Abstract
There now exists a resurgence of interest in the role of intermediary metabolism in medicine; especially in relation to medical disorders. Coupled with this is the contemporary focus on molecular biology, genetics and proteomics and their integration into studies of regulation and alterations in cellular metabolism in health and disease. This is a marriage that has vast potential for elucidation of the factors and conditions that are involved in cellular metabolic and functional changes, which heretofore could not be addressed by the earlier generations of biochemists who established the major pathways of intermediary metabolism. The achievement of this present potential requires the appropriate application and interpretation of genetic and proteomic studies relating to cell metabolism and cell function. This requires knowledge and understanding of the principles, relationships, and methodology, such as biochemistry and enzymology, which are involved in the elucidation of cellular regulatory enzymes and metabolic pathways. Unfortunately, many and possibly most contemporary molecular biologists are not adequately trained and knowledgeable in these areas of cell metabolism. This has resulted in much too common inappropriate application and misinformation from genetic/proteomic studies of cell metabolism and function. This presentation describes important relationships of cellular intermediary metabolism, and provides examples of the appropriate and inappropriate application of genetics and proteomics. It calls for the inclusion of biochemistry, enzymology, cell metabolism and cell physiology in the graduate and postgraduate training of molecular biology and other biomedical researchers.
Keywords: Cellular intermediary metabolism, Genetics and proteomics, Geneticist and biochemist approach, Medicine and medical disorders, Regulatory enzymes and transporters
1. Introduction
A renewed contemporary interest has arisen in the role of cellular intermediary metabolism in health and medicine; particularly in the development and progression of medical disorders (Costello and Franklin, 2005, 2006a; German et al., 2005). This is well illustrated in the recent evolution of specialty areas such as metabolomics, mitochondrial medicine, and metabolic medicine. Clinical areas such as cancer, diabetes, aging, neurological conditions, nutrition, and virtually all health-associated conditions are now focusing on the metabolic disorder implications. This rejuvenation of interest and focus in cellular metabolism has evolved in large measure from the contemporary development and advancements in molecular biology, genetics, and proteomics; and the advancements in areas such as molecular technology and nanotechnology. This is a welcomed marriage that can address and elicit metabolic relationships in medicine that heretofore were not achievable. Most unfortunately, it has also resulted in serious issues of misinformation and inappropriate translational implications, which have not been addressed and have been counter-productive and problematic to the interests of the medical community. This presentation describes important basic principles and approaches involved in elucidation of cell metabolism, and the requirement for appropriate integration of genetic and proteomic studies. The discussion brings attention to issues of inappropriate application of genetics and proteomics; and the adverse impact on the direction of clinical application and the direction of biomedical research. The resolution of this problem and the optimizing of the advancements from the integration of molecular genetics/proteomics with cell metabolism will be dependent upon the appropriate inclusion of the principles and methods of biochemistry, enzymology, and cell physiology in the graduate and post-graduate molecular biology training programs.
Although this presentation focuses on cancer and metabolism, it is equally applicable to all areas of medicine.
2. In the beginning and now
The hallmark studies of Otto Warburg and colleagues reported in 1926 (Warburg et al., 1929) sparked the era of tumor cell metabolism. From then until around 1980, studies of intermediary metabolism of normal and malignant cells were dominant areas of research and of graduate and post-graduate training in biomedical sciences. This period culminated in the outstanding discoveries of the operation of the Emden–Meyerhoff (glycolytic) pathway, the operation of the Krebs cycle, the pathway of terminal oxidation, the coupling of energy production through oxidation–phosphorylation, and the myriad of associated pathways involved in the “thoroughfare” of cellular intermediary energy metabolism. The evolution of these discoveries resulted from the pioneering efforts of earlier generations of outstanding, dedicated, and tireless scientists to which the contemporary and future generations owe an enormous degree of respect, admiration, and appreciation.
The contemporary era of genetics, proteomics, molecular biology, molecular technology, nanotechnology, computers, and other advances in analytical methods were non-existent and unavailable to the earlier generations, and were not employed in those great discoveries. One could not identify, visualize, and quantify the existence of an enzyme in a cell or cell extract as can now rapidly and easily be achieved through methods such as Western blot analysis, immunohistochemistry, and mass spectrometry. Instead, enzyme isolation and identification required days of preparations such as ammonium sulfate fractionations; and each step had to be accompanied by enzyme assay of each fraction. One could not conduct multiple rapid microassays of cell reactions as can be achieved by instrumental analysis such as fluorescent microplate readers. Substrate and product analysis of enzyme activities andmetabolic sequences were performed one at a time; and multiple spectral analyses and automation did not exist.
Moreover, the research support services that facilitate the researchers of today were not available to the earlier generations. Assays, data analyses and statistical analyses were performed manually or at best with the aid of a “sophisticated” calculator. Each scientist was also a trained statistician, and had to subject the raw data to stepwise calculations to obtain the means, standard errors, and statistical probability of the results of an experiment. To achieve this would take hours and days; so a ‘simple’ experiment for identification of an enzyme and its activity would takes days and even weeks to complete.
Literature searches for publications of methods, research reports, and other relevant information had to be conducted at the library. This was achieved by the tedious and time-consuming process of looking through the index of volumes of issues of Chemical Abstracts, Biological Abstracts, Index Medicus, and other such index/abstract publications. The researcher had to manually transcribe pages of abstracts and citations since copying machines and services were not available. Today, a computer-generated PubMed search from one's office or home will generate within minutes and with a hardcopy printout of information that our predecessors could not obtain even within weeks of library “work”. These are the conditions under which the great advances and discoveries were achieved by the past generations of outstanding scientists. Today's researchers have all the technological advances, instrumentation and tools that make research in cell metabolism (and all other areas) much simpler, more rapid, more efficient, and more sensitive than available to their predecessors. In addition, this provides for new advances that can result from investigations into issues that were not previously possible.
Beginning around 1980, the advent, development, and subsequence dominance of molecular biology, molecular genetics, proteomics, and molecular technology in clinical and experimental biomedical application occurred. However, this was accompanied by the nearly complete submersion of interest and training in areas of cellular intermediary metabolism, biochemistry, enzymology, and cell physiology. Notwithstanding the contemporary technological and informational advances, the experimental approach and the requirements to establish the cellular reactions and activities of enzymes and the operation of a cellular metabolic pathway have not changed. There is no contemporary alternative to the essential biochemical/metabolic approaches that were employed by our predecessors. Better, simpler, more rapid, more sensitive assay procedures and methods now exist; but the required approach remains the same. The earlier pioneers were expert in the areas of biochemistry, enzymology, enzyme reaction kinetics, metabolic principles, cellular physiology, and the methods required to investigate and to establish cellular metabolic pathways and relationships. This expertise is largely absent in the contemporary generation ofmolecular biologists/geneticists that now dominate clinical and biomedical research. This is the issue that must be appreciated, addressed, and reconciled as we enter the rejuvenated era of the role of intermediary metabolism in normal cellular function and in the development and progression of cancers and other disorders.
3. Is there really a problem; and why does it exist?
Yes, there is a serious problem with serious implications. Unfortunately, although the problem is widespread, the issues are largely unrecognized and unidentified by many, and likely most, of the contemporary clinical and biomedical research investigators. In our commentary in 2006 (Costello and Franklin, 2006a), we first attempted to bring attention to the evolving and impending issues that were arising in published reports involving genetic and proteomic implications in altered cellular metabolism and cell function. Since that commentary, a noticeable continuation and seemingly increase in such reports has occurred. This has resulted in published misinformation and inappropriate translational interpretations of the implications of genetic and proteomic studies on cell metabolism and functional relationships in cancer and in other disorders. This is exacerbated by the lack of understanding and recognition of the limitations associatedwith such reports; thus leading to the acceptance and validity of such misinformation. A major contributing factor is the insufficient background, training, and understanding of fundamental principles and relationships of biochemistry, cell metabolism, enzymology, and other required knowledge (Costello, 2009; Costello and Franklin, 2006a). This applies to the authors of the reported studies, the reviewers and editors of the journals; and also applies to the peer reviewers of grant proposals. This has serious consequences for the translational application that dictates the direction of clinical application and biomedical research. The following discussions will identify such issues, present some examples, and provide the background and basis associated with the issues. In the hope of assisting with some resolution to this problem, this presentation describes important relationships that one must consider for investigations and interpretations concerning the integration and application of molecular biology, genetics, and proteomics to the role of altered metabolism in cancer as well as other medical conditions.
4. Important relationships of regulatory enzymes in cell metabolism
The in situ activity of an enzyme in a cell is dependent upon two factors: 1) the concentration (abundance) of the active form of the enzyme; and 2) the kinetics of the enzyme activity. The former is determined by the gene expression and biosynthesis of the enzyme in its active form, and also the turnover rate of the enzyme. The latter is dependent upon the conditions of the cellular environment of the enzyme; such as substrate concentration, pH, cofactor requirements, allosteric effects of modulators, interconversion of active and inactive forms. The most common way to determine the activity and kinetic properties of a specific enzyme is to employ cell preparations and extracts, often with additional purification, followed by appropriate assay of the enzyme's activity (Michaelis–Menton kinetics). Generally, the conditions employed result in maximal activity of the enzyme. While the potential activity of the enzyme can be obtained, such information does not likely represent the activity of the enzyme under the conditions that exist in situ in the cell. Therefore, other additional approaches must be employed to determine the cellular enzyme activity. Such approaches include cell studies with substrate analysis, radioactive tracer analysis, and specific inhibitor effects.
In any series of reactions that comprise a metabolic pathway, the overall rate of the pathway is governed by the slowest reaction within the pathway (the ‘master reaction’). Fig. 1 exemplifies a sequence of enzymes comprising a metabolic pathway leading to the following product:
Fig. 1.

A representation of the relationships of a series of enzyme reactions that comprise a metabolic pathway.
Enzyme activities 1,2,4 are in excess, and enzyme 3 is rate limiting. The product of the pathway ‘E’ is low despite the fact that enzyme 4 is in excess. Reaction 4 is low because the substrate D concentration is lower than the optimal Km for the reaction 4 enzyme. Therefore, the up regulation of enzyme 4 gene expression will have little, if any, effect on increasing the pathway for conversion of substrate ‘A’ to product ‘E’. Moreover, the accumulation of intermediate C could induce a product inhibition of reaction 2, which then decreases product C, even if enzyme 2 is in excess. In such an example, the identification of altered expression of “metabolic” genes (a terminology that will be described below) and of changes in the level of the corresponding enzymes does not establish changes in the cellular activity of the enzyme or the associated metabolic pathway. Conversely, the identification of altered enzyme activity of metabolic pathways does not identify the factors and cause of the altered metabolism. This is when the genetic/proteomic approach becomes an important tool for understanding mechanisms of regulation of cellular metabolism.
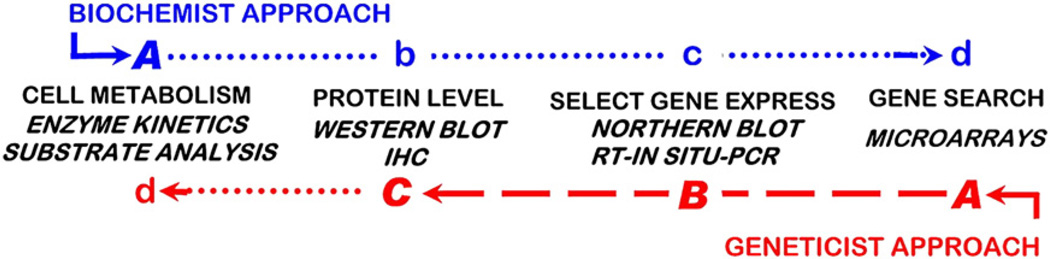
5. The geneticist approach versus the Biochemist approach
For studies of cellular metabolism and operating enzymes and pathways, the new molecular technology and methods do not replace or eliminate the requirement for the biochemical/enzymology approach of the past. To address this issue we identify two approaches: the “Biochemist approach” versus the “Geneticist Approach” (Costello and Franklin, 2006a).
5.1. The Biochemist approach
This is the approach of investigators who were trained to study cellular intermediary metabolism by application of the principles and methodology of biochemistry, enzymology, cellular physiology, metabolic pathways, and related areas. Those investigators fully understand that molecular genetics and proteomics cannot identify the operation of cellular pathways of metabolism and/or specific enzyme activities; which can only be established by the traditional methods of substrate analysis and enzyme kinetics.
The Biochemist approach (Fig. 2) first seeks to identify the operation and alteration in the cellular intermediary metabolism (step A) such as a change in the specific enzyme activity and/or the operation of a metabolic pathway. If an alteration in the cellular enzyme activity and/or associated metabolic pathway is not identified, its involvement in a metabolic transformation is unlikely. The need to proceed with genetic and proteomic studies (steps b, c, d), seemingly becomes unnecessary. However, pursuant genetic/proteomic studies might reveal altered expression and level of the enzyme. Then an important issue is revealed. “What are the cellular conditions that prevent the change in the activity of the altered enzyme level?” This would dictate the need for further investigation.
Fig. 2.
The comparison of the application of genetic, proteomic, and metabolic steps in the Biochemist versus the Geneticist approaches in cell metabolism.
Alternatively, step A might reveal a cellular alteration in the enzyme activity and associated metabolic pathway. Then, the issue becomes the identification of the mechanism of altered enzyme and metabolic activity. The application of the contemporary molecular tools of proteomics and gene expression (b, c, d) are then applied, accompanied by biochemical examination of cellular conditions that can alter the activity of an enzyme. For example, a kinetic change in the enzyme Vmax with no change in the substrate Km value would suggest that the level of enzyme is altered. This could correlate with a corresponding change in the gene expression and/or protein level (steps b–d); and define a critical role of altered gene expression in the metabolic transformation. Conversely, the enzyme kinetic change might not be mimicked by genetic/proteomic changes. Then one must consider alternative reasons for the change in Vmax as described above.
There are other scenarios that exist. However, the Biochemist approach is essentially devoid of potential false-positive and false-negative results relative to identifying the presence of enzyme activities and metabolic pathways in metabolic transformations. The application of genetic/proteomic studies is essential for the elucidation of the mechanisms of altered enzyme activity and the regulation of metabolic transformations.
5.2. The Geneticist approach
This approach focuses on employing the principles and technology of molecular genetics, and proteomics that are generally applied to all protein products of gene expressions; among which the enzymes of intermediary metabolism are included. The Geneticist approach (Fig. 2) focuses initially on identification of genetic changes (steps A, B). If a “significant” difference in a gene expression is revealed, studies proceed to the proteomic identification of corresponding changes in the relative level of the enzyme protein (step C). When the proteomic change corroborates the gene change; this is often determined to be presumptive and even conclusive evidence of a corresponding change in the cellular enzyme activity and associated pathway of metabolism. This often leads to the Geneticist approach ending at step C. The reasons for ending at this point are: the lack of the knowledge regarding cellular metabolic relationships as described above; the lack of knowledge of the biochemical/enzymological methods that need to be employed; and/or because, unlike the rapid and low-intensity labor involved with the molecular/genetic technology, the cellular biochemistry/metabolic methods are labor intense and tedious.
The Geneticist approach (steps A–C) in the absence of the cellular metabolic studies (step d) should not be interpreted or extrapolated as demonstrating a cellular enzyme activity status or a metabolic pathway status. If steps A–C demonstrate a change in enzyme gene expression and abundance, it is possible that cellular conditions could impose kinetic effects that prevent the presumed change in the enzyme activity, or the presumed effect on the etabolic pathway, for reasons described above. Thus, in the absence of proceeding with step d, the Geneticist approach will elicit a “false-positive” interpretation. Conversely, if steps A, B and/or C reveal no significant change in the expression of a gene, the presumption is made that its associated enzyme and/or metabolic pathway is not involved in a metabolic transformation, and step d is eliminated. This then becomes a “false negative” result of the Geneticist approach.
This type of issue is well represented by our studies of the maconitase and citrate oxidation relationship in prostate cancer. The major metabolic transformation associated with the development of prostate malignant cells is the change from citrate-producing normal epithelial cells to citrate-oxidizing malignant cells (Costello and Franklin, 2006b). The key enzyme responsible for this altered metabolism is m-aconitase, which activity is low in normal cells and high in malignant cells. This was established by kinetic studies of the maconitase reaction with prostate preparations; by 14C-citrate oxidation and 14CO2 production by prostate epithelial cells; and by determination the steady state citrate/isocitrate ratio (the m-aconitase reaction substrate and product) in the prostate tissues. Then the issue becomes ‘What is the cause of this change in m-aconitase activity?' An obvious likelihood from the geneticist's view is a change in the abundance of the m-aconitase enzyme that would result from a change in the expression of the m-aconitase gene. This could be revealed by application of the Geneticist approach steps A–C. In so doing, m-aconitase immunohistochemical analysis of normal and malignant prostate tissue sections reveals no difference in the abundance of the enzyme (Singh et al., 2006). In the absence of the information established by the cell metabolism analysis, the Geneticist approach without proceeding to the tedious studies required for step d would have lead to the erroneous conclusion that m-aconitase is not important in the metabolic transformation that is essential for the development of prostate malignancy. The question still remains as to the cause of the altered m-aconitase activity. The explanation is the important relationship of zinc as an inhibitor of m-aconitase. Normal prostate epithelial cells accumulate high levels of zinc that inhibit m-aconitase activity so that citrate is produced for secretion into prostatic fluid. The malignant cells have lost the capability to accumulate high zinc levels so that m-aconitase activity is not inhibited and citrate is oxidized via the Krebs cycle. The reason for this is the down regulation of expression of ZIP1 zinc uptake transporter that occurs in themalignant cells. The Geneticist approach-would not have revealed this coupling of altered ZIP1 gene expression to m-aconitase and citrate oxidation in prostate cancer; but would lead to inappropriate conclusions regarding genetic/proteomic relationships in prostate cancer.
6. The concept of ‘metabolic” genes
Another problem resides in the concept in molecular genetics that gene products are essentially a homogeneous group of proteins, with no recognition of the different cellular functional relationships of the protein products. This introduces serious consequences and misinterpretations of the role and existence of altered cellular intermediary metabolism based on such genetic and proteomic analysis. To address this issue we identify those genes that are involved in the expression of regulatory enzymes of intermediary metabolism as “metabolic” genes as differing from those genes that are involved in the expression of other proteins such as structural/skeletal proteins and secretory/digestive enzymes (Costello and Franklin, 2002, 2006a). The latter group can be classified as “abundant” proteins that require expression levels and protein abundance over a manyfold range. Enzymes of intermediary metabolism are not abundant proteins and exist in micro-abundant levels. In most instances the alterations in the level of regulatory enzymes of intermediary metabolism in the range of 1–2-fold will exhibit significant changes in the cellular enzyme activity until the concentration for Vmax is attained. Thereafter, several-fold increases in enzyme expression and concentration above the Vmax level will be superfluous relative to the impact on the cell's metabolism. One must recognize this important distinction between regulatory enzymes of intermediary metabolism and other enzymes/proteins.
The statistical parameters that are applied to microarrays and to RT-PCR for identification of significant changes in the expression of a gene are of serious consequence for the application to “metabolic genes”. The statistical stringency that is applied to the analysis of typical microarray data is somewhat arbitrary and designed to separate signal from noise. Inorder to reduce the rate at which significant differences in expression are falsely identified, the threshold for designating differences as being significant is often set higher (e.g., two-fold or greater) than might be expected for significant functional differences in metabolic enzyme activity. Then, these potentially highly relevant genetic changes are erroneously eliminated as important genes involved in cancer; or in other conditions. This introduces a potential for “false-negative” results that is more probable for metabolic genes than for other genes. In fact the concept of a quantitative relationship of the level of expression and its product abundance to the cellular relevancy of the level is misguided. A 50-fold change in expression of gene X is not necessarily more relevant than a 10-fold change in gene Y regarding the effects on cellular activities. A 50-fold increase in gene X expression is not necessarily more significant than a 5-fold increase in gene X expression in relation to the effects at the cellular level. A 2-fold increase in a metabolic regulatory gene and its regulatory enzymeproduct can bemore relevant than a 20-fold change in a structural protein or secretory enzyme gene expression.
7. Transporters are guided by similar relationships and issues as enzymes
Many cell transporters such as plasma membrane transporters are closely linked to cellular metabolism. Cellular transport activities are guided by the same Michaelis–Menton kinetic principles and considerations as enzymes. As described above, there is no kinetic/functional purpose to an increase in the transporter abundance that is many fold greater than the level required to attain membrane saturation and maximal activity. Yet, the geneticist focus on quantitative level and change in expression dominates the interpretation of the significance of change in transporter relationships.
To exemplify this problem, we will continue with the prostate example described above. The change in ZIP1 expression that occurs in prostate cells is of the magnitude of ~2-fold,which has been established to have a significant effect on the transport of zinc into the cells. Nevertheless, the following is a typical comment from reviewers of a grant proposal; “The experiments are based on modest effects of a 2-fold change in ZIP1.” Similarly, the following comment was elicited by a reviewer of a manuscript for publication, “the effect on expression resulted in only a 1-fold change in ZIP1.” These comments were made although evidence that the cellular functional and kinetic activities exhibits highly significant and important change in the cellular uptake and accumulation of zinc. Such comments reveal a geneticist's view and misunderstanding of the application of genetics and proteomics to the metabolic, and functional relationships of cells; and the serious consequences of this lack of knowledge.
In addition, the cellular location of a transporter is a major factor relating to its cellular function. As an example, for a transporter to function as a cellular zinc uptake transporter it must be localized at the plasma membrane; and at the correct in situ location of the plasma membrane, such as the basilar membrane of epithelium for uptake from blood-derived interstitial fluid. Consequently, determination of genetic expression and proteomic levels of the transporter do not establish the in situ cellular functionality of the transporter. Moreover, uptake studies in isolated cells and cell lines cannot be translated into the status of the transporter in the cell in its natural tissue environment. Isolated cells in vitro do not exhibit the polarity that exists in the cells in situ; and do not reflect the in situ conditions that can influence the localization of the transporter. Therefore one cannot employ cell lines and engineered cells to represent the functional relationships of the in situ status, unless the status is also identified in the in situ condition of the cell.
Illustrative of such issues is the observation that ZIP2 and ZIP3 transporters, as with ZIP1 transporter, are expressed in normal prostate cells in situ, and are down-regulated in the malignant cells (Desouki et al., 2007). However, ZIP2 and ZIP3 are localized at the apical membrane; whereas ZIP1 is localized at the basolateral membrane. Therefore, ZIP2 and ZIP3 do not function as transporters for the uptake of zinc from circulation. In contrast to prostate cells, ZIP3 is expressed in pancreatic epithelial cells in situ; but the transporter is localized at the basilar membrane and functions for cellular uptake of zinc from circulation. These important cellular relationships and differences cannot be established be genetic expression and proteomic protein abundance determinations. Extrapolations from the status in one cell type to another cell type, and in different cellular environments, are not appropriate.
Another issue arises from the interpretation and translational application of the constitutive genetic expression and proteomic status in cell lines as being representative of the in vivo status of the parental cell in its natural tissue environment. This ignores relationships that can alter gene expression under the in vivo conditions of cell's tissue environment, which do not exist under the in vitro conditions of the cell lines. This is illustrated by the fact that ZIP1 gene expression is silenced in situ in prostate malignancy, but the malignant cell lines (PC-3, LNCaP, DU-145,) derived from the in situ malignant loci exhibit constitutive expression of ZIP1 transporter. This is likely due to the removal of in vivo conditions that result in epigenetic silencing conditions, which do not exist under the in vitro conditions of the cell lines. Thus, identification of the in vivo status and importance of altered ZIP1 expression in prostate cancer is not revealed by cell lines; and the latter leads to misrepresentation of the important genetic/metabolic events in prostate carcinogenesis.
Asimilar problemis represented in a report (for collegial reasons, the source and the specifics will not be identified) that purported to show with tissue microarray study that expression of a specific transporter ‘X’ is increased in cancer ‘A’. Transporter ‘X’ is a cellular uptake transporter for substrate ‘Z’. Immunohistochemistry of tissue sections revealed an increase in transporter ‘X’ abundance in the malignant cells. This was taken as presumptive evidence that substrate ‘Z’ levels are increased in cancer ‘A’, although no measurements of substrate ‘Z’ levels were provided. Examination of the tissue section immunohistochemical staining reveals that the increase in transporter ‘X’ protein exhibited no localization at the plasma membrane, which would be required for the functional uptake transport of substrate ‘Z’. In lieu of this information, the authors performed uptake studies with genetically-engineered cell lines to show that transporter ‘X’ increases the uptake of substrate ‘Z’. This provided their evidence that an increase in substrate ‘Z’ is important in the development of cancer ‘A’; and that up-regulation of gene expression of transporter ‘X’ is an important event responsible for the increase in substrate ‘Z’. For the reasons described above, this is an inappropriate conclusion derived fromgenetic/proteomic studies, in the absence of the required in situ status that exists in the natural tissue environment and conditions. Nevertheless, this report has been accepted by the journal, which gives credibility to the study and its conclusions; and which sets the direction for clinical and research application regarding cancer ‘A’ and other cancers. The problem is exacerbated by the fact that recent evidence has surfaced with direct measurements of substrate ‘Z’, which shows that substrate ‘Z’ is markedly decreased, not increased, in cancer ‘A’.
Subsequent to the above report, another study purported to show that transporter ‘Y’ for the same substrate ‘Z’ is increased in cancer ‘B’. This was based on RT-PCR analysis of RNA extracts of malignant and normal tissues, and immunohistochemistry analysis of tissue sections. No measurements of ‘substrate ‘Z’ were provided. Nevertheless, the authors' concluded that substrate ‘Z’ is likely to be increased in cancer ‘B’ because transporter ‘Y’ is known to be an uptake transporter in some cells. However, their tissue section immunohistochemistry does not exhibit any plasma membrane localization of transporter ‘Y’. As in the previous example, presumptive and inappropriate extrapolations were based on genetic and proteomic profiles that were not established and confirmed in the in situ tissue conditions. Nevertheless this report passed the scrutiny of the journal review editorial process. Moreover, evidence has established that substrate ‘Z’ is decreased, not increased, in cancer ‘B’.
8. The principles (axioms) of the relationship between cell activity and cell metabolism
The preceding descriptions lead to the importance of understanding the relationships of cell intermediary metabolism to cell activity, which we describe as “axioms” (Costello and Franklin, 2005, 2006a, 2006b). The following are axioms that are particularly appropriate to this presentation.
Axiom 1. The existing intermediary metabolism of a cell provides the bioenergetic/synthetic/catabolic requirements that are essential for the manifestation of the cell's current activity (e.g. function, growth, proliferation, differentiation).
Axiom 2. When the activity of a cell changes, its metabolism must also be altered to provide new bioenergetic/synthetic/catabolic requirements for the cell's changing activity.
Axiom 3. Genetic transformations and proteomic changes have little relevancy if the genetic/proteomic alterations are not manifested as changes in cell metabolism and function. The absence of identified genetic transformations and proteomic changes does not demonstrate the absence of changes in cell metabolism and function.
Axioms 1 and 2 are irrefutable universal principles that apply to all cells. “Metabolism” is one of the attributes of life; and these axioms describe its relationship to cell activity.Axiom 3 integrates the application of genetic and proteomic relationships to the principles represented in axiom 1 and 2.
9. “What is the resolution?”
This presentation, we believe, has revealed important issues and representative examples of the misinformation arising from the inappropriate application of molecular biology, genetics, and proteomics studies to cell metabolism and cell function. This is a serious problem with widespread adverse implications on the present and future directions of clinical applications and biomedical research; and is not in the best interest of the public welfare. Consequently, once identified and brought to light, it becomes a critical issue that needs to be addressed and resolved. So, “why does the problem exist?”; and “what is the resolution?”
The problem exists largely as a result of the contemporary graduate and post-graduate training programs; which is described in an earlier commentary (Costello, 2009). The dominance of focus on molecular biology and molecular technology in contemporary biomedical science graduate/postgraduate programs has created a generation of researchers who are deep in molecular biology and shallow in the integrated processes of normal bodily function and disease. Their didactic, technological, and experimental experiences are focused on molecular genetics, cell signaling, proteomics, microarrays, etc. Conversely, there is a limited, if any, understanding of human physiology and pathophysiology, or in integrated organ systems function, or in biochemical principles and cell metabolism, and even in cell physiology. In our view, an understanding of these integrated structural, functional, metabolic processes is essential to all biomedical scientists; regardless of the specialty research track that one wishes to pursue. There needs to be a return to the purpose of graduate/postgraduate training programs to produce scientists with a broad understanding of the living system, and its application to the human relationships. The issues that this presentation has identified make it obvious that exclusion of areas such as biochemistry/enzymology, cell metabolism, cell physiology, and pathophysiology from molecular biology training programs results in ill-prepared, narrowly-focused researchers; and the consequences that we have described. For further and more extensive discussion see Costello(2009); Costello and Franklin(2006a).
10. Concluding comments
It is highly appropriate to apply to molecular biology/genetic/proteomics, the admonitions regarding epidemiology studies that “There would be few drawbacks to publishing weak, uncertain associations if epidemiologists operated in a vacuum, but they do not. .... By the time the information reaches the public mind, via print or screen, the tentative suggestion (of association) is likely to be interpreted as a fact …” (MacMahon, 1994); and also “The first one or two papers about a suspected association spring into the general public consciousness…And once a possible link is in the public eye, it can be virtually impossible to discredit” (Taubes, 1995). This is the situation that must be avoided in the application and integration of genetics and proteomics to cell metabolism and function.
With the methodology, technology, and informational resources that existed, the earlier generations of biochemists provided the contemporary generation with the elucidation of major pathways of metabolism. The present and future generations now have methodology, technology, informational resources that did not exist for earlier generations; and also have the resources from the earlier generations. Thus, the opportunity now exists to advance the understanding of cell metabolism and cell function. This especially applies to implications of genetics, signaling factors, and proteomics in the regulation and alteration of cell metabolism in health and disease. Taking advantage of this marriage requires the training of scientists who know “How to proceed; How not to proceed!”
Acknowledgments
This report and the studies of LCC and RBF cited here-in were supported in part by NIH grants CA79903, DK076783, and DK42839.
Abbreviations
- IHC
immunohistochemistry
- Km
the substrate concentration at which the reaction rate is half of Vmax
- Vmax
the maximum rate of an enzyme that is achieved at saturating substrate concentrations
- m-aconitase
mitochondrial aconitase
- ZIP
family of “zinc/iron transporter proteins”
- RNA
ribonucleic acid.
References
- Costello LC. The effect of contemporary education and training of biomedical scientists on present and future medical research. Acad. Med. 2009;84:459–463. doi: 10.1097/ACM.0b013e31819a7c6b. [DOI] [PubMed] [Google Scholar]
- Costello LC, Franklin RB. Testosterone and prolactin regulation of metabolic genes and citrate metabolism of prostate epithelial cells. Horm. Metabol. Res. 2002;34:417–424. doi: 10.1055/s-2002-33598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB. “Why do tumor cells glycolyze?”: from glycolysis through citrate to lipogenesis. Mol. Cell. Biochem. 2005;280:1–8. doi: 10.1007/s11010-005-8841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB. Tumor cell metabolism: the marriage of molecular genetics and proteomics with cellular intermediary metabolism; proceed with caution! Mol. Cancer. 2006a doi: 10.1186/1476-4598-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol. Cancer. 2006b doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol. Cancer. 2007 doi: 10.1186/1476-4598-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German JB, Hammock BD, Watkins SM. Metabolomics: building on a century of biochemistry to guide human health. Metabolom. 2005;1:3–9. doi: 10.1007/s11306-005-1102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon B. Editorial: pesticide residues and breast cancer? J. Nat. Cancer Inst. 1994;86:572–573. doi: 10.1093/jnci/86.8.572. [DOI] [PubMed] [Google Scholar]
- Singh KK, Desouki MM, Franklin RB, Costello LC. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol. Cancer. 2006 doi: 10.1186/1476-4598-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubes G. Epidemiology faces its limits. Science. 1995;269:164–169. doi: 10.1126/science.7618077. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1929;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]