Abstract
Current research suggests that mood varies from season to season in some individuals, in conjunction with light-modulated alterations in chronobiologic indices like melatonin and cortisol. The primary aim of this study was to evaluate the effects of seasonal variations in darkness on mood in depressed antepartum women, and to determine the relationship of seasonal mood variations to contemporaneous blood melatonin and cortisol measures; a secondary aim was to evaluate the influence of seasonal factors on measures of melancholic versus atypical depressive symptoms. We obtained measures of mood and overnight concentrations of plasma melatonin and serum cortisol in 19 depressed patients (DP) and 12 healthy control (HC) antepartum women, during on-going seasonal variations in daylight/darkness, in a cross-sectional design. Analyses of variance showed that in DP, but not HC, Hamilton Depression Rating Scale (HRSD) scores were significantly higher in women tested during seasonally longer vs. shorter nights. This exacerbation of depressive symptoms occurred when the dim light melatonin onset, the melatonin synthesis offset and the time of maximum cortisol secretion (acrophase) were phase-advanced (temporally shifted earlier), and melatonin quantity was reduced, in DP but not HC. Serum cortisol increased across gestational weeks in both the HC and DP groups, which did not differ significantly in cortisol concentration. Nevertheless, serum cortisol concentration correlated positively with HRSD score in DP but not HC; notably, HC showed neither significant mood changes nor altered melatonin and cortisol timing or quantity in association with seasonal variations. These findings suggest that depression severity during pregnancy may become elevated in association with seasonally-related phase-advances in melatonin and cortisol timing and reduced melatonin quantity that occur in DP, but not HC. Thus, women who experience antepartum depression may be more susceptible than their non-depressed counterparts to phase alterations in melatonin and cortisol timing during seasonally longer nights. Interventions that phase delay melatonin and/or cortisol timing -- for example, increased exposure to bright evening light -- might serve as an effective intervention for antepartum depressions whose severity is increased during seasonally longer nights.
Keywords: Pregnant depression, chronobiology, season, circadian rhythm, darkness
INTRODUCTION
Although some early work suggested that depression risk was reduced during pregnancy (Paffenbarger, 1964) recent studies indicate that the risk of a major depressive episode (MDE) during pregnancy may be greater than previously recognized, particularly in women with a previous personal or family history of depression (Cohen et al., 2010). Antepartum MDE risk appears to increase in conjunction with co-morbidities such as physical and emotional abuse, poverty, limited education, lack of social support, single marital status and HIV seropositivity (Coelho et al., 2013; Makara-Studzinska et al., 2013; Manikkam and Burns, 2012). Some recent studies estimate depression prevalence during pregnancy in the range of 8–16% (Bowen et al., 2012; Colvin et al., 2013). Antidepressant medication is reported to be prescribed to more than 13% of pregnant women in the United States (Rosenquist, 2013), and El Marroun et al. (2012) found that 8.7% of 7027 pregnant women studied had clinically relevant depressive symptoms, with 15% of those women receiving selective serotonin re-uptake inhibitors (SSRIs) to relieve depression symptoms during pregnancy. Notably, the prevalence of suicidality in a prospective study of 1,066 women was found to be 6.9–12.0% during the last six antepartum months (Mauri et al., 2012).
Some lines of contemporary research identify dysregulation of circadian rhythms in clinical depression (Bunney and Bunney, 2000; Terman and Terman, 2005; Terman and Terman, 2010). We recently reviewed evidence implicating melatonin in antepartum depression, as well as in depressions associated with other reproductive epochs (Parry et al., 2006a; Parry et al., 2006b). We also reported (Parry et al., 2008a) that relative to non-depressed women, nocturnal plasma melatonin in pregnant depressed women was reduced, in contrast to postpartum women in whom it was elevated. Also, melatonin timing measures were phase-advanced in pregnant women with a personal or family history of depression, relative to women without such a history.
Abundant evidence also implicates dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in clinical depression. In fact, HPA dysregulation with accompanying cortisol elevation is regarded as the most consistent neuroendocrine abnormality in depression (Dinan and Scott, 2005; Rubin et al., 2001), and may be particularly prevalent in women (Young and Korszun, 2010). Cortisol secretion is usually suppressed after dexamethasone administration, but resistance to dexamethasone suppression of cortisol occurs more commonly among patients with major depression than among non-depressed persons (Carroll et al., 1968). Further, Jokinen & Nordstrom (2009) found that depressed participants who had attempted suicide were more likely to be non-suppressors in the dexamethasone suppression test than non-attempters. Women are especially prone to cortisol elevation in response to stress because ovarian hormones modulate the HPA axis and cortisol responses (Young and Korszun, 2010). During pregnancy, free cortisol is elevated approximately three-fold (Mastorakos and Ilias, 2003), producing levels comparable with those in Cushing’s syndrome (Kammerer et al., 2006), which may be associated with increased melancholic symptoms. Nevertheless, while cortisol elevation is a frequent feature of clinical depression, not all studies support this finding. For example, a recent meta-analysis (Knorr et al., 2010) found no reliable differences in cortisol in HC vs. DP. The discrepancy in cortisol findings may be due, in part, to the fact that cortisol elevation may be a correlate of melancholic, rather than atypical depression (Kammerer et al., 2006; Young et al., 2001). The HRSD score is often described as reflecting more melancholic features of depression (e.g., insomnia, decreased appetite, weight loss) while the atypical score reflects primarily the hypersomnia and increased appetite features seen in more atypical depressions. Depression with atypical features may affect 15–40% of depressed individuals (Quitkin, 2002), and women are believed to be more susceptible to atypical depressive symptoms than men (Grigoriadis and Robinson, 2007).
Finally, mood alterations associated with seasonal changes, e.g., reduced positive affect with shorter day lengths, occur globally (Golder and Macy, 2011). Seasonal affective disorder (SAD) occurring in winter (“winter depression”), an increase in depressive symptoms during the winter months, also occurs globally, but generally with greater frequency in higher latitudes where winter ambient daylight is reduced (Rosen et al., 1990). Levitt et al. (2000) estimated that the seasonal subtype of depression plays a role in 11% of all cases of major depression. Lewy et al. (1987) proposed a “phase shift hypothesis” suggesting that SAD was a manifestation of circadian dysregulation involving a phase-delay in melatonin secretion in most cases, but noted that a phase-advance in melatonin secretion occurred in some instances. This hypothesis was supported by studies showing that bright morning light, which phase-advanced (shifted to an earlier time) melatonin timing produced antidepressant effects. Subsequent work (Lam and Levitan, 2000; Terman and Terman, 2005; Wirz-Justice, 2003) provided further evidence for the efficacy of bright light therapy, typically administered in the morning, for SAD as well as for non-seasonal depressions, including the treatment of depression during pregnancy (Crowley and Youngstedt, 2012; Oren et al., 2002; Parry and Maurer, 2003; Wirz-Justice et al., 2011).
The present study extends our earlier work (Parry et al., 2008a) by examining the effect of seasonal changes in day length on mood alterations in antepartum women. The primary aim was to elucidate the effects of seasonal changes in darkness on depression severity during pregnancy, as related to circadian parameters of melatonin and cortisol rhythms. A secondary aim was to contrast the effects of seasonal and circadian parameters on melancholic versus atypical antepartum depression symptoms. We hypothesized that seasonal changes in nocturnal darkness would influence mood, melatonin, and cortisol phase timing and amplitude in depressed, but not non-depressed antepartum women. We also expected to find seasonal differences in severity of melancholic vs. atypical depressive symptoms.
MATERIALS AND METHODS
Subjects
Data for this report were collected between November 1989 and July 2010, and includes observations collected from some subjects reported on previously (Parry et al., 2008a). Details of subject recruitment procedures are described elsewhere (Parry et al., 2008a). In brief, we telephone screened 20–45 year-old San Diego women who were pregnant (up to 34 weeks, estimated). To be eligible, women had to not smoke and not use medications, herbs or over-the-counter preparations that would interfere with neuroendocrine measures. Participants took part in multiple overnight hospital stays in the General Clinical Research Center (GCRC), where they were allowed to bring a child with them if needed. Subjects had laboratory tests for clinical chemistry, thyroid indices, and complete blood count, urinalysis and urine toxicology screens. They were without significant medical illness and without medication that would interfere with study measures. Depressed subjects were required to be without antidepressant medication ≥ two weeks (four weeks for fluoxetine) before study. Patients with bipolar or primary anxiety disorders were excluded. The DP and HC participants were without alcohol abuse within the last year.
To establish DSM-IV-TR (APA, 2000) entrance and baseline criteria, trained clinicians gave each participant a structured psychiatric interview, a Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995) and at least two baseline evaluation ratings scheduled one week apart using the Structured Interview Guide for the 21-item Hamilton Depression Rating Scale (HRSD), Seasonal Affective Disorders (SIGH-SAD) version (Williams et al., 1994) that included an 8-item atypical depressive symptom inventory; the Beck Depression Inventory (BDI) (Beck et al., 1961); and the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., 1987) also validated for use during pregnancy (Hewitt et al., 2009).
From a pool of 42 pregnant volunteers who completed the study, we obtained mood data on 12 essentially asymptomatic, healthy control (HC) women with baseline ratings of ≤ 8 on the Structured Interview Guide for the 21-item Hamilton Depression Rating Scale (HRSD), Seasonal Affective Disorders (SIGH-SAD) version. We compared the HC with 19 antepartum women with ratings of ≥ 14, identified hereafter as depressed patients (DP). Complete melatonin and cortisol data were obtained on 12 HC and 17 DP subjects.
Methods
Subjects meeting entrance criteria were admitted to the University of California General Clinical Research Center (GCRC) at 16:00 h local (Pacific standard) time (PST). After a night of adaptation to the sleep room, licensed nurses inserted an intravenous catheter at 17:00 h and drew blood (3cc) every 30 minutes from 18:00–11:00 h for measurement of nocturnal plasma melatonin and serum cortisol. Subjects remained at bed rest in a single room with double doors and heavy drapery over the windows to block extraneous light from 16:00–11:00 h. Light panels kept daytime light exposure relatively dim (< 30 lux). We considered this light intensity too dim to substantially suppress melatonin in undilated pupils, disrupt sleep or shift circadian rhythms, yet not so dim that it might serve as a dark pulse (Benloucif et al., 2008). Subjects slept in the dark with an eye mask. Nurses or sleep technicians entered the room only when necessary (recorded by infrared camera), using a pen-size dim red flashlight. During sleep times, GCRC nurses threaded the intravenous catheter through a porthole in the wall and drew samples from an adjoining room to minimize sleep disturbances.
The UCSD Institutional Review Board approved the protocol. All subjects gave written informed consent after procedures had been explained fully.
Melatonin Assay
Blood samples for melatonin and cortisol were placed in plastic tubes containing ethylenediaminetetracetic acid, centrifuged, frozen immediately, and then stored at −70° C until assayed. Samples for the same subject were run in the same assay. Initial assays for melatonin were described in previously published manuscripts (Anderson et al., 1976; Brzezinski et al., 1988). We assayed plasma melatonin concentrations of the first 44 subjects by radioimmunoassay (RIA) with kits manufactured by IBL Immuno-Biological Laboratories, Hamburg, Germany. As the manufacturer changed this kit, plasma for the last five subjects was assayed with Direct Melatonin RIA kits manufactured by Bühlmann Laboratories (ALPCO Diagnostics, Windham NH). This widely used RIA kit uses calibrators ranging from 1–81 pg/ml and reports intra- and inter-assay CVs of 6.7 % and 10.4 %, respectively. The standard range is from 1.0–81 pg/ml, with an analytical sensitivity of 0.8 pg/ml.
Cortisol Assay
Serum cortisol concentration was determined using solid-phase radioimmunoassay kits (Diagnostic Products Corporation, Los Angeles, CA) with reported intra-assay coefficient of variation of c. 4 % and inter-assay coefficient of variation of c. 6 %; the standard range is from 0.5–50 μg/dl, with an assay sensitivity of 0.3 μg/dl. The manufacturer reports highest antibody cross-reactivities were for: Prednisolone (76%); methylprednisolone (12%); and 11-Deoxycortisol (11.4%). Cross-reactivities of all other reported compounds were below 1%.
Analyses
Melatonin Parameters
For this study we converted all local time measures obtained during Pacific Daylight Time (PDT) to PST prior to analyses of temporal effects on melatonin and cortisol parameters. As described previously (Parry et al., 2008b), we defined the dim light melatonin onset (DLMO) as the first time that the slope (dy/dt) of the log-transformed melatonin concentration curve became steeply positive for at least three consecutive time points relative to the slope of the points immediately preceding it; synthesis offset (SynOff) as the first time after the melatonin peak when the slope of the descending log-transformed melatonin curve became steeply negative for three consecutive time points; dim light melatonin offset/return to baseline (DLMOff) as the first time when the slope of the descending log-transformed melatonin curve approached zero for at least three consecutive time points; synthesis duration as (SynOff – DLMO); synthesis AUC (SynAUC) as the integrated area under the melatonin curve between DLMO and SynOff.
For SIGH-SAD analyses, we did separate analyses on the 21-item HRSD and the 8-item atypical subscale, averaged over 2–4 administration. For tests of the effects of seasonally longer vs. seasonally shorter nights, we calculated the difference (Sunset Time – Sunrise Time) as reported for San Diego, CA (Location: W117° 08′, N32° 45′) in online tables from the U. S. Naval Observatory web site http://aa.usno.navy.mil/data/docs/RS_OneYear.php). Based on the Naval Observatory tables we defined darkness duration as the number of hours between sunset and sunrise; at this latitude darkness duration varies from a minimum of 9.7 decimal h in summer to a maximum of 14.0 decimal h in winter.
Statistics
Cortisol and melatonin concentrations were subjected to a modified cosine analysis yielding estimates of the circadian rhythm-adjusted mean cortisol or melatonin quantity (mesor) based on 17 hours of samples collected every 30 min from 6:00 pm to 11:00 am, PST; the peak excursion above the mesor value (amplitude); plus the time of the peak of the rhythm of cortisol secretion (acrophase). For some statistical analyses we assigned subjects to either “shorter” or “longer” darkness duration categories based on a median split on darkness durations (Sunset Time – Sunrise Time) prevailing at the time they were studied. Using this bimodal darkness split, we analyzed the melatonin quantity profile with Time X Diagnosis X Bimodal Darkness ANCOVA, covarying on PST vs. PDT prevailing at the time of data collection. Timing and amplitude measures for plasma melatonin (as described above) and serum cortisol were also analyzed with multivariate analyses of covariance (MANCOVA), followed by univariate ANCOVA when the MANOVA was significant. Gestation week was applied as a covariate for the cortisol analyses.
RESULTS
Demographic Characteristics
None of the women studied met SCID criteria for SAD. Of those meeting screening criteria, one was dropped as an outlier because her delayed melatonin onset (24:30 h) and advanced baseline melatonin offset (05:30 h) differed from the group mean by more than three standard deviations. We studied the remaining 31 pregnant women (12 HC and 19 DP): 17 Caucasian, 10 Hispanic, 2 African American and 2 Multiethnic. Table 1 summarizes demographic data for HC and DP groups. With the exception of SIGH-SAD scores, the groups differed little on demographic characteristics; however, a personal history of depression was more common in DP (p = .006), which also had marginally more children, on average, than HC (p = .051).
Table 1.
Means (± SD) of Subject Characteristics in Healthy Control and Depressed Women Studied during Pregnancy. MEQ = Morningness-Eveningness Questionnaire.
| Healthy Control (N = 12) | Depressed (N = 19) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | S.D. | Range | Mean | S.D. | Range | P | |
| Age | 24.2 | 4.8 | 19–36 yr | 26.5 | 5.8 | 20–38 yr | .228 |
| Weeks Pregnant | 29.9 | 8.9 | 8–37 wk | 29.1 | 7.6 | 11–36 wk | .292 |
| Body Mass Index | 28.4 | 3.8 | 24–36 | 29.9 | 5.8 | 22–41 | .325 |
| MEQ Score | 54.4 | 12.5 | 36–69 | 55.5 | 9.6 | 41–73 | .799 |
| Children | 0.8 | 0.6 | 1–2 | 1.4 | 1.2 | 0–5 | .051 |
| SIGH-SAD score | 4.9 | 2.3 | 1–8 | 21.4 | 5.9 | 14–34 | < .001 |
| Family History of Depression (%) | 25.0 (3/12) | - | - | 36.8 (7/19) | - | - | .492 |
| Personal History of Depression (%) | 8.3 % (1/12) | - | - | 57.9 (11/19) | - | - | .006 |
| Duration Current Depressed Episode | 12.1 | 3.8 | 3–28 wk | NA | NA | NA | NA |
Seasonal Distributions in HC vs. DP
The DP were somewhat more likely to have enrolled for study in Fall/Winter months than in Spring/Summer months (10/19 = 53% in October – March vs. 9/19 = 47% in April – September); HC were somewhat less likely to have enrolled in Fall/Winter than Spring/Summer (3/12 = 25% vs. 9/12 = 75%, respectively). However, this difference in frequencies did not attain statistical significance (p = .158, Fisher exact test). Along with that finding, based on a median split on hours of darkness, DP were somewhat more likely than HC to have entered the study during seasons with longer darkness (12/19 = 63.2% vs. 4/12 = 33.3%, p = .149, Fisher exact test), and thus, hours of darkness exposure (Sunset time – Sunrise time) was somewhat higher in DP vs. HC (11.9 ± 1.4 vs. 11.1 ± 1.3 h, F(1,29) = 2.234, p = .146). Contrary to expectation, among women for whom data were available, 50.0% of HC vs. 38.5% of DP reported that Fall or Winter was their “worst season” (p = .448); further, 20.0% of the HC and 53.8% of the DP also identified Spring or Summer as their “best season” (p = .197)
Relationship of Darkness Duration to Mood, Melatonin and Cortisol Timing and Quantity
HRSD Score vs. Atypical Score
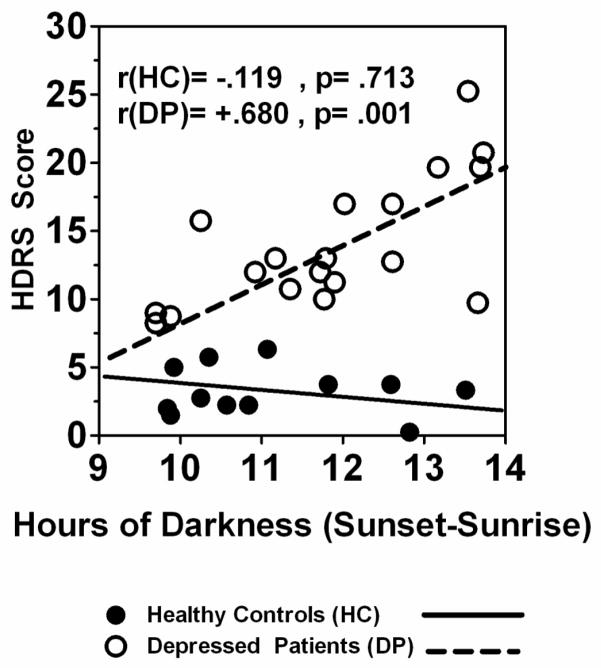
Hours of darkness was significantly and positively correlated with depressive symptoms as measured by the HRSD score in DP (r = .680, p = .001), but not HC (r = −.119, p = .713); see Fig. 1. In contrast, darkness duration was not significantly correlated with the atypical depression scale score of the SIGH-SAD in either DP (r = −.174, p = .476) or HC (r = −.244, p = .445). Thus, in DP, depressive symptom severity as measured by the HRSD was greater during periods of seasonally longer nights than during seasonally shorter nights. (N.B.: Correlations between atypical score and other variables of interest were not significant (p > .05) except as noted, below).
Fig. 1.
Pearson correlations of hours of darkness (Sunset – Sunrise) with depressive symptom severity (HRSD score) in pregnant HC (n = 12) and DP (n = 19) groups.
Melatonin Timing and Quantity
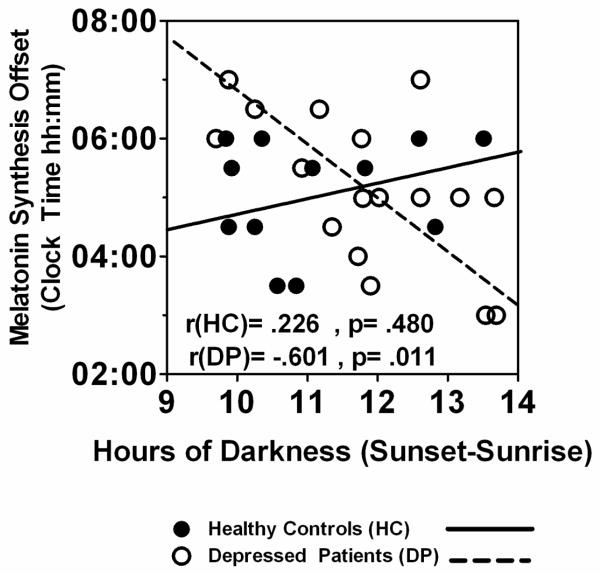
As shown in Table 2, in DP, but not HC, darkness duration (Sunset – Sunrise) was significantly and negatively correlated with SynOff (r = −.601, p = .011) (See Fig. 2) and synthesis duration (r = −.489, p = .047), but not DLMO (r = .100, p = .701); the correlation between hours of darkness and melatonin synthesis AUC was also negative, but marginal (r = −.480, p = .051) in DP. Thus, longer darkness was associated with earlier SynOff and shorter synthesis duration, and a somewhat lower melatonin synthesis AUC in DP; notably, in HC, darkness duration was not significantly correlated with any melatonin timing or quantity measures.
Fig. 2.
Pearson correlations of hours of darkness (Sunset – Sunrise) with melatonin synthesis offset in pregnant HC (n = 12) and DP (n = 17) groups.
In partial confirmation of the correlation results, analyses based on a median split on hours of darkness showed that greater seasonal darkness had significant effects on melatonin timing and quantity in DP, but not HC (see Table 3): e.g., DLMO (p = .050) and SynOff (p = .036) occurred significantly earlier in DP (but not HC) on nights with longer vs. shorter darkness; the DLMOff and Synthesis Duration were not significantly affected by darkness duration (p > .05). The melatonin synthesis AUC also was significantly smaller under conditions of longer vs. shorter darkness in DP (p = .018). In contrast, none of these darkness-related differences were significant in the HC group (all p > .05) (see Table 3). Furthermore, ANOVA on melatonin AUC showed a significant Darkness X Diagnosis interaction, F(1,25) = 6.678, p= .016. Analyses of simple effects showed synthesis AUC was significantly smaller in DP vs. HC under conditions of longer darkness, (mean = 927.3 ± 272.2 vs. 1870.5 ± 1166.0 pg/ml, F(1,13) = 7.038, p = .020), but DP vs. HC was not significantly different under conditions of shorter darkness (mean = 1782.5 ± 1027.2 vs. 1256.5 ± 618.5 pg/ml, F(1,12) = 1.431, p = .255). Thus, AUC was reduced rather than increased during periods of seasonally longer darkness in DP vs. HC. Fig. 3A illustrates the minimal differences in melatonin secretion profiles obtained under conditions of longer vs. shorter darkness in HC. In contrast, Fig. 3B shows the reduced AUC and the phase-advances (shifts leftward) in melatonin timing in longer vs. shorter darkness in DP.
Table 3.
Seasonal Darkness Effects on Mean (± SD) Melatonin and Cortisol Timing and Quantity in Pregnant Healthy Control (HC) Subjects and Depressed Patients (DP). Shorter vs. Longer Darkness was based on a median split on darkness durations (Sunset Time – Sunrise Time) in HC and DP groups, combined. Significant differences between Longer vs. Shorter Darkness are highlighted in boldface. [-] designates Effect Sizes in which HC and DP are numerically in opposite directions.
| Melatonin Synthesis | Cortisol Synthesis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median Split Darkness |
Sunrise (hh:mm) |
Sunset (hh:mm) |
Darkness (Sunset– Sunrise) (h) |
DLMO (hh:mm) |
Offset (hh:mm) |
DLMOff (hh:mm) |
Duration (h) |
AUC (pg/ml/h) |
Acrophase (hh:mm) |
Mesor (ug/dL) |
Amplitude (ug/dL) |
|
| HC | Shorter (N=8) | 05:01 (00:12) | 18:40 (00:17) | 10.30 (0.46) | 19:38 (1:27) | 04:53 (1:02) | 9:58 (1:09) | 9.25 (1.10) | 1256.5 (618.5) | 9:28 (1:09) | 19.16 (6.54) | 10.10 (3.47) |
| Longer (N=4) | 06:03 (00:30) | 17:22 (00:17) | 12.70 (0.70) | 20:15 (0:41) | 05:30 (0:42) | 9:52 (1:45) | 9.25 (0.50) | 1870.5 (1166.0) | 9:16 (0:44) | 18.14 (12.51) | 8.98 (5.78) | |
| Effect Size | [-]0.496 | [-]0.658 | 0.069 | 0.000 | [-]0.731 | 0.210 | [-]0.122 | [-]0.271 | ||||
| F(1,10) | 27.779 | 58.493 | 49.91 | 0.646 | 1.174 | 0.013 | 0.000 | 1.488 | 0.267 | 0.067 | 0.182 | |
| P | <.0001 | <.0001 | <.0001 | 0.440 | 0.304 | 0.913 | 1.000 | 0.251 | 0.617 | 0.801 | 0.679 | |
| DP | Shorter (N=6) | 05:08 (00:20) | 18:35 (00:22) | 10.54 (0.70) | 20:04 (00:31) | 06:00 (0:54) | 9:55 (1:24) | 9.54 (1.08) | 1782.5 (1027.9) | 10:34 (0:21) | 16.14 (5.56) | 9.02 (2.78) |
| Longer (N=11) | 06:01 (00:22) | 17:26 (00:30) | 12.59 (0.80) | 19:00 (1:05) | 04:40 (1:14) | 9:11 (1:11) | 9.71 (1.96) | 927.3 (272.2) | 9:28 (0:53) | 16.78 (5.60) | 9.48 (3.36) | |
| Effect Size | 1.080 | 1.038 | 0.581 | 0.097 | 1.149 | 1.203 | 0.118 | 0.149 | ||||
| F(1,15) | 23.725 | 24.24 | 27.379 | 4.620 | 5.297 | 1.322 | 0.035 | 7.078 | 8.096 | 0.051 | 0.081 | |
| P | <.0001 | <.0001 | <.0001 | 0.050 | 0.036 | 0.268 | 0.854 | 0.018 | 0.012 | 0.824 | 0.78 | |
Fig. 3.
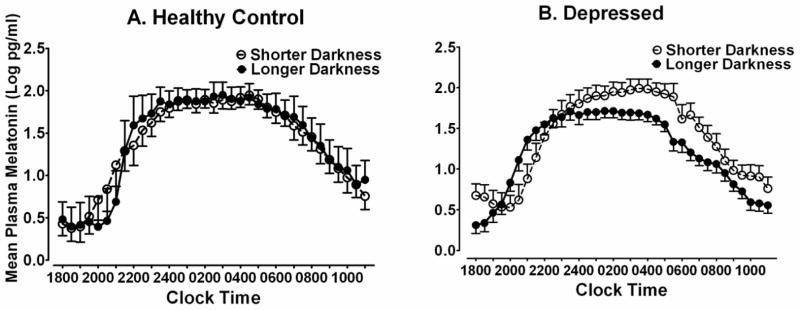
Nocturnal plasma melatonin profiles at 30-minute intervals under conditions of shorter vs. longer darkness in pregnant (A) healthy controls (n = 12) and (B) depressed patients (n = 17). Data points represent means (±SEM) of 30-minute collection intervals.
Cortisol timing and quantity
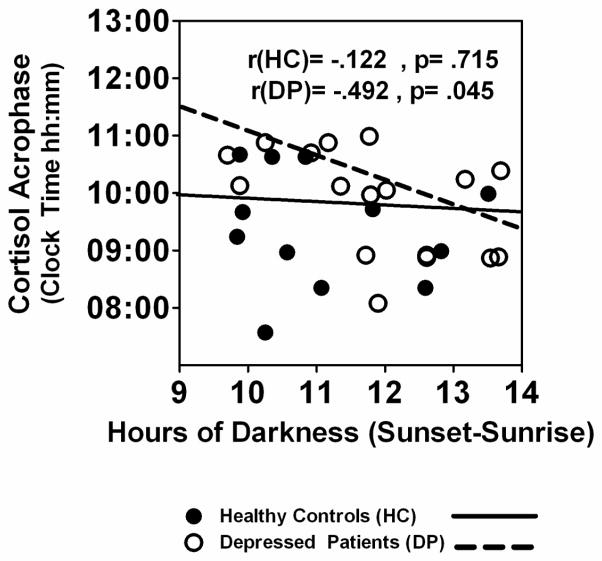
Cortisol acrophase was negatively correlated with hours of darkness in DP (r = −.492, p = .045) but not HC (r = −.122, p = .715), see Fig. 4. Thus, cortisol secretion peaked earlier during periods of seasonally-longer darkness in DP, but not HC. As might be expected in light of the significant correlations between darkness and melatonin timing (Fig. 2), and darkness and cortisol timing (Fig. 4), cortisol acrophase also was correlated positively with melatonin SynOff in DP (r = .665, p = .004) but not HC (r = −.079, p = .808). The cortisol mesor increased linearly across weeks of gestation in both HC and DP, and correlations between cortisol mesor and gestation week were nearly identical in DP (r = .619, p = .009) and HC (r = .618, p = .032). The cosine-derived cortisol amplitude was not correlated significantly with gestation week in either HC or DP (p > .05). A MANCOVA with gestation week as a covariate showed HC vs. DP were not significantly different in cortisol mesor (mean HC vs. DP = 18.8 ± 8.4 vs. 16.6 ± 5.4 ug/dL, F(1,26) = 0.036, p = .851), amplitude (mean HC vs. DP = 9.7 ± 4.1 vs. 9.3 ± 3.1, F(1,26) = 0.001, p = .999), acrophase (mean HC vs. DP = 10:32 ± 0:19 vs. 10:30 ± 0:16 hh:mm, F(1,27) = 0.004, p = .949) or lowest nighttime cortisol value (nadir) (mean HC vs. DP = 9.1 ± 5.3 vs. 7.2 ± 4.2), F(1,26) = 0.104, p = .750. “Darkness,” however, was a key element: Cortisol acrophase was phase-advanced under conditions of longer vs. shorter darkness in DP (p = .012), but not in HC (see Table 3). Cortisol mesor and amplitude were not affected significantly by longer vs. shorter darkness (p > .05) in either DP or HC. Thus, in DP but not HC, the cortisol acrophase was phase-advanced concurrent with the phase-advance in melatonin timing. The cortisol mesor and amplitude were unaffected by increased seasonal darkness during periods when seasonal darkness was greatest in both HC and DP. Fig. 5A illustrates the minimal differences in cortisol secretion profiles obtained under conditions of longer vs. shorter darkness in HC. In contrast, Fig. 5B shows the phase-advance (shift leftward) in cortisol timing in longer vs. shorter darkness in DP.
Fig. 4.
Relationship of hours of darkness to cortisol acrophase in pregnant HC (n = 12) and DP (n = 17) groups
Fig. 5.
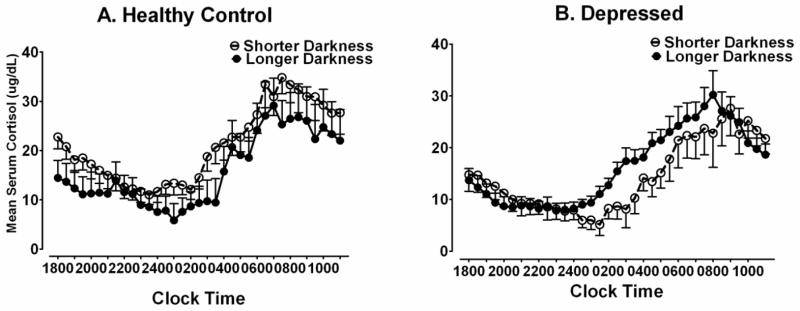
Serum cortisol profiles at 30-minute intervals under conditions of shorter vs. longer darkness in pregnant (A) healthy controls (n = 12) and (B) depressed patients (n = 17). Data points represent means (±SEM) of 30-minute collection intervals.
Relationship of HRSD scores to melatonin and cortisol timing and quantity
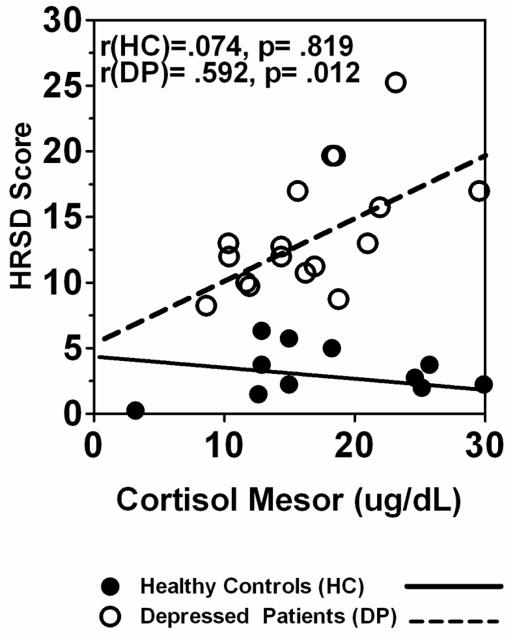
The HRSD score was correlated negatively with melatonin synthesis offset in DP (r = −.603, p = .010), but the correlation was not significant and in the opposite (positive) direction in HC (r = .564, p = .056). Thus, increased depression severity was associated with phase-advanced melatonin synthesis offset in DP, but not HC. The HRSD score also was positively correlated with cortisol mesor in DP (r = .592, p = .012), but not HC (r = .074, p = .819) (See Fig. 6). The correlation between atypical score and cortisol mesor in DP was not significant (r = .277, p = .282).
Fig. 6.
Relationship of depressive symptom severity (HRSD) score to cortisol mesor in pregnant HC (n = 12) and DP (n = 17) groups.
Summary of Results
Depressed and non-depressed pregnant women differed significantly in their responses to seasonal changes in daylight: In DP but not HC, seasonally longer nights were associated with (1) higher HRSD (greater depression) scores; (2) phase-advances in DLMO and melatonin SynOff; (3) reduced melatonin synthesis AUC; and (4) a phase-advance in cortisol acrophase. Overall, cortisol mesor and amplitude did not differ significantly in the HC vs. DP groups or in response to seasonal changes in darkness. Despite the absence of difference between groups, cortisol mesor was correlated positively with HRSD score in DP, but not in HC. Thus, during periods of seasonally longer darkness, the increased cortisol mesor and phase-advance in melatonin and cortisol timing were associated with greater HRSD depression scores in DP but not HC. In contrast to the HRSD score, the SIGH-SAD atypical depression score was unrelated to seasonal darkness, or to melatonin or cortisol timing and quantity in pregnant DP.
DISCUSSION
The present results confirm major findings of our earlier work on antepartum depression (Parry et al., 2007; Parry et al., 2008a) while providing new evidence regarding seasonal influences on depression severity during pregnancy. To our knowledge, our study is the first to report increased symptoms of antepartum depression in association with seasonally reduced daylight. Although investigators have reported an increased risk of postpartum depression in women whose babies were born during fall and winter months (Corral et al., 2007a; Hiltunen et al., 2004; Sylven et al., 2011; Yang et al., 2011), a large cross-sectional analysis of 67,079 births detected no significant relationship between postpartum depression and season of birth or length of daylight (Jewell et al., 2010).
Melatonin Timing Effects
Although seasonal differences in month of study enrollment were non-significant, the seasonal differences in melatonin timing and quantity we found might be interpreted as evidence that women of a certain chronotype were simply more likely to volunteer for this study in winter than in summer. A plausible explanation for why this could occur might be that pregnant women with phase-advanced melatonin timing during winter were more likely to volunteer for a study on women’s depressions because that was the time when their symptoms were most intense. Furthermore, our finding that these women were more likely to have symptoms whose intensity was correlated with length of nocturnal darkness directly confirms that their depressive symptoms were exacerbated during the winter months.
Equally important, the exacerbation of depression symptoms during longer nights was associated with phase-advanced melatonin timing in DP, but not in HC. Wehr et al. (1993) studied the effects of long vs. short nights on melatonin secretion in healthy individuals exposed to artificial “days” that differed in light duration, under controlled laboratory conditions. Duration of nocturnal melatonin secretion was longer after exposure to long nights than after short nights. In a subsequent study, Wehr et al. (1995) studied melatonin secretion in 21 healthy men, under naturalistic light conditions prevailing in both summer and winter. In contrast to their earlier findings, they found that melatonin timing was unchanged in association with seasonal changes in ambient daylight in these subjects. The authors attributed the lack of seasonal effects to the fact that their subjects were exposed to the combined effects of ambient sunlight plus artificial light. Thus, rather than varying with day length, melatonin onset, offset and secretion duration remained essentially unchanged in the healthy participants, despite seasonal changes in day length. In a follow up study in which SAD patients were compared with healthy subjects, Wehr et al. (2001) found a seasonal change in melatonin secretion in the SAD patients, but not in healthy volunteers. Thus, under naturalistic conditions of ambient sunlight plus artificial light, melatonin secretion patterns were maintained in non-depressed subjects despite seasonal changes in day length, but were altered in association with seasonal changes in SAD patients. This suggests that with respect to melatonin and cortisol profiles, DP were more sensitive to changes in the seasonal light/dark cycle than HC. These outcomes are consistent with our findings in pregnant HC, whose mood and melatonin profiles did not vary with seasonal changes, whereas mood and melatonin timing were altered in association with seasonal changes in DP. Together, these results suggest that women who become depressed during pregnancy are more likely to present with depression during periods of longer nights/shorter days, presumably because their temporally associated symptoms are more intense then, and occur in association with significant alterations in melatonin timing. A plausible mechanism for these results could be that pregnant depressed women stay in-doors, and thus are exposed to less ambient sunlight, than their non-depressed counterparts, making their melatonin rhythms more like those of subjects in controlled studies of ambient light restriction (e.g., (Wehr et al., 1993). Alternately, women who experience depression during pregnancy may have less stable, more photo-sensitive, or more photo-responsive circadian rhythms that make them more vulnerable to environmental perturbations (e.g., changes in light/dark cycle) than non-depressed women. This pattern is analogous to seasonal changes in circadian waveform and circadian photic responses found in hamsters exposed to the long nights of winter-like photoperiods (Evans et al., 2012; Glickman et al., 2012). A recent study (Menegazzi et al., 2012) likewise showed that mutant Drosophila melanogaster whose circadian clocks were impaired responded more strongly to environmental changes than did wild type flies.
Although an impressive body of evidence (Kripke, 1983; Lewy, 2007; Lewy, 2009; Lewy et al., 2007; Lewy et al., 1987; Wehr et al., 1979; Wirz-Justice et al., 1995) shows that chronobiologic alterations are associated with both seasonal and non-seasonal depressions, not all studies have confirmed this association (Karadottir and Axelsson, 2001; Van Dongen et al., 1998; Youngstedt et al., 2005). Our results indicate that during seasonally longer nights, changes in melatonin and cortisol timing and quantity were associated with altered depressive symptom severity in pregnant women. However, the seasonal worsening of mood we found in pregnant DP occurred in conjunction with phase-advanced melatonin timing measures, while most studies of the chronobiology of winter depression report opposite effects -- i.e., phase-delayed melatonin timing (Emens et al., 2009; Lewy, 2012). These earlier findings provided the basis for the hypothesis, confirmed in several studies (Terman and Terman, 2005; Wirz-Justice, 2003) that by phase-advancing melatonin timing, exposure to early morning bright light provides the best antidepressant treatment for SAD, while evening bright light is ineffective or exacerbates depression symptoms (Lewy et al., 1998; Lewy et al., 1987; Terman and Terman, 2005; Wehr et al., 1979; Wirz-Justice, 2003). Nevertheless, consistent with the present findings, some studies have shown that increased symptom severity in some winter depressions may occur in conjunction with a phase-advance, rather than a phase-delay, in melatonin timing (Lewy et al., 2006; Lewy et al., 1987).
Furthermore, some studies report equal or superior antidepressant benefit from exposure to evening vs. morning light in non-seasonal depressions ((Wirz-Justice et al., 1993); see (Lam and Levitan, 2000) for review). A phase-advance model of mood disorders which proposes that circadian markers are advanced in some depressions, either in relation to clock time or relative to other circadian markers (e.g., the sleep-wake cycle), has been advanced previously (Emens et al., 2009; Kripke, 1983; Wehr and Goodwin, 1983; Wehr and Wirz-Justice, 1982; Wirz-Justice et al., 1995). The disparity between our results and those of some earlier studies suggests that with respect to seasonal and chronobiologic features, depression in pregnant women may be chronobiologically different from depressions occurring in non-pregnant individuals. Depending on the reproductive epic (menstrual, pregnant, postpartum or menopausal), either phase-advances or phase-delays in melatonin timing, as well as increases or decreases in melatonin amplitude, may occur in depressed women. For example, we found decreased melatonin amplitudes along with phase-advanced melatonin timing in association with premenstrual depression (Parry et al., 1990) and during pregnancy (current findings and (Parry et al., 2008a)), but increased melatonin amplitude without significant phase change in postpartum depression (Parry et al., 2008a), and an increased amplitude and phase-delayed melatonin offset in depression during the peri-menopausal period (Parry et al., 2008b).
Melatonin Quantity Effects
In contrast to postpartum depressed patients, in whom morning melatonin levels were elevated, we found a reduced melatonin AUC, primarily in the morning hours, in pregnant DP vs. HC (Parry et al., 2008a). In the present study synthesis AUC was significantly reduced in conjunction with longer vs. shorter darkness, but only in DP, not in HC. Thus, despite an abundant literature showing that increased darkness enhances melatonin duration, we found melatonin synthesis AUC was reduced in DP vs. HC under conditions of seasonally longer vs. shorter darkness. This finding suggests that pregnant DP respond anomalously to seasonal changes in darkness. Souetre et al. (1989) also found a reduced melatonin amplitude in depressed patients, and a significant negative correlation between HRSD scores and melatonin amplitude. Sandyk & Awerbuch (1993a) detected a reduced mean melatonin level and a phase advance in the melatonin secretion offset in multiple sclerosis patients with histories of affective illness. These authors (1993b) also found a lower mean melatonin level in psychiatric patients who had attempted suicide and/or expressed suicidal ideation. Kripke et al. (2007) found that increased light during the day was associated with increased melatonin secretion at night in post-menopausal women.
Cortisol Timing Effects
Like melatonin SynOff, cortisol acrophase was phase-advanced in DP during periods of increased darkness, and the melatonin SynOff and cortisol acrophase were positively correlated. Furthermore, the median split analyses showed a highly reliable phase-advance in cortisol acrophase of approximately 98 min (p = .001) under conditions of longer vs. shorter darkness in DP. That these alterations were absent in HC supports the interpretation that the significant phase-advances we observed in cortisol timing during pregnancy reflect primarily timing, not amplitude differences between DP and HC in their responses to seasonally increased darkness.
An early study (Lohrenz et al., 1969) described a phase-advance in cortisol secretion in depression. To our knowledge, ours is the first study to report a significant phase-advance in cortisol acrophase in pregnant DP, occurring in conjunction with seasonally longer nights and a phase-advance in melatonin timing. In a sample of depressed women who were not pregnant, Young et al. (2001) found no reliable difference in the timing of the cortisol maximum in DP vs. HC. Koenigsberg et al. (2004), however, found a phase-advance in the cortisol circadian rhythm in a group of 14 male and 8 female depressed patients. These authors interpreted their results as consistent with a circadian dysregulation hypothesis of depression. Our findings of advances in cortisol and melatonin timing are consistent with these findings and this hypothesis. In light of the disparate findings regarding the chronobiology of depression in different populations (see (Parry et al., 2006a; Parry et al., 2006b) – e.g., phase-delayed chronobiologic parameters in SAD, phase-advanced parameters in pregnant depression – a dysregulation hypothesis as applied to receptor sensitivity by Siever & Davis (1985), which proposes that either advances or delays in melatonin and cortisol timing may be associated with increased depression severity, seems more appropriate than a unitary, directional hypothesis.
Cortisol Quantity Effects
Although cortisol elevation in association with major depression is widely reported (Dinan and Scott, 2005; Rubin et al., 2001), a recent meta-analysis (Knorr et al., 2010) found no reliable differences between HC vs. DP in the case of salivary cortisol. A central question we sought to address was whether serum cortisol quantity was elevated in association with antepartum depression. We found cortisol mesor was highly correlated with gestation week in HC as well as DP, but cortisol amplitude was not. We also detected no significant differences in HC vs. DP in cortisol mesor or amplitude. King, et al. (2010) compared a group of pregnant women experiencing medical problems during pregnancy with healthy controls. Although those women with medical disorders were significantly more anxious and depressed than controls, there were no significant differences between the groups in cortisol levels, as we found for HC vs. DP. Suzuki et al. (1993) reported a trend towards decreased amplitude in the cortisol rhythm during healthy pregnancy, in association with a suppression of the early morning cortisol rise.
Published studies of cortisol during pregnancy are rare and results are inconsistent. Salacz et al. (2012) found depression and anxiety were associated with subjective distress in pregnant women, but not with elevated plasma cortisol. In contrast, Field et al. (2006a) reported a high degree of inter-correlation among cortisol levels, depression, and problems during pregnancy such as back pain, leg pain and sleep disturbances during the third trimester. Focusing on the second trimester, O’Keane et al. (2010) found elevated cortisol in the evening – i.e., at the time when cortisol levels are normally low – in depressed vs. normal control pregnant women. We found no significant difference in DP vs. HC in the evening cortisol nadir, and in preliminary tests (data not shown) we found no significant group differences at any time intervals between 18:00 and 11:00 h of the following morning; therefore we opted to focus on the cortisol mesor as an index of total cortisol during the test interval. The discrepancy between our results and those of O’Keane et al. (2010), and of Field et al. (2006a) could relate to the differences in sampling times, and the fact that our data were collected across all three trimesters, rather than only the second or third. In depressed women who were not pregnant, Young, et al. (2001) found only a non-significant trend toward elevated cortisol, and a clear cortisol elevation in only 24% of their depressed patients. Cortisol elevation may be present in only c. 25–30% of patients with depression (Young et al., 2001).
Notably, we found that although cortisol was elevated during pregnancy to comparable degrees in both DP and HC, cortisol mesor was positively correlated with HRSD score in DP, but not HC (Fig. 6). An important implication of this finding is that the ubiquitous cortisol elevation occurring during pregnancy (Field et al., 2006a; Field et al., 2006b; Mastorakos and Ilias, 2003) is not uniformly associated with increased depression severity. For some pregnant women, cortisol elevation is dissociated from untoward mood consequences; for others, it is a biomarker of depression severity.
Melancholic vs. Atypical Symptoms
The fact that increased cortisol mesor in DP was associated with an elevated HRSD score, but not the SIGH-SAD atypical depression score supports the hypothesis that cortisol elevation may be a correlate of melancholic, rather than an atypical depression (Kammerer et al., 2006; Young et al., 2001). Lamers et al. (2012) compared cortisol AUCs of 111 chronically depressed people diagnosed with melancholic depression vs. 122 diagnosed with atypical depression. Cortisol was significantly higher in subjects with melancholic depression compared to non-depressed controls, or patients with atypical depression. The authors interpreted their findings as evidence of increased HPA-axis activity in melancholic vs. atypical depression. A recent review (Harald and Gordon, 2012) suggests that cortisol may be reduced in atypical depression, relative to control or melancholic depression. Some defining clinical features of SAD (hypersomnia, hyperphagia, irritability) are thought to be more common in atypical depression than in melancholia (Howland, 2009; Wehr et al., 1991).
Conclusion
Our findings indicate that antepartum depressions share some features with winter depressions, e.g., greater depressed mood during periods of seasonally longer darkness in association with altered melatonin and/or cortisol timing and quantity. Notably, Wehr (1998) found that non-depressed urban women, on average, were more likely than men to show alterations in melatonin duration during winter months; roughly 1/3 of women vs. 1/8 of men evidenced changes in winter. That women with antepartum major depression may be more sensitive than non-depressed women to seasonal variations in melatonin and cortisol chronobiology suggests there may be two groups of pregnant women: (a) those who respond to seasonally reduced daylight with phase-advances in melatonin and cortisol timing, diminished melatonin quantity and increased symptoms of melancholic depression vs. (b) those whose melatonin and cortisol timing and quantity remain relatively invariant with seasonally increased darkness, and in whom depression severity does not increase. Kripke (1983) proposed that a biological predisposition toward internal desynchronization of circadian rhythms could sensitize some individuals to depression. Evans et al. (2012) recently showed that individual differences in susceptibility to light-modulated alterations in circadian wave form occur in the Siberian hamster.
Finally, our data imply that seasonality contributes to existing, especially melancholic, depression severity, rather than being a primary etiologic factor in antenatal depression. Factors like genetic vulnerability, personal and family history of depression, anxiety, chronotype, and social zeitgebers and must also be recognized as potential determinants of depressive mood.
Implications for Treatment and Research
These findings suggest important implications for our understanding of the relationship of chronobiological variables to mood, and to potential antidepressant chronotherapies during pregnancy. Circadian rhythm dysregulation as reported here may contribute to antepartum depression severity, and interventions that normalize circadian rhythms might relieve depressive symptoms. Although significant benefits relative to placebo light have not been obtained in all studies (Corral et al., 2007b; Even et al., 2008; Lanfumey et al., 2013; Parry et al., 1993), critically-timed exposure to light (“bright light therapy”) has been shown to ameliorate some non-seasonal depressions, possibly by synchronizing circadian rhythms (Dallaspezia and Benedetti, 2011; Dallaspezia et al., 2012; Lieverse et al., 2011; Wirz-Justice et al., 2011; Wirz-Justice et al., 2005; Wirz-Justice et al., 2004). Light treatment represents a potentially viable option for women who do not wish to use pharmacologic means to alter mood during pregnancy (Yonkers et al., 2009). In cases where depressive symptoms are associated with phase-advances in melatonin and cortisol timing, such as might occur with antepartum depression during seasonally longer nights, evening bright light would be the indicated treatment as it would be expected to phase-delay, and thereby normalize, circadian parameters. In a recent study in non-seasonally depressed pregnant women, however, Wirz-Justice et al. (2011) found that five weeks of bright morning light decreased depression symptoms significantly better than a dim light placebo. Chronobiologic correlates of the mood improvement were not evaluated. Notably, Oren et al. (2002) found reduced antepartum depression in women exposed to morning light, as did Epperson et al. (2004), who found a significant antidepressant effect in pregnant women in conjunction with a phase-advance in melatonin timing after 10 weeks of bright morning light.
LIMITATIONS
Our use of small sample sizes in this study opens the possibility that the study may have been underpowered to detect some differences, e.g., those associated with longer vs. shorter darkness in the HC group (Table 3). Indeed, power analyses showed effect sizes for some melatonin comparisons of approximately 0.50 or greater in the HC group. Nevertheless, these differences were in the direction opposite to those found in the DP; i.e., while DP showed significant phase advances in DLMO and synthesis offset under conditions of increased darkness, the HC exhibited (non-significant) differences in the direction of phase delays. Thus, all other things being equal, increasing the HC sample sizes could only be expected to further increase the differences between HC and DP in their responses to seasonally-mediated longer vs. shorter darkness. However, replication with larger samples is necessary to insure the reliability of these results. Furthermore, a more complete design which included non-pregnant healthy and non-pregnant depressed women would have been preferable, as this would have enhanced the generalizability of the results.
The cross-sectional nature of the study is a limitation, since subjects were accepted for study only during seasons in which they volunteered, rather than being assigned for study equally across all seasons. Additionally, since all participants resided in San Diego County, 32° 45′ north latitude, they were exposed to only modest fluctuations (c. 4.5 h) in daylight from summer to winter. Different outcomes could occur in locations with greater seasonal variations in daylight between winter and summer.
Finally, the HRSD was not designed for use with healthy subjects, but rather was designed to be used as an index of symptom severity in individuals already diagnosed with depression.
Table 2.
Pearson Correlations: Hours of Darkness (Sunset – Sunrise) with Dim Light Melatonin Onset (DLMO), Synthesis Offset (SynOff), Dim Light Melatonin Offset/Return to Baseline (DLMOff), Area under the melatonin curve (AUC) and Cortisol Acrophase, Mesor and Amplitude in Pregnant Healthy Control (HC; n = 12) and Depressed Patients (DP; n = 17). P-values for HC and DP correlations appear in parentheses, with statistically significant correlations (p < .05) highlighted in boldface.
| Melatonin | Cortisol | |||||||
|---|---|---|---|---|---|---|---|---|
| DLMO | SynOff | DLMOff | Synthesis Duration | Synthesis AUC | Acrophase | Mesor | Amplitude | |
|
HC n = 12 |
.183 (ns) | .226 (ns) | .114 (ns) | −.016 (ns) | .429 (ns) | −.122 (ns) | −.072 (ns) | −.216 (ns) |
| DP n = 17 |
.100 (ns) | −.601 (.011) | −.394 (ns) | −.489 (.047) | −.480 (.051) | −.492 (.045) | .228 (ns) | .277 (ns) |
Footnotes
DECLARATION OF INTEREST: The authors report no conflicts of interest.
References
- Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28:179–96. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition - Text Revision (DSM-IV-TR) 4. American Psychiatric Association; Washington, D. C: 2000. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–9. [PMC free article] [PubMed] [Google Scholar]
- Bowen A, Bowen R, Butt P, Rahman K, Muhajarine N. Patterns of depression and treatment in pregnant and postpartum women. Can J Psychiatry. 2012;57:161–7. doi: 10.1177/070674371205700305. [DOI] [PubMed] [Google Scholar]
- Brzezinski A, Lynch HJ, Seibel MM, Deng MH, Nader TM, Wurtman RJ. The circadian rhythm of plasma melatonin during the normal menstrual cycle and in amenorrheic women. J Clin Endocrinol Metab. 1988;66:891–5. doi: 10.1210/jcem-66-5-891. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Molecular clock genes in man and lower animals: possible implications for circadian abnormalities in depression. Neuropsychopharmacology. 2000;22:335–45. doi: 10.1016/S0893-133X(99)00145-1. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Martin FI, Davies B. Resistance to suppression by dexamethasone of plasma 11-O.H.C.S. levels in severe depressive illness. Br Med J. 1968;3:285–7. doi: 10.1136/bmj.3.5613.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FM, Pinheiro RT, Silva RA, de Quevedo LA, Souza LD, Castelli RD, Matos MB, Pinheiro KA. Major depressive disorder during teenage pregnancy: socio-demographic, obstetric and psychosocial correlates. Rev Bras Psiquiatr. 2013;35:51–6. doi: 10.1016/j.rbp.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Wang B, Nonacs R, Viguera AC, Lemon EL, Freeman MP. Treatment of mood disorders during pregnancy and postpartum. Psychiatr Clin North Am. 2010;33:273–93. doi: 10.1016/j.psc.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Colvin L, Slack-Smith L, Stanley FJ, Bower C. Are women with major depression in pregnancy identifiable in population health data? BMC Pregnancy Childbirth. 2013;13:63. doi: 10.1186/1471-2393-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral M, Wardrop A, Zhang HB. Seasonality of symptoms in women with postpartum depression. Arch Womens Ment Health. 2007a;10:9–13. doi: 10.1007/s00737-006-0160-x. [DOI] [PubMed] [Google Scholar]
- Corral M, Wardrop AA, Zhang H, Grewal AK, Patton S. Morning light therapy for postpartum depression. Arch Womens Ment Health. 2007b;10:221–4. doi: 10.1007/s00737-007-0200-1. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Crowley SK, Youngstedt SD. Efficacy of light therapy for perinatal depression: a review. J Physiol Anthropol. 2012;31:15. doi: 10.1186/1880-6805-31-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaspezia S, Benedetti F. Chronobiological therapy for mood disorders. Expert Rev Neurother. 2011;11:961–70. doi: 10.1586/ern.11.61. [DOI] [PubMed] [Google Scholar]
- Dallaspezia S, Benedetti F, Colombo C, Barbini B, Fulgosi MC, Gavinelli C, Smeraldi E. Optimized light therapy for non-seasonal major depressive disorder: Effects of timing and season. J Affect Disord. 2012 doi: 10.1016/j.jad.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Scott LV. Anatomy of melancholia: focus on hypothalamic-pituitary-adrenal axis overactivity and the role of vasopressin. J Anat. 2005;207:259–64. doi: 10.1111/j.1469-7580.2005.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Jaddoe VW, Hudziak JJ, Roza SJ, Steegers EA, Hofman A, Verhulst FC, White TJ, Stricker BH, Tiemeier H. Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Arch Gen Psychiatry. 2012;69:706–14. doi: 10.1001/archgenpsychiatry.2011.2333. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168:259–61. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Terman M, Terman JS, Hanusa BH, Oren DA, Peindl KS, Wisner KL. Randomized clinical trial of bright light therapy for antepartum depression: preliminary findings. J Clin Psychiatry. 2004;65:421–5. doi: 10.4088/jcp.v65n0319. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Individual differences in circadian waveform of Siberian hamsters under multiple lighting conditions. J Biol Rhythms. 2012;27:410–9. doi: 10.1177/0748730412455915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even C, Schroder CM, Friedman S, Rouillon F. Efficacy of light therapy in nonseasonal depression: a systematic review. J Affect Disord. 2008;108:11–23. doi: 10.1016/j.jad.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Field T, Hernandez-Reif M, Diego M, Figueiredo B, Schanberg S, Kuhn C. Prenatal cortisol, prematurity and low birthweight. Infant Behav Dev. 2006a;29:268–75. doi: 10.1016/j.infbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Stability of mood states and biochemistry across pregnancy. Infant Behav Dev. 2006b;29:262–7. doi: 10.1016/j.infbeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- research version. Biometerics Research Dept., New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Glickman G, Webb IC, Elliott JA, Baltazar RM, Reale ME, Lehman MN, Gorman MR. Photic sensitivity for circadian response to light varies with photoperiod. J Biol Rhythms. 2012;27:308–18. doi: 10.1177/0748730412450826. [DOI] [PubMed] [Google Scholar]
- Golder SA, Macy MW. Diurnal and seasonal mood vary with work, sleep, and daylength across diverse cultures. Science. 2011;333:1878–81. doi: 10.1126/science.1202775. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–55. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- Harald B, Gordon P. Meta-review of depressive subtyping models. J Affect Disord. 2012;139:126–40. doi: 10.1016/j.jad.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Hewitt C, Gilbody S, Brealey S, Paulden M, Palmer S, Mann R, Green J, Morrell J, Barkham M, Light K, Richards D. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13:1–145. 147–230. doi: 10.3310/hta13360. [DOI] [PubMed] [Google Scholar]
- Hiltunen P, Jokelainen J, Ebeling H, Szajnberg N, Moilanen I. Seasonal variation in postnatal depression. J Affect Disord. 2004;78:111–8. doi: 10.1016/s0165-0327(02)00239-2. [DOI] [PubMed] [Google Scholar]
- Howland RH. An overview of seasonal affective disorder and its treatment options. Phys Sportsmed. 2009;37:104–15. doi: 10.3810/psm.2009.12.1748. [DOI] [PubMed] [Google Scholar]
- Jewell JS, Dunn AL, Bondy J, Leiferman J. Prevalence of self-reported postpartum depression specific to season and latitude of birth: evaluating the PRAMS data. Matern Child Health J. 2010;14:261–7. doi: 10.1007/s10995-009-0498-6. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Nordstrom P. HPA axis hyperactivity and attempted suicide in young adult mood disorder inpatients. J Affect Disord. 2009;116:117–20. doi: 10.1016/j.jad.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Kammerer M, Taylor A, Glover V. The HPA axis and perinatal depression: a hypothesis. Arch Womens Ment Health. 2006;9:187–96. doi: 10.1007/s00737-006-0131-2. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Axelsson J. Melatonin secretion in SAD patients and healthy subjects matched with respect to age and sex. Int J Circumpolar Health. 2001;60:548–51. [PubMed] [Google Scholar]
- King NM, Chambers J, O’Donnell K, Jayaweera SR, Williamson C, Glover VA. Anxiety, depression and saliva cortisol in women with a medical disorder during pregnancy. Arch Womens Ment Health. 2010;13:339–45. doi: 10.1007/s00737-009-0139-5. [DOI] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35:1275–86. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Teicher MH, Mitropoulou V, Navalta C, New AS, Trestman R, Siever LJ. 24-h Monitoring of plasma norepinephrine, MHPG, cortisol, growth hormone and prolactin in depression. J Psychiatr Res. 2004;38:503–11. doi: 10.1016/j.jpsychires.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kripke DF. Phase-advance theories for affective illnesses. In: Wehr TA, Goodwin FK, editors. Circadian Rhythms in Psychiatry. Pacific Grove, CA: Boxwood Press; 1983. pp. 41–70. [Google Scholar]
- Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Levitan RD. Pathophysiology of seasonal affective disorder: a review. J Psychiatry Neurosci. 2000;25:469–80. [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Mongeau R, Hamon M. Biological rhythms and melatonin in mood disorders and their treatments. Pharmacol Ther. 2013;138:176–84. doi: 10.1016/j.pharmthera.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Levitt AJ, Boyle MH, Joffe RT, Baumal Z. Estimated prevalence of the seasonal subtype of major depression in a Canadian community sample. Can J Psychiatry. 2000;45:650–4. doi: 10.1177/070674370004500708. [DOI] [PubMed] [Google Scholar]
- Lewy AJ. Melatonin and human chronobiology. Cold Spring Harb Symp Quant Biol. 2007;72:623–36. doi: 10.1101/sqb.2007.72.055. [DOI] [PubMed] [Google Scholar]
- Lewy AJ. Circadian misalignment in mood disturbances. Curr Psychiatry Rep. 2009;11:459–65. doi: 10.1007/s11920-009-0070-5. [DOI] [PubMed] [Google Scholar]
- Lewy AJ. Depressive disorders may more commonly be related to circadian phase delays rather than advances: time will tell. Sleep Med. 2012;11:117–8. doi: 10.1016/j.sleep.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Cutler NL, Sack RL, Ahmed S, Thomas KH, Blood ML, Jackson JM. Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998;55:890–6. doi: 10.1001/archpsyc.55.10.890. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–9. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Rough JN, Songer JB, Mishra N, Yuhas K, Emens JS. The phase shift hypothesis for the circadian component of winter depression. Dialogues Clin Neurosci. 2007;9:291–300. doi: 10.31887/DCNS.2007.9.3/alewy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235:352–4. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, Hoogendijk WJ. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2011;68:61–70. doi: 10.1001/archgenpsychiatry.2010.183. [DOI] [PubMed] [Google Scholar]
- Lohrenz FN, Fullerton DT, Wenzel FJ, Chosy JJ, Dickson KB. Circadian rhythm of adrenal cortical activity in depression. Behav Neuropsychiatry. 1969;1:10–3. [PubMed] [Google Scholar]
- Makara-Studzinska M, Morylowska-Topolska J, Sygit K, Sygit M, Gozdziewska M. Socio-demographical and psychosocial determinants of anxiety symptoms in a population of pregnant women in the regions of central and eastern Poland. Ann Agric Environ Med. 2013;20:195–202. [PubMed] [Google Scholar]
- Manikkam L, Burns JK. Antenatal depression and its risk factors: an urban prevalence study in KwaZulu-Natal. S Afr Med J. 2012;102:940–4. doi: 10.7196/samj.6009. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–49. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- Mauri M, Oppo A, Borri C, Banti S. SUICIDALITY in the perinatal period: comparison of two self-report instruments. Results from PND-ReScU. Arch Womens Ment Health. 2012;15:39–47. doi: 10.1007/s00737-011-0246-y. [DOI] [PubMed] [Google Scholar]
- Menegazzi P, Yoshii T, Helfrich-Forster C. Laboratory versus Nature: The Two Sides of the Drosophila Circadian Clock. J Biol Rhythms. 2012;27:433–42. doi: 10.1177/0748730412463181. [DOI] [PubMed] [Google Scholar]
- O’Keane V, Lightman S, Marsh M, Pawlby S, Papadopoulos AS, Taylor A, Moore R, Patrick K. Increased pituitary-adrenal activation and shortened gestation in a sample of depressed pregnant women: a pilot study. J Affect Disord. 2010;130:300–5. doi: 10.1016/j.jad.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Oren DA, Wisner KL, Spinelli M, Epperson CN, Peindl KS, Terman JS, Terman M. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry. 2002;159:666–9. doi: 10.1176/appi.ajp.159.4.666. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS., Jr Epidemiological Aspects of Parapartum Mental Illness. Br J Prev Soc Med. 1964;18:189–95. doi: 10.1136/jech.18.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Berga SL, Kripke DF, Klauber MR, Laughlin GA, Yen SS, Gillin JC. Altered waveform of plasma nocturnal melatonin secretion in premenstrual depression. Arch Gen Psychiatry. 1990;47:1139–46. doi: 10.1001/archpsyc.1990.01810240059010. [DOI] [PubMed] [Google Scholar]
- Parry BL, Mahan AM, Mostofi N, Klauber MR, Lew GS, Gillin JC. Light therapy of late luteal phase dysphoric disorder: an extended study. Am J Psychiatry. 1993;150:1417–9. doi: 10.1176/ajp.150.9.1417. [DOI] [PubMed] [Google Scholar]
- Parry BL, Martinez LF, Maurer EL, Lopez AM, Sorenson DL, Meliska CJ. Sleep rhythms and women’s mood. Part I: Menstrual cycle, pregnancy and postpartum. Sleep Med Rev. 2006a;10:129–144. doi: 10.1016/j.smrv.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Parry BL, Martinez LF, Maurer EL, Lopez AM, Sorenson DL, Meliska CJ. Sleep, rhythms and women’s mood. Part II: Menopause. Sleep Med Rev. 2006b;10:197–208. doi: 10.1016/j.smrv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Parry BL, Maurer EL. Light treatment of mood disorders. Dialogues in Clinical Neuroscience. 2003;5:353–365. doi: 10.31887/DCNS.2003.5.4/bparry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Meliska CJ, Martinez LF, Maurer EL, Lopez AM, Sorenson DL. Neuroendocrine abnormalities in women with depression linked to the reproductive cycle. In: Sibley D, Hanin I, Kuhar M, Skolnick P, editors. The Handbook of Contemporary Neuropharmacology. New York: John Wiley and Sons; 2007. pp. 843–857. [Google Scholar]
- Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, Elliott JA, Hauger RL, Kripke DF. Plasma melatonin circadian rhythm disturbances during pregnancy and postpartum in depressed women and women with personal or family histories of depression. Am J Psychiatry. 2008a;165:1551–8. doi: 10.1176/appi.ajp.2008.08050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, Hauger RL, Elliott JA. Increased melatonin and delayed offset in menopausal depression: role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass index. J Clin Endocrinol Metab. 2008b;93:54–60. doi: 10.1210/jc.2006-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitkin FM. Depression With Atypical Features: Diagnostic Validity, Prevalence, and Treatment. Prim Care Companion J Clin Psychiatry. 2002;4:94–99. doi: 10.4088/pcc.v04n0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen LN, Targum SD, Terman M, Bryant MJ, Hoffman H, Kasper SF, Hamovit JR, Docherty JP, Welch B, Rosenthal NE. Prevalence of seasonal affective disorder at four latitudes. Psychiatry Res. 1990;31:131–44. doi: 10.1016/0165-1781(90)90116-m. [DOI] [PubMed] [Google Scholar]
- Rosenquist SE. When the bough breaks: rethinking treatment strategies for perinatal depression. Am J Clin Hypn. 2013;55:291–323. doi: 10.1080/00029157.2012.723284. [DOI] [PubMed] [Google Scholar]
- Rubin R, Dinan TG, Scott LV. The neuroendocrinology of affective disorders. In: Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, Moss RL, Rubin RT, editors. Hormones, Brain and Behavior. New York: Academic Press; 2001. pp. 467–514. [Google Scholar]
- Salacz P, Csukly G, Haller J, Valent S. Association between subjective feelings of distress, plasma cortisol, anxiety, and depression in pregnant women. Eur J Obstet Gynecol Reprod Biol. 2012;165:225–30. doi: 10.1016/j.ejogrb.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Sandyk R, Awerbuch GI. Nocturnal melatonin secretion in multiple sclerosis patients with affective disorders. Int J Neurosci. 1993a;68:227–40. doi: 10.3109/00207459308994278. [DOI] [PubMed] [Google Scholar]
- Sandyk R, Awerbuch GI. Nocturnal melatonin secretion in suicidal patients with multiple sclerosis. Int J Neurosci. 1993b;71:173–82. doi: 10.3109/00207459309000602. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. Overview: toward a dysregulation hypothesis of depression. Am J Psychiatry. 1985;142:1017–31. doi: 10.1176/ajp.142.9.1017. [DOI] [PubMed] [Google Scholar]
- Souetre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, Ardisson JL, Darcourt G. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28:263–78. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Dennerstein L, Greenwood KM, Armstrong SM, Sano T, Satohisa E. Melatonin and hormonal changes in disturbed sleep during late pregnancy. J Pineal Res. 1993;15:191–8. doi: 10.1111/j.1600-079x.1993.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Sylven SM, Papadopoulos FC, Olovsson M, Ekselius L, Poromaa IS, Skalkidou A. Seasonality patterns in postpartum depression. Am J Obstet Gynecol. 2011;204:413 e1–6. doi: 10.1016/j.ajog.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–63. doi: 10.1017/s1092852900019611. quiz 672. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS. Circadian rhythm phase advance with dawn simulation treatment for winter depression. J Biol Rhythms. 2010;25:297–301. doi: 10.1177/0748730410374000. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Kerkhof GA, Souverijn JH. Absence of seasonal variation in the phase of the endogenous circadian rhythm in humans. Chronobiol Int. 1998;15:623–32. doi: 10.3109/07420529808993198. [DOI] [PubMed] [Google Scholar]
- Wehr TA. Effect of seasonal changes in daylength on human neuroendocrine function. Horm Res. 1998;49:118–24. doi: 10.1159/000023157. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Duncan WC, Jr, Sher L, Aeschbach D, Schwartz PJ, Turner EH, Postolache TT, Rosenthal NE. A circadian signal of change of season in patients with seasonal affective disorder. Arch Gen Psychiatry. 2001;58:1108–14. doi: 10.1001/archpsyc.58.12.1108. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Giesen HA, Moul DE, Turner EH, Schwartz PJ. Suppression of men’s responses to seasonal changes in day length by modern artificial lighting. Am J Physiol. 1995;269:R173–8. doi: 10.1152/ajpregu.1995.269.1.R173. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Giesen HA, Schulz PM, Anderson JL, Joseph-Vanderpool JR, Kelly K, Kasper S, Rosenthal NE. Contrasts between symptoms of summer depression and winter depression. J Affect Disord. 1991;23:173–83. doi: 10.1016/0165-0327(91)90098-d. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Goodwin FK. Biological rhythms in manic depressive illness. In: Wehr TA, Goodwin FK, editors. Circadian Rhythms in Psychiatry. Pacific Grove, CA: Boxwood Press; 1983. pp. 129–201. [Google Scholar]
- Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993;265:R846–57. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A. Circadian rhythm mechanisms in affective illness and in antidepressant drug action. Pharmacopsychiatria. 1982;15:31–9. doi: 10.1055/s-2007-1019506. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A, Goodwin FK, Duncan W, Gillin JC. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206:710–3. doi: 10.1126/science.227056. [DOI] [PubMed] [Google Scholar]
- Williams JB, Link MJ, Rosenthal NE, Amira L, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD) New York Psychiatric Institute; New York: 1994. revised edition. [Google Scholar]
- Wirz-Justice A. Chronobiology and mood disorders. Dialogues Clin Neurosci. 2003;5:315–325. doi: 10.31887/DCNS.2003.5.4/awirzjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, Bader A, Frisch U, Stieglitz RD, Alder J, Bitzer J, Hosli I, Jazbec S, Benedetti F, Terman M, Wisner KL, Riecher-Rossler A. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry. 2011;72:986–93. doi: 10.4088/JCP.10m06188blu. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Berger M, Lam RW, Martiny K, Terman M, Wu JC. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35:939–44. doi: 10.1017/s003329170500437x. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Graw P, Krauchi K, Gisin B, Jochum A, Arendt J, Fisch HU, Buddeberg C, Poldinger W. Light therapy in seasonal affective disorder is independent of time of day or circadian phase. Arch Gen Psychiatry. 1993;50:929–37. doi: 10.1001/archpsyc.1993.01820240013001. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Krauchi K, Brunner DP, Graw P, Haug JH, Leonhardt G. Circadian rhythms and sleep regulation in seasonal affective disorder. Acta Neuropsychiatrica. 1995;7:41–43. doi: 10.1017/S0924270800037522. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Terman M, Oren DA, Goodwin FK, Kripke DF, Whybrow PC, Wisner KL, Wu JC, Lam RW, Berger M, Danilenko KV, Kasper S, Smeraldi E, Takahashi K, Thompson C, van den Hoofdakker RH. Brightening depression. Science. 2004;303:467–9. doi: 10.1126/science.303.5657.467c. [DOI] [PubMed] [Google Scholar]
- Yang SN, Shen LJ, Ping T, Wang YC, Chien CW. The delivery mode and seasonal variation are associated with the development of postpartum depression. J Affect Disord. 2011;132:158–64. doi: 10.1016/j.jad.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31:403–13. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E, Korszun A. Sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15:23–8. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- Young EA, Carlson NE, Brown MB. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25:267–76. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, Kripke DF, Elliott JA, Rex KM. Circadian phase-shifting effects of a laboratory environment: a clinical trial with bright and dim light. J Circadian Rhythms. 2005;3:11. doi: 10.1186/1740-3391-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]