Abstract
Genome-wide association studies (GWAS) have shown that single nucleotide polymorphisms (SNPs) in G6PC2 are the most important common determinants of variations in fasting blood glucose (FBG) levels. Molecular studies examining the functional impact of these SNPs on G6PC2 gene transcription and splicing suggest that they affect FBG by directly modulating G6PC2 expression. This conclusion is supported by studies on G6pc2 knockout (KO) mice showing that G6pc2 represents a negative regulator of basal glucose-stimulated insulin secretion that acts by hydrolyzing glucose-6-phosphate, thereby reducing glycolytic flux and opposing the action of glucokinase. Suppression of G6PC2 activity might therefore represent a novel therapy to lower FBG and the risk of cardiovascular associated mortality. GWAS and G6pc2 KO mouse studies also suggest that G6PC2 affects other aspects of beta cell function. The evolutionary benefit conferred by G6PC2 remains unclear but it is unlikely to be related to its ability to modulate FBG.
Keywords: Islet, Glucose, Glucose-6-Phosphatase, Insulin, Secretion, Mouse
Introduction
Over the past five years, through the power of genome-wide association studies (GWAS), there has been an explosion in our knowledge with respect to the identity of the genes that confer enhanced risk for the development of type 2 diabetes; over 60 loci have been linked to type 2 diabetes risk [1, 2]. GWAS data have also provided insight into the genes controlling fasting blood glucose (FBG) levels [3, 4], glycated hemoglobin A1C (HbA1c) levels [5] as well as the genes associated with increased risk for the development of obesity [6]. Elevated FBG, HbAlc and body mass are all important traits that are correlated with the risk of developing type 2 diabetes [7–10] and cardiovascular-associated mortality (CAM) [11, 12, 13], though, as will be discussed later, the precise relationships between FBG and type 2 diabetes [14] and between FBG and CAM [15] are still under investigation.
At this point multiple investigators are performing follow-up studies on the genes implicated by GWAS in an effort to understand how particular genes contribute to disease risk. This is a difficult proposition because in many instances the single nucleotide polymorphisms (SNPs) that have been linked to disease risk fall in intergenic regions. As such, identifying the disease-related gene(s) associated with these SNPs is a significant challenge. In some cases these intergenic SNPs may impact the function of transcriptional control structures, such as enhancers and silencers [16], such that the genes whose expression are affected may be located a considerable distance from the SNP, though in the literature the nearest gene is often assumed to be the likely candidate.
In relation to the GWAS data for type 2 diabetes, several of the disease-associated SNPs fall within specific genes [1]. Some of these genes had already been implicated by other genetic approaches to disease risk (PPARG, KCNJ11, TCF7L2) or had already been shown or were suspected to have a role in metabolism (HNF1B), obesity (FTO) or beta cell biology (SLC30A8) through molecular studies. Several excellent recent reviews have highlighted the progress made in understanding the molecular and physiological mechanisms whereby the FTO, SLC30A8 and TCF7L2 genes contribute to disease risk [17–20]. However, for most of the genes linked by GWAS to type 2 diabetes the mechanisms by which the encoded proteins modulate disease risk remain unclear [1].
With respect to the genes linked to variations in FBG, multiple GWAS have shown that the G6PC2 locus harbors the strongest common genetic determinant of FBG levels in terms of significance and effect size with a common SNP, rs560887, explaining ~1% of the total variance in FBG [3, 4, 21–26]. Common variants in the GCK gene, which encodes glucokinase, have also been linked to variations in FBG, but the influence of these common GCK variants on FBG is less than that of the common variants in G6PC2 [3]. This observation highlights a critical point, namely that the magnitude of the effect of common gene variants identified through GWAS does not necessarily correlate with the importance of the gene in relation to the parameter under investigation. With respect to G6PC2 and GCK, deletion of the G6pc2 gene in mice has a mild metabolic phenotype [27, 28] and rare mutations in G6PC2 are not a cause of monogenic forms of diabetes [29]. In contrast, deletion of the Gck gene in mice is lethal [30] and rare heterozygous inactivating mutations in GCK are a cause of maturity-onset diabetes of the young, which is characterized by mild fasting hyperglycemia, whereas homozygous inactivating glucokinase mutations result in permanent neonatal diabetes mellitus, which is characterized by severe hyperglycemia [31]. In contrast, glucokinase activating mutations result in hyperinsulinemia leading to hypoglycemia [31]. These rare GCK mutations have provided fascinating molecular insights into the function of glucokinase [31] and, along with mouse models of Gck overexpression [32] and tissue-specific deletion [30, 33], have contributed greatly to the recognition that glucokinase is the pancreatic islet beta cell glucose sensor [34]. Far less is known about the G6PC2 gene, which is the focus of this review.
G6PC2 Encodes a Glucose-6-Phosphatase Catalytic Subunit
Glucose-6-phosphatase catalyzes the hydrolysis of glucose-6-phosphate (G6P) to glucose and inorganic phosphate [35–39]. It exists as a multi-component system located in the endoplasmic reticulum and is comprised of several integral membrane proteins, namely a catalytic subunit (G6PC), a glucose transporter and a G6P/inorganic phosphate antiporter [35–39]. Three G6PC isoforms have been identified, designated G6PC, G6PC2 and G6PC3 [39]. Each isoform is encoded by a separate gene with a distinct pattern of tissue-specific expression [39]. G6PC2 was originally named IGRP, which stands for islet-specific glucose-6-phosphatase catalytic subunit related protein [40, 41]. The gene is expressed exclusively in pancreatic islet beta cells [42]. G6PC2 is a major autoantigen in both mouse [43–45] and human [46, 47] type 1 diabetes, but interestingly GWAS data have not linked G6PC2 SNPs to type 1 diabetes risk [48].
Correlation Between GWAS and Knockout Mouse Data with Respect to the Regulation of FBG by G6PC2
Taneera et al. [49] have suggested that rs560887, which is located in the third intron of G6PC2, acts in trans to modulate the expression of multiple other genes but more recent molecular studies [50], that will be described later, show that the ‘A’ allele of rs560887, that is associated with reduced FBG, leads directly to a reduction in G6PC2 expression. As such, these molecular data are consistent with the ~15% decrease in FBG observed following a global knockout (KO) of G6pc2 in mice [27, 28]. This decrease in FBG is observed when G6pc2 KO mice are studied on a mixed [27] or pure C57BL/6J [28] genetic background. These mouse data strongly support the hypothesis that genetic variation within the G6PC2 gene, rather than surrounding genes, directly contributes to variations in FBG in humans.
The Mechanism of FBG Regulation by G6PC2
A comparison of glucose-6-phosphatase activity in islets isolated from wild type and G6pc2 KO mice indicates that activity is abolished in the latter [28]. These data led to the simple hypothesis that G6pc2 acts as a negative regulator of basal glucose-stimulated insulin secretion (GSIS) by hydrolyzing G6P and thereby opposing the action of the glucose sensor, glucokinase [51, 52] (Figure 1). This glucokinase/G6pc2 futile substrate cycle is predicted to reduce glycolytic flux and hence insulin secretion [28]. Consistent with this model and human GWAS data, a reduction in G6pc2 expression results in a leftward shift in the dose response curve for GSIS explaining why, under fasting conditions, blood glucose levels are reduced [28].
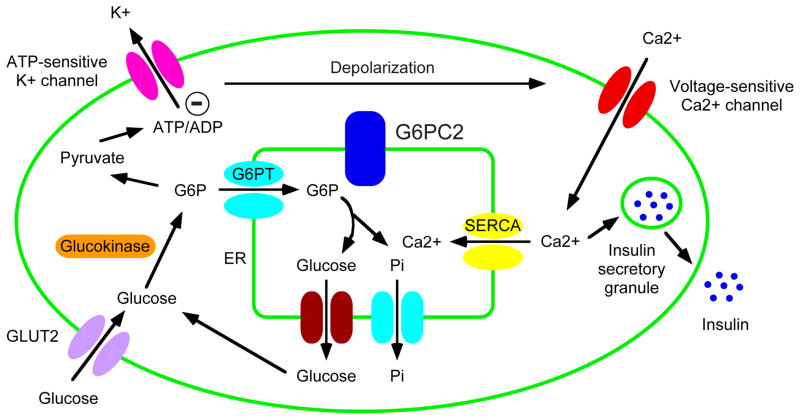
Figure 1. G6PC2 regulates GSIS by opposing the action of glucokinase.
The model shows the best-characterized pathway for GSIS, though studies on potassium channel mutations indicate that other pathways clearly contribute [96]. The glucose-6-phosphate (G6P) transporter (G6PT) and inorganic phosphate transporter are shown in the same color to indicate the fact that G6PT is actually a G6P:Pi antiporter [97]. The model proposes that G6PC2 regulates GSIS by opposing the action of glucokinase but it also suggests that G6PC2 might modulate islet calcium metabolism through its ability to promote the generation of inorganic phosphate in the endoplasmic reticulum lumen resulting in the retention of calcium [77]. (Modified from: Pound LD, Oeser JK, O’Brien TP, Wang Y, Faulman CJ, Dadi PK, Jacobson DA, Hutton JC, McGuinness OP, Shiota M, O’Brien RM: G6PC2: A Negative Regulator of Basal Glucose-Stimulated Insulin Secretion. Diabetes 2013;62:1547–1556) [28•].
Do GWAS and Knockout Mouse Data Resolve the Historical Controversy Over Islet Glucose-6-Phosphatase?
Historically the role of glucose-6-phosphatase activity in pancreatic islets has been highly controversial. Initially there was debate over whether such activity even existed in islets though over time the majority of studies found that activity was detectable, but at a lower level than that found in liver [53, 36, 40, 54–56]. However the harder issue to resolve is whether the level of activity is enough to affect GSIS and therefore be of physiological significance. This question has been investigated by several groups. Sweet et al. [56] concluded that, while glucose-6-phosphatase activity is present in rat islets, the level of activity is not enough to result in sufficient G6P hydrolysis so as to affect GSIS. However, the relevance of these rat islet data to the human GWAS data are unclear because, in contrast to all other vertebrate species examined (see http://genome.ucsc.edu/), G6PC2 is a pseudogene in rats [57].
The observations of Sweet et al. [56] and the absence of G6PC2 in rats raises the question as to what benefit islet glucose-6-phosphatase activity confers to mice and humans that is dispensable to rats. However, three observations suggest that the premise of this question is not well founded. First, rat islets express low levels of the G6PC isoform whereas microarray data show no expression of G6pc or G6pc3 in mouse islets (unpublished data). G6PC is predominantly expressed in liver and kidney where it catalyzes the final step in the gluconeogenic and glycogenolytic pathways [35–39] but in various rat models associated with impaired glucose tolerance, G6PC expression is induced [58–60]. Second, Pedersen et al. [61] have demonstrated that the rat G6PC promoter is activated strongly by glucose, much more so than the mouse or human G6PC promoters. This implies that the estimates of G6P hydrolysis in rat islets will be very dependent on the glucose concentration in the culture medium and hence the level of induction of G6PC. Third, G6PC is ~20 fold more active than G6PC2 [62] such that much less G6PC is required in rat islets to catalyze an equivalent rate of G6P hydrolysis as observed in mouse islets. It therefore appears that G6PC may play the same role in rat islets as G6PC2 does in human and mouse islets.
Several groups have also examined G6P hydrolysis in mouse, rather than rat islets. One early study suggested that, even though glucose-6-phosphatase activity is present in mouse islets, G6P hydrolysis does not occur [63]. This counterintuitive conclusion was challenged by later studies, which showed that the measurement of G6P hydrolysis within islets is critically dependent on experimental conditions [64, 65]. The more recent GWAS and G6pc2 KO mouse data described above, especially the demonstration that G6pc2 accounts for the low glucose-6-phosphatase enzyme activity detected in mouse islets [28], would seem to resolve the historical controversy over the importance of glucose-6-phosphatase activity in islets. However, two caveats remain.
The first caveat relates to the fact that estimates of glucose cycling in mouse pancreatic islets are very low [66]. However, these estimates of glucose cycling were generated using radioisotopes and the methodology involved is associated with a number of assumptions [64, 65]. To avoid these assumptions it will be essential to reassess the level of glucose cycling in pancreatic islets using more recently developed stable isotope methodology [67]. If the rates of glucose cycling calculated using this technology are greater than previously estimated using radioisotopes then this would support the hypothesis that G6PC2 directly influences GSIS through its ability to hydrolyze G6P. Importantly, because the glucose-6-phosphatase activity of G6PC2 is ~20 fold lower than that of G6PC [62, 28] it heightens the concern that G6PC2 may be influencing GSIS through a mechanism independent of its ability to hydrolyze G6P. Indeed all three G6PC isoforms possesses a phosphatidic acid phosphatase domain [68] raising the possibility that they may also have lipid substrates.
Human GWAS Data Provide Novel Insights into the Influence of G6PC2 on GSIS
The second caveat concerning the function of G6PC2 in pancreatic islets relates to the unexpected effects of altered G6PC2 expression on insulin secretion during glucose tolerance tests. This issue was initially uncovered through the analysis of GWAS data rather than the analysis of G6pc2 KO mice. This provides an interesting example of how GWAS data can not only provide insight into genes linked to the initial parameter under investigation but also provide insight into other functions of the genes identified. As mentioned above, a reduction in G6pc2 expression results in a leftward shift in the dose response curve for GSIS explaining why, under fasting conditions, blood glucose levels are reduced [28]. But under conditions of elevated blood glucose this same leftward shift arising from a reduction in G6pc2 expression should result in increased GSIS. Indeed, in perfused pancreas studies comparing pancreata from wild type and G6pc2 KO mice, GSIS is increased in the KO pancreata at sub-maximal glucose concentrations [28]. Likewise, in isolated islet studies comparing islets from wild type and G6pc2 KO mice, GSIS is increased in the KO islets at sub-maximal glucose concentrations [28]. This increased insulin secretion at sub-maximal glucose concentrations was predicted to result in improved glucose tolerance in G6pc2 KO mice. However, neither intraperitoneal and oral glucose tolerance tests show major differences in glucose tolerance or insulin secretion between WT and G6pc2 KO mice over a range of glucose concentrations [28].
These observations in G6pc2 KO mice are consistent with earlier human GWAS data showing no association between G6PC2 and glucose tolerance [69–72]. GWAS data also showed that G6PC2 is not associated with variations in insulin sensitivity in humans [69–72], an observation that was confirmed in G6pc2 KO mice [28].
To further complicate the situation, human GWAS data show that the SNP within the G6PC2 gene that is associated with reduced G6PC2 expression [50] and reduced FBG [3] is actually associated with a reduction in insulin secretion during glucose tolerance tests rather than the expected increase [69–72]. So in humans reduced G6PC2 expression appears to promote glycolytic flux leading to reduced FBG but this enhanced flux not only fails to enhance glucose tolerance during a glucose challenge but it is actually associated with a decrease in insulin secretion during that glucose challenge. The data with G6pc2 KO mice appear slightly different to the human GWAS data in that a similar reduction in insulin secretion was not observed in G6pc2 KO mice during glucose tolerance tests [28]. However, this may simply be due to the relatively low number of animals analyzed [28] relative to the vast number of humans analyzed in GWAS studies [69]. Thus mouse data are inherently noisy in that significant variations in insulin sensitivity, and hence insulin secretion, are observed even within inbred C57BL/6J mice [73]. The key consistent observation is that in both mice and humans reduced G6PC2 expression does not lead to an improvement in glucose tolerance.
The unexpected association between reduced G6PC2 expression and reduced insulin secretion during glucose tolerance tests in humans has been hypothesized to indicate that either G6PC2 affects the pulsatility of insulin secretion [69] or that it affects hepatic glucose production rather than beta cell function [72]. The latter explanation appears highly unlikely since human G6PC2 [57, 3] and mouse G6pc2 [40, 39] are only expressed in islets and not in liver. In contrast a change in the pulsatility of insulin secretion, and hence the efficacy of insulin signaling, would provides an elegant explanation as to how reduced G6PC2 expression could lead to a reduction in insulin secretion that is not associated with a counterbalancing change in glucose tolerance or insulin sensitivity [69–72].
Whether the absence of G6pc2 in mice affects the pulsatility of insulin secretion is a key question that remains to be addressed. However, if this is the explanation for the reduced insulin secretion during glucose tolerance tests in humans, then the question arises as to whether the ability of G6PC2 to influence the pulsatility of insulin secretion is dependent on its ability to hydrolyze G6P or some other function, perhaps connected with the phosphatidic acid phosphatase domain mentioned above [68]. The generation of a transgenic model in which a mutated form of G6pc2 lacking glucose-6-phosphatase activity is expressed in the G6pc2 KO mice might provide insight into this question. Merrins et al. [74] have elegantly shown that pulsatile insulin secretion is driven by metabolic oscillations and that the magnitude of the pulses can be amplified by raising intracellular calcium levels. This then raises the question as to whether G6PC2 might affect metabolic oscillations, intracellular calcium levels or both. Merrins et al. [75] have recently shown that metabolic oscillations in islets are initiated at an early stage in glycolysis with the mechanism likely involving the autocatalytic feedback of fructose 1, 6-bisphosphate onto phosphofructokinase 1, with phosphofructokinase 1 being activated by its product resulting in the subsequent depletion of its substrate [76]. Based on these data it would appear more likely that G6PC2 affects pulsatile insulin secretion through an action on intracellular islet calcium levels rather than metabolic oscillations. Indeed, basal cytoplasmic calcium levels are enhanced in islets isolated from G6pc2 KO mice [28]. This increase was interpreted as a secondary event resulting from the enhanced rate of glycolytic flux [28] but, based on the studies of Merrins et al. [74], if this difference was instead, at least in part, a primary consequence of G6pc2 deletion, then a difference in intracellular calcium between wild type and G6pc2 KO islets would be predicted to be associated with altered pulsatile insulin secretion. Indeed G6PC2 might modulate islet calcium metabolism through its ability to promote the generation of inorganic phosphate in the endoplasmic reticulum lumen resulting in the retention of calcium [77] (Figure 1).
Human GWAS Data Uncover an Unexpected Connection Between G6PC2 and Body Weight
In addition to uncovering the complex relationship between G6PC2 and glucose tolerance, human GWAS data show that G6PC2 is also unexpectedly associated with altered body fat and BMI [69]. The ‘A’ allele of rs560887, that is associated with reduced FBG [3] and with reduced G6PC2 expression [50], is also associated with reduced body fat and BMI [69]. Subsequently, studies on female G6pc2 KO mice demonstrated a reduction in body fat and body weight on both chow fed and high-fat fed diets, consistent with the human GWAS data [28]. The reason why similar differences are not observed in male mice is unclear but the key question is how G6PC2, which is selectively expressed in islets [42, 3], could impact body fat and body weight. There appear to be three possibilities. The first, as suggested by Li et al. [69] is that changes in body weight are secondary to altered efficacy of insulin signaling arising from altered pulsatile insulin secretion. But a second possibility is that G6PC2 is expressed elsewhere in the body in a tissue that can influence body weight. The obvious candidate would perhaps be the hypothalamus given its well-established role in body weight regulation and the fact that glucokinase is also expressed there [78]. Goh et al. [79] have reported very low G6pc2 expression in mouse hypothalamus but only at very high template concentrations and PCR cycles. Furthermore, the catalytically more active G6pc3 isoform was expressed at much higher levels [79] such that the loss of barely detectable G6pc2 expression would likely have little impact on total hypothalamic glucose-6-phosphatase activity. Of note, an analysis of multiple G6pc2-LacZ transgenes failed to detect hypothalamic expression [80, 27, 81]. Finally, a third possibility to explain the connection between G6PC2 and body weight relates to the kinetics of GSIS. The same model that predicts a leftward shift in the dose response curve for GSIS following a reduction in G6PC2 expression [28] would predict a faster rise in plasma insulin levels as glucose levels rise following a meal. Since insulin is a satiety factor [82] the more rapid rise in plasma insulin might lead to an earlier cessation of feeding and hence a reduction in food intake and body mass. Further studies in G6pc2 KO mice should be able to address this hypothesis.
Evolution and the Function of G6PC2
An intriguing aspect of the human GWAS data discussed above is the conclusion that the absence of G6PC2 would be beneficial to several aspects of human health. In relation to FBG, it would be lowered by the absence of G6PC2, which would be predicted to reduce the risk of cardiovascular-associated mortality and type 2 diabetes (but see below). Similarly, in relation to body fat and BMI, both would be lowered by the absence of G6PC2, with the expected health benefits. Finally, the human GWAS data imply that, if G6PC2 is affecting the pulsatility of insulin secretion and hence the efficacy of insulin signaling, then this pulsatility is actually enhanced by the absence of G6PC2, explaining the reduced insulin secretion during glucose tolerance tests [69].
Since the presence of G6PC2 has been retained though mammalian evolution, with the exception of rats, this implies that there must be beneficial effects conferred by G6PC2 that are not apparent from the human GWAS data. The health benefits conferred by reduced FBG and BMI are unlikely to be relevant to reproductive potential since the diseases associated with elevated FBG and BMI typically occur later in life long after an individual has passed on their genetic material to their offspring. Furthermore, such diseases are only prevalent to the modern world and would not even have been a factor during the course of evolution.
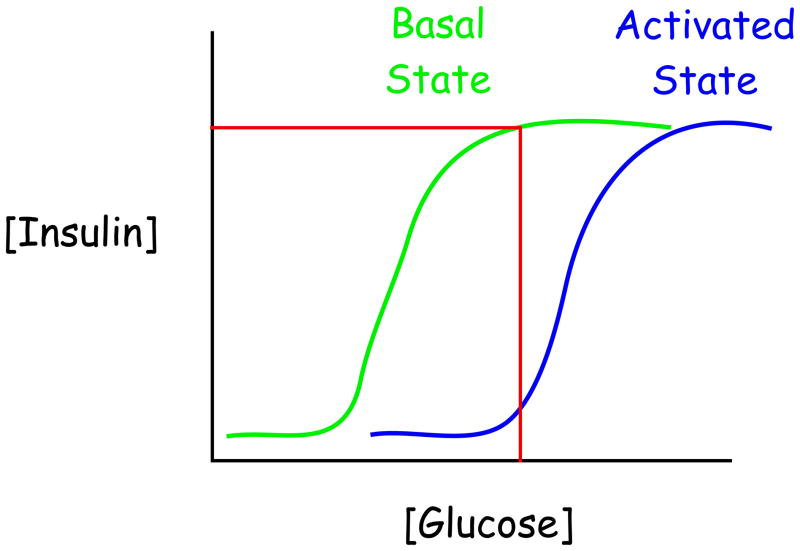
The biological benefit(s) conferred by the presence of G6PC2 are currently unknown but one possibility is that G6PC2 expression or G6PC2 enzyme activity are activated under specific physiological conditions. This would have the effect of shifting the dose response curve for GSIS to the right, resulting in reduced insulin secretion (Figure 2). A number of studies have suggested that the activity of hepatic G6PC is altered by insulin signaling [83], though because the mechanisms involved are unknown it is unclear whether the same signaling pathway might regulate G6PC2 enzyme activity. Similarly, while multiple transcription factors that contribute to the islet beta cell-specific expression of G6PC2 have been identified [39], there is currently no evidence that G6PC2 expression is modulated in vivo under different physiological conditions. Nonetheless, there is circumstantial data to support the potential impact of altered G6PC2 expression. First, in various rat models associated with impaired glucose tolerance, G6PC expression is induced such that G6P hydrolysis would be elevated and GSIS blunted [58–60]. This induction may play a protective role against excessive stimulation of the beta cells, which is a concern given their susceptibility to ER [84] and oxidative stress [85]. Second, experiments in which G6PC was overexpressed in pancreatic islet beta cell-derived cell lines using adenovirus [86] or stable transfection [87] have directly demonstrated that altered expression of this single gene is sufficient to inhibit insulin secretion.
Figure 2. Elevated G6PC2 expression or enzyme activity would alter the sensitivity of GSIS.
The evolutionary function of G6PC2 is unknown but is unlikely to be related to the control of FBG. The model speculates that under specific physiological conditions G6PC2 expression or enzyme activity are elevated changing the sensitivity of GSIS. This model is consistent with the observations that a reduction in G6PC2 expression [28] or Gck overexpression [98, 99] augments glycolytic flux and causes a leftward shift in the dose response curve for GSIS.
The Relationship Between G6PC2, FBG and Type 2 Diabetes Risk
Since G6PC2 is associated with variations in FBG and HbA1c [3, 4, 21–26] and because the accepted dogma is that elevated FBG and HbA1c are associated with an increased risk for the development of type 2 diabetes [7–9] one would logically have expected that G6PC2 would also be associated with increased risk for the development of type 2 diabetes. Indeed studies on Chinese individuals have shown such an association [24, 25], though the sample sizes used in these studies were relatively small. Other studies, with large sample sizes, have shown that in European populations G6PC2 is linked to variations in FBG but not risk for the development of type 2 diabetes [23, 22]. In contrast, a recent meta-analysis contradicted these conclusions and described an association between G6PC2 and type 2 diabetes risk in Caucasians but not Asians [88]. Adding to the confusion, this potential discordance in the connection between G6PC2, FBG and type 2 diabetes risk does not apply to other genes that have been linked to variations in FBG. For example, variations in GCK are linked to both FBG and type 2 diabetes risk [23].
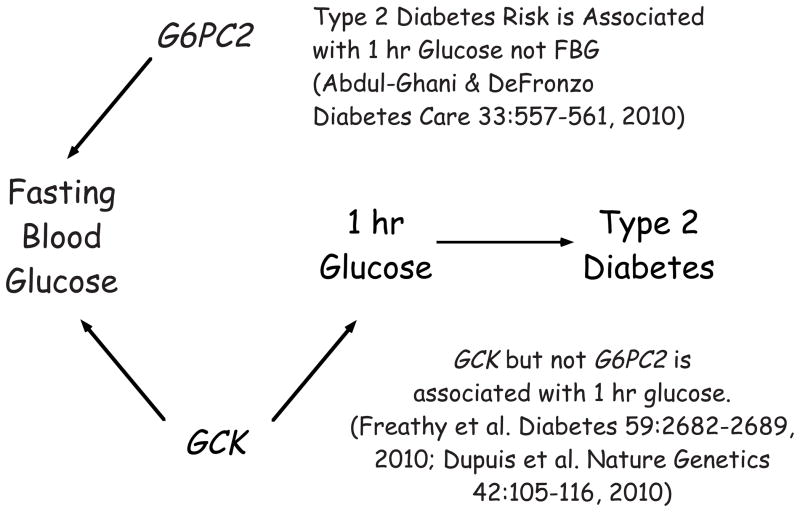
A study by Abdul-Ghani et al. [14•] provides a potentially elegant resolution to this paradox. They have reported that the 1 hour glucose level in a glucose tolerance test is a better predictor of type 2 diabetes risk than FBG such that, after correcting for this variable, the association between FBG levels and type 2 diabetes risk is lost. The authors suggest that the apparent correlation between elevated FBG and type 2 diabetes risk is not due to the increase in FBG per se but is due instead to the correlation between FBG and 1 hour glycemia [14]. Based on the observations of Abdul-Ghani et al. [14], one would predict that variations in GCK would affect both 1 hr glucose levels in a glucose tolerance test, in addition to FBG, whereas variations in G6PC2 would affect only the latter (Figure 3). Indeed, the rs1799884 GCK variant is associated with higher 1 hr glucose levels [89]. In some populations [89], though not others [69], this variant is also associated with higher 2 hr glucose levels. In contrast, the rs560887 G6PC2 variant is not associated with altered glucose tolerance [69–72]. The observations of Abdul-Ghani et al. [14] also lead to the conclusion that the observed decrease in G6PC2 expression in islets from donors with type 2 diabetes [49] is likely to be a secondary event, specifically a response to the diabetic environment, rather than a causative event that contributes to the development of type 2 diabetes. Thus based on G6pc2 knockout mouse data [27, 28], a decrease in G6PC2 expression would lead to enhanced insulin secretion, which would make sense in terms of a compensatory attempt by unhealthy islets to maintain insulin secretion.
Figure 3. Variations in GCK but not G6PC2 affect type 2 diabetes risk.
GWAS data show that SNPs in both G6PC2 and GCK are associated variations in FBG but only SNPs in GCK are associated with type 2 diabetes risk. The model proposes that this observation is explained by the fact that SNPs in GCK are associated variations in 1 hr glucose levels during a glucose tolerance test whereas SNPs in G6PC2 are not. This concept is based on the studies of Abdul-Ghani et al. [14] who showed that the 1 hour glucose level in a glucose tolerance test is a better predictor of type 2 diabetes risk than FBG such that, after correcting for this variable, the association between FBG levels and type 2 diabetes risk is lost.
As described below, the rs560887 G6PC2 variant linked by GWAS to FBG but not type 2 diabetes risk is predicted to have only a small effect on G6PC2 expression [50]. However, even though small changes in G6PC2 expression are not associated by GWAS with the risk for type 2 diabetes, this would not exclude the possibility that rare variants that markedly elevate G6PC2 expression or increase activity may be associated with altered risk for type 2 diabetes.
Functional Analysis of SNPs that Modulate G6PC2 Splicing and Gene Transcription
Several studies have examined the molecular effects of SNPs on G6PC2 splicing and gene transcription. Two SNPs in the G6PC2 promoter, rs13431652 and rs2232316, were shown to affect G6PC2 fusion gene expression by modulating NF-Y and Foxa2 binding, respectively [90, 50]. In addition, two SNPs in the third G6PC2 intron, rs560887 and rs2232321, were shown to affect G6PC2 RNA splicing [50], likely by modulating the strength of a branch point sequence, a key element in RNA splicing [91, 92]. The in vitro and in situ molecular data suggest that all four SNPs are potentially causative since the allele that results in elevated G6PC2 expression is associated with elevated FBG [90, 50]. In contrast, for another G6PC2 promoter SNP, rs573225, that also affects G6PC2 fusion gene expression by modulating Foxa2 binding, the allele that results in elevated G6PC2 expression is associated with reduced FBG [90, 50], suggesting that rs573225 is a functional SNP that opposes the action of causative SNPs on G6PC2 expression [90, 50], a conclusion that contrasts with an earlier study [93].
Challenges in the Identification of Causative G6PC2 SNPs
There are several key limitations in the analysis of G6PC2 causative SNPs. First, because these SNPs are in high linkage disequilibrium, it is difficult to definitely determine whether one or all of these SNPs are truly causative [90, 50]. Second, because the G6PC2 gene is only expressed in pancreatic islet beta cells and because the effects of these SNPs are subtle, the lack of sufficient human samples has limited the ability to correlate genotypes with endogenous G6PC2 expression. Finally, multiple caveats are associated with analyzing G6PC2 promoter SNPs using fusion genes in islet-derived cell lines [90, 50]. Furthermore, most of these cell lines are derived from rodent islets and recent studies suggest the existence of significant differences between rodent and human islets [94].
Conclusions and Future Directions
The GWAS and molecular studies described above strongly suggest that G6PC2 modulates FBG by hydrolyzing G6P thereby opposing the action of the beta cell glucose sensor, glucokinase. However, these studies suggest that G6PC2 has other unexplained effects on islet beta cell function that merit further investigation. The evolutionary benefit conferred by G6PC2 remains unclear but it is unlikely to be related to its ability to modulate FBG. The analysis of rare SNPs that markedly affect G6PC2 enzyme activity and the analysis of the biological impact of these SNPs might provide further insight into G6PC2 function, as have similar studies with glucokinase. Finally, because G6PC2 opposes the action of glucokinase, suppression of G6PC2 activity might represent a novel therapy to lower FBG and HbA1c levels and hence the risk of CAM. It is noteworthy that the alternate strategy, the use of GCK activators, has shown promise with several compounds currently in clinical trials [95, 52].
Acknowledgments
I would like to thank Les Satin (University of Michigan) for helpful comments on the mechanism of pulsatile insulin secretion. Research in the laboratory of R.O’B. was supported by NIH grant DK92589. This review is dedicated to the memory of my long-time colleague and friend, John C. Hutton.
References
- 1.Yaghootkar H, Frayling TM. Recent progress in the use of genetics to understand links between type 2 diabetes and related metabolic traits. Genome biology. 2013;14(3):203. doi: 10.1186/gb-2013-14-3-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres JM, Cox NJ, Philipson LH. Genome wide association studies for diabetes: perspective on results and challenges. Pediatric diabetes. 2013;14(2):90–6. doi: 10.1111/pedi.12015. [DOI] [PubMed] [Google Scholar]
- 3.Bouatia-Naji N, Rocheleau G, Van Lommel L, Lemaire K, Schuit F, Cavalcanti-Proenca C, et al. A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science. 2008;320(5879):1085–8. doi: 10.1126/science.1156849. [DOI] [PubMed] [Google Scholar]
- 4.Chen WM, Erdos MR, Jackson AU, Saxena R, Sanna S, Silver KD, et al. Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest. 2008;118:2620–28. doi: 10.1172/JCI34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, et al. Common variants at ten genomic loci influence hemoglobin A1C levels via glycemic and non-glycemic pathways. Diabetes. 2010;59(12):3229–39. doi: 10.2337/db10-0502. db10-0502 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Sayed Moustafa JS, Froguel P. From obesity genetics to the future of personalized obesity therapy. Nature reviews Endocrinology. 2013 doi: 10.1038/nrendo.2013.57. [DOI] [PubMed] [Google Scholar]
- 7.Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, et al. Use of HbA1c in predicting progression to diabetes in French men and women: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2006;29(7):1619–25. doi: 10.2337/dc05-2525. [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Ghani MA, DeFronzo RA. Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care. 2009;32 (Suppl 2):S194–8. doi: 10.2337/dc09-S309. 32/suppl_2/S194 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ. Utility of hemoglobin A1c in predicting diabetes risk. J Gen Intern Med. 2004;19(12):1175–80. doi: 10.1111/j.1525-1497.2004.40178.x. JGI40178 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye J. Mechanisms of insulin resistance in obesity. Frontiers of medicine. 2013;7(1):14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22(2):233–40. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 12.Lawes CM, Parag V, Bennett DA, Suh I, Lam TH, Whitlock G, et al. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27(12):2836–42. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 13.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(5):968–76. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 14•.Abdul-Ghani MA, Stern MP, Lyssenko V, Tuomi T, Groop L, Defronzo RA. Minimal contribution of fasting hyperglycemia to the incidence of type 2 diabetes in subjects with normal 2-h plasma glucose. Diabetes Care. 2010;33(3):557–61. doi: 10.2337/dc09-1145. dc09-1145 [pii] This paper challenges the long held connection between FBG and type 2 diabetes risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9. S0140-6736(10)60484-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cecchini KR, Raja Banerjee A, Kim TH. Towards a genome-wide reconstruction of cis-regulatory networks in the human genome. Seminars in cell & developmental biology. 2009;20(7):842–8. doi: 10.1016/j.semcdb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurray F, Moir L, Cox RD. From mice to humans. Curr Diab Rep. 2012;12(6):651–8. doi: 10.1007/s11892-012-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frayling TM, Ong K. Piecing together the FTO jigsaw. Genome biology. 2011;12(2):104. doi: 10.1186/gb-2011-12-2-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson O, Zhou Y, Renstrom E, Osmark P. Molecular function of TCF7L2: Consequences of TCF7L2 splicing for molecular function and risk for type 2 diabetes. Curr Diab Rep. 2010;10(6):444–51. doi: 10.1007/s11892-010-0149-8. [DOI] [PubMed] [Google Scholar]
- 20.van de Bunt M, Gloyn AL. From genetic association to molecular mechanism. Curr Diab Rep. 2010;10(6):452–66. doi: 10.1007/s11892-010-0150-2. [DOI] [PubMed] [Google Scholar]
- 21.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2008;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiling E, van ’t Riet E, Groenewoud MJ, Welschen LM, van Hove EC, Nijpels G, et al. Combined effects of single-nucleotide polymorphisms in GCK, GCKR, G6PC2 and MTNR1B on fasting plasma glucose and type 2 diabetes risk. Diabetologia. 2009;52:1866–70. doi: 10.1007/s00125-009-1413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–16. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu C, Zhang R, Wang C, Ma X, Wang C, Fang Q, et al. A genetic variant of G6PC2 is associated with type 2 diabetes and fasting plasma glucose level in the Chinese population. Diabetologia. 2009;52(3):451–6. doi: 10.1007/s00125-008-1241-3. [DOI] [PubMed] [Google Scholar]
- 25.Hu C, Zhang R, Wang C, Yu W, Lu J, Ma X, et al. Effects of GCK, GCKR, G6PC2 and MTNR1B variants on glucose metabolism and insulin secretion. PLoS One. 2010;5(7):e11761. doi: 10.1371/journal.pone.0011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tam CH, Ho JS, Wang Y, Lee HM, Lam VK, Germer S, et al. Common polymorphisms in MTNR1B, G6PC2 and GCK are associated with increased fasting plasma glucose and impaired beta-cell function in Chinese subjects. PLoS One. 2010;5(7):e11428. doi: 10.1371/journal.pone.0011428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Martin CC, Oeser JK, Sarkar S, McGuinness OP, Hutton JC, et al. Deletion of the Gene Encoding the Islet-Specific Glucose-6-Phosphatase Catalytic Subunit-Related Protein Autoantigen Results in a Mild Metabolic Phenotype. Diabetologia. 2007;50:774–8. doi: 10.1007/s00125-006-0564-1. [DOI] [PubMed] [Google Scholar]
- 28•.Pound LD, Oeser JK, O’Brien TP, Wang Y, Faulman CJ, Dadi PK, et al. G6PC2: A Negative Regulator of Basal Glucose-Stimulated Insulin Secretion. Diabetes. 2013;62:1547–56. doi: 10.2337/db12-1067. This study complements the related GWAS data by demonstrating that G6PC2 directly regulates FBG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnefond A, Bouatia-Naji N, Simon A, Saint-Martin C, Dechaume A, de Lonlay P, et al. Mutations in G6PC2 do not contribute to monogenic forms of early infancy diabetes and beta cell dysfunction. Diabetologia. 2009;52(5):982–5. doi: 10.1007/s00125-009-1299-6. [DOI] [PubMed] [Google Scholar]
- 30.Grupe A, Hultgren B, Ryan A, Ma YH, Bauer M, Stewart TA. Transgenic knockouts reveal a critical requirement for pancreatic beta cell glucokinase in maintaining glucose homeostasis. Cell. 1995;83(1):69–78. doi: 10.1016/0092-8674(95)90235-x. [DOI] [PubMed] [Google Scholar]
- 31.Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanne-Chantelot C, Ellard S, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009;30(11):1512–26. doi: 10.1002/humu.21110. [DOI] [PubMed] [Google Scholar]
- 32.Magnuson MA, She P, Shiota M. Gene-altered mice and metabolic flux control. J Biol Chem. 2003;278(35):32485–8. doi: 10.1074/jbc.R300020200. [DOI] [PubMed] [Google Scholar]
- 33.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274(1):305–15. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 34.Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep. 2005;5(3):171–6. doi: 10.1007/s11892-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 35.Mithieux G. New knowledge regarding glucose-6 phosphatase gene and protein and their roles in the regulation of glucose metabolism. Eur J Endocrinol. 1997;136(2):137–45. doi: 10.1530/eje.0.1360137. [DOI] [PubMed] [Google Scholar]
- 36.Foster JD, Pederson BA, Nordlie RC. Glucose-6-phosphatase structure, regulation, and function: an update. Proc Soc Exp Biol Med. 1997;215(4):314–32. doi: 10.3181/00379727-215-44142. [DOI] [PubMed] [Google Scholar]
- 37.van de Werve G, Lange A, Newgard C, Mechin MC, Li Y, Berteloot A. New lessons in the regulation of glucose metabolism taught by the glucose 6-phosphatase system. Eur J Biochem. 2000;267(6):1533–49. doi: 10.1046/j.1432-1327.2000.01160.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Schaftingen E, Gerin I. The glucose-6-phosphatase system. Biochem J. 2002;362(Pt 3):513–32. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutton JC, O’Brien RM. The glucose-6-phosphatase catalytic subunit gene family. J Biol Chem. 2009;284:29241–5. doi: 10.1074/jbc.R109.025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arden SD, Zahn T, Steegers S, Webb S, Bergman B, O’Brien RM, et al. Molecular cloning of a pancreatic islet-specific glucose-6-phosphatase catalytic subunit-related protein. Diabetes. 1999;48(3):531–42. doi: 10.2337/diabetes.48.3.531. [DOI] [PubMed] [Google Scholar]
- 41.Ebert DH, Bischof LJ, Streeper RS, Chapman SC, Svitek CA, Goldman JK, et al. Structure and promoter activity of an islet-specific glucose-6- phosphatase catalytic subunit-related gene. Diabetes. 1999;48(3):543–51. doi: 10.2337/diabetes.48.3.543. [DOI] [PubMed] [Google Scholar]
- 42.Hutton JC, Eisenbarth GS. A pancreatic beta-cell-specific homolog of glucose-6-phosphatase emerges as a major target of cell-mediated autoimmunity in diabetes. Proc Natl Acad Sci U S A. 2003;100(15):8626–8. doi: 10.1073/pnas.1633447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2003;100(14):8384–8. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han B, Serra P, Amrani A, Yamanouchi J, Maree AF, Edelstein-Keshet L, et al. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med. 2005;11(6):645–52. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee R, Wagar D, Stephens TA, Lee-Chan E, Singh B. Identification of CD4+ T cell-specific epitopes of islet-specific glucose-6-phosphatase catalytic subunit-related protein: a novel beta cell autoantigen in type 1 diabetes. J Immunol. 2005;174(9):5306–15. doi: 10.4049/jimmunol.174.9.5306. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Danke NA, Berger D, Reichstetter S, Reijonen H, Greenbaum C, et al. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J Immunol. 2006;176(5):2781–9. doi: 10.4049/jimmunol.176.5.2781. [DOI] [PubMed] [Google Scholar]
- 47.Jarchum I, Nichol L, Trucco M, Santamaria P, DiLorenzo TP. Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin Immunol. 2008;127(3):359–65. doi: 10.1016/j.clim.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet. 2011;12(11):781–92. doi: 10.1038/nrg3069. [DOI] [PubMed] [Google Scholar]
- 49.Taneera J, Lang S, Sharma A, Fadista J, Zhou Y, Ahlqvist E, et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 2012;16(1):122–34. doi: 10.1016/j.cmet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Baerenwald DA, Bonnefond A, Bouatia-Naji N, Flemming BP, Umunakwe OC, Oeser JK, et al. Multiple functional polymorphisms in the G6PC2 gene contribute to the association with higher fasting plasma glucose levels. Diabetologia. 2013;56(6):1306–16. doi: 10.1007/s00125-013-2875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45(2):223–41. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 52.Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66(1):27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waddell ID, Burchell A. The microsomal glucose-6-phosphatase enzyme of pancreatic islets. Biochem J. 1988;255(2):471–6. doi: 10.1042/bj2550471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perales MA, Sener A, Malaisse WJ. Hexose metabolism in pancreatic islets: the glucose-6-phosphatase riddle. Mol Cell Biochem. 1991;101(1):67–71. doi: 10.1007/BF00238439. [DOI] [PubMed] [Google Scholar]
- 55.Trandaburu T. Fine structural localization of glucose-6-phosphatase activity in the pancreatic islets of two amphibian species (Salamandra salamandra L. and Rana esculenta L.) Acta Histochem. 1977;59(2):246–53. doi: 10.1016/S0065-1281(77)80046-9. [DOI] [PubMed] [Google Scholar]
- 56.Sweet IR, Najafi H, Li G, Grodberg J, Matschinsky FM. Measurement and modeling of glucose-6-phosphatase in pancreatic islets. Am J Physiol. 1997;272(4 Pt 1):E696–711. doi: 10.1152/ajpendo.1997.272.4.E696. [DOI] [PubMed] [Google Scholar]
- 57.Martin CC, Bischof LJ, Bergman B, Hornbuckle LA, Hilliker C, Frigeri C, et al. Cloning and Characterization of the Human and Rat Islet-Specific Glucose-6-Phosphatase Catalytic Subunit-Related Protein (IGRP) Genes. J Biol Chem. 2001;276(27):25197–207. doi: 10.1074/jbc.M101549200. [DOI] [PubMed] [Google Scholar]
- 58.Khan A, Chandramouli V, Ostenson CG, Low H, Landau BR, Efendic S. Glucose cycling in islets from healthy and diabetic rats. Diabetes. 1990;39(4):456–9. doi: 10.2337/diab.39.4.456. [DOI] [PubMed] [Google Scholar]
- 59.Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, et al. Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem. 2003;278(5):2997–3005. doi: 10.1074/jbc.M210581200. [DOI] [PubMed] [Google Scholar]
- 60.Tokuyama Y, Sturis J, DePaoli AM, Takeda J, Stoffel M, Tang J, et al. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44(12):1447–57. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- 61.Pedersen KB, Zhang P, Doumen C, Charbonnet M, Lu D, Newgard CB, et al. The promoter for the gene encoding the catalytic subunit of rat glucose-6-phosphatase contains two distinct glucose-responsive regions. Am J Physiol Endocrinol Metab. 2007;292(3):E788–801. doi: 10.1152/ajpendo.00510.2006. 00510.2006 [pii] [DOI] [PubMed] [Google Scholar]
- 62.Petrolonis AJ, Yang Q, Tummino PJ, Fish SM, Prack AE, Jain S, et al. Enzymatic characterization of the pancreatic islet-specific glucose-6-phosphatase-related protein (IGRP) J Biol Chem. 2004;279:13976–83. doi: 10.1074/jbc.M307756200. [DOI] [PubMed] [Google Scholar]
- 63.Ashcroft SJ, Randle PJ. Glucose-6-phosphatase activity of mouse pancreatic islets. Nature. 1968;219(5156):857–8. doi: 10.1038/219857a0. [DOI] [PubMed] [Google Scholar]
- 64.Khan A, Chandramouli V, Ostenson CG, Ahren B, Schumann WC, Low H, et al. Evidence for the presence of glucose cycling in pancreatic islets of the ob/ob mouse. J Biol Chem. 1989;264(17):9732–3. [PubMed] [Google Scholar]
- 65.Chandramouli V, Khan A, Ostenson CG, Berggren PO, Low H, Landau BR, et al. Quantification of glucose cycling and the extent of equilibration of glucose 6-phosphate with fructose 6-phosphate in islets from ob/ob mice. Biochem J. 1991;278 (Pt 2):353–9. doi: 10.1042/bj2780353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan A, Chandramouli V, Ostenson CG, Berggren PO, Low H, Landau BR, et al. Glucose cycling is markedly enhanced in pancreatic islets of obese hyperglycemic mice. Endocrinology. 1990;126(5):2413–6. doi: 10.1210/endo-126-5-2413. [DOI] [PubMed] [Google Scholar]
- 67.Jazmin LJ, Young JD. Isotopically nonstationary 13C metabolic flux analysis. Methods Mol Biol. 2013;985:367–90. doi: 10.1007/978-1-62703-299-5_18. [DOI] [PubMed] [Google Scholar]
- 68.Martin CC, Oeser JK, Svitek CA, Hunter SI, Hutton JC, O’Brien RM. Identification and Characterization of a Human cDNA and Gene Encoding a Ubiquitously Expressed Glucose-6-Phosphatase Catalytic Subunit-Related Protein. Journal of molecular endocrinology. 2002;29:205–22. doi: 10.1677/jme.0.0290205. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Shu YH, Xiang AH, Trigo E, Kuusisto J, Hartiala J, et al. Additive Effects of Genetic Variation in Gck and G6pc2 on Insulin Secretion and Fasting Glucose. Diabetes. 2009;58:2946–53. doi: 10.2337/db09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rose CS, Grarup N, Krarup NT, Poulsen P, Wegner L, Nielsen T, et al. A variant in the G6PC2/ABCB11 locus is associated with increased fasting plasma glucose, increased basal hepatic glucose production and increased insulin release after oral and intravenous glucose loads. Diabetologia. 2009;52(10):2122–9. doi: 10.1007/s00125-009-1463-z. [DOI] [PubMed] [Google Scholar]
- 71.Ingelsson E, Langenberg C, Hivert MF, Prokopenko I, Lyssenko V, Dupuis J, et al. Detailed physiologic characterization reveals diverse mechanisms for novel genetic Loci regulating glucose and insulin metabolism in humans. Diabetes. 2010;59(5):1266–75. doi: 10.2337/db09-1568. db09-1568 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heni M, Ketterer C, t Hart LM, Ranta F, van Haeften TW, Eekhoff EM, et al. The Impact of Genetic Variation in the G6PC2 Gene on Insulin Secretion Depends on Glycemia. J Clin Endocrinol Metab. 2010;95:E479–84. doi: 10.1210/jc.2010-0860. jc.2010-0860 [pii] [DOI] [PubMed] [Google Scholar]
- 73.Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, et al. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS genetics. 2006;2(5):e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merrins MJ, Fendler B, Zhang M, Sherman A, Bertram R, Satin LS. Metabolic oscillations in pancreatic islets depend on the intracellular Ca2+ level but not Ca2+ oscillations. Biophysical journal. 2010;99(1):76–84. doi: 10.1016/j.bpj.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merrins MJ, Bertram R, Sherman A, Satin LS. Phosphofructo-2-kinase/fructose-2,6-bisphosphatase modulates oscillations of pancreatic islet metabolism. PLoS One. 2012;7(4):e34036. doi: 10.1371/journal.pone.0034036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bertram R, Sherman A, Satin LS. Electrical bursting, calcium oscillations, and synchronization of pancreatic islets. Adv Exp Med Biol. 2010;654:261–79. doi: 10.1007/978-90-481-3271-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolf BA, Colca JR, Comens PG, Turk J, McDaniel ML. Glucose 6-phosphate regulates Ca2+ steady state in endoplasmic reticulum of islets. A possible link in glucose-induced insulin secretion. J Biol Chem. 1986;261(35):16284–7. [PubMed] [Google Scholar]
- 78.Jetton TL, Liang Y, Pettepher CC, Zimmerman EC, Cox FG, Horvath K, et al. Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut. J Biol Chem. 1994;269(5):3641–54. [PubMed] [Google Scholar]
- 79.Goh BH, Khan A, Efendic S, Portwood N. Expression of glucose-6-phosphatase system genes in murine cortex and hypothalamus. Horm Metab Res. 2006;38(1):1–7. doi: 10.1055/s-2006-924964. [DOI] [PubMed] [Google Scholar]
- 80.Frigeri C, Martin CC, Svitek CA, Oeser JK, Hutton JC, Gannon M, et al. The Proximal Islet-Specific Glucose-6-Phosphatase Catalytic Subunit Related Protein (IGRP) Autoantigen Promoter is Sufficient to Initiate but not Maintain Transgene Expression in Mouse Islets In Vivo. Diabetes. 2004;53:1754–64. doi: 10.2337/diabetes.53.7.1754. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y, Flemming BP, Martin CC, Allen SR, Walters J, Oeser JK, et al. Long-range enhancers are required to maintain expression of the autoantigen islet-specific glucose-6-phosphatase catalytic subunit-related protein in adult mouse islets in vivo. Diabetes. 2008;57(1):133–41. doi: 10.2337/db07-0092. [DOI] [PubMed] [Google Scholar]
- 82.Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1219–35. doi: 10.1098/rstb.2006.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barzilai N, Rossetti L. Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem. 1993;268(33):25019–25. [PubMed] [Google Scholar]
- 84.Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annual review of biochemistry. 2012;81:767–93. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karunakaran U, Park KG. A systematic review of oxidative stress and safety of antioxidants in diabetes: focus on islets and their defense. Diabetes & metabolism journal. 2013;37(2):106–12. doi: 10.4093/dmj.2013.37.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trinh K, Minassian C, Lange AJ, O’Doherty RM, Newgard CB. Adenovirus-mediated expression of the catalytic subunit of glucose-6- phosphatase in INS-1 cells. Effects on glucose cycling, glucose usage, and insulin secretion. J Biol Chem. 1997;272(40):24837–42. doi: 10.1074/jbc.272.40.24837. [DOI] [PubMed] [Google Scholar]
- 87.Iizuka K, Nakajima H, Ono A, Okita K, Miyazaki J, Miyagawa J, et al. Stable overexpression of the glucose-6-phosphatase catalytic subunit attenuates glucose sensitivity of insulin secretion from a mouse pancreatic beta-cell line. J Endocrinol. 2000;164(3):307–14. doi: 10.1677/joe.0.1640307. [DOI] [PubMed] [Google Scholar]
- 88.Wang H, Liu L, Zhao J, Cui G, Chen C, Ding H, et al. Large Scale Meta-Analyses of Fasting Plasma Glucose Raising Variants in GCK, GCKR, MTNR1B and G6PC2 and Their Impacts on Type 2 Diabetes Mellitus Risk. PLoS One. 2013;8(6):e67665. doi: 10.1371/journal.pone.0067665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freathy RM, Hayes MG, Urbanek M, Lowe LP, Lee H, Ackerman C, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: common genetic variants in GCK and TCF7L2 are associated with fasting and postchallenge glucose levels in pregnancy and with the new consensus definition of gestational diabetes mellitus from the International Association of Diabetes and Pregnancy Study Groups. Diabetes. 2010;59(10):2682–9. doi: 10.2337/db10-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bouatia-Naji N, Bonnefond A, Baerenwald DA, Marchand M, Bugliani M, Marchetti P, et al. Genetic and Functional Assessment of the Role of the rs13431652-A and rs573225-A Alleles in the G6PC2 Promoter that Strongly Associate With Elevated Fasting Glucose Levels. Diabetes. 2010;59(10):2662–71. doi: 10.2337/db10-0389. db10-0389 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharp PA. Split genes and RNA splicing. Cell. 1994;77(6):805–15. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 92.Solis AS, Shariat N, Patton JG. Splicing fidelity, enhancers, and disease. Front Biosci. 2008;13:1926–42. doi: 10.2741/2812. [DOI] [PubMed] [Google Scholar]
- 93.Dos Santos C, Bougneres P, Fradin D. An SNP in a methylatable Foxa2 binding site of the G6PC2 promoter is associated with insulin secretion in vivo and increased promoter activity in vitro. Diabetes. 2009;58:489–92. doi: 10.2337/db08-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, et al. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2012;55(3):707–18. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8(5):399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 96.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2008;295(6):E1287–97. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen SY, Pan CJ, Nandigama K, Mansfield BC, Ambudkar SV, Chou JY. The glucose-6-phosphate transporter is a phosphate-linked antiporter deficient in glycogen storage disease type Ib and Ic. Faseb J. 2008;22:2206–13. doi: 10.1096/fj.07-104851. [DOI] [PubMed] [Google Scholar]
- 98.Wang H, Iynedjian PB. Modulation of glucose responsiveness of insulinoma beta-cells by graded overexpression of glucokinase. Proc Natl Acad Sci U S A. 1997;94(9):4372–7. doi: 10.1073/pnas.94.9.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang H, Iynedjian PB. Acute glucose intolerance in insulinoma cells with unbalanced overexpression of glucokinase. J Biol Chem. 1997;272(41):25731–6. doi: 10.1074/jbc.272.41.25731. [DOI] [PubMed] [Google Scholar]