Abstract
Surgical resection remains the standard of care for functionally operable early-stage non-small-cell lung cancer (NSCLC) and resectable stage IIIA disease. The role of invasive staging and restaging techniques is currently being debated, but they provide the largest biopsy samples which allow for precise mediastinal staging. Different types of operative procedures are currently available to the thoracic surgeon, and some of these interventions can be performed by video-assisted thoracic surgery (VATS) with the same oncological results as those by open thoracotomy. The principal aim of surgical treatment for NSCLC is to obtain a complete resection which has been precisely defined by a working group of the International Association for the Study of Lung Cancer (IASLC). Intraoperative staging of lung cancer is of utmost importance to decide on the extent of resection according to the intraoperative tumour (T) and nodal (N) status. Systematic nodal dissection is generally advocated to evaluate the hilar and mediastinal lymph nodes which are subdivided into seven zones according to the most recent 7th tumour-node-metastasis (TNM) classification. Lymph-node involvement not only determines prognosis but also the administration of adjuvant therapy.
In 2011, a new multidisciplinary adenocarcinoma classification was published introducing the concepts of adenocarcinoma in situ and minimally invasive adenocarcinoma. This classification has profound surgical implications. The role of limited or sublobar resection, comprising anatomical segmentectomy and wide wedge resection, is reconsidered for early-stage lesions which are more frequently encountered with the recently introduced large screening programmes. Numerous retrospective non-randomised studies suggest that sublobar resection may be an acceptable surgical treatment for early lung cancers, also when performed by VATS.
More tailored, personalised therapy has recently been introduced. Quality-of-life parameters and surgical quality indicators become increasingly important to determine the short-term and long-term impact of a surgical procedure. International databases currently collect extensive surgical data, allowing more precise calculation of mortality and morbidity according to predefined risk factors. Centralisation of care has been shown to improve results. Evidence-based guidelines should be further developed to provide optimal staging and therapeutic algorithms.
1. Introduction
Thoracic surgery remains a major diagnostic and therapeutic modality for patients with non-small-cell lung cancer (NSCLC). However, many controversial issues remain regarding its precise role and application. Invasive staging and restaging procedures are applied more selectively with the introduction of endosonographic techniques. When discussing the different types of operative procedures that are available to the thoracic surgeon, a distinction has to be made between early-stage disease (stages IA/B and IIA/B), locoregionally advanced disease (stages IIIA/B), and metastatic disease (stage IV). Indications for surgical treatment of NSCLC are tailored according to the most recent 7th tumour-node-metastasis (TNM) classification, taking into account that surgery for locoregionally advanced disease remains a highly controversial topic. Intraoperative staging of lung cancer is extremely important to determine the extent of resection according to the intraoperative tumour (T) and nodal (N) status. Systematic nodal dissection is generally advocated to determine the precise nodal involvement. In 2011, a new adenocarcinoma classification was published with adenocarcinoma in situ and minimally invasive adenocarcinoma as new categories. The surgical implications of this relate mainly to the role of limited, sublobar resection. Especially when part of a combined modality regimen, surgical resection has a profound influence on quality of life. Prospective data on short- and long-term effects have recently become available, allowing better counselling of our patients.
In this review invasive mediastinal staging and restaging are discussed, as well as indications for surgical resection, intraoperative staging, the new adenocarcinoma classification and its surgical implications, quality of life after lung resection, and finally surgical quality indicators, including the relationship between volume and outcome.
2. Invasive mediastinal staging and restaging of lung cancer
2.1. Importance of lymph-node staging
In the absence of distant metastases, the prognosis of a patient with lung cancer largely depends on locoregional lymph-node involvement. Pathological staging remains the gold standard in quantifying the extent of locoregional and mediastinal lymph-node involvement. Patients with ipsilateral hilar or intrapulmonary lymph-node metastases (N1) are not precluded from surgery as complete resection provides a good long-term outcome when combined with adjuvant chemotherapy. Patients with ipsilateral mediastinal lymph-node metastases (N2) are currently treated with combined modality therapy, mostly chemoradiation. Only patients with limited N2 disease, in whom down-staging is obtained after induction therapy, may be considered for surgical resection. Patients with contralateral mediastinal or supraclavicular lymph-node involvement (N3) are currently considered unsuitable candidates for surgery due to poor long-term prognosis with multimodality therapy, including surgery.
Currently available invasive staging techniques are summarised in Table 1. Due to refinements in non-invasive and minimally invasive, endosonographic staging techniques, the role of surgical invasive staging and restaging has been redefined.
Table 1.
Invasive mediastinal staging and restaging techniques.
| Cervival mediastinoscopy |
| Repeat mediastinoscopy, remediastinoscopy |
| Anterior mediastinoscopy (mediastinotomy) |
| Extended mediastinoscopy (combination cervical + anterior) |
| Scalene lymph-node biopsy |
| Video-assisted mediastinal lymphadenectomy (VAMLA) |
| Transcervical extended mediastinal lymphadenectomy (TEMLA) |
| Video-assisted thoracic surgery (VATS), thoracoscopy |
2.2. Invasive mediastinal staging
Mediastinoscopy was introduced by Carlens in 1959 as a method for inspection and tissue biopsy in the superior mediastinum; it still holds true today [1]. With the routine use of mediastinoscopy, the rate of exploratory thoracotomies could be drastically reduced. Mediastinoscopy is associated with low morbidity (2%) and low mortality (0.08%) but remains an invasive procedure requiring general anaesthesia [2]. With the subsequent advent of new imaging techniques – initially computed tomography (CT), later on positron emission tomography (PET) and integrated PET–CT followed by the minimally invasive endosonographic techniques, endoscopic ultrasound (EUS) and endobronchial ultrasound (EBUS) – the precise role of mediastinoscopy is a matter of constant debate.
The different staging techniques that belong to the surgical armamentarium are listed in Table 1. Comparison of mediastinal lymph-node stations that can be reached by endosonography and invasive surgical staging is given in Table 2. Mediastinoscopy provides a thorough exploration of the superior mediastinum, allowing not only large biopsy samples of the different nodal stations in the superior mediastinum, but also evaluation of possible mediastinal extension of a primary lung cancer. In this way, sufficient tissue becomes available for detailed molecular analysis.
Table 2.
Mediastinal staging.
| LN | EBUS | EUS | Cervical mediastinoscopy | VAMLA TEMLA | VATS |
|
|---|---|---|---|---|---|---|
| L | R | |||||
| 1 | + | + | + | + | – | – |
| 2R | + | – | + | + | – | + |
| 2L | + | + | + | + | – | – |
| 4R | + | – | + | + | – | + |
| 4L | + | + | + | + | – | – |
| 5 | – | – | – | + | + | – |
| 6 | – | – | – | + | + | – |
| 7 | + | + | + | + | + | + |
| 8 | – | + | – | + | + | + |
| 9 | – | + | – | – | + | + |
EBUS, endobronchial ultrasound; EUS, oesophageal ultrasound; LN, lymph-node station, L, left; R, right; TEMLA, transcervical extended mediastinal lymphadenectomy; VAMLA, video-assisted mediastinal lymphadenectomy; VATS, video-assisted thoracic surgery.
Anterior mediastinoscopy, which is called anterior mediastinotomy when a rib cartilage has to be removed, provides access to the anterior mediastinum and lymph-node stations 5 and 6 on the left side. Some centres have experience with extended mediastinoscopy which is a combination of cervical and anterior mediastinoscopy by the same cervical incision [3]. Also evaluation of the supraclavicular lymph-node station 1 is possible by the latter incision. In many centres anterior mediastinoscopy is now replaced by thoracoscopy or video-assisted thoracic surgery (VATS), allowing a complete exploration of the ipsilateral pleural cavity.
The reported positive predictive value (PPV) and negative predictive value (NPV) of mediastinoscopy for staging of NSCLC are 100% and 96%, respectively [4]. When an extensive lymph-node dissection is performed by video-assisted mediastinal lymphadenectomy (VAMLA) or transcervical extended mediastinal lymphadenectomy (TEMLA) procedures, the rate of false negatives becomes extremely low [5–7].
Combination of EBUS and EUS – so-called ‘medical mediastinoscopy’ – provides access to a larger number of lymph-node stations than classical cervical mediastinoscopy. Both EBUS and EUS can be performed under local anaesthesia, which is a major advantage. Rapid on-site examination (ROSE) by the pathologist definitely increases accuracy. When a positive result is obtained, surgical invasive staging is avoided. For this reason EBUS and EUS are currently the preferred examinations after non-invasive tests, and in experienced hands a high accuracy is reported.
In the randomised Assessment of Surgical Staging versus Endosonographic Ultrasound in Lung Cancer: a Randomized Clinical Trial (ASTER) study, 241 patients with resectable, suspected or proven NSCLC, in whom mediastinal staging was indicated on the basis of CT or PET findings, were enrolled into a randomised controlled multicentre study comparing different strategies for mediastinal lymph-node staging [8]. Nodal metastases were found in 35% by surgical staging alone, 46% by endosonography (EBUS and EUS) and 50% by endosonography followed by surgical staging. NPVs were 86, 85 (P = 0.47) and 93% (P = 0.18), respectively [8].
In a prospective study of 153 patients, Yasufuku and colleagues chose EBUS as the initial investigation for mediastinal lymph-node staging followed by mediastinoscopy [9]. If both were negative, a thoracotomy was performed. Prevalence of N2/N3 disease was 35%. EBUS had an NPV of 91% and mediastinoscopy of 90%, so the conclusion of this study was that EBUS may replace mediastinoscopy. In a recent retrospective study from the same centre, the sensitivities and NPVs of EBUS in evaluating clinical N0 or N1 disease were 76% and 96%, respectively [9]. The role of endobronchial ultrasound-guided transbronchial needle aspiration for differentiating stage I from stage II lung cancer: poster presentation at the 49th Annual Meeting of the Society of Thoracic Surgeons, Los Angeles, California, January 26–30, 2013). However, it should be mentioned that this group comprises thoracic surgeons having a large experience with endosonographic and invasive staging techniques. Can these results from a high-volume dedicated centre be duplicated in everyday practice?
Cerfolio published a retrospective review of 234 patients with NSCLC who were staged by EBUS or EUS for suspected N2 disease on CT or PET–CT [10]. Mediastinoscopy was performed when EBUS/EUS were negative. NPVs for detecting N2 disease by EBUS, EUS and mediastinoscopy were 79%, 80% and 93%, respectively. EBUS was found to be falsely negative in 28%, and EUS in 22% of the cases. In a retrospective study from a single institution by Defranchi, 494 patients, suspected of lung cancer, underwent EBUS [11]. A negative result was followed by mediastinoscopy. Of the patients with suspicious mediastinal lymph nodes, 28% still had N2 disease confirmed by mediastinoscopy despite a negative EBUS. In this way, negative EBUS/EUS results should still be confirmed by mediastinoscopy.
The current indications for surgical mediastinal staging are a matter of judgment and precise knowledge of the various staging modalities and their results. None of the available techniques can be expected to provide perfect results. The main question becomes what false-negative rate one is willing to accept. In patients with suspected mediastinal lymph-node involvement by non-invasive techniques, evaluation by EBUS/EUS followed by mediastinoscopy in cases where no positive lymph nodes are found by endosonographic techniques has produced excellent results, with a reported increase in sensitivity for detection of mediastinal nodal disease of up to 93% [8]. In concordance with the European Society of Thoracic Surgeons (ESTS) guidelines, positive CT, PET or PET–CT findings should be cytologically or pathologically confirmed [12]. EBUS and EUS are complementary to surgical invasive staging techniques with a high specificity but low NPV. Therefore, an invasive surgical technique is still indicated if EBUS/EUS yield a negative result. Fig. 1 provides a flow chart of mediastinal staging of NSCLC that is currently used at the Antwerp University Hospital in Belgium.
Fig. 1.
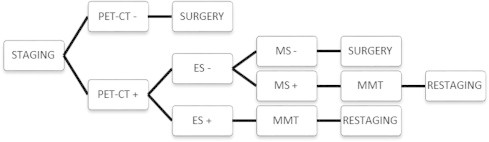
Flow chart for mediastinal staging of non-small-cell lung cancer (NSCLC) in the Antwerp University Hospital. ES, endosonographic technique (endobronchial or endoscopic ultrasound); MS, mediastinoscopy; MMT, multimodality treatment; PET–CT, integrated positron emission tomography and computed tomography.
2.3. Invasive mediastinal restaging
Most patients with pathologically proven N2 disease detected during preoperative work-up will be treated by induction therapy. The mediastinum can be principally restaged by the same techniques as applied in primary staging. At the present time, CT, PET and PET–CT are not accurate enough to make final further therapeutic decisions after induction therapy. The accuracy of CT in restaging after induction therapy is only 58% [13]. PET scanning is more accurate than CT for mediastinal restaging, with a reported PPV to detect persisting nodal disease of 73% [14]. In detecting residual N2 disease, however, PPV was less than 20%. The use of PET–CT fusion images significantly increases the accuracy through better localisation of focal isotope uptake in mediastinal nodes [15]. However, 20% false-negative and 25% false-positive rates have been reported [16]. In cases where there is a suspicion of residual mediastinal disease, invasive biopsies are still required.
Endosonographic techniques are also used for restaging. However, their false-negative rates remain high, ranging between 20% and 30% [17,18]. Therefore, negative findings should still be confirmed by surgical restaging.
Results of recent series of repeat mediastinoscopies after induction therapy are summarised in Table 3 [15,19–24]. Remediastinoscopy offers the advantage of providing pathological evidence of response after induction therapy. In this way, it remains a valuable tool to select patients for surgical resection [25]. Survival clearly depends on the findings of remediastinoscopy, patients with a positive repeat mediastinoscopy having a poor prognosis compared to those with a negative remediastinoscopy [21]. In a combined series of 104 patients, nodal status was the only significant factor related to survival in multivariate analysis [22].
Table 3.
Results of remediastinoscopy after induction therapy.
| Author, year | Ref. | n | IT | Morbidity (%) | Mortality (%) | Sensitivity (%) | Negative predictive value (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| Pitz, 2002 | 19 | 15 | CT | 0 | 0 | 50 | 71 | 78 |
| Stamatis, 2005 | 20 | 165 | CT–RT | 2.5 | 0 | 74 | 86 | 93 |
| De Waele, 2006 | 21 | 32 | CT (n = 26) CT–RT (n = 6) | 3.1 | 0 | 71 | 75 | 84 |
| De Leyn, 2006 | 15 | 30 | CT | 0 | 0 | 29 | 52 | 60 |
| De Waele, 2008a | 22 | 104 | CT (n = 79) CT–RT (n = 25) | 3.9 | 1 | 70 | 73 | 84 |
| Marra, 2008b | 23 | 104 | CT–RT | 1.9 | 0 | 61 | 85 | 88 |
| Call S, 2011c | 24 | 84 | CT (n = 49) CT–RT (n = 35) | 4.0 | 1 | 74 | 79 | 87 |
Ref., reference; n, number of patients; IT, induction therapy; CT, chemotherapy; CT–RT, chemoradiotherapy.
Combined, updated series.
Subset of patients of Stamatis, 2005 [20].
Results of restaging after induction therapy.
An alternative approach consists of the use of minimally invasive, endosonographic procedures to obtain an initial proof of mediastinal nodal involvement. Mediastinoscopy is subsequently performed after induction therapy to evaluate response [26]. In this way, a technically more difficult remediastinoscopy can be avoided.
Only one study has reported the results of VATS for restaging after induction therapy [27]. In this Cancer and Leukemia Group B (CALGB) 39803 trial a negative result of VATS was defined as negative lymph-node biopsies from at least three lymph-node stations, whereas a positive result consisted of a pathological proof of persisting N2 disease in the mediastinum or the demonstration of pleural carcinomatosis. Sensitivity, specificity and NPV of VATS for restaging were 67%, 100% and 73%, respectively.
In the restaging guidelines published by the ESTS an invasive technique providing cytological or histological information is also recommended [12]. Endoscopic or surgical invasive procedures may be utilised, the precise choice depending on the availability of the technique and expertise of the centre [12]. This policy was confirmed in a recent systematic review on restaging after induction therapy for stage IIIA NSCLC [26].
Current restaging algorithms used at the Antwerp University Hospital are depicted in Fig. 2.
Fig. 2.
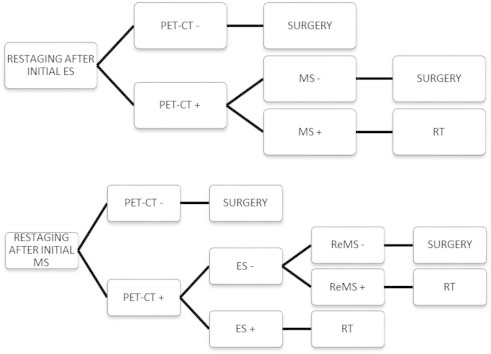
Flow chart for mediastinal restaging of non-small-cell lung cancer (NSCLC) in the Antwerp University Hospital depending on whether a minimally invasive procedure or mediastinoscopy was initially performed. ES, endosonographic technique (endobronchial or endoscopic ultrasound); MS, mediastinoscopy; PET–CT, integrated positron emission tomography and computed tomography; RT, radiotherapy; ReMS, repeat mediastinoscopy
3. Surgery for non-small cell lung cancer (NSCLC)
3.1. Complete R0 resection
The final aim of surgical treatment for non-small-cell lung cancer (NSCLC) is complete (R0) resection. In this respect, specific criteria have been established by a working group of the International Association for the Study of Lung Cancer (IASLC) [28]. Complete resection is defined as complete removal of the primary tumour with no residual macroscopic or microscopic tumour left behind; moreover, a systematic or lobe-specific nodal dissection must have been performed, and the highest mediastinal lymph node must be negative.
Although no prospective, randomised trial exists to compare surgery versus radiotherapy in the treatment of early-stage NSCLC, surgical resection has traditionally been considered the treatment of choice. Markedly improved survival rates are reported in surgical series in comparison to patients who did not undergo surgical resection for a variety of reasons [29]. Early-stage disease and T3N1 NSCLC are considered definite indications for surgery.
Resectability and operability of a primary NSCLC depend not only on the clinical and intraoperative staging of the tumour, but also on the functional capacity of the patient. So, detailed cardiopulmonary evaluation to determine the functional status is equally important as this might impact on the extent of resection [30]. After definitive pathological examination, a distinction can be made between R0 resections when there is no residual tumour, R1 with microscopic residual tumour and R2 with macroscopic residual tumour.
3.2. Types of lung cancer resection
Lung cancer resections can be divided into three major groups (Table 4).
Table 4.
Types of operative procedures
| Standard: | |
| Lobectomy | |
| Bilobectomy | |
| Pneumonectomy | |
| Lung-parenchyma-sparing operations: | |
| Proximal | Bronchotomy |
| Rotating bronchoplasty | |
| Bronchial or tracheal wedge excision | |
| Bronchial or tracheal sleeve resection | |
| Distal | Anatomical segmentectomy |
| (wide) Wedge excision | |
| Extended procedures (lung + other structure): | |
| Pericardium (intrapericardial pneumonectomy) | |
| Diaphragm | |
| Chest wall (ribs, vertebrae) | |
| Superior sulcus (Pancoast tumour) | |
Group 1 – standard resections: standard resections include lobectomy (removal of a lobe), bilobectomy (removal of two lobes on the right side) and pneumonectomy (removal of an entire lung). Pneumonectomy was initially considered as the treatment of choice in the years 1940–1950, whilst lobectomy was reserved for patients with diminished pulmonary or cardiac reserve. In later years, lobectomy was found to provide a similar survival rate as pneumonectomy if the lesion could be totally resected by lobectomy.
Group 2 – lung parenchyma saving operations: these operations can be divided into proximal and distal procedures. The proximal interventions comprise all bronchoplastic and tracheoplastic operations. The most frequently performed bronchoplastic procedure is a sleeve resection of the right upper lobe for a lung cancer invading the upper lobe orifice. The very first sleeve resection was performed in 1947 for a carcinoid tumour in the right upper-lobe orifice in an Air Force cadet to avoid a pneumonectomy which would have precluded his career as a pilot [31]. Distal procedures include segmentectomies and wedge resections.
Regarding the extent of resection, lobectomy is generally considered the procedure of choice in cancers confined to a single lobe. This attitude resulted from a prospective randomised trial from the Lung Cancer Study Group comparing lobectomy to lesser resections for peripheral clinical T1N0 lesions [32]. Patients were randomised to standard lobectomy or lesser resection during thoracotomy. Noteworthy in this study was that nearly half of the patients had a contraindication to randomisation, mostly because of location of the tumour or unexpected N1 or N2 disease. Patients who underwent a limited resection were found to have a tripling of local recurrence rate, a 30% increase in overall death rate and a 50% increase in cancer-related death rate in comparison to lobectomy patients. However, these results were only significant at a P-value level of 0.10.
The role of sublobar resection, anatomical segmentectomy or wide-wedge resection is being reconsidered for very early lung cancer following large screening programmes for lung cancer. This is due to the findings of non-solid or part-solid ground glass opacities, so-called GGOs [33]. This will be further discussed with the newly introduced adenocarcinoma classification.
Group 3 – extended operations: extended operations involve resection of lung parenchyma with an adjacent organ or structure invaded by the tumour. Examples include resection of the chest wall, diaphragm, pericardium, left atrium, superior vena cava and apex of the chest in superior sulcus tumours. En bloc resection of the locally involved extrapulmonary structure is advised to avoid tumour spillage and to ensure a complete R0 resection with negative margins.
3.3. Different thoracic approaches
A posterolateral thoracotomy incision is the classical incision performed for lung cancer resection. If feasible, a muscle-sparing thoracotomy is preferred to preserve the latissimus dorsi muscle. Sternotomy may be used in patients requiring bilateral procedures, especially bilateral upper-lobe lung cancers. An extended incision such as a hemi-clamshell incision is utilised in selected patients requiring an extended resection. At the present time, VATS is increasingly being used as specific access to the thoracic cavity. In a series of 1100 VATS lobectomies, excellent results were reported, with an operative mortality of 0.8% [34]. Morbidity generally appears to be lower with the VATS approach, although in a nationwide database of 13,619 patients who underwent lobectomy by thoracotomy or VATS, patients who underwent VATS lobectomy were 1.6 times more likely to have intraoperative complications [35]. A recent systematic review and meta-analysis of randomised and non-randomised trials concluded that VATS lobectomy is an appropriate procedure for selected patients with early-stage NSCLC [36]. Currently, VATS has become a standard approach for peripheral wedge resections and lobectomy for stage I tumours. VATS segmentectomy is much less widely performed and its potential benefits and limitations still require further evaluation [37,38].
Although VATS seems to be equal or even beneficial in terms of morbidity, length of stay and survival in comparison to an open approach, further evaluation in large, prospective randomised trials is necessary [39].
3.4. Intraoperative staging – systematic nodal dissection
Detailed intraoperative systematic lymph node dissection is important to provide an accurate pathological TNM staging. The different intrathoracic lymph-node stations were originally described by Naruke et al. in 1978 [40] and were recently updated in the 7th TNM classification where the concept of nodal zones was introduced [41]. The nodal zones and stations are listed in Table 5 [41,42].
Table 5.
Regional lymph-node mapping into zones and stations according to the 7th tumour-node-metastasis (TNM) edition [41].
| Supraclavicular zone: |
| 1. Low cervical, supraclavicular and sternal notch |
| Upper zone: |
| 2. Upper paratracheal |
| 3. a. Prevascular |
| b. Retrotracheal |
| 4. Lower paratracheal |
| Aortopulmonary (AP) zone: |
| 5. Subaortic or Botallo’s |
| 6. Para-aortic (ascending aorta or phrenic) |
| Subcarinal zone: |
| 7. Subcarinal |
| Lower zone: |
| 8. Para-oesophageal (below carina) |
| 9. Pulmonary ligament |
| Hilar/interlobar zone: |
| 10. Hilar |
| 11. Interlobar |
| Peripheral zone: |
| 12. Lobar: upper, middle and lower lobe |
| 13. Segmental |
| 14. Subsegmental |
Thoracotomy provides the final investigation and determination of resectability.
Non-resectable tumours include T4 tumours with invasion into important adjacent structures or tumours with extensive mediastinal metastases. These include involvement of vital mediastinal structures or extracapsular N2 and N3 diseases. For resected N2 disease, invasion of the highest mediastinal lymph node heralds a poor prognosis. Massive involvement of hilar structures is generally a contraindication unless an intrapericardial pneumonectomy can be performed. Pleural metastases are also a contraindication to resection due to a poor long-term survival.
When deciding on the type of operation to be performed, the surgeon should first perform a careful intraoperative exploration, taking into account several strategic points. He/she should determine whether the tumour is peripheral or central, which lymph nodes are involved, and whether or not there is transgression of the fissure. Frozen-section analysis of suspicious lymph nodes or margins can be helpful in determining the extent of resection. Whenever possible, lobectomy remains the procedure of choice. Pneumonectomy is considered ‘a disease in itself’ due to its profound respiratory and haemodynamic implications and associated higher complication rates in comparison to lobectomy. Most patients, however, have adapted to living with just one lung [43]. A sleeve lobectomy should be considered as an alternative whenever technically feasible, providing that a complete resection can be obtained [44].
In a large series of 334 patients operated for lung cancer invading the chest wall, 5-year survival rate was 32% in patients who underwent a complete resection, in contrast to only 4% for incomplete resections, and 0% for exploration only [45]. Long-term survival was mainly dependent on nodal involvement and complete resection, and less dependent on the depth of chest wall invasion.
For precise N staging during thoracotomy, a systematic nodal dissection is performed as advocated by Graham et al. [46]. In this technique, dissection of the mediastinal, hilar and lobar lymph nodes proceeds in a systematic fashion. In their classical paper Graham et al. reviewed 240 patients with clinical T1–3 N0–1 NSCLC [46]. Preoperative mediastinoscopy was performed when lymph nodes larger than 1.5 cm were present on chest CT. The rate of exploratory thoracotomy without further resection was only 3%. Following surgical resection pathological N2 disease was found in 20% of patients. There was no subgroup with 0% incidence of N2 involvement and skip metastases were found in 34% of patients with N2 disease. Peripheral tumours less than 2 cm had a 24% incidence of lymph-node metastases. Systematic lymph-node dissection is currently considered the gold standard for the accurate staging of nodal (N) disease and should be routinely performed, also when a minimally invasive approach is chosen.
In a non-randomised study of 373 patients, complete mediastinal lymph-node dissection identified more levels of N2 disease in patients with stages II and IIIA NSCLC, and was associated with improved survival in comparison to systematic nodal sampling but only for right-sided lesions [47]. A survival advantage of complete mediastinal lymph-node dissection has only been demonstrated in one prospective randomised trial [48]. In a recently published multicentre prospective clinical trial, patients with intraoperatively staged T1-2N0-non-hilar N1 NSCLC were randomised to lymph-node sampling versus systematic nodal dissection. The latter identified occult disease in 3.8% of patients but was not associated with a benefit in overall survival [49]. However, all patients in this trial were carefully staged with invasive, pathological analysis of four lymph-node stations. These results should not be generalised to higher-stage tumours.
The technique of systematic lymph-node dissection on the right side includes the dissection of the upper (level 2R) and lower (level 4R) paratracheal nodes, subcarinal (level 7), para-oesophageal (level 8R) and inferior pulmonary ligament (level 9R) lymph-node stations. On the left side, the aortopulmonary, para-aortic and lower paratracheal nodes (levels 5, 6, 4L), and levels 7, 8L and 9L should be resected.
N1 disease also represents a heterogeneous group of diseases. This was demonstrated by Riquet et al., who reported a series of 1174 patients with NSCLC; 22% of the patients had N1 disease, with a 5-year survival of 47.5% [50]. A distinction was made between intralobar N1 (levels 12 and 13) and extralobar hilar N1 (levels 10 and 11) diseases. Five-year survival rate for intralobar N1 was 54% and for hilar N1 39%. This difference was highly significant. The prognosis of intralobar N1 is similar to N0 disease, and extralobar N1 is more closely related to N2 with single-station involvement.
4. Surgical implications of the new adenocarcinoma classification
4.1. New categories
In early 2011, a new adenocarcinoma classification was published by a common working group of the IASLC, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) [51–53]. This classification is listed in Table 6.
Table 6.
IASLC/ATS/ERS classification of lung adenocarcinoma in resection specimens [51–53]. Table reproduced with permission from Wolters Kluwer Health.
| Preinvasive lesions: |
| Atypical adenomatous hyperplasia |
| Adenocarcinoma in situ (⩽3 cm, formerly BAC) |
| - non-mucinous |
| - mucinous |
| - mixed mucinous/non-mucinous |
| Minimally invasive adenocarcinoma (⩽3 cm lepidic predominant tumour with ⩽5 mm invasion): |
| - non-mucinous |
| - mucinous |
| - mixed mucinous/non-mucinous |
| Invasive adenocarcinoma: |
| Lepidic predominant (formerly non-mucinous BAC pattern, with >5 mm invasion) |
| Acinar predominant |
| Papillary predominant |
| Micropapillary predominant |
| Solid predominant with mucin production |
| Variants of invasive adenocarcinoma |
| Invasive mucinous adenocarcinoma (formerly mucinous BAC) |
| Colloid |
| Foetal (low and high grade) |
| Enteric |
BAC, bronchioloalveolar carcinoma.
Of special interest to thoracic surgeons are the new categories adenocarcinoma in situ (AIS) which represents small (⩽3 cm) solitary adenocarcinomas consisting purely of lepidic growth without invasion, and minimally invasive adenocarcinoma (MIA) with ⩽0.5 cm invasion. AIS and MIA were introduced because the 5-year disease-free survival approaches 100% if the tumours are completely resected. The term bronchioloalveolar carcinoma (BAC) is no longer utilised as it applies to five different categories in the new classification, which gave rise to much confusion [51].
With the advent of helical CT and screening trials in high-risk populations, there is a renewed interest in small nodules, especially those with ground-glass opacity (GGO). Recently, results of the National Lung Screening Trial were published [54]. In this trial, 53,454 people at high risk for lung cancer were randomised between screening with low-dose CT or chest radiography. In the CT group, there was a relative reduction in mortality from lung cancer of 20.0% and a reduction in death from any cause of 6.7% [54].
Whether some of these lesions can be treated by limited resection – so-called sublobar resection comprising anatomical segmentectomy or wedge excision – is currently the subject of intensive investigation [53]. For a limited resection to be oncologically valid, a precise pre- and intraoperative diagnosis becomes imperative. In terms of preoperative diagnosis, specific criteria on chest CT as percentage GGO, tumour shadow disappearance rate and histogram analysis have been shown to have a high predictive value [55]. The role of PET and integrated PET–CT scanning and specific tumour markers is currently being evaluated [56].
4.2. Sublobar (limited) resection for lung cancer
The detection rate of smaller lung cancers in recent times is increasing, and therefore the appropriateness of lobectomy for stage I lung cancer, especially those tumours ⩽2 cm (clinical T1a disease), is again being questioned [33,57]. Recently, there have been numerous publications suggesting that sublobar resection for early lung cancers may be an adequate surgical treatment. Many of these studies are retrospective and not randomised [58–60]. Most reports showed no difference in survival or in locoregional recurrence between lobectomy and sublobar resection for tumours ⩽2 cm in size. Patients with GGO tumours on CT have been reported to have a 100% survival at 5 years after resection [61–64]. However, possible delayed cut-end recurrences have been described after limited resection of GGO lesions [65].
Two recent reviews and one meta-analysis of sublobar resection concluded that the well-selected use of sublobar resection, especially for pure AIS ⩽2 cm, yielded survival and recurrence rates comparable to those of lobectomy [66–68]. Thus, sublobar resection is generally considered acceptable for GGO lesions or adenocarcinomas with minimal invasion. Lobectomy is still considered the standard surgical treatment for tumours ⩽2 cm in size that have a solid appearance on CT because such tumours are invasive carcinomas. Definite recommendations can only be made when the results of large randomised trials such as Japan Clinical Oncology Group (JCOG) 0802 in Japan, CALGB 140503 in North America and European Institute of Oncology (IEO) S638/311 in Italy become available. These trials randomise patients with tumours ⩽2 cm between lobectomy and sublobar resection.
Whether a purely anatomical segmentectomy provides a similar or better result to a wide-wedge resection has not yet been clearly determined. When correlating CT findings of GGO with histopathology, many of these lesions correspond to non-invasive forms of neoplastic growth. [61–64,69,70]. In a recent prospective study from Japan (JCOG 0201), radiological non-invasive peripheral lung adenocarcinoma was defined as an adenocarcinoma ⩽2.0 cm with ⩽0.25 consolidation [71].
Recent guidelines and a large, randomised screening trial state that small nodules ⩽10 mm or ⩽500 mm3 that are clearly 100% pure GGO lesions on chest CT, which are suspected to be AIS or MIA, be considered for close follow-up rather than immediate surgical resection [72,73]. Specific CT characteristics to be considered are size, attenuation, shape and growth rate.
4.3. Systematic lymph-node dissection for early-stage adenocarcinoma
In some specific subsets of very early-stage adenocarcinoma, especially pure GGO lesions, systematic lymph-node dissection may not always be required [74]. Recent analysis of the Italian COSMOS screening study showed that systematic nodal dissection can be avoided in the early stage – clinically N0 lung cancer when the maximum standardised uptake value on PET scanning is <2.0 and the pathological nodule size is ⩽10 mm – as the risk of nodal involvement is very low in this subset of patients [75].
In a Japanese prospective study, a specific treatment algorithm has been proposed [76]. Lesions ⩽10 mm of any type or pure GGO nodules were initially observed and discussed with each specific patient. When size or density increased, they were subsequently resected. GGO lesions 11–15 mm were treated by segmentectomy and lymph-node sampling. Solid lesions of 11–15 mm and GGO lesions of 16–20 mm were removed by segmentectomy combined with lymph-node dissection. Solid lesions of 16–20 mm were resected by lobectomy with lymph-node dissection. Applying this algorithm, an excellent 5-year disease-free survival rate of 98% was observed for limited resection [76].
5. Quality of life after lung cancer resection
Although mortality and major morbidity rates offer a patient valuable information, these data alone are inadequate in meeting the growing needs for detailed comparison of surgical approaches and rising expectations of patients. Patients may regard immediate postoperative complications as an acceptable risk, but are not prepared to accept significant postoperative quality of life (QoL) impairments [77]. Several publications focus on predictors of QoL after early-stage lung cancer resection. Extent of resection [78–80], surgical approach [81,82], age [83–85] and smoking status [86–88] are considered to be significant.
The extent of resection has a significant influence on the QoL evolution. Several publications evaluated QoL after lobectomy and pneumonectomy [78–80]. Literature data agree that the initial limitations in the physical QoL component observed after both resections are more pronounced after pneumonectomy. Depending on the specific publication, both resections yielded comparable results after 3–6 months. Sublobar resections, indicated in stage IA patients with a tumour located in the periphery of the lung and <2 cm in diameter, are currently often performed. Although these procedures imply a parenchyma-sparing intent, QoL has rarely been reported after sublobar resections. Pompeo et al. evaluated patients with severe emphysema undergoing a sublobar resection for stage I lung cancer [89]. Significant improvements were reported in the domains of the physical QoL component, presumably because of a lung volume reduction effect. Saad prospectively described the evolution in QoL after different lung resections [90]. A comparable improvement in the physical QoL was seen after lobectomy as well as after sublobar resection.
The effect on QoL not only of the muscle-sparing versus the non-muscle-sparing thoracotomy, but also of minimally invasive thoracic surgery was recently evaluated. The effect of the muscle-sparing thoracotomy is mostly seen on the physical QoL component, with improved shoulder function and less thoracic pain compared to the non-muscle sparing thoracotomy [91]. The advantages of VATS over thoracotomy in terms of QoL are found in the immediate postoperative period. After postoperative day 4, no significant differences in QoL are seen [92]. After a VATS procedure Landreneau et al. found that patients experienced significantly less pain only on the first 2 days in comparison to a muscle-sparing thoracotomy [93].
Conclusions of QoL research in younger patients cannot be transferred blindly to septuagenarians. Several authors prospectively evaluated QoL after lung-cancer surgery in an elderly patient population. In general, age >70 years is an important risk factor for impairment of the physical QoL component, and recovery is not guaranteed until ⩾24 postoperative months [78]. After lobectomy as well as pneumonectomy, the emotional component returns to baseline the first 3 months after surgery in elderly patients and may reflect a so-called response shift whereby patients adapt their standards and perceptions to their expectations and rate their personal situations better than would otherwise be expected [94].
Several authors compared QoL between patients aged less versus more than 70 years. Salati et al. compared QoL after lobectomy [83]. Preoperatively, elderly patients scored worse on the physical component of QoL, but scored higher values on the emotional component. At 3 months after surgery, no significant differences were seen between the two patient groups. Burfeind et al. evaluated the effect of lobectomy on patients who were younger versus older than 70 years [84]. Both groups demonstrated a similar decrement in QoL with a parallel return to baseline. The one notable exception was in the physical QoL component, which had returned to baseline by 6 months in young patients and stayed impaired in patients ⩾70 years at 6 and 12 months postoperatively.
The effect of smoking habits on postoperative QoL at 6 months was evaluated by Myrdal et al. [87]. Patients who continued smoking after lung-cancer surgery had significantly lower scores for the emotional QoL component than former smokers and those who had never smoked. In contrast, Sarna et al. could not withhold the smoking status as predictive for postoperative QoL [88]. In a recent study, we concluded that smoking cessation is beneficial at any time point prior to lung-cancer resection [86]. Current smoking at the time of surgery is associated with a longer impairment of QoL functioning as well as symptom scores. Since smoking status is one of the few prognostic factors in the direct control of the patient, this study offers valuable information to promote smoking cessation before lung-cancer surgery.
There is an ongoing debate on the effect of induction and adjuvant chemotherapy or radiotherapy on QoL after lung-cancer surgery [95–97]. Several authors evaluated the effect of chemotherapy on the QoL of lung-cancer patients, in both non-surgical and surgical patients. In non-surgical patients, chemotherapy was associated with worse QoL, unless the patient responded to treatment [98]. Paull et al. reported that exposure to postoperative chemotherapy was a risk factor for poor QoL after surgery for early-stage lung cancer [97]. These results are not consistent with the study of Fiedler et al. who evaluated QoL after pneumonectomy comprising early- as well as advanced-stage lung cancer [96]. Adjuvant chemotherapy had no significant influence on QoL at 6 months.
Although many questions remain concerning QoL evolution after lung-cancer surgery, QoL data are essential in proper patient counselling and may create realistic postoperative objectives for patients. The real challenge in the management of lung-cancer patients consists not only in improving prognosis but also in maintaining or increasing QoL.
6. Surgical quality control
Thoracic surgery comprises a large variety of different procedures which may prove to be technically challenging, such as extended resections of the superior sulcus, sleeve resections, intrapericardial procedures and extensive operations after induction therapy. For this reason uniform judgment of surgical quality is difficult to perform. Overall mortality is only a crude parameter and risk stratification is necessary. Moreover, dedicated anaesthesiological, intensive-care and nursing management is required to obtain the best postoperative results; thus team management will not only determine the short-term results but also long-term outcome. This also implies that hospital volume may be a critical determinant.
Is there a clear relationship between surgeon or hospital volume and final outcome? In a seminal paper Luft et al. demonstrated that mortality after open-heart surgery, vascular surgery and prostatectomy decreases with increasing number of procedures performed [99]. When analysing data from university reports, Hillner et al. showed a relationship between volume and outcome for complex intra-abdominal and lung-cancer interventions [100]. When looking at the specific number of pulmonary resections, mortality was lower in those centres performing more than 24 interventions on a yearly basis. Mortality of lobectomy was significantly lower when performed by a thoracic surgeon compared to a general surgeon. The latter finding was confirmed in a more recent study [101]. In another analysis of more than 2000 pulmonary resections, low-volume centres were compared to high-volume institutions. Morbidity was lowest and survival highest in those centres performing more than 67 resections per year [102]. Also in a Flemish, multicentre, hospital-based lung-cancer registry, mortality decreased when hospital volume increased [103]. The same holds true for oesophagectomy [104]. In the latter paper, mortality after lung resection was not related to technical factors but mainly to severe postoperative complications, underlining the importance of a dedicated multidisciplinary team in taking care of this patient population.
Are results different in teaching hospitals with specific thoracic surgical residents? In a recent analysis of 498,099 lung resections a superior outcome was found in hospitals with a thoracic surgery residency programme [105]. The odds ratio of death in patients undergoing pneumonectomy was reduced by more than 30% compared with hospitals providing training in general surgery.
Although evidence-based minimal volume standards are currently lacking, a recent systematic review and meta-analysis concluded that hospital volume and surgeon specialty are important determinants of outcome in lung-cancer resections [106].
So, centralisation of care seems to be the logical consequence in improving short-term and long-term results. However, most of the presented data come from North America with established training programmes in thoracic and cardiothoracic surgery. What is the current situation in Europe?
In many European countries, general thoracic surgery currently exists as a separate specialty. However, the precise number of centres performing thoracic surgical procedures is unknown and accurate figures on the total number of interventions are not currently available. Certification in thoracic surgery is not uniform throughout Europe. As an example, thoracic surgery is not a specifically defined entity in Belgium, where it falls within the discipline of general surgery together with abdominal, cardiac, vascular, paediatric and trauma surgery.
There is also no uniform European training programme in thoracic or cardiothoracic surgery. To establish a more precise structure of general thoracic surgery a working group has been established by the European Association for Cardio-thoracic Surgery (EACTS) and the ESTS. Recently, the Union Européenne des Médecins Spécialistes (UEMS) has created a specific thoracic surgical division related to the general and cardiothoracic surgical sections. Specific criteria for training and accreditation in thoracic surgery are currently being developed.
To obtain more precise data on the number of general thoracic surgical procedures in Europe, several large databases have been created. The ESTS created a voluntary database for general thoracic surgery. In 2011 a total of 24,574 lung resections were reported, including all diagnoses. Lobectomy represented 57.5% of cases, and pneumonectomy 9.5%. A total of 16,710 cases of primary lung cancer were reported, lobectomy and bilobectomy being performed in 76% of cases. In this database mortality and morbidity are calculated according to specific risk scores, allowing benchmarking between different units and countries.
To ensure high-quality patient care in thoracic surgery, the EACTS/ESTS working group felt that it should be performed within the logistical and economical framework of specialised units. These units should be designed to allow patient care and treatment according to recommended standards, as well as education of surgical trainees, continuous development and research in thoracic surgery. The working group proposed two types of thoracic surgical centres: highly specialised centres within or associated with a university, performing at least 250 major thoracic procedures per year, and standard units which are free-standing or combined with cardiac, vascular or general surgery. In a standard unit at least 100 major interventions should be performed annually. Lung transplantation and its alternative procedures should be performed only in centres with special interest and with cardiac surgical facilities. According to well-defined criteria in combination with an on-site visit, dedicated thoracic units can obtain an institutional quality certification in general thoracic surgery.
In order to raise the profile of thoracic surgery in Europe, further harmonisation is necessary. Unified databases should become available, detailing not only mortality but also specific outcome measures related to morbidity, survival and quality of life. Postgraduate education remains necessary to ensure a high quality of thoracic surgical interventions as has recently been demonstrated by a study from the Netherlands evaluating completeness of lymph-node dissection in dedicated thoracic surgical centres [107,108].
Thoracic surgeons should be further involved in randomised clinical trials comparing newly introduced treatment modalities – such as stereotactic radiotherapy or radiofrequency ablation – to classical surgical procedures. They should also be prepared to adapt to a new, constantly changing environment. Multidisciplinary collaboration and large-scale prospective studies are necessary to update current diagnostic and therapeutic algorithms ensuring optimal patient care in thoracic surgery [109].
Conflict of interest statement
None declared.
References
- 1.Carlens E. Mediastinoscopy: a method for inspection and tissue biopsy in the superior mediastinum. Dis Chest. 1959;36:343–352. doi: 10.1378/chest.36.4.343. [DOI] [PubMed] [Google Scholar]
- 2.Kirschner P.A. Cervical mediastinoscopy. Chest Surg Clin N Am. 1996;6:1–20. [PubMed] [Google Scholar]
- 3.Obiols C., Call S., Rami-Porta R. Extended cervical mediastinoscopy: mature results of a clinical protocol for staging bronchogenic carcinoma of the left lung. Eur J Cardiothorac Surg. 2012;41:1043–1046. doi: 10.1093/ejcts/ezr181. [DOI] [PubMed] [Google Scholar]
- 4.Gdeedo A., Van Schil P., Corthouts B., Van Mieghem F., Van Meerbeeck J., Van Marck E. Prospective evaluation of computed tomography and mediastinoscopy in mediastinal lymph node staging. Eur Respir J. 1997;10:1547–1551. doi: 10.1183/09031936.97.10071547. [DOI] [PubMed] [Google Scholar]
- 5.Witte B., Hurtgen M. Video-assisted mediastinoscopic lymphadenectomy (VAMLA) J Thorac Oncol. 2007;2:367–369. doi: 10.1097/01.JTO.0000263725.89512.d7. [DOI] [PubMed] [Google Scholar]
- 6.Leschber G., Holinka G., Linder A. Video-assisted mediastinoscopic lymphadenectomy (VAMLA) – a method for systematic mediastinal lymphnode dissection. Eur J Cardiothorac Surg. 2003;24:192–195. doi: 10.1016/s1010-7940(03)00253-7. [DOI] [PubMed] [Google Scholar]
- 7.Zielinski M., Hauer L., Hauer J., Pankowski J., Szlubowski A., Nabialek T. Transcervical extended mediastinal lymphadenectomy (TEMLA) for staging of non-small-cell lung cancer (NSCLC) Pneumonol Alergol Pol. 2011;79:196–206. [PubMed] [Google Scholar]
- 8.Annema J.T., van Meerbeeck J.P., Rintoul R.C. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304:2245–2252. doi: 10.1001/jama.2010.1705. [DOI] [PubMed] [Google Scholar]
- 9.Yasufuku K., Pierre A., Darling G. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142 doi: 10.1016/j.jtcvs.2011.08.037. [1393–400 e1] [DOI] [PubMed] [Google Scholar]
- 10.Cerfolio R.J., Bryant A.S., Eloubeidi M.A. The true false negative rates of esophageal and endobronchial ultrasound in the staging of mediastinal lymph nodes in patients with non-small cell lung cancer. Ann Thorac Surg. 2010;90:427–434. doi: 10.1016/j.athoracsur.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 11.Defranchi S.A., Edell E.S., Daniels C.E. Mediastinoscopy in patients with lung cancer and negative endobronchial ultrasound guided needle aspiration. Ann Thorac Surg. 2010;90:1753–1757. doi: 10.1016/j.athoracsur.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 12.De Leyn P., Lardinois D., Van Schil P.E. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 13.Mateu-Navarro M., Rami-Porta R., Bastus-Piulats R., Cirera-Nogueras L., Gonzalez-Pont G. Remediastinoscopy after induction chemotherapy in non-small cell lung cancer. Ann Thorac Surg. 2000;70:391–395. doi: 10.1016/s0003-4975(00)01437-5. [DOI] [PubMed] [Google Scholar]
- 14.Port J.L., Kent M.S., Korst R.J., Keresztes R., Levin M.A., Altorki N.K. Positron emission tomography scanning poorly predicts response to preoperative chemotherapy in non-small cell lung cancer. Ann Thorac Surg. 2004;77:254–259. doi: 10.1016/s0003-4975(03)01457-7. [discussion 9] [DOI] [PubMed] [Google Scholar]
- 15.De Leyn P., Stroobants S., De Wever W. Prospective comparative study of integrated positron emission tomography–computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 non-small-cell lung cancer: a leuven lung cancer group study. J Clin Oncol. 2006;24:3333–3339. doi: 10.1200/JCO.2006.05.6341. [DOI] [PubMed] [Google Scholar]
- 16.Cerfolio R.J., Bryant A.S., Ojha B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg. 2006;131:1229–1235. doi: 10.1016/j.jtcvs.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 17.Annema J.T., Veselic M., Versteegh M.I., Willems L.N., Rabe K.F. Mediastinal restaging: EUS–FNA offers a new perspective. Lung Cancer. 2003;42:311–318. doi: 10.1016/s0169-5002(03)00364-7. [DOI] [PubMed] [Google Scholar]
- 18.Herth F.J., Annema J.T., Eberhardt R. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol. 2008;26:3346–3350. doi: 10.1200/JCO.2007.14.9229. [DOI] [PubMed] [Google Scholar]
- 19.Pitz C.C., Maas K.W., Van Swieten H.A., de la Riviere A.B., Hofman P., Schramel F.M. Surgery as part of combined modality treatment in stage IIIB non-small cell lung cancer. Ann Thorac Surg. 2002;74:164–169. doi: 10.1016/s0003-4975(02)03647-0. [DOI] [PubMed] [Google Scholar]
- 20.Stamatis G., Fechner S., Hillejan L., Hinterthaner M., Krbek T. Repeat mediastinoscopy as a restaging procedure. Pneumologie. 2005;59:862–866. doi: 10.1055/s-2005-919103. [DOI] [PubMed] [Google Scholar]
- 21.De Waele M., Hendriks J., Lauwers P. Nodal status at repeat mediastinoscopy determines survival in non-small cell lung cancer with mediastinal nodal involvement, treated by induction therapy. Eur J Cardiothorac Surg. 2006;29:240–243. doi: 10.1016/j.ejcts.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 22.De Waele M., Serra-Mitjans M., Hendriks J. Accuracy and survival of repeat mediastinoscopy after induction therapy for non-small cell lung cancer in a combined series of 104 patients. Eur J Cardiothorac Surg. 2008;33:824–828. doi: 10.1016/j.ejcts.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Marra A., Hillejan L., Fechner S., Stamatis G. Remediastinoscopy in restaging of lung cancer after induction therapy. J Thorac Cardiovasc Surg. 2008;135:843–849. doi: 10.1016/j.jtcvs.2007.07.073. [DOI] [PubMed] [Google Scholar]
- 24.Call S., Rami-Porta R., Obiols C. Repeat mediastinoscopy in all its indications: experience with 96 patients and 101 procedures. Eur J Cardiothorac Surg. 2011;39:1022–1027. doi: 10.1016/j.ejcts.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Rami-Porta R., Call S. Invasive staging of mediastinal lymph nodes: mediastinoscopy and remediastinoscopy. Thorac Surg Clin. 2012;22:177–189. doi: 10.1016/j.thorsurg.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 26.de Cabanyes Candela S., Detterbeck F.C. A systematic review of restaging after induction therapy for stage IIIa lung cancer: prediction of pathologic stage. J Thorac Oncol. 2010;5:389–398. doi: 10.1097/JTO.0b013e3181ce3e5e. [DOI] [PubMed] [Google Scholar]
- 27.Jaklitsch M.T., Gu L., Demmy T. Prospective phase II trial of pre-resection thoracoscopic mediastinal restaging following neoadjuvant therapy for IIIA (N2) non-small cell lung cancer: results of CALGB protocol #39803. J Thorac Cardiovasc Surg. 2013;146:9–16. doi: 10.1016/j.jtcvs.2012.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rami-Porta R., Wittekind C., Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49:25–33. doi: 10.1016/j.lungcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Reif M.S., Socinski M.A., Rivera M.P. Evidence-based medicine in the treatment of non-small-cell lung cancer. Clin Chest Med. 2000;21 doi: 10.1016/s0272-5231(05)70011-3. [107–20, ix] [DOI] [PubMed] [Google Scholar]
- 30.Brunelli A., Charloux A., Bolliger C.T. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 31.Naef A.P. The mid-century revolution in thoracic and cardiovascular surgery. Part 2: prelude to 20th century cardio-thoracic surgery. Interact Cardiovasc Thorac Surg. 2003;2:431–449. doi: 10.1016/S1569-9293(03)00190-7. [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg R.J., Rubinstein L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [discussion 22–3] [DOI] [PubMed] [Google Scholar]
- 33.Asamura H., Suzuki K., Watanabe S., Matsuno Y., Maeshima A., Tsuchiya R. A clinicopathological study of resected subcentimeter lung cancers: a favorable prognosis for ground glass opacity lesions. Ann Thorac Surg. 2003;76:1016–1022. doi: 10.1016/s0003-4975(03)00835-x. [DOI] [PubMed] [Google Scholar]
- 34.McKenna R.J., Jr., Houck W., Fuller C.B. Video-assisted thoracic surgery lobectomy: experience with 1100 cases. Ann Thorac Surg. 2006;81:421–425. doi: 10.1016/j.athoracsur.2005.07.078. [discussion 5–6] [DOI] [PubMed] [Google Scholar]
- 35.Gopaldas R.R., Bakaeen F.G., Dao T.K., Walsh G.L., Swisher S.G., Chu D. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg. 2010;89:1563–1570. doi: 10.1016/j.athoracsur.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Yan T.D., Black D., Bannon P.G., McCaughan B.C. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2553–2562. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 37.Swanson S.J. Segmentectomy for lung cancer. Semin Thorac Cardiovasc Surg. 2010;22:244–249. doi: 10.1053/j.semtcvs.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Schuchert M.J., Pettiford B.L., Pennathur A. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg. 2009;138 doi: 10.1016/j.jtcvs.2009.08.028. [1318–25 el] [DOI] [PubMed] [Google Scholar]
- 39.Swanson S.J. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg. 2010;89:S2096–S2097. doi: 10.1016/j.athoracsur.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 40.Naruke T., Suemasu K., Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg. 1978;76:832–839. [PubMed] [Google Scholar]
- 41.Rusch V.W., Asamura H., Watanabe H., Giroux D.J., Rami-Porta R., Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 42.Goldstraw P., Crowley J., Chansky K. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 43.Deslauriers J., Ugalde P., Miro S. Adjustments in cardiorespiratory function after pneumonectomy: results of the pneumonectomy project. J Thorac Cardiovasc Surg. 2011;141:7–15. doi: 10.1016/j.jtcvs.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Van Schil P.E., Brutel de la Riviere A., Knaepen P.J. Long-term survival after bronchial sleeve resection: univariate and multivariate analyses. Ann Thorac Surg. 1996;61:1087–1091. doi: 10.1016/0003-4975(96)00006-9. [DOI] [PubMed] [Google Scholar]
- 45.Downey R.J., Martini N., Rusch V.W., Bains M.S., Korst R.J., Ginsberg R.J. Extent of chest wall invasion and survival in patients with lung cancer. Ann Thorac Surg. 1999;68:188–193. doi: 10.1016/s0003-4975(99)00456-7. [DOI] [PubMed] [Google Scholar]
- 46.Graham A.N., Chan K.J., Pastorino U., Goldstraw P. Systematic nodal dissection in the intrathoracic staging of patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 1999;117:246–251. doi: 10.1016/S0022-5223(99)70419-8. [DOI] [PubMed] [Google Scholar]
- 47.Keller S.M., Adak S., Wagner H., Johnson D.H. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg. 2000;70:358–365. doi: 10.1016/s0003-4975(00)01673-8. [discussion 65–6] [DOI] [PubMed] [Google Scholar]
- 48.Wu Y., Huang Z.F., Wang S.Y., Yang X.N., Ou W. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer. 2002;36:1–6. doi: 10.1016/s0169-5002(01)00445-7. [DOI] [PubMed] [Google Scholar]
- 49.Darling G.E., Allen M.S., Decker P.A. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141:662–670. doi: 10.1016/j.jtcvs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riquet M., Manac’h D., Le Pimpec-Barthes F., Dujon A., Chehab A. Prognostic significance of surgical-pathologic N1 disease in non-small cell carcinoma of the lung. Ann Thorac Surg. 1999;67:1572–1576. [PubMed] [Google Scholar]
- 51.Travis W.D., Brambilla E., Noguchi M. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Travis W.D., Brambilla E., Van Schil P. Paradigm shifts in lung cancer as defined in the new IASLC/ATS/ERS lung adenocarcinoma classification. Eur Respir J. 2011;38:239–243. doi: 10.1183/09031936.00026711. [DOI] [PubMed] [Google Scholar]
- 53.Van Schil P.E., Asamura H., Rusch V.W. Surgical implications of the new IASLC/ATS /ERS adenocarcinoma classification. Eur Respir J. 2012;39:478–486. doi: 10.1183/09031936.00027511. [DOI] [PubMed] [Google Scholar]
- 54.Aberle D.R., Adams A.M., Berg C.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikeda K., Awai K., Mori T., Kawanaka K., Yamashita Y., Nomori H. Differential diagnosis of ground-glass opacity nodules: CT number analysis by three-dimensional computerized quantification. Chest. 2007;132:984–990. doi: 10.1378/chest.07-0793. [DOI] [PubMed] [Google Scholar]
- 56.Lee H.Y., Han J., Lee K.S. Lung adenocarcinoma as a solitary pulmonary nodule: Prognostic determinants of CT, PET, and histopathologic findings. Lung Cancer. 2009;66:379–385. doi: 10.1016/j.lungcan.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Henschke C.I., McCauley D.I., Yankelevitz D.F. Early lung cancer action project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 58.El-Sherif A., Gooding W.E., Santos R. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg. 2006;82:408–415. doi: 10.1016/j.athoracsur.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura H., Kawasaki N., Taguchi M., Kabasawa K. Survival following lobectomy vs limited resection for stage I lung cancer: a meta-analysis. Br J Cancer. 2005;92:1033–1037. doi: 10.1038/sj.bjc.6602414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okada M., Koike T., Higashiyama M., Yamato Y., Kodama K., Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–775. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 61.Kodama K., Higashiyama M., Yokouchi H. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer. 2001;33:17–25. doi: 10.1016/s0169-5002(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki K., Asamura H., Kusumoto M., Kondo H., Tsuchiya R. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg. 2002;74:1635–1639. doi: 10.1016/s0003-4975(02)03895-x. [DOI] [PubMed] [Google Scholar]
- 63.Takamochi K., Nagai K., Yoshida J. Pathologic N0 status in pulmonary adenocarcinoma is predictable by combining serum carcinoembryonic antigen level and computed tomographic findings. J Thorac Cardiovasc Surg. 2001;122:325–330. doi: 10.1067/mtc.2001.114355. [DOI] [PubMed] [Google Scholar]
- 64.Sakurai H., Maeshima A., Watanabe S. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol. 2004;28:198–206. doi: 10.1097/00000478-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida J., Ishii G., Yokose T. Possible delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J Thorac Oncol. 2010;5:546–550. doi: 10.1097/JTO.0b013e3181d0a480. [DOI] [PubMed] [Google Scholar]
- 66.Rami-Porta R., Tsuboi M. Sublobar resection for lung cancer. Eur Respir J. 2009;33:426–435. doi: 10.1183/09031936.00099808. [DOI] [PubMed] [Google Scholar]
- 67.Blasberg J.D., Pass H.I., Donington J.S. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol. 2010;5:1583–1593. doi: 10.1097/jto.0b013e3181e77604. [DOI] [PubMed] [Google Scholar]
- 68.Fan J., Wang L., Jiang G.N., Gao W. Sublobectomy versus lobectomy for stage i non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol. 2012;19:661–668. doi: 10.1245/s10434-011-1931-9. [DOI] [PubMed] [Google Scholar]
- 69.Adler B., Padley S., Miller R.R., Muller N.L. High-resolution CT of bronchioloalveolar carcinoma. Am J Roentgenol. 1992;159:275–277. doi: 10.2214/ajr.159.2.1321558. [DOI] [PubMed] [Google Scholar]
- 70.Aoki T., Tomoda Y., Watanabe H. Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology. 2001;220:803–809. doi: 10.1148/radiol.2203001701. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki K.K.T., Asakawa T., Kusumoto M. A prospective study to evaluate radiological diagnostic criteria by thin-section computed tomography to predict pathological non-invasiveness in peripheral clinical IA lung cancer (JCOG 0201) J Thorac Oncol. 2011;6:751–756. doi: 10.1097/JTO.0b013e31821038ab. [DOI] [PubMed] [Google Scholar]
- 72.van Klaveren R.J., Oudkerk M., Prokop M. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–2229. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
- 73.Godoy M.C., Naidich D.P. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology. 2009;253:606–622. doi: 10.1148/radiol.2533090179. [DOI] [PubMed] [Google Scholar]
- 74.Nomori H., Iwatani K., Kobayashi H., Mori A., Yoshioka S. Omission of mediastinal lymph node dissection in lung cancer: its techniques and diagnostic procedures. Ann Thorac Cardiovasc Surg. 2006;12:83–88. [PubMed] [Google Scholar]
- 75.Veronesi G., Maisonneuve P., Pelosi G. Screening-detected lung cancers: is systematic nodal dissection always essential? J Thorac Oncol. 2011;6:525–530. doi: 10.1097/JTO.0b013e318206dbcc. [DOI] [PubMed] [Google Scholar]
- 76.Kodama K., Higashiyama M., Takami K. Treatment strategy for patients with small peripheral lung lesion(s): intermediate-term results of prospective study. Eur J Cardiothorac Surg. 2008;34:1068–1074. doi: 10.1016/j.ejcts.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 77.Cykert S., Kissling G., Hansen C.J. Patient preferences regarding possible outcomes of lung resection: what outcomes should preoperative evaluations target? Chest. 2000;117:1551–1559. doi: 10.1378/chest.117.6.1551. [DOI] [PubMed] [Google Scholar]
- 78.Schulte T., Schniewind B., Dohrmann P., Kuchler T., Kurdow R. The extent of lung parenchyma resection significantly impacts long-term quality of life in patients with non-small cell lung cancer. Chest. 2009;135:322–329. doi: 10.1378/chest.08-1114. [DOI] [PubMed] [Google Scholar]
- 79.Brunelli A., Socci L., Refai M., Salati M., Xiume F., Sabbatini A. Quality of life before and after major lung resection for lung cancer: a prospective follow-up analysis. Ann Thorac Surg. 2007;84:410–416. doi: 10.1016/j.athoracsur.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 80.Zieren H.U., Muller J.M., Hamberger U., Pichlmaier H. Quality of life after surgical therapy of bronchogenic carcinoma. Eur J Cardiothorac Surg. 1996;10:233–237. doi: 10.1016/s1010-7940(96)80144-8. [DOI] [PubMed] [Google Scholar]
- 81.Li W.W., Lee T.W., Lam S.S. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest. 2002;122:584–589. doi: 10.1378/chest.122.2.584. [DOI] [PubMed] [Google Scholar]
- 82.Sugiura H., Morikawa T., Kaji M., Sasamura Y., Kondo S., Katoh H. Long-term benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparo Endo Per. 1999;9:403–408. [PubMed] [Google Scholar]
- 83.Salati M., Brunelli A., Xiume F., Refai M., Sabbatini A. Quality of life in the elderly after major lung resection for lung cancer. Interact Cardiovasc Thorac Surg. 2009;8:79–83. doi: 10.1510/icvts.2008.184986. [DOI] [PubMed] [Google Scholar]
- 84.Burfeind W.R., Jr., Tong B.C., O’Branski E. Quality of life outcomes are equivalent after lobectomy in the elderly. J Thorac Cardiovasc Surg. 2008;136:597–604. doi: 10.1016/j.jtcvs.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 85.Balduyck B., Hendriks J., Lauwers P., Sardari Nia P., Van Schil P. Quality of life evolution after lung cancer surgery in septuagenarians: a prospective study. Eur J Cardiothorac Surg. 2009;35:1070–1075. doi: 10.1016/j.ejcts.2009.01.050. [discussion 5] [DOI] [PubMed] [Google Scholar]
- 86.Balduyck B., Sardari Nia P., Cogen A. The effect of smoking cessation on quality of life after lung cancer surgery. Eur J Cardiothorac Surg. 2011;40:1432–1437. doi: 10.1016/j.ejcts.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 87.Myrdal G., Valtysdottir S., Lambe M., Stahle E. Quality of life following lung cancer surgery. Thorax. 2003;58:194–197. doi: 10.1136/thorax.58.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sarna L., Cooley M.E., Brown J.K., Chernecky C., Elashoff D., Kotlerman J. Symptom severity 1 to 4 months after thoracotomy for lung cancer. Am J Crit Care. 2008;17:455–467. quiz 68. [PMC free article] [PubMed] [Google Scholar]
- 89.Pompeo E., De Dominicis E., Ambrogi V., Mineo D., Elia S., Mineo T.C. Quality of life after tailored combined surgery for stage I non-small-cell lung cancer and severe emphysema. Ann Thorac Surg. 2003;76:1821–1827. doi: 10.1016/s0003-4975(03)01302-x. [DOI] [PubMed] [Google Scholar]
- 90.Saad I.A., Botega N.J., Toro I.F. Evaluation of quality of life of patients submitted to pulmonary resection due to neoplasia. J Brasil Pneumol. 2006;32:10–15. doi: 10.1590/s1806-37132006000100005. [DOI] [PubMed] [Google Scholar]
- 91.Balduyck B., Hendriks J., Lauwers P., Van Schil P. Quality of life evolution after lung cancer surgery: a prospective study in 100 patients. Lung Cancer. 2007;56:423–431. doi: 10.1016/j.lungcan.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 92.Forster R., Storck M., Schafer J.R., Honig E., Lang G., Liewald F. Thoracoscopy versus thoracotomy: a prospective comparison of trauma and quality of life. Langenbeck Arch Surg/Deutsche Gesellschaft fur Chirurgie. 2002;387:32–36. doi: 10.1007/s00423-002-0287-9. [DOI] [PubMed] [Google Scholar]
- 93.Landreneau R.J., Hazelrigg S.R., Mack M.J. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56:1285–1289. doi: 10.1016/0003-4975(93)90667-7. [DOI] [PubMed] [Google Scholar]
- 94.Sprangers M.A., Van Dam F.S., Broersen J. Revealing response shift in longitudinal research on fatigue – the use of the thentest approach. Acta Oncol. 1999;38:709–718. [PubMed] [Google Scholar]
- 95.Handy J.R., Jr., Asaph J.W., Skokan L. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest. 2002;122:21–30. doi: 10.1378/chest.122.1.21. [DOI] [PubMed] [Google Scholar]
- 96.Fiedler R., Neef H., Hennig H., Rosendahl W., Lautenschlager C. Quality of life after pneumonectomy for bronchial carcinoma. ZBL Chir. 1997;122:327–331. [PubMed] [Google Scholar]
- 97.Paull D.E., Thomas M.L., Meade G.E. Determinants of quality of life in patients following pulmonary resection for lung cancer. Am J Surg. 2006;192:565–571. doi: 10.1016/j.amjsurg.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 98.Montazeri A., Gillis C.R., McEwen J. Quality of life in patients with lung cancer: a review of literature from 1970 to 1995. Chest. 1998;113:467–481. doi: 10.1378/chest.113.2.467. [DOI] [PubMed] [Google Scholar]
- 99.Luft H.S., Bunker J.P., Enthoven A.C. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- 100.Hillner B.E., Smith T.J., Desch C.E. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000;18:2327–2340. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 101.Farjah F., Flum D.R., Varghese T.K., Jr., Symons R.G., Wood D.E. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg. 2009;87:995–1004. doi: 10.1016/j.athoracsur.2008.12.030. [discussion 5–6] [DOI] [PubMed] [Google Scholar]
- 102.Bach P.B., Cramer L.D., Schrag D., Downey R.J., Gelfand S.E., Begg C.B. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 103.van Meerbeeck J.P., Damhuis R.A., Vos de Wael M.L. High postoperative risk after pneumonectomy in elderly patients with right-sided lung cancer. Eur Respir J. 2002;19:141–145. doi: 10.1183/09031936.02.00226202. [DOI] [PubMed] [Google Scholar]
- 104.Birkmeyer J.D., Stukel T.A., Siewers A.E., Goodney P.P., Wennberg D.E., Lucas F.L. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 105.Bhamidipati C.M., Stukenborg G.J., Ailawadi G., Lau C.L., Kozower B.D., Jones D.R. Pulmonary resections performed at hospitals with thoracic surgery residency programs have superior outcomes. J Thorac Cardiovasc Surg. 2013;145:60–66. doi: 10.1016/j.jtcvs.2012.10.015. [7 e1–2; discussion 6–7] [DOI] [PubMed] [Google Scholar]
- 106.von Meyenfeldt E.M., Gooiker G.A., van Gijn W. The relationship between volume or surgeon specialty and outcome in the surgical treatment of lung cancer: a systematic review and meta-analysis. J Thorac Oncol. 2012;7:1170–1178. doi: 10.1097/JTO.0b013e318257cc45. [DOI] [PubMed] [Google Scholar]
- 107.Verhagen A.F., Schoenmakers M.C., Barendregt W. Completeness of lung cancer surgery: is mediastinal dissection common practice? Eur J Cardiothorac Surg. 2012;41:834–838. doi: 10.1093/ejcts/ezr059. [DOI] [PubMed] [Google Scholar]
- 108.Van Schil P.E. Action point: intraoperative lymph node staging. Eur J Cardiothorac Surg. 2012;41:839–840. doi: 10.1093/ejcts/ezr061. [DOI] [PubMed] [Google Scholar]
- 109.Van Schil P.E. The present and future of thoracic surgery within the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2013;43:219–222. doi: 10.1093/ejcts/ezs425. [DOI] [PubMed] [Google Scholar]


