Abstract
Saccharomyces cerevisiae is one of the most studied model organisms for the identification of genes and mechanisms that affect aging. The chronological lifespan (CLS) assay which monitors the survival of a non-dividing population, is one of the two methods to study aging in yeast. To eliminate potential artifacts and identify genes and signaling pathways that may also affect aging in higher eukaryotes, it is important to determine CLS by multiple methods. Here, we describe these methods as well as the assays to study macromolecular damage during aging in yeast, with a focus on genomic instability.
Keywords: Aging, Caloric restriction, Genomic instability, Mutation frequency
1. Introduction
Studies using the Saccharomyces cerevisiae aging model have uncovered lifespan regulatory pathways that are partially conserved in higher eukaryotes. Here, we describe the standard procedures used to study chronological aging/senescence in yeast. We high-light the importance of utilizing multiple approaches to analyze yeast chronological lifespan (CLS) to rule out system bias and identify genes and signaling pathways that may also affect aging in higher eukaryotes. The methods described here provide straight-forward approaches to determine CLS and genomic integrity while avoiding the artifacts that can be caused by the accumulation of toxic levels of acids in the medium or the regrowth of subpopulation of cells within the nondividing cultures.
2. Materials
2.1.Common Materials
Dextrose (20%, w/v) stock solution.
Sterile 18.2 MΩ.cm ultrapure water; used for media preparation, serial dilution, and survival analysis in water experiments.
Synthetic complete dextrose (SDC) medium (Table 1). Medium is prepared without glucose. After sterilization by autoclaving, glucose is added at the desired level. For SDC solid medium, add 20 g/L Bacto™ Agar.
Synthetic complete (SC) medium with various carbon sources: SC medium without carbon source; SC medium supplemented with low glucose (e.g., 0.5%, w/v) or othcr carbon sources such as ethanol (e.g., 0.8%, w/v), glycerol (1–3%, w/v), or acetic acid (10–l00 mM).
- Amino acid dropout media:
-
-SDC Arg− Can+ medium for Canr frequency assay: SDC without arginine, with l-canavanine sulfate (60 μg/mL).
-
-SDC Arg− Can+ 5FOA+ medium for gross chromosomal rearrangement (GCR) frequency assay: SDC without arginine, l-canavanine sulfate (60 μg/ mL), and 5-fluoroorotic acid (5FOA, 1 mg/mL).
-
-SDC Trp– medium for Trp– reversion and in situ viability assay: SDC without tryptophan.
-
-SDC Lys– medium for frame-shift mutation frequency assay: SDC without lysine.
-
-SC His− Gal+ medium for homologous/homeologous recombination frequency assay: SC without histidine, with galactose (2%, w/v).
-
-
Yeast extract peptone dextrose (YPD) medium: l0 g/LBacto™ Yeast Extract,20 g/LBactor™ Peptone, 20 g/L Bactor™ Agar. Dissolve in water (up to 900 mL). Autoclave and add glucose stock (20%) to a final concentration of 2%. Mix well and pour the plates.
Yeast strain: DBY746 MATα leu2-3,112 his3Δ1 trp1-289a ura3-52 GAI+.
Table 1. Synthetic complete glucose (SDC) medium.
| Component | g/L |
|---|---|
| d-Glucose | 20 |
| Ammonium sulfate | 5 |
| Nitrogen base (−AS/−AA) | 1.8 |
| NaH2P04 | 1.4 |
| mg/L | |
| Adenine | 80 |
| l-Arginine | 40 |
| l-Aspartic acid | 100 |
| l-Glutamic acid | 100 |
| l-Histidine | 80 |
| l-Isoleucine | 60 |
| l-Leucine | 120 |
| l-Lysine | 60 |
| l-Methionine | 80 |
| l-Phenylalanine | 60 |
| l-Serine | 400 |
| l-Threonine | 200 |
| l-Tryptophan | 80 |
| l-Tyrosine | 40 |
| l-Valine | 150 |
| Uracil | 80 |
SDC (18) is supplemented with a four-fold excess of histidine, leucine, tryptophan, and uracil to compensate the auxotrophies in the DBY746 strain. Similar supplementations should be carried out based on the autotrophics of the genetic background used. Adjust to pH 6.0 with NaOH.
3. Methods
3.1. Yeast CLS in Liquid Culture
From the frozen stock, streak strain of interest onto a YPD plate. Incubate the plate at 30°C for 2–3 days. Inoculate a single colony into 1–2 mL synthetic complete dextrose medium (SDC)and incubate overnight with shaking (220 rpm) at 30°C see Note 1).
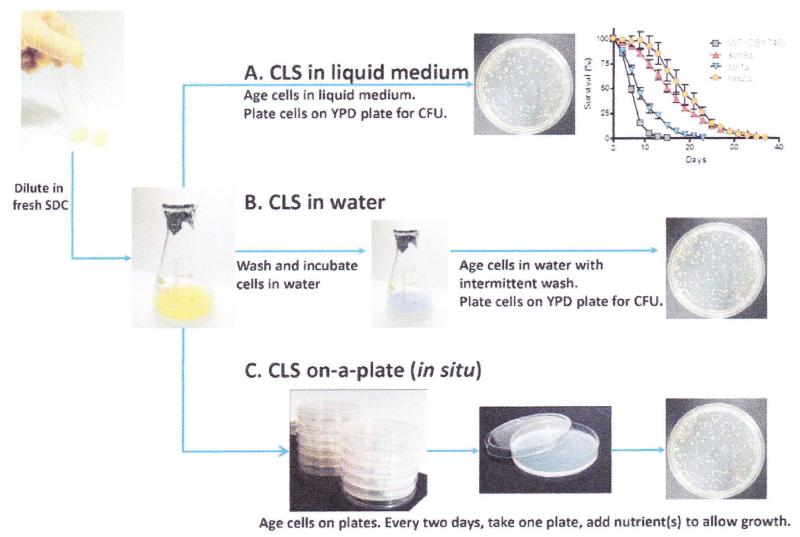
Dilute the overnight culture into fresh SDC medium (usually 10 mL) to OD600: ~0.1 (~1:100 dilution) and incubate with shaking (220 rpm) at 30°C (see Notes 2 and 3). This time point is considered day 0 ofthe chronological aging. Note that the use of aluminum foil caps (Fig. 1) minimizes evaporation and also reduces exposure of culture to oxygen (see Notes 4 and 5).
Starting at day 3, remove two aliquots of 10 μL from the flask, dilute 10,000 times in sterile water, plate 10 μL of the diluted culture (i.e ., 104–10 μL) onto YPD plates every 2 days (see Note 6). Incubate plates at 30°C for 2–3 days and count colony forming unit (CFU).
The day 3 CFU number is considered to be the 100% survival. Dilution factors of aging culture in the subsequent days should be adjusted to ensure ~20–100 colonies per plate can be counted (e.g., 104–10, 104–30, and 103–10 μL). For wild-type cells (DBY746 background), the culture reaches the 50% survival by days 6–7 and 1% survival around day 11; for the 8Y4741 background the 50% survival is reached after approximately 15 days (see Note 7).
Fig. 1.
Yeast chronological lifespan (CLS) analyses, lnoculate freshly streaked colony into 1 mL SDC medium and let grow overnight. (a) For a typical liquid SDC CLS analysis, the overnight cultures are diluted (1:100) in to fresh SDC medium to a final volume of 10 mL (with flask to culture volume of (5;1). This time point is considered day 0. Every 2 days, aliquots from the culture are properly diluted and plated onto YPD plates for Colony Forming Units (CFUs) evaluation. Viability at day 3, when the yeast had reached the stationary phase, is considered to be the initial survival (100%). Representative results of chronological survival of the wild-type (DBY746), sch9Δ, torlΔ, and ras2Δ are shown (replotted from Wei (1)). (b) For extreme CR/starvation, cells from 3-day-old SDC culture are washed three times with sterile water and resuspended in water. Every 2–4 days, cells from the water cultures are washed to remove nutrients released from dead cells. (c) Chronological survival on solid medium. Day 1 SDC cultures are diluted and plated onto agar plates (extreme calorie restriction) or tryptophan dropout (SC-TRP) plates. Plates are incubated at 30°C for the duration of the assay. Every 2 days, one plate from each set is retrieved and appropriate nutrients are added to allow growth.
3.2. Yeast CLS in Water
This water assay for CLS serves three purposes: (1) it prevents any regrowth (GASP) which would invalidate the CLS assay (see Note 7); (2) it allows the assessment of CLS independently of the composition and age-dependent modification of the extracellular medium; (3) it serves as a way to assess survival under extreme calorie restriction condition, which represents a starvation environment frequency encountered by microorganisms.
Proceed as in steps 1–3 in Subheading 3.1.
Remove conditional medium by centrifuging (l,4000 × g for 5 min) at room temperature.
Wash the cell pellet with sterile water (equal volume as the original culture) three times.
Resuspend cell pellet in water (equal volume as the original culture), incubate with shaking (220 rpm) at 30°C.
Every 2 days, aging culture must be washed with sterile water and resuspended in equal volume ofwater to remove any nutrients or metabolites (see Note 8). Attention should be paid to aseptic practices as water cultures are particularly prone to contamination during the advanced phases of survival.
Perform viability assay by sampling the aging culture as described in Subheading 3.1. This starvation condition will lead to a doubling of mean CLS in the wild-type DBY746 strain (1).
3.3. Yeast CLS on Solid
The in situ viability system that utilizes the auxotrophy of the Medium DBY746 strain (trp1) circumvents the regrowth/GASP problem mentioned above and can be used to test the effect of constant exposure to the selected external nutrients or stimuli on yeast CLS (2). This in situ viability assay is also more closely related to the yeast replicative lifespan (RLS) model where cells are constantly exposed to abundant nutrients for the duration of lifespan analysis (see Note 9). The tryptophan deficiency was selected since it is one of the deficiencies that does not result in reduction of CLS due to starvation.
Proceed as in steps 1–3 in Subheading 3.1.
Dilute the culture with sterile water to 100–200 cell/10 μL (usually 104-fold dilution of the day 3 culture) and 1,000 cells/1O μL (103-fold dilution).
Plate two aliquots of 10–30 μL of diluted culture onto one tryptophan dropout SDC plate. For each strain, prepare a set of 8–20 plates and labele according to plating density (e.g., 104-fold diluted, plate 10 μL or 104-10, 104–30, 103–10, and 103–30 μL), which ensures that 10–100 colonies can be counted in anticipation of decreasing viability during aging.
Incubate the plate set at 30°C for the duration of the lifespan analysis (see Note 10).
On the day of plating and every 2 days subsequently, remove one plate and slowly and drop-wise add 0.5 mL tryptophan (2 mg/mL) to allow viable cells to grow. Incubate the plate at 30°C for additional 2–3 days. Record the CFU as the viability of the day of tryptophan addition. The CFU on day 3 is considered 100% survival.
Variations of in situ viability assay:
For extreme calorie restriction conditions, age cells on agar plates (see SubheadingS 2). Add 1 mL 2× YPD instead of tryptophan on subsequent days to allow growth and CFU count.
For carbon source-specific growth conditions, age cells on plates with tryptophan dropout synthetic complete medium but with various carbon sources, e.g., various concentrations of glucose (calorie restriction by glucose limitation, e.g., 0.5 or 0.05% vs. the standard 2% glucose), various carbon sources such as ethanol, glycerol, acetic acid, can be used in place of glucose (3). Add 1 mL glucose/tryptophan solution (20% glucose and 1 mg/mL tryptophan) to allow growth and CFU count.
3.4. Nuclear DNA Mutation Frequency During Chronological Aging
Canavanine resistance (Canr)
Mutations in the plasma membrane arginine permease (CAN1/YEL063) render cells resistant to the arginine analogue l-canavanine. Spontaneous frequency of a wide variety of mutations can be evaluated by monitoring the frequency of canavanine resistance (Canr) in chronologically aging cultures. Sequencing of the CAN1 gene in Canr colonies collected at different time points can provide the age-dependent mutation spectrum data (with Mutation Surveyor, SoftGenetics) (4).
Base substitutions (Trp+ reversion)
Strains with trp1-289 contain an amber mutation (C403T) in the TRP1/YDR007W coding sequence . The frequency of trp1-289/wild-type reversion allows the estimation of the frequency in base substitutions during yeast chronological aging (5).
Frame-shift mutations
The Lys− strain EH150 (MATα, lys2ΔBglII, trpl-Δ, his3-Δ200, ura3-52, ade2-lo) harbors a lys2ΔBglII mutation that was constructed by inserting four nucleotides to create a BglII restriction enzyme site in the LγS2 gene. The resulting +4 shift in the open reading frame results in auxotrophy for lysine drat can be reversed by small insertion/deletion mutations (6,7).
Gross chromosomal rearrangements (GCRs)
HXT13/YEL069C is located 7.5 kb telomeric to CAN1 (YEL063C) on chromosome V. Stains carrying hxt13::URA3 can be used to measure mutation frequency in both CAN1 and URA3 genes) which render cells resistance to l-canavanine and 5-fluoroorotic acid (5FOA), respectively. Considering the low frequency of point mutations that occur in both genes, analysis of the Canr 5FOA, frequency provides an estimation of GCRs by assessing the lack of both genes (8).
Homologous and. Homologous recombinations
To monitor the level of homologous (100%) and homeologous (91%) recombinations during chronological aging, strains harboring HIS3::intron-IR-URA3 with either l00% homologous inverted repeats (IRs) (pSR406) or 91% homeologous IRs (pSR407) at the HIS3 locus are studied (9). Recombination between the IRs allows the expression of functional His3 protein.
- In parallel to normal viability assay (see Subheading 3.1), remove an appropriate arnount of cells from the aging cultures (see Notes 11 and 12).
- For Canr mutation, start with ~2 ×107 cells (~200 μL of day 3 SDC culture).
- For Trp+ reversion, start with ~108 cells (500–1,000 μL of day 3 culture).
- For Lys+ frame-shift mutation, start with ~108 cells (500–1,000 μL of day 3 culture).
- For GCRs, start with 2–3 × 108 cells (2–3 mL of day 3 culture).
- For homologous and homeologous recombination, start with ~5 × 107 cells (200–500 μL of day 3 culture).
Pellet the cells in a bench-top centrifuge (1400 × g for 5 min).
Resuspend cells in 1 mL sterile water) and pellet the cells again.
- Resuspend cell pellet in 100 μL of water. Plate cells,
- For Canr mutation, on SDC Arg− Can+ plates.
- For Trp+ reversion, on SDC Trp– plates.
- For Lys+ frame-shift mutation, on SDC Lys– plates.
- For GCRs, on SDC Arg− 5FOA+ plates.
- For homologous and homeologous recombination, on SC His− Gal+ plates (see Note 13).
Count CFUs after a 3–4 days’ incubation at 30°C. The mutation frequency is normalized to the number of viable cells (as described in Subheading 3.1 ).
Complementary to the in situ viability assay (Subheading 3.2), age-dependent Trp+ reversion, Lys+ frame-shift mutation, recombination, or Canr can also be studied in cells aged on plates.
Proceed as step 1–3 in Subheading 3.2.
Suspend cells in water at ~108 cells/100 μL. Plate 20–200 μL of cells suspension on to Trp-, Lys-, His-dropout, or Can+ SDC plates.
Incubate plates at 30°C for the duration of the CLS assay.
Every 2 days (or at the time point of interest), score the newly emerging colonies.
Mutation frequency is estimated by normalizing newly emerging colonies to the total number of viable cells of the specific day (as described in Subheading 3.2).
Footnotes
CLS assay should be carried out using freshly streaked cells. Master plates should be kept at 4°C and plates >1 week old should be discarded. Three inoculums from independent colonies should be prepared for each strain to provide biological replicas.
Maintain a 1:5 ratio of culture/flask volume (e.g., 10 mL culture in a 50 mL flask) to ensure proper mixing and aeration.
The standard CLS in liquid culture can be augmented to study the effects of various nutrients on chronological aging. For example, dilution of overnight culture in glucose-limited SC medium (e.g., 0.5 or 0.05% glucose instead of the standard 2% in SDC) leads to lower population saturation density on day 3, but much extended mean (50% survival) and maximal (10% survival) lifespans.
The uses of different flask closures greatly affect aeration, evaporation rate, and metabolism of microorganism cultures (10-12). The commonly used loose plastic cap and cotton plugs result in high evaporation rate under the standard shaking speed. Due to the long-lasting nature of the chronological aging study we chose to use aluminum foil cap (Fig. 1), which leads to less than 15% volume loss at the end of a typical 2-weeks long CLS experiment and still allows a level of oxygen sufficient for cell growth on non-fermentable carbon sources. Moreover, the use of plastic caps that allow maximum aeration will cause early depletion of ethanol and increased accumulation of acetic acid.
Special attention should be paid to culture conditions, such as culture density (due to evaporation), acidification, and accumulation of various metabolites when interpreting experimental results. In a typical CLS cxperiment, accumulation of metabolites leads to medium acidification (13, 14). Depending on the culture volume, shake speed, type of flask closure, and strain of interest, the pH of the medium normally drops from 6 to 3–4. Constant adjustment of pH by addition of NaOH or buffer solution (e.g., MES) is not recommended since it often leads to regrowth/GAsP.
For short-lived strains, daily assessment of viability is recommended. Viability can also be confirmed by live/dead staining, e.g., FUN1 (Invitrogen). Dot assay could also be used to obtain the approximate survival rate of an aging culture as an initial estimation of viability and lifespan (15).
In the liquid culture, a small fraction of the surviving cells may reenter the cell cycle using the remaining nutrients or those released from lysed dead cells to proliferate, a phenotype termed regrowth or GASP (grollth advantage in stationary phase). This phenotype is often associated with increased oxidative stress and/ or decreased protection in the cell (14, 16). We define regrowth as an increase in viability or stabilization in viability for three consecutive samplings in the high mortality phase during chronological aging. CLS analysis in water or on plate can eliminate the complication of regrowth (see Subheadings 3.2 and 3.3).
During yeast chronological aging, dead cells lyse and release nutrients. Accumulation of various metabolites affects extra-and intracellular PH, signaling, and viability of yeast. Intermittent wash of aging culture removes metabolites whose generation may be strain-specific and reduces potential systematic. for such as selecting cells resistant to media acidification or acetic acid cYtotoxicity.
The survival curve of approximately 200 wild-type DBY746 cells plated on SC Trp– plates supplemented with 2% glucose is reminiscent of that in the standard liquid SDC medium (3). However, certain amino acid auxotrophy may lead to a dramatic reduction of chronological survival, which may be strain specific. For example, survival of DBY746 is much reduced on SDC Leu–plates.
water loss due to evaporation could pose a problem for the long-term in situ chronological aging study. Every 2–4 days, dropwise add 0.5–1.0 mL sterile water to each plate and return the plates to incubator without disturbance.
Percoll density gradient fractionation can be used to isolate quiescent and non-quiescent cells to evaluate their contribution in nuclear DNA mutations in chronologically aged cells (4, 17).
Age-dependent mutation frequency varies greatly depending on the strain background, genetic manipulation, culture conditions, as well as regrowth/gasp and extremely low survival. Preliminary experiments should be carried out to determine the mutation frequency range before performing the full-scale lifespan analysis. Multiple biological replicas should be included in the study; and, both liquid and in situ viability/mutation assays should be carried out to corroborate the results.
Galactose is used to induce the expression of HIS3 in the recombination frequency assay. For strains with slow growth on galactose, after overnight incubation at 30°C, drop-wise add 1 mL 20% glucose onto the plates, return plates to 30°C incubator.
References
- 1.Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:el3. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madia F, Gattazzo C, Fabrizio P, Longo VD. Mech Ageing Dev. 2007;128:45–49. doi: 10.1016/j.mad.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei M, Fabrizio P, Madia F, Hu J, Ge H, Li LM, Longo VD. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet. 2009;5:e1000467. doi: 10.1371/journal.pgen.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madia F, Wei M, Yuan V, Hu J, Gattazzo C, Pham P, Goodman MF, Longo VD. Oncogene homologue Sch9 promotes age-dependent mutations by a superoxide and Revl/Polzeta-dependent mechanism. J Cell Biol. 2009;186:509–523. doi: 10.1083/jcb.200906011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capizzi RL, Jameson JW. A table for the estimation of the spontaneous mutation rate of cells in culture. Mutat Res. 1973;17:147–148. doi: 10.1016/0027-5107(73)90265-0. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich E, Novotny R, Kneidinger B, Holzmann V, Wintersberger U. Non-homologous end joining as an important mutagenic process in cell cycle-arrested cells. EMBO J. 2003;122:2274–2283. doi: 10.1093/emboj/cdg203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidenreich E, Wintersberger U. Replication-dependent and selection-induced mutations in respiration-competent and respiration-deficient strains of Saccharomyces cerevisiae. Mol Gen Genet. 1998;260:395–400. doi: 10.1007/s004380050909. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Kolodner RD. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat Genet. 1999;23:81–85. doi: 10.1038/12687. [DOI] [PubMed] [Google Scholar]
- 9.Datta A, Adjiri A, New L, Crouse GF, Jinks Robertson S. Mitotic crossovers between diverged sequences are regulated by mismatch repair proteins in Saccharomyces cerevisiae. Mol Cell Biol. 1996;l6:1085–1093. doi: 10.1128/mcb.16.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chain EB, Gualandi G. Aeration studies. II. Rend Ist Sup Sanit. 1954;17:1109–1163. [PubMed] [Google Scholar]
- 11.Schultz JS. Cotton closure as an aeration barrier in shaken flask fermentations. Appl Microbiol. 1964;12:305–310. doi: 10.1128/am.12.4.305-310.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDaniel LE, Bailey EG. Effect of shaking speed and type of closure on shake flask cultures. Appl Microbiol. 1969;17:286–290. doi: 10.1128/am.17.2.286-290.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8:1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, Diaspro A, Dossen JW, Gralla EB, Longo VD. Superoxide is a mediator of an altruistic aging program it Saccharomyces cerevisiae. J Cell Biol. 2004;166:1055–1067. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith DL, Jr, McClure JM, Matecic M, Smith JS. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently the Sirtuins. Aging Cell. 2007;6:649662. doi: 10.1111/j.1474-9726.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 16.Fabrizio P, Pletcher SD, Minois N, Vaupel JW, Longo VD. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 2004;557:136–142. doi: 10.1016/s0014-5793(03)01462-5. [DOI] [PubMed] [Google Scholar]
- 17.Allen C, Buttner S, Aragon AD, Thomas JA, Meirelles O, jaetao JE, Benn D, Ruby SW, Veenhuis M, Madeo F, Werner-Washburne M. Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. J Cell Biol. 2006;174:89–100. doi: 10.1083/jcb.200604072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guthrie C, Fink GR. Guide to yeast genetics and molecular biology. Methods Enzlmol. 1991;194:1–863. [PubMed] [Google Scholar]