Abstract
Objectives
This study sought to investigate plasma levels of circulating cardiac natriuretic peptides, atrial natriuretic peptide (ANP) and B-type or brain natriuretic peptide (BNP), in the general community, focusing on their relative differences in worsening human hypertension.
Background
Although ANP and BNP are well-characterized regulators of blood pressure in humans, little is known at the population level about their relationship with hypertension. The authors hypothesized that hypertension is associated with a lack of activation of these hormones or their molecular precursors.
Methods
The study cohort (N = 2,082, age>45 years) was derived from a random sample from Rochester, Minnesota, and each subject had a medical history, clinical examination, and assessment of different plasma forms of ANP and BNP. Patients were stratified by blood pressure. Multivariable linear regression was used to assess differences in natriuretic peptide levels in worsening hypertension.
Results
Compared to normotensive, BNP1–32 and N-terminal proBNP1–76 (NT-proBNP1–76) were significantly decreased in pre-hypertension (p < 0.05), with BNP1–32 significantly decreased in stage 1 as well (p < 0.05). Although proBNP1–108 remained unchanged, the processed form was significantly increased only in stage 2 hypertension (p < 0.05). ANP1–28 remained unchanged, while NT-ANP1–98 was reduced in pre-hypertension (p < 0.05).
Conclusions
The authors demonstrated the existence of an impaired production and/or release of proBNP1–108 along with a concomitant reduction of BNP1–32 and NT-proBNP1–76 in the early stages of hypertension, with a significant elevation only in stage 2 hypertension. Importantly, they simultaneously demonstrated a lack of compensatory ANP elevation in advanced hypertension.
Keywords: ANP, BNP, hypertension, natriuretic peptide, NT-proBNP, proBNP
Atrial natriuretic peptide 1–28 (ANP) and B-type natriuretic peptide 1–32 (BNP1–32) are 28- and 32-amino acid hormones, respectively, that are normally produced and secreted by the human heart in response to cardiac injury and mechanical stretch. Both cardiac hormones regulate blood pressure (BP) homeostasis via their pleiotropic actions, including natriuresis, vasodilation, and renin-angiotensin-aldosterone system suppression, but their mechanisms of secretion and processing into active forms differ greatly (1,2). ANP is secreted and circulates as an active hormone, whereas a growing body of evidence indicates that the major secreted and circulating immunoreactive form of BNP is actually its 108-amino acid precursor, pro–B-type natriuretic peptide 1–108 (proBNP 1–108), and not BNP 1–32 (3–6). Current evidence supports that this circulating prohormone, which has significantly reduced biological activity, is cleaved by corin and/or furin into the biologically active BNP1–32 and the inactive N-terminal proBNP1–76 (NT-proBNP1–76) in plasma or at the target tissue level (3,7,8). Although the ANP and BNP systems have been studied extensively, their simultaneous critical role in BP regulation has not yet been described at the population level.
Prior studies have reported that BNP1–32 and NT-proBNP1–76 are elevated in hypertension, especially when left ventricular hypertrophy (LVH) is present (9). However, Belluardo et al. (10) challenged this common view by showing in clinically and biochemically well-characterized subjects with essential hypertension that BNP 1–32 and NT-proBNP1–76 are actually reduced in the early stages of human hypertension. Furthermore, the importance of ANP and BNP in BP regulation and in risk for development of hypertension was recently confirmed by the elegant studies of Newton-Cheh et al. (11). In that population study, genetic variants of the ANP and BNP gene, resulting in higher plasma concentrations of ANP and/or BNP, were associated with lower BP and reduced risk for hypertension. These findings are consistent with earlier animal studies in which ANP or natriuretic peptide receptor type A (NPR-A) gene deletion in mice resulted in hypertension and marked LVH and hypertension (12). Nonetheless, it is unknown whether decreased BNP 1–32 is accompanied by reduced circulating proBNP1–108 and ANP, which, if present, would indicate that inadequate natriuretic peptide synthesis or secretion is a significant mechanism contributing to hypertension development. A lack of large, complete biomarker studies in hypertensive patients, with and without end-organ damage, has limited our understanding of the role of these 2 cardiac peptides (ANP and BNP) in worsening hypertension.
Using a large study from Olmsted County, Minnesota, derived from a population-based sample known as the PAVD (Prevalence of Asymptomatic Ventricular Dysfunction) cohort (N=2,082, age >45 years), our objective was to confirm and extend in the general adult population our previous assessment of circulating levels of several molecular forms of BNP and ANP through simultaneous measurement of proBNP1–108, BNP1–32, NT-proBNP1–108, ANP1–28, and NT-ANP1–98. As a corollary to the first observation of Belluardo et al. (10), we hypothesized that human hypertension is characterized by a lack of activation of these cardiac hormones, indicating the existence of a relative deficiency of their endogenous cardiorenal protective actions in the morbid condition. This finding would underscore an opportunity to intervene in hypertension with improved therapeutics, including natriuretic peptides and their chimerics, which are currently in the pipeline for clinical trials.
Methods
Study population
This study was approved by the Mayo Clinic institutional review board, and informed consent was obtained from all subjects. Participating subjects came from the PAVD cohort (N = 2,082, age >45 years), a study population derived from a random sample of subjects from the Rochester Epidemiology Project (13–15). PAVD investigators have previously reported each subject’s medical history, clinical examination, detailed 2-dimensional echocardiography, and procedures for storage and measurement of BNP forms, including BNP1–32 (16), NT-proBNP1–76 (17), and proBNP1–108 (5). Although prior human studies used assays that measured BNP1–32 and NT-proBNP1–76, which cross-reacted with proBNP1–108 (3), our novel approach used a newly available radioimmunoassay that is highly specific for proBNP1–108 (18). Furthermore, we simultaneously measured 2 different forms of mature circulating ANP (ANP1–28 and NT-proANP1–98). Subjects were classified according to office systolic BP and/or diastolic BP as normotensive or belonging to 1 of 3 grades of hypertension: pre-hypertensive, stage 1 hypertension, and stage 2 hypertension, in accordance with the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) guidelines (19). BP was measured 3 times at 5-min intervals at an outpatient clinic. A complete cardiovascular evaluation, including 2-dimensional Doppler echocardiograms, was performed the day of blood collection. Heart failure was defined as previously described in the publication from Redfield et al. (14).
LVH was defined as a LV mass index >134 for males and >110 for females, where the LV mass index was defined as the quotient of left ventricular mass (by echocardiogram) divided by the body surface area (20).
Radioimmunoassay
Natriuretic peptide levels were determined using the Shionogi (Florham Park, New Jersey) and Biosite (San Diego, California) assays for BNP1–32, Roche (Indianapolis, Indiana) for NT-proBNP1–76, Bio-Rad (Hercules, California) for proBNP1–108, and Phoenix Pharmaceuticals (Mountain View, California) for ANP1–28 and for NT-proANP1–98 (21,22). The specific characteristics of these assays have previously been reported. The BNP1–32 and NT-proBNP1–76 assays have been reported to display cross-reactivities for precursor BNP forms, including proBNP1–108 (3). However, they have been found to be highly specific with regard to processed BNP1–32 and NT-proBNP1–76, respectively.
Statistical methods
Summary statistics were separated by JNC 7 category as defined by BP at the time of clinical exam and displayed as frequencies (percentages) for categorical variables, and median, Q1 to Q3 range, and frequency (percentage) of missing entries for continuous variables. Categorical variables were compared by chi-square test, and continuous variables (including levels of natriuretic peptide forms) were compared by the Kruskal-Wallis rank sum test. Multivariable linear regression was used to determine which variables were independently associated with plasma BNP-form levels using a step-wise model-building process. The variables for each multivariable linear regression model were selected from among the variables with p < 0.10 in univariate analysis (not shown). With the exception of age, continuous variables were classified into categories. Body mass index (BMI) was included as a 3-level variable: <25 kg/m2 (reference), 25 to 30 kg/m2, and >30 kg/m2. Creatinine was defined as a dichotomous variable: <1.2 (reference) and >1.2. To account for potential non-linearity of NP effects with age, a quadratic term for age was also included. Progressive models added variables that were associated with the plasma concentration of the hormones and a final model included age, age2, sex, BMI, LVH, creatinine level, and the number of antihypertensive medications (except for the analysis that was performed in the subjects who were not on antihypertensive medications). In order to satisfy modeling and regression assumptions, BNP and ANP values were transformed by taking the natural logarithm of the raw values. In order to visually display effects, the least squares adjusted means were calculated from the final model and estimates and 95% CI were back-transformed to original scale. Homogeneity, across hypertension groups, of age and gender effects were tested by inclusion of interaction terms with stage of hypertension. Age effects were found to be homogeneous. Due to some evidence that gender effects were non-homogeneous, a sensitivity analysis was conducted to compare normotensive and pre-hypertensive subjects. All tests were 2-sided, and for all analyses, p < 0.05 was considered statistically significant.
Results
General characteristics
Table 1 displays the characteristics of our study population stratified by JNC 7 BP categories at initial visit. The covariates that were significantly different between the stages of hypertension were age, sex, BMI, chronic renal insufficiency defined as Cockroft-Gault calculated glomerular filtration rate <60 ml/min, diabetes, coronary artery disease, valvular heart disease, cerebrovascular accidents, LV mass index, LV geometry, proBNP1–108, NT-proBNP1–76, BNP1–32, ANP1–28, and NT-proANP1–98. Of the 2,082 subjects, 1,465 (72%) had elevated BP (systolic BP >120 mm Hg and/or diastolic BP >80 mm Hg), further classified into 755 pre-hypertensive subjects (51% of all hypertensive subjects), 492 stage 1 hypertension (34%) subjects, and 218 stage 2 hypertension (15%) subjects. The overall prevalence of LVH in our study population was 12.4%. As expected, the percentage of subjects with LVH increased over each stage of hypertension, from 7.3% in normotensive subjects to 32.9% in subjects with stage 2 hypertension.
Table 1.
Demographics of the Study Population by BP
| Variable | Normotensive | Pre-Hypertensive | Stage 1 Hypertensive | Stage 2 Hypertensive |
|---|---|---|---|---|
| Age, yrs* | 56.5 (50.3–64.8) | 61.0 (53.9–69.2) | 65.0 (56.9–72.7) | 70.1 (62.6–77.1) |
| Females | 320 (56%) | 360 (48%) | 251 (51%) | 126 (58%) |
| BMI, kg/m2 | 26.2 (23.5–29.1) | 27.9 (25.4–31.2) | 28.9 (25.7–33.0) | 28.3 (25.3–33.2) |
| CRF | 109 (20%) | 168 (23%) | 155 (33%) | 86 (41%) |
| Diabetes | 28 (5%) | 53 (7%) | 50 (10%) | 19 (9%) |
| CAD | 62 (11%) | 78 (10%) | 68 (14%) | 37 (17%) |
| HF | 13 (2%) | 16 (2%) | 15 (3%) | 7 (3%) |
| Atrial fibrillation | 17 (3%) | 39 (5%) | 31 (6%) | 12 (6%) |
| Creatinine >1.2 | 128 (23%) | 194 (26%) | 148 (31%) | 55 (26%) |
| Valvular disease | 62 (11%) | 100 (13%) | 83 (17%) | 47 (22%) |
| CVA | 6 (1%) | 12 (2%) | 3 (1%) | 13 (6%) |
| LV mass index* | 90.4 (78.5–101.4) | 93.8 (81.5–108.1) | 98.4 (86.1–113.7) | 103.0 (87.6–125.3) |
| LV geometry | ||||
| Normal | 394 (78%) | 387 (67%) | 211 (59%) | 59 (40%) |
| Antihypertensive medications | 130 (25%) | 260 (37%) | 226 (49%) | 112 (55%) |
| Antihypertensive medications, n | ||||
| 0 | 441 (77%) | 495 (66%) | 266 (54%) | 106 (49%) |
| 1 | 69 (12%) | 163 (22%) | 126 (26%) | 57 (26%) |
| 2 | 53 (9%) | 74 (10%) | 68 (14%) | 31 (14%) |
| 3 | 6 (1%) | 18 (2%) | 26 (5%) | 22 (10%) |
| 4 | 2 (0%) | 5 (1%) | 6 (1%) | 2 (1%) |
| Beta-blocker | 68 (13%) | 96 (14%) | 87 (19%) | 44 (21%) |
| Calcium channel blocker | 16 (3%) | 51 (7%) | 48 (10%) | 33 (16%) |
| ACE inhibitor | 35 (7%) | 61 (9%) | 55 (12%) | 30 (15%) |
| Diuretic | 55 (11%) | 118 (17%) | 120 (26%) | 53 (26%) |
| Nitrate | 15 (3%) | 27 (4%) | 17 (4%) | 15 (7%) |
| Vasodilator | 10 (2%) | 19 (3%) | 22 (5%) | 12 (6%) |
| ARB | 2 (0.4%) | 13 (2%) | 15 (3%) | 6 (3%) |
| ProBNP1–108 (Bio-Rad)* | 17.5 (8.0–36.5) | 18.0 (8.0–41.0) | 21.0 (9.0–43.0) | 31.0 (14.0–64.0) |
| BNP3–32 (Shionogi)* | 12.9 (5.1–28.6) | 13.7 (5.4–28.2) | 15.8 (5.8–32.7) | 23.6 (10.0–52.3) |
| BNP1–32 (Biosite Triage)* | 20.7 (8.5–45.3) | 21.2 (8.4–44.9) | 24.6 (9.7–57.2) | 51.9 (22.6–106.8) |
| NT-proBNP1–76 (Roche)* | 56.3 (24.3–120.0) | 59.2 (24.5–118.0) | 78.1 (31.9–166.9) | 142.3 (62.8–332.7) |
| ANP128 (Phoenix)* | 11.0 (7.2–15.2) | 11.1 (7.2–15.5) | 12.3 (7.6–16.8) | 14.2 (8.7–18.7) |
| NT-ANP198 (Phoenix)* | 2,079.0 (1,420.0–3,023.0) | 2,066.5 (1,297.0–3,112.0) | 2,384.0 (1,543.0–3,512.0) | 2,753.5 (1,725.5– 4,242.0) |
| 2,212.5 (1,415.0–3,281.0) | <0.001 |
Values are n (%) or median (interquartile range).
Kruskal-Wallis test used for continuous variables; all other variables were categorical and were compared by chi-square.
ACE = angiotensin-inhibiting enzyme; ANP = A-type natriuretic peptide; ARB = angiotensin receptor blocker; BMI = body mass index; BNP = B-type natriuretic peptide; BP = blood pressure; CAD = coronary artery disease; CRF = chronic renal failure; CVA = cerebrovascular accident; HF = past heart failure diagnosis; LV = left ventricular; NT = N-terminal; ProBNP = pro–B-type natriuretic peptide
Natriuretic peptides in normotensive and in hypertensive subjects
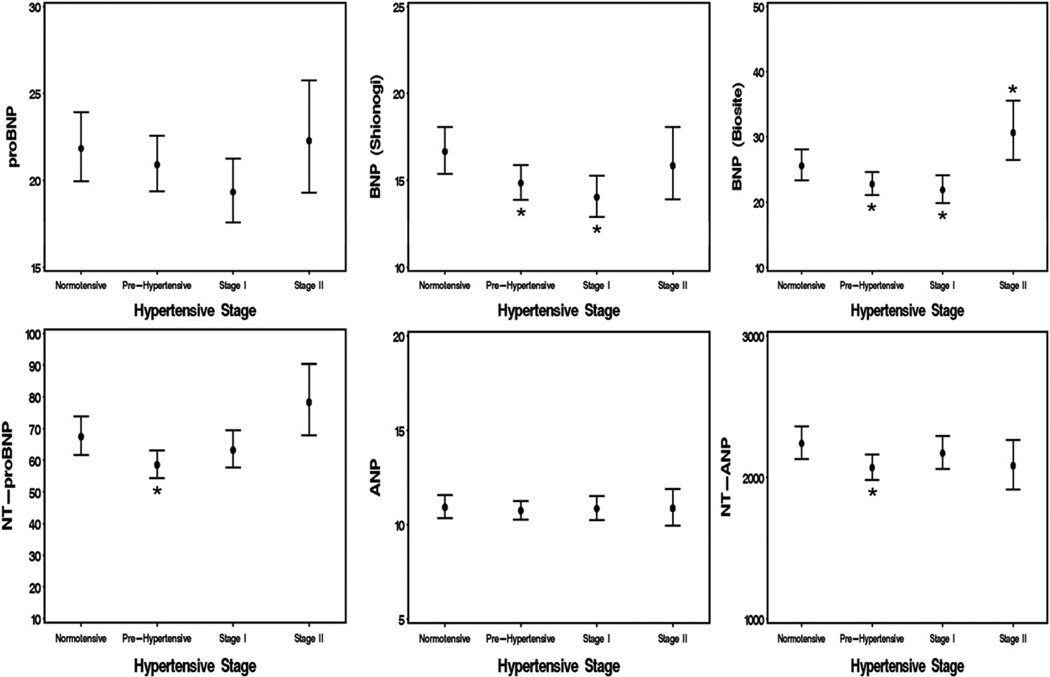
Table 2 displays the effects of hypertension stage on natriuretic peptides levels, with no adjustment and, consistent with previous reports, shows progressive elevation of all NPs with the severity of hypertension. Progressive multivariable adjustments were performed on the entire study population, including those on antihypertensive medications. This regression accounted for important covariates that were previously identified in univariate analysis, including age, sex, BMI, LVH, and creatinine (Fig. 1). Although, proBNP1–108 remained unchanged in the different stages of hypertension, processed BNP forms, including BNP1–32 and NT-proBNP1–76, were significantly decreased in prehypertension (p < 0.05), with BNP1–32 significantly decreased in stage 1 as well (p < 0.05). The NT-proBNP1–75 tended to increase, while BNP1–32 was elevated in stage 2 hypertension (p < 0.05). ANP1–28 levels, after adjustment, showed no association with stage of hypertension, whereas NT-proANP1–98 was significantly decreased in pre-hypertension (p < 0.05), and showed no change with stages 1 and 2 hypertension. These results were similar when analyses were restricted to only normotensive and pre-hypertensive subjects (Table 3). Because the effect of antihypertensive medications on natriuretic peptides levels cannot be completely corrected by multivariate adjustment, we reanalyzed the association between natriuretic peptide levels and hypertension, limiting our analysis to subjects who were not on antihypertensive medications (Table 3). After adjustment, we found that none of the BNP forms were elevated and that BNP1–32 (Roche) and NT-proBNP1–76 showed a slight trend downward in early hypertension. ANP1–28 levels were not associated with stage of hypertension, whereas NT-proANP1–98 levels were significantly decreased in pre-hypertensive subjects (p < 0.05) in models 1 to 3.
Table 2.
Univariate Analysis of Biomarkers and Stage of Hypertension
| Hypertensive Stage | proBNP1–108 Bio-Rad (� ± SE) |
BNP1–32 Shionogi (� ± SE) |
BNP1–32 Biosite (� ± SE) |
NT-proBNP1–76 Roche (� ± SE) |
ANP1–28 Phoenix Pharmaceuticals (� ± SE) |
NT-ANP1–98 Phoenix Pharmaceuticals (� ± SE) |
|
|---|---|---|---|---|---|---|---|
| All subjects | |||||||
| Normotensive | Reference | Reference | Reference | Reference | Reference | Reference | |
| Pre-hypertensive | 0.06 ±0.07 | −0.00 ±0.06 | −0.02 ±0.07 | −0.01 ±0.07 | 0.02 ±0.04 | −0.03 ±0.04 | |
| Stage I hypertensive | 0.16 ±0.07* | 0.12 ±0.07 | 0.17 ±0.08* | 0.33 ±0.08† | 0.10 ±0.04* | 0.12 ±0.04* | |
| Stage II hypertensive | 0.61 ±0.09† | 0.52 ±0.08† | 0.86 ±0.10† | 0.96 ±0.11† | 0.20 ±0.05† | 0.24 ±0.06† | |
| Subjects not on antihypertensive medications | |||||||
| Normotensive | Reference | Reference | Reference | Reference | Reference | Reference | |
| Pre-hypertensive | 0.03 ±0.07 | 0.04 ±0.07 | −0.06 ±0.08 | −0.06 ±0.08 | 0.02 ±0.05 | −0.08 ±0.05 | |
| Stage I hypertensive | 0.13 ±0.09 | 0.07 ±0.08 | 0.11 ±0.10 | 0.23 ±0.10* | 0.04 ±0.05 | 0.04 ±0.05 | |
| Stage II hypertensive | 0.50 ±0.12† | 0.49 ±0.11† | 0.62 ±0.14† | 0.68 ±0.14† | 0.11 ±0.08 | 0.20 ±0.08* | |
Values are 1 ± SE in contrast to normotensive subjects. Logarithm of BNP/ANP values used to satisfy regression assumptions.
p < 0.05,
p < 0.001 compared with normotensive subjects.
Abbreviations as in Table 1.
Figure 1.
Results of Multivariable Analysis of All Subjects Adjusted for Age, Gender, BMI, LVH, Creatinine, and Number of Antihypertensive Medications
Figure displays least squares adjusted means for each stage of hypertension and each biomarker. ANP = atrial natriuretic peptide; BNP = B-type natriuretic peptide; NT-ANP = N-terminal atrial natriuretic peptide; NT-proBNP = N-terminal pro–B-type natriuretic peptide; ProBNP = pro–B-type natriuretic peptide.
Table 3.
Univariate and Multivariate Analysis in Normotensive and Pre-Hypertensive Subjects
| Biomarker | Univariate Model (� ± SE) |
Model 1 (� ± SE) |
Model 2 (� ± SE) |
Model 3 (� ± SE) |
Model 4 (� ± SE) |
Model 5 (� ± SE) |
|---|---|---|---|---|---|---|
| Log proBNP1–108 | 0.06 ± 0.07 | −0.07 ± 0.06 | −0.04 ± 0.06 | −0.04 ± 0.06 | −0.04 ± 0.06 | −0.04 ± 0.06 |
| Log BNP1–32 (Shionogi) | −0.00 ± 0.06 | −0.12 ± 0.05* | −0.11 ± 0.05* | −0.11 ± 0.05* | −0.11 ± 0.05* | −0.11 ± 0.05* |
| Log BNP1–32 (Biosite) | −0.02 ± 0.07 | −0.13 ± 0.06* | −0.12 ± 0.06 | −0.12 ± 0.06 | −0.12 ± 0.06 | −0.12 ± 0.06* |
| Log NT-proBNP1–76 (Roche) | −0.01 ± 0.07 | −0.15 ± 0.06* | −0.13 ± 0.06* | −0.14 ± 0.06* | −0.13 ± 0.06* | −0.14 ± 0.06* |
| Log ANP1–28 (Phoenix Pharmaceuticals) | 0.02 ± 0.04 | −0.01 ± 0.04 | −0.00 ± 0.04 | −0.01 ± 0.04 | −0.01 ± 0.04 | −0.01 ± 0.04 |
| Log NT-ANP1–98 (Phoenix Pharmaceuticals) | −0.03 ± 0.04 | −0.11 ± 0.03* | −0.09 ± 0.03* | −0.08 ± 0.04* | −0.08 ± 0.03* | −0.08 ± 0.03* |
Values are 1 ± SE in contrast to normotensive subjects. Model 1 = age+age2+sex; Model 2 = age+age2+sex+BMI; Model 3 = age+age2+sex+BMI+LVH; Model 4 = age+age2+sex+BMI+LVH+creatinine; Model 5 = hypertension+age+age2+sex+BMI+LVH+creatinine+number of antihypertensive medications.
Logarithm of BNP and ANP values used to satisfy regression assumptions.
p < 0.05,
p < 0.001 compared with normotensive subjects.
Abbreviations as in Table 1.
Discussion
The purpose of the current investigation was to evaluate the hypothesis that there is a lack of elevation of circulating ANP and BNP in hypertension in the general population. By characterizing hypertension as a relative deficiency state of these cardiac peptides, we imply their key role in the development of human hypertension and their ability, if augmented, potentially to reduce disease burden. To test this hypothesis, we used the PAVD population, a well-characterized adult population from Olmsted County, Minnesota. We classified subjects according to their BP levels into normotensive, pre-hypertensive, and stage 1 and stage 2 hypertensive. Indeed, our results, for the first time, support the existence of such a relative deficiency state, as indicated by the simultaneous lack of increase of proBNP1–108 and ANP1–28 in any of the hypertensive stages, concomitant with reduced levels of BNP1–32 and NT-proBNP1–76 in early stages of hypertension and with reduced NT-ANP in stage 1 hypertension (Fig. 1). Importantly, we are the first to our knowledge to show that the absence of an increase in all BNP forms was also not accompanied by a compensatory increase in ANP forms. A significant elevation of a mature BNP (Biosite) form was observed only in stage 2 hypertension.
These findings extend our previous study of a selected group of hypertensive patients (10). That study also indicated the presence of a BNP deficiency state in early stages of hypertension. However, the original investigation of Belluardo et al. (10) did not have access to proBNP1–108 levels, so it was not possible to investigate whether reduced levels of processed forms of BNP, namely NT-proBNP1–76 and BNP1–32, in early hypertensive stages were also associated with an inadequate production/release of their precursor. Importantly, in the current study, when we looked at the entire population and adjusted for antihypertensive medications, we demonstrated that the reduced levels of mature BNP1–32 in early stages of hypertension were accompanied by a lack of activation of its precursor, proBNP1–108, and by a similar reduction of NT-ANP1–98. All together these findings support the existence of a relative deficiency of such protective hormonal system possibly due to an impaired release and/or to an elevated metabolism of the mature and biologically active forms of these cardiac hormones.
Cardiac endocrine function in human hypertension
For the first time to our knowledge, both arms of the cardiac endocrine system were assessed in worsening hypertension in a large sample of the general community, adjusting for end-organ cardiac effects of hypertension, such as ventricular hypertrophy. Adjustment for important variables associated with natriuretic peptide levels reveals that most NP forms tend to decrease or are significantly decreased in early hypertension and then tend to increase only in late hypertension, when we consider early and late hypertension independently. Furthermore, using the JNC 7 classifications, proBNP1–108, the precursor BNP form, was not significantly increased in any stage of hypertension. These observations point to a problem of production of substrate (proBNP1–108), rather than a problem of downstream processing in hypertension.
Our conclusion regarding the presence of a relative deficiency of the entire BNP system in hypertension is also supported by recent evidence that some individuals in the community who have an elevated level of processed BNP forms have reduced, rather than elevated, BP. Specifically, polymorphisms in minor alleles of the NPPA gene were associated with lower BP and higher natriuretic peptide levels in subjects with coronary artery disease, although no correlation was seen with clinical outcome (23). Furthermore, Newton-Cheh et al. (11) also found polymorphisms of NPPA associated with higher natriuretic peptide levels and lower rates of hypertension in the general community. We have built on these important studies by characterizing levels of the precursor proBNP1–108. At this time, it is unknown whether NPPA polymorphisms are associated with higher levels of proBNP1–108 production. Thus, this study confirms that in human hypertension, there is a lack of activation of both BNP and ANP.
The question remains as to why there are inadequate or failed metabolic compensatory mechanisms, specifically proBNP1–108 production and processing, in hypertensive patients. Therefore, there is a need for studies at the molecular and tissue levels of human myocardium to elucidate where proBNP1–108 processing occurs in humans and whether chronically elevated BP affects the heart’s production of proBNP1–108 (such as through LVH) more than the peripheral processing to BNP (in plasma or at the level of a target organ, such as the kidney, heart, or adipose tissue). Finally, it may be possible to prevent late changes in the processing pathway by therapeutic supplementation of natriuretic peptides, which could reduce BP and improve cardiorenal structure and function. Indeed, a recent study by Cataliotti et al. (24) demonstrated that early vector delivery of a proBNP gene in spontaneously hypertensive rats, which resulted in constitutive overexpression of BNP, was associated with decreased BP and improved cardiac structure and function. The beneficial effect on controlling BP was recently confirmed in a patient with uncontrolled hypertension despite treatment with conventional anti-hypertensive medication, where a small dose of subcutaneously delivered BNP induced sustained normalization of BP without the need for further therapy (25).
Study limitations
An important limitation of our study is that it is epidemiological in nature and does not describe the molecular abnormalities that result in aberrant natriuretic peptide levels in hypertension. Another limitation of the current study is that residual confounding factors cannot be ruled out. We used multiple linear regression to attempt to control this cross-sectional comparison for marked age and gender differences between hypertension categories, and indeed some differences which were not apparent in unadjusted analyses became apparent only after adjustment. This statistical adjustment procedure makes assumptions of linearity relating age and sex to log transformed peptide levels. We tested for non-linearity of age effects as well as for non-homogenous effects of these variables across hypertension categories. Although gender/hypertension category interactions were seen, a meta analysis based on gender stratified analysis gave very similar results to those presented using the simpler assumption. When we examined all hypertensive groups for evidence of interaction with age and gender, there was some evidence of gender effects that were differential by hypertensive stage. There was no evidence of differences by age, however. Table 1 of the Supplementary Data compares results of a simple gender adjustment with the results of combining separate male and female analyses with meta-analysis. These data indicate that circulating levels of NPs may be differently affected by gender.
Our study raises an important question regarding which part of the BNP system, the production of proBNP1–108 or the processing into mature BNP1–32, is more deficient in hypertension, and therefore, which of the 2 could be a better novel therapeutic target for the treatment of hypertension. Another important limitation is that we did not differentiate between essential hypertension and secondary hypertension. We also did not differentiate between the types of antihypertensive medications used in our analysis, and it may be that some individuals respond differently to different anti-hypertensive agents or may seek treatment at different points in the course of disease, making them more or less likely to benefit from their own endogenous natriuretic system. There is no definitive way to correct for medication usage, and we have, therefore, performed a variety of analyses to attempt to adjust for this factor. Specifically, we performed the analysis without adjusting for medications (Table 3, models 1 to 4), and adjusting for the medications (Fig. 1, Table 3, model 5), and also in the group of subjects who were not taking any medication (Table 4).
Table 4.
Univariate and Multivariate Analysis in Normotensive and Pre-Hypertensive Subjects Not on Antihypertension Medications
| Biomarker | Univariate Model (� ± SE) | Model 1 (� ± SE) | Model 2 (� ± SE) | Model 3 (� ± SE) | Model 4 (� ± SE) |
|---|---|---|---|---|---|
| Log proBNP1–108 | 0.03 ±0.07 | −0.00 ±0.07 | 0.02 ±0.07 | 0.02 ±0.07 | 0.02 ±0.07 |
| Log BNP1–32 (Shionogi) | 0.04 ±0.07 | −0.01 ±0.06 | −0.00 ±0.06 | 0.00 ±0.06 | 0.00 ±0.06 |
| Log BNP1–32 (Biosite) | −0.06 ±0.08 | −0.07 ±0.07 | −0.05 ±0.07 | −0.05 ±0.07 | −0.05 ±0.07 |
| Log NT-proBNP1–76 (Roche) | −0.06 ±0.08 | −0.08 ±0.07 | −0.06 ±0.07 | −0.06 ±0.07 | −0.06 ±0.07 |
| Log ANP1–28 (Phoenix Pharmaceuticals) | 0.02 ±0.05 | 0.01 ±0.05 | 0.03 ±0.05 | 0.03 ±0.05 | 0.03 ±0.05 |
| Log NT-ANP1–98 (Phoenix Pharmaceuticals) | −0.08 ±0.05 | −0.12 ±0.04* | −0.10 ±0.04* | −0.09 ±0.04* | −0.08 ±0.04 |
Values are 1 ± SE in contrast to normotensive subjects. Model 1 = age+age2+sex; Model 2 = age+age2+sex+BMI; Model 3 = age+age2+sex+BMI+LVH; Model 4 = age+age2+sex+BMI+LVH+creatinine. Logarithm of BNP and ANP values used to satisfy regression assumptions.
p < 0.05.
Abbreviations as in Table 1.
Perspective
Recent reports have described an underactivation of BNP forms in early hypertension. A seminal study by Newton-Cheh et al. (11) has now established in a cohort of 48,939 subjects that basal BP and risk for hypertension is associated with common genetic variants of the ANP (NPPA) and BNP (NPPB) genes that affect ANP and BNP levels. In that study, reduced ANP and BNP levels were characterized by elevated BP and increased risk for hypertension, leading the authors to propose the use of novel natriuretic peptides to treat hypertension. Furthermore, the presence of the single nucleotide polymorphism rs5068, characterized by a significantly higher level of circulating ANP and BNP, was associated with a 15% reduction in odds of hypertension. Thus, our data from a general adult population and the genetic studies from Newton-Cheh et al. support the existence of genetic predisposition to hypertension that is characterized by lower circulating levels of ANP and BNP. Newton-Cheh et al. have further proposed the beneficial effects of exogenous natriuretic peptides administration in the regulation of BP and support the need for their supplementation in hypertension. Recent studies from our group have confirmed such hypothesis both in animal models of hypertension and in a pilot study in human with uncontrolled hypertension (24,25). Indeed, the lack of ANP and BNP functionality, whether due to impaired processing or production, in patients in whom their biological properties would be highly beneficial, may have clinical and therapeutic implications that favor the use of such peptides for the treatment of this morbid condition. In this regard, Cataliotti et al. recently reported the favorable BP-lowering effects of subcutaneously (SQ) administered BNP in a patient with difficult to control hypertension and resistance to multiple drug regiment. Here a small dose of SQ BNP (10 J.g/kg, twice daily) completely normalized BP without the need of further supplementation with conventional antihypertensive drugs (25). This study is the first clinical evidence of the efficacious blood pressure control with a cardiac hormone in a patient with difficult to control hypertension and opens to the possible use of BNP for the treatment of resistant hypertension.
Conclusions
We reported that although there appears to be no increase in mature forms of ANP and BNP in early hypertension, multivariable modeling instead shows significant deficiency of mature forms with a trend toward reduced proBNP1–108. We conclude that secretion of proBNP1–108 is spared in early hypertension, and that it does not increase with the severity of hypertension, thus decreased levels of mature forms of BNP are better explained by either an impaired peripheral processing or an accelerated metabolism in this early stage, although a relative deficiency of proBNP1–108 production does emerge as hypertension progresses, with compensatory processing likely only in late-stage hypertension. Because LVH represents reversible end-organ damage due to essential hypertension, which is likely the single biggest modifiable risk factor for cardiac and renal disease (26), the current findings encourage further studies to evaluate whether the use of natriuretic peptides or their chimeric derivates can be useful in the treatment of human hypertension.
Acknowledgments
The authors thank the Cardiorenal Research Laboratory. Additionally, they also thank Drs. Ivan Nenadic, Wael Salem, Rishi Wadhera, Eddie Greene, Tony Windebank, and Thomas Wang for their most helpful and thoughtful discussions of this work. This work was supported by grants from the National Institutes of Health (R01 HL36634, R01-HL55502, and R01-HL76611), the Stanley J. Sarnoff Endowment for Cardiovascular Science, and the Mayo Division of Cardiology. Funding for this study was also provided in part by Bio-Rad. Dr. Cataliotti was supported by the Doris Duke Charitable Foundation (CSDA 2006064) and by the MIUR Progetto Riento dei Cervelli. Drs. Chen and Burnett have received royalties from Nile Therapeutics, Anexon, and Up-to-Date; and has patent and licensed designer natriuretic peptides with the Mayo Clinic.
Abreviations
- ANP1–28
atrial natriuretic peptide1–28
- BMI
body mass index
- BNP1–32
B-type natriuretic peptide1–32
- BP
blood pressure
- NT-ANP1–98
N-terminal atrial natriuretic peptide1–98
- NT-proBNP1–76
N-terminal pro–B-type natriuretic peptide1–76
- LV
left ventricular
- LVH
left ventricular hypertrophy
- proBNP1–108
pro–B-type natriuretic peptide1–108
Footnotes
All other authors have reported that they have no relationships relavant to the contents of this paper to disclose.
REFERENCES
- 1.Cataliotti A, Chen HH, Redfield MM, Burnett JC., Jr Natriuretic peptides as regulators of myocardial structure and function: pathophysiologic and therapeutic implications. Heart Fail Clin. 2006;2:269–276. doi: 10.1016/j.hfc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Mukoyama M, Nakao K, Saito Y, et al. Human brain natriuretic peptide, a novel cardiac hormone. Lancet. 1990;335:801–802. doi: 10.1016/0140-6736(90)90925-u. [DOI] [PubMed] [Google Scholar]
- 3.Heublein DM, Huntley BK, Boerrigter G, et al. Immunoreactivity and guanosine 3’,5’-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension. 2007;49:1114–1119. doi: 10.1161/HYPERTENSIONAHA.106.081083. [DOI] [PubMed] [Google Scholar]
- 4.Lam CS, Burnett JC, Jr, Costello-Boerrigter L, Rodeheffer RJ, Redfield MM. Alternate circulating pro-B-type natriuretic peptide and B-type natriuretic peptide forms in the general population. J Am Coll Cardiol. 2007;49:1193–1202. doi: 10.1016/j.jacc.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Macheret F, Boerrigter G, McKie P, et al. Pro-B-type natriuretic peptide(1–108) circulates in the general community: plasma determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2011;57:1386–1395. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonne JM, Campbell JM, Cataliotti A, et al. Secretion of glycosylated pro-B-type natriuretic peptide from normal cardiomyocytes. Clin Chem. 2011;57:864–873. doi: 10.1373/clinchem.2010.157438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawada Y, Suda M, Yokoyama H, et al. Stretch-induced hypertrophic growth of cardiocytes and processing of brain-type natriuretic peptide are controlled by proprotein-processing endoprotease furin. J Biol Chem. 1997;272:20545–20554. doi: 10.1074/jbc.272.33.20545. [DOI] [PubMed] [Google Scholar]
- 8.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hildebrandt P, Boesen M, Olsen M, Wachtell K, Groenning B. N-terminal pro brain natriuretic peptide in arterial hypertension—a marker for left ventricular dimensions and prognosis. Eur J Heart Fail. 2004;6:313–317. doi: 10.1016/j.ejheart.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Belluardo P, Cataliotti A, Bonaiuto L, et al. Lack of activation of molecular forms of the BNP system in human grade 1 hypertension and relationship to cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H1529–H1535. doi: 10.1152/ajpheart.00107.2006. [DOI] [PubMed] [Google Scholar]
- 11.Newton-Cheh C, Larson MG, Vasan RS, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishimoto I, Rossi K, Garbers DL. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci U S A. 2001;98:2703–2706. doi: 10.1073/pnas.051625598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 14.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen SJ, Mahoney DW, Redfield MM, Bailey KR, Burnett JC, Jr, Rodeheffer RJ. Participation bias in a population-based echocardiography study. Ann Epidemiol. 2004;14:579–584. doi: 10.1016/j.annepidem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 17.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliani I, Rieunier F, Larue C, et al. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clin Chem. 2006;52:1054–1061. doi: 10.1373/clinchem.2005.061770. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 21.Burnett JC, Jr, Kao PC, Hu DC, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 22.Lerman A, Gibbons RJ, Rodeheffer RJ, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet. 1993;341:1105–1109. doi: 10.1016/0140-6736(93)93125-k. [DOI] [PubMed] [Google Scholar]
- 23.Ellis KL, Newton-Cheh C, Wang TJ, et al. Association of genetic variation in the natriuretic peptide system with cardiovascular outcomes. J Mol Cell Cardiol. 2011;50:695–701. doi: 10.1016/j.yjmcc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Cataliotti A, Tonne JM, Bellavia D, et al. Long-term cardiac pro-B-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation. 2011;123:1297–1305. doi: 10.1161/CIRCULATIONAHA.110.981720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cataliotti A, Costello-Boerrigter LC, Chen HH, Textor SC, Burnett JC., Jr Sustained blood pressure–lowering actions of subcutaneous B-type natriuretic peptide (nesiritide) in a patient with uncontrolled hypertension. Mayo Clin Proc. 2012;87:412–415. doi: 10.1016/j.mayocp.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maisel AS. Cardiovascular and renal surrogate markers in the clinical management of hypertension. Cardiovasc Drugs Ther. 2009;23:317–326. doi: 10.1007/s10557-009-6177-4. [DOI] [PubMed] [Google Scholar]