Abstract
Idiopathic pulmonary fibrosis (IPF) is characterized by the progressive and ultimately fatal accumulation of fibroblasts and extracellular matrix in the lung that distorts its architecture and compromises its function. IPF is now thought to result from wound-healing processes that, although initiated to protect the host from injurious environmental stimuli, lead to pathological fibrosis due to these processes becoming aberrant or over-exuberant. Although the environmental stimuli that trigger IPF remain to be identified, recent evidence suggests that they initially injure the alveolar epithelium. Repetitive cycles of epithelial injury and resultant alveolar epithelial cell death provoke the migration, proliferation, activation and myofibroblast differentiation of fibroblasts, causing the accumulation of these cells and the extracellular matrix that they synthesize. In turn, these activated fibroblasts induce further alveolar epithelial cell injury and death, thereby creating a vicious cycle of pro-fibrotic epithelial cell-fibroblast interactions. Though other cell types certainly make important contributions, we focus here on the “pas de deux” (steps of two), or perhaps more appropriate to IPF pathogenesis, the “folie à deux” (madness of two) of epithelial cells and fibroblasts that drives the progression of pulmonary fibrosis. We describe the signaling molecules that mediate the interactions of these cell types in their “fibrosis of two”, including transforming growth factor-β, connective tissue growth factor, sonic hedgehog, prostaglandin E2, angiotensin II and reactive oxygen species.
Keywords: pulmonary fibrosis, epithelial cells, apoptosis, fibroblasts, myofibroblasts, extracellular matrix
1. Introduction
Fibrosis characterizes many chronic diseases that result in end-stage organ failure, and consequently is a major cause of morbidity and mortality. The pathogenesis of fibrosis in many of these diseases is thought to involve aberrant or over-exuberant wound-healing processes initiated to protect the host from injurious stimuli [1]. In response to noxious stimuli of many different types, aberrant repair processes can produce the common result of excessive deposition of extracellular matrix that disrupts normal tissue homeostasis. Repair processes involve multiple cell types, including epithelial cells, fibroblasts, endothelial cells, pericytes and leukocytes, all of which potentially interact with each other. Interactions between two cell types in particular, alveolar epithelial cells and fibroblasts, appear to be central to the pathogenesis of idiopathic pulmonary fibrosis (IPF) [1].
IPF is characterized by progressive fibrosis, with excessive matrix deposition leading to destruction of lung architecture and ultimately fatal impairment of lung function. IPF has a heterogenous clinical course, but the median survival after diagnosis is only 2.5 – 3.5 years [2]. Although much of the pathogenesis of IPF remains to be elucidated, fibroblasts and epithelial cells have emerged as principal players in this disease, in particular myofibroblasts and type II alveolar epithelial cells. Fibroblasts and myofibroblasts accumulate in IPF lungs in “fibroblastic foci” that, as the predominant sites of excess matrix production, can be thought of as the leading edge of active fibrosis [3]. Fibroblast activation and accumulation in IPF, however, appears to be fundamentally driven by recurrent and/or non-resolving injury to the alveolar epithelium, and therefore in another sense, the injured alveolar epithelium can be thought of as the leading edge of active fibrosis. With fibroblasts and alveolar epithelial cells being in close apposition in the lung, it is not surprising that the interactions between these two key cellular players contribute to the development of pulmonary fibrosis. Though other cell types certainly make important contributions, we will focus on the “pas de deux” (steps of two), or perhaps more appropriate to IPF pathogenesis, the “folie à deux” (madness of two) of epithelial cell-fibroblast interactions as critical drivers of pulmonary fibrosis. Whereas the source of the fibroblasts and myofibroblasts that accumulate in the lung as fibrosis develops – whether these cells arise from resident fibroblasts, resident epithelial cells or circulating precursors – has been an area of controversy, the pro-fibrotic effects of the interactions of fibroblasts and myofibroblasts with resident lung epithelial cells has become increasingly clear. We describe the role of several important mediators in orchestrating the pro-fibrotic interactions of epithelial cells and fibroblasts in their “fibrosis of two”, including transforming growth factor-β, connective tissue growth factor, sonic hedgehog, prostaglandin E2, angiotensin II and reactive oxygen species (Figure 1).
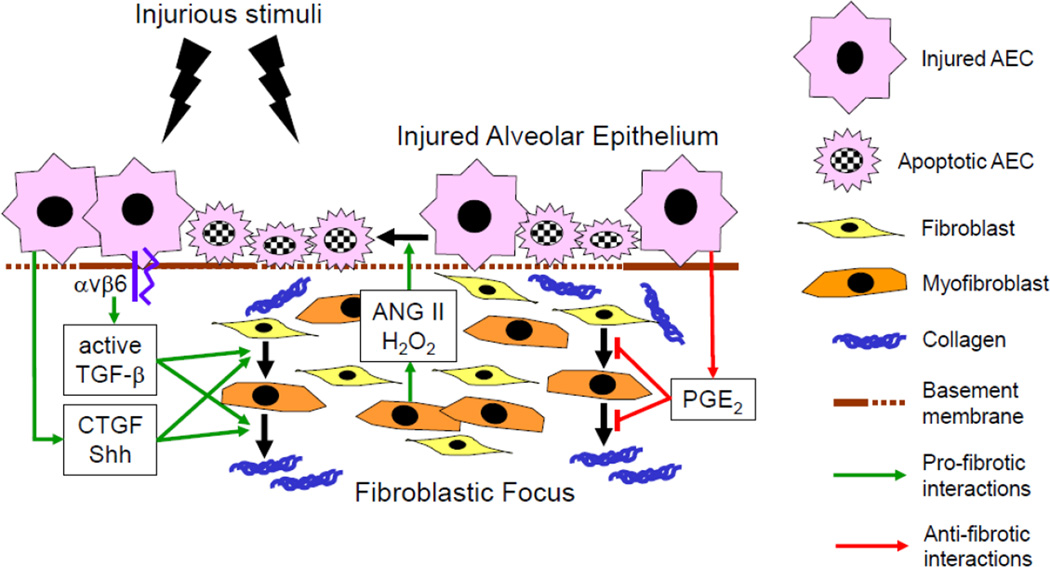
Figure 1. Epithelial-fibroblast interactions drive the progression of idiopathic pulmonary fibrosis.
Environmental stimuli initially injure alveolar epithelial cells (AECs), inducing their apoptosis and their production and/or activation of pro-fibrotic mediators, including TGF-β, CTGF and Shh. These AEC-derived mediators direct fibroblast migration, proliferation, activation and myofibroblast differentiation, resulting in the accumulation myofibroblasts and extracellular matrix in the lung. Myofibroblasts in turn secrete mediators that amplify AEC injury and apoptosis, creating a vicious cycle of pro-fibrotic epithelial cell-fibroblast interactions that drives the progression of IPF. PGE2 normally mediates anti-fibrotic interactions between epithelial cells and fibroblasts, but its production by AECs is reduced in IPF, as is fibroblast PGE2-responsiveness. Green arrows indicate pro-fibrotic epithelial cell-fibroblast interactions; red arrows indicate anti-fibrotic interactions. TGF-β, transforming growth factor-β; CTGF, connective tissue growth factor; Shh, sonic hedgehog; ANG II, angiotensin II; H2O2, hydrogen peroxide; PGE2, prostaglandin E2.
2. Epithelial cells: targeted cells in IPF
Accumulating evidence points toward recurrent and/or non-resolving injury to the lung epithelium as the “prime mover” of pulmonary fibrosis. Although the cause of this injury in IPF remains enigmatic, the footprints of lung epithelial injury are manifest, both in the form of (1) increased epithelial cell death, and (2) phenotypic alterations of the surviving epithelial cells. Increased numbers of apoptotic and necrotic cells have been observed in both the alveolar and bronchial epithelia of IPF patients [4–6]. Surviving epithelial cells in IPF lungs demonstrate several altered phenotypes [7]. Cuboidal epithelial cells representing type II alveolar epithelial cell (AEC) hyperplasia and/or bronchiolar basal cell proliferation line thickened fibrotic alveolar septa; single layers of flattened epithelial cells suggestive of squamous metaplasia frequently are present overlying fibroblastic foci; and single-layered columnar or pseudostratified columnar epithelial cells often line the abnormally enlarged, restructured air spaces of honeycomb lung. These morphological changes are associated with modifications of epithelial cell expression of specific cytokeratins, suggesting that in addition to their morphology, the differentiation states and functions of epithelial cells are likely profoundly altered in IPF. Of note, although IPF has been traditionally viewed as affecting the parenchymal lung rather than the airways, a potentially central role for the bronchial epithelium in addition to the alveolar epithelium in IPF pathogenesis has been suggested by the recent association of a genetic variant in mucin 5B (MUC5B) with both familial and sporadic IPF [8].
The potential of epithelial injury in general to cause pulmonary fibrosis has been demonstrated in several mouse models. Induction of pulmonary epithelial cell death in mice, either by pulmonary delivery of anti-Fas antibody [9–10] or transgenic overexpression of transforming growth factor-β (TGF-β) [11], results in the development of fibrosis, as does genetically targeting diptheria toxin-induced injury to alveolar epithelial cells [12]. Additionally, inhibition of apoptosis attenuates the fibrosis induced by bleomycin challenge, the most commonly used mouse model of pulmonary fibrosis [13].
Finally, in addition to noxious stimuli in the external environment, alterations in the internal environment of epithelial cells can also lead to their death and promote pulmonary fibrosis. For example, the mutations in the gene encoding surfactant protein C (SFTPC) that have been associated with familial pulmonary fibrosis (familial interstitial pneumonia) cause SFTPC misfolding, leading to protein accumulation and endoplasmic reticulum (ER) stress [14–17]. Unresolved or prolonged ER stress activates cellular apoptotic pathways, and the resulting epithelial cell death may cause the pulmonary fibrosis that affects these SFTPC mutation kindreds [18]. Thus epithelial cell injury and death, albeit due to a variety of causes and through a variety of mechanisms, appears to be a common initiating pathway to fibrosis in the lung.
3. Epithelial cell-to-fibroblast interactions: how injured epithelial cells activate fibroblasts
Areas of AEC apoptosis and foci of α-smooth muscle actin (αSMA)-positive myofibroblasts colocalize in the lungs of IPF patients [6], making it plausible for these two cell types to directly influence each other as fibrosis develops. The ability of injured epithelial cells to affect local fibroblast behavior in a paracrine fashion has been demonstrated by in vitro co-culture experiments. In these experiments, mechanical injury to epithelial cells induced the expression of α-SMA and type I and III collagen in cocultured fibroblasts by stimulating the activation of TGF-β in the extracellular matrix [19]. In addition to TGF-β, a growing list of mediators has been found to affect the ability of injured epithelial cells activate fibroblasts, including other cytokines/growth factors, such as connective tissue growth factor (CTGF); morphogens, such as sonic hedgehog (Shh), and lipid mediators, such as prostaglandin E2 (PGE2) [20–24]. Many of these mediators, such as TGF-β, CTGF and Shh promote fibroblast activation, whereas others such as PGE2 are suppressive (Figure 1). In the sections that follow, TGF-β, CTGF, Shh and PGE2 are discussed as important examples of mediators through which injured epithelial cells regulate fibroblast activation, but are certainly not a complete list of the molecules involved in epithelial cell-to-fibroblast interactions in fibrosis.
3.1 Transforming growth factor-beta (TGF-β)
TGF-β is a member of the TGF-β super-family, which in addition to TGF-β includes related cytokines such as bone morphogenic proteins, activins and inhibins [25]. Mammals have three different forms of TGF-β (TGF-β1, -β2, and -β3), each of which are widely expressed throughout the body [26]. All three isoforms initiate their cellular effects using the same high-affinity cell surface receptors (TGF-β type I and type II receptors) [27–28]. But despite their common receptor usage, the isoforms have differing biological functions, as indicated by the differing phenotypes of mice deficient for each. TGF-β1 null mice develop severe multi-focal inflammation and die within 3 weeks of birth [29–30], whereas TGF-β2 null mice die in the perinatal period due to cyanotic heart disease and pulmonary insufficiency [31], and TGF-β3 null mice die of craniofacial defects, most notably cleft palate [32]. Taken together, these phenotypes indicate that TGF-β signaling is important for tissue growth and morphogenesis during embryonic development, and for tissue homeostasis thereafter. When tissue homeostasis is perturbed by injury, however, TGF-β acts as a major pro-fibrotic cytokine, potently increasing fibroblast recruitment, proliferation, differentiation into myofibroblasts and production of extracellular matrix [33]. Delivery of this cytokine by itself to the rodent lung through intratracheal transfer of active TGF-β1 in an adenoviral vector is sufficient to induce pulmonary myofibroblast accumulation and fibrosis [34–35]. Conversely, inhibiting TGF-β with neutralizing antibodies or a type I receptor inhibitor suppresses experimental pulmonary fibrosis [36–37]. Importantly, increased endogenous expression of TGF-β is not by itself sufficient to increase TGF-β function, because all three TGF-β isoforms are generated and are present in tissues as inactive latent precursors. As discussed below, epithelial cells have been shown to mediate the activation of latent TGF-β in the lung, and consequently to play a critical role in the presentation of active TGF-β to fibroblasts in the pathogenesis of pulmonary fibrosis.
3.1.1 Activation of TGF-β by epithelial cell integrins
The three TGF-β isoforms are produced in the form of small latent complexes (SLCs), in which the bioactive TGF-β peptides form non-covalent associations with a latency-associated peptide (LAP) [26]. Further, SLCs are usually secreted in association with latent TGF-β binding proteins (LTBPs) as large latent complexes (LLCs). LLCs are sequestered in the extracellular matrix through the covalent binding of LTBPs to extracellular matrix proteins, such as fibrillin and fibronectin [26]. For TGF-β to exert biological effects, it must be activated from these latent complexes. The changes in TGF-β’s interactions with its LAP required for TGF-β activity can be accomplished by either non-proteolytic or proteolytic mechanisms that result in conformational changes or cleavage of the LAP respectively [26, 38–39]. The non-proteolytic activation of TGF-β by epithelial cells, and the presentation of the active TGF-β produced to fibroblasts, appears to be an epithelial cell-to-fibroblast interaction that is central to the development of pulmonary fibrosis.
Epithelial cells induce activating conformational changes in latent TGF-β complexes through their integrins. Integrins are cell adhesion molecules and transmembrane receptors that link the cytoskeleton to the extracellular matrix, and in addition to adhesion, regulate multiple fundamental cell processes including cell migration, proliferation and differentiation [40–41]. Integrins are composed of α and β subunits (18 α and 8 β subunits) that heterodimerize to form 24 αβ combinations [42]. Eight of these, including all five αν-containing integrins (ανβ1, ανβ3, ανβ5, ανβ6 and ανβ8), are capable of binding ligands through an arginine-glycine-aspartate (RGD) motif. The LAPs of both TGF-β1 and TGF-β3 have RGD sequences, and these two TGF-β isoforms can be activated in vitro by at least four of the αν-containing integrins (ανβ3, ανβ5, ανβ6, ανβ8) [42].
Activation of latent TGF-β specifically by the ανβ6 integrin appears to centrally important in the development of pulmonary fibrosis. Genetic deletion of the β6 subunit, or antibody blockade of the ανβ6, suppress TGF-β signaling in the lung after injury, and protect mice from the development of pulmonary fibrosis induced by bleomycin or radiation [43–45]. Lung expression of ανβ6 appears to be restricted to epithelial cells, underscoring the fundamental involvement of epithelial cell-to-fibroblast interactions in the pro-fibrotic behaviors induced in fibroblasts by TGF-β during the development of pulmonary fibrosis. The ability of epithelial cell ανβ6 to mediate latent TGF-β activation is dependent on epithelial cell cytoskeletal function. The cytoplasmic tail of the β6 subunit binds to the actin cytoskeleton, and disruption of this binding by mutation of the β6 cytoplasmic domain, or inhibition of actin polymerization with cytochalasin D, abolish epithelial cell activation of latent TGF-β [43].
Consistent with the activation of this pathway in pulmonary fibrosis, markedly increased lung epithelial ανβ6 expression is present in mouse lungs post-bleomycin challenge, and in human lungs with usual interstitial pneumonia (UIP) pattern pulmonary fibrosis [45–46]. This increased ανβ6 expression may itself be driven by active TGF-β. TGF-β induces the expression of the β6 subunit gene (itgb6) through the transcription factor Ets1 [47–48], and this process is inhibited by neutralizing β6 antibody [49]. ανβ6 integrin-mediated activation of latent TGF-β may therefore be amplified in pulmonary fibrosis by a feed-forward loop of increased TGF-β activation and increased ανβ6 expression.
3.1.2 Pro-fibrotic effects of TGF-β on fibroblasts
Once freed from its latent complexes by epithelial cell integrins, active TGF-β interacts with its receptors expressed by fibroblasts to induce multiple pro-fibrotic behaviors. Active TGF-β first binds to TGF-β receptor type II (TBRII), which phosphorylates and heterodimerizes with TGF-β receptor type I (TBRI) to form an active ligand–receptor complex. This TGF-β–TBRII/I complex initiates pro-fibrotic responses in fibroblasts through both canonical and non-canonical signaling pathways. In the canonical TGF-β signaling pathway, activated TBRI phosphorylates effector Smad proteins (Smad2 and Smad3), which heterodimerize with Smad4 to form Smad2/4 or Smad3/4 complexes [50]. These complexes translocate to the nucleus, where they bind to Smad response elements located in the promoter regions of pro-fibrotic genes such as type I collagen, fibronectin and αSMA [51]. Consistent with canonical TGF-β signaling having pro-fibrotic effects, Smad3-deficient mice are protected from bleomycin-induced lung fibrosis [52].
Both the c-Abelson tyrosine kinase (c-Abl) and mitogen-activated protein kinases (MAPKs) have been implicated in non-canonical TGF-β signaling. c-Abl is directly activated by TGF-β in fibroblasts, and signals through Egr-1 [53]. The small molecule imatinib mesylate potently inhibits c-Abl, as well as the platelet-derived growth factor receptor tyrosine kinase. Imatinib was demonstrated to prevent the development of bleomycin-induced lung fibrosis in mice [54], but a recently completed randomized placebo-controlled clinical trial of imatinib in IPF patients showed no benefit [55].
Mitogen-activated protein kinases (MAPKs) have also been shown to be involved in non-canonical TGF-β signaling. The MAPK family of serine-threonine protein kinases include extracellular signal–regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38, which mediate a wide variety of cellular responses, including proliferation, differentiation, and apoptosis [56–57]. In lung fibroblasts, TGF-β induction of αSMA and collagen expression has been shown to be dependent on ERK and p38 MAPK [56–57], and p38 MAPK activation by TGF-β has been shown to contributes to fibroblast resistance to apoptosis through the pro-survival phosphatidyl inositol 3-kinase (PI3K)/AKT pathway [58].
3.2 Connective tissue growth factor (CTGF/CCN2)
CTGF is as a member of the CCN protein family, containing Cysteine rich protein 61 (cyr61/CCN1), CTGF/CCN2, and Nephroblastoma overexpressed gene (NOV/CCN3), as well as three Wnt-1-induced proteins (WISPs), WISP-1/CCN4, WISP-2/CCN5 and WISP-3/CCN6. These proteins are characterized by an extraordinarily high content of cysteine residues, with the position of 38 of these cysteines being conserved almost entirely across the six family members [59–60]. CCN family proteins including CTGF contain four structural modules: an insulinlike growth factor binding protein module (module I), a von Willebrand factor type C module (module II), a thrombospondin type I homology module (module III) and a carboxy-terminal cysteine knot motif, heparin-binding module (module IV). These modules each have specific binding partners, including insulin-like growth factor for module I, TGF-β for module II, specific integrins (α4β1, α5β1, α6β1 and ανβ3) and sulfated glycoconjugates for module III, and heparin sulfate-containing proteoglycans (HSPGs) such as syndecan 4 and perlecan for module IV [59–61].
3.2.1 Synthesis of CTGF by epithelial cells
CTGF is principally regulated at the level of transcription [62]. Its promoter region contains binding sites for multiple transcription factors, including Smads, AP-1, Sp1, Ets-1, hypoxia-inducible factor and serum response factor [62–66]. Stimuli able induce CTGF transcription, dependent on the cell type, include TGF-β, thrombin, lysophosphatidic acid, and mechanical stress [60–61]. Signaling pathways involved in the regulation of CTGF expression by these stimuli include MAPK, protein kinase C, the small GTPase RhoA, and PI3K [61, 66–68]. Interestingly, most of the stimuli able to induce CTGF induction have also been implicated in the pathogenesis of pulmonary fibrosis [36, 69–72]. CTGF was originally discovered as a ‘PDGF-related mitogen’ in the medium of cultured human umbilical vein endothelial cells [73], but has subsequently been shown to be expressed by other cell types, including alveolar epithelial cells [21, 73]. In lung tissues obtained from IPF patients, CTGF mRNA and protein are both increased, localizing predominantly to AECs and activated fibroblasts. In contrast, CTGF-expressing AECs are sparse in the normal lung [21].
3.2.2 Pro-fibrotic effects of CTGF on fibroblasts
CTGF regulates multiple fibroblast behaviors that contribute to fibrosis, including fibroblast adhesion, migration, proliferation, differentiation, matrix production and apoptosis [74]. CTGF can induce these behaviors either by binding to fibroblast cell surface molecules directly, or by acting as an “adapter” molecule that brings other mediators into contact with their receptors on fibroblasts [74]. CTGF can directly affect fibroblast behaviors by binding to integrins, HSPGs and the low density lipoprotein receptor-related protein/p2-macroglobulin receptor (LRP) [60, 75–77]. For example, CTGF binding to fibroblast HSPGs induces fibroblast adhesion and proliferation [77–78]. Specific CTGF binding to the insulin-like growth factor-II (IGF-II)/mannose 6-phosphate (M6P) receptor expressed on lung fibroblasts has also recently been shown to induce fibroblast proliferation [79]. CTGF has also been shown to directly stimulate lung fibroblast expression of multiple proteins known to be elevated in IPF, including type I collagen, the cytoskeletal proteins vinculin, moesin and ezrin, and IQ motif containing GTPase activating protein 1 (IQGAP1), a scaffold protein that regulates cell migration and is elevated in lung fibroblasts isolated from scleroderma patients with pulmonary fibrosis [80].
In its role as an adapter molecule [74], the cytokines and growth factors that CTGF can “present” to their specific receptors on fibroblasts include TGF-β [81], epidermal growth factor, and insulin-like growth factor-II [82]. This adapter function appears to be required for at least some of the pro-fibrotic effects of TGF-β on fibroblasts. Although CTGF-deficient fibroblasts show intact TGF-β-induced Smad signaling, the ability of TGF-β to induce the expression of multiple pro-fibrotic mRNAs and proteins, including αSMA and type I collagen, is impaired in these cells [83].
Pro-fibrotic effects of CTGF have also been demonstrated in vivo. Whereas BALB/c mice are resistant to bleomycin-induced pulmonary fibrosis [84], transient overexpression of CTGF in the lungs of these “fibrosis-resistant” mice by adenoviral gene transfer CTGF enabled bleomycin to induce pulmonary myofibroblast accumulation and fibrosis similar in extent to that produced in “fibrosis-prone” C57Bl/6 mice [85]. The pro-fibrotic effects of CTGF that is specifically expressed by pulmonary epithelial cells were demonstrated in a transgenic mouse model with doxycycline-inducible overexpression of CTGF in respiratory epithelial cells directed by the clara cell secretory protein. Overexpression of CTGF from postnatal days 1–14 resulted in increased αSMA expression, collagen deposition and dramatic thickening in the peribronchial/peribronchiolar and perivascular regions of the lungs [86]. Cooperation between the pro-fibrotic effects of TGF-β and CTGF has also been noted in vivo. Intraperitoneal co-administration of CTGF and TGF-β2 induced multiorgan fibrosis in the lungs, liver and kidneys, whereas administration of either cytokine alone failed to elicit a fibrotic response [87]. Conversely, anti-CTGF antibodies have been shown to mitigate the increases in αSMA and type I collagen protein expression, as well as total collagen content, induced in lung in the bleomycin model of pulmonary fibrosis [88–89].
3.3 Sonic hedgehog (Shh)
Some of the pathways that regulate embryological development but are subsequently quiescent appear to re-emerge during repair responses to tissue injury [90]. Consistent with re-activation of developmental pathways in response to injury, microarray analyses of IPF gene expression revealed enriched expression of genes associated with lung development, including Patched-1, a receptor in the hedgehog pathway [91]. The hedgehog (Hh) family in mammals consists of sonic hedgehog (Shh), desert hedgehog (Dhh), and indian hedgehog (Ihh), which play critical roles in embryonic development, tissue patterning, and organogenesis [92–93]. Of these three mammalian Hh homologues, Shh has been shown to be responsible for tissue patterning the lung, regulating branching morphogenesis [94–96]. During lung development, Shh is produced in the distal epithelium and stimulates mesenchymal cell proliferation. Shh overexpression in mice pre-natally results in lethally excessive accumulation of lung interstitial mesenchyme [96], emphasizing that the Shh pathway mediates important epithelial cell-to-mesenchymal cell interactions during embryological lung development.
3.3.1 Epithelial cell synthesis of Shh
In both lung development and pulmonary fibrosis, epithelial cells appear to be the major source of Shh. During embryological development, low levels of Shh mRNA are present throughout the epithelium, and high levels are present at the tips of the terminal buds that ultimately form alveoli [23, 97]. Several studies of lung tissues obtained from IPF patients have demonstrated high Shh expression in reactive AECs, as well as in the epithelial cells that line honeycomb cysts, compared with little or no Shh expression in normal adult lung tissues [98–100]. In contrast, another recent study found that most of the components of the Shh system were expressed in normal adult alveolar epithelium, but that this pathway was activated specifically in IPF tissue [101]. Of potential relevance to the re-expression or activation of the Shh pathway in pulmonary fibrosis, both TGF-β and hydrogen peroxise (H2O2) have been reported to modulate Shh production by AECs [102– 103]. Although short-term incubation with TGF-β has been noted to suppress the secretion of Shh protein without significantly changing Shh mRNA expression [102], chronic TGF-β exposure dramatically increases expression of Shh both at the mRNA and protein levels [103]. In contrast, H2O2 enhances the release of Shh protein from epithelial cells without changing Shh mRNA expression, suggesting that H2O2 induces release of pre-formed Shh from intracellular stores [102].
3.3.2 Pro-fibrotic effects of Shh on fibroblasts
Shh signaling is transduced by 2 transmembrane proteins, patched-1 (PTCH-1) and smoothened (SMO). PTCH-1 is an Shh-binding receptor, which in the absence of Shh constitutively blocks SMO activity. Shh binding to PTCH-1 activates SMO, and activated SMO directs the biochemical processing and subsequent nuclear translocation of the transcription factors glioma-associated oncogene homolog (GLI)1, GLI2, and GLI3 Target genes regulated by these transcription factors include PTCH-1 itself, as well as regulators of the cell cycle, enabling the Shh pathway to regulate cell proliferation [104–106]. One recent immunohistochemical analysis of Shh pathway components in IPF reported fibroblast expression of PTCH-1, SMO, and GLI1 in the lungs of IPF patients, whereas no staining was detected in normal lungs [100]. PTCH-1 mRNA was also upregulated in primary lung fibroblasts grown from IPF versus normal lung tissue, and in fibroblasts assessed by laser capture microdissection in IPF lung tissues versus lung tissues from patients with cryptogenic organizing pneumonia [100]. In vitro, recombinant SHH has recently been shown to increase fibroblast proliferation and migration, to increase fibroblast expression of collagen and fibronectin but not αSMA, and to protect fibroblasts from apoptosis [100]. Additionally, TGF-β1’s ability to drive and maintain myofibroblast differentiation was recently demonstrated to require SMO/GLI pathway activity [101].
3.4 Prostaglandin E2 (PGE2)
The paucity of fibroblasts in normal alveolar septae compared with the greater abundance of fibroblasts in the connective tissue surrounding pulmonary bronchi, arteries, and veins led some investigators to hypothesize that under homeostatic conditions, alveolar epithelial cells suppress fibroblast accumulation. Media conditioned by AEC-fibroblast co-culture was in fact demonstrated to inhibit fibroblast proliferation, and this inhibition in subsequent studies was determined to be attributable to PGE2 synthesized by AECs [24, 107]. In contrast to the pro-fibrotic epithelial cell-to-fibroblast interactions mediated by TGF-β, CTGF and Shh, PGE2 therefore carries anti-fibrotic signals from epithelial cells to fibroblasts.
3.4.1 Epithelial cell synthesis of PGE2
AECs have a large capacity for synthesizing PGE2, the most abundant arachidonic acid metabolite that these cells produce. Following the liberation of arachidonic acid from membrane phospholipids, the synthesis of prostanoids, including prostaglandins, thromboxane and prostacyclin, is initiated by two cyclooxygenase (COX) enzymes, COX-1 and COX-2. COX-1 is constitutively expressed in most cells and tissues, whereas COX-2 is expressed when induced by inflammatory or mitogenic stimuli [108]. The pulmonary epithelium represents an exception to this usual pattern, in that AECs constitutively express both COX isoforms [109]. Experiments using COX-2-deficient AECs demonstrated that the PGE2 synthetic capacity of these cells is predominantly COX-2-dependent [24].
PGE2 synthesis is reduced in IPF, limiting the anti-fibrotic epithelial cell-to-fibroblast interactions that are mediated by PGE2. PGE2 levels in the epithelial lining fluid of individuals with IPF were found to be 50% lower than those in normal subjects [110]. AEC production of PGE2 may be suppressed by increased levels of plasminogen activation inhibitor-1 (PAI-1) and CC chemokine ligand 2 (CCL2) in IPF lungs. Plasmin has recently been shown to upregulate AEC PGE2 expression [111]. Plasmin upregulates AEC COX-2 expression, potentially through its ability to proteolytically activate and release hepatocyte growth factor (HGF) from these cells and/or the extracellular matrix. In bronchial epithelial cells, HGF has been shown to increases COX-2 gene expression through an Akt-, MAPK-, and β-catenin-dependent pathway [112]. PAI-1, which is markedly upregulated in fibrotic lungs [113], prevents the generation of plasmin by inhibiting urokinase-type plasminogen activator (uPA). By reducing the generation of plasmin, increased levels of PAI-1 could therefore reduce AEC PGE2 synthesis in IPF. In support of this hypothesis, PAI-1-deficient mice demonstrate increased lung production of PGE2, and are protected from bleomycin-induced pulmonary fibrosis [111]. This protection was abrogated by a selective inhibitor of the HGF receptor c-Met, which reduced lung COX-2 and PGE2 levels. In contrast to its upregulation by plasmin, AEC PGE2 synthesis is downregulated by CCL2. CCL2 is present in increased amounts in the bronchoalveolar lavage fluid of IPF patients [114], and may be induced in the AECs themselves by thrombin activation of the major thrombin receptor, proteinase-activated receptor-1 (PAR1) [115]. CCL2 and PAR1 are co-expressed and co-upregulated on the activated epithelium in fibrotic areas in IPF, and thrombin potently induces CCL2 expression in lung epithelial cells in vitro in a PAR1-dependent manner.
3.4.2 Anti-fibrotic effects of PGE2 on fibroblasts
PGE2 has been demonstrated to have multiple activities on fibroblasts that could suppress fibrosis, including inhibition of fibroblast migration [116], proliferation [117–118], collagen synthesis [119], and myofibroblast differentiation [120]. Of the 4 E prostanoid (EP) receptors, designated EP1, EP2, EP3, and EP4 [120], these inhibitory effects of PGE2 on fibroblasts are mediated by EP2 [120–122]. Lung fibroblast EP2 expression, however, is downregulated in pulmonary fibrosis. Diminished EP2 levels in fibroblasts isolated from mouse lungs following bleomycin challenge reduced the ability of PGE2 to inhibit their proliferation and collagen secretion [121]. Fibroblasts isolated from patients with IPF also exhibit decreased EP2 expression, and are similarly refractory to the anti-fibrotic effects of PGE2 [123]. The diminished EP2 expression levels in fibroblasts from fibrotic lungs are maintained by hypermethylation of the PGE receptor 2 gene (PTGER2) promoter [124]. Treatment of these fibroblasts with DNA methylation inhibitors or DNA methyltransferase-specific siRNA decreased PTGER2 methylation, increased EP2 mRNA and protein expression, and restored PGE2 responsiveness [124]. The anti-fibrotic epithelial cell-to-fibroblast interactions mediated by PGE2 therefore appear to be limited in IPF by decreased fibroblast EP2 expression as well as by decreased AEC PGE2 synthesis. Whereas epithelial-to-fibroblast interactions mediated by TGF-β, CTGF and Shh are attractive targets for new IPF therapies to inhibit, restoring PGE2-mediated interactions, by increasing AEC PGE2 production and fibroblast EP2 expression, represents an attractive therapeutic strategy for pulmonary fibrosis.
4. Fibroblast-to-epithelial cell interactions: how activated fibroblasts injure epithelial cells
As noted above, recurrent and/or non-resolving injury to the lung epithelium now appears to be the “prime mover” of pulmonary fibrosis. Increased numbers of apoptotic cells have been observed in the alveolar and bronchial epithelia of IPF patients [4–5], and the specific induction of epithelial injury and/or apoptosis has been shown to be sufficient to cause pulmonary fibrosis in several mouse models [9–12]. Although the initiating causes of epithelial injury in IPF remain enigmatic, activated fibroblasts appear to be able to amplify the epithelial apoptosis that results. As also noted above, the co-localization of αSMA-positive myofibroblast foci with areas of AEC apoptosis in IPF lungs [6] makes the paracrine interaction of these two cell types plausible in the development of fibrosis in vivo. The ability of activated fibroblasts to affect epithelial cells in a paracrine fashion has been demonstrated in vitro. In these experiments, media conditioned by fibroblasts isolated from the lungs of IPF patients markedly induced apoptosis of AECs in culture, whereas media conditioned by fibroblasts from control subjects did not [125]. Several mediators have been found to be responsible for this ability of activated fibroblasts to induce epithelial cell apoptosis, including angiotensin II and hydrogen peroxide (H2O2) (Figure 1).
4.1 Angiotensin II
The renin-angiotensin system consists of renin, angiotensinogen (AGT), angiotensin I (ANG I), angiotensin converting enzyme (ACE) and angiotensin II (ANG II). The octapeptide ANG II is the primary effector molecule of this pathway, and is formed by enzymatic cleavage of AGT to ANG I by the aspartyl protease renin, followed by the conversion of ANG I to ANG II by ACE. Although best know for its role in blood pressure regulation due to its ability to mediate vasoconstriction, ANG II has been implicated in the pathogenesis of fibrotic diseases affecting multiple organs, including the lung [126].
4.1.1 Fibroblast synthesis of ANG II
ANG II was determined to be the soluble factor responsible for inducing AEC apoptosis in media conditioned by IPF lung fibroblasts [127]. Lung tissue from IPF patients and bleomycin-challenged mice demonstrate upregulation of AGT and ANG peptides specifically in myofibroblasts [128–129]. In vitro, human lung fibroblasts have been demonstrated to upregulate AGT expression in response to TGF-β, through a mechanism involving activation of AGT transcription by hypoxia-inducible factor-1α and Jun D [130]. Myofibroblasts, especially under hypoxic conditions, are consequently thought to be an important source of ANG II production in the fibrosing lung.
4.1.2 Pro-fibrotic effects of angiotensin II on alveolar epithelial cells
The biological effects of ANG II are mediated through its two specific G protein-coupled seven transmembrane domain receptors, ANG II type 1 receptor (AT1R) and ANG II type 2 receptor (AT2R) [126]. Although AECs express both AT1R and AT2R, experiments with AT1R- and AT2R-selective antagonists indicate that AT1R mediates ANG II-induced AEC apoptosis [131–132]. An AT1R-selective antagonist was able to prevent mouse AEC apoptosis and lung fibrosis in the bleomycin model, as was an antisense oligonucleotide targeting AGT mRNA and an ACE inhibitor [128, 133–134]. AT1R-deficient mice similarly demonstrated reduced AEC apoptosis and collagen accumulation in the bleomycin model [134]. Further implicating the renin-angiotensin system in IPF, a single-nucleotide polymorphism (SNP) in the promoter region of the AGT gene that increases AGT transcription has been associated with more rapid disease progression [135].
4.2 Reactive Oxygen Species (ROS)
Experiments co-culturing small airway epithelial cells with TGF-β-stimulated fibroblasts isolated from IPF patients identified hydrogen peroxide (H2O2) as another diffusible paracrine signal produced by activated myofibroblasts that is able to induce epithelial cell death [136]. Production of ROS, such as H2O2, superoxide anions (· O2-) and hydroxyl radicals (·OH), in excess of the capacity of cells and tissues to detoxify or scavenge them is referred to as “oxidative stress”, and has been implicated in fibrotic diseases of multiple organs, including the lung [137]. Multiple lines of evidence indicate the presence of oxidative stress in IPF lungs. H2O2 concentrations are significantly higher in the exhaled breath condensates of IPF patients than control subjects, and correlate with disease severity [138]. Proteins in the bronchoalveolar lavage fluid of IPF patients demonstrate elevated levels of oxidative changes, such as oxidation of methionine residues to methionine sulfoxide, and introduction of carbonyl groups into other amino acid side-chains [139–140]. Additionally, epithelial cells in the lungs of IPF patients demonstrate the presence of ROS-induced DNA modifications [141].
4.2.1 Fibroblast synthesis of ROS
ROS are formed by the univalent reduction of oxygen, generally mediated by several ROS-producing enzymes, such as mitochondrial respiratory oxidases, xanthine oxidase, myeloperoxidase and NADPH oxidases [137, 142]. NADPH oxidases comprise a seven member family, including NOX1, 2, 3, 4 and 5, Duox1 and 2. Both NOX1 and NOX2 generate superoxide anions, whereas NOX4 produces H2O2 [143]. Recent evidence has implicated NOX4 expressed by fibroblasts in the development of lung fibrosis. TGF-β induces expression of this NOX isoform in IPF lung fibroblasts in vitro. Further, the expression αSMA and extracellular matrix proteins induced in lung fibroblast by TGF-β, as well as the proliferation of these cells induced by serum, require NOX4-dependent generation of H2O2 [144]. In IPF lung sections, H2O2 localizes specifically to αSMA-expressing myofibroblasts [136], and NOX4 similarly localizes to myofibroblastic foci [144]. A critically important role for fibroblast NOX4, and the H2O2 it produces, in the pathogenesis of pulmonary fibrosis was demonstrated in experiments in which siRNA-mediated knockdown of NOX4 expression suppressed fibrosis in vivo in both bleomycin- and FITC-induced mouse models of pulmonary fibrosis [144].
4.2.2 Pro-fibrotic effects of ROS on alveolar epithelial cells
In cultures of confluent primary distal lung epithelial cells, exogenous H2O2 inhibits monolayer closure after scratch wounding by inducing epithelial cell apoptosis [145]. As noted above, co-culture experiments demonstrated that endogenous generation of H2O2 by TGF-β-stimulated IPF lung fibroblasts induces apoptosis of small airway epithelial cells in a paracrine manner [136]. In the bleomycin mouse model of pulmonary fibrosis, alveolar epithelial apoptosis induced in vivo by bleomycin injury was demonstrated to be NOX4-dependent. The dramatic increase in apoptotic alveolar epithelial cells observed in wild type mice at early time points post-bleomycin challenge was abrogated in NOX4-deficient mice, and the development of fibrosis in these mice was markedly reduced at later time points [146]. A pathogenic role for ROS in pulmonary fibrosis in vivo studies has been further underscored by the amelioration of bleomycin-induced fibrosis in mice administered the antioxidant superoxide dismutase, and the exacerbation of bleomycin-induced pulmonary fibrosis in mice genetically deficient for this enzyme [147–148].
5. Bidirectional epithelial cell-fibroblast interactions in pulmonary fibrosis
Many important mediators of pulmonary fibrosis may be produced by both epithelial cells and fibroblasts, and may exert important effects on both cell types as well. Several of the signaling molecules discussed above carry signals both from epithelial cells to fibroblasts and from fibroblasts to epithelial cells, thus mediating bidirectional epithelial cell-fibroblast interactions in pulmonary fibrosis.
5.1 TGF-β
As described above, TGF-β is activated during the development of pulmonary fibrosis by epithelial cell integrins, and induces multiple pro-fibrotic activities in fibroblasts, including their recruitment, proliferation, myofibroblast differentiation and extracellular matrix production [33]. In addition to mediating these pro-fibrotic effects on fibroblasts, TGF-β delivers pro-fibrotic signals to the alveolar epithelium. In contrast to its induction of resistance to apoptosis in lung fibroblasts, TGF-β promotes apoptosis of lung epithelial cells in vitro [149–150]. A pathogenic role for TGF-β-induced epithelial cell apoptosis is suggested by observations of mice with lung-specific overexpression of active TGF-β. In these mice, a transient wave of epithelial apoptosis precedes the development of lung fibrosis, and blocking the TGF-β-induced epithelial apoptosis by administration of a general or a 3/7- selective caspase inhibitor markedly ameliorated the subsequent fibrosis. A pathogenic role for TGF-β-induced epithelial cell apoptosis is further suggested by the protection from bleomycin-induced fibrosis observed in mice with epithelial cell-specific deletion of TGF-β type II receptor [151]. Lung fibroblasts produce TGF-β latent complexes, and have been shown to be able to activate these complexes themselves by the ανβ5 integrins that they express [152]. TGF-β therefore could very plausibly mediate pro-fibrotic fibroblast-to-epithelial cells interactions, in which TGF-β produced and activated by lung fibroblasts mediates epithelial cell apoptosis. TGF-β thus could be an important mediator of bidirectional interactions between epithelial cells and fibroblasts in pulmonary fibrosis.
5.2 ANG II
As noted above, ANG II produced by myofibroblasts induces apoptosis in alveolar epithelial cells in a paracrine fashion. In addition to mediating this pro-fibrotic effect of fibroblasts on epithelial cells, ANG II may also carry pro-fibrotic signals from epithelial cells to fibroblasts. Apoptotic AECs have been shown to produce AGT and convert it to ANG II both in vitro and in the lung [129, 153]. Lung fibroblasts express AT1R and AT2R, and ANG II-AT1R signaling induces multiple pro-fibrotic activities in cells, including their proliferation, migration, and extracellular matrix synthesis [129]. ANG II therefore could also mediate pro-fibrotic effects of epithelial cells on fibroblasts, in which case it would also be an important mediator of bidirectional interactions between these two cell types in pulmonary fibrosis.
6. Other mediators of epithelial cell-fibroblast interactions in pulmonary fibrosis
The signaling molecules discussed in this review are meant to serve as important examples, rather than as a complete list, of mediators that direct epithelial cell-fibroblast interactions in lung fibrosis. Multiple other pathways that can contribute to the pro-fibrotic interactions of these two cell types have been described. Additional examples of important such signaling molecules include members of the WNT pathway and of the found in inflammatory zone (FIZZ) / resistin-like molecule (RELM) family. The WNT pathway was recently demonstrated to deliver pro-fibrotic signals from epithelial cells to fibroblasts in the development of lung fibrosis [154]. Expression of canonical (β-catenin-dependent) WNT signaling components and the WNT pathway target molecule, WNT-1-inducible signaling protein-1 (WISP1, another member of the CCN family of matricellular proteins), are strongly upregulated in type II AECs in both mice challenged with bleomycin and humans with IPF [154]. Alveolar epithelial cell WISP1 can exert pro-fibrotic effects on lung fibroblasts: WISP1 stimulates fibroblast extracellular matrix synthesis and myofibroblast differentiation in vitro, and its neutralization suppresses bleomycin-induced lung fibrosis in mice in vivo [154]. FIZZ/RELM family members, including FIZZ1/RELM-α and FIZZ2/RELM-β, similarly have been shown to be expressed by pulmonary epithelial cells during the development of fibrosis, and to exert pro-fibrotic effects on fibroblasts. FIZZ1 is strongly induced in mouse AECs following bleomycin challenge [155], and induces fibroblast extracellular matrix production, myofibroblast differentiation, and resistance to apoptosis [155–157]. FIZZ2 can also be induced in lung epithelial cells, is highly expressed in the lungs of bleomycin-challenged mice and IPF patients, and stimulates fibroblast type I collagen synthesis and myofibroblast differentiation; genetic deletion of FIZZ2 significantly attenuates pulmonary fibrosis in the bleomycin mouse model [158]. We expect that the number of pathways found to contribute to the pro-fibrotic interactions between epithelial cells and fibroblasts will continue to grow as investigators continue to unravel the complex pathogenesis of pulmonary fibrosis.
7. Epithelial cell-fibroblast interaction as a common theme in the development of organ fibrosis
Epithelial cell-fibroblast (and mesothelial cell-fibroblast) interactions may importantly contribute to the pathogenesis of fibrotic diseases in multiple organs. These interactions may be particularly relevant in organs in which fibroblasts and epithelial cells (or mesothelial cells) normally reside in close proximity, such as the lung, kidney, liver and peritoneum.
Progressive and potentially lethal renal fibrosis occurs in diverse kidney diseases. Renal fibrosis most often involves the accumulation of fibroblasts and extracellular matrix in the tubular interstitium, and atrophy of the tubular epithelium. The degree of renal fibrosis correlates well with the prognosis of the renal diseases it is found in, independent of their etiologies [159]. Accumulating evidence indicates that injured renal tubular epithelial cells activate and/or upregulate mediators such as TGF-β and CTGF that deliver pro-fibrotic signals to neighboring fibroblasts [160]. Apoptosis of renal tubular epithelial cells contributes to the progression of renal fibrosis [161], and both ANG II and ROS have been shown to induce apoptosis in these epithelial cells [162–164], as they do in alveolar epithelial cells in pulmonary fibrosis.
Epithelial cell-fibroblast interactions analogous to those occurring in IPF also appear to be important in the pathogenesis of hepatic and biliary fibrosis [165–167]. Hepatocyte injury and death leading to hepatic stellate cell activation in viral or toxin-induced hepatic fibrosis is analogous in many ways to AEC injury activating myofibroblasts in pulmonary fibrosis. In the case of biliary fibrosis, it is injury to cholangiocytes that leads to the activation and/or upregulation of mediators such TGF-β and CTGF that promote the activation and recruitment of hepatic stellate cells and portal fibroblasts [165].Moreover, ανβ6 integrin expression is upregulated by cholangiocytes in mouse models of biliary fibrosis, and contributes to the ability of these cells to activate latent TGF-β [168]. Biliary fibrosis produced in mice in vivo by bile duct ligation was significantly reduced in β6 integrin-deficient mice, and by administration of a blocking antibody to ανβ6 [169].
Peritoneal fibrosis is an important problem following acute peritoneal injury, as in the development of adhesions post-abdominal surgery, and following chronic peritoneal injury, as in the development of peritoneal fibrosis post-chronic peritoneal dialysis [170–171]. In the normal peritoneum, fibroblasts reside in a thin interstitial layer adjacent to the mesothelial cell monolayer. In the case of peritoneal dialysis-induced fibrosis, dialysis solutions that are hyperosmotic, hyperglycemic and/or acidic chronically injure the mesothelial cell layer, causing these cells to elaborate pro-fibrotic mediators including CTGF that drives peritoneal fibroblast proliferation and matrix deposition, resulting in progressive fibrotic expansion of the peritoneal interstitium [170, 172–173].
Concluding remarks
IPF remains a devastating disease that as yet is without effective pharmacological therapy, despite intensive scientific and clinical investigation. Although the stimuli that initiate epithelial injury in IPF have yet to be identified, substantial evidence supports the hypothesis that progression of IPF is driven by a series of pro-fibrotic epithelial cell-fibroblast interactions. In a “pas de deux”, or “folie à deux”, of epithelial cells and fibroblasts, interactions between these two cell types create a vicious cycle in which repetitive cycles of AEC injury provoke the activation of fibroblasts, and these activated fibroblasts in turn induce further AEC injury. The mediators of these interactions represent attractive therapeutic targets for treatment of pulmonary fibrosis, as well as other organs such as the kidney and liver where epithelial cell-fibroblast interactions appear to be central to the pathogenesis of fibrosis. Several of the mediators of these interactions discussed in this review are already being targeted by drugs in various stages of evaluation for IPF and/or other fibrotic diseases: TGF-β signaling is the target of the anti-ανβ6 integrin antibody STX-100 (Stromedix/Biogen Idec), the anti-TGF-β antibodies GC-1008 (Genzyme) and LY2382770 (Lilly), and one of the targets of pirfenidone (InterMune); CTGF is the target of the anti-CTGF antibody FG-3019 (FibroGen); ROS are the target of N-acetylcysteine given to augment lung anti-oxidant defense; and Ang II signaling is the target of the angiotensin II receptor antagonist losartan [174–175]. An even greater understanding of epithelial cell-fibroblast interactions in fibrosis will facilitate the development of additional strategies to therapeutically target the signaling molecules that mediate these interactions. Given the complexities of these interactions, with multiple mediators involved and at least some of which carrying bi-directional signals to and from each cell type, a better mapping of the steps that epithelial cells and fibroblasts take in their “fibrosis of two” will increase the likelihood of success of the mounting efforts to develop effective new anti-fibrotic therapies.
Highlights.
Interactions between alveolar epithelial cells and fibroblasts are central to IPF.
Injured epithelial cells regulate fibroblasts through TGF-β, CTGF, Shh and PGE2.
Activated fibroblasts injure epithelial cells by producing angiotensin II and ROS.
Epithelial cell-fibroblast interaction may be a common theme in organ fibrosis.
Acknowledgements
The authors gratefully acknowledge support from NIH R01-HL095732 and R01- HL108975, and Scleroderma Research Foundation, Coalition for Pulmonary Fibrosis, Pulmonary Fibrosis Foundation and Nirenberg Center for Advanced Lung Disease Grants to A.M.T., and from the Japan Society for the Promotion of Science (JSPS) Excellent Young Researcher Overseas Visit Program to N.S.
Abbreviations
- IPF
idiopathic pulmonary fibrosis
- AEC
alveolar epithelial cell
- TGF-β
transforming growth factor
- CTGF
connective tissue growth factor
- Shh
sonic hedgehog
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- 1.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King TE, Jr., Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 3.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 4.Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, Nakanishi Y, Hara N. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154:477–483. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- 5.Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest. 2005;127:266–274. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- 6.Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol. 1998;275:L1192–L1199. doi: 10.1152/ajplung.1998.275.6.L1192. [DOI] [PubMed] [Google Scholar]
- 7.Pardo A, Selman M. Molecular mechanisms of pulmonary fibrosis. Front Biosci. 2002;7:d1743–d1761. doi: 10.2741/pardo. [DOI] [PubMed] [Google Scholar]
- 8.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagimoto N, Kuwano K, Miyazaki H, Kunitake R, Fujita M, Kawasaki M, Kaneko Y, Hara N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol. 1997;17:272–278. doi: 10.1165/ajrcmb.17.3.2893. [DOI] [PubMed] [Google Scholar]
- 10.Matute-Bello G, Wurfel MM, Lee JS, Park DR, Frevert CW, Madtes DK, Shapiro SD, Martin TR. Essential role of MMP-12 in Fas-induced lung fibrosis. Am J Respir Cell Mol Biol. 2007;37:210–221. doi: 10.1165/rcmb.2006-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwano K, Kunitake R, Maeyama T, Hagimoto N, Kawasaki M, Matsuba T, Yoshimi M, Inoshima I, Yoshida K, Hara N. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2001;280:L316–L325. doi: 10.1152/ajplung.2001.280.2.L316. [DOI] [PubMed] [Google Scholar]
- 14.Nogee LM, Dunbar AE, 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 15.Thomas AQ, Lane K, Phillips J, 3rd, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, Roberts R, Haines J, Stahlman M, Loyd JE. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 16.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 17.Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol. 2005;32:521–530. doi: 10.1165/rcmb.2005-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanjore H, Lawson WE, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbadis.2012.11.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morishima Y, Nomura A, Uchida Y, Noguchi Y, Sakamoto T, Ishii Y, Goto Y, Masuyama K, Zhang MJ, Hirano K, Mochizuki M, Ohtsuka M, Sekizawa K. Triggering the induction of myofibroblast and fibrogenesis by airway epithelial shedding. Am J Respir Cell Mol Biol. 2001;24:1–11. doi: 10.1165/ajrcmb.24.1.4040. [DOI] [PubMed] [Google Scholar]
- 20.Khalil N, O'Connor RN, Flanders KC, Unruh H. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol. 1996;14:131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- 21.Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, Sawai T. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001;17:1220–1227. doi: 10.1183/09031936.01.00074101. [DOI] [PubMed] [Google Scholar]
- 22.Ingham PW. Localized hedgehog activity controls spatial limits of wingless transcription in the Drosophila embryo. Nature. 1993;366:560–562. doi: 10.1038/366560a0. [DOI] [PubMed] [Google Scholar]
- 23.Urase K, Mukasa T, Igarashi H, Ishii Y, Yasugi S, Momoi MY, Momoi T. Spatial expression of Sonic hedgehog in the lung epithelium during branching morphogenesis. Biochem Biophys Res Commun. 1996;225:161–166. doi: 10.1006/bbrc.1996.1147. [DOI] [PubMed] [Google Scholar]
- 24.Lama V, Moore BB, Christensen P, Toews GB, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am J Respir Cell Mol Biol. 2002;27:752–758. doi: 10.1165/rcmb.4857. [DOI] [PubMed] [Google Scholar]
- 25.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 26.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 27.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 28.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 29.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3:413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenyon NJ, Ward RW, McGrew G, Last JA. TGF-beta1 causes airway fibrosis and increased collagen I and III mRNA in mice. Thorax. 2003;58:772–777. doi: 10.1136/thorax.58.9.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, Protter AA, Gauldie J. Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med. 2005;171:889–898. doi: 10.1164/rccm.200405-612OC. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol. 2009;175:1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi H, Sakai T. Biological Significance of Local TGF-beta Activation in Liver Diseases. Front Physiol. 2012;3:1–11. doi: 10.3389/fphys.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 41.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 42.Coward WR, Saini G, Jenkins G. The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2010;4:367–388. doi: 10.1177/1753465810379801. [DOI] [PubMed] [Google Scholar]
- 43.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 44.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, Violette SM, Grant KS, Colarossi C, Formenti SC, Munger JS. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O’Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 46.Aluwihare P, Munger JS. What the lung has taught us about latent TGF-beta activation. Am J Respir Cell Mol Biol. 2008;39:499–502. doi: 10.1165/rcmb.2008-0003ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang A, Yokosaki Y, Ferrando R, Balmes J, Sheppard D. Differential regulation of airway epithelial integrins by growth factors. Am J Respir Cell Mol Biol. 1996;15:664–672. doi: 10.1165/ajrcmb.15.5.8918373. [DOI] [PubMed] [Google Scholar]
- 48.Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, Sheppard D, Oettgen P, Mercurio AM. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, Barzcak A, Xiao Y, Erle DJ, Nishimura SL. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santibanez JF, Quintanilla M, Bernabeu C. TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2011;121:233–251. doi: 10.1042/CS20110086. [DOI] [PubMed] [Google Scholar]
- 51.Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther. 2010;12:213. doi: 10.1186/ar3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr., Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L585–L593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- 53.Bhattacharyya S, Wu M, Fang F, Tourtellotte W, Feghali-Bostwick C, Varga J. Early growth response transcription factors: key mediators of fibrosis and novel targets for anti-fibrotic therapy. Matrix Biol. 2011;30:235–242. doi: 10.1016/j.matbio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 56.Hu Y, Peng J, Feng D, Chu L, Li X, Jin Z, Lin Z, Zeng Q. Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-beta1-induced alpha smooth muscle actin expression in human fetal lung fibroblasts in vitro. Lung. 2006;184:33–42. doi: 10.1007/s00408-005-2560-5. [DOI] [PubMed] [Google Scholar]
- 57.Caraci F, Gili E, Calafiore M, Failla M, La Rosa C, Crimi N, Sortino MA, Nicoletti F, Copani A, Vancheri C. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008;57:274–282. doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, Cui Z, Thannickal VJ. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem. 2004;279:1359–1367. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- 60.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Gressner OA, Gressner AM. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008;28:1065–1079. doi: 10.1111/j.1478-3231.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 62.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- 63.Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21:473–482. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- 64.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- 65.Holmes A, Abraham DJ, Chen Y, Denton C, Shi-wen X, Black CM, Leask A. Constitutive connective tissue growth factor expression in scleroderma fibroblasts is dependent on Sp1. J Biol Chem. 2003;278:41728–41733. doi: 10.1074/jbc.M305019200. [DOI] [PubMed] [Google Scholar]
- 66.Muehlich S, Cicha I, Garlichs CD, Krueger B, Posern G, Goppelt-Struebe M. Actin-dependent regulation of connective tissue growth factor. Am J Physiol Cell Physiol. 2007;292:C1732–C1738. doi: 10.1152/ajpcell.00552.2006. [DOI] [PubMed] [Google Scholar]
- 67.Leivonen SK, Hakkinen L, Liu D, Kahari VM. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-beta-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol. 2005;124:1162–1169. doi: 10.1111/j.0022-202X.2005.23750.x. [DOI] [PubMed] [Google Scholar]
- 68.Chen Y, Blom IE, Sa S, Goldschmeding R, Abraham DJ, Leask A. CTGF expression in mesangial cells: involvement of SMADs, MAPkinase and PKC. Kidney Int. 2002;62:1149–1159. doi: 10.1111/j.1523-1755.2002.kid567.x. [DOI] [PubMed] [Google Scholar]
- 69.Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, Chambers RC. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol. 2005;166:1353–1365. doi: 10.1016/S0002-9440(10)62354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hocher B, Schwarz A, Fagan KA, Thone-Reineke C, El-Hag K, Kusserow H, Elitok S, Bauer C, Neumayer HH, Rodman DM, Theuring F. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am J Respir Cell Mol Biol. 2000;23:19–26. doi: 10.1165/ajrcmb.23.1.4030. [DOI] [PubMed] [Google Scholar]
- 71.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 72.Villar J, Cabrera NE, Valladares F, Casula M, Flores C, Blanch L, Quilez ME, Santana-Rodriguez N, Kacmarek RM, Slutsky AS. Activation of the Wnt/beta-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS One. 2011;6:e23914. doi: 10.1371/journal.pone.0023914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 75.Ball DK, Moussad EE, Rageh MA, Kemper SA, Brigstock DR. Establishment of a recombinant expression system for connective tissue growth factor (CTGF) that models CTGF processing in utero. Reproduction. 2003;125:271–284. doi: 10.1530/rep.0.1250271. [DOI] [PubMed] [Google Scholar]
- 76.Ball DK, Rachfal AW, Kemper SA, Brigstock DR. The heparin-binding 10 kDa fragment of connective tissue growth factor (CTGF) containing module 4 alone stimulates cell adhesion. J Endocrinol. 2003;176:R1–R7. doi: 10.1677/joe.0.176r001. [DOI] [PubMed] [Google Scholar]
- 77.Chen CC, Chen N, Lau LF. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- 78.Brigstock DR, Steffen CL, Kim GY, Vegunta RK, Diehl JR, Harding PA. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem. 1997;272:20275–20282. doi: 10.1074/jbc.272.32.20275. [DOI] [PubMed] [Google Scholar]
- 79.Blalock TD, Gibson DJ, Duncan MR, Tuli SS, Grotendorst GR, Schultz GS. A connective tissue growth factor signaling receptor in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2012;53:3387–3394. doi: 10.1167/iovs.12-9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bogatkevich GS, Ludwicka-Bradley A, Singleton CB, Bethard JR, Silver RM. Proteomic analysis of CTGF-activated lung fibroblasts: identification of IQGAP1 as a key player in lung fibroblast migration. Am J Physiol Lung Cell Mol Physiol. 2008;295:L603–L611. doi: 10.1152/ajplung.00530.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 83.Shi-wen X, Stanton LA, Kennedy L, Pala D, Chen Y, Howat SL, Renzoni EA, Carter DE, Bou-Gharios G, Stratton RJ, Pearson JD, Beier F, Lyons KM, Black CM, Abraham DJ, Leask A. CCN2 is necessary for adhesive responses to transforming growth factor-beta1 in embryonic fibroblasts. J Biol Chem. 2006;281:10715–10726. doi: 10.1074/jbc.M511343200. [DOI] [PubMed] [Google Scholar]
- 84.Kolb M, Bonniaud P, Galt T, Sime PJ, Kelly MM, Margetts PJ, Gauldie J. Differences in the fibrogenic response after transfer of active transforming growth factor-beta1 gene to lungs of "fibrosis-prone" and "fibrosis-resistant" mouse strains. Am J Respir Cell Mol Biol. 2002;27:141–150. doi: 10.1165/ajrcmb.27.2.4674. [DOI] [PubMed] [Google Scholar]
- 85.Bonniaud P, Martin G, Margetts PJ, Ask K, Robertson J, Gauldie J, Kolb M. Connective tissue growth factor is crucial to inducing a profibrotic environment in "fibrosis-resistant" BALB/c mouse lungs. Am J Respir Cell Mol Biol. 2004;31:510–516. doi: 10.1165/rcmb.2004-0158OC. [DOI] [PubMed] [Google Scholar]
- 86.Wu S, Platteau A, Chen S, McNamara G, Whitsett J, Bancalari E. Conditional overexpression of connective tissue growth factor disrupts postnatal lung development. Am J Respir Cell Mol Biol. 2010;42:552–563. doi: 10.1165/rcmb.2009-0068OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, Brenner M, Guo G, Zhang W, Oliver N, Lin A, Yeowell D. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, Hoyles RK, Bou-Gharios G, Black CM, Denton CP, Abraham DJ, Leask A, Lindahl GE. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009;60:2142–2155. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Wu G, Gou L, Liu Z, Fan X, Wu L, Liu N. A novel single-chain-Fv antibody against connective tissue growth factor attenuates bleomycin-induced pulmonary fibrosis in mice. Respirology. 2011;16:500–507. doi: 10.1111/j.1440-1843.2011.01938.x. [DOI] [PubMed] [Google Scholar]
- 90.Studer SM, Kaminski N. Towards systems biology of human pulmonary fibrosis. Proc Am Thorac Soc. 2007;4:85–91. doi: 10.1513/pats.200607-139JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ingham PW. Hedgehog signaling. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 94.Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol. 2003;258:169–184. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 95.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- 96.Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124:53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- 97.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- 98.Stewart GA, Hoyne GF, Ahmad SA, Jarman E, Wallace WA, Harrison DJ, Haslett C, Lamb JR, Howie SE. Expression of the developmental Sonic hedgehog (Shh) signalling pathway is up-regulated in chronic lung fibrosis and the Shh receptor patched 1 is present in circulating T lymphocytes. J Pathol. 2003;199:488–495. doi: 10.1002/path.1295. [DOI] [PubMed] [Google Scholar]
- 99.Coon DR, Roberts DJ, Loscertales M, Kradin R. Differential epithelial expression of SHH and FOXF1 in usual and nonspecific interstitial pneumonia. Exp Mol Pathol. 2006;80:119–123. doi: 10.1016/j.yexmp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Bolanos AL, Milla CM, Lira JC, Ramirez R, Checa M, Barrera L, Garcia-Alvarez J, Carbajal V, Becerril C, Gaxiola M, Pardo A, Selman M. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L978–L990. doi: 10.1152/ajplung.00184.2012. [DOI] [PubMed] [Google Scholar]
- 101.Cigna N, Farrokhi Moshai E, Brayer S, Marchal-Somme J, Wemeau-Stervinou L, Fabre A, Mal H, Leseche G, Dehoux M, Soler P, Crestani B, Mailleux AA. The Hedgehog System Machinery Controls Transforming Growth Factor-beta-Dependent Myofibroblastic Differentiation in Humans: Involvement in Idiopathic Pulmonary Fibrosis. Am J Pathol. 2012;181:2126–2137. doi: 10.1016/j.ajpath.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 102.Fitch PM, Howie SE, Wallace WA. Oxidative damage and TGF-beta differentially induce lung epithelial cell sonic hedgehog and tenascin-C expression: implications for the regulation of lung remodelling in idiopathic interstitial lung disease. Int J Exp Pathol. 2011;92:8–17. doi: 10.1111/j.1365-2613.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maitah MY, Ali S, Ahmad A, Gadgeel S, Sarkar FH. Up-regulation of sonic hedgehog contributes to TGF-beta1-induced epithelial to mesenchymal transition in NSCLC cells. PLoS One. 2011;6:e16068. doi: 10.1371/journal.pone.0016068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 105.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 106.Wilson CW, Chuang PT. Mechanism and evolution of cytosolic Hedgehog signal transduction Development. 2010;137:2079–2094. doi: 10.1242/dev.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R, 3rd, Toews GB. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol. 2003;284:L342–L349. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]
- 108.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 109.Wardlaw SA, March TH, Belinsky SA. Cyclooxygenase-2 expression is abundant in alveolar type II cells in lung cancer-sensitive mouse strains and in premalignant lesions. Carcinogenesis. 2000;21:1371–1377. [PubMed] [Google Scholar]
- 110.Borok Z, Gillissen A, Buhl R, Hoyt RF, Hubbard RC, Ozaki T, Rennard SI, Crystal RG. Augmentation of functional prostaglandin E levels on the respiratory epithelial surface by aerosol administration of prostaglandin E. Am Rev Respir Dis. 1991;144:1080–1084. doi: 10.1164/ajrccm/144.5.1080. [DOI] [PubMed] [Google Scholar]
- 111.Bauman KA, Wettlaufer SH, Okunishi K, Vannella KM, Stoolman JS, Huang SK, Courey AJ, White ES, Hogaboam CM, Simon RH, Toews GB, Sisson TH, Moore BB, Peters-Golden M. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest. 2010;120:1950–1960. doi: 10.1172/JCI38369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee YH, Suzuki YJ, Griffin AJ, Day RM. Hepatocyte growth factor regulates cyclooxygenase-2 expression via beta-catenin, Akt, and p42/p44 MAPK in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L778–L786. doi: 10.1152/ajplung.00410.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chapman HA, Allen CL, Stone OL. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis. 1986;133:437–443. doi: 10.1164/arrd.1986.133.3.437. [DOI] [PubMed] [Google Scholar]
- 114.Moore BB, Paine R, 3rd, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- 115.Mercer PF, Johns RH, Scotton CJ, Krupiczojc MA, Konigshoff M, Howell DC, McAnulty RJ, Das A, Thorley AJ, Tetley TD, Eickelberg O, Chambers RC. Pulmonary epithelium is a prominent source of proteinase-activated receptor-1-inducible CCL2 in pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:414–425. doi: 10.1164/rccm.200712-1827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 117.Elias JA, Rossman MD, Zurier RB, Daniele RP. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis. 1985;131:94–99. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- 118.Bitterman PB, Wewers MD, Rennard SI, Adelberg S, Crystal RG. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986;77:700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goldstein RH, Polgar P. The effect and interaction of bradykinin and prostaglandins on protein and collagen production by lung fibroblasts. J Biol Chem. 1982;257:8630–8633. [PubMed] [Google Scholar]
- 120.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 121.Moore BB, Ballinger MN, White ES, Green ME, Herrygers AB, Wilke CA, Toews GB, Peters-Golden M. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol. 2005;174:5644–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- 122.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol. 2008;39:82–489. doi: 10.1165/rcmb.2008-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang SK, Wettlaufer SH, Hogaboam CM, Flaherty KR, Martinez FJ, Myers JL, Colby TV, Travis WD, Toews GB, Peters-Golden M. Variable prostaglandin E2 resistance in fibroblasts from patients with usual interstitial pneumonia. Am J Respir Crit Care Med. 2008;177:66–74. doi: 10.1164/rccm.200706-963OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang SK, Fisher AS, Scruggs AM, White ES, Hogaboam CM, Richardson BC, Peters-Golden M. Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am J Pathol. 2010;177:2245–2255. doi: 10.2353/ajpath.2010.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uhal BD, Joshi I, True AL, Mundle S, Raza A, Pardo A, Selman M. Fibroblasts isolated after fibrotic lung injury induce apoptosis of alveolar epithelial cells in vitro. Am J Physiol. 1995;269:L819–L828. doi: 10.1152/ajplung.1995.269.6.L819. [DOI] [PubMed] [Google Scholar]
- 126.Uhal BD, Li X, Piasecki CC, Molina-Molina M. Angiotensin signalling in pulmonary fibrosis. Int J Biochem Cell Biol. 2012;44:465–468. doi: 10.1016/j.biocel.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang R, Ramos C, Joshi I, Zagariya A, Pardo A, Selman M, Uhal BD. Human lung myofibroblast-derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. Am J Physiol. 1999;277:L1158–1164. doi: 10.1152/ajplung.1999.277.6.L1158. [DOI] [PubMed] [Google Scholar]
- 128.Li X, Zhuang J, Rayford H, Zhang H, Shu R, Uhal BD. Attenuation of bleomycin-induced pulmonary fibrosis by intratracheal administration of antisense oligonucleotides against angiotensinogen mRNA. Curr Pharm Des. 2007;13:1257–1268. doi: 10.2174/138161207780618867. [DOI] [PubMed] [Google Scholar]
- 129.Li X, Molina-Molina M, Abdul-Hafez A, Ramirez J, Serrano-Mollar A, Xaubet A, Uhal BD. Extravascular sources of lung angiotensin peptide synthesis in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2006;291:L887–L895. doi: 10.1152/ajplung.00432.2005. [DOI] [PubMed] [Google Scholar]
- 130.Abdul-Hafez A, Shu R, Uhal BD. JunD and HIF-1alpha mediate transcriptional activation of angiotensinogen by TGF-beta1 in human lung fibroblasts. FASEB J. 2009;23:1655–1662. doi: 10.1096/fj.08-114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 132.Papp M, Li X, Zhuang J, Wang R, Uhal BD. Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am J Physiol Lung Cell Mol Physiol. 2002;282:L713–L718. doi: 10.1152/ajplung.00103.2001. [DOI] [PubMed] [Google Scholar]
- 133.Wang R, Ibarra-Sunga O, Verlinski L, Pick R, Uhal BD. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2000;279:L143–L151. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- 134.Li X, Rayford H, Uhal BD. Essential roles for angiotensin receptor AT1a in bleomycin-induced apoptosis and lung fibrosis in mice. Am J Pathol. 2003;163:2523–2530. doi: 10.1016/S0002-9440(10)63607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Molina-Molina M, Xaubet A, Li X, Abdul-Hafez A, Friderici K, Jernigan K, Fu W, Ding Q, Pereda J, Serrano-Mollar A, Casanova A, Rodriguez-Becerra E, Morell F, Ancochea J, Picado C, Uhal BD. Angiotensinogen gene G-6A polymorphism influences idiopathic pulmonary fibrosis disease progression. Eur Respir J. 2008;32:1004–1008. doi: 10.1183/09031936.00015808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 137.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Psathakis K, Mermigkis D, Papatheodorou G, Loukides S, Panagou P, Polychronopoulos V, Siafakas NM, Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur J Clin Invest. 2006;36:362–367. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 139.Maier K, Leuschel L, Costabel U. Increased levels of oxidized methionine residues in bronchoalveolar lavage fluid proteins from patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;143:271–274. doi: 10.1164/ajrccm/143.2.271. [DOI] [PubMed] [Google Scholar]
- 140.Lenz AG, Costabel U, Maier KL. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur Respir J. 1996;9:307–312. doi: 10.1183/09031936.96.09020307. [DOI] [PubMed] [Google Scholar]
- 141.Kuwano K, Nakashima N, Inoshima I, Hagimoto N, Fujita M, Yoshimi M, Maeyama T, Hamada N, Watanabe K, Hara N. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J. 2003;21:232–240. doi: 10.1183/09031936.03.00063203. [DOI] [PubMed] [Google Scholar]
- 142.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 146.Geiser T, Ishigaki M, van Leer C, Matthay MA, Broaddus VC. H(2)O(2) inhibits alveolar epithelial wound repair in vitro by induction of apoptosis. Am J Physiol Lung Cell Mol Physiol. 2004;287:L448–L453. doi: 10.1152/ajplung.00177.2003. [DOI] [PubMed] [Google Scholar]
- 147.Tanaka K, Ishihara T, Azuma A, Kudoh S, Ebina M, Nukiwa T, Sugiyama Y, Tasaka Y, Namba T, Sato K, Mizushima Y, Mizushima T. Therapeutic effect of lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;298:L348–L360. doi: 10.1152/ajplung.00289.2009. [DOI] [PubMed] [Google Scholar]
- 148.Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med. 2003;35:763–771. doi: 10.1016/s0891-5849(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 149.Hagimoto N, Kuwano K, Inoshima I, Yoshimi M, Nakamura N, Fujita M, Maeyama T, Hara N. TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J Immunol. 2002;168:6470–6478. doi: 10.4049/jimmunol.168.12.6470. [DOI] [PubMed] [Google Scholar]
- 150.Solovyan VT, Keski-Oja J. Proteolytic activation of latent TGF-beta precedes caspase-3 activation and enhances apoptotic death of lung epithelial cells. J Cell Physiol. 2006;207:445–453. doi: 10.1002/jcp.20607. [DOI] [PubMed] [Google Scholar]
- 151.Li M, Krishnaveni MS, Li C, Zhou B, Xing Y, Banfalvi A, Li A, Lombardi V, Akbari O, Borok Z, Minoo P. Epithelium-specific deletion of TGF-beta receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest. 2011;121:277–287. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou Y, Hagood JS, Lu B, Merryman WD, Murphy-Ullrich JE. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382–22393. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li X, Zhang H, Soledad-Conrad V, Zhuang J, Uhal BD. Bleomycin-induced apoptosis of alveolar epithelial cells requires angiotensin synthesis de novo. Am J Physiol Lung Cell Mol Physiol. 2003;284:L501–L507. doi: 10.1152/ajplung.00273.2002. [DOI] [PubMed] [Google Scholar]
- 154.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Gunther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164:1315–1326. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, Lowe JB, Phan SH. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol. 2009;174:1745–1755. doi: 10.2353/ajpath.2009.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chung MJ, Liu T, Ullenbruch M, Phan SH. Antiapoptotic effect of found in inflammatory zone (FIZZ)1 on mouse lung fibroblasts. J Pathol. 2007;212:180–187. doi: 10.1002/path.2161. [DOI] [PubMed] [Google Scholar]
- 158.Liu T, Baek HA, Yu H, Lee HJ, Park BH, Ullenbruch M, Liu J, Nakashima T, Choi YY, Wu GD, Chung MJ, Phan SH. FIZZ2/RELM-beta induction and role in pulmonary fibrosis. J Immunol. 2011;187:450–461. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970;1:631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- 160.Prunotto M, Budd DC, Gabbiani G, Meier M, Formentini I, Hartmann G, Pomposiello S, Moll S. Epithelial-mesenchymal crosstalk alteration in kidney fibrosis. 2012 doi: 10.1002/path.4049. [DOI] [PubMed] [Google Scholar]
- 161.Kim SH, Yu MA, Ryu ES, Jang YH, Kang DH. Indoxyl sulfate-induced epithelial-to-mesenchymal transition and apoptosis of renal tubular cells as novel mechanisms of progression of renal disease. Lab Invest. 2012;92:488–498. doi: 10.1038/labinvest.2011.194. [DOI] [PubMed] [Google Scholar]
- 162.Bhaskaran M, Reddy K, Radhakrishanan N, Franki N, Ding G, Singhal PC. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol. 2003;284:F955–F965. doi: 10.1152/ajprenal.00246.2002. [DOI] [PubMed] [Google Scholar]
- 163.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009;297:F461–F470. doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 164.Brezniceanu ML, Lau CJ, Godin N, Chenier I, Duclos A, Ethier J, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol. 2010;21:943–954. doi: 10.1681/ASN.2009030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jensen K, Marzioni M, Munshi K, Afroze S, Alpini G, Glaser S. Autocrine regulation of biliary pathology by activated cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2012;302:G473–G483. doi: 10.1152/ajpgi.00482.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361–368. [PMC free article] [PubMed] [Google Scholar]
- 167.Jensen K, Marzioni M, Munshi K, Afroze S, Alpini G, Glaser S. Autocrine regulation of biliary pathology by activated cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2012;302:G473–G483. doi: 10.1152/ajpgi.00482.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Popov Y, Patsenker E, Stickel F, Zaks J, Bhaskar KR, Niedobitek G, Kolb A, Friess H, Schuppan D. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol. 2008;48:453–464. doi: 10.1016/j.jhep.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 169.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. 2000;20(Suppl 4):S43–S55. [PubMed] [Google Scholar]
- 171.van der Wal JB, Jeekel J. Biology of the peritoneum in normal homeostasis and after surgical trauma. Colorectal Dis. 2007;9(Suppl 2):9–13. doi: 10.1111/j.1463-1318.2007.01345.x. [DOI] [PubMed] [Google Scholar]
- 172.Mizutani M, Ito Y, Mizuno M, Nishimura H, Suzuki Y, Hattori R, Matsukawa Y, Imai M, Oliver N, Goldschmeding R, Aten J, Krediet RT, Yuzawa Y, Matsuo S. Connective tissue growth factor (CTGF/CCN2) is increased in peritoneal dialysis patients with high peritoneal solute transport rate. Am J Physiol Renal Physiol. 2010;298:F721–F733. doi: 10.1152/ajprenal.00368.2009. [DOI] [PubMed] [Google Scholar]
- 173.Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 2013 doi: 10.1096/fj.12-219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol. 2011;163:141–172. doi: 10.1111/j.1476-5381.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr1. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]