1998;119:761–767. doi: 10.1016/s0305-0491(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Spotila JR, Spotila LD, Kaufer NF. Molecular mechanisms of TSD in reptiles: a search for the magic bullet. J Exp Zool. 1994;270:117–127. doi: 10.1002/jez.1402700113. [DOI] [PubMed] [Google Scholar]
- Spotila LD, Spotila JR, Hall SH. Sequence and expression analysis of Wt1 and Sox9 in the red-eared slider turtle, Trachemys scripta. J Exp Zool. 1998;281:417–427. doi: 10.1002/(sici)1097-010x(19980801)281:5<417::aid-jez7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Vidal V, Schedl A. Requirement of WT1 for gonad and adrenal development: insights from transgenic animals. Endocr Res. 2000;26:1075–1082. doi: 10.3109/07435800009048640. [DOI] [PubMed] [Google Scholar]
- Western PS, Sinclair AH. Sex, genes, and heat: triggers of diversity. J Exp Zool. 2001;290:624–631. doi: 10.1002/jez.1113. [DOI] [PubMed] [Google Scholar]
- Western PS, Harry JL, Marshall Graves JA, Sinclair AH. Temperature-dependent sex determination in the American alligator: expression of SF1, WT1 and DAX1 during gonadogenesis. Gene. 2000;241:223–232. doi: 10.1016/s0378-1119(99)00466-7. [DOI] [PubMed] [Google Scholar]
- Wibbels T, Bull J, Crews D. Chronology and morphology of temperature-dependent sex determination. J Exp Zool. 1991;260:371–381. doi: 10.1002/jez.1402600311. [DOI] [PubMed] [Google Scholar]
- Yntema CL. A series of stages in the embryonic development of Chelydra serpentina. J Morphol. 1968;125:219–251. doi: 10.1002/jmor.1051250207. [DOI] [PubMed] [Google Scholar]