Abstract
Objective
Soluble fms-like tyrosine kinase (sFlt-1) is an important mediator in the pathogenesis of preeclampsia. We sought to determine if platelet-monocyte aggregates (PMAs) produced sFlt-1 and if PMAs contributed to sFlt-1 production in preeclampsia.
Study Design
Case-control study of sFlt-1 release from PMAs using blood samples from women with preeclampsia matched by gestational age to pregnant controls. A third group of nonpregnant, reproductive-age women comprised an additional control group. Experiments were also performed using blood from non-pregnant women to elucidate if inducing PMAs could stimulate sFlt-1 production, and if so, to determine the necessary receptors and pathways.
Results
Women with preeclampsia had increased total Flt-1 concentrations in platelets and monocytes at baseline compared to pregnant controls (25 vs. 10 pg/ml, p=0.0003). sFlt-1 production was elicited from monocytes incubated with thrombin-activated platelets from non-pregnant women. sFlt-1 production was regulated at the transcriptional level by p38 and NF-κB dependent pathways.
Conclusion
Activated platelets in preeclampsia bind monocytes to generate sFlt-1. PMAs are a previously unrecognized source of sFlt-1 that may contribute to endothelial dysfunction and systemic inflammation commonly observed in preeclampsia.
Keywords: platelet-monocyte aggregates, preeclampsia, sFlt-1
Introduction
Preeclampsia is a major contributor to maternal and neonatal morbidity and mortality worldwide. The inciting cause is unknown, and it is likely this disorder represents a final common pathway of multiple pathologic processes. Maternal endothelial dysfunction, platelet activation, and a systemic inflammatory response are hallmarks of this final pathway.1-3 Although there is considerable evidence to support these features of preeclampsia, the underlying pathogenesis uniting these findings is not understood.
Soluble fms-like tyrosine kinase (sFlt-1) is an important mediator in preeclampsia. Circulating levels of sFlt-1 are elevated in the serum of women with preeclampsia, and this elevation precedes the development of clinical signs by 4-6 weeks.4 The source of circulating sFlt-1 in women with preeclampsia is thought to be primarily placental.5-6 Placental trophoblast produces sFlt-1 in response to a variety of stimuli, particularly hypoxia.7 However, the amount of sFlt-1 produced by trophoblast in response to placental hypoxia is thought to be insufficient to explain circulating levels seen in preeclampsia. Release of sFlt-1 from monocytes has been previously demonstrated.8 Subsequently, Rajakumar et al reported sFlt-1 production from mononuclear cells in women with preeclampsia.9
The stimulus resulting in sFlt-1 production from monocytes in women with preeclampsia is unknown; however, platelet activation is a well-recognized feature of preeclampsia.10 Interaction between activated platelets and monocytes is known to induce gene expression pathways in monocytes, resulting in increased production of inflammatory modulators by the target monocyte.11-13 This interaction may also explain the observation that monocytes from women with preeclampsia release increased amounts of interleukin-6 (IL-6), interleukin-8 (IL-8), and interleukin-1 beta (IL-1β) compared to women with normal pregnancies and non-pregnant women.14 We hypothesized that PMAs generate sFlt-1, which may contribute to endothelial dysfunction and systemic inflammatory response associated with preeclampsia.
Materials and Methods
Participant selection
A convenience sample of women with preeclampsia and women with uncomplicated, normotensive pregnancies (pregnant controls) matched by gestational age at the time of enrollment were recruited from the Labor and Delivery units, antenatal testing units, and prenatal clinics at the University of Utah Health Sciences Center and Intermountain Medical Center in Salt Lake City, UT. Non-pregnant women ages 18-45 with previous normal pregnancies comprised an additional control group.
Women were included in the preeclampsia group if they had a singleton fetus ≥ 24 weeks gestation and met criteria for diagnosis of preeclampsia as defined by the American Congress of Obstetrics and Gynecology (ACOG).15 Women with preeclampsia were excluded if they had any of the following conditions: multiple gestation, active labor, current or recent infection, any known platelet or coagulation disorder, heparin or aspirin use, tobacco use, current substance abuse, diabetes, renal disease, or autoimmune disease. Pregnant controls were matched 1:1 to women with preeclampsia and met the same exclusion criteria. In addition, pregnant controls were excluded if they had hypertension, a history of preeclampsia in a previous pregnancy, or fetal growth restriction.
Non-pregnant women were included if they were aged 18-45 years, with no chronic medical conditions, no known disorder of platelets or coagulation, and no current tobacco use. Experiments performed to characterize sFlt-1 expression and release from PMA utilized cells from women meeting the same criteria as non-pregnant controls. All participants gave written informed consent. Protocols for participant enrollment, venipuncture, and other study procedures were approved by the Institutional Review Board at each institution (IRB #1009177, Intermountain Healthcare, and IRB #29586, University of Utah).
Whole blood and plasma studies
Whole blood was obtained from each participant through standard venipuncture and collected into syringes containing acid citrate dextrose. Whole blood flow cytometry was used to determine the relative ratio of circulating PMA and surface expression of P-selectin on platelets at baseline. To assess circulating PMA, 50 μl of whole blood was added to 20 μl of Fluorescein isothiocyanate (FITC)-labeled anti-CD14 and 20 μl of either phycoerythrin (PE)-labeled anti-CD41 or IgG isotype control (BD Biosciences, San Jose, CA). After incubation at 37°C for 15 minutes, cells were fixed with FACS lysis buffer (BD BioSciences) for 10 minutes at room temperature, and stored at 4°C until analysis within 24 hours. P-selectin surface expression was evaluated by diluting whole blood 1:10 using HEPES-Tyrode's buffer. 10 μl of diluted blood was then incubated with 10 μl of PE-labeled anti-CD41 and 10 μl of either FITC-labeled anti-P-selectin or IgG isotype control (BD BioSciences) for 15 minutes at 37°C. Cells were fixed with FACS lysis buffer for 10 minutes at room temperature, and then stored at 4°C until being read within 24 hours. All flow cytometry analysis was performed using a BD FACScan flow cytometer. Platelet poor plasma (PPP) from the remaining whole blood was obtained by centrifuging whole blood initially at 150 × g to remove RBC/WBC. Platelet-rich plasma (PRP) was removed and centrifuged at 300 × g to remove platelets. PPP was stored at -80 C until analysis for sFlt-1, which was performed using a commercially available ELISA (R&D Systems, Minneapolis, MN).
Purified platelet and monocyte studies
Platelets were isolated as described above. Contaminating leukocytes were removed from platelet preparations by CD45 positive selection (Miltenyi Biotec Bergisch Gladbach, Germany) and purified platelets were resuspended in M199 culture medium (BioWhitaker, Walkersville, MD).
Monocytes were isolated from whole blood, which was centrifuged at 150 × g for 20 minutes to separate platelet-rich plasma (PRP) from red and white blood cells (RBC/WBC). The PRP was removed and the remaining RBC/WBC mixture was resuspended with 0.9% sterile saline back to the original volume and layered over an equal volume of Ficoll-Paque Plus (GE Healthcare Biosciences, Piscataway, NJ). The layered cells were then centrifuged for 30 minutes at 250 × g at 20°C. After 30 minutes, the mononuclear leukocyte layer was removed and washed with Hank's Balanced Salt Solution (Sigma-Aldrich, St. Louis, MO) with 1% human serum albumin (HBSS/A) (University of Utah Hospital, Salt Lake City, UT) and centrifuged for 10 minutes at 300 × g at 20°C. The cell pellet was then resuspended in 400 μl of HBSS/A and 100 μL of CD14 microbeads (Miltenyi Biotec) and incubated at 4°C for 15 minutes. Cells were washed with HBSS/A to remove any free CD14 microbeads, resuspended in 500 μL of HBSS/A, and monocytes were isolated using an autoMACs cell separator (Miltenyi Biotec). Cells were then washed with HBSS/A, re-suspended in M199 (BioWhitaker) and counted.
Freshly isolated platelets and monocytes were mixed together at a ratio of 100:1. This ratio approximates ratios of platelets to monocytes in whole blood. Preliminary studies found this ratio induces marked release of sFlt-1 from PMAs compared to substantially lower or higher ratios of platelets to monocytes (data not shown). Thrombin activation was used to stimulate PMA formation, at a dose of 0.1 U/ml (Sigma-Aldrich). Thrombin-stimulated monocytes alone and lipopolysaccharide (LPS)-stimulated monocytes were used in control experiments. Cells were incubated at 37°C and then centrifuged at 13,000 × g for 2 minutes to obtain cell culture supernatants. Cell culture supernatants were collected and frozen at -80 C until further analysis. Cells were lysed in Trizol (Invitrogen, Grand Island, NY) for mRNA extraction or in cell lysis buffer for ELISA (R&D Systems). sFlt-1 and IL-8 in cell culture supernatants, and total Flt-1 in cell lysates were determined by ELISA (R&D Systems). Platelets, monocytes, and existing PMA present in the monolayer obtained after centrifugation over Ficoll were fixed in 2% paraformaldehyde and stained using fluorescent wheat germ agglutinin (WGA) (Invitrogen) with and without AlexaFluor 488-conjugated antibody to Flt-1 (Santa Cruz Biotechnology, Santa Cruz, CA), sFlt-1 (Invitrogen), or IgG control (Santa Cruz Biotechnology). Cells were imaged using confocal microscopy.
mRNA studies
RNA was isolated with Trizol reagent following the manufacturer's instructions. Isolated RNA was DNAse-treated with Ambion DNA-free. cDNA was synthesized using oligo-dT primers (Invitrogen). Real time PCR was used to determine the level of sFlt-1 expression using primers specific for the portion of intron 14 that is specific for sFlt-1. The sequence for the forward primer is as follows: TGAGCACTGCAACAAAAAGG; the reverse primer sequence is AGAGGTTGGCATCAAAATGG. Results were normalized to GAPDH and compared to baseline expression using the ΔΔCT method.
Inhibition of sFlt-1 release from PMA
Freshly isolated platelets and monocytes were preincubated for 30 minutes with 5 μg/ml actinomycin-D or 5 μg/ml cycloheximide (Sigma-Aldrich), to inhibit RNA transcription and translation, respectively, before the addition of thrombin. Specific pathway inhibitors were also added to thrombin-stimulated platelets and monocytes: U0126 (EMD Millipore, Billerica, MA), SB203580 (EMD Millipore, Billerica, MA), Bay-11-7082 (Sigma-Aldrich), and SP600125 (Sigma-Aldrich). To inhibit binding between P-selectin on the surface of activated platelets and P-selectin glycoprotein ligand-1 (PSGL-1) on the monocyte surface, an anti-P-selectin antibody 20 μg/ml or IgG isotype control (R&D Systems) was added to monocytes before addition of platelets and thrombin.
Statistical analysis
Experiments examining release of sFlt-1 from PMA were compared to appropriate controls using independent samples t-test. The results presented represent mean ± standard error of the mean (SEM) for 3-5 experiments.
For participants in the case-control study, paired t-tests were used to compare categorical variables and chi-square was used for dichotomous variables between women with preeclampsia and pregnant controls. An independent t-test and chi square were used for comparison of continuous and dichotomous variables, respectively, between nonpregnant controls and both pregnant groups. A p<0.05 was considered significant for all tests.
Results
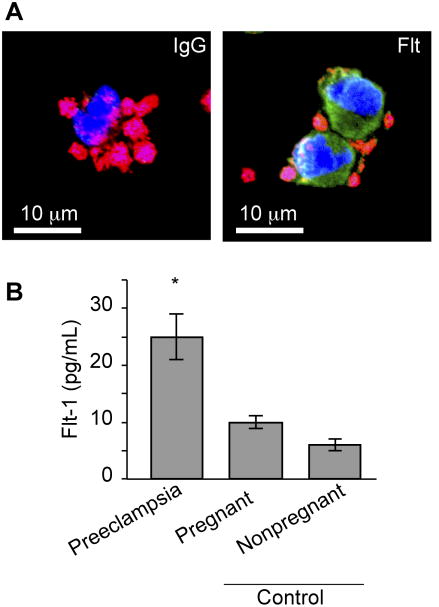
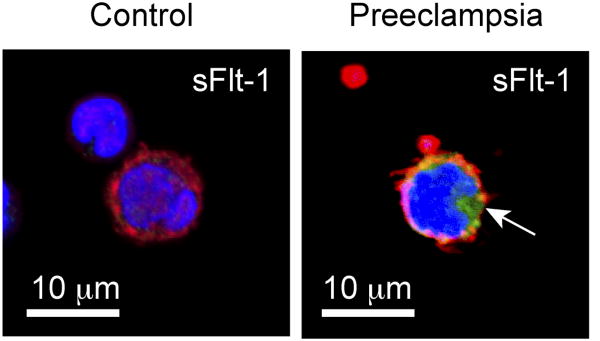
To evaluate if PMAs are a potential source of sFlt-1 in preeclampsia, we initially examined PMAs in mononuclear cells isolated from women with preeclampsia. Platelets commonly formed rosettes around monocytes, but not lymphocytes, and clusters of PMAs expressed Flt-1 (Figure 1A). Consistent with these results, freshly-isolated monocytes, which contain PMAs, had significantly higher levels of total Flt-1 in women with preeclampsia compared to pregnant controls and non-pregnant controls (Figure 1B). A portion of the positive staining for Flt-1 can be attributed to the presence of sFlt-1 based on further staining using an antibody specific for this splice variant (Figure 2). Twenty women with preeclampsia, twenty matched pregnant controls, and seventeen nonpregnant, reproductive-age controls were included in these comparisons, with clinical and demographic characteristics summarized in Table 1. No participant had HELLP syndrome at the time of enrollment. Plasma levels of sFlt-1 were elevated in women with preeclampsia, with a mean level of 25.16 ng/ml, while pregnant controls had a mean level of 4.35 ng/ml and nonpregnant controls 0.07 ng/ml. Together, these data suggest that PMAs generate total Flt-1 and release its splice variant, sFlt-1, into the circulation.
Figure 1. Flt-1 expression is increased in PMAs from women with preeclampsia.
(A) PMAs present in freshly-isolated mononuclear cells from women with preeclampsia were stained with WGA (red), which stains sialic acids in platelets and mononuclear cells and TOPRO-3 (blue), which stains nuclei. In parallel, an antibody specific for the N-terminus of Flt-1 (green) or its IgG control was used to identify Flt-1 in monocytes. (B) Freshly-isolated monocytes (including PMAs) were immediately lysed and total (soluble and membrane-bound) Flt-1 levels were determined by ELISA. The bars in this graph represent the mean ± SEM for all participants in each group, *p=0.003 compared to pregnant and nonpregnant controls.
Figure 2. sFlt-1 expression is found only in monocytes from women with preeclampsia.
Freshly isolated mononuclear cells from women with preeclampsia were stained with WGA and TOPRO-3 as described previously. An antibody specific for the soluble form of Flt-1 (sFlt-1, green) was used to identify sFlt-1 in monocytes. sFlt-1 was not found in monocytes from pregnant controls (left), but was seen in monocytes with platelet(s) attached from women with preeclampsia (right, arrow). This figure is representative of 10 cases and controls imaged.
Table 1. Clinical and Demographic Characteristics of Study Participants.
| Characteristic | Preeclampsia (n=20) | Pregnant controls (n=20) | Nonpregnant controls (n=17) |
|---|---|---|---|
| Age (years) | 27.3 + 6.2 | 28.4 + 3.8 | 33.4 + 6.2* |
| White race (n,%) | 19, 95% | 19, 95% | 17,100% |
| Nulliparous (n,%) | 13, 65% | 6,30% | 0 |
| SBP at enrollment | 158.3 + 12.4* | 113.1 + 11.6 | NA |
| DBP at enrollment | 100.1 + 8.8* | 69.2 + 10.2 | NA |
| Proteinuria/24 hours (grams) | 2.2 + 3.2 | NA | NA |
| Gestational age at enrollment (weeks) | 32.4 + 4.7 | 32.3 + 4.8 | NA |
| Gestational age at delivery (weeks) | 32.7 + 4.6* | 39.4 + 1.0 | NA |
| Infant birth weight (grams) | 1945 + 1004* | 3461 + 301 | NA |
SBP = systolic blood pressure, DBP = diastolic blood pressure,
denotes statistically significant difference between groups, p<0.05.
To determine if PMAs produce sFlt-1, platelets and monocytes from healthy, non-pregnant women were co-incubated with one another in the presence of thrombin. LPS, a known stimulus for sFlt-1 release from monocytes, was used as a positive control. The release of sFlt-1 and IL-8, generated by both PMAs and LPS stimulated monocytes, was examined. Thrombin-activated platelets induced similar sFlt-1 production in monocytes when compared to LPS (Figure 3). This differed from IL-8 release, which was robust from monocytes stimulated with LPS and more modest from PMAs.
Figure 3. Activated platelets induce sFlt-1 production by monocytes.
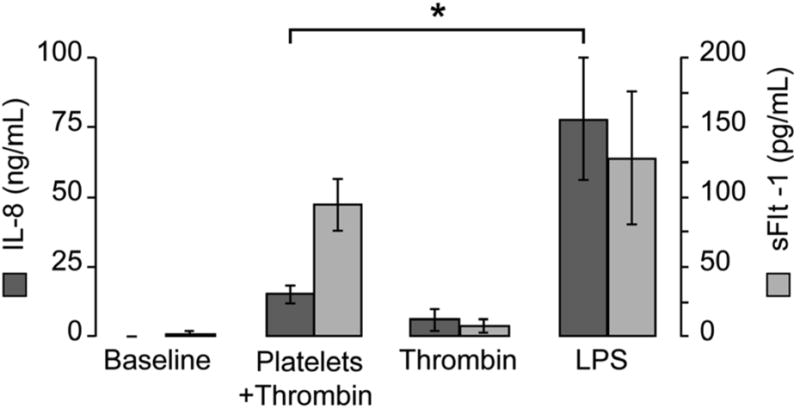
Thrombin-activated platelets or LPS were added to freshly isolated monocytes for 18 hours. IL-8 (dark-gray bars) and sFlt-1 (light gray bars) were measured in cell-free supernatants by ELISA. The bars in this graph represent the mean ± SEM of three independent experiments, *p<0.05 for IL-8 release from monocytes stimulated with LPS compared to thrombin-activated platelets.
Next we confirmed that sFlt-1 was synthesized de novo by PMAs. Pretreatment of monocytes with the transcriptional inhibitor actinomycin-D abolished transcription of Flt-1 mRNA (Figure 4A) and the release sFlt-1 protein (Figure 4B). Similarly, cycloheximide, a translational inhibitor that globally blocks protein synthesis, abolished sFlt-1 protein accumulation (Figure 4B). Consistent with transcriptional regulation, we found that inhibition of NF-κB signaling with Bay 11-7082 completely inhibited sFlt-1 release in our model of PMA formation (Figure 4C). The addition of U0126, a specific inhibitor of MEK 1 and 2 (both MAP kinase kinases) similarly repressed sFlt-1 release as did a specific inhibitor of the p38 pathway (Figure 4C). In contrast, SP600125, which inhibits JNK signaling, did not alter sFlt-1 production.
Figure 4. Synthesis of sFlt-1 is regulated at the transcriptional level in PMAs.
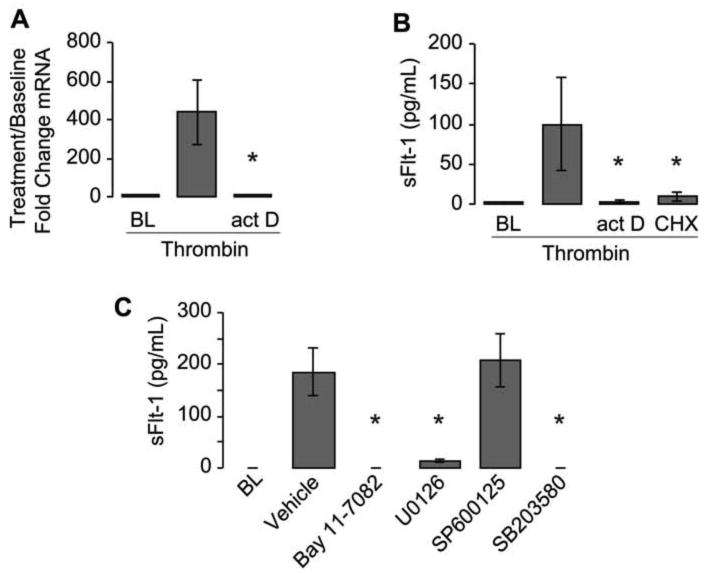
Thrombin-activated platelets were added to monocytes pretreated with actinomycin-D (actD) or cycloheximide (CHX) and incubated together for either 2 or 18 hours to assess sFlt-1 mRNA (A) or protein in the supernatant (B). In panel C, thrombin-activated platelets were added to monocytes pretreated with specific inhibitors against NF- κB (Bay 11-7082), MEK 1 and 2 (U0126), JNK (SP600125), or p-38 MAPK (SB203580). The bars in this graph represent the mean ± SEM of 3-5 independent experiments. *p<0.05 compared to thrombin alone (A,B) or vehicle (C).
Comment
Activated platelets from women with preeclampsia bind monocytes and induce the generation of sFlt-1, an important mediator in the pathogenesis of the disease. Maynard et al showed that sFlt-1 not only produced endothelial dysfunction in an in vitro model, but that overexpression of sFlt-1 in pregnant rats led to the development of hypertension, proteinuria, and glomerular endotheliosis, which are hallmarks of preeclampsia in humans.5 Levine and colleagues demonstrated that women with preeclampsia develop elevated serum levels of sFlt-1 compared to women with normal pregnancy outcomes, and that this elevation preceded the onset of clinical disease by approximately 5 weeks.4,16 Although placental sFlt-1 production is an accepted source of sFlt-1 in preeclampsia, other sources of sFlt-1 may contribute. To our knowledge, these studies are the first to link production of sFlt-1 to PMAs that are commonly observed in preeclampsia. We have shown sFlt-1 production can be induced from monocytes by interaction with activated platelets. This interaction has also been shown to result in release of other inflammatory cytokines, including IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), and IL-1β.17 Of note, circulating levels of these cytokines are elevated in women with preeclampsia.18
Our study demonstrates elevated levels of total Flt-1 in monocytes and PMAs of women with preeclampsia at presentation, suggesting these cells contribute to the elevated levels of circulating sFlt-1 in this disease. In our cohort of women with preeclampsia, we found a 1.9-fold increase in P-selectin expression on the surface of platelets compared to pregnant controls. Expression of P-selectin on the platelet surface is necessary for formation of PMAs, and this finding suggests an increased propensity to form PMAs in women with preeclampsia. Consistent with our findings, Increased numbers of circulating PMAs in women with preeclampsia19 have been reported by other investigators. Previous studies from our group have shown that interactions between P-selectin and PSGL-1, which are expressed on the surface of platelets and monocytes respectively, regulate the expression of inflammatory cytokines.11-13 Blockade of P-selectin resulted in a modest (30%) but consistent reduction in sFlt-1 production. Incomplete blockade may be due to decay in the inhibitory properties of P-selectin neutralizing antibodies over time, with 90% blockade of PMA formation at 2 hours falling to less than 30% blockade by 8 hours (data not shown). An alternative explanation is that other receptor-ligand interactions besides P-selectin/PSGL-1 contribute to sFlt-1 production.
Our data clearly indicate that regulation of sFlt-1 release is transcriptionally mediated, through mechanisms that involve NF-κB. Indeed, activation of NF-κB in placentas and leukocytes of women with preeclampsia has been reported.20-21 We also found that activation of the MAPK pathways, specifically p38 kinase, is essential for sFlt-1 production from PMAs. Others have investigated the MAPK pathways in placentas taken from women with preeclampsia.22-4 Activation of p38 seems to be essential in production of sFlt-1 from placental explants in response to a variety of stimuli, including IL-6 and hypoxia. Phosphorylation of p38 kinase is seen in placentas of women with preeclampsia, but not in placentas of women with no hypertensive complications. Inhibition of p38 kinase using the same inhibitor used in our study abrogated sFlt-1 release from placental explants. Thus, pathways contributing to sFlt-1 generation in the placenta are similar to those that control sFlt-1 production by PMAs. Thus, PMAs may be an excellent model for further investigation of the mechanism of sFlt-1 production in this disorder.
In summary, our studies demonstrate that PMAs generate sFlt-1, which may contribute to endothelial dysfunction and inappropriate inflammatory responses seen in preeclampsia. Future directions for study should address the origin(s) of platelet activation in preeclampsia, as this may be a downstream effect of several different inciting events. Abrogating the monocyte response to activated platelets may also have clinical benefit in reducing sFlt-1 level and inflammatory cytokines, and is worthy of further investigation.
Clinical Implications.
Activated platelets from women with preeclampsia bind monocytes and induce the synthesis of soluble fms-like tyrosine kinase (sFlt-1), an important mediator in the disease process.
Platelet-monocyte aggregates (PMAs) generate inflammatory cytokines and may contribute to the endothelial dysfunction and inflammatory response seen in preeclampsia.
Regulation of sFlt-1 synthesis by PMAs is transcriptionally mediated through mechanisms involving nuclear factor kappa-light-chain-enhancer of activated B cells and mitogen-activated protein kinase pathways, making PMAs an appropriate primary cell model for studying the mechanism of sFlt-1 production in preeclampsia.
Acknowledgments
We thank Ms. Diana Lim for preparing the figures in this manuscript, and for her consultation regarding responsible and effective display of images. We also wish to thank the Vice President's Clinical and Translational Scholars Program of the University of Utah for their support of this research and Guy Zimmerman, MD for his thoughtful input and contributions. Ms. Diana Lim and Dr. Guy Zimmerman are employed by the University of Utah.
The work in this report was supported by grants from Primary Children's Medical Center Foundation (Dr. Major), the American Heart Foundation grant 11POST7290019 (Dr. Campbell), and the National Institutes of Health grant numbers 1U54HL112311 and 2RO1HL066277 (Dr. Weyrich).
Footnotes
The authors report no conflict of interest.
Neither have been compensated for their work on this manuscript, and have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–16. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Oron G, Ben-Haroush A, Hod M, Orvieto R, Bar J. Serum-soluble CD40 ligand in normal pregnancy and in preeclampsia. Obstet Gynecol. 2006;107:896–900. doi: 10.1097/01.AOG.0000206206.99212.9e. [DOI] [PubMed] [Google Scholar]
- 3.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16:216–29. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 5.Maynard SE, Min HY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark DE, Smith SK, Licence D, Evans AL, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–8. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta. 2005;26:210–7. doi: 10.1016/j.placenta.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert JS, Bauer AJ, Gilbert SA, Banek CT. The opposing roles of antiangiogenic factors in cancer and preeclampsia. Front Biosci. 2012;4:2752–69. doi: 10.2741/e581. [DOI] [PubMed] [Google Scholar]
- 9.Rajakumar A, Michael HM, Rajakumar PA, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–73. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533–7. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 11.Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95:2297–303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weyrich AS, Elstad MR, McEver RP, et al. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–34. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon DA, Tolley ND, Bemis-Standoli K, et al. Expression of COX-2 in platelet-monocyte interactions occurs via combinatorial regulation involving adhesion and cytokine signaling. J Clin Invest. 2006;116:2727–38. doi: 10.1172/JCI27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luppi P, Deloia JA. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol. 2006;118:268–75. doi: 10.1016/j.clim.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 16.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 17.Franks ZG, Campbell RA, Weyrich AS, Rondina MT. Platelet-leukocyte interactions link inflammatory and thromboembolic events in ischemic stroke. Ann N Y Acad Sci. 2010;1207:11–7. doi: 10.1111/j.1749-6632.2010.05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tosun M, Celik H, Avci B, Yavuz E, Alper T, Malatyalioğlu E. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med. 2010;23:880–6. doi: 10.3109/14767051003774942. [DOI] [PubMed] [Google Scholar]
- 19.Lukanov TH, Bojinova SI, Popova VS, et al. Flow cytometric investigation of CD40-CD 40 ligand system in preeclampsia and normal pregnancy. Clin Appl Thromb Hemost. 2010;16:306–12. doi: 10.1177/1076029608331229. [DOI] [PubMed] [Google Scholar]
- 20.Vaughan JE, Walsh SW. Activation of NF-kappaB in placentas of women with preeclampsia. Hypertens Pregnancy. 2012;31:243–51. doi: 10.3109/10641955.2011.642436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luppi P, Tse H, Lain KY, Markovic N, Piganelli JD, Deloia JA. Preeclampsia activates circulating immune cells with engagement of the NF-kappaB pathway. Am J Reprod Immunol. 200(56):135–44. doi: 10.1111/j.1600-0897.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 22.Luo X, Yao ZW, Qi HB, et al. Gadd45alpha as an upstream signaling molecule of p38 MAPK triggers oxidative stress-induced sFlt-1 and sEng upregulation in preeclampsia. Cell Tissue Res. 2011;344:551–65. doi: 10.1007/s00441-011-1164-z. [DOI] [PubMed] [Google Scholar]
- 23.Xiong Y, Liebermann DA, Holtzman EJ, Jeronis S, Hoffman B, Gelfman-Holtzman O. Preeclampsia-associated stresses activate Gadd45a signaling and sFlt-1 in placental explants. J Cell Physiol. 2013;228:362–70. doi: 10.1002/jcp.24139. [DOI] [PubMed] [Google Scholar]
- 24.Xiong Y, Liebermann DA, Tront JS, et al. Gadd45a stress signaling regulates sFlt-1 expression in preeclampsia. J Cell Physiol. 2009;220:632–9. doi: 10.1002/jcp.21800. [DOI] [PubMed] [Google Scholar]