Abstract
Adolescence is an important period for brain development. White matter growth is influenced by sex hormones such as testosterone, and the corpus callosum—the largest white matter structure in the human brain—may change structurally during the hormone-laden period of adolescence. Little is known about puberty’s relationship to structural brain development, even though pubertal stage may better predict cognitive and behavioral maturity than chronological age. We therefore aimed to establish the presence and direction of pubertal effects on callosal anatomy. For this purpose, we applied advanced surface-based mesh-modeling to map correlations between callosal thickness and pubertal stage in a large and well-matched sample of 124 children and adolescents (62 female and 62 male) aged 5–18 years from a normative database. When linking callosal anatomy to pubertal status, only positive correlations reached statistical significance, indicating that callosal growth advances with puberty. In tests of differences in callosal anatomy at different stages of puberty, callosal growth was concentrated in different locations depending on the pubertal stage. Changing levels of circulating sex hormones during different phases of puberty likely contributed to the observed effects, and further research is clearly needed. Direct quantification of sex hormone levels and regional fiber connectivity—ideally using fiber tractography—will reveal whether hormones are the main drivers of callosal change during puberty. These callosal findings may lead to hypotheses regarding cortical changes during puberty, which may promote or result from changes in interhemispheric connectivity.
Keywords: corpus callosum, development, gender, pubertal status, sex
1. Introduction
Adolescence is a critical period for human brain development, and much research has been devoted to the anatomical study of teenage brains. Hu et al., for example, linked pubertal status to volumetric changes in medial temporal lobe structures (Hu et al., 2013). Bramen et al. addressed puberty effects on medial temporal lobe, thalamic, caudate, and cortical gray matter volumes as well as on cortical thickness (Bramen et al., 2011; Bramen et al., 2012). These brain measures were related to measures of physical sexual maturity and also to measures of testosterone. Similarly, Perrin et al. (2008) and Paus et al. (2010) obtained actual testosterone measures from the adolescent brain while also genotyping a functional polymorphism in the androgen receptor gene to see if it moderated the effect of testosterone on different tissue types (Perrin et al., 2008; Paus et al., 2010). The latter two studies revealed that characteristics of the androgen receptor gene moderated the impact of testosterone not only on gray matter, but also on white matter. Thus, it stands to reason that the corpus callosum—the largest white matter structure in the human brain (Lebel et al., 2010)—may display morphological changes during the hormone-laden period of adolescence.
The corpus callosum connects the cerebral hemispheres through over 200 million fibers of varying diameter and degree of myelination (Aboitiz et al., 1992). Numerous studies have found that dramatic changes occur in callosal micro- and macro-anatomy throughout childhood and adolescence (Allen et al., 1991; Giedd et al., 1996; Rajapakse et al., 1996; Giedd et al., 1997; Giedd et al., 1999; Thompson et al., 2000; DeBellis et al., 2001; Chung et al., 2001; Lenroot et al., 2007; Hasan et al., 2008; Muetzel et al., 2008; Hasan et al., 2009; Luders et al., 2010; Lebel et al., 2010; Lebel et al., 2012; Dennis and Thompson, 2013). Rather than uniformly changing over time and/or across the entire structure, the corpus callosum seems to display both temporally and spatially distinct changes. These region-specific growth patterns may reflect a permanent adjustment and fine-tuning of fibers (Luders et al., 2010), perhaps due to hormonal surges during adolescence. Recently, Blakemore et al. advocated more extensive research on puberty’s relationship to structural brain development (Blakemore et al., 2010), suggesting that pubertal stage may better account for changes in cognition and behavior than simple chronological age.
To our knowledge, research linking callosal morphology to pubertal maturation status (rather than chronological age) is currently lacking. This study, therefore, was designed to explore the degree to which pubertal status was associated with changes in callosal anatomy. For this purpose, we applied advanced surface-based mesh-modeling methods and mapped correlations between callosal thickness and pubertal stage in a large and well-matched sample of 124 children and adolescents (62 female and 62 male) aged 5–18 years from a normative participant database (Evans, 2006). As sex differences in brain development have been found in white matter in general (Reiss et al., 1996; DeBellis et al., 2001; Wilke et al., 2007; Perrin et al., 2008; Paus et al., 2010) and callosal measures in particular (DeBellis et al., 2001; Lenroot et al., 2007; Luders et al., 2010), we also set out to assess the degree to which sex moderates the link between pubertal status and callosal thickness.
2. Experimental Procedures
2.1 Participants
Scans were selected from “The NIH MRI Study of Normal Brain Development” database (Evans, 2006), which excluded participants who met criteria “established or highly suspected to adversely impact healthy brain development” (see Evans, 2006). Informed consent was obtained from parents and adolescents; and assent was obtained from child participants. All protocols and procedures were approved by the relevant Institutional Review Board at each evaluation site and at each coordinating center (Evans, 2006).
Given the aims of the study, only scans that included existing callosal contours (Luders et al., 2010; Luders et al., 2011; Kurth et al., 2012), data on sexual development (Hu et al., 2013), and puberty scores of less than 4—as measured by the Pubertal Development Scale (Petersen et al., 1988)—were included. The Pubertal Developmental Scale has been found to be both valid and reliable in discerning subjects’ pubertal status (Petersen et al., 1988). It is a noninvasive, self-reported measure of the physical presentations of puberty that develop concomitantly with underlying changes in circulating sex hormones. As previously described (Hu et al., 2013), the Pubertal Development Scale is based on five categories for each sex (male/female), ranked 1 through 4, with 1 = “not started,” 2 = “barely started,” 3 = “definitely under way,” 4 = “completed.” For girls, the categories include height growth spurt, body hair growth, skin changes, breast growth, and menstruation started (1 = no; 4 = yes). For boys, the categories include height growth spurt, body hair growth, skin changes, voice deepening, and facial hair. Averaging the values across all categories results in the puberty score (i.e., a continuous variable between 1 and 4). For the purpose of this study, we excluded individuals with an average score of 4 because having “completed” puberty, they were outside our scope of interest. Consequently, the final sample consisted of 124 participants (62 males; 62 females) between ages 5 and 18 (mean ± SD: 11.8 ± 3.2 years).
We subsequently devised three subgroups based on puberty score: Group 1 (G1) included 62 participants with puberty scores of less than 2 (puberty score < 2), Group 2 (G2) contained 30 participants with puberty scores including 2 but less than 3 (2 ≤ puberty score < 3), and Group 3 (G3) included 32 participants scoring 3 and above but less than 4 (3 ≤ puberty score < 4). Within each group (G1; G2; G3), we balanced the number of boys and girls (31:31; 15:15; 16:16) while matching them closely for the proportion of non-right-handed participants (3:2; 1:2; 4:3), as summarized in Table 1. As previously described (Kurth et al., 2012), to determine handedness, subjects were asked to perform eight different activities, modified from the Edinburgh Handedness Inventory (Oldfield, 1971). The use of the right hand for each activity was scored as 1; the use of the left hand was scored as 0. Participants with a total score of <7 were classified as non-right-handed. There were no significant differences between the three groups with respect to parental education (using six distinct categories for the mother and the father separately) or the combined annual household income (using ten distinct categories). In contrast, as expected, there were significant differences with respect to chronological age between the three groups (Table 1), with the mean age being smallest in the groups with the lowest maturation status, and largest in the groups with the highest maturation status (G1<G2<G3).
Table 1.
Subgroup composition and characteristics
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Combined Sample | n = 62 (5) | n = 30 (3) | n = 32 (7) |
| Boys | n = 31 (3) | n = 15 (1) | n = 16 (4) |
| Girls | n = 31 (2) | n = 15 (2) | n = 16 (3) |
| Mean age ± SD | 9.80 ± 2.59 years | 12.95 ± 1.68 years | 15.48 ± 1.65 years |
The number of non-right-handers is indicated in the parentheses; SD = standard deviation
2.2 Image Acquisition
Images were obtained on 1.5 T systems from General Electric (GE) or Siemens Medical Systems (Siemens) using a 3D T1-weighted spoiled gradient recalled (SPGR) echo sequence with the following parameters: TR = 22–25 ms, TE = 10–11 ms, excitation pulse = 30°, refocusing pulse = 180°, orientation: sagittal; field of view: AP = 256 mm; LR = 160–180 mm (whole-head coverage). The voxel size was set to 1 mm3, except on GE scanners, where the maximum number of slices was 124, and hence the slice thickness was increased to 1.5 mm in the sagittal plane (Evans, 2006). Importantly, the three groups were comparable with respect to the systems used to acquire the scans. More specifically, 17 out of the 62 subjects (Group 1), 8 out of the 30 subjects (Group 2), and 10 out of the 32 subjects (Group 3) received a Siemens scan. Scanner-specific information for 4 subjects (all in Group 1) was not available. Thus, all three groups (G1; G2; G3) show a similar percental distribution with respect to the underlying scanning system (Siemens: 27%; 27%; 31% and GE: 66%; 73%; 69%).
2.3 Image Preprocessing
Automated radio-frequency bias field corrections were applied to correct image volumes for intensity drifts caused by magnetic field inhomogeneities (Shattuck et al., 2001). In addition, all image volumes were placed into the same standard space by co-registering them to the ICBM-152 template using automated 6-parameter rigid-body transformations (Woods et al., 1998). That is, images were corrected for differences in brain position and orientation while preserving their native dimensions. The corpus callosum was outlined automatically based on the Chan-Vese model for active contours (Chan and Vese, 2001) using the LONI pipeline processing environment (Rex et al., 2003; Dinov et al., 2009). This resulted in two midsagittal callosal segments (i.e., the upper and lower callosal boundary) for each participant, as detailed elsewhere (Luders et al., 2006; Luders et al., 2007). Subsequently, each callosal segment was overlaid onto the MR image from which it had been extracted and visually inspected to ensure that automatically generated callosal outlines precisely followed the natural course and boundaries of the corpus callosum.
2.4 Callosal Thickness Measurements
To obtain highly localized measures of callosal thickness, anatomical surface-based mesh modeling methods were employed (Thompson et al., 1996a; Thompson et al., 1996b). That is, the upper and lower callosal boundaries were re-sampled at regular intervals to render the discrete points comprising the boundaries spatially uniform. Then, a new segment (i.e., the medial core) was automatically created by calculating a spatial average 2D curve from 100 equidistant surface points representing the upper and lower callosal boundaries. Finally, the distances between 100 surface points of the medial core and the 100 corresponding surface points of both the upper and the lower callosal boundaries were computed. These regional distances indicate callosal thickness with a high spatial resolution (i.e., at 100 locations distributed evenly over the callosal surface).
2.5 Statistical Analyses
First, we tested whether there was a link between callosal anatomy and pubertal status. For this purpose, we mapped the correlations between puberty scores and callosal thickness at 100 equidistant surface points within the combined sample (n=124). Second, we examined if there were differences in callosal anatomy between groups defined by pubertal status. For this purpose, we used group 1 (n=62), group 2 (n=30) and group 3 (n=32), as detailed above, and tested if there was a significant main effect of group with respect to callosal thickness. In addition, we tested if sex had a significant impact on the group differences (group-by-sex interaction). Significant main effects of group as well as significant group-by-sex interactions were followed by post hoc comparisons between groups, within the combined sample and also within boys and girls separately.
For all analyses, we used an uncorrected two-tailed alpha level of p≤0.05 as the threshold for projecting significance values (p) onto the group-averaged callosal surface models. In addition, we generated callosal maps corrected for multiple comparisons using False Discovery Rate (FDR) thresholded at 0.05 (Benjamini and Hochberg, 1995). Note, since age and pubertal status are naturally very closely linked, we abstained from co-varying for age, as this would not just remove age-specific variance but also the variance shared between age and pubertal status (and thus with it large parts of the effects we are interested in).
3. Results
3.1 Linking Callosal Thickness and Pubertal Status
As shown in Figure 1, both positive and negative correlations were detected between the point-wise callosal distance measures and puberty scores. However, only positive correlations reached statistical significance. Specifically, significant positive correlations were detected within the genu, anterior midbody, posterior midbody, and splenium1 at p≤0.05, uncorrected. When using FDR-corrected thresholds at p≤0.0104, effects remained significant within the anterior midbody, posterior midbody and splenium.
Figure 1. Correlations between callosal thickness and pubertal status.
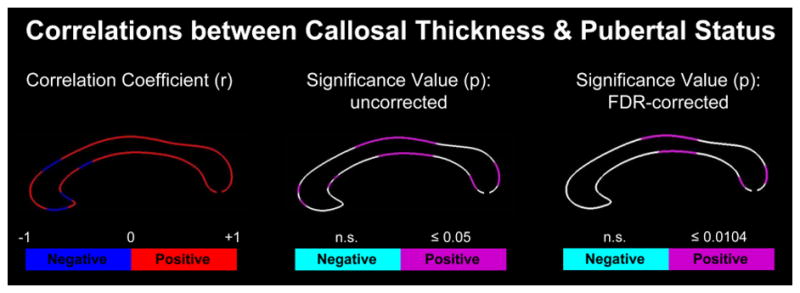
Left Panel: Illustrated are r-maps (correlation coefficients) indicating the direction of the correlation (blue = negative; red = positive). Middle Panel: Illustrated are uncorrected p-maps (significance values) indicating in pink where positive correlations are significant at p≤0.05. Negative correlations were not significant (n.s.). Right Panel: Illustrated are FDR-corrected p-maps (significance values) indicating in pink where positive correlations survived corrections for multiple comparisons at the more stringent significance thresholds. The anterior callosal section is located on the left; the posterior callosal section points to the right.
3.2 Differences in Callosal Thickness between Groups defined by Pubertal Status
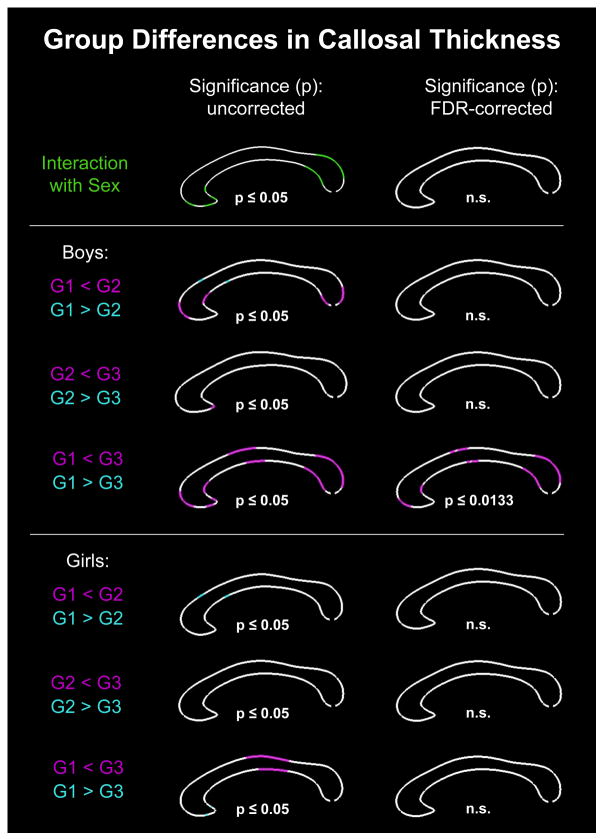
As shown in Figure 2, there was a significant main effect of group (i.e., significant differences in callosal thickness among the three groups), which survived corrections for multiple comparisons at p≤0.0039. As further illustrated in Figure 2, follow-up post hoc comparisons revealed several regions where callosal thickness increases as puberty advances (significant decreases were fully absent). More specifically, at p≤0.05, uncorrected, the corpus callosum was significantly thicker in Group 2 than Group 1 (G1 < G2) within the genu, at the border between anterior and posterior midbody, and the splenium. When using FDR-corrected thresholds at p≤0.0137, group differences remained significant within these callosal areas but significance clusters were a bit less extended. The corpus callosum was also significantly thicker in Group 3 than Group 2 (G2 < G3), but only within a small region of the genu as well as in a larger region covering the anterior and posterior midbody (p≤0.05, uncorrected). Only the effect within the callosal midbody survived FDR-corrections at p≤0.0055. As shown in Figure 3, a significant group-by-sex interaction was evident using uncorrected significance thresholds within rostrum, genu, and splenium. However, none of the significance clusters survived corrections for multiple comparisons. Nevertheless, we conducted the post hoc comparisons contrasting all three groups (G1, G2, and G3), within boys and girls separately, to generate sex-specific spatial profiles in order to provide a framework against which outcomes from future studies can be compared.
Figure 2. Group differences in callosal thickness (combined sample).
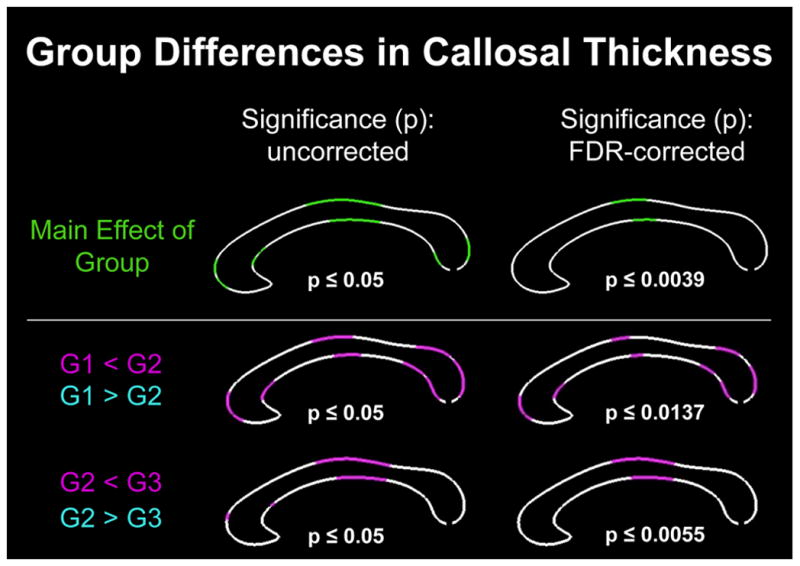
Top Row: Illustrated is the main effect of group. Bottom Rows: Illustrated are the outcomes of the post hoc comparisons between groups, with thinner regions in group 1 than group 2 (G1<G2) and thinner regions in group 2 than group 3 (G2<G3). There were no regions detected as being thicker in group 1 than group 2 (G1>G2) nor as being thicker in group 2 than group 3 (G2>G3). Left Panel: Illustrated are p-maps indicating in green and pink where effects occurred at p≤0.05 (uncorrected significance values). Right Panel: Illustrated are p-maps indicating where effects survived corrections for multiple comparisons (FDR-corrected significance values).
Figure 3. Group differences in callosal thickness (separated by sex).
Top Row: Illustrated is the group-by-sex interaction. Middle and Bottom Rows: Illustrated are the outcomes of the post hoc comparisons, separately within boys and girls, contrasting group 1 and group 2 (G1<G2; G1>G2), group 2 and group 3 (G2<G3; G2>G3), as well as group 1 and group 3 (G1<G3; G1>G3). Left Panel: Illustrated are p-maps indicating in green and pink/cyan where effects occurred at p≤0.05 (uncorrected significance values). Right Panel: Illustrated are p-maps indicating where effects survived corrections for multiple comparisons (FDR-corrected significance values) or were not significant (n.s.).
4. Discussion
As numerous brain structures undergo significant morphological changes during puberty and androgen receptors appear to mediate testosterone’s effect on white matter growth (Perrin et al., 2008; Paus et al., 2010), it is likely that the corpus callosum also changes during the hormone-laden period of adolescence (Muetzel et al., 2008; Lebel et al., 2010; Blakemore et al., 2010; Lebel et al., 2012). In fact, several studies have tracked the development of the corpus callosum against chronological age during this period (Allen et al., 1991; Giedd et al., 1996; Rajapakse et al., 1996; Giedd et al., 1997; Giedd et al., 1999; Thompson et al., 2000; DeBellis et al., 2001; Chung et al., 2001; Lenroot et al., 2007; Hasan et al., 2008; Muetzel et al., 2008; Hasan et al., 2009; Luders et al., 2010; Lebel et al., 2010; Lebel et al., 2012). However, it was recently suggested that the stage of sexual development is more important than chronological age when evaluating cognition and brain development in adolescents (Blakemore et al., 2010), and recent neuroimaging research seems to support this proposition (Perrin et al., 2008; Paus et al., 2010; Bramen et al., 2011; Bramen et al., 2012; Hu et al., 2013).
Thus, we set out to assess the degree to which callosal thickness changes throughout puberty. Overall, our analyses revealed that a higher puberty score is associated with a thicker corpus callosum—reflected not only as positive correlations between callosal thickness and pubertal status but also as thicker corpora callosa in more sexually mature groups (i.e., G1 < G2 < G3). While this is the overall trend, the locations of effects found when comparing G1 and G2 differ from those found when comparing G2 and G3. Specifically, when separating out group differences, the significant effects for G1 < G2 are located in the genu, at the border between anterior and posterior midbodies, and in the splenium. However, for G2 < G3, the significant effects straddle the anterior and posterior midbodies. These differences suggest that during different stages of puberty, callosal morphology undergoes distinct regional changes.
Region-specific callosal changes may be related to region-specific cortical changes as callosal tracts are organized topographically. In the following commentary on the FDR-corrected findings of our study, we will refer to a recent parcellation scheme based on diffusion tensor imaging (DTI) and fiber tractography (Hofer and Frahm, 2006), which complements, refines, and slightly revises the well-established Witelson’s parcellation scheme (Witelson, 1989). As shown in Figure 1, relating callosal thickness and pubertal status, significant positive correlations are evident in callosal regions connecting premotor and supplementary motor cortices, the primary motor cortex, and the parietal, temporal, and occipital cortices. Furthermore, as shown in Figure 2, callosal thickness is significantly greater in G2 than in G1 in callosal regions connecting the prefrontal cortex; the premotor and supplementary motor cortices; and the parietal, temporal, and occipital cortices. In contrast, callosal thickness is significantly greater in G3 than in G2 only within callosal regions connecting the premotor, supplementary motor and primary motor cortices (Hofer and Frahm, 2006; Zarei et al., 2006). Some of these aforementioned cortical regions (i.e., parts of the prefrontal cortex, the primary motor cortex, the supplementary motor cortex, the posterior parietal cortex, the primary auditory cortex in the temporal lobe, and the occipital pole) are noted as containing a high density of estrogen and/or androgen receptors (Goldstein et al., 2001). This may perhaps suggest that throughout puberty, not only does the connectivity of these regions undergo significant changes, but also that cortical regions change per se. In support of this, other studies link physical sexual maturity, circulating sex hormones, and cerebral cortex features, including cortical thickness and cortical gray matter volume (Bramen et al., 2011; Bramen et al., 2012).
Adolescence is a sensitive period of brain development. The surge of gonadal hormones associated with puberty continues to organize the nervous system beyond the organization that occurs during prenatal development (Schulz, Molenda-Figueira, & Sisk, 2009). For instance, studies on animal models have demonstrated that pubertal hormones are necessary to maintain and/or further differentiate sexually dimorphic brain regions, including the anteroventral periventricular nucleus of the hypothalamus, the sexually dimorphic nucleus of the preoptic area, and the medial amygdala (Ahmed et al., 2008). Future studies may reveal the interplay between callosal and cortical changes during puberty by relating both region-specific cortical morphology and indicators of inter-hemispheric connectivity to pubertal status. Moreover, direct quantification of sex hormone levels—perhaps using a longitudinal design— will serve to test the hypothesis that hormones are the main propellants of callosal change during puberty. As pubertal progression was the main influence observed here, there must be some variable characteristic that defines this progression. Direct evidence for the hormonal impact on callosal anatomy is sparse. However, one study (Chura et al., 2010) reported a significant positive correlation between the rightward asymmetry of the callosal isthmus in 8–11 year old-boys and their fetal testosterone (i.e., previously measured in utero via amniocentesis during the second trimester), although there was no relationship detected between fetal testosterone and midsagittal corpus callosum size per se. Evidence for the powerful impact of sex hormones on brain anatomy in general also comes from studies in clinical populations with, for example, disorders of sex development, such as congenital adrenal hyperplasia, where neurodevelopment was shown to be significantly affected due to in utero exposure to elevated levels of androgens (Nass et al., 1997; Merke et al., 2003; Bergamaschi et al., 2006). Additionally, studies have documented the role of the menstrual cycle in the interplay of brain structure, brain function, and behavior (see Sacher et al., 2013).
Our study detected no significant sex effects on the relationship between pubertal status and callosal thickness when using appropriate corrections for multiple comparisons. This was surprising, as sex differences in brain development have been reported, both with respect to white matter in general (Reiss et al., 1996; DeBellis et al., 2001; Wilke et al., 2007; Perrin et al., 2008; Paus et al., 2010) and callosal measures in particular (DeBellis et al., 2001; Lenroot et al., 2007; Luders et al., 2010). These studies, however, related their cerebral measures to chronological age rather than to pubertal status2. Consequently, those findings are not directly comparable to the current findings. A few other studies specifically assessed puberty effects correlation analyses were only conducted within males, but not females. (alone or in combination with age effects) by determining physical sexual maturity and/or quantifying actual levels of circulating sex hormones. These studies reported significant interactions between sex and the effect of puberty in several cortical and subcortical regions (Bramen et al., 2011; Bramen et al., 2012). Rising levels of testosterone (in boys) and estrogen (in girls) might have opposite effects in both sexes in some medial temporal lobe structures (Hu et al., 2013). While this seems to contradict our findings indicating no significant sex interactions, prior sex interactions were found for brain regions consisting of gray matter and not white matter (e.g., the corpus callosum). To our knowledge, this is the first study relating pubertal status to a refined measure of callosal anatomy. As no sex interactions survived corrections for multiple comparisons, our findings suggest that the relationship between pubertal status and callosal thickness are similar in boys and girls.
5. Conclusion, Limitations, and Outlook
Our study provides striking evidence that the corpus callosum undergoes significant changes during puberty. Positive correlations between pubertal status and callosal thickness suggest that advancing sexual maturity is linked to callosal growth. Interestingly, callosal growth occurs in different locations depending on the stage of puberty, which may suggest that different cortical regions (connected through the respective callosal sections) also show temporally distinct changes during the course of puberty. Varying levels of circulating sex hormones during different phases of puberty likely contribute to the observed effects. However, no actual hormone measures were obtained in this study and thus, it remains to be established if (and how) hormones modulate the thickness of the corpus callosum. Moreover, we have examined callosal morphology based on computing regional callosal distances, where statistical analyses were conducted at corresponding surface points (equidistant from each other) along the callosal boundaries. Although this is reasonable given the approximate topographic ordering of callosal projections to the cortex, the simple matching of callosal boundaries might be slightly conservative for detecting systematic effects, albeit unlikely to detect spurious associations. Alternative approaches based on DTI may be able to provide additional, higher-order constraints that allow a more precise matching of anatomical tracts within the corpus callosum across subjects. On a related note, DTI-based fiber tractography might aid to resolve if (and how) the observed callosal effects are associated with cortical changes during puberty. This, in turn, would help to elucidate the exact mechanisms underlying the associations between pubertal status and neurodevelopment, in general.
Highlights.
Positive correlations suggest that advancing puberty is linked to callosal growth.
Callosal growth occurs in different locations depending on the stage of puberty.
Changing levels of sex hormones are likely contributors for the observed effects.
Acknowledgments
Data used in the preparation of this article were obtained from the Pediatric MRI Data Repository created by the NIH MRI Study of Normal Brain Development. This is a multi-site, longitudinal study of typically developing children, from ages newborn through young adulthood, conducted by the Brain Development Cooperative Group and supported by the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, -2315, -2316, -2317, -2319 and -2320). This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH.
Footnotes
Regions are defined according to the traditional Witelson classification scheme (Witelson, 1989).
While Paus et al. (2010) and Perrin et al. (2008) also obtained measures of testosterone, their sex hormone-related
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Allen LS, Richey MF, Chai YM, Gorski RA. Sex differences in the corpus callosum of the living human being. J Neurosci. 1991;11:933–942. doi: 10.1523/JNEUROSCI.11-04-00933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nature Neuroscience. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Pratical and Powerful Approach to Multiple Testing. J R Statist Soc. 1995;57:289–300. [Google Scholar]
- Bergamaschi R, Livieri C, Uggetti C, Candeloro E, Egitto MG, Pichiecchio A, Cosi V, Bastianello S. Brain white matter impairment in congenital adrenal hyperplasia. Arch Neurol. 2006;63:413–416. doi: 10.1001/archneur.63.3.413. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Dinov ID, Worthman CM, Sowell ER. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS ONE. 2012;7:e33850. doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TF, Vese LA. Active contours without edges. IEEE Trans Image Process. 2001;10:266–277. doi: 10.1109/83.902291. [DOI] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Paus T, Cherif C, Collins DL, Giedd JN, Rapoport JL, Evans AC. A unified statistical approach to deformation-based morphometry. Neuroimage. 2001;14:595–606. doi: 10.1006/nimg.2001.0862. [DOI] [PubMed] [Google Scholar]
- Chura LR, Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Bullmore ET, Baron-Cohen S. Organizational effects of fetal testosterone on human corpus callosum size and asymmetry. Psychoneuroendocrinology. 2010;35:122–132. doi: 10.1016/j.psyneuen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. Mapping connectivity in the developing brain. Int J Dev Neurosci. 2013;31:525–542. doi: 10.1016/j.ijdevneu.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinov ID, Van Horn JD, Lozev KM, Magsipoc R, Petrosyan P, Liu Z, kenzie-Graham A, Eggert P, Parker DS, Toga AW. Efficient, Distributed and Interactive Neuroimaging Data Analysis Using the LONI Pipeline. Front Neuroinformatics. 2009;3:22. doi: 10.3389/neuro.11.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, Vauss YC, Hamburger SD, Rapoport JL. A quantitative MRI study of the corpus callosum in children and adolescents. Brain Res Dev Brain Res. 1996;91:274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal Sexual Dimorphism of the Adult Human Brain Assessed by In Vivo Magnetic Resonance Imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Iftikhar A, Kramer LA, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Res. 2009;1249:91–100. doi: 10.1016/j.brainres.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Kramer LA, Papnicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor quantification of the human midsagittal corpus callosum subdivisions across the lifespan. Brain Res. 2008;1227:52–67. doi: 10.1016/j.brainres.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hu S, Pruessner JC, Coupe P, Collins DL. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. Neuroimage. 2013;74:276–287. doi: 10.1016/j.neuroimage.2013.02.032. [DOI] [PubMed] [Google Scholar]
- Kurth F, Mayer EA, Toga AW, Thompson PM, Luders E. The right inhibition? Callosal correlates of hand performance in healthy children and adolescents callosal correlates of hand performance. Hum Brain Mapp. 2013 Sep;34(9):2259–65. doi: 10.1002/hbm.22060. Epub 2012 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Caverhill-Godkewitsch S, Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage. 2010;52:20–31. doi: 10.1016/j.neuroimage.2010.03.072. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Di Paola M, Tomaiuolo F, Thompson PM, Toga AW, Vicari S, Petrides M, Caltagirone C. Callosal morphology in Williams syndrome: a new evaluation of shape and thickness. Neuroreport. 2007;18:203–207. doi: 10.1097/WNR.0b013e3280115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Narr KL, Zamanyan A, Chou YY, Gutman B, Dinov ID, Toga AW. The link between callosal thickness and intelligence in healthy children and adolescents. Neuroimage. 2011;54:1823–1830. doi: 10.1016/j.neuroimage.2010.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010;30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88:1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, Schissel M, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage. 2008;39:1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Heier L, Moshang T, Oberfield S, George A, New MI, Speiser PW. Magnetic resonance imaging in the congenital adrenal hyperplasia population: increased frequency of white-matter abnormalities and temporal lobe atrophy. J Child Neurol. 1997;12:181–186. doi: 10.1177/088307389701200306. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paus T, Nawaz-Khan I, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Susman E, Veillette S, Pausova Z. Sexual dimorphism in the adolescent brain: Role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm Behav. 2010;57:63–75. doi: 10.1016/j.yhbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rumsey JM, Vaituzis AC, Hamburger SD, Rapoport JL. Regional MRI measurements of the corpus callosum: a methodological and developmental study. Brain Dev. 1996;18:379–388. doi: 10.1016/0387-7604(96)00034-4. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119 ( Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Sacher J, Okon-Singer H, Villringer A. Evidence from neuroimaging for the role of the menstrual cycle in the interplay of emotion and cognition. Front Hum Neurosci. 2013;7:374. doi: 10.3389/fnhum.2013.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones & Behavior. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW. Three-dimensional statistical analysis of sulcal variability in the human brain. J Neurosci. 1996a;16:4261–4274. doi: 10.1523/JNEUROSCI.16-13-04261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996b;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Wilke M, Krageloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112 ( Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209(3):311–20. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]