Abstract
Background
Pain behavior in response to skin incision is developmentally regulated but little is known about the underlying neuronal mechanisms. We hypothesize that the spatial activation and intensity of dorsal horn neuron responses to skin incision differs in immature and adult spinal cord.
Methods
Single wide dynamic range dorsal horn cell spike activity was recorded for a minimum of 2 hours from anesthetized rat pups 7 and 28 days of age. Cutaneous pinch and brush receptive fields were mapped and von Frey hair thresholds determined on the plantar hindpaw before and 1-hour after a skin incision was made.
Results
Baseline receptive field areas for brush and pinch were larger and von Frey thresholds lower in the younger animals. One hour after the incision, brush and pinch receptive field area, spontaneous firing and evoked spike activity had significantly increased in the 7 day old animals, but not in the 28 day old animals. Von Frey hair thresholds decreased at both ages.
Conclusions
Continuous recording from single dorsal horn cells both pre- and post-injury shows that sensitization of receptive fields and of background and afferent evoked spike activity at 1 hour is greater in younger animals. This difference is not reflected in von Frey mechanical thresholds. These results highlight the importance of studying the effects of injury upon sensory neuron physiology. Injury in young animals induces a marked and rapid increase in afferent evoked activity in second order sensory neurons which may be important when considering long term effects and analgesic interventions.
Introduction
Acute pain resulting from surgery in patients of all ages is commonly encountered in clinical practice. Advances in understanding mechanisms of acute postoperative pain in adults have brought about a better understanding of treatment options and their impact on morbidity. Despite these advances, acute pain, and postoperative pain in particular, continues to result in increased health care costs in children and adults by increasing morbidity and prolonging hospital stay directly effecting health care resources.1 Postoperative pain and its treatment in infants and children is of particular concern as it has received much less attention, and our understanding of acute pain mechanisms during development remains poor.
In the United Kingdom there are approximately 500,000 surgical procedures requiring anesthesia in children each year, while in the United States it is estimated to be well over 3 million.2 A direct result of children having procedures is the subsequent acute pain associated with tissue trauma. Many of these children are very young at the time of tissue injury. Children who undergo painful procedures have been shown to have altered responses to stimuli long after the initial procedure.3 This occurs as a generalized response and is not necessarily restricted to the area where the previous pain was initiated.4,5 Other important potential effects of acute pain in early life may include long-lasting changes in behaviors such as have been observed in animal models and which may be permanent.6–8 There is no known correlate of these sequela in adults.
The study of central nociceptive processing has highlighted important differences in the neurobiology of nociceptive circuits in the young9,10 Developmental differences in the pattern and intensity of dorsal horn cell responses to noxious and inflammatory stimuli have been observed,11,12 and it is becoming clear that in many cases pain and analgesia in young patients is likely to differ substantially from that in adults. Whether this holds true for the pain of surgical trauma, which is a major source of noxious nociceptive input in children, is not clear. Surgical trauma is initiated by the scalpel incision which will immediately activate and damage cutaneous nerve terminals in the region, thereby altering the pattern of postsynaptic activity in central sensory circuits. This pattern of activation can be analyzed by studying the receptive field (RF) properties and the spike activity of individual dorsal horn sensory neurons that receive input from the skin in and around the area of damage. Understanding the developmental profile of this pattern of activation is likely to improve postoperative pain management in children.
The paw incision model has been extremely useful in the study of incision pain.13–15 In this model, pain from incision, as measured by an increased behavioral response to mechanical and thermal stimuli, occurs in all ages.13 However, the duration of mechanical hyperalgesia is much shorter in the young when compared to adults, and there are substantial differences in the sensitivity to preoperative local anesthetic block and cox-1 inhibition.16–18 These findings suggest a postnatal developmental regulation of key factors involved in the initiation, maintenance, and resolution of nociceptive processing following skin incision that requires investigation. In the adult, the activity of individual primary afferents and dorsal horn cells has been studied at various times post-incision in the rat.19–24 The first hours after incision are marked by a modest sensitization in mechanosensitive A delta and C fibres22 accompanied by increased background activity of subpopulations of dorsal horn cells,23 changes that are likely to underlie the observed pain behavior.13 This post-incisional central sensitization differs from other types of central sensitization25 in that it requires a continuous afferent barrage from the periphery21 and is not NMDA dependent.26 Here we have used this model of incisional pain to study the postnatal developmental regulation of dorsal horn activity following skin incision in young and adult rats. We have focused upon the dorsal horn cell cutaneous RF size and mechanical evoked activity in the first hours after incision. By performing defined incisions within a known RF we have been able to analyze how dorsal horn cell activity is altered in different spatial regions of the field. The data provide important insights into how the immediate postsynaptic effects of incision in dorsal horn nociceptive circuits differ with postnatal age and illustrate important developmental differences in responses to different stimulus modalities following surgery.
Materials and Methods
Sprague-Dawley rats of both sexes and aged postnatal day (P) 7 (1 week) and P28 (4 weeks) were used in this study. All experiments electrophysiology experiments were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986. Behavior experiments were done after approval from the Animal Care and Use Committee (Wake Forest University, Winston-Salem, NC).
In vivo electrophysiology
Rats were anesthetized with a single inhalational anesthetic isoflurane 3.5% via nose cone. The animals were tracheotomized and placed on a ventilator with 95% O2 and 5% CO2 and placed in a stereotaxic frame and held with ear and hip bars. Temperature was maintained with a heated blanket and the heart rate was monitored throughout by electrocardiogram. The lumbar spinal cord was exposed by laminectomy, held stable with a rostral vertebral clamp, and the dura removed. Once the animal was stabilized, the isoflurane was reduced and kept constant at 1.4%.
Extracellular recordings of dorsal horn cell activity were made in the L4–5 spinal cord with 10-μm-tip glass-coated tungsten microelectrodes, lowered onto the cord surface under microscopic vision. Vertical tracks were made through the dorsal horn in 2- or 10-μm steps using a microdrive. The average depths of cells in this study were 216 μm from the surface of the spinal cord in the P7 and 353 μm in the P28 animals and were therefore classified as cells from deep laminae III, IV, and V.12 Figure 1 shows a scatter plot of the depth of the cells at the different ages. Recordings were fed into Chart software (AD Instruments, Chalgrove, Oxfordshire, UK), and data were analyzed using Chart software spike histogram. All cells were wide dynamic range (WDR) neurons responding to the initial search stimulus of stroking the paw and subsequently to both brush and pinch. Single spikes were isolated and background activity noted. Any cell that was not a WDR (cell responding to only brush or only pinch) was discarded and any cell that could not be carried throughout the entire testing paradigm was omitted. Throughout the recording period, spike shape was scrutinized to ensure that the same cell was still being recorded. Only one cell was studied per animal. Animals were euthanized under isoflurane anesthesia with an overdose of sodium pentobarbital at the end of the experiment.
Figure 1.
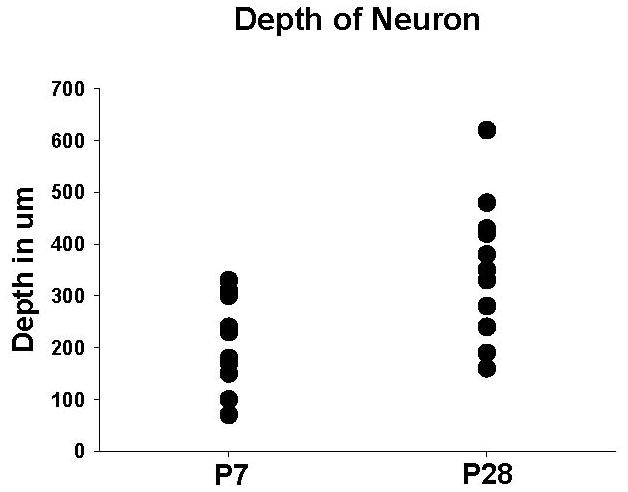
Scatter plot of the depth of the cells from the surface of the spinal cord at the different age animals. The average depth of the neuron from the P7 animals was 216 μm from the surface of the spinal cord and 353 μm in the P28 animals (P7 = postnatal day 7, P28 = postnatal day 28, μm=micrometers).
Establishing RF boundaries
All RFs were located in the middle of the plantar hindpaw in both P7 and P28 animals so the center of the RF was as close to the center of the paw as possible (see Figure 2). The spatial extent of the cutaneous RF was mapped with a standard fine artist paint brush for light touch and rounded blunt forceps for pinch. The edge of the field was defined as the area where no spikes were evoked by skin stimulation.
Figure 2.

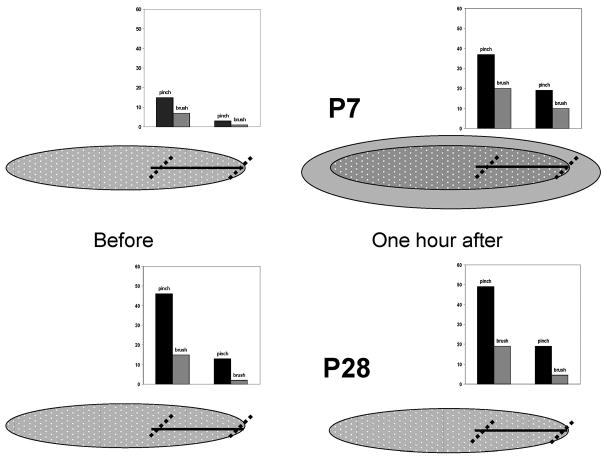
The paw of the rat and the experimental protocol. After isolation of the cell with the entire receptive field (RF) in the plantar surface of the paw, the RFs to low threshold brush and high threshold pinch were mapped. A marker was used to draw the length of the incision (straight black line down the center of the foot) relative to the size of the foot from the center of the RF to just outside the RF. The threshold and the responses to threshold and suprathreshold were determined at the center of the RF adjacent to the incision. Brush and pinch RF were mapped and responses were recorded in the center of the RF across the incision and at the extent of the incision inside at the outer edge of the RF (the small circles for the thresholds and arrows for the brush and the pinch at the limits of the incision). An incision was made along the line and closed with one suture. One hour later the same testing paradigm was again repeated.
Quantitative spatial RF mapping
To map the gradient of cutaneous sensitivity across the RF, pinch and brush stimuli were applied at 2 defined sites within the RF, and the spike activity recorded. One site was near the RF center (inner) and the other was at the edge of the RF (outer); both were across the skin mark for the incision (Figure 1). The number of spikes evoked by a 1 sec pinch and 3 consecutive brush strokes was recorded at each site.
Measuring responses to von Frey hairs
Mechanical RF thresholds were established by sequential application of calibrated von Frey hair filaments (vFh) of increasing strength to the center of the RF (see Figure 1). The RF mechanical sensitivity was also tested at this site by measuring spike activity evoked by a 3-sec skin application of both threshold and suprathreshold (3 hairs above threshold) vFh.
Experimental procedure
Upon isolation of single unit spikes, the background activity was noted for 15 min before mapping the boundaries of the cutaneous RF. When the RF boundaries had been established, a line was drawn on the skin that extended from the center of the field to just beyond the outer edge of the pinch field (Figure 2). Quantitative spatial mapping of the RF was then performed by measuring the sensitivity to brush and pinch at two defined site near this line, one in the center of the RF (inner) and one on the RF boundary (outer). A skin incision was then made along the line of the skin mark, such that it went across the center of the RF and reached the outer edge of the RF boundary (see Figure 2). The incision was standardized to be one-half the size of the original incision used in a previous study, or slightly less than the half of the distance from the toe pads to the heel, and was closed using one inverted mattress suture with 5.0 braided silk on an FS2 needle.15 One hour later, the background activity was again noted, and the above stimulation procedure was repeated.
Behavioral von Frey hair testing of mechanical threshold
Animals were placed on a mesh floor in a plastic cage and acclimatized to the environment for 20 min prior to testing. Withdrawal to mechanical stimulation was assessed on the hind foot with application of calibrated von Frey filaments to the footpad just anterior and lateral to the incision until the filaments bent. The von Frey filaments used were 3.84, 4.08, 4.31, 4.56, 4.74, 4.93, 5.18, 5.46, 5.88, 6.10 corresponding to 0.5, 0.9, 1.7, 3.7, 5.5, 8.0, 12.4, 21.5, 53.0, and 84 g, respectively. This was done 3 times with a positive response determined by brisk withdrawal of the paw. The force resulting in withdrawal with a 50% probability was determined using the up-down method. Withdrawal threshold was determined before surgery and 1 hour after surgery. All animals were included in the data analysis, and no animal in the study had a wound dehiscence or infection during the study.
Data Analysis
Baseline and background spikes were counted with a fixed window length of 30 sec. The total number of spikes fired in response to each stimulus was counted, with the length of the window for spike counting kept at the duration of the stimulus so as to exclude after discharge. This time was consistent over all cells; the window lengths were approximately 3 sec for vFh threshold and suprathreshold, 0.5 sec for pinch, and 1 sec for brush. Recordings were fed into Chart software (AD Instruments, Chalgrove, Oxfordshire, UK), and data were analyzed using Chart software spike histogram. Receptive field areas were mapped on paper and then digitized, and the area calculated as percent of the surface area of the paw (surface area being age-dependent). The average absolute area of the RF at baseline in P7 animals was 72 mm2, while in the P28 animals it was 220 mm2.
Data were analyzed using the paired t-test and adjusted for multiple comparisons using the Bonferroni correction where appropriate for within groups comparison. For comparison between groups, a change score was calculated by taking the difference between the response before and after incision. This was analyzed using an unpaired t-test and is presented as the change score with a 95% confidence interval where significant. There is inconsistency in the literature regarding presentation and analysis of von Frey filament withdrawal thresholds. Under many circumstances non-parametric assumptions should be considered with von Frey filament withdrawal threshold analysis and presentation. This is the case for the von Frey filaments threshold response based on WDR neuron response and therefore threshold is a given filament where the median and range are presented. The Wilcoxon Signed Rank was used to test for differences in threshold from incision. This is different from the behavioral withdrawal threshold. The behavior 50% withdrawal threshold in grams is derived from patterns of withdrawal to different von Frey filaments using the up-down method for behavior and these data achieve interval (continuous) level measurement status. Additionally, the results for the inference testing were confirmed using non-parametric testing, Wilcoxon signed rank and Mann-Whitney where appropriate, and significance holds up under these circumstances. Therefore, we consider our data on 50% withdrawal threshold to satisfy the assumptions for parametric statistics based on normal distribution and interval (continuous) measurement and as a result means and standard error of the means are presented and analyzed with parametric statistics. A paired t-test was used for analysis of mechanical thresholds and the group differences were compared using a change score and an unpaired t-test. Statistics were done using JMPIN Version 5.1 (SAS Institute, Cary, NC). No formal power calculation was performed prior to doing this study as the effects of incision had not been studied before. Effect size was determined using standard estimates of the Cohen d whereby the average mean difference was divided by the standard deviation. Significance is p<0.05. Data are presented as the mean ± the standard error (SE) of the mean.
Results
These experiments required stable extracellular recording of single WDR deep dorsal horn cells for over 2 h in order to map RF properties before and after skin incision with precision. This was achieved in 12 cells at postnatal day (P)7 and 11 cells at P28. Spike shape and amplitude were compared before and 1 hour after the skin incision to ensure that the same WDR cell was recorded throughout the experiment. Representative spike traces from P7 and P28 WDR neurons are shown in Figure 3.
Figure 3.
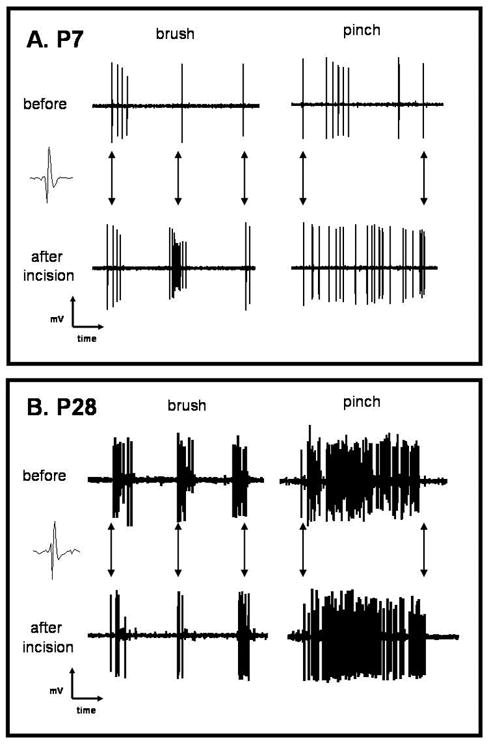
Representative raw tracings of elicited responses from a single cell in P7 and P28 animals before and 1-h after incision (P7 = postnatal day 7, P28 = postnatal day 28). Multiple spikes are elicited from the stimuli at both ages. The baseline response was more robust in the older animals. A greater increase can be seen for both the brush and the pinch after incision in the P7 (A), but only a slight difference if any can be seen in the P28 cell (B). Arrows show the three brush strokes and arrows for the pinch show the beginning of the stimulus and the end of the pinch. The responses of a single cell can readily be seen to pinch with multiple spikes, while the pinch is in place and then an end to spikes with removal of the stimulus (A and B) and the multiple spikes elicited with 3 strokes of the brush (A and B).
Background firing increases after skin incision in P7 but not P28 rats
Table 1 shows the mean background firing frequency of WDR neurons before and 1-h after skin incision at P7 and P28. While there was no difference in background firing frequency at the two ages before the incision, at P7 there was a significant (~10-fold) increase in background spike activity 1-h after the incision. This was in contrast to P28, where skin incision had no effect upon background firing.
Table 1.
Background Firing and Evoked Responses to Brush and Pinch Stimulation before and 1-hour after Incision at P7 and P28
| Background | Pinch Inner | Pinch Outer | Brush Inner | Brush Outer | |
|---|---|---|---|---|---|
| P7 (N=12) | |||||
| Before | 0.2 ± 0.1 | 15.2 ± 3.9 | 3.15 ± 1.5 | 6.7 ± 1.4 | 0.9 ± 0.3 |
| After | 1.9 ± 1.2 | 37.3 ± 9.3 | 20.3 ± 4.8 | 18.9 ± 6.4 | 9.5 ± 3.6 |
| p-value | 0.02 | 0.05 | 0.01 | 0.04 | 0.03 |
| P28 (N=28) | |||||
| Before | 0.3 ± 0.1 | 46.0 ± 11.3 | 13.2 ± 3.6 | 15.1 ± 6.2 | 1.8 ± 0.6 |
| After | 0.4 ± 0.2 | 49.4 ± 6.5 | 19.3 ± 4.6 | 19.0 ± 6.8 | 4.6 ± 1.1 |
| p-value | ns | ns | ns | ns | 0.03 |
| p-value for Change Score between ages | ns | 0.03 | ns | 0.04 | ns |
All numbers are spikes/second during stimulus
ns = not statistically significant
P7 = postnatal day 7
P28 = postnatal day 28
Innocuous brush and noxious pinch RFs enlarge 1 hour after skin incision at P7 but not at P28
The mean brush RF size of WDR neurons at the two ages, at baseline and 1-h after incision, are shown in Figure 4A. Consistent with previous reports,12, 27 we observed that the mean baseline RF size for innocuous brush is significantly larger at P7 (31.6 ± 5% of the surface area of the foot) than at P28 (17.5 ± 3.4%). Despite this, 1-h post-incision the brush RF had significantly increased in the P7 rats to 55.5 ± 7% of the surface area of foot. In contrast, there was no significant difference in the brush RF in P28 rats (20.8 ± 3.9%) after the incision.
Figure 4.
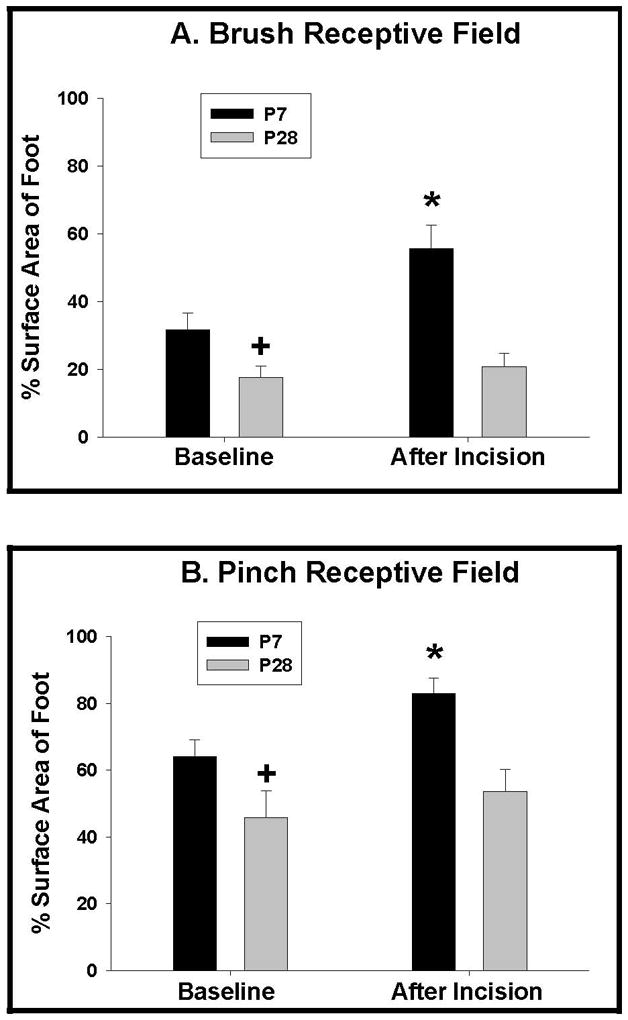
Receptive field (RF) size for brush (low threshold) and pinch (high threshold) before and 1-h after incision in P7 and P28 animals (P7 = postnatal day 7, P28 = postnatal day 28). A) Brush RF is larger in the P7 animals at baseline (+) (p<0.05). Brush RF increases 1-h after the incision in the P7 animals (*) (p<0.05), but does not increase significantly in the P28 animals. B) Pinch RF size before and 1-hour after incision in P7 and P28 animals. Pinch RF is larger in the P7 animals at baseline (+) (p<0.05). Pinch RF increases 1-hour after the incision in the P7 animals (*) (p<0.05), but does not increase significantly in the P28 animals.
The same increase was observed in pinch RF size. Figure 4B shows that, like brush RFs, the mean baseline RF size for noxious pinch in P7 WDR cells is significantly larger (64 ± 5% of the surface are of the foot) than that of P28 rats (45.7 ± 8%, p<0.05). One hour after incision, the pinch RF size in P7 pups increased significantly to 83 ± 5% (P<0.05) of the surface area of the foot after the incision, while the mean pinch RF size at P28 after incision was not significantly different from baseline (53.6 ± 5%).
A change score was calculated for both brush and pinch to directly compare the differences between the P7 and the P28 RF. The increase in RF for brush and pinch were significantly larger in the P7 with a change score for brush of 22.6 (95% C.I. 14.7, 30.5) compared to 2.9 (95% C.I. 2.6, 3.2) in the P28 animals and a change score in the P7 for pinch of 19.2 (95% C.I. 15.1, 23.3) compared to 7.9 (95% C.I. 5.9, 9.9) in the P28 animals.
Spatial analysis across RFs reveals a marked increase in brush and pinch evoked spikes at the edges of the RF one hour after skin incision at P7
To test whether the increase in RF size after skin incision at P7 was accompanied by increased cutaneous sensitivity at the RF boundaries, neuronal spike activity evoked by low-threshold brush and the high-threshold pinch was measured at inner and outer zones in the RF before and 1-h after skin incision. The results are shown in Table 1. At both ages, the WDR cells show a gradient across their RF, such that testing in the inner part of the RF always produces more spikes than testing in the outer part, and this gradient is maintained after skin incision. However, Table 1 also shows that in P7 animals, both brush and pinch responses at both sites increased significantly 1-h after skin incision. This increase was especially marked at the outer site, where brush responses increased ~10-fold and pinch responses ~6-fold, thereby significantly reducing the sensitivity gradient between the inner and outer RF. In P28 animals, skin incision had no effect at 1-h upon pinch-evoked spike activity at either the inner or outer site. There was also no difference in the neuronal response to the low-threshold brush inside the RF next to the incision (inner). The only increase in evoked activity observed at P28 animals was to brush next to the incision at the edge (outer) of the initial RF (from 1.8 spike/s before the incision, to 4.6 spikes/s after the incision).
Magnitude of RF effects
The magnitude of the effect was analyzed using the Cohen d. The effect size was very large in the change in the RF size in the P7 animals with a Cohen d value of 1.0 for the low-threshold brush RF and 0.7 for the high-threshold pinch RF. However, the effect size in the P28 was small in the older P28 adult animal with a Cohen d of 0.2 for the low-threshold brush RF and 0.3 for the high-threshold pinch RF. This relationship was similar across all outcome measures reported. The significance of this is that there is a much greater amount of afferent activity generated on any single WDR from nociceptive input in the young animal because the area causing activation is much greater. Although, there appears also to be increased activity in the older animals following incision, the study is not powered to detect the difference. In addition, the relative change in electrical signal with more spikes arriving at the WDR from a given stimulus in the RF of any given peripheral neuron after the incision leads to a large relative increase in electrical activity in the WDR in the P7 that is not matched at P28, at least in the initial hour after incision. It is the ubiquity of the response in the P7 animals that is of note when considering the increased amount of neural input arriving at the spinal cord with a given insult and the effect size of the changes after incision.
Mechanical von Frey hair responses in the center of the RF increases one hour after skin incision at both ages
While the increases in WDR cell spontaneous activity and RF size were significantly greater at P7 compared to P28, this was not true of responses to single punctuate von Frey hair stimulation. The vFh thresholds and spike responses in the center of the RF before and after a skin incision are shown in Table 2. The baseline mean vFh threshold at P7 was lower than at P28 (4.0 g (0.6–8.5) versus 7.5 g (0.4–8.5)), although this difference was not statistically significant. One-hour after incision, the threshold decreased significantly at both ages (p<0.05). but the effect was greater in the P28 animals. The number of spikes evoked by neurons to both threshold and suprathreshold vFh stimulus was significantly increased 1-h after incision in both the P7 and the P28 animals. The threshold spike response and the suprathreshold responses nearly doubled at P7 and at P28.
Table 2.
Mechanical Thresholds and Responses to Threshold and Suprathreshold von Frey Hair Stimulation before and 1-hour after Incision
| Threshold (g) | Threshold Response (spikes/sec) | Suprathreshold Response (spikes/sec) | |
|---|---|---|---|
| P7 | |||
| Before | 4 (0.6 – 8.5) | 4.8 ± 1.5 | 13.7 ± 2.9 |
| After | 2.5 (0.4 – 8.5) | 8.2 ± 1.4 | 23.1 ± 5.7 |
| p-value | <0.05 | <0.05 | <0.05 |
| P28 | |||
| Before | 7.5 (0.4 – 8.5) | 3.4 ± 0.8 | 11.0 ± 2.4 |
| After | 1.8 (0.4 – 6.4) | 8.7 ± 1.7 | 22.4 ± 6.2 |
| p-value | <0.05 | <0.05 | <0.05 |
| p-value for Change score between ages | ns | ns | ns |
Threshold and suprathreshold (ST) responses are elicited spikes to threshold von Frey hair and suprathreshold von Frey hair.
ns = not statistically significant
P7 = postnatal day 7
P28 = postnatal day 28
This result was reflected in behavioral studies. Withdrawal thresholds in awake animals before and 1 hour after incision are presented in Figure 5. As with individual WDR cells, mechanical thresholds at baseline were lower in young animals (5.7 + 0.1 grams in P7 and 18.8 + 0.8 grams in P28 (p<0.05). After surgery threshold decreased significantly at both ages (p<0.05) with the thresholds being different between the ages (p<0.05), but the effect was greater at P28 than at P7 (p<0.05). The difference between the 2 ages was significant (p<0.05), with a threshold change score in grams for the P7 of 3.9 (95% CI 3.8, 4.0) compared to 20.7 (95% CI 16.4, 25.1) in the P28 animals.
Figure 5.
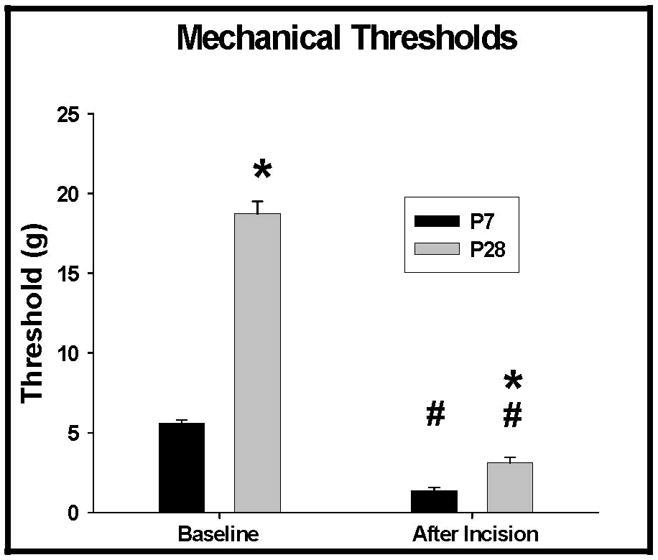
Mechanical thresholds in grams for P7 and P28 animals before surgery and 1 hour after incision (P7 = postnatal day 7, P28 = postnatal day 28). Baseline and 1 hour thresholds are different between the 2 ages (*) (p<0.05). Both ages had a significant decrease in threshold 1 hour after incision (#) (p<0.05). However, there was a greater decrease in threshold in the P28 animals compared to the P7 animals (83.4 % versus 72.4%, respectively). The difference between the 2 ages was significant (p<0.05), with a change score for the P7 of 3.9 (95% CI 3.8, 4.0) compared to 20.7 (95% CI 16.4, 25.1) in the P28 animals.
Discussion
In this paper we have shown that there is a marked difference in the acute initial effects of surgical incision on RF size and evoked responses of dorsal horn WDR cells in young and mature rats. As might be expected, dorsal horn WDR cells at both ages rapidly become more sensitive to mechanical von Frey hair stimulation at the center of the RF following an incision across the field, presumably as a result of peripheral afferent terminal sensitization. A delta and C fibers adjacent to a skin incision are known to become sensitized after one hour in adults 21 and while this has not be directly tested in newborns, the ability of nociceptors to sensitize has been reported from early stages of development.28,29 In contrast, other changes, such as in RF size and RF sensitivity gradient to brush and pinch, which result from central integration of afferent input across wider areas of the RF, were substantially different at the two ages. At P7 there is a rapid expansion of both brush and pinch RF size which is not observed at P28. In addition brush and pinch evoked activity at the inner and outer edge of the incision is markedly increased at P7, reducing the sensitivity gradient across the RF. This is in contrast to the modest change in brush responses at the outer edge only at P28. Evidently then, the response to tissue trauma from surgical incision in young animals has a different temporal and spatial pattern from adults. The results suggests a more immediate integration of afferent input leading to an intense activation across the RF of the WDR neurons in the young spinal cord which is not observed in older animals. Figure 6 summarizes these differences in the initial RF response of WDR cell to skin incisions at P7 and P28.
Figure 6.
Diagram illustrating the change in brush and pinch responses across the inner and outer receptive field (RF) zones (dotted lines) at P7 and P28 before and one hour after an incision is made in the RF (solid line within oval field) (P7 = postnatal day 7, P28 = postnatal day 28). Average data are plotted in spikes per sec.
The results were obtained using precise skin incisions within the RF of individual dorsal horn neurons combined with careful analysis of responses to standardized cutaneous stimuli at different sites within the RF. As reported elsewhere baseline RF sizes to brush stimuli were relatively larger in the paw of the younger animal when compared to the older animals.12, 27 We show that this is also true of pinch RFs. Despite their relatively larger size, the RF of the P7 WDR neurons increased to both the high-threshold and the low-threshold stimuli in response to incision, while in the P28 animals the RF size did not acutely change in response to the incision. Previous studies in adult animals have shown an increase in RF in 15 of 29 of WDR neurons 1-h after an adjacent skin incision.23 There are differences in methodology from our study. These studies did not map the entire cutaneous RF; rather the increase in RF was characterized as a response in an area that was previously unresponsive with examples of increases in RF provided. In our current study, the actual size of the RF was calculated before and after incision from the same WDR neuron. The difference in the brush evoked response at the edge of the RF in the P28 animals, the only RF parameter that was different for the P28 animals, suggests that RF changes were taking place but that these were modest in comparison to those at P7. It is important to note that the response appears to be increasing after incision in the older animals in the other modalities tested, but the study is not powered to evaluate this. The Cohen d presented suggests the magnitude of the effect is greater in the young on the RF. There is in fact an effect on RF in the older animals from the Cohen d, but the smaller effect size is not powered to pick up this difference either.
The mechanism behind the rapid and marked change in RF size and responsiveness in the young animal is unclear. Rapid expansion of RF size and increased sensitivity occurs because dorsal horn WDR RFs are surrounded by “low probability” firing zones, where stimulation normally results in subthreshold depolarization. In adult rats, intense nociceptor stimulation outside the RF causes rapid inclusion of these zones into the RF thus increasing their spatial extent, amplifying their responsiveness, and reducing their thresholds.30 The fact that these rapid RF changes following skin injury are restricted to younger animals suggests that young WDR neurons are subject to much greater injury induced nociceptor barrage than adults.
One possibility is that more afferent activity is evoked from the damaged area in young animals but this is unlikely as afferent firing frequencies in immature A ∂ and C fibres are, if anything, lower in immature animals compared to adults.31 In addition, the comparable increase in vFh evoked activity at P7 and P28 suggests similar peripheral sensitization at the two ages. A more likely reason, therefore, lies in the immaturity in central processing in dorsal horn circuits, especially in inhibitory interneurons. Stimulation of both low and high threshold afferents excites both excitatory and inhibitory interneurons in the dorsal horn and the balancing action of inhibition is an essential part of sensory processing. Much evidence suggests that central inhibitory processing is less effective in the young dorsal horn leading to larger baseline RFs and sensitization to repetitive low threshold stimulation.10,32 This may be due immature connections at the circuit level33 or to functional immaturity of inhibitory synapses.34,35 The relative lack of brainstem inhibitory descending controls of spinal nociceptive circuits are also likely to play a role.36,37 One explanation, therefore, for the early exaggerated sensitization of P7 RFs after skin incision, is that the balancing action of inhibitory interneuronal activity that is normally rapidly recruited upon noxious stimulation in adults,38 is not present at younger ages. Consistent with this proposal is the relative delay in the maturation of glycinergic synaptic activity in the newborn dorsal horn compared to GABA (gamma-amino butyric acid) ergic synaptic activity.34 Strong tonic glycinergic inhibition with characteristic fast kinetics is a feature of the adult dorsal horn but is absent in the newborn, where slower GABA inhibition dominates.39
The RF response to skin incision at P7 differs from the response to experimental carageenan inflammation where increases in RF were not found until after the second postnatal week.12 Pain signaling differs depending upon the nature of the injury and it is clear that that the mechanisms of surgically-induced tissue trauma pain have unique qualities40 and may represent a complex combination of both inflammatory and neuropathic pain as suggested for postoperative pain.41
The behavioral von Frey hair withdrawal data demonstrated a decrease in threshold after incision in both age animals, the decrease being greater in the older animal, as previously reported in slightly older animals 2–4 hours after incision.15–17 Previous studies have suggested that enhanced dorsal horn responses to punctate mechanical stimuli code for the decreased von Frey hair withdrawal thresholds21,23 and this is supported here. What is also clear from this study, however, is that a different picture of sensory sensitization emerges when examining integrated responses to brush and pinch across whole neuronal RFs compared to simply measuring von Frey thresholds (either with behaviour or electrophysiology). Receptive field analysis shows that surgical incision has a greater impact on the sensory neuronal traffic in the young dorsal horn than is apparent from von Frey hair thresholds tests alone.
The findings presented in this paper have revealed fundamental differences that occur during development in response to tissue trauma from surgical incision. Sensory circuits in the young dorsal horn respond more rapidly to the afferent barrage from skin incision than adult circuits, leading to a striking enlargement of RFs within an hour of the tissue damage. Further studies to determine the mechanisms of the rapid changes, the time course, and the long-term implications of early spinal cord activation will be essential. With greater understanding of the underlying differences in responses during development, better and more directed interventions can be designed to reduce unwanted short- and long-term consequences of skin damage in young patients.
Summary.
Paw incision immediately increases evoked neuronal activity to a greater extent in very young animals. These developmental differences may impact short- and long-term responses to surgery and alter therapeutic considerations in the young
Acknowledgments
Funding sources: The Medical Research Council of the United Kingdom, the University College London Wellcome Trust Neuroscience PhD programme (LB), London, United Kingdom and the United States National Institutes of Health, National Institute of General Medical Science Grant No. GM72105 Grant, Bethesda, Maryland, USA
References
- 1.Strassels SA, Chen C, Carr DB. Postoperative analgesia: economics, resource use, and patient satisfaction in an urban teaching hospital. Anesth Analg. 2002;94:130–7. doi: 10.1097/00000539-200201000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Tanner S. Trends in children’s surgery in England. Arch Dis Child. 2007;92:664–7. doi: 10.1136/adc.2006.099705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taddio A, Goldbach M, Ipp M, Stevens B, Koren G. Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet. 1995;345:291–2. doi: 10.1016/s0140-6736(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 4.Schmelzle-Lubiecki BM, Campbell KA, Howard RH, Franck L, Fitzgerald M. Long-term consequences of early infant injury and trauma upon somatosensory processing. Eur J Pain. 2007;11:799–809. doi: 10.1016/j.ejpain.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Hermann C, Hohmeister J, Demirakça S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125:278–85. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–37. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–96. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Torsney C, Fitzgerald M. Spinal dorsal horn cell receptive field size is increased in adult rats following neonatal hindpaw skin injury. J Physiol. 2003;550:255–61. doi: 10.1113/jphysiol.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7:246–57. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–20. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 11.Walker SM, Meredith-Middleton J, Lickiss T, Moss A, Fitzgerald M. Primary and secondary hyperalgesia can be differentiated by postnatal age and ERK activation in the spinal dorsal horn of the rat pup. Pain. 2007;128:157–68. doi: 10.1016/j.pain.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Torsney C, Fitzgerald M. Age-dependent effects of peripheral inflammation on the electrophysiological properties of neonatal rat dorsal horn neurons. J Neurophysiol. 2002;87:1311–7. doi: 10.1152/jn.00462.2001. [DOI] [PubMed] [Google Scholar]
- 13.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 14.Zahn PK, Gysbers D, Brennan TJ. Effect of systemic and intrathecal morphine in a rat model of postoperative pain. Anesthesiology. 1997;86:1066–77. doi: 10.1097/00000542-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Ririe DG, Vernon TL, Tobin JR, Eisenach JC. Age-dependent responses to thermal hyperalgesia and mechanical allodynia in a rat model of acute postoperative pain. Anesthesiology. 2003;99:443–8. doi: 10.1097/00000542-200308000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Ririe DG, Barclay D, Prout H, Tong C, Tobin JR, Eisenach JC. Preoperative sciatic nerve block decreases mechanical allodynia more in young rats: is preemptive analgesia developmentally modulated? Anesth Analg. 2004;99:140–5. doi: 10.1213/01.ANE.0000114181.69204.72. [DOI] [PubMed] [Google Scholar]
- 17.Ririe DG, Prout HM, Eisenach JC. Effect of cyclooxygenase-1 inhibition in postoperative pain is developmentally regulated. Anesthesiology. 2004;101:1031–5. doi: 10.1097/00000542-200410000-00034. [DOI] [PubMed] [Google Scholar]
- 18.Ririe DG, Prout HD, Barclay D, Tong C, Lin M, Eisenach JC. Developmental differences in spinal cyclooxygenase 1 expression after surgical incision. Anesthesiology. 2006;104:426–31. doi: 10.1097/00000542-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–72. doi: 10.1097/00000542-199903000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Zahn PK, Brennan TJ. Incision-induced changes in receptive field properties of rat dorsal horn neurons. Anesthesiology. 1999;91:772–85. doi: 10.1097/00000542-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol. 2002;87:721–31. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 22.Hämäläinen MM, Gebhart GF, Brennan TJ. Acute effect of an incision on mechanosensitive afferents in the plantar rat hindpaw. J Neurophysiol. 2002;87:712–20. doi: 10.1152/jn.00207.2001. [DOI] [PubMed] [Google Scholar]
- 23.Vandermeulen EP, Brennan TJ. Alterations in ascending dorsal horn neurons by a surgical incision in the rat foot. Anesthesiology. 2000;93:1294–302. doi: 10.1097/00000542-200011000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Banik RK, Brennan TJ. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain. 2004;112:204–13. doi: 10.1016/j.pain.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain. 2005;114:499–510. doi: 10.1016/j.pain.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J Physiol. 1985;364:1–18. doi: 10.1113/jphysiol.1985.sp015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koltzenburg M, Lewin GR. Receptive properties of embryonic chick sensory neurons innervating skin. J Neurophysiol. 1997;78:2560–8. doi: 10.1152/jn.1997.78.5.2560. [DOI] [PubMed] [Google Scholar]
- 29.Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–50. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- 30.Woolf CJ, King AE. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci. 1990;10:2717–26. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzgerald M. Cutaneous primary afferent properties in the hind limb of the neonatal rat. J Physiol. 1987;383:79–92. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proc Natl Acad Sci U S A. 1999;96:7719–22. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremner L, Fitzgerald M. Postnatal tuning of cutaneous inhibitory receptive fields in the rat. J Physiol. 2008;586:1529–37. doi: 10.1113/jphysiol.2007.145672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci. 2004;24:4749–57. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordero-Erausquin M, Coull JA, Boudreau D, Rolland M, De Koninck Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: impact of chloride extrusion capacity. J Neurosci. 2005;25:9613–23. doi: 10.1523/JNEUROSCI.1488-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hathway G, Harrop E, Baccei M, Walker S, Moss A, Fitzgerald M. A postnatal switch in GABAergic control of spinal cutaneous reflexes. Eur J Neurosci. 2006;23:112–8. doi: 10.1111/j.1460-9568.2005.04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res. 1986;389:261–70. doi: 10.1016/0165-3806(86)90194-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhou HY, Zhang HM, Chen SR, Pan HL. Increased nociceptive input rapidly modulates spinal GABAergic transmission through endogenously released glutamate. J Neurophysiol. 2007;97:871–82. doi: 10.1152/jn.00964.2006. [DOI] [PubMed] [Google Scholar]
- 39.Chéry N, de Koninck Y. Junctional versus extrajunctional glycine and GABA(A) receptor-mediated IPSCs in identified lamina I neurons of the adult rat spinal cord. J Neurosci. 1999;19:7342–55. doi: 10.1523/JNEUROSCI.19-17-07342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America. 2005;23:1–20. doi: 10.1016/j.atc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]