Abstract
The expansion of immunosuppressive cells represents a cardinal strategy deployed by tumors to escape from detection and elimination by the immune system. Regulatory T lymphocytes (Treg) and myeloid-derived suppressor cells (MDSC), major components of these inhibitory cellular networks, have drawn intense scrutiny in recent years. In cancer patients and in tumor animal models, these suppressor cells accumulate in the tumor microenvironment, secondary lymphoid tissues and in the blood. Equipped with the ability to suppress innate and adaptive anticancer immunity, these cells also foster disease development by promoting tumor neo-angiogenesis and enhancing cancer metastasis. They therefore represent major impediments for anticancer therapies, particularly for immune-based interventions. Recent work has provided evidence that, beyond their direct cytotoxic or cytostatic effects on cancer cells, several conventional chemotherapeutic drugs and agents used in targeted therapies can promote the elimination or inactivation of suppressive Treg or MDSC, resulting in enhanced antitumor immunity. We analyze findings pertinent to this concept, discuss the possible molecular bases underlying the selective targeting of these immunosuppressive cells by antineoplastic agents and consider current challenges and future prospects related to the integration of these molecules into more efficient anticancer chemoimmunotherapeutic strategies.
Keywords: Chemotherapy, Molecular targeted therapy, Immunosuppressive cells, Myeloid-derived suppressor cells, Regulatory T cells, Chemoimmunotherapy
The immune system, cancer and chemotherapeutic agents: “The Good, the Bad and the Ugly”?
For decades, the field of cancer biology has almost entirely centered upon malignant cells themselves, with limited consideration (if any) given to non-neoplastic cellular elements of the tumor microenvironment. However, compelling evidence has emerged that this network of non-transformed cells (mainly endothelial, stromal or immune cells) considerably influences tumor development. Ignored for many years in oncology, the complex interactions between cancer cells and immune cells have more recently been subjected to intensive scrutiny. It has become clear that the immune system is endowed with the paradoxical ability to exert both tumor-suppressing and tumor-promoting effects, well-described in the seminal concept of cancer immunoediting (1). The demonstration that immune–mediated mechanisms can lead to the specific recognition and killing of tumor cells has opened the door to the field of cancer immunotherapy. However, it has also become evident that developing tumors commonly avoid immune destruction by inducing an immunosuppressive environment permissive to the emergence of immune-resistant cancer cell variants. As a result, in cancer-bearing hosts, immune cells are profoundly altered phenotypically and functionally and are therefore incapable of orchestrating efficient antitumoral immune responses (2, 3). The recognition of tumor escape from immune elimination as an emerging additional hallmark of cancer has constituted one of the major paradigm shift in the field (1–3). Multiple mechanisms of cancer immune evasion and immunoinhibitory pathways have been identified (3). In the context of the current discussion, it has been well-established that tumors can promote immunosuppressive cells, primarily regulatory T lymphocytes (Treg), myeloid-derived suppressor cells (MDSC), alternatively activated macrophages (M2) or immature/tolerogenic dendritic cells (DC). These cells inhibit anticancer immunity and significantly compromise the efficacy of immune-based therapies (3–5). They also play a role in tumor angiogenesis, cancer cell survival, proliferation and metastatic potential. Their elimination and/or inactivation has become a major therapeutic objective to alter the immunosuppressive tumor microenvironment and uncover anticancer immunity.
As initial research efforts almost entirely focused on decrypting the biological and genetic characteristics of malignant cells, it is not surprising that most anticancer therapies were originally developed based on the exclusive targeting of tumor cells. In this perspective, conventional chemotherapeutic drugs were selected for their direct cytotoxic effects against highly proliferative cancer cells. These antineoplastic molecules, such as anthracyclines, DNA-alkylating agents, or anti-microtubes compounds, are however associated with significant toxicities and their efficacy is limited by the emergence of drug-resistant tumor cell clones (6). With the growing understanding of cancer cell biology at the molecular level, more specific antineoplastic agents were developed and referred to as targeted therapeutic agents (6, 7). Typically, molecular targeted therapies are designed to inhibit oncogenic pathways essential for the proliferation and survival of tumor cells. This approach has sparked considerable hope and has led to the development of many small-molecule inhibitors, with imatinib mesylate, a BCR-ABL tyrosine kinase inhibitor, as an archetype (6). The therapeutic efficacy of these molecules as single agents is however limited by the acquisition of secondary mutations leading to drug resistant tumor clones and patient relapse.
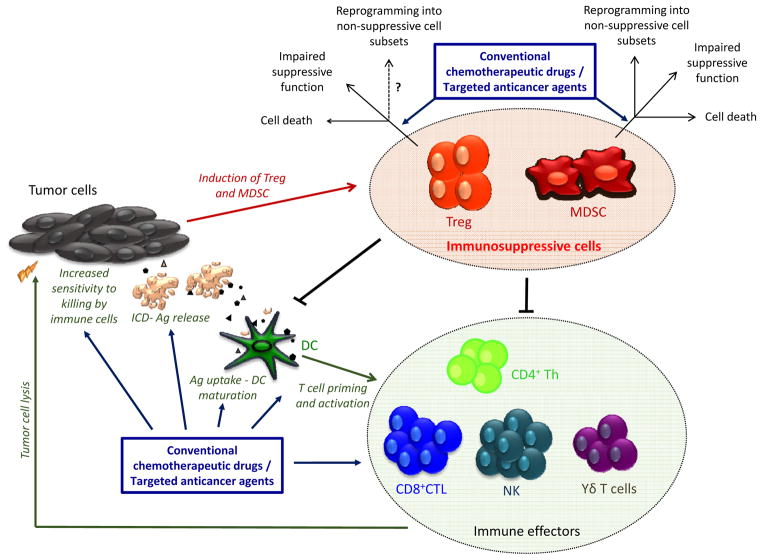
Recent studies have lent support to the notion that the antitumoral efficacy of many chemotherapeutic drugs or targeted agents may be partially or substantially mediated by immune-dependent mechanisms (6). For instance, the promotion of anti-cancer immune responses by anthracyclines has been related to the induction of an immunogenic type of tumor cell death associated with immune-activating “danger” signals. This fosters the antigen processing and presenting function of DC (responsible for the initiation and regulation of immune responses), leading to enhanced priming and activation of tumor-specific T lymphocytes. Several anticancer agents (for instance, platinum-based molecules, alkylating agents, anthracyclines or nucleoside analogues) are also capable of enhancing the proliferation and function of antitumoral cytotoxic T lymphocytes (CTL), natural killer (NK) and T helper (Th) cells (6). Additionally, drugs such as doxorubicin or cisplatin may sensitize tumor cells to CTL-mediated killing. Cisplatin has also recently been shown to promote the accumulation of CD11c+ DC in the tumor beds, which after intratumoral delivery of tumor antigens are capable to induce tumor-specific CTL (8). The aforementioned immunomodulatory effects of antineoplastic agents have been extensively reviewed elsewhere (6). Evidence has emerged that these molecules may also enhance anticancer immunity by blocking immunoinhibitory cellular networks (Figure 1). In the current review, we examine this concept, focusing on two major immunosuppressive cell populations, Treg and MDSC. The possible molecular mechanisms underlying the selective targeting of these suppressive cells and the prospect of exploiting the multifaceted properties of anticancer agents in more efficient chemoimmunotherapeutic strategies is discussed.
Figure 1. Elimination, inactivation or reprograming of tumor-induced Treg and MDSC by conventional chemotherapeutic drugs or targeted therapeutic agents.
Treg and MDSC critically contribute to the immunosuppressive environment associated with developing cancers. These cells blunt antitumor immunity by suppressing effector CD4+ Th, CD8+ CTL, NK, macrophages or dendritic cells. A selective list of conventional chemotherapeutic drugs or targeted therapeutic molecules are endowed with the ability to promote antitumor immunity and foster the efficacy of immunotherapeutic interventions by enhancing the function of immune effector cells or by reversing immunosuppression induced by Treg and MDSC. Antineoplastic agents can selectively trigger death of suppressive cells, inhibit their function or foster their differentiation into proinflammatory subsets devoid of inhibitory properties. Ag, Tumor-derived antigens; CTL, Cytotoxic T Lymphocytes; DC, Dendritic Cells; ICD, Immunogenic Cell Death; Th, T helper lymphocytes.
Targeting Treg and MDSC using antineoplastic agents
Treg elimination or inactivation
CD4+CD25+ Treg represent a subpopulation of T cells characterized by the expression of FoxP3, a forkhead/winged helix transcription factor essential for their development and function. Treg control immune responses by suppressing conventional effector T lymphocytes, NK, DC or macrophages through different mechanisms (9). Treg are produced during T cell development in the thymus or are generated in the periphery from naïve CD4+ T lymphocytes. Compelling studies in mice and human have demonstrated that many cancers can induce the proliferation of Treg and/or promote their generation from naïve T cells, resulting in the accumulation of these cells in the tumor beds and in the periphery (5). Importantly, the elimination and/or functional inactivation of tumor-induced Treg can promote antitumor immunity and enhance the efficacy of immunotherapy.
Treg targeting using chemotherapeutic drugs
Cyclophosphamide (an alkylating agent used to treat some lymphoma, leukemia, multiple myeloma, neuroblastoma, breast or ovarian cancers) was one of the first drugs reported for its ability to interfere with suppressive T cells and to improve immunotherapy (10). We demonstrated in a rat colon cancer model that cyclophosphamide eliminates CD4+CD25+ Treg and improves overall survival when combined with immunotherapy (11). However, in this model, cyclophosphamide administration results in a non-selective elimination of both suppressor and effector T cells. Of therapeutic relevance, effector T lymphocytes reconstitute earlier than Treg, and for a defined window of time low Treg to effector CD4+ and CD8+ T lymphocyte ratios allow efficient immunotherapy (11). A later report indicated that low dose cyclophosphamide triggers mouse Treg apoptosis in vitro and in vivo without affecting conventional CD4+CD25− T cell viability. In this study, cyclophosphamide also impairs Treg suppressive function by down-regulating FoxP3 and glucocorticoid-induced TNFR-related protein (GITR) (12). The possibility of combining cyclophosphamide-mediated elimination of Treg with tumor-specific vaccination was further confirmed in different mouse models (13). Interestingly, the association of this drug with an agonistic antibody against the co-stimulatory receptor OX40 was shown to eliminate intratumoral, but not peripheral, Treg (14). Along these lines, cyclophosphamide was proven effective in reducing Treg number and restoring T and NK cell effector functions in patients with different types of advanced stage cancers (15). The mechanism(s) behind the preferential elimination of Treg by cyclophosphamide remain to be fully elucidated. Since this alkylating agent primarily affects highly proliferative cells, it is possible that the higher proliferation rate of Treg (as compared to other cells) in the context of growing tumor makes them ideal targets. Similarly, the preferential depletion of intratumoral Treg (as opposed to peripheral Treg) observed in some studies may reflect differential characteristics of Treg (such as their proliferation status) depending on their location. It should also be underlined that in most studies the immunostimulatory effects of cyclophosphamide were observed only at low, “metronomic” doses, while at high doses this drug is immunosuppressive. In addition, cyclophosphamide-mediated depletion of Treg is usually transient and a new Treg pool, with antigen recognition specificities skewed towards cancer antigens, eventually reconstitutes, mainly as a result of tumor-driven conversion of CD4+CD25−FoxP3− T cells (5). Therefore, in a chemoimmunotherapeutic perspective, careful considerations should be given to the dosing of this agent and to the timing of immunotherapy performed post-cyclophosphamide administration. These parameters are still in need of further optimization.
Paclitaxel, a mitotic inhibitor, has been reported to selectively reduce Treg number and function while sparing effector T lymphocytes in patients with advanced non-small cell lung cancer (NSCLC) (16). The selective induction of Treg apoptosis by paclitaxel was attributed to the up-regulation of the cell death receptor Fas on Treg but not on effector cells (16). In a mouse lung carcinoma model, paclitaxel was shown to induce Treg but not effector cell apoptosis, possibly by selectively inducing the down-regulation of the anti-apoptotic molecule Bcl-2 and the up-regulation of Bax (a pro-apoptotic factor) (17). The anticancer molecules lenalidomide and pomalidomide may also alter Treg proliferation and function by reducing FoxP3 expression through unclear molecular mechanisms (18). Lenalidomide has also recently been shown to reduce both Treg and MDSC number in the A20 lymphoma model (19). Additionally, low dose metronomic regimens of temozolomide, an alkylating agent, can reduce Treg to total CD4+ T lymphocyte ratios and impair Treg suppressive activity in glioma-bearing rats (20). Importantly, high doses of this agent have no effect on Treg, further underlining that chemotherapeutic drugs exert distinct immunomodulatory effects depending on their concentration. Temozolomide has also been reported to specifically reduce Treg in advanced melanoma patients (21)
Influence of agents used in targeted therapies on Treg
Several tyrosine kinase inhibitors (TKI) have been described for their ability to impair Treg. Sunitinib, a multi-tyrosine kinase inhibitor, has been reported to decrease the number of peripheral and tumor-infiltrating Treg in patients with renal cell carcinoma (RCC) (22). Sunitinib also reduces Treg in the mouse CT26 tumor model through targeting of the VEGF-VEGFR axis (23). Similar effects of the multi-kinase inhibitor sorafenib on Treg were observed (24). We have reported that imatinib mesylate (a BCR-ABL TKI, which also blocks c-KIT, a oncogenic tyrosine kinase receptor and platelet-derived growth factor receptor) reduces Treg number, impairs their suppressive function and promotes the efficacy of dendritic cell-based vaccination in a lymphoma mouse model (25). Imatinib interferes with early TCR/CD28 signaling events, leading to the down-regulation of FoxP3. In line with this result, it has recently been demonstrated that dasatinib (a second-generation tyrosine kinase inhibitor used against imatinib-resistant leukemia and malignancies characterized by c-KIT mutations) decreases the frequency of circulating Treg in a mastocytoma mouse model (26). Importantly, the reduction of Treg number was transient and these cells reconstituted quickly after drug removal. In this study, dasatinib efficiently synergizes with immunotherapy (agonistic anti-OX40) resulting in improved antitumor effects (26). The increased sensitivity of Treg to imatinib and dasatinib compared to effector T cells may be explained by a higher dependence of Treg on Lck, an essential tyrosine kinase in TCR signaling, and potential target of these TKI.
The apparent ability of the aforementioned antineoplastic agents to affect multiple immunological processes has sparked some criticism as it relates to their usefulness as direct Treg targeting agents. Whether the improvement of anticancer immunity may result from Treg depletion or from the concomitant enhancement of effector immune cell function by these molecules remains indeed difficult to ascertain. A more refined identification of the molecular bases underlying the mode of action of these agents on Treg will certainly help address this issue. Nonetheless, although antibody-based approaches such as anti-CTLA4 or anti-OX40 have shown promise to impair Treg, a universal optimized strategy to selectively target these cells is still needed. In this context, the possibility of using conventional chemotherapeutic molecules or targeted therapeutic agents to interfere with these immunosuppressive lymphocytes remains an appealing strategy.
MDSC elimination, inactivation, and/or differentiation into pro-inflammatory cells
MDSC have been described as a heterogeneous population of immature myeloid cells endowed with the capacity to inhibit innate and adaptive immunity (4). In mice, two main populations equipped with dedicated mechanisms of suppression and functions can be distinguished: monocytic CD11b+Ly6G−Ly6Chigh and granulocytic CD11b+Ly6G+Ly6Clow MDSC (4). Typically, solid tumors mainly induce the expansion of the granulocytic subset. Human MDSC are primarily defined by a CD33+CD11b+HLA-DRneg/low phenotype. The accumulation of these cells in the periphery and tumor microenvironment results from the stimulation of myelopoiesis and from the blockade of the differentiation of immature into mature myeloid cells by several tumor-derived factors which also promote MDSC suppressive function (4). Multiple studies conducted in rodents and patients with different types of cancer have demonstrated that MDSC elimination, inhibition, or the induction of their differentiation into pro-inflammatory cells using anticancer agents enhance antitumor immunity and foster the response to immunotherapy.
Targeting MDSC with conventional chemotherapeutic drugs
Gemcitabine, an anti-metabolite drug (nucleoside analogue) used for the treatment of pancreatic, breast, ovarian, and lung cancers, has been reported to deplete MDSC in tumor-bearing mice, resulting in enhanced antitumor immunity (27). Another anti-metabolite, 5-Fluorouracil (5-FU), used at low doses, has also been shown to induce MDSC apoptosis (27). Interestingly, MDSC are more sensitive to these molecules than other immune cells or than tumor cells. This selective effect was explained by a lower expression of thymidylate synthase by MDSC (27). The DNA demethylating agent 5-azacytidine may also reduce the accumulation and function of MDSC induced in the mouse TC-1/A9 and TRAMP-C2 tumor models (28). Docetaxel, (a mitotic inhibitor, semi-synthetic analogue of paclitaxel) was shown to impair MDSC suppressive function, predominantly by blocking Stat3 phosphorylation and by promoting MDSC differentiation into M1 macrophages (29). Similarly, it has also been reported that low-dose paclitaxel promotes MDSC differentiation into dendritic cells in vitro (30).
The anthracycline doxorubicin has been described for its plethoric immunostimulatory effects (6). We have recently demonstrated in different mouse cancer models that this drug selectively, but however transiently, eliminates and inactivates MDSC (31). This preferential targeting of MDSC translates into increased effector lymphocyte to immunosuppressive MDSC ratios and is associated with enhanced CD4+, CD8+ and NK cell activation and pro-inflammatory cytokine production, fundamental prerequisites to efficient immunotherapy. A chemoimmunotherapeutic regimen consisting of doxorubicin and adoptive transfer of CD4+ Th lymphocytes impairs tumor development, metastatic spreading and improves survival. It is noteworthy that MDSC number remains low over time in animals receiving the combination treatment, whereas these cells expanded de novo post-therapy in mice administered with doxorubicin alone. This observation further underlines the possibility, and importance, of combining chemotherapeutic approaches with immmunostimulation (31). The selective effect of doxorubicin may again be partly explained by the preferential targeting of highly proliferative cells (in untreated tumor-bearing mice MDSC proliferation status is higher than that of T or NK cells). Another possibility is that doxorubicin may enhance the already high production of reactive oxygen species (ROS) by MDSC (compared to other cell subsets), leading to ROS-dependent apoptosis.
Impact of molecules used in targeted therapies on tumor-induced MDSC
Sunitinib, has been shown to reduce circulating MDSC number and suppressive function in RCC patients, which correlates with improved T cell function (32). Again, this effect was selective since T cells exposed to concentrations of sunitinib that impair MDSC were viable and functional. Although the molecular mechanisms remain to be fully deciphered, it has been speculated that this agent, known to interfere with tyrosine kinases of the VGEFR1-3 or c-KIT family, may inhibit VEGF and/or c-KIT-mediating signaling in MDSC, pathways that participate in the tumor-driven expansion of these cells (32, 33). An additional mode of action of sunitinib may include Stat3 inhibition (34). Since stem-cell factor (c-KIT ligand) contributes to MDSC accumulation, one should expect that other c-KIT-targeting TKI such as dasatinib, imatinib or sunitinib may also effectively interfere with this suppressive cell population.
Reticences raised by the absence of definitive mechanistic grounds explaining the selectivity of antineoplastic agents on MDSC have eroded the exploitation of this immunomodulatory strategy. In addition, MDSC elimination with these molecules is, as for Treg, usually transient and a narrow therapeutic window is available for immunostimulatory interventions. Many pathways involved in MDSC generation and suppressive activity can be targeted with several other inhibitors such as Celecoxib (cyclooxygenase-2 inhibitor) or sildenafil (phosphodiesterase-5 inhibitor). A systematic comparison of the efficacy of these reagents to that of low non-cytotoxic doses of antineoplastic agents to impact the multiple aspects of MDSC immunobiology is undeniably needed.
Future directions and concluding remarks
The discovery that many conventional chemotherapeutic drugs and molecules used in targeted therapies can blunt the immunosuppressive cellular networks induced by tumors has sparked a new promising area of research, at the interface between traditional cancer therapies and immune-based strategies. It is however noteworthy that the long-term immunological consequences of anticancer agent administration are not always therapeutically beneficial and essentially depend on parameters such as the nature of the considered molecule(s), the dosing and scheduling, which all require further optimization.
In this context, the identification of additional specific anticancer agents endowed with the ability to impede suppressive cells such as Treg and MDSC while sparing (or promoting) antitumoral immune effectors is of paramount importance. Along these lines, a more refined understanding of the interference(s) of these compounds with specific molecular pathways in suppressive cells should help decipher the mechanisms underlying the selective targeting of these cells. It should also be underlined that in most cases antineoplastic molecules only curtail suppressive cells for a transient period of time. This imposes a regular monitoring to identify a specific window of time associated with high effector to suppressor ratios, permissive for efficient immunotherapeutic interventions. Conceivably, as different molecules may affect different suppressive cell populations (for instance, cyclophosphamide primarily targets Treg, while doxorubicin mainly impact MDSC), the identification of the immunoinhibitory cell subset predominantly expanded in a considered cancer patient should guide the choice of the optimal agent(s).
An additional important question relates to the notion of Treg “reprogramming”. The plasticity of FoxP3+ Treg and the possibility to induce their trans-differentiation into effector non-suppressive cells such as T helper 17 (Th17) has been described (35). Although a recent study has indicated that cyclophosphamide-induced generation of Th17 does not depend on Treg reprogramming (36), the possibility that other agents may foster the conversion of already polarized Treg into effector subsets, or reroute the differentiation of naïve T cells towards effector T lymphocytes instead of Treg remains to be addressed.
A significant advance in our understanding of the plethoric immunomodulatory properties of many conventional chemotherapeutic drugs and targeted therapeutic agents has been a driving force behind the design and development of chemoimmunotherapeutic strategies. Selected antineoplastic agents, by inducing rapid tumor shrinkage, reversing cancer-induced immunosuppression and promoting antitumor cytotoxic immune effectors may create a favorable environment, allowing immune-based therapies to elicit long-lasting memory immune responses capable of controlling relapse due to drug-resistant disease and metastatic spreading (Figure 1). Although the dose for each therapeutic arm and the sequence of administration require additional refinements, the combination of these two modalities, operating by distinct but complementary and overlapping mechanisms, represents arguably a promising emerging anticancer strategy.
Acknowledgments
Grant support: Supported in part by the Cancer Biology Training Grant T32CA009213, AZ Cancer Center Support Grant CA023074, NIH grant R01 CA104926.
We apologize to all the authors whose work could not be cited due to space limitation.
Footnotes
Conflict of Interest: No potential conflicts of interest.
Authors’ contribution
Concept and design: D. Alizadeh, N. Larmonier
Development and methodology: D. Alizadeh, N. Larmonier
Analysis and interpretation of data: D. Alizadeh, N. Larmonier
Writing, review, and/or revision of the manuscript: D. Alizadeh, N. Larmonier
References
- 1.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–7. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nature reviews Drug discovery. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 7.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang TH, Mao CP, Lee SY, Chen A, Lee JH, Kim TW, et al. Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res. 2013;73:2493–504. doi: 10.1158/0008-5472.CAN-12-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 10.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. The Journal of experimental medicine. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 12.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 13.Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–9. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 14.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–16. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Dermawan K, Jin M, Liu R, Zheng H, Xu L, et al. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol. 2008;129:219–29. doi: 10.1016/j.clim.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu N, Zheng Y, Zhu Y, Xiong S, Chu Y. Selective impairment of CD4+CD25+Foxp3+ regulatory T cells by paclitaxel is explained by Bcl-2/Bax mediated apoptosis. International immunopharmacology. 2011;11:212–9. doi: 10.1016/j.intimp.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakamaki I, Kwak LW, Cha SC, Yi Q, Lerman B, Chen J, et al. Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas. Leukemia. 2014;28:329–37. doi: 10.1038/leu.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer immunology, immunotherapy: CII. 2009;58:1627–34. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridolfi L, Petrini M, Granato AM, Gentilcore G, Simeone E, Ascierto PA, et al. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J Transl Med. 2013;11:135. doi: 10.1186/1479-5876-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother. 2010;33:991–8. doi: 10.1097/CJI.0b013e3181f4c208. [DOI] [PubMed] [Google Scholar]
- 23.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–49. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 24.Desar IM, Jacobs JH, Hulsbergen-vandeKaa CA, Oyen WJ, Mulders PF, van der Graaf WT, et al. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. Int J Cancer. 2011;129:507–12. doi: 10.1002/ijc.25674. [DOI] [PubMed] [Google Scholar]
- 25.Larmonier N, Janikashvili N, LaCasse CJ, Larmonier CB, Cantrell J, Situ E, et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL- tumors. J Immunol. 2008;181:6955–63. doi: 10.4049/jimmunol.181.10.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Liu C, Peng W, Lizee G, Overwijk WW, Liu Y, et al. Antitumor T-cell responses contribute to the effects of dasatinib on c-KIT mutant murine mastocytoma and are potentiated by anti-OX40. Blood. 2012;120:4533–43. doi: 10.1182/blood-2012-02-407163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil Selectively Kills Tumor-Associated Myeloid-Derived Suppressor Cells Resulting in Enhanced T Cell-Dependent Antitumor Immunity. Cancer Research. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 28.Mikyskova R, Indrova M, Vlkova V, Bieblova J, Simova J, Parackova Z, et al. DNA demethylating agent 5-azacytidine inhibits myeloid-derived suppressor cells induced by tumor growth and cyclophosphamide treatment. J Leukoc Biol. 2014 doi: 10.1189/jlb.0813435. [DOI] [PubMed] [Google Scholar]
- 29.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–94. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michels T, Shurin GV, Naiditch H, Sevko A, Umansky V, Shurin MR. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. Journal of immunotoxicology. 2012;9:292–300. doi: 10.3109/1547691X.2011.642418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–18. doi: 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 33.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey-Bucktrout SL, Bluestone JA. Regulatory T cells: stability revisited. Trends Immunol. 2011;32:301–6. doi: 10.1016/j.it.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 2011;71:661–5. doi: 10.1158/0008-5472.CAN-10-1259. [DOI] [PubMed] [Google Scholar]