Abstract
Virtually every eukaryotic cell has an endogenous circadian clock and a biological sex. These cell-based clocks have been conceptualized as oscillators whose phase can be reset by internal signals such as hormones, and external cues such as light. The present review highlights the inter-relationship between circadian clocks and sex differences. In mammals, the suprachiasmatic nucleus (SCN) serves as a master clock synchronizing the phase of clocks throughout the body. Gonadal steroid receptors are expressed in almost every site that receives direct SCN input. Here we review sex differences in the circadian timing system in the hypothalamic-pituitary-gonadal axis (HPG), the hypothalamicadrenal-pituitary (HPA) axis, and sleep-arousal systems. We also point to ways in which disruption of circadian rhythms within these systems differs in the sexes and is associated with dysfunction and disease. Understanding sex differentiated circadian timing systems can lead to improved treatment strategies for these conditions.
Keywords: Circadian, Suprachiasmatic Nucleus, Sex Differences, Hormones, Reproduction, HPG, Stress, HPA, Sleep
1. Introduction and Rationale
Evidence of the inter-relationships between the circadian timing system and sex differences cannot be ignored. Virtually every cell in the body has a circadian clock and a biological sex. It appears that no matter what one studies, the measured response is likely influenced by the circadian timing system, and many of these variables differ between the sexes. As noted by Simerly (2005), “The same basic neural pathways are present in each sex, but they are represented differentially (e.g. differing numbers of neurons, projections, dendritic spines, and differing synapse densities); thus, the transmission and processing of sensory information through sexually dimorphic neural networks are likely to be distinct in males and females.” Moreover, there is substantial evidence that sex differences in the circadian timing system are important in determining responses to both endogenous and exogenous factors. This not so inconvenient truth (McCarthy et al., 2012) has implications for understanding behavior and physiology at many different levels of analysis, including: genes, cells, tissues, and whole organisms.
Female-male differences in regulatory events at the level of individual brain cells can arise from many factors, including: sex chromosome differences, specializations in receptor expression, ion channels, or as a result of differences in circulating hormones. In turn, these cell based sex differences can give rise to male-female differences in brain networks, organs, and behavior. Such effects have substantial implications for the application of basic research findings to practical problems and investigating the causes of sex differences in disease incidence. The former can lead to optimizing the timing of drug delivery; the latter can provide clues to both protective and susceptibility mechanisms that differ between the sexes (IOM [Institute of Medicine] 2011). As noted in a series of papers in Nature magazine on sex differences in 2010 (Zucker and Beery, 2010; Kim et al., 2010), there is a dearth of research with female animals, and in some instances the sex of the subject is not even reported. This is especially relevant to circadian rhythms research where a small fraction of work (<20%) includes females (Kuljis et al., 2013).
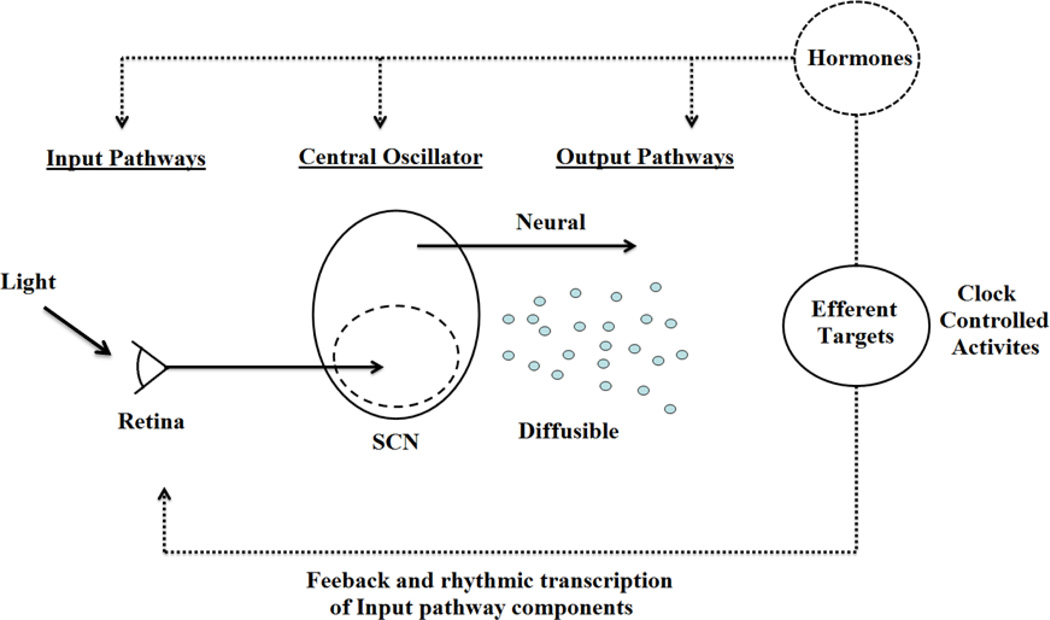
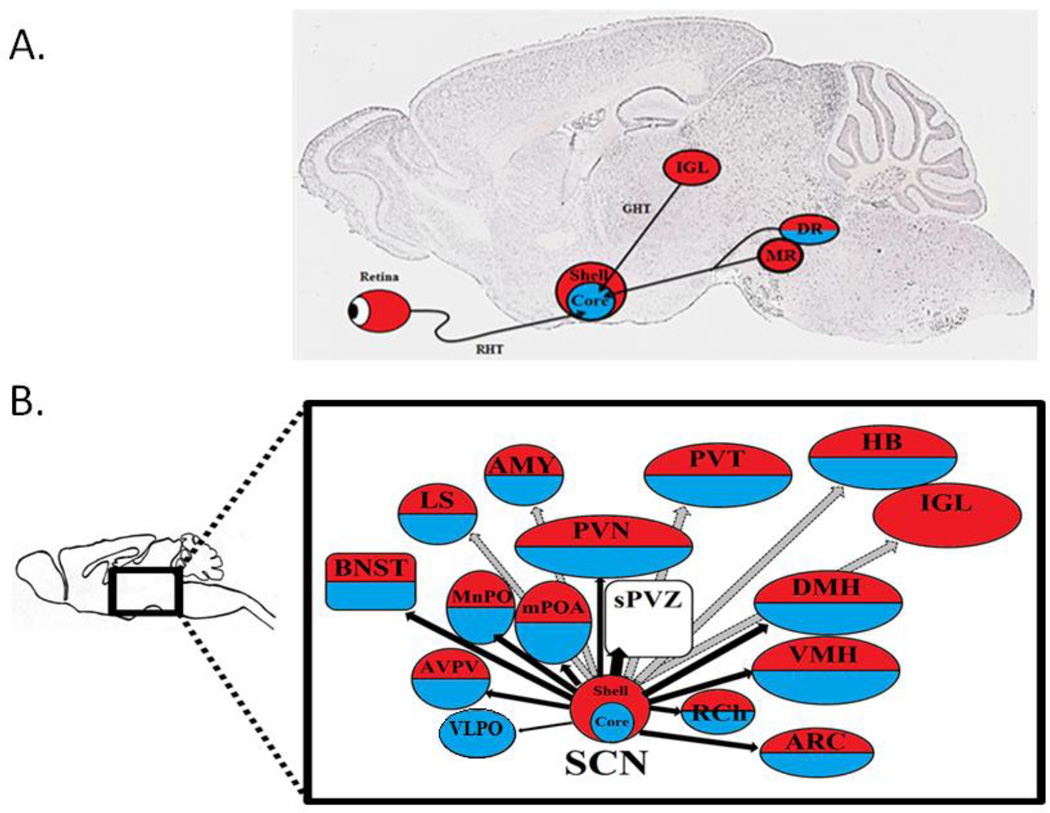
Daily rhythms exist in virtually every behavioral and physiological response that one can measure. These are orchestrated by a brain clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Three distinct components are involved in the SCN’s ability to function as a brain clock (Figure 1). These include: input from the environment via a direct retinohypothalamic tract (RHT), an oscillating clock in the SCN, and output pathways to various target areas in nearby hypothalamic regions. Important in the present context is the fact that brain nuclei at each stage in this system - namely, input, clock and targets, all bear estrogen receptors (ER), androgen receptors (AR), or both. This allows for feedback from circulating hormones to act on each of these components of the circadian timing system. Sex differences in the steroid receptor expressing brain regions set the stage for the present review of sex differences in the circadian timing system. Such differences can arise from organizational actions of hormones in the pre- and perinatal period, and/or from activational effects of hormones during the pubertal period and adulthood. Furthermore, as noted by Arnold (2012), some sex differences antecede gonadal differentiation and are determined by non-gonadal effects such as the number and type of sex chromosomes. These influences on sex differences in circadian timing have not been examined.
Figure 1. Representation of circadian timing system.
The circadian clock has been represented as having three components: input pathways, a central oscillator (or pacemaker), and output pathways. Input pathways such as photic signals from the retina, or temperature, can influence the master oscillator in the suprachiasmatic nucleui (SCN) of the hypothalamus which produces the endogenous biological rhythm that synchronize the rest of the body. Output pathways to target sites entail both neural connections and diffusible signals, and these regulate clock-controlled biological processes. Additional pathways (shown as dotted lines) include multiple interlocking positive or negative feedback from clock controlled activities. One prominent feedback mechanism are the systemically secreted hormones which can then influence the circadian system at all levels, including input pathways, the central oscillator, and output pathways. Adapted from Kriegsfeld et al. (2002).
To explore sex differences in the circadian timing system and resulting neuroendocrine consequences, we first characterize circadian phenomena and define terminology used in the field of chronobiology. In turning to the current literature, we focus on the SCN and its organization, the history of research in circadian timing, and some of the terminology and experimental paradigms used in the field. We next provide a brief overview of the literature on sex differences in circadian timing. We then focus attention on sex differences and the relationships between circadian rhythms and several physiological and behavioral systems. In the hypothalamic-pituitary-gonadal axis (HPG) the differences between the sexes are highly salient; the hypothalamic-adrenal axis (HPA) has broad impact on circadian regulation of cells and clock genes throughout the body. Next, we examine sleep-wake cycles, which constitute the most salient circadian phenomena experienced by most people and discuss their importance in understanding environmental disturbances related to jet lag and shift work which can increase one’s susceptibility for numerous disease states. Finally, we consider a very practical issue: the critical importance of sex differences in circadian rhythmicity in disease and drug administration using findings from the treatment of cancer as a case in point.
2. Overview of the circadian timing system
2.1 Circadian terminology and experimental paradigms
To explore research on circadian rhythms we review some of the terminology, key concepts, and experimental paradigms used by chronobiologists. Circadian rhythms are biological processes which have an endogenous oscillation of about (circa) a day (diem) and persist in the absence of any external temporal cues. In fact, the body’s circadian clocks are generally viewed as oscillators with a period of approximately 24 hours.
In order to distinguish endogenous circadian rhythms from daily variations that are driven by external cues, chronobiologists study responses in the presence and absence of external signals. Proof that a given response is under endogenous circadian control requires that it can be detected in the complete absence of time cues from the environment. In contrast, some daily rhythms which are not circadian in nature occur in the presence of environmental cues but disappear in constant conditions (e.g. constant darkness (DD) or constant light (LL)). These externally driven changes are said to be diurnal rather than circadian.
2.1.1 Period
The period of a circadian rhythm is the duration of time it takes to complete one cycle. The period is typically measured from peak to peak or trough to trough but could be measured from any specific position on the curve (Figure 2A). Circadian rhythms with a period of approximately 24 hours are said to be “free-running.” In the absence of external time cues, they appear to drift a little each day - starting either progressively earlier (short circadian free-running period) or progressively later (long circadian period) from day to day. The period of the free-running rhythm reflects the cycle length of the endogenous clock, which is generally either somewhat shorter or longer than 24 hours. Under normal conditions, the endogenous rhythm is synchronized by environmental time cues known as zeitgebers (from the German word “time giver”).
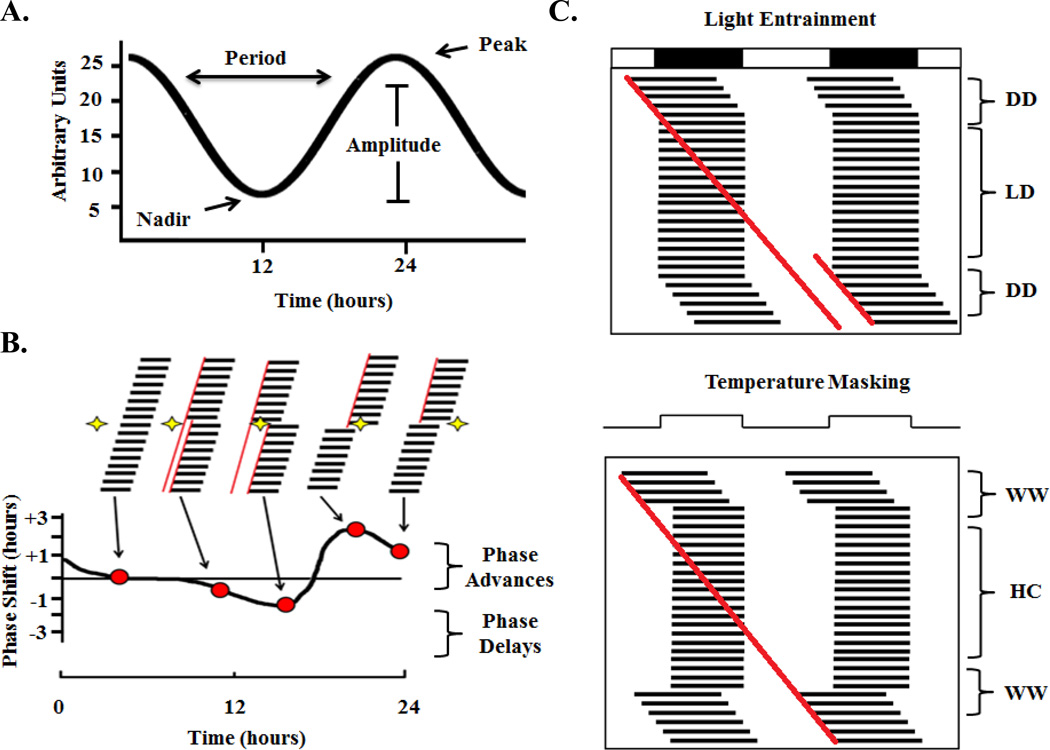
Figure 2. Experimental Paradigms in circadian rhythms research.
(A). Shows the parameters used to measurea circadian rhythms, including the lowest point (nadir), the highest point (peak), the distance from peak to trough (amplitude), the time taken to complete one full cycle (period). (B). Top half of the figure shows the activity record of a representative individual animal. The vertical black bars represent when the animal is active, occurring primarily during the night or subjective night. When a light pulse is given in the middle of the subjective day occurring (white region of behavioral profile), there is no effect on the timing of the daily locomotor behavior. When a light pulse is given early in the subjective night is a small phase delay in activity onset and when a light pulse is given slightly later in the subjective night, there ensues a larger phase delay. When a light pulse is given in the latter half of the subjective night there is a large phase advance in the daily rhythm, and when a light pulse is given at the end of the subjective night there is smaller phase advance. Bottom Half, shows the phase response curve corresponding to the animal receiving a light pulse at one of the previously mentioned times. Red dots correspond to the phase shift corresponding to the light pulse condition. Adapted from Moore-Ede and Czeisler (1982). (C). Top panel Shows an entrainment paradigm of a diurnal animal exposed to either constant darkness (DD) or a light-dark (LD) cycle of 14 hours light, 10 hours darkness (indicated by black and white bars at the top). This double plotted actogram has two consecutive days plotted side by side and consecutive days on each subsequent row (dark bars indicate activity output). To visually inspect an actogram for entrainment, a line is draw at the start of activity each day, and if the line fits the activity after the constant environmental conditions resume (after LD), masking has occurred. Here, the line does not overlap (two red lines) so the light condition entrained the animal. Bottom panel Shows a masking paradigm of same animal in either constant warm (WW) conditions of 200C, or hot-cold (HC) conditions of 250C or 150C for 10:14 hours; respectively. Here, because the line drawn at the start of each day’s activity perfectly fits with the activity after the return to constant conditions (single red line), masking is said to have occurred.
2.1.2 Phase and amplitude of rhythm
Phase is the relative position on the curve of an oscillation with reference to a particular time (Figure 2A). The peak is the highest point in the rhythm, and the nadir is the point at which the rhythm is the lowest. Amplitude is the measurement of the recorded output from peak to nadir.
2.1.3 Phase response curves and phase shifts
The phase response curve depicts the effect of a brief light pulse on activity onset in a free-running response (Figure 2B). A discrete exposure to an environmental cue (input) causes the level of clock-controlled response to reset, resulting in a phase shift of the rhythm. Positive shifts are termed phase advances while negative phase shifts are termed phase delays. A brief light pulse given to an organism during the middle of the subjective day produces little to no phase response, while the same light pulse in the early subjective night produces phase delay shifts, and light in the late subjective night produce phase advance shifts.
2.1.4 Entrainment
Entrainment is the synchronization of a circadian response by an external stimulus or zeitgeber, such as a light/dark (LD) cycle. Key to the determination of the occurrence of entrainment is the persistence of the entrained response from the appropriate entrained phase reference point, with a near 24-hour period following removal of the entraining signal creating an absence of environmental cues, as is the case in DD.
2.1.5 Masking
A free-running rhythm may be masked by external factors. Masking describes the phenomenon whereby a circadian response that is being measured (e.g. locomotor activity) is obscured, but not altered, by an exogenous factor. An experimental protocol for distinguishing light entrainment from masking is shown in (Figure 2C). In this figure, it can be seen that the presence of light-dark cues entrain the circadian rhythms of daily activity (Figure 2C, top), whereas temperature cues mask the endogenous ~24 hour free running period of daily activity (Figure 2C, bottom). When the masking signal is removed, it can be seen that the free-running period has not been reset by the masking cue but continues from the point that would be expected under conditions of free running. In the case of entrainment, however, the removal of cues results in the rhythm starting at a time which is different than when the cues were present.
2.2 Clock genes and clock controlled genes
To understand the broad impact of the circadian timing system, it is helpful to look to some of the experiments in this field. A genetic basis for the generation of circadian rhythms was first reported in flies following the discovery that a single gene mutation in the Period (Per) gene accounted for lengthened, shortened or arrhythmic circadian behavior (Konopka and Benzer, 1971). At the time of this discovery, it was wholly unexpected that a single gene mutation could disrupt such a complex behavior. Major breakthroughs in our understanding of circadian rhythms have been built on this initial discovery.
Today, many clock genes and modulators of clock genes have been discovered in prokaryotic and eukaryotic organisms ranging from cyanobacteria to mammals, including humans. The conserved unifying mechanism that emerges from examination of clock mechanisms across species is that organisms have evolved transcriptional/posttranslational feedback loops which allow circadian rhythms to be maintained at the level of the individual cell. The trajectory of our understanding of these feedback loops has gone from a simple negative feedback loop to more complex multi-element negative and positive feedback loops (Zhang et al., 2010; Figure 3).
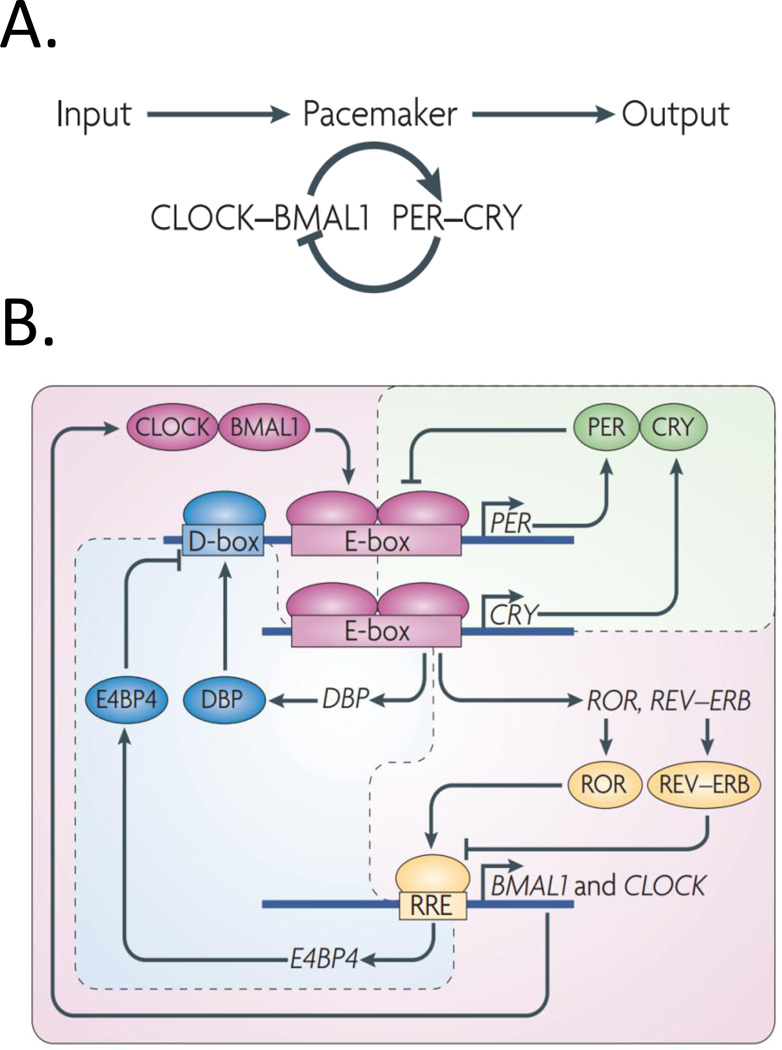
Figure 3. Circadian clock genes: Historical perspective.
(A). Represents our understanding of the circadian pacemaker around a decade ago. This understanding was limited to a single ‘core loop’ in which clock genes and their protein products are regulated by negative and positive feedback loops. Briefly, CLOCK and BMAL1 hetero-dimerize and activate the expression of period genes (Per1, 2, and 3) and Cry1 and 2. PER and CRY proteins enter the nucleus and repress CLOCK-BMAL1-driven transcription (Reviewed in Antle and Silver, 2005). (B). Represents the understanding in the past half-decade, where a more complex model has been developed based on triple interlocking loops, which include the PER–CRY loop, which is the primary loop, and the retinoid-related orphan receptor (ROR)–REV–ERB- and DBP–E4 promoter-binding protein 4 (E4BP4)-associated loops. In addition to the primary loop, which was previously recognized as the core loop (A), transcriptional regulation of CLOCK and BMAL1 is controlled by ROR transcriptional activators and the dimeric REV–ERB repressors (by binding to REV response element (RRE)), the expression of which is governed by CLOCK–BMAL1 activity (through binding to E-box-containing DNA elements). Furthermore, the transcriptional activator DBP (the expression of which is controlled by an E-box) and E4BP4 (the expression of which is controlled by RRE) synergistically regulate the expression of D-box containing genes, including PER. Reprinted from Zhang et al. (2010).
Another tremendous leap in our understanding of the importance of circadian rhythms arose from the discovery that cellular “clocks” regulate global gene expression patterns in many tissues, and that many of these clock controlled genes are tissue specific (Doherty & Kay, 2010). For example, extensive circadian cycling in the transcriptome is reported in Arabidopsis (6%), Drosophila (1–5%), and in mice (5–10%). These numbers are even higher for specific tissues: a microarray study indicates that over 8% of the mRNA transcripts in the mouse liver are rhythmically expressed and similar percentages are found in other mammalian tissues, ranging from connective tissue to the cerebral cortex (Akhtar et al., 2002; Yang et al., 2007). Importantly, perturbations of clock genes can lead to profound disruptions in many aspects of physiology and behavior. Clock gene mutations with relevance to sex differences and circadian rhythms are discussed below.
2.3 The SCN as the master clock
The idea that the SCN functions as a master clock in the body has been supported since its discovery in 1972, confirmed by many converging lines of evidence, in many laboratories, over many years (summarized in Table 1). The discovery of clock genes in the SCN of mammals, and the subsequent finding that the same clock genes are present in fibroblasts and other cells throughout the body has led to an explosion of research on these genes. These findings led to the question of how the SCN is able to serve as a master pacemaker and how the SCN differs from other bodily cells/tissues that express clock genes. The answer rests in the network organization of the SCN itself, in the inputs and outputs of the SCN, and in the hierarchical organization of the circadian timing system. Specifically, the SCN coordinates the phase of extra-SCN clocks in the rest of the brain as well as in the periphery. There have been substantial advances in our understanding of the brain clock - from the conceptual origins of a “black box” to its current status as a heterogeneous, multicellular, plastic, multifunctional network of interconnected cells. Much information about the SCN is beyond the scope of the present review, and the reader is directed to the following reviews for topics not covered here (Welsh, 2010; Butler and Silver 2009; Mohawk et al., 2012). The history of discovery of the SCN as the master clock, and its role in coordinating bodily oscillators, is summarized in Table 1.
Table 1.
| 1972: Direct retinal input to the SCN via the retinohypothalamic tract (Moore and Lenn, 1972) |
| 1972: SCN lesions result in arrhythmicity (Stephan and Zucker, 1972; Moore and Eichler, 1972) |
| 1979: Clock resides in the SCN in the hypothalamic island (Inouye and Kawamura, 1979) |
| 1982: Circadian rhythm in SCN firing rate in vivo and in vitro (Groos and Hendriks, 1982; Green and Gillette, 1982) |
| 1987: Clock is transplantable and restores behavioral rhythm in host (Lehman et al., 1987) |
| 1990: Transplanted SCN determines circadian phenotype (Ralph et al., 1990) |
| 1995: Individual SCN neurons bear a circadian clock (Welsh et al., 1995) |
| 1996: Diffusible signals from the SCN control behavioral but not hormonal rhythms (Silver et al., 1996) |
| 1997: Discovery of mammalian Period genes in SCN (Tei et al., 1997; Sun Z et al ., 1997) |
| 1998: Fibroblast cells have circadian clocks (Balsalobre et al., 1998) |
| 2002: SCN vs. peripheral clocks, SCN is not the only clock (Yamazaki et al., 2002) |
| 2004: SCN orchestrates timing in peripheral clocks (Yoo et al ., 2004) |
| 2006: Following SCN ablation, synchrony of peripheral clocks within organs is lost (Guo et al., 2006) |
To summarize, the aforementioned experimental paradigms have facilitated the discovery and characterization of many of the circadian rhythms we will discuss below. These include circadian rhythms in behavior (locomotor activity and sleep), physiology (reproductive hormones and stress hormones), and gene expression. The discovery that the SCN functions as the master clock regulating all of these rhythms was a major advance. Many of the observed sex differences in behavior and physiology are tied to sex differences within the SCN and its input and output pathways.
3. Sex differences in the circadian timing system
3.1 Characterization of the SCN
The SCN is a bilaterally symmetrical nucleus located just above the optic chiasm. It is made up of a relatively small number of neurons in humans (~50,000), rats (~20,000) and mice (~8,000 to 10,000) (Klein et al., 1991). The SCN is heterogeneous, as it is composed of clusters of cells bearing distinct neurochemicals, afferent/efferent connections, and regional specializations of function (reviewed in Antle and Silver 2005; Butler and Silver 2009; Morin, 2013). SCN peptidergic cells are clustered within subregions of the nucleus and include: arginine vasopressin (AVP), calbindin, calretinin, cholecystokinin, enkephalin, gastrin-releasing peptide (GRP), neurotensin (NT), substance P (SP), somatostatin (SS), and vasoactive intestinal polypeptide (VIP). All of these neurons are thought to contain GABA. Most commonly, the SCN is classified into two main subregions: the core (which receives the greatest density of retinal input) and the shell (where retinal input is sparse). The core and shell regions were initially termed “ventrolateral” and “dorsomedial,” respectively, based on the anatomy of the rat, but the latter spatial designations proved confusing, as subsequent research showed that other species had somewhat different SCN landscapes.
The greatest experimental attention has been devoted to core neurons containing VIP and GRP and shell neurons containing AVP. The direct retinorecipient neurons of the core are the first to respond with Period 1 and Period 2 (Per1 and Per2, respectively) clock gene and protein expression following a light pulse (Yan and Silver 2002; 2004). Many cells in the core do not oscillate detectably, perhaps because of a very low amplitude oscillation with respect to the clock gene expression or electrical activity. In contrast, a large proportion of cells located within the shell are rhythmic (Jobst and Allen, 2002; Hamada et al., 2001; Foley et al., 2011). These core-shell differences are significant with respect to sex differences in steroid receptors (further discussed in section 3.4).
3.2 Morphological and peptidergic sex differences in the SCN
Morphological sex differences in the SCN are well established in both animals and humans. For example, electron microscopic analysis indicates that the SCN of male rats has more axo-spinal synapses, postsynaptic density material, asymmetrical synapses, neurons that contain more nucleoli than females (Güldner, 1982; Güldner, 1983; Güldner, 1984), and that the overall volume is also greater as well (Robinson et al., 1986). In gerbils, the volume of the SCN is sexually dimorphic, as is the organization of astroglia (Collado et al., 1995). Sex differences in SCN morphology have been described in humans as well, as both the relative volume and length of rostrocaudal axis of the SCN are greater in females (Hofman et al., 1988).
Cells which produce VIP are located in the SCN core. VIP is a key element in orchestrating rhythmicity through its actions in coupling SCN neurons (Maywood et al., 2011) and in sustaining circadian rhythms of single cells. This was initially demonstrated in studies of KO animals, such that mice deficient in either the VPAC2 receptor (vipr2−/−) (Harmar et al. 2002) or VIP the peptide (vip−/−) (Colwell et al. 2003) had weak locomotor activity rhythms. Subsequent work indicated that the expression of VIP and its receptor, VPAC2R, is necessary for maintaining synchronous daily rhythms among neurons in the SCN. Importantly in both rodents and humans, sexual dimorphisms are reported in this SCN peptide, with more VIP expressing neurons in the human SCN of males, and with sexually dimorphic rhythms of Vip mRNA expression in the SCN (Zhou et al., 1995; Hofman et al., 1996; Mahoney et al., 2009; Krajnak et al., 1998). The sex difference in VIP expression has consequences. For example, activity-dependent neuroprotective protein is a VIP-responsive gene with significantly higher mRNA expression in males than females (Furman et al., 2004).
3.3 Sex differences in SCN electrical activity
Sex differences in electrical activity parallel the foregoing anatomical differences. Sex differences in VIP-driven coupling among neurons are considered a possible contributor to characteristic spontaneous firing rate of SCN neurons and damping of the SCN molecular rhythms. This was assessed by whole-cell patch-clamp electrophysiology and PER2::luciferase-driven bioluminescence in an SCN brain slice preparation (Kuljis et al., 2013). More specifically, in neurons of the SCN shell region, higher rates of spontaneous activity are seen in males than in females during the light phase of the LD cycle. During the dark phase, however; neurons in the shell in the female SCN have higher thresholds for evoking action potentials (AP), whereas in males the action potential after hyperpolarization area is larger in the night than in the day (Kuljis et al., 2013). During the light phase, sex differences in electrical activity were not observed in neurons of the SCN core.
During the dark phase, however, SCN cells from the core of males had higher AP thresholds, faster rise rates, and greater amplitude compared to females (Kuljis et al., 2013). It has been proposed that lower amplitude oscillators are more easily phase shifted (Abraham et al., 2010) and if so, the data suggests that females might be able to phase shift more readily to resetting environmental cues than males.
3.4 Gonadal steroid receptors
3.4.1 within the SCN
Androgen Receptors
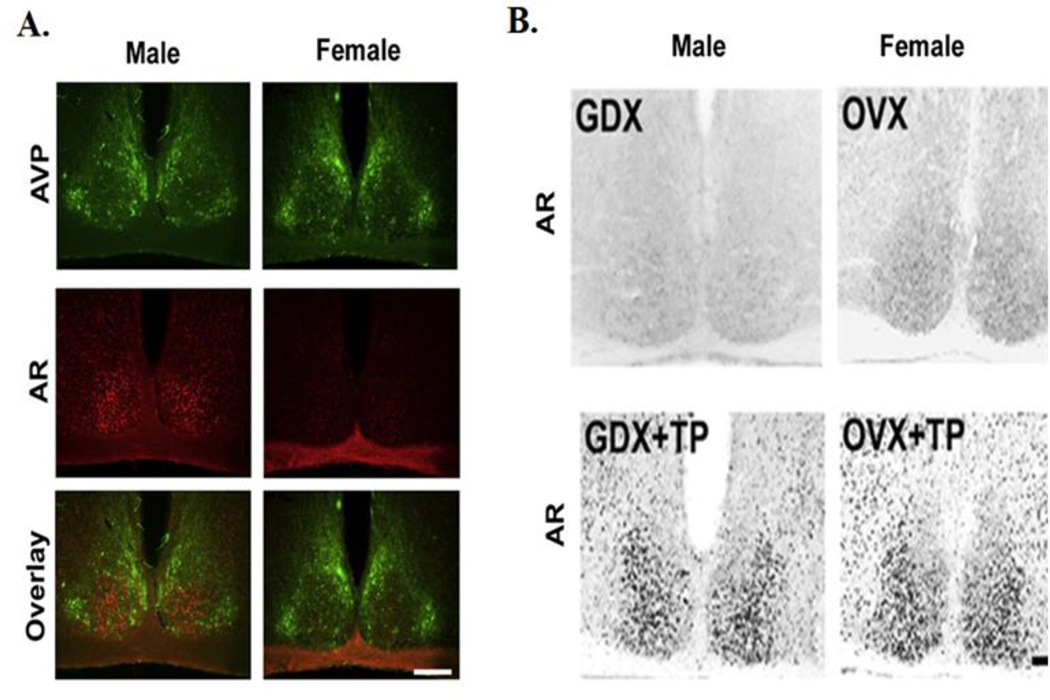
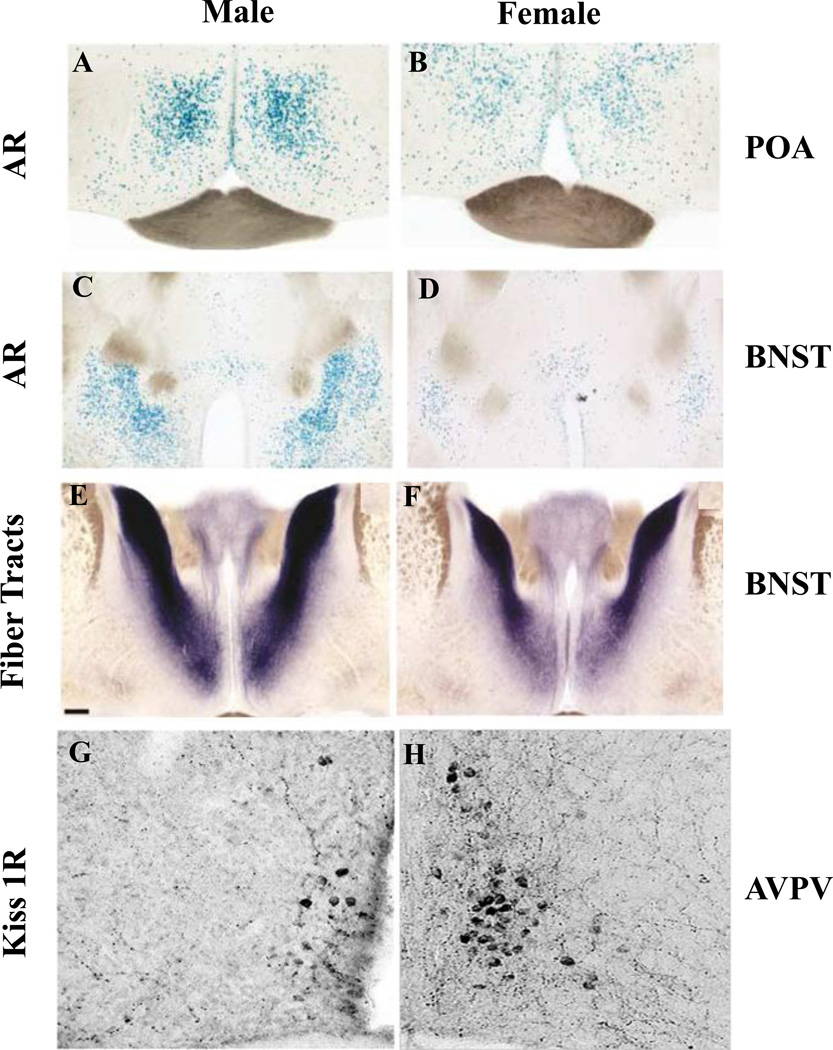
The presence of AR within the SCN has been identified in several species, including: mice, rats, and humans (Karatsoreos et al., 2007; Zhou et al., 1994; Fernandez-Guasti et al., 2000). In humans, males have higher levels of AR expression in the SCN (Fernandez-Guasti et al., 2000). Similar patterns of AR expression are observed in mice (Figure 4A), as Western blots and immunochemistry show males have 2-fold higher AR expression in the SCN than females (Iwahana et al., 2008). Neurons expressing AR are localized within the SCN core and are primarily expressed in GRP neurons (>85%), and expressed in VIP (~12%) and AVP neurons (< 7%) to a much lesser extent (Karatsoreos et al., 2007a). Gonadectomy (GDX) eliminates these sex differences, and androgen treatment restores AR expression in both sexes (Figure 4B), as treatment of females with testosterone propionate (TP), or with the non-aromatizable androgen dihydrotestosterone (DHT) results in male-like patterns of SCN expression (Iwahana, et al., 2008).
Figure 4. Sex difference in AR expression in the SCN.
(A) Sex difference in androgen receptor (AR) expression in the SCN ventromedial core. Top panel showed AVP-ir cells (green) which defines the core of the SCN, middle shows AR-ir cell expression (red), and lower panel shows overlay of AVP and AR. Males have higher expression of AR-ir cells than females. (B) Photomicrographs show AR-ir in GDX/OVX (upper panel) and GDX/OVX+TP (lower panel). AR expression is regulated by TP in both males and females. Reprinted from Iwahana et al. (2008).
Estrogen Receptors
Sex differences in ER expression appear to be both species and receptor subtype specific. In humans, there are greater levels of estrogen receptor alpha (ER α) expression in women, but levels of estrogen receptor beta (ERβ) expression do not differ (Kruijver and Swaab, 2002). The sex difference in ER expression in the SCN of mice is also receptor subtype specific. While few SCN neurons express ERα in female (~4.5%) or male mice (~3%), the levels of ERβ expression are 5-fold higher in female mice (Vida et al., 2008). Treatment of GDX mice (either sex) with 17β-estradiol (E2) for 1 day reduces the number of neurons expressing ERβ but does not alter the number of ERα expressing cells (Vida et al., 2008). ERα expression is localized in calretinin (12%) or calbindin (10%) containing neurons of the SCN. High proportions of calbindin D28K cells contain ERβ (~38%), while few (2%) calretinin or vasopressin cells express ERβ (Vida et al., 2008). Neither VIP- nor GRP-IR neurons express ER. Thus, the primary action of direct estrogenic influence on the SCN likely occurs through ERβ within the shell sub region of the nucleus in mouse.
In Summary, sex difference exist in both AR and ER expression levels within the SCN, and the expression levels of these receptors can be influenced by circulating hormone levels. This represents a direct mechanism through which gonadal hormone levels (well known to differ between the sexes) can interact with the brain’s master clock to result in sex differences in a wide range of physiological and behavioral processes controlled by the circadian timing system.
3.4.2 In SCN afferent pathways
Three major pathways send direct afferents to the SCN: the RHT via the retina, the geniculohypothalamic tract (GHT) via the intergeniculate leaflet (IGL), and the serotonergic (5-HT) inputs from the dorsal raphe (DR) nucleus and medial raphe (MR) nuclei. These three input pathways can all be influenced by gonadal hormones as the retina, IGL and raphe nuclei each express either AR, ER, or some combination of both (Figure 5A).
Figure 5. Gonadal hormone receptor expression in SCN afferents and efferents.
(A) The main afferent pathways of the SCN, with nuclei expressing estrogen receptors (ER), red, or androgen receptors (AR), blue, or both ER and AR, red/blue. Light information from the Retina is sent to the SCN core via the retinothypothalamic tract (RHT). Non-photic information is sent to the SCN core from the intergenculate leaflet (IGL) via the geniculohypothalamic tract (GHT), and the dorsal and medial raphe nuclei (DR and MR). (B) Main efferent pathways of the SCN to hypothalamic nuclei (black arrows) and extra hypothalamic sites (grey arrowss), which express ER, red, AR, blue, or both, red/blue Nuclei receiving inputs from the SCN core and shell include: the pre optic area (POA) - specifically the medial preoptic area (mPOA), the median preoptic area (MnPO), and the anteroventral periventricular nucleus (AVPA); as well as sparse connections with the ventrolateral peroptic area (VLPO). Projections extending dorsally heavily innervate the sub paraventricular zone (sPVZ) - including ventral sPVZ; as well as the paraventricular nucleus of the hypothalamus (PVN). Caudal projections innervate the the retrochiasmatic area (Rch), the arcuate nucleus (ARC), ventromedial nucleus of the hypothalamus (VMH), the dorsomedial hypothalamus (DMH). Extra hypothalamic regions receiving direct SCN input include: the lateral septum (LS), the bed nucleus of the stria terminalis (BNST), the anterior paraventricular thalamic nuclei (PVA), the amygdala (AMY), the habenula (HB), and the intergeniculate leaflet (IGL). (Simerly et al., 1990; Simerly, 2002; Zhang et al., 2002; Shughrue et al., 1992; Shughrue et al., 1997; Shughrue et al., 2001; Orikasa and Sakuma, 2004). (Kriegsfeld et al., 2004; Abrahamson et al., 2001; Leak el al., 2001; Morin, 2013; Dibner et al., 2010).
The RHT is the input pathway by which the SCN receives photic information from non-image forming photoreceptors known as intrinsically photosensitive retinal ganglion cells (Berson et al., 2002). The greatest density of retinal input to the SCN reaches the core region of the nucleus with some species differences (Morin, 2006; Morin, 2013). In rats, as well as humans, ER and AR are expressed in the retina (Munaut et al., 2001; Wickham et al., 2000), which raises the possibility that gonadal steroids may be able to modulate the SCN by influencing the origin of its major input pathway.
The GHT originates in the IGL, a region which also receives direct photic information from the retina (Morin, 2013). The GHT is thought to convey information about both day length and arousal to the SCN through the direct input that the IGL receives from the hypocretin arousal-promoting system (Mintz et al., 2001). Moreover, IGL neurons show enhanced C-FOS expression levels following arousal promoting procedures (i.e. running in a novel wheel or gentle handling) which are sufficient to induce phase advances in circadian rhythms (Webb et al., 2008). Gonadal hormones may be able to influence the SCN by acting at this input pathway as well as ERβ is expressed in the IGL (Horvath et al., 1999).
Finally, the MR (hamsters) and both the MR and DR (mice and rats) comprise the third major input pathway, sending 5-HT projections to the SCN. This pathway is thought to convey information about activity/exercise as blocking this 5-HT input pharmacologically affects locomotor activity at any time of the LD cycle (van Esseveldt et al., 2000). Once again, gonadal hormones may influence the SCN through this input pathway as well, as both ERα and ERβ are expressed in the MR and DR (Sheng et al., 2004). In both rats and mice, a sex difference in AR expression levels is present in the DR as males express AR in this region, whereas females do not (Sheng et al., 2004).
To summarize, in addition to sex steroid receptors directly in the SCN, each of the three major input pathways to the SCN express either AR’s, ER’s, or both. Not only can sex steroids act directly within the SCN, but also they modulate information reaching the brain’s master clock. Sex differences in modulation of these SCN targets represent a fruitful opportunity for future work.
3.4.3 SCN efferent target sites
The efferent connections of the SCN have been described for the mouse, rat and hamster (reviewed in Buijs et al., 2001; Dibner et al., 2010; Morin, 2013). As noted in his thorough review of the extended circadian system Morin (2013) points out that, “The number of anatomical routes that could theoretically be involved in rhythm regulation is enormous, with the SCN projecting to 15 regions and being directly innervated by about 35. If multisynaptic afferents to the SCN are included, the number expands to approximately 85 brain areas providing input to the SCN.”
There is general consensus on the major projection patterns from SCN to most target sites (Figure 5B). The major efferent connections to hypothalamic nuclei include rostral projections to the preoptic area (POA) - specifically the medial preoptic area (mPOA), the median preoptic area (MnPO), and the anteroventral periventricular nucleus (AVPV); as well as sparse connections with the ventrolateral peroptic area (VLPO). Projections extending dorsally heavily innervate the sub paraventricular zone (sPVZ), as well as the paraventricular nucleus of the hypothalamus (PVN). Caudal projections innervate the retrochiasmatic area (Rch), the arcuate nucleus (ARC), ventromedial nucleus of the hypothalamus (VMH), and the dorsomedial hypothalamus (DMH). Extra hypothalamic regions receiving direct SCN input include: the lateral septum (LS), the bed nucleus of the stria terminalis (BNST), the anterior paraventricular thalamic nuclei (PVA), the amygdala (AMY), the habenula (HB), and the IGL.
There is a substantial literature on the distribution of gonadal steroid receptors throughout the brain, the relative density of these receptors in subregions of specific nuclei, and species differences. The earliest studies examining ER distribution in the brain employed autoradiographic techniques (Pfaff, 1968), and immunohistochemistry and in situ hybridization experiments have confirmed and extended these findings (Amandusson and Blomqvist, 2013). An observation which has received little recent attention or discussion is of particular relevance to the current review: almost all of the sites that receive direct input from the SCN express AR, ER or some combination of both (Figure 5B). Moreover, there are sex differences in the expression levels of these gonadal steroid receptors in some of these sites (summarized in Table 2). Briefly, areas receiving direct SCN input which express AR include: the POA (mPOA, AVPV, MnPO), ARC, DMH, the VMH, PVN, as well as LS, the BNST, the PVA, HB, and the AMY. Of these areas, those in which a sex difference exists (the expression of AR is greater in males than females) include the mPOA (Figure 6A-B), the BNST (Figure 6C-F), the VMH, and the PVN.
Table 2.
| Brain Region |
Phenotype | Sex Difference | Mechanism | Reference |
|---|---|---|---|---|
| mPOA | ER α Expression | Female > Male | Early brain exposure to T/E in males | Kudwa, et al. (2006); Yokosuka, at al., (1997) |
| ER β Expression | Male > Female | Circulating E lowers expression | Kudwa, et al. (2006); Yokosuka, at al., (1997) | |
| AR Expression | Male > Female | Postnatal T acting through ER | McAbee & DonCalrlos (1999); Roselli (1991) | |
| AVPV | ER β | Female > Male | ---------------------- | Orikasa et al., (2002) |
| BNST | AR expression | Male>Female | Postnatal T acting through ER | Herbison (1995); McAbee & DonCalrlos (1999); Roselli (1991) |
| VMH | ER α Expression | Female>Male | ---------------------- | Cao & Patisaul, (2011) |
| ER β Expression | Female>Male | Perinatal estrogen leads to irreversible masculinization | Cao & Patisaul, (2011); Orikasa & Sakuma (2004) | |
| AR Expression | Male>Female | ---------------------- | Roselli (1991) | |
| PVN | AR Expression | Male>Female | ---------------------- | Herbison (1995); Roselli (1991) |
| SCN | ER α Expression | Female=Male (low levels) | ---------------------- | Vida et al., (2008) |
| ER β Expression | Female>Male in Shell | ---------------------- | Vida et al., (2008) | |
| AR Expression | Male>Female in Core | Circulating testosterone | Iwahana et al., (2008) | |
| DR | ER α Expression | Female = Male | ---------------------- | Shengn et al., (2004) |
| ER β Expression | Female = Male | ---------------------- | ||
| AR Expression | Male only | ----------------------- | ||
Figure 6. Sex differences in SCN efferent sites.
Sex difference have been demonstrated in the medial pre optic area (mPOA) as AR expression is greater in males (A) than females (B). AR expression in the BNST is also greater in males (C) than females (D). The fiber tracts of AR expressing neurons projecting from the BNST are denser in males (E) than females (F). Reprinted from Shah et al. (2004). The number of Kisspetin- (Kiss1) expressing neurons in the AVPV is greater in females (H) than in males (G). Image in (B) provided by (L. Kriegsfeld, L., and A. Geserich).
Both ERα and ERβ are expressed in a number of the efferent targets of the SCN, and some regions express both subtypes. Areas expressing both ERα and ERβ include: the POA (mPOA and AVPV), RCh the DMH, and ARC, as well as the LS, BNST, HB and AMY. The VMH expresses ERα, and the PVN, SCN, and PVT primarily express ERβ. Of these locations, females have higher levels of ERα expression than males in the mPOA, and VMH and have higher levels of ERβ in the VMH; whereas males have higher levels of ERβ within the mPOA.
In summary, almost every brain region receiving direct SCN input expresses either or both AR’s and ER’s, with sex differences in the expression levels of these receptors (see Table 2). The influence of gonadal hormones on circadian rhythms at these SCN target sites remain to be examined.
3.5 Sex Differences in targets of SCN efferents
In addition to sex differences in the expression level of gonadal steroid receptors (Figure 6A-C), there are multiple structural and functional sex differences in many of the efferent target sites receiving direct SCN input (see Simerly, 2002; Forger et al., 2004 for a review). Sex typical gonadal hormones are associated with a multitude of sex differences in neurotransmitter systems, which presumably contribute to differences in neural morphoplogy, function and behavior.
In males, the sexually dimorphic nucleus of the preoptic area (SDN-POA) and the BNST are substantially larger than in females (Simerly, 2002; Forger et al., 2004). Males also have a denser projection from the BSNT to the AVPV (Shah et al., 2004; Polston et al., 2004), and more VP neurons in the BNST and medial AMY nucleus, as well as more dense projections from these areas than do females in many mammalian species (De Vries et al., 2008).
In females, the AVPV is larger in terms of cell number (Forger et al., 2004), and the projection from this region to the ARC is more robust (Simerly, 2002). There is also a greater expression of tyrosine hydroxylase (TH) (Simerly et al., 1985), kisspeptin (Kauffman et al., 2007), and GABA/glutamate expressing neurons (Ottem et al., 2004) in females relative to males (summarized in Table 3). As noted in the introduction to this review, the differential numbers of neurons, projections, dendritic spines, etc. can result in sex differences in the transmission and processing of information in sexually dimorphic neural networks.
Table 3.
| Brain Region |
Phenotype | Sex Difference | Mechanism | Reference |
|---|---|---|---|---|
| mPOA | Cell Number/ size | Male > Female | T prevents cell loss in males | Simerly et al., (2002; Forger et al. (2004) |
| AVPV | Cell Number/ size | Female > Male | T leads to ↓ cell # in males | Forger et al.,(2004) ; Sumida et al. (1993) |
| Kiss1 expression | Female > Male | Postnatal Testosterone | Kauffman et al.,(2007); Clarkson et al. (2006) | |
| TH expression | Female > Male | T or E leads to ↓ cell # through ER α in males | Simerly (1998); Simerly et al., (1997); Simerly et al., (2008) | |
| GABA/glutamate Expression | Female > Male | (ER dependent) | Ottem et al., (2004) | |
| Projection to ARC | Female > Male | ---------------------- | Simerly et al., (2002) | |
| BNST | Cell Number/ size | Male > Female | T prevents cell loss in males via estrogenic metabolites acting though ER α and ER β | Forger et al. (2004); Hines et al., (1992) |
| Projection to AVPV | Male > Female | Perinatal exposure to T | Shah et al., (2004), Hutton et al., (1998); Polston et al.,(2004) ; DeVries & Milller (1985) | |
| VP expression | Male > Female | Circulating T | De Vries et al., (2008) | |
| SCN | Calbindin Expression | Female > Male | Prenatal Testosterone | Abizaid et al., (2004) |
| VIP Expression | Female > Male | Perinatal steroid exposure | Simerly et al., (2002) | |
The foregoing discussion enumerated numerous sex within the SCN, its inputs and efferent target sites. These in turn underlie sex differences in the HPG axis and reproduction, the HPA axis and stress, and the sleep-arousal networks of the brain.
4. Sex differences in circadian gating of the reproductive axis
The functions of sex differences in the circadian timing system are most salient in the regulation of reproduction. The SCN clock ensures the precise temporal organization of numerous neuroendocrine events required for successful puberty, mating, and birth. Many sex differences are readily evident in the hypothalamic-pituitary-gonadal (HPG) axis. In rodents, the SCN plays a crucial role in the regulation of the 4–5 day estrous cycle. In spontaneously ovulating females, there is a preovulatory surge of luteinizing hormone (LH) during the afternoon of proestrus. This LH surge is followed by ovulation around 6 hours later, just after the onset of the dark phase (Williams and Kriegsfeld, 2012). In a now classic study, Everett and Sawyer (1950) were the first to implicate a neural signal as being involved in the timing of the LH surge. By anesthetizing female rats with a barbiturate at specific times of day, they identified a 2 hour window (during the afternoon preceding ovulation) in which the anesthetic delayed the LH surge for 24 hours, rather than the few hours it took for the anesthetic to wear off.
It is now known that the SCN initiates a neural signal around the same time every 24 hours which creates a small temporal window during which the LH surge can occur (reviewed in Williams and Kriegsfeld, 2012). The LH surge occurs around the time of activity onset in animals housed under various LD cycle conditions, and both the LH surge and ovulation persists close to the expected time in animals housed in DD or LL conditions (Christian and Moenter, 2010). This SCN gated window is crucial for successful reproductive function, as it is the only time that E2 can act to stimulate the surge of LH necessary for ovulation. Estradiol levels must be above a critical threshold in order for the LH surge to occur. Under normal conditions, the levels of E2 secreted by the developing ovarian follicles are too low to surpass this threshold except on the afternoon of proestrus. By ovarectomizing (OVX) females and implanting them with E2 capsules (which constantly maintain E2 levels above this critical threshold), it has been shown that female hamsters, rats, and mice produce a daily LH surge around the same time each day (reviewed in Christian and Moenter, 2010). This experimental manipulation successfully unmasks the circadian rhythm of the SCN’s neural signal, which temporally gates the window of time during which high levels of E can induce the LH surge.
In women, LH secretion is not as tightly gated by the circadian system, but there does seem to be a circadian influence on LH secretion. Many women have a surge of LH between 4:00am and 8:00am (37%) or between 8:00am and 12:00pm (48%), (Cahill et al., 1998). Furthermore, the LH surge in women tends to occur at the same time as their daily peak in cortisol (Kerdelhué, 2002), which suggests circadian control of both of these hormone rhythms.
In males, there is a circadian rhythm in plasma testosterone (T) levels. Plasma T levels show a trough in the late evening and a peak in early morning (Kriegsfeld et al., 2002). Generally speaking, the SCN determines the phase of circadian rhythms which controls the timing of the release of various reproductive hormones, which then act at various levels of the neuroendocrine axis to influence circadian rhythmicity. As an example, castration reduces the cohesion of daily running bouts, decreases the precision of activity onset in male hamsters (Morin and Cummings, 1981), and leads to a lengthened period and loss of the early evening bout onset in male mice (Daan et al., 1975; Karatsoreos et al, 2007a). Androgen replacement restores normal functioning in both species.
4.1 Sex differences in the hypothalamic nuclei of the HPG axis
Brain regions known to be sexually dimorphic include the SDN-POA, the AVPV, and the BNST (Simerly, 2002; Shah et al., 2004). In each of these nuclei, sex differences are modulated by the circadian timing system.
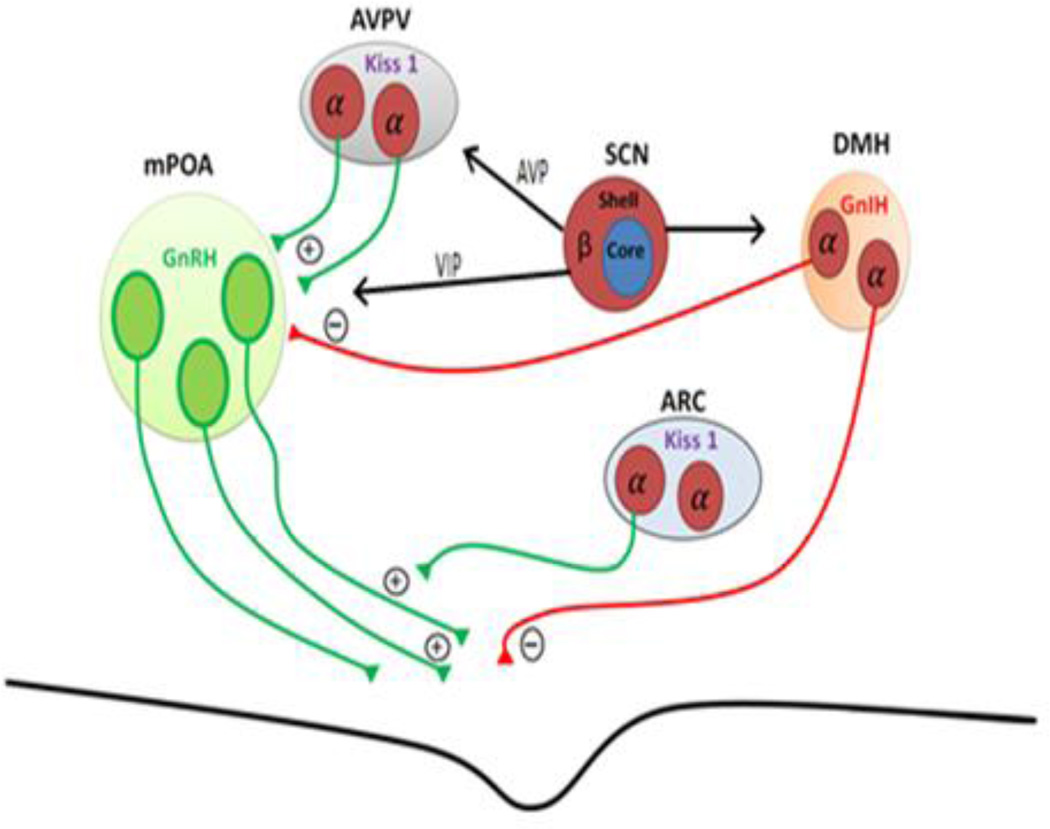
4.1.1 mPOA and GnRH neuronal secretion
Gonadotropin releasing hormone (GnRH) secreting neurons are located within the mPOA and serve as the final output pathway regulating the LH surge. GnRH neurons send axons to the median eminence of the hypothalamus where they release GnRH into the pituitary portal system, thereby triggering LH secretion and ovulation (Figure 7A; reviewed in Christenson et al., 2012; Williams and Kriegsfeld, 2012). Females, but not males, are able to produce an LH surge even though there is no sex difference in the GnRH neurons themselves. The difference in ability to generate a GnRH/LH surge is believed to be upstream of the GnRH neurons and is the result of organizational processes shaped by gonadal steroid exposure during neonatal development. Support for this view comes from studies showing that males castrated during the neonatal period are able to generate a GnRH/LH surge, and females that are exposed to T in the critical developmental period lack the LH surge in adulthood (reviewed in Kaufman, 2007). As described below, the circadian timing system (by acting on sexually differentiated neurons of the AVPV) regulates the dynamics of the neural circuits leading to the rhythmic generation of the GnRH/LH surge and ultimately ovulation.
Figure 7. Sex steroids and hypothalamic control of the HPG-axis.
Hypothalamic nuclei involved in regulation of gonadotropin release at the level of the hypothalamus. Neurons expressing estrogen receptors (ER), red, with ER subtype (α or β) indicated. Axons depicted in green represent neuronal inputs promoting the release of gonadotropins and axons depicted in red represent neuronal inputs inhibiting their release. The gonadotropin releasing hormone (GnRH) neurons, green, of the medial pre optic area (mPOA), light green, release gonadotropins into the medial eminence. Excitatory inputs upstream of the GnRH neurons include Kisspeptin (Kiss1) producing neurons in the anteroventral paraventricular nucleus (AVPV), grey, which project onto the somas of GnRH neurons, as well as Kisspeptin neurons of the arcuate nucleus (ARC), silver, which project onto the axons of GnRH neurons. The major inhibitory signals come from gonadotropin inhibiting hormone (GnIH) releasing neurons of the dorsomedial hypothalamus (DMH), light orange. The SCN influences multiple sites involved in control of gonadotropin release throughout the hypothalamus. Black arrows indicate monosynaptic projections from the shell of the SCN, to the positive (kisspeptin, and GnRH) as well as the negative signal of the GnIH. Adapted from Williams and Kriegsfeld (2012).
4.1.2 AVPV influence of GnRH neurons
The AVPV sits upstream of GnRH neurons and is characterized by multiple sex differences. It is important for the regulation of HGP axis, as lesions of the AVPV prevent spontaneous and steroid induced preovulatory surges of LH (reviewed in Herbison, 2008). Females have more AVPV neurons than males, as well as a more dense projection from the AVPV to GnRH neurons (Sumida et al., 1993). There are also a number of differences in neurochemical properties of these neurons. For example, the AVPV neurons of females have a greater TH expression and GABA/glutamate expression than males, however, the extent to which these neurochemical differences are involved in the generation of the LH surge remains to be determined (reviewed in Kauffman, 2010).
One sex difference which has been unequivocally implicated in the generation of the LH surge is the expression of the kisspeptin (KiSS1) gene in AVPV neurons. Females have up to 25-times greater AVPV kisspetin expressing neurons compared to males (Figure 6 G-H; reviewed in Williams and Kriegsfeld, 2012). Moreover, both AVPV KiSS1 and C-FOS expression levels increase during the preovulatory LH surge (Smith et al., 2006; Robertson et al., 2009). Treatment with E2 produces a surge of LH in females but not males, and central infusion of kisspeptin receptor (Kiss1 R) antagonist into the mPOA blocks the E2 induced LH surge as well as estrus rhythmicity (Kinosheta et al., 2005). Finally, Kiss1 knock out (KO) mice cannot generate an LH surge in response to E2 (Clarkson et al., 2008; Williams and Kriegsfield, 2012).
The sex difference in AVPV kisspeptin is not due to adult levels of circulating hormones, but results from exposure to hormones at critical times in development, suggesting that hormones have organizational effects on the AVPV in early development. Females treated postnatally with T or E2 have fewer kisspeptin neurons than untreated females (Kaufman et al., 2007a), and they are unable to produce the E2 induced LH surge in adulthood (Barraclough and Groski, 1961; Gogan et al., 1980). Further, males who undergo castration shortly after birth have greater numbers of AVPV kisspeptin neurons than intact males (Homma et al., 2009), and they can produce an E2 induces surge of LH (Gogan et al., 1980; Corbier, 1985). The evidence for AVPV kisspeptin stimulation of GnRH neurons and the generation of an LH surge includes: a) Kisspeptin stimulates LH and FSH secretion (in vivo and in vitro), and the effect is blocked by combining kisspeptin treatment with GnRH receptor antagonists; b) Kisspeptin induces C-FOS expression and prolonged evoked action potentials in GnRH neurons, and c) AVPV kisspeptin neurons make appositions with GnRH cell bodies - which themselves express the Kiss1 R (reviewed in Williams and Kriegsfeld, 2012; Kauffman, 2010).
The aforementioned AVPV-GnRH circuit is under circadian control. In female mice, KiSS1 gene expression shows a circadian pattern of expression as KiSS1 gene expression of E2 treated mice kept in DD exhibit circadian rhythms (Robertson et al., 2009). In the presence of E2, these AVPV KiSS1 expressing neurons also show a circadian pattern of C-FOS activation (Robertson et al., 2009; Williams et al., 2011). Recent work has shown that both the circadian rhythms in AVPV KiSS1 expression and activation of GnRH neurons are dependent upon the intact synaptic input of the ipsilateral SCN (Smarr et al., 2012). Further, the role of the ventrolateral and dorsomedial SCN have been dissociated with regards to HPG regulation. In a manner independent of the LD cycle, the dorsomedial SCN, but not the ventrolateral SCN, appears to control the circadian rhythms of KiSS1 expression in the AVPV which is always in phase with the LH surge. Moreover, the amplitude of the LH surge is dependent on the phase coherence between the dorsomedial and ventrolateral SCN (Smarr et al., 2012). This indirect evidence for a role of the dorsomedial SCN in kisspeptin regulation is reinforced in studies of mice and hamsters demonstrating a role of SCN-derived AVP in mediating AVPV kisspeptin cells (discussed below in section 4.2.2.)
4.1.3 DMH and GnIH neuronal secretion
A subset of neurons within the DMH express gonadotropin inhibiting hormone (GnIH), a neuropeptide that has been shown to act as a robust inhibitor of the reproductive access (Kriegsfeld et al., 2006a). These neurons project directly to GnRH neurons of the mPOA (Kriegsfeld et al., 2006a). In all mammals studied to date, GnIH rapidly suppresses LH release (reviewed in Bentley et al., 2010; Kriegsfeld et al., 2010). There does not appear to be a sex difference with regards to GnIH, as adult males and females (hamsters and mice) have been shown to have equal expression of GnIH (Kriegsfeld et al., 2006a; Poling et al., 2012). During the time of the LH surge, C-fos expression in these neurons is maximally reduced, and this temporal regulation is dependent on E2 (Gibson et al., 2008). The SCN sends projections to a large portion of GnIH cells, suggesting circadian regulation of this population of neurons (Gibson et al., 2008; Williams and Kriegsfeld, 2012).
Finally, gonadal hormones likely modulate the activity of GnIH neurons as female mice and hamsters express ERα and male hamsters express AR (Williams and Kriegsfeld, 2012). There is an induction of C-FOS expression in the ERα positive GnIH cells of female hamsters following the administration of E2 (Kriegsfeld et al., 2006). Further, GnIH expression within the DMH is regulated by E2 in both female and male mice as E2 treatment of GDX mice leads to lower expression of GnIH relative to GDX animals (Molnár et al., 2011). Treatment with non-aromatizable DHT does not have the same effect implying that the response is estrogenic (Poling et al., 2012).
4.1.4 The BNST and HPG axis stimulation
The BNST projects to both GnRH neurons and to the AVPV (Hahn and Cohen, 2006). Electrical stimulation of this region leads to an immediate LH surge (Beltramino and Taleisnik, 1980). The BNST is larger in males than females (Hines et al., 1992), as neurons in females undergo increased cell death during development (Chung et al., 2000). Perinatal hormones control cell numbers in the BNST as treating females with T leads to a complete masculinization of the female BNST. Both ERα and ERα are thought to be involved as treating females with agonists for either receptor masculinizes the BNST (Forger, 2009). While the levels of ER expression are similar, there is a large sex difference in the number of AR expressed (see Figure 6C-D), as well as AVP (DeVreis et al. 1985; Shah et al., 2004). The AVP efferent projections of the BNST are denser in males, and this is dependent upon the presence of gonadal steroids (Van Leeuwen et al., 1985). There is also a sex difference in the density of GABAergic fibers that project from the BNST to the AVPV neurons, with males having more dense projections as a result of perinatal hormone exposure (Polston et al., 2004).
Electrical rhythmicity in the BNST, a major target of SCN efferents, is strongly coupled to the SCN. This was shown by monitoring the phase relationship of multiple unit neural activity (MUA) in the BNST and the SCN of freely moving male hamsters housed under both LD cycles and constant darkness DD (Yamazaki et al., 1998). Circadian rhythmicity in BNST was always in-phase with the SCN. In contrast, rhythmic MUA outside of the SCN (in the ventrolateral thalamic nucleus, the caudate putamen, the nucleus accumbens, the medial septum, the LS, the VMH, the mPOA, and the stria medullaris) were always out of phase (Yamazaki et al., 1998). Unfortunately, females were not examined, and the occurrence of sex differences is not known, though the neuroendocrinology indicates that sex differences are likely.
4.2 Sex differences in SCN efferents in regulation of reproduction
The stage for circadian regulation of the HPG axis is set through direct efferents from SCN on GnRH neurons. Specifically, both AVP- and VIP-ergic SCN projections extend rostrally to contact GnRH cells directly (Mahoney, 2005; van der Beek, 1993).
4.2.1 VIP efferents
VIP neurons, which are located in the SCN core sub-region, have monosynaptic connections with GnRH neurons that express the VIP receptor VPCA2 (Smith et al., 2000). VIP neurons of the SCN core likely play a role in modulating the sexually dimorphic LH surge generated only in females (see Williams and Kreigsfeld, 2012 for a review). The SCN of females rats, compared to males have significantly greater VIP innervation of GnRH neurons (Horvath et al., 1998). The number of VIP-GnRH contacts increases in females from prepubertal ages to adulthood further implicating this sex difference as underlying the sex difference in LH surge (Kriegsfeld and Silver, 2006b). Further, GnRH neurons which receive input from VIP neurons show enhanced C-FOS expression during the afternoon of proestrus during the LH surge (Van der Beck et al., 1994), and this can be blocked using antisense antagonism of VIP in OVX females primed with E2 (Harney et al, 1996; Gerhold et al., 2005). Finally, VPAC2 antagonists decrease the firing rates of GnRH neurons during proestrus (Christian and Moenter, 2008). Together, these findings strongly implicate SCN regulation of GnRH neuronal influence on LH surge generation.
4.2.2 AVP efferents
Neurons within the shell sub-region of the SCN which produce AVP have also been suggested to play a role in modulating the LH surge observed in females. The SCN sends AVP projections to the AVPV, Rch, the anterior PVN, and the DMH, and the projections to Rch and DMH are denser in female mice than males (Rood et al., 2013). In both hamsters and mice, the SCN sends AVP projections to AVPV kisspeptin neurons, and a large percentage of these Kiss1 neurons express the vasopressin V1a receptor (Williams et al., 2011; Vida et al,. 2010). This is the same population of AVPV Kiss1 cells which sends projections to the GnRH neurons involved in the generation of the LH surge. The strength of the SCN AVP-AVPV connections increases following E2 treatment (Vida et al., 2010). While the mechanism has yet to be fully worked out, of note is the fact that many of these AVPV neurons which express the V1a receptor are also known to express ERα (Hoonrenman and Buijs, 1982; DeVries et al., 1985; de la Iglesia et al., 1995; Watson et al., 1995). Lesioning the SCN leads to the elimination of AVP fibers in the AVPV kisspeptin neurons suggesting the only AVP input to this region is provided by the SCN (Williams et al., 2011). Moreover, the SCN input to the AVPV kisspeptin neurons is highly specific to AVP as no VIP-IR is detected in this region in either mice or in hamsters (Vida et al., 2010; Williams et al., 2011). Finally, there is a clear sex difference with regards to the overall density of the projection as males have far fewer AVPV Kisspeptin neurons than females, which results in females receiving more AVP input from the SCN relative to males (Vida et al., 2010).
Vassopresin gene expression is controlled in a circadian manner by molecular clocks, and the release of the peptide itself is also regulated in a circadian manner (Shinohara et al., 1994), which coincides with the timing of the LH surge (Schwartz and Reppert, 1985; Kalsbeek et al, 1995). When AVP is injected into animals, it produces LH like surges in SCN lesioned OVX E2 treated females (Palm et al.,1999). Further support for the circadian nature of this interaction comes from the observation that central injections of AVP are only able to activate the LH surge during a small time window in the afternoon in intact females (Palm et al., 2001). Recent studies have attempted to determine if the mechanism responsible for the temporal gating of AVP’s ability to induce an LH surge occurs at the level of the AVPV V1a expressing neurons, or at the level of the GnRH neurons. It was found that treating OVX E2 treated female hamsters with AVP leads to increased activation of AVPV Kiss1 neurons even outside the window during which the LH surge can occur. In contrast, AVP treatment did not increase cellular activity in GnRH neurons outside the window of time during which the LH surge can occur (Williams et al., 2011). Thus, it appears that the SCN can stimulate the activity of AVPV Kiss1 neurons via AVP projections at any time throughout the day, but the temporal window during which AVP can induce the excitability of GnRH neurons and the subsequent LH surge occurs through a gating mechanism occurring at the level of the GnRH neurons.
4.3 Sex differences in gonadal oscillators
Ovarian tissue shows 24 hour rhythms in the gene expresses of core clock genes important for circadian timing (reviewed in Kennaway, 2012). Briefly, Brain and Muscle Arnt-like protein (BMAL1) mRNA levels peak around the time of lights on, and Per2 transcript peaks around the time of dark onset in the ovaries (Karman and Tischkau, 2006). The actual timing of the ovarian rhythms and SCN differ, as the phase of the SCN is 8 hours earlier than the ovarian rhythm. The hormones secreted by the ovaries are involved in the rhythmic expression of ovarian clock genes. Hyophysectomized rats which have never experienced an LH surge do not show rhythmic ovarian expression of Bmal1 or Per2. Following treatment with exogenous gonadotropin, rhythms subsequently appear in both Per2 and Bmal1, suggesting that gonadal hormones are important in driving the rhythmic clock gene expression (Karman and Tischkau, 2006). There also appears to be a circadian rhythm in the sensitivity of the ovarian tissue in response to both LH and FSH, as following chronic endogenous LH suppression, there are specific times during a 24 hours period where the ovarian tissue shows differential sensitivity to these hormones, with the greatest sensitivity occurring during the night of proestrus (Sellix, 2010). In contrast to the ovary, testicular tissue does not show circadian expression of clock genes in male mice (Alvarez et al., 2003; Bittman et al., 2003). There is, however, evidence of clock gene rhythms in testes of some hamster species (reviewed in Kennaway, 2012).
4.4 Sex differences in behavioral effects of gonadal hormones
4.4.1 Testicular hormones influence circadian rhythms
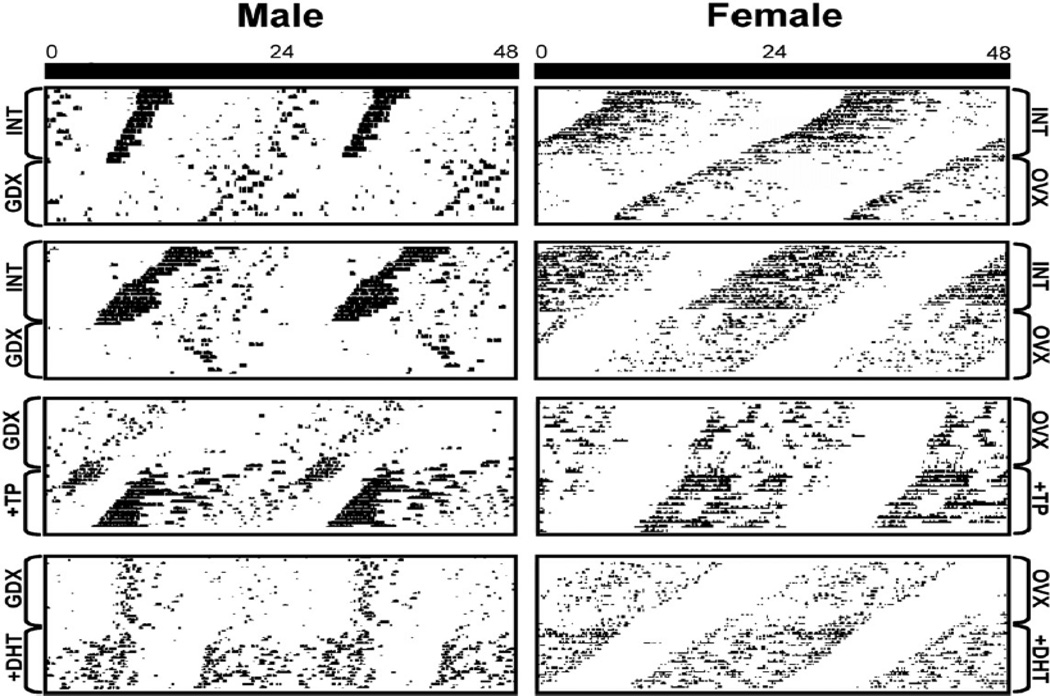
Gonadectomy in male mice lengthens the period of circadian rhythms of activity and increases the day-to-day variability of activity onset time in mice and hamsters (Butler et al., 2012). Both of these responses are rescued by treatment with either T or DHT (Figure 8), indicating that androgen aromatization is not necessary for this effect (Iwahana et al., 2008).
Figure 8. Sex differences in GDX effects on free-runs.
Actograms depicting the free running locomotor activity of male (left) and female (right) mice housed under conditions of constant darkness (horizontal axis show 48 consecutive hours; vertical axis show consecutive days). Each box shows an individual animal before (top) and after (bottom) an experimental manipulation. Top two boxes shows behavior of intact male and female at the top, and following gonadectomized (GDX) and overectomized (OVX); respectively, at the bottom. The bottom boxes show GDX/OVX animals at the top that are then treated with either testosterone propionate (TP) and dihydrotestosterone propionate (DHT); respectively, at the bottom. Reprinted from Iwahana et al. (2008).
Photic inputs
A critical function of the circadian system is to gate and respond to environmental stimuli. Interestingly, the response to androgens depends on the photic conditions in which the animal is held. GDX lengthens circadian period in male mice housed in dim light, but not DD (Butler et al., 2012). Increasing the intensity of the constant light parametrically increases circadian period in nocturnal animals (Aschoff rule). In androgen treated GDX animals housed in DD, T concentration is positively correlated with precision of activity onset and AR expression in the SCN and negatively correlated with duration of activity (Butler et al., 2012). The effect is specific to the circadian system as androgen exposure does not alter light-induced pupil constriction, a non-circadian response. Given that period is a property of the SCN (Daan et al., 1975), this work suggests that androgens modulate photic responses to light by direct effects within SCN.
Light also has acute effects on androgen mediated SCN responses. Following a light pulse during the subjective night, there is an increase in C-FOS expression in male mice in GRP cells of the SCN core, cells which bear AR receptors (Mong et al., 2011). GDX also leads to changes in the synaptic architecture and exerts effects on the molecular responses to light such that glial fibrillary acidic protein increases, while the expression of the synaptic proteins synaptophysin and postsynaptic density 95 decreases (Karatsoreos et al., 2011; Mong, 2011). In parallel, in late night [circadian time (CT) 21], GDX increases light-induced mPer1 gene expression but not mPer2, compared with intact controls. In contrast, in early night (CT 13.5), GDX decreases light induced mPer2 but has no effect on mPer1. At this time, GDX animals show larger phase delays than controls. Treatment of GDX animals with DHT restores SCN glial fibrillary acidic protein, postsynaptic density 95, and synaptophysin, and reinstates the normal pattern of responses to light (Karatsoreos et al., 2011). Thus, androgens alter responses to light, changing SCN clock gene expression. Androgens also alter synaptic properties, with likely consequences for the organization of SCN circuits that in turn produce the alterations seen in behavioral responses to photic stimuli. In summary, several lines of converging evidence suggest that AR-containing cells respond to both internal (gonadal hormone) and external (photic) cues, and are positioned to integrate endogenous and exogenous cues used in timing days and seasons.
4.4.2 Ovarian hormones influence circadian rhythms
Early studies in hamsters demonstrated a link between estrogen and behavioral circadian rhythms. Estradiol treatment of female hamsters shortens free running periods and advances the phase angle of entrainment (Morin et al., 1977). In mice, GDX of female mice held in constant dim red light (~1 lux) does not change the period of free-running locomotor rhythms (reviewed in Karatsoreos and Silver, 2007b). Consistent with this finding, in SCN slices harvested from PER2::LUC knockin mice, E2 applied to explanted cultures did not change the period of PER2::LUC expression in the SCN (Nakamura et al., 2008).
4.4.3 Clock gene mutations and fertility
Several clock gene mutant mice are available, though many of the mutations studied to date do not disrupt reproductive function (Boden and Kennaway, 2006). Homozygous null (−/−) mutant Per1−/− and Per2−/− mutant mice do not differ from wild type (WT) mice in young adulthood in terms of reproductive fecundity or estrus cyclicity. There does, however, appear to be a difference as these mice age, as middle aged Per1−/− and Per2 −/− mice have reduced reproductive success and have higher percentage of irregularity and acyclicity of their estrous cycles (Pilorz and Steinlechner, 2008). The authors of these studies suggest this might mean disrupted Per1 and Per2 genes result in a more rapid aging of the reproductive system.
The Clock mutant mouse, which carries a 51 amino acid deletion of the transcriptional-activation domain of the CLOCK protein, has been shown to have extended and irregular estrous cycles, lack of a coordinated LH surge, increased fetal reabsorption, and higher rates of full term pregnancy failures (Miller et al., 2004). Further, SCN-mediated AVP signaling is disrupted in these mice as they have reduced AVP expression in SCN and AVP1 receptor expression in the hypothalamus. Intra-cerebral infusion of AVP leads to an LH surge in only 50% of Clock mutant mice (Miller et al., 2006).
A clock gene disruption which has more profound deleterious effects on reproduction is seen in Bmal1 KO mice. Here, rhythmic gene expression in the ovaries is disrupted, a high percentage of mice have irregular estrus cycles (75%) and these mice cannot support pregnancy past implantation (Boden et al., 2010; Kennaway, 2012). While numerous studies have examined the role of clock genes in females, there have been no systemic studies of fertility in males. That said, this is a promising topic: Bmal1 null males are infertile as multiple aspects of their sexual development are abnormal and they have reduced sperm counts relative to WT mice ((Kennaway, 2012, Alvarez et al., 2008).
5. The Hypothalamic – Adrenal – Pituitary (HPA) Axis: circadian rhythms, sex differences, and stress
5.1 Circadian Rhythms and the HPA- axis
Several aspects of the circadian rhythms in adrenal function are salient (reviewed in Kalsbeek et al., 2012). Plasma glucocorticoids (GC) produced by the adrenal gland peak just before awakening in all species studied to date, and the circadian rhythms in plasma concentrations of the GC are highly predictable throughout the day. Specifically, GC levels peak in the early morning prior to awakening and reach their trough in the late evening for diurnal species. The converse is true of nocturnal species, such that the increase in GC is coupled to the time of arousal of a given species. Moreover, in addition to their functions in stress responses, energy metabolism, blood pressure, and synchronizing oscillators (delineated above), rhythms in adrenal hormones are also important in physiological functions throughout the body (Dallman, 1993). GC’s help regulate homeostasis through shifting the gene expression of several clock-related genes in the liver, heart, and kidney (Oishi, 2005; Balsalobre et al., 2000), as well as through their ability to alter mRNA expression of ~ 20% of the expressed genome (Chrousos and Kino, 2005).
Maintaining proper HPA axis circadian rhythmicity is unquestionably important, and we will discuss the mounting evidence that suggests a disrupted regulation of this axis is implicated in a host of human diseases (Chung et al., 2011). Finally, circadian rhythms in adrenal hormone secretions are different between the sexes. These sex differences have major implications in the diseases associated with HPA dysfunction. Understanding the origins of these sex differences may potentially aid our understanding of some of the diseases related to HPA dysfunction, as the prevalence of a number of these diseases differs between the sexes.
5.2 SCN regulation of the HPA
The SCN has long been known to regulate circadian rhythms in plasma levels of GC (Figure 9A), and it is now known that both neural and humoral signals are involved (discussed below). The earliest studies of the brain clock showed that lesions to the SCN disrupt circadian rhythms of corticosterone secretion (Moore and Eichler, 1972). While transplanting an SCN graft into SCN ablated animal was sufficient to restore circadian rhythms of locomotor behavior, this was not sufficient to restore circadian rhythms in GC release (reviewed in Kriegsfeld et al., 2002). This observation points to the importance of neuronal efferents from the SCN to sites involved in the regulation of these GC circadian rhythms. The SCN controls these daily rhythms through two distinct mechanisms. The first involves neuronal connections with hypothalamic neurosecretory neurons; the second involves multi-synaptic connections to the adrenal cortex via the autonomic nervous system (ANS). (see Dickmeis, 2009; Kahlsbeek et al., 2012 for reviews).
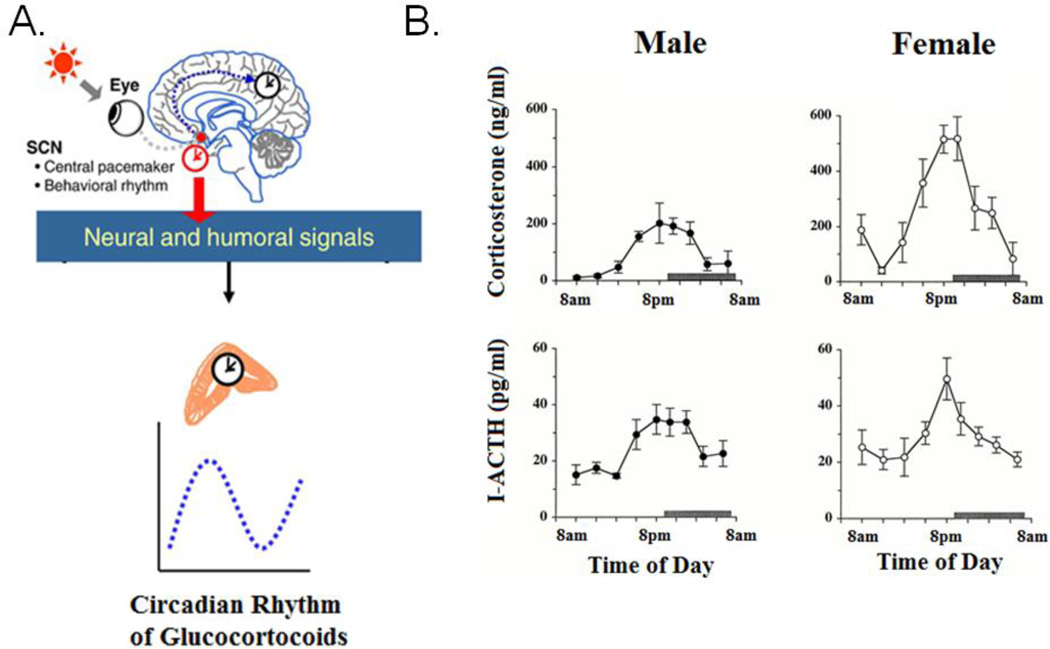
Figure 9. Sex difference in HPA-axis steroid secretion rhythms.
(A) (A) The SCN controls the phase of the circadian rhythms in the hypothalamicpituitary- adrenal axis (HPA) secretion of glucocorticoids through both neural and humoral output signals. Adapted from Chung et al. (2011). (B) Depicts the sex difference in circadian rhythms of stress related hormones. Female rodents have a circadian rhythm in plasma levels of stress related hormones as observed by the higher peak of both corticosterone (top) and immunoreactive ACTH (I-ACTH), (bottom) than males. Reprinted from Atkinson (1997) with permission from Endocrinology.
5.2.1 SCN regulation of HPA through the CNS
Corticotropin releasing hormone (CRH) is synthesized by CRH neurons within the medial parvocellular PVN (mpPVN) of the hypothalamus. The activity of these neurons and the secretion of CRH is the major determinant of the basal functioning of the HPA axis (Watts, 2005). Approximately half of all CRH neurons co-express AVP, and these CRH/AVP neurons send their axonal projections to the median eminence where the release of CRH and AVP can stimulate the anterior pituitary to release adrenocorticotropic hormone (ACTH). ACTH then acts upon the adrenal cortex to stimulate the release of GC. The SCN exerts a strong inhibitory influence on the HPA axis and the release of GC by modulating the CRH/AVP neurons of the mpPVN. The SCN sends a modest number of direct connections to the mpPVN, but it also sends projections to neighboring areas of the PVN that are thought to be able to influence the hypophysiotropic neurons (reviewed in Dickmeis, 2009). The SCN also influences the CRH/AVP neurons of the mpPVN through its connections with the sPVZ and the DMH, both regions which provide direct input to the CRH/AVP neurons (Kalsbeek, 2010).
The PVN displays circadian rhythms in cell firing rates which are lost when the SCN was removed from a hypothalamic slice preparation. The rhythms are restored if the tissue is then co-cultured with a rhythmic SCN, possibly as a result of AVP signaling from the co-cultured SCN graft (Tousson and Meissl, 2004). AVP is one of the main neurotransmitters in the projections of the SCN to the PVN and the DMH. AVP in the SCN is a clock-controlled gene. Further, a diurnal rhythm in the release of AVP from the SCN is observed in cerebrospinal fluid (Reppert et al., 1981; Schwartz et al., 1985) as well as within the SCN (Kalsbeek et al., 1995), and this rhythmicity is lost in Clock gene mutant animals (Jin et al., 1999; Silver et al., 1999). It has been suggested that AVP plays two opposite roles in the HPA, dependent on the location of its release. When released into the median eminence from the mpPVN CRH/AVP neurons, AVP stimulates ACTH secretion from the anterior pituitary. At sites upstream of the median eminence, however, AVP has an inhibitory effect. In both the PVN and the DMH, micro infusions of AVP leads to an inhibition of GC release in SCN ablated rats (Kalsbeek et al., 1992), indicating that AVP can mimic the inhibitory effect of the SCN when released in these two hypothalamic nuclei. Moreover, GC release is stimulated when AVP is blocked by an antagonist infused into either one of these regions during the peak time of AVP release (Dickmeis, 2009). It has been recently proposed that the most likely mechanism of AVP induced inhibition is the activation of GABA-ergic interneurons within the DMH and sPVZ/PVN (Kalsbeek et al., 2012), an idea supported by in vitro electrophysiology experiments performed in slices of hypothalamic tissue (Hermes et al., 2000).
5.2.2 SCN regulation of the adrenal gland via the ANS
The SCN regulates circadian changes in adrenal activity through routes other than the hypothalamic connections previously described. For example, hypophysectomised rats lose rhythms of ACTH, yet they maintain rhythms in corticosterone secretion (Meier, 1976). These corticosterone rhythms are lost when these hypophysectomised rats have their adrenal glands denervated (Ottenweller, 1982), suggesting neural control through the ANS (reviewed in Engeland, 2005). Experiments using trans-neuronal retrograde viral tracers injected into the adrenal gland reveal multi-synaptic connections between the SCN and the adrenal gland via pre-autonomic PVN neurons as a first relay station, and then these connect to the intermediolateral column (IML) of the spinal cord which serves as the second relay station. These pre-autonomic PVN neurons contact the sympathetic preganglionic neurons of the IML, which innervate the adrenal gland through the splanchnic nerve (Buijs et al., 1999). This second pathway appears to be entirely non-overlapping with the SCN efferent projections to the mpPVN, sPVZ, and DMH.
A first line of evidence for the non-hypothalamic SCN regulation of the adrenal gland comes from studies showing that light can alter the adrenal gland’s release of GC without any changes occurring in the HPA axis (reviewed in Dickmeis, 2009). Briefly, light exposure at the start of rat’s subjective night leads to a rapid suppression of corticosterone release (Buijs et al., 1999); in mice there is no decrease, but an increase after 1 hour when the light is given in the subjective night only (Ishida, 2005). The changes were unrelated to any corresponding changes in levels of ACTH. SCN ablation prevents these light induced glucocorticoid release changes in both species (Buijs et al., 1999, Ishida et al., 2005). Finally, adrenal denervation blocks this light induced change in corticosterone release (Niijma et al., 1993; Ishida et al., 2005).
A second line of evidence for the non-hypothalamic SCN regulation of the adrenal gland comes from the observation that the SCN modulates the gating of a temporal window during which the adrenal cortex is most sensitive to ACTH. There is a clear circadian rhythm in the sensitivity of the adrenal cortex to ACTH (Kaneko et al., 1981), such that ACTH sensitivity is highest in the evening. As a result, higher levels of corticosterone release occur in the evening given the same amount of ACTH (Dallman et al, 1978). These circadian rhythms in adrenal ACTH sensitivity are lost following SCN ablation (Sage et al., 2002). Evidence for the SCN-ANS modulation of this circadian rhythm in ACTH sensitivity comes from experiments showing that the ACTH hypersensitivity in the evening is lost when the adrenal gland is denervated, along with decreases in corticosterone secretion (reviewed in Englend, 2005; Ulrich-Lai et al., 2006).
In summary, the SCN can modulate the HPA axis control of GC release through two distinct mechanisms: through neuronal connections with hypothalamic CRH neurons, and through the multi-synaptic connections to the adrenal cortex via the autonomic nervous system (ANS). SCN mediation of the HPA axis is sexually differentiated (discussed below), and this has implications for diseases related to HPA dysfunction.
5.2.3 Clock genes and adrenal gland function
Steroid secretion by the adrenal gland is intrinsically rhythmic (Andrews and Folk, 1964; O’Hare and Hornsby, 1975), and there are differences in clock gene expression between the adrenal cortex and medulla (Dickmeis, 2009). For example, within the adrenal cortex all clock genes except Clock and Cry2 show robust patterns of circadian rhythms (Oster et al., 2006). The adrenal medulla shows circadian rhythmicity in the expression of only Bmal, Per1, and Per3, while Per2, Cry1, and Clock are weakly expressed (Bittman, 2003; Oster et al., 2006), suggesting that the corticoid producing outer layers of the cortex are the main sites of circadian adrenal pacemaker. There have been reports of sex differences in the adrenal gland of mice. For example, the the zona faciculata, the cortical layer which is responsive to ACTH and responsible for producing glucocorticoids, is larger in females than males after 7 weeks of age (Bielohuby et al., 2007). There have not, however, been any systematic studies investigating whether or not sex differences in adrenal gland clock gene expression exist.
5.2.4 Clock genes and HPA function
A number of different mouse models with clock gene mutations have been studied to determine the consequence of the clock gene disruption on GC circadian rhythms (reviewed in Dickmeis, 2009; Nader et al., 2010). Male Per1Brd mice which are deficient in Per1 have a short circadian cycle (Zheng, 2001). These mice also lack circadian rhythms in circulating glucocorticoids secretion, and the average circulating levels of GC are elevated relative to wild type mice (Dallmann, 2006). Male Per2 Brd mice also have a short circadian cycle with a loss of persistent circadian rhythms when maintained in DD (Zheng, 1999). These mice have elevated average GC levels relative to wild types, but the circadian rhythms of circulating glucocorticoids remain intact (Dallmann, 2006). Male Per2tm1Brd mice lack circadian rhythms in GC (Yang et al., 2009), but have intact ACTH rhythms (Yang et al., 2009). Male Per2 Brd/Cry1−/− mice, deficient in both Per2 and Cry1, show no behavioral circadian rhythm, and have a loss of both GC and ACTH circadian rhythms (Oster et al., 2006). Further, the adrenals of these mice do not show circadian rhythms in the expression of clock genes (Bmal1, Per1, Per3, Rev-ERBα) and also a loss in the circadian rhythm in their sensitivity to ACTH.
Female Per2drmd1 mice have a short circadian cycle with a loss of persistent circadian rhythms, and show dampened circadian rhythms in GC excretion (Pilorz et al., 2009). As evident from this brief overview, the majority of the studies done looking at the effect of clock gene mutations on circadian rhythms in corticosteroid secretion have been done in male mice, and sex differences have not been a major focus of these studies. This presents an area of opportunity for future work.
5.3 Sex differences in the HPA
5.3.1 Sex differences in response to stress: role of PVN and CRH
A potential explanation for the observed differences in the prevalence of certain diseases related to HPA dysfunction may be due to sex differences in the HPA axis response to stress (Handa and McGivern, 2009). Evidence for such a sex difference comes from observations that the increases in basal plasma ACTH and CRH mRNA expression in the PVN in proestrus female rodents are higher than in males. Further, in response to a stress induced by foot shocks for 1 hour, females have a more rapid increase in both plasma ACTH and CRF mRNA expression increase in the PVN (Iwasaki-Sekino et al., 2009). In response to a milder form of stress, psychological stress, females show an increase in both plasma levels of ACTH and CRF mRNA expression in the PVN, during both proestrus and diestrus, but males do not show an increased HPA response to this milder form of stress (Iwasaki-Sekino et al., 2009).
5.3.2 Gonadal hormones, stress and HPA functioning
There are sex differences in the circadian rhythms of HPA-axis hormone secretion of laboratory rodents under basal conditions, as well as in response to stress. The circadian rhythms of GC secretion has a higher peak in female rats such that plasma and adrenal corticosterone levels are higher than in males (Figure 9B), and the average corticosterone levels over a 24 hour period are higher in females compared to males as well (Critchlow et al., 1963, Hanada et al., 1994).
In both sexes, E2 treatment leads to an increase in basal corticosterone and basal ACTH secretion and also leads to increased levels of corticosterone secretion in response to physical and psychological stressors (Burgess and Handa, 1992; Handa et al., 1994). In females, corticosterone levels are correlated with E levels across the estrus cycle, such that when E2 is highest the levels of corticosterone secretion are greatest, and the responsiveness of the HPA-axis to stress is greatest as well (Raps et al., 1971; Viau and Meany, 1991). Estrogen is known to affect ACTH release by prolonging the duration of its secretion (Burgess and Handa, 1992), which suggests a possible mechanism of E2 influence on the HPA is the impairment of glucocorticoid receptor (GR)-mediated negative feedback (Young, 1995a; Young, 1995b). This is supported by the observation that GC feedback is altered and auto-regulation of GR mRNA levels are altered when E2 is present (Burgess and Handa, 1992; Burgess and Handa, 1993). Finally, there are also changes in levels of CRH and CRH mRNA within the PVN when E2 is present (Bohler et al., 1990).
Androgens also influence the HPA, but in contrast to E2 it exerts an inhibitory effect on the magnitude of ACTH release in response to stress (Bingaman et al., 1994; Handa et al., 1994; Viau and Meaney, 1996). For example, prepubertal castration of male rats and hamsters leads to an increased response of the adrenal cortex following physical stress, making ACTH levels after such a stress more similar to those observed in females, and even a single injection of T was sufficient to bring the corticosterone response back down to levels of intact males (Gaskin and Kitay, 1971). Androgen receptors are implicated in mediating this effect as treatment with a non-aromatizable androgen (DHT) has the same effect as T (Handa et al., 1994). Further, castration leads to increases in hypothalamic CRH neurons (Bingaman et al., 1994; Almeida et al., 1992).
A prototypical example of the influence of daily rhythms in HPA hormones on depression and anxiety like behaviors which are impacted with stress is seen in a study of male and female rodents during the light and dark phase by Verma et al. (2010). Males had higher levels of anxiety on the elevated plus maze than females, but this sex difference was only present during the light phase. The open:closed arm ratio (which measures anxiety) was lower for females in the dark phase, but this time of day effect was only present for females. Further, females were affected by the light phase in the forced swim test as they showed more “despair” (quantified by immobility) when tested in the dark, whereas males were unaffected by phase in this task. Finally, when tested in the open field following the stressor of a brief physical restraint both sexes showed a stress induced increase in their open field activity in the light, but not dark phase. In line with previous work (Critchlow et al., 1963), females showed higher levels of circulating corticosterone than males in baseline conditions, and the magnitude of this sex difference was even more pronounced in the dark phase relative to the light phase (Verma et al., 2010). Studies such as these highlight the importance of photic conditions and time of day in behavioral testing, especially when animal housing is maintained in LD cycles convenient for caretakers, and animals are tested in their normally inactive daytime.
While studies in animals have shown that females have higher basal GC peaks and basal GC levels than males this finding has been inconsistent in the literature on humans, likely constrained by small sample sizes and heterogeneous population of subjects in the older literature (Balbo et al., 2010). In one extensive study, however, 200 temporal profiles of plasma cortisol were taken from men and women with ages ranging from young adulthood to late senescence. This study reported both sex differences and age-related differences in the circadian rhythms of cortisol levels (Van Cauter et al., 1996). In men and women the mean 24 cortisol levels increase by 20 to 50% between ages 20 to 80. Young (premenopausal) women had smaller 24-h mean cortisol levels than age matched men, and this difference is not present in post-menopausal women compared to older age matched men. Premenopausal women also have lower and less variable peak cortisol levels during the morning phase, a longer quiescent period (that started earlier and ended later), and a lower absolute amplitude of the circadian variation. Thus, the female response to the circadian signal appeared to be slower and of a lesser magnitude than in men, and recovered from the morning peak more rapidly with a longer quiescent period. Finally, a sex difference in the effects of aging on HPA function was apparent, as an increase in the level of the peak concentration of cortisol in the morning was observed in women, but not in men and the reduction in the duration of the quiescent period was sharper in women than in men (Van Cauter et al., 1996).
There are also systematic age related changes which occur in humans in both sexes. There was an age-related progressive increase in 24-hour mean cortisol levels associated with a shorter quiescent period, and higher nadir values consistent with the association of aging and impairment in the inhibitory feedback mechanisms (Seeman and Robbins, 1994). In addition, the timing of the nadir and onset of the nocturnal cortisol rise was advanced in older adults, and the circadian amplitude was decreased: hence, during senescence a progressive advance of circadian phase seems to occur, associated with a loss of strength of the circadian signal.
5.3.3 Sex differences in HPA desynchrony
The role of the HPA axis in response to stress, risk of disease, and psychological disruption is further exemplified in sex differences in mice null for urocortin 2. Urocortin 2 is a member of the corticotropin-releasing factor family and is expressed in discrete neuroendocrine and stress-related nuclei of the rodent CNS. Female, but not male, mice lacking urocortin 2 exhibit a significant increase in the basal daily rhythms of ACTH and corticosterone. They also show a significant decrease in fluid intake and depressive-like behavior which implicates urocortin 2 in sex differences in HPA responses (Chen et al., 2006).
A number of different diseases are associated with disruptions of the HPA-axis. Both hyper and hypo-activity of the HPA increases the risk of developing certain diseases, and men and women appear to have different vulnerabilities to different types of HPA related dysfunction (reviewed in Kudielka and Kirschbaum, 2005). Hyperactivity of the HPA axis is associated with susceptibility to infectious disease (Mason, 1991), cardiovascular problems (McEwen, 1998), and major depression (Bjorntorp, 1996). Hypoactivity of the HPA has been associated with autoimmune processes such as lupus erythematosis (Weiner, 1991), multiple sclerosis (Adams and Victor, 1997), neurodermatitis (Buske-Kirschbaum et al., 1997) fibromyalgia, chronic fatigue syndrome, and rheumatoid arthritis (Tsigos and Chrousos, 2002). Of the somatic diseases associated with HPA dysfunction, women more commonly suffer from autoimmune diseases, while men have a greater risk of developing coronary heart disease and infectious disease (Kudielka et al., 2000a, Kudielka et al., 2000b). Of the psychiatric disorders associated with HPA dysfunction, women have higher prevalence rates of depression and anxiety disorders, while men are more likely to develop antisocial behavioral problems and substance abuse problems (Bebbington, 1996; Weich et al., 2001). Understanding the nature of sex differences in HPA-dysfunction provides an opportunity by which to better understand the cause of certain diseases, and potentially offer new, sex appropriate strategies by which to treat them.
6.0 Sleep and the circadian timing system
6.1 Homeostatic and circadian components of sleep regulation
Daily rhythms in sleep and arousal are the most noticeable manifestation of the circadian timing system and the circadian modulation of sleep differs in women and men. The conceptualization that sleep-wake cycles are regulated by a widely distributed neural system that involves both circadian and non-circadian (homeostatic) aspects was first proposed by Borbely (1982). In this view, sleep is determined by two components: a “C Process”, or a circadian rhythm of sleep-wake oscillations that is independent of the duration one has been awake, and an “S Process” which represents a need-based homeostatic drive mechanism that builds up sleep pressure with increasing durations of wakefulness, thereby enhancing the propensity to sleep (reviewed in Morris et al., 2012; Venkataraman et al., 2013). The circadian “C Process” is thought to influence sleep by acting as a gating mechanism, whose role is to promote sleep onset at the ecologically appropriate time of day. Thus, diurnal animals sleep at night and are awake during the day, while the converse holds true for nocturnal animals.
Several lines of evidence support the view that C and S processes involve different neural mechanisms. SCN lesions lead to a loss in circadian rhythmicity of sleep, but cause little or no change in the homeostatic response to sleep deprivation (Mistlberger, 2005; Venkataraman et al., 2013). In the diurnal squirrel monkey, even after up to 1.5 days of complete sleep deprivation, they do not sleep until the next “circadian night” suggesting that the circadian system dominates homeostatic processes in determining the timing of sleep (Edgar et al., 1993). In contrast to the well characterized network of SCN efferents, the neural basis (bases) of the homeostatic “S” process is not established.
6.2 Anatomy of SCN efferents to sleep-wake brain regions
6.2.1 Arousal/Wakefulness and sleep promoting circuits
The major components of the sleep/arousal network have been well characterized in many species (reviewed in Rihel and Schier, 2013; Rosenwasser, 2009). The ascending arousal system projects to the thalamus and neocortex and is comprised of several wake-promoting circuits including: the cholinergic basal forebrain, the histaminergic (HA) tuberomammillary nucleus (TMN), the 5-HT dorsal raphe, and the noradrenaline (NA) locus coeruleus (LC). Orexin neurons (also known as hypocretin) of the LHA, which are active during wakefulness, project to all sites of the ascending arousal system (Saper, 2005). In turn, the GABAergic neurons of the sleep promoting VLPO send inhibitory connections to both the LHA orexin neurons and ascending arousal systems (Saper, 2005). The TMN, raphe, and LC make mutual inhibitory connections with the VLPO and are thought to form a ‘flip-flop’ circuit (Saper, 2013), enabling the shift between sleep/wake states. The pineal gland (a source of melatonin during the night time hours), whose secretion is under circadian control by the SCN, can in turn signal SCN and the GABA-ergic reticular thalamus.
6.2.2 SCN influence on sleep-active and wake active areas
The SCN influences sleep-wake areas through both direct and indirect connections, many of which contain gonadal steroid hormone receptors (Figure 5B; reviewed in Saper et al., 2005; Rosenwasser, 2009). As seen in Table 4, the SCN projects directly to various hypothalamic nuclei involved in sleep-wake regulation. While SCN projections to the VLPO are extremely sparse, the SCN’s influence on sleep promoting brain regions is thought to mainly occur via projections to the sPVZ, DMH, and MnPO as each of these regions send direct efferent projections to the VLPO (Deurveilher et al., 2002; Chou et al., 2002; Novak et al., 1999; Sun et al., 2001). The sPVZ is believed to play a particularly prominent role in the SCN’s regulation of sleep as lesions to ventral sPVZ, which don’t damage the SCN, disrupt sleep-wake activity rhythms (Rosenwasser, 2009). The SCN influences wake promoting regions via multi-synaptic connections with the sPVZ, and DMH. Lesions to the DMH disrupt a number of circadian rhythms, including: sleep-wake rhythms, feeding rhythms, locomotor activity rhythms, corticosterone secretion rhythms, as well as the rhythms in neural activity elicited by the LC (Aston-Jones et al, 2001; Bellinger et al., 1976; Chou et al., 2003). The sPVZ, and DMH primarily convey SCN influence on wake promoting regions through projections to the LHA, specifically with the orexin/CRHT neurons (Chou et al., 2002, Deurveilher and Semba, 2005). These LHA orexin/CRHT neurons send excitatory efferents to all of the monoaminergic arousal system neural sites (HA in TMN, 5-HT in DR and MR, NA in the LC) and can modulate their activity (Siegel et al., 2004; Saper et al., 2001).
Table 4.
| Brain region | Function | SCN input | State specific neuronal activity | Efferent targets | Lesion effects | Express AR/ER ? | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Waking | NREM | REM | |||||||
| LC | Waking/ Arousal | Indirect via LH,DMH,sPVZ | Fastest Firing | Slow Firing | No Firing | LH, BF | Profound Sleep/Coma | AR/ER | Saper et al., 2001; Saper et al., 2005; Gerashchenko et al., 2003; Aston-Jones and Bloom, 1981; Fornal et al., 1985 |
| DR | Waking/ Arousal | Indirect via LH, DMH, sPVZ | Fastest Firing | Slow Firing | No Firing | LH, BF | Profound Sleep/Coma | AR/ER | |
| MR | Waking/ Arousal | Indirect via LH, DMH, sPVZ | Fastest Firing | Slow Firing | No Firing | LH, BF | Profound Sleep/Coma | ER | |
| TMN | Waking/ Arousal | Indirect via LH, DMH, sPVZ | Fastest Firing | Slow Firing | No Firing | LH, BF | Profound Sleep/Coma | AR | |
| (Ach) pons/ tegmentum | Waking/ Arousal | Indirect via LH, DMH, sPVZ | Yes | Slow Firing | Yes | Thalamus | Saper et al., 2005; Strecker et al., 2007; McCormick, 1989 | ||
| LH | Modulates waking sites | Indirect via DMH, sPVZ | -------- | ------- | ------- | LC,DR,MR,TMN | Sleepiness, coma | AR/ER | Estabrooke et al, 2001; Lee et al., 2005; Peyron et al., 1998; España et al., 2003; Hagan et al., 1999; Kiyashchenko et al., 2003; Chemelli et al., 1999; Verret et al.,2003 |
| VLPO | Sleep-Active | Direct (sparse) | No Firing | Yes | Yes | LC,DR,MR, TMN,LH,vPAG | Insomnia, 50% ↓ NREM/REM | AR** | Lu et al., 2002; Lu et al., 2000; Sherin et al., 1996; Gaus et al., 2002; Sherin et al., 1998; Szymusiak et al. 1998; Saper et al., 2005; Novak and Nunez, 2000; |
| Core | Gating of REM | TMN | Disrupts REM | ||||||
| Extended | Gating of NREM | LC, DR,MR | Disrupts NREM | ||||||
| MnPO | Sleep-Active | Direct and Indirect | ------- | Yes | Yes | Insomnia, modest Δ S-W CR | AR/ER | Rosenwasser, 2009; Suntsova et al., 2002; Lu et al., 2000, Schmidt et al., 2000; Deurveilher and Semba, 2003 | |
| sPVZ | Relay of SCN to sleep/wake sites | Direct (Dense) | All SCN targets: VLPO,DMH,LH | Deurveilher et al., 2002; Chou et al., 2002; Novak et al., 1999; Sun et al., 2001; Lu et al., 2001; Moore et al., 2002 | |||||
| Ventral | Sleep-wake | ------- | ------- | ------- | VLPO,DMH,LH | Δ CR in S-W, and feeding | |||
| Dorsal | Body temp | mPOA | Δ Body temp CR | ||||||
| DMH | Sleep-wake, cort Locomotion, feeding rhythms | Direct | ------- | ------- | ------- | LH,VLPO,PVH | Δ CR: S-W, cort, feeding, locomotor | AR/ER | Deurveilher et al., 2002; Chou et al., 2002; Novak et al., 1999; Sun et al., 2001; Abrahamson et al., 2001; Deurveilher and Semba, 2005 |
Unreported in literature; determined through Allen Brain Atlas
©2012 Allen Institute for Brain Science. Allen Mouse Brain Atlas [Internet]. Available from: http://mouse.brain-map.org/
In summary, the SCN can exert an influence on the sleep promoting VLPO primarily through projections to the sPVZ, DMH, and MnPO, which all send direct projections to the VLPO. The SCN can exert an influence on the arousal system via the sPVZ and DMH as these regions both project to orexin neurons of the LHA, neurons which project to all major nodes of the ascending arousal system of the brain.
6.3 Circadian Rhythms in the Sleep-Wake promoting hormone melatonin
The pineal hormone melatonin is said to be the “hormone of the night,” and is strongly associated with sleep. Specifically, plasma melatonin peaks at night and reaches a trough during the day (Cain et al., 2010; Gooley et al., 2011). Melatonin rhythms are under circadian control as the act of sleeping has minimal influence on the circulating melatonin levels (Gooley et al., 2011). This is in contrast to cortisol levels which are influenced by sleep as cortisol levels drop during the first few hours of sleep regardless of the time of day or circadian phase (Weitzman, 1983). The SCN controls melatonin production via the autonomic nervous system. Briefly, the SCN projects to the pineal gland through a multisynaptic pathway to the PVN to the IML of the upper thoracic spinal cord (Morris et al., 2012). Lesions of this pathway in rodents disrupt melatonin rhythms (Hastings and Herbert, 1986). Spinal cord injury patients with a complete severing of the cervical spinal cord (transecting the pathway) have no circadian rhythms in melatonin production whereas people with spinal cord injuries below the third thoracic vertebra (leaving this pathway intact) do have daily melatonin rhythms (Zeiter, 2000).
Melatonin’s role in modulating sleep in humans comes from three lines of evidence. First, administration of melatonin during times outside the endogenous phase it gets produced leads to increases in total sleep time and sleep maintenance (Sack and Lewy, 1997; Aeschbach, 2009). Second, administration of exogenous melatonin alters patterns of electroencephalography (EEG) patterns during sleep by increasing sleep spindle frequency (Aeschbach, 2009). Finally, blocking melatonin during the night with beta-blockers increases the time spent awake during the normal sleeping hours, and administration of exogenous melatonin can reverse this effect (van den Heuvel, 1997).
There are sex differences in melatonin rhythms as women have a higher melatonin amplitude than men as well as an earlier onset of peak melatonin levels (Cain et al., 2010). The timing of circadian phases of melatonin and body temperature (another measure of circadian phase) occurs earlier in women than in men, as compared to self-selected sleep time. This implies that women and men are sleeping at the same “clock time,” but at a different “biological time,” relative to their circadian cycle. In a study where men and women were matched for chronotype preference, total sleep times did not differ between the sexes (Cain et al., 2010), suggesting that perhaps the sex difference in melatonin secretion may serve to equalize their sleep times.
6.4 Circadian Rhythms and Sex Differences in Sleep across the Lifespan
6.4.1 Infancy
Certain rhythms like heart rate and breathing exist even as early as in utero, but there seems to be a period of development required for the rhythmicity of sleep-wake patterns after birth (Mirmiran et al., 2003). Infants do not exhibit regular sleep-wake rhythms until around three months of age (Kleitman, 1953).There are subtle sex differences in sleep even in infancy, but this is not a highly researched area (Paul et al., 2008). Sex differences in EEG waveforms are seen soon after birth, and at around ten months of age girls begin to spend more total time sleeping than boys (Thordstein, 2006). At this time sex differences in the underlying sleep architecture begin to more fully emerge (Menna-Barreto, 1989). Interestingly, baby boys are slightly delayed in the development of coherent sleeping rhythms relative to girls of the same age (Nagy, 2001).
6.4.2 Adolescence
One sex difference in sleeping which emerges during adolescence is the change in chronotype. Chronotype is defined as the preferential time frame of daily activity. People are generally classified as having either a morning or evening preference (morning larks or night owls, respectively (Figure 10A). A dramatic shift in chronotype is seen during adolescence in both girls and boys (reviewed in Hagenauer, 2012). During adolescence, both sexes experience a change in chronotype, marked by going to bed at increasingly later times (Figure 10B). It is unlikely that this shift in chronotype is due entirely to social or environmental factors as similar patterns have been observed in adolescents in several countries around the world including: Korea, Brazil, Italy, The United States (Hagenauer, 2012), and has been observed in both pre-industrial and industrial cultures (reviewed in Carskadon, 2008).
Figure 10. Sex differences in sleep onset with age.
Assessment of chronotype (N≈25,000 people). (A) Shows the distribution of chronotypes based on midpoint to sleep on free days (MSF) with 0 corresponding to earliest and 12 corresponding to the latest. (B) Shows the age-dependent changes in average chronotype (±SD) shift to later chronotypes around the time of puberty. (C) Shows sex difference in the age-dependent changes in chronotype (filled circles and black line: females; open circles and gray line: males). Gray areas indicate significant male–female differences (t-test, p < 0.001). Reprinted from Roenneberg et al. (2004).
The changes which occur in adolescent sleeping behavior are thought to be driven by pubertal hormonal changes (see Hagenauer, 2012). On average, puberty begins one year earlier in girls than in boys. Concomitantly, girls begin to experience the chronotype shift and begin staying up later one year earlier than boys which implicates gonadal hormones (Figure 10C). Further, in girls, the peak delay in sleep onset also occurs one year earlier than it does in boys (Ronneberg, 2004). In addition to a sex difference in the time of chronotype onset, there also appears to be a sex difference in the magnitude of the chronotype shift as well. The overall change in chronotype is more pronounced in boys than it is in girls (Figure 10C). Finally, there is a correlation between the development of secondary sexual characteristics and age-related changes in time of sleep onset, which further implicates gonadal hormones in chronotype changes observed in adolescence (Hagenauer, 2012; Sadeh, 2009).
The hormonally driven changes in chronotype that occur in human adolescence have parallels in rats, mice, and macaques (for review, see Hagenauer, 2012). For example, there are sex differences in the magnitude of chronotype shifts, such that male rats showed a greater shift than females as seen in humans (Hagenauer, 2011). In accordance with human data in which females experience chronotype shifts at younger ages than males, both rats and macaque females go through the puberty related chronotype shifts earlier than males in both of these species as well (Hagenauer, 2011; Golub, 2002). Taken together, these observations of chronotype shifts across mammalian species support the notion that reproductive hormones are responsible for the change. This should not be surprising, as reproductive hormones also affect sleep in adults, as ovarian hormones influence sleep in women (Hartmann, 1966; Schiff, 1979), and testosterone levels are related to sleep in men (Luboshitzky, 1999).
6.4.3 Adulthood
Sex differences in several aspects of adult sleep behavior have been reported. On average, women go to bed earlier than men, spend more total time in bed than men, and get more total sleep (Manber, 2006). Interestingly, the tendency for women to go to bed earlier than men at all ages throughout adulthood disappears with the onset of menopause (Ronneberg, 2007), once again implicating hormonal influence on the circadian and sleep systems.
Evidence of a sex difference in chronotype in adulthood comes from the finding that women are also more likely to rate themselves as preferring the morning (morning larks) than men (Adan, 2002), see Figure 10C. Interestingly, women also report poorer quality of sleep than men despite getting more total sleep, and the risk of women developing insomnia is 41% higher than men (Zhang, 2006). This risk ratio difference increases with age (Figure 11A). The precise reasons for this large discrepancy are unknown. In a study comparing EEG activity between insomnia patients and healthy controls, the EEG of men with insomnia did not significantly differ from that of healthy male controls, whereas women with insomnia had different EEG activity than healthy female controls through all of NREM sleep phases (Buysee et al., 2008), (Figure 11B). This finding may be related to the increased incidence of insomnia in women.
Figure 11. Sex difference in Insomnia: Prevalence and cortical activity.
(A) Shows the sex difference in the prevalence of insomnia complaints at different ages in a general population between women (grey) and men (black). The sex difference begins around puberty, implicating gonadal hormones. Figure adapted and data re-plotted from Mong et al. (2011). (B) Shows the sex difference in cortical activity between primary insomnia patients and controls. Plot depicts the F ratio of differences in EEG activity from F tests conducted between healthy females and females with primary insomnia (grey) and healthy males and males with primary insomnia (black) at each 1-Hz frequency bin for all-night NREM data (dashed grey (female) and black (male) line = p<.05, dotted grey (female) and black (male) line = p<.01) (see Buysse et al., 2008 for data across all NREM stages). A sex difference exists as cortical brain activity during sleep is significantly different between healthy females and female insomnia patients, but the same is not true for males. This may be related to the sex difference in prevalence of insomnia, see (A), observed between males and females. Figure adapted and data replotted from Buysse et al. (2008).
In adulthood, sex differences in sleep are present in mice as well. Female mice spend approximately 1.5 hours more awake per day than males, resulting from females spending less time in NREM sleep during the dark phase compared to males (Paul, 2006). These differences are diminished following gonadectomy (GDX) in mice (Paul, 2006), and in rats OVX decreases amount of time awake and increases NREM sleep (Li, 1995). Treatment of OVX female mice with E2 decreases total amounts of REM sleep (Colvin, 1969). Finally, treatment of OVX animals with both E2 and progesterone (P) leads to reductions in both REM and NREM sleep (Branchey, 1971). Taken together, these results in female mice and rats support the notion that female reproductive hormones lead to decreased sleep and increased wakefulness, suggesting sex differences seen in humans may likely be modulated by reproductive hormones.
6.5 Sex differences in sleep homeostasis in response to sleep deprivation
There is a growing literature on sex differences in sleep homeostasis and response to sleep deprivation in humans. A sex difference seems to exist in response to sleep deprivation as the consequences of sleep debt accumulation seems to differentially affect men and women (reviewed in Mong et al., 2011; Paul et al., 2008). Women appear to accumulate sleep debt more rapidly than men do, and the consequences of sleep debt seem to be more debilitating in women (Armitage, 2001), having greater negative impact on their health (reviewed in Mong et al., 2011). Objective measures of sleep show that, even at baseline, sex differences exist as women have more Slow Wave Activity (SWA; also called δ activity) during NREM sleep than men (Dijk, 1989; Ehlers, 1997). Supporting the idea that the S Process may differ between the sexes is the observation that women exhibit a greater SWA rebound in response to sleep deprivation than men (Manber, 1999; Armitage, 2001). For a two hour interval following sleep deprivation, female mice also show more NREM sleep compared to males (Paul, 2006). This result suggests that there may be a sex difference in the homeostatic sleep process in rodents as well as humans.
In recent years numerous studies in rodents have examined how ovarian hormones influence various sleep parameters (REM sleep, NREM sleep, and sleep intensity) under baseline condition and following a brief Sleep-Deprivation (SD) procedure (reviewed in Mong et al., 2011; Paul et al., 2009). To assess the effects of estrogen and progesterone under stable conditions, female rats were ovarectomized (OVX) and given subcutaneous hormone implants of E2, P, or Estradiol plus Progesterone (EP) which stabilize hormone levels for ~ 2 weeks (Deurveihler, 2009). Under baseline conditions, OVX female rats treated with E2 or EP spend more time awake with less time spent in NREM or REM sleep, whereas OVX females treated with P alone spend less time in REM sleep than oil treated control animals during baseline (Deurveihler, 2009). These effects are most pronounced during the dark (active) phase (Schwartz and Mong, 2012). Following SD, all subjects show REM increases relative to baseline, and treatment with either E or EP increases total REM sleep, but this is restricted to the light (inactive phase) (Schwartz and Mong, 2012; Deurveihler, 2009).
In one study of male rats, GDX males treated with E2 spent more time awake during dark phase under baseline conditions, whereas after sleep deprivation GDX males treated with E2 spent more time in REM recovery than did oil treated controls (Wibowo et al., 2011). Thus, in males E2 promotes baseline wakefulness during dark phase and facilitates REM recovery following sleep deprivation, which is similar to the results found in females treated with E2. Accumulating evidence now suggests that rather than altering the homeostatic response to sleep deprivation, ovarian hormones are actually improving circadian rhythms in sleep-wake cycles by facilitating sleep during the light phase and suppressing sleep during the dark phase (Mong et al., 2011).
6.6 Clock genes and Circadian and homeostatic interactions in sleep
Mice with mutations in various clock genes have altered sleep homeostasis in addition to a disrupted circadian rhythmicity. A number of studies have looked at the effects of these clock gene mutations on sleep (see Franken, 2009; Landgraf, 2012 for a review). Table 5 summarizes many of these finding. As can be seen in the table, clock gene mutations can influence a number of different sleep parameters (both circadian and non-circadian). For example, Bmal1−/− mice have disrupted circadian rhythmicity, and show normal amounts of total sleep, REM sleep, and NREM sleep during the light phase, but all three of these parameters are elevated during the dark phase (Laposky et al., 2005). Under baseline conditions, the δ power in NREM sleep is elevated in the light phase, reduced in the dark phase, and attenuated in both the light and dark phase following sleep deprivation (Laposky et al., 2005).
Table 5.
| Mouse Mutant |
Circadian Phenotype |
Wheel Running |
Sleep Amount Light/ Dark |
REM Light/ Dark |
NREM Light/ Dark |
δ Power in NREM Light/ Dark |
Response to sleep deprivation |
Sex of Mice Sex |
References |
|---|---|---|---|---|---|---|---|---|---|
| Bmal1−/− | Arhythmic | Arhythmic | Normal/ elevated | Normal/ elevated | Normal/ elevated | Elevated/ Reduced | Attenuated NREM/REM | --------- | Laposky et al., (2005) |
| Npas2−/− | Short Period | Rhythmic | Normal/ reduced | Normal/ reduced | Normal/ reduced | Normal/ normal | Attenuated NREM/ reduced delta power | Male; M/F SD = yes | Dudley et al., (2003); Franken et al., (2006) |
| ClockΔ19 | Long Period | Rhythmic | Reduced/ reduced | Normal/ normal | Reduced/ reduced | Attenuated REM | Male | Naylor et al., (2000) | |
| Per1/2m/m | Arhythmic | Arhythmic | Reduced/ normal | Normal/ normal | Reduced/ normal | Normal/ normal | Increased delta power | Male | Shiromani et al., (2004) |
| Cry1/2−/− | Arhythmic | ------ | Normal/ elevated | Reduced/ elevated | Normal/ elevated | Elevated/ elevated | Attenuated NREM/REM, reduced delta power | Male | Wisor et al., (2002) |
| Dec2P385R | Normal | ------ | Reduced/ normal | Reduced/ normal | Reduced/ normal | Normal/ normal | Attenuated NREM/ REM, reduced delta power | Male/ Female | He et al., (2009) |
| Dpb−/− | Short Period | Rhythmic | Normal/ normal | Reduced/ normal | Normal/ normal | Normal/ reduced | Attenuated REM | Male | Franken et al., (2000) |
| Pk2−/− | Attenuated amplitude | ------ | Reduced/ normal | Normal/ elevated | Reduced/ normal | Normal/ normal | Attenuated NREM/ REM, reduced delta power | --------- | Hu et al., (2007) |
| Vipr2−/− | Arrhythmic | ------ | Reduced/ elevated | Reduced/ elevated | Reduced/ elevated | Normal/ normal | -------------- | --------- | Sheward et al., (2010) |
Where sex is not listed it was not reported
Adapted from: Landgraf, D., A. Shostak, et al. (2012). Clock genes and sleep. European Journal of Physiology 463(1): 3-14. [Pending Perimision]
Mice which lack both Cry1 and Cry2 (Cry1,2−/−) have an arrhythmic circadian phenotype. They also show normal amounts of time spent in NREM in the light phase but have reduced REM in the light phase. During the dark phase, however, Cry1,2−/− mice show elevated amounts of REM and NREM (Wisor et al., 2002). The δ power in NREM in these mice is elevated in both the light and dark phase, and they have attenuated NREM and REM sleep as well as reduced in response to sleep deprivation (Wisor et al., 2002; Franken, 2009).
While most studies performed to date have been done only in males, mice with disrupted Neuronal PAS domain-containing protein 2 (Npas2) function have been studied in both sexes. Npas2 is a paralouge of the CLOCK, and these two proteins serve overlapping functions within the intracellular transcription-translation feedback loop of the molecular clock (see Debruyne, 2008 for review). Npas2−/− mice have a shortened circadian period. They show normal levels of total sleep time, REM, and NREM in the light phase, but all three of these parameters are reduced relative to WT mice during the dark phase. These mice have normal δ power in NREM sleep under normal conditions in both phases. In response to sleep deprivation; however, they show attenuated amounts of NREM sleep and reduced δ power in NREM sleep (Dudley et al., 2003; Franken et al., 2006). A sex difference has been reported in response to sleep deprivation, as Npas2−/− males showed a reduced compensation of NREMS time compared to Npas2−/− female mice (Franken, 2006). See Table 5 for a more comprehensive summary.
The role of clock genes in circadian rhythms and sleep is an area of opportunity for future researchers as sex differences have not been systematically examined and could potentially shed light on some of the sex differences observed in human sleep problems.
6.7 Disruptions to daily sleep rhythm and disease propensity
A number of deleterious consequences, both acute and chronic, are associated with the failure to keep the body’s biological clocks and sleep-wake cycles in synchrony with the external environment. Multiple studies have demonstrated negative consequences of such asynchrony on health in both males and females. A number of such cases have been documented in night shift workers and similar professions. Compared to people who do not work during the night, male nightshift workers have been shown to suffer from higher rates of prostate cancer (Conlon et al., 2007), type2 diabetes is higher in both males and females (Morikawa et al., 2005; Pan et al., 2011), hypertension and cardiovascular disorder is higher in females (Kivimaki et al., 2006), both males and females are at increased risk of psychological disorders (Bildt and Michelsen, 2002), and male overnight truck drivers have higher incidence of ulcers (Koda et al., 2000). Moreover, the impact of an irregular sleep schedule and repeated asynchrony has been shown to adversely impact cognition in female flight attendants who travel across time zones on a frequent basis over many years. It was found that working these schedules is associated with decreased spatial learning and memory, increased cortisol levels, and reduced temporal lobe volume (Cho, 2001; Cho et al., 2000).
These adverse consequences have also been modeled in mice and hamsters. A study by Davidson et al., (2006) exposed male mice to an 8 week light cycle intended to produce circadian rhythm disruptions equivalent to a weekly flight from Washington to Paris. In aged mice, profound deleterious consequences resulted as over half of the mice died from the continual disruption to their body’s natural circadian sleep-wake cycle (Davidson, 2006). The deleterious effects of chronic jet lag has also been shown to impair learning and hippocampal neurogenesis in female hamsters, and the negative consequences persist well after the mice are returned to a normal LD schedule (Gibson et al., 2010). Clearly, maintaining synchrony is important for normal healthy functioning, and this is an area of study for which understanding how the circadian system and sex differences interact will be important for improving the treatment of the associated health problems.
7.0 Circadian rhythms and disease
The circadian transcriptome and proteome are thought to encompass more than 10% of known genes and proteins (Reddy et al., 2006; Panda et al., 2002; Hughes et al., 2009). In this context, it is perhaps not surprising that disrupted synchrony of circadian genes and the circadian timing system is linked to a broad range of human conditions, including sleep disorders, cancer, obesity, cardiovascular disease, immune system function, and a wide spectrum of neuro-behavioral disorders including drug and alcohol addiction, and other psychiatric conditions (Turek et al., 2001; Wirz-Justice, 2003; Rosenwasser, 2010; Takahashi et al., 2008).
7.1 Sleep disorders and circadian clock genes
One example of circadian disruption is seen in the study of familial sleep disorders where Familial Advanced Sleep Phase Syndrome (FASPS), Delayed Sleep Phase Syndrome (DSPS), and a gene mutation leading to short sleep duration have been discovered. A Mendelian sleep disorder known as FASPS is a condition in which there is a persistent (3–4hr) advanced sleep onset and early morning awakenings (Jones et al., 1999). Two clock gene mutations have been linked to this disorder in two separate families, as PER2, and CK1 (Xu, 2005; Dudley, 2003) mutations were discovered in these two cases. Both of these mutations ultimately result in a disruption of the normal post translational modification of Per2.
In the human condition DSPS, the individual experiences a chronic inability to fall asleep or awaken relative to normal time of day. A person with DSPS will typically experience sleep onset around (3–6am) and will awaken around (11–2pm), but unlike FASPS, a specific gene mutation contributing to this condition has yet to be identified. Gene association studies have identified the CLOCK, CSNK1E, PER2, PER 3 genes as being associated with DSPS (reviewed in Takahashi, 2008). A variable nucleotide tandem repeat (VNTR) for the PER 3 gene has also been implicated with sleep disturbances, diurnal preference, and DSPS (Franken, 2009). The VNTR in PER 3 and TIMELESS appears to increase both physiological and behavioral markers of sleep debt accumulation compared to individuals lacking this polymorphism. These include: sleep latency, SWA, and a decrement in waking performance (in particular executive function) following sleep deprivation of a day and a night without sleep. (Viola et al., 2007; Groeger et al., 2008).
Finally, one mutation in a gene called Dec2 (which is involved with the negative regulation of the circadian clock) seems to decrease the need for sleep. It was identified in a pedigree that segregates a short sleep length phenotype. Carriers of the Dec2 mutation had a typically sleep duration of 6.25 hours compared to the average sleep duration of 8.06 in non-carriers of the Dec2 mutation (Landgaf et al., 2012). A mouse model with the human Dec2 mutation gene was generated, and while this mouse has normal circadian rhythms, it spends smaller amount of time sleeping, in line with observations in humans with the mutation (He et al., 2009). While these circadian effects are widely documented, there has been relatively little work done on sex differences in these responses or in the underlying mechanisms.
7.2 Sex differences in cancer and cancer treatment
The circadian timing system gates cell division, thereby regulating apoptosis and DNA repair as well as several signaling and metabolic pathways relevant for cancer and its treatment (Figure 12A). Such phenomena form the rationale underlying the search for chronotherapeutic effects in cancer treatment. In fact, it appears that circadian timing of chemotherapy influences both the tolerability the drugs and optimal antitumor efficacy, which coincides with best tolerability and least disruption of the circadian timing system (Figure 12B; Reviewed in Levi et al., 2010). Both experimental and clinical evidence support an important role for circadian disruption in carcinogenesis and cancer progression, while circadian rhythm regulation enhances cancer inhibition (Innominato et al., 2010; Li et al., 2010).
Figure 12. Circadian timing and anticancer drugs.
(A) The circadian timing system determines the optimal circadian timing of anticancer medications through actions on drug transport, bioactivation, detoxification, metabolism, targets, and elimination. All of these are important determinants of the chronopharmacology of anticancer agents at various levels (cellular, tissue, and whole organism levels) (left). Various cell-cycle-related events that gate G1/S or G2/M transitions as well as DNA repair and apoptosis are regulated by the circadian clock. This influences the chronopharmacodynamics of anticancer drugs (right). The relationship between chronopharmacokinetics and chronopharmacodynamics influence the optimal chronomodulated drug delivery schedules (middle). (B) Circadian timing determines the tolerability of anticancer drugs. Circadian timing (in ZT) associated with optimal tolerability, and relative magnitude of survival benefit from optimal to worst timing, ranging from 0 to 200%. The diagram illustrates chronotolerance for 16 anticancer drugs in male mice, synchronized with LD12:12. The average circadian rhythm in body temperature is shown in the internal circle and provides a CTS biomarker, an endogenous reference for optimal drug timing. Reprinted from Levi et al. (2010).
The mediating mechanisms are thought to involve circadian changes in enzymatic activity, gene expression responsible for drug transport, bioactivation, detoxification, and pharmacodynamics (Levi and Schibler, 2007). Important in the present context is evidence for sex differences in chronotherapeutic effects. Thus, in preclinical chronotherapeutic studies of 5-fluorouracil (5-FU) and oxaliplatin, male mice were used as the basis for the time modulated schedule of drug treatments. Also, male patients predominated in the human translational studies of colorectal cancer that supported the concept of timed 5-FU and oxaliplatin effects. This regimen was subsequently used in clinical trials. Surprisingly, Levi and collaborators found that chronomodulated infusion of chemotherapy drugs significantly improved survival in males when compared with flat rate of infusion, while an opposite effect was found in females (Giacchetti, 2006). This example makes it quite clear that sex differences in circadian timing systems cannot be ignored.
8.0 Conclusion and future research directions
While there has been much work over the past few decades exploring the interrelationship between sex differences and circadian rhythms, this is an area with numerous opportunities and unexplored questions which can surely expand in the coming years. Systematic explorations of clock gene mutant mice with regards to phenotypes known to differ between the sexes (i.e. HPA axis functioning and response to stress and sleep) will potentially yield novel insights into sex differences and the circadian clock at the molecular level. Further, gonadal hormone receptors are expressed at nearly every site which receives direct SCN input and the role of gonadal steroids with regards to the functioning of these regions and circadian timing is another area for future investigation.
Finally, exciting new discoveries in circadian rhythm research will likely also benefit from a systematic investigation of sex differences. The study of metabolic circadian clocks is one such area of circadian rhythms research on the frontier of our knowledge. In the midst of the outburst of clock gene research, there has been an important new fork in the path toward understanding circadian oscillation. In both single cell and mammalian systems there is evidence of circadian clocks that work independently of gene activity (O'Neill et al., 2011). Human red blood cells, for example, lack a nucleus and cannot synthesize new proteins, but nevertheless show circadian oscillations (O'Neill and Reddy, 2011). The enzyme peroxiredoxin, is part of an evolutionarily ancient metabolic circadian clock (Loudon, 2012; Edgar et al., 2012). This metabolic clock does not depend on other circadian clocks, which rely on feedback loops. Mutations that disabled other transcription dependent clocks in fruitflies, plants, fungi, and algae did not stop peroxiredoxin from cycling between its two states across the day. The interconnectivity between the metabolic clock and transcription-translation feedback loops of the circadian clockwork is a frontier research area today. How sex differences might differ in these circadian systems is unexamined, although the lessons learned from other aspects of the circadian timing system suggests that sex is likely to prove salient in understanding these phenomena.
Highlights.
-
-
A general overview of circadian rhythms research and the suprachasmatic nucleus (SCN) is provided.
-
-
The SCN sends direct efferent projections to multiple sexually dimorphic brain regions.
-
-
Gonadal steroid receptors are expressed at most targets sites receiving direct SCN input.
-
-
Sex differences exist in the circadian timing of reproduction, stress hormone rhythms, and sleep.
-
-
Several diseases with sex differences in incidence are associated with disruptions to circadian systems.
Acknowledgments
Funding: The research from our lab reported here was supported by Grants NS37919 and MH 075045 (to RS)
Abbreviations
- 5-FU
5-fluorouracil
- 5-HT
serotonergic
- E2
17β-estradiol
- ACTH
adrenocorticotropic hormone
- AMY
amygdala
- ANS
autonomic nervous system
- AP
action potential
- AR
androgen receptors
- ARC
arcuate nucleus
- AVP
arginine vasopressin
- AVPV
anterolateral paraventricular nucleus
- BMAL1
brain and muscle ARNT-like protein1
- BNST
bed nucleus of the stria terminalis
- c-fos
(denoting the gene or mRNA)
- C-FOS
(denoting the protein)
- CRH
corticotropin releasing hormone
- DD
constant darkness
- DHT
dihydrotestosterone
- DMH
dorsomedial hypothalamus
- DR
dorsal raphe
- DSPS
Delayed Sleep Phase Syndrome
- EEG
electroencephalography
- EP
estradiol plus progesterone
- ER
estrogen receptors
- ERα
estrogen receptor alpha
- FASPS
Familial Advanced Sleep Phase Syndrome
- GC
glucocortocoids
- GDX
gonadectomy
- GHT
geniculo-hypothalamic tract
- GnIH
gonadotropin inhibiting hormone
- GnRH
gonadotropin releasing hormone
- GR
Glucocortocoid receptor
- GRP
gastrin-releasing peptide
- HA
histamine
- HB
habenula
- HPA
hypothalamic-adrenal
- HPG
hypothalamic-pituitary-gonadal
- IGL
intergeniculate leaflet
- IML
intermediolateral column
- Kiss1
Kisspeptin
- Kiss1 R
Kiss1 receptor
- KO
knock out
- LC
locus coeruleus
- LD
light-dark
- LH
luteinizing hormone
- LHA
lateral hypothalamic area
- LS
lateral septum
- MnPO
median preoptic area
- mPOA
medial preoptic area
- mpPVN
medial parvocellular PVN
- MR
medial raphe
- MUA
multi-unit neural activity
- NA
norandrenergic
- Npas2
Neuronal PAS domain-containing protein 2
- P
progesterone
- PER1
Period1 protein
- Per1
Period1 gene or mRNA
- PER2
Period2 protein
- Per2
Period2 gene or mRNA
- POA
preoptic area
- PVA
anterior paraventricular thalamic nuclei
- PVN
paraventricular nucleus of the hypothalamus
- Rch
retrochiasmatic area
- RHT
retinohypothalamic tract
- SCN
suprachiasmatic nuclei
- SD
sleep deprivation
- sPVZ
sub paraventricular zone
- SWA
slow wave activity
- T
testosterone
- TH
Tyrosine hydroxylase
- TMN
tuberomamillary neurons
- VIP
vasoactive intestinal polypeptide
- VMH
ventromedial nucleus of the hypothalamus
- VNTR
variable nucleotide tandem repeat
- VLPO
ventrolateral preoptic area
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abizaid A, Mezei G, Sotonyi P, Horvath TL. Sex differences in adult suprachiasmatic nucleus neurons emerging late prenatally in rats. European Journal of Neuroscience. 2004;19(9):2488–2496. doi: 10.1111/j.0953-816X.2004.03359.x. [DOI] [PubMed] [Google Scholar]
- Abraham U, Granada AE, et al. Coupling governs entrainment range of circadian clocks. Molecular systems biology. 2010;6:438. doi: 10.1038/msb.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson EE, Leak RK, et al. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12(2):435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- Adams RD, Victor M, Ropper AH. Principles of Neurology. New York: McGraw-Hill; 1997. Multiple sclerosis and allied demyelinative diseases; pp. 902–927. [Google Scholar]
- Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiology International. 2002;19(4):709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- Aeschbach D. Use of transdermal melatonin delivery to improve sleep maintenance during daytime. Clinical pharmacology and therapeutics. 2009;86(4):378–382. doi: 10.1038/clpt.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar RA, Reddy AB, et al. Circadian Cycling of the Mouse Liver Transcriptome, as Revealed by cDNA Microarray, Is Driven by the Suprachiasmatic Nucleus. Current Biology. 2002;12(7):540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- Alvarez JD, Hansen A, et al. The Circadian Clock Protein BMAL1 Is Necessary for Fertility and Proper Testosterone Production in Mice. Journal of Biological Rhythms. 2008;23(1):26–36. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amandusson Å, Blomqvist A. Estrogenic influences in pain processing. Frontiers in Neuroendocrinology. doi: 10.1016/j.yfrne.2013.06.001. (0) [DOI] [PubMed] [Google Scholar]
- Andrews RV, Edgar Folk G., Jr Circadian metabolic patterns in cultured hamster adrenal glands. Comparative Biochemistry and Physiology. 1964;11(4):393–409. doi: 10.1016/0010-406x(64)90006-4. [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends in Neurosciences. 2005;28(3):145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Armitage R, Smith C, Thompson S, Hoffmann R. Sex differences in slow-wave activity in response to sleep deprivation. Sleep Res Online. 2001;4(1):33–41. [Google Scholar]
- Arnold AP. The end of gonad-centric sex determination in mammals. Trends in Genetics. 2012;28(2):55–61. doi: 10.1016/j.tig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom F. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. The Journal of Neuroscience. 1981;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, et al. A neural circuit for circadian regulation of arousal. Nature Neuroscience. 2001;4(7):732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ. Circadian Variation in Basal Plasma Corticosterone and Adrenocorticotropin in the Rat: Sexual Dimorphism and Changes across the Estrous Cycle. Endocrinology. 1997;138(9):3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- Balbo M, Leproult R, et al. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. International journal of endocrinology. 2010;2010:759234. doi: 10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, et al. A Serum Shock Induces Circadian Gene Expression in Mammalian Tissue Culture Cells. Cell. 1998;93(6):929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, et al. Resetting of Circadian Time in Peripheral Tissues by Glucocorticoid Signaling. Science. 2000;289(5488):2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Barraclough CA, Gorski RA. Evidence That The Hypothalamus Is Responsible For Androgen-Induced Sterility In The Female Rat. Endocrinology. 1961;68(1):68–79. doi: 10.1210/endo-68-1-68. [DOI] [PubMed] [Google Scholar]
- Bebbington P. The origins of sex differences in depressive disorder: Bridging the gap. International Review of Psychiatry. 1996;8(4):295–332. [Google Scholar]
- Bellinger LL, Bernardis LL, et al. Effect of Ventromedial and Dorsomedial Hypothalamic Lesions on Circadian Corticosterone Rhythms. Neuroendocrinology. 1976;22(3):216–225. doi: 10.1159/000122628. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Dual Action of Electrochemical Stimulation of the Bed Nucleus of the Stria terminalis on the Release of LH. Neuroendocrinology. 1980;30(4):238–242. doi: 10.1159/000123007. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Tsutsui K, et al. Recent studies of gonadotropin-inhibitory hormone (GnIH) in the mammalian hypothalamus, pituitary and gonads. Brain Research. 2010;1364(0):62–71. doi: 10.1016/j.brainres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bildt C, Michélsen H. Gender differences in the effects from working conditions on mental health: a 4-year follow-up. International Archives of Occupational and Environmental Health. 2002;75(4):252–258. doi: 10.1007/s00420-001-0299-8. [DOI] [PubMed] [Google Scholar]
- Bielohuby M, Herbach N, et al. Growth analysis of the mouse adrenal gland from weaning to adulthood: time- and gender-dependent alterations of cell size and number in the cortical compartment. American Journal of Physiology - Endocrinology And Metabolism. 2007;293(1):E139–E146. doi: 10.1152/ajpendo.00705.2006. [DOI] [PubMed] [Google Scholar]
- Bingaman EW, Magnuson DJ, et al. Androgen Inhibits the Increases in Hypothalamic Corticotropin-Releasing Hormone (CRH) and CRH-lmmunoreactivity following Gonadectomy. Neuroendocrinology. 1994;59(3):228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Doherty L, et al. Period gene expression in mouse endocrine tissues. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2003;285(3):R561–R569. doi: 10.1152/ajpregu.00783.2002. [DOI] [PubMed] [Google Scholar]
- Björntorp P. Behavior and metabolic disease. International journal of behavioral medicine. 1996;3(4):285–302. doi: 10.1207/s15327558ijbm0304_1. [DOI] [PubMed] [Google Scholar]
- Bohler HCL, Jr, Thomas Zoeller R, et al. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Molecular Brain Research. 1990;8(3):259–262. doi: 10.1016/0169-328x(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction. 2006;132(3):379–392. doi: 10.1530/rep.1.00614. [DOI] [PubMed] [Google Scholar]
- Boden MJ, Varcoe TJ, et al. Reproductive biology of female Bmal1 null mice. Reproduction. 2010;139(6):1077–1090. doi: 10.1530/REP-09-0523. [DOI] [PubMed] [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Human Neurobiology. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Branchey M, Branchey L, Nadler RD. Effects of estrogen and progesterone on sleep patterns of female rats. Physiology & behavior. 1971;6(6):743–746. doi: 10.1016/0031-9384(71)90267-8. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, et al. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. European Journal of Neuroscience. 1999;11(5):1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Buijs RM. Hypothalamic integration of central and peripheral clocks. Nature reviews. Neuroscience. 2001;2(7):521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131(3):1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Estrogen-Induced Alterations in the Regulation of Mineralocorticoid and Glucocorticoid Receptor Messenger RNA Expression in the 80 Female Rat Anterior Pituitary Gland and Brain. Molecular and Cellular Neuroscience. 1993;4(2):191–198. doi: 10.1006/mcne.1993.1023. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Hall ML, Moul DE, Nofzinger EA, Begley A, Kupfer DJ. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31(12):1673. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Silver R. Basis of Robustness and Resilience in the Suprachiasmatic Nucleus: Individual Neurons Form Nodes in Circuits that Cycle Daily. Journal of Biological Rhythms. 2009;24(5):340–352. doi: 10.1177/0748730409344800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Karatsoreos IN, et al. Dose-Dependent Effects of Androgens on the Circadian Timing System and Its Response to Light. Endocrinology. 2012;153(5):2344–2352. doi: 10.1210/en.2011-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill DJ, Wardle PG, et al. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertility and Sterility. 1998;70(1):56–59. doi: 10.1016/s0015-0282(98)00113-7. [DOI] [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SBS, Santhi N, Duffy JF. Sex differences in phase angle of entrainment and melatonin amplitude in humans. Journal of Biological Rhythms. 2010;25(4):288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors α and β and Kiss1 in neonatal male and female rats. Journal of Comparative Neurology. 2011;519(15):2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Annals of the New York Academy of Sciences. 2004;1021(1):276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, et al. Narcolepsy in orexin Knockout Mice: Molecular Genetics of Sleep Regulation. Cell. 1999;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen A, Zorrilla E, et al. Urocortin 2-Deficient Mice Exhibit Gender-Specific Alterations in Circadian Hypothalamus–Pituitary–Adrenal Axis and Depressive-Like Behavior. The Journal of Neuroscience. 2006;26(20):5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A, Ennaceur JC, et al. Chronic Jet Lag Produces Cognitive Deficits. J. Neurosci. 2000;20:66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Chronic 'jet lag' produces temporal lobe atrophy and spatial cognitive deficits. Nature Neuroscience. 2001;4(6):567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, et al. Critical Role of Dorsomedial Hypothalamic Nucleus in a Wide Range of Behavioral Circadian Rhythms. The Journal of Neuroscience. 2003;23(33):10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, et al. Afferents to the Ventrolateral Preoptic Nucleus. The Journal of Neuroscience. 2002;22(3):977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A. Hormonal regulation of female reproduction. Hormone and metabolic research. 2012;44(8):587–591. doi: 10.1055/s-0032-1306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. Vasoactive Intestinal Polypeptide Can Excite Gonadotropin-Releasing Hormone Neurons in a Manner Dependent on Estradiol and Gated by Time of Day. Endocrinology. 2008;149(6):3130–3136. doi: 10.1210/en.2007-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The Neurobiology of Preovulatory and Estradiol-Induced Gonadotropin-Releasing Hormone Surges. Endocrine Reviews. 2010;31(4):544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Science Signaling. 2005;2005(304):pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- Chung WC, Swaab DF, et al. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. Journal of Neurobiology. 2000;43(3):234–243. [PubMed] [Google Scholar]
- Chung S, Son GH, et al. Circadian rhythm of adrenal glucocorticoid: Its regulation and clinical implications. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2011;1812(5):581–591. doi: 10.1016/j.bbadis.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, et al. Kisspeptin–GPR54 Signaling Is Essential for Preovulatory Gonadotropin-Releasing Hormone Neuron Activation and the Luteinizing Hormone Surge. The Journal of Neuroscience. 2008;28(35):8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado P, Beyer C, et al. Hypothalamic distribution of astrocytes is gender-related in Mongolian gerbils. Neuroscience Letters. 1995;184(2):86–89. doi: 10.1016/0304-3940(94)11175-i. [DOI] [PubMed] [Google Scholar]
- Colvin GB, Whitmoyer DI, Sawyer CH. Circadian sleep-wakefulness patterns in rats after ovariectomy and treatment with estrogen. Experimental neurology. 1969;25(4):616–625. doi: 10.1016/0014-4886(69)90104-6. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Disrupted circadian rhythms in VIP-and PHI-deficient mice. American journal of physiology. Regulatory, integrative and comparative physiology. 2003;285(5):R939. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Conlon M, Lightfoot N, et al. Rotating Shift Work and Risk of Prostate Cancer. Epidemiology. 2007;18(1):182–183. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- Corbier P. Sexual Differentiation of Positive Feedback: Effect of Hour of Castration at Birth on Estradiol-Induced Luteinizing Hormone Secretion in Immature Male Rats. Endocrinology. 1985;116(1):142–147. doi: 10.1210/endo-116-1-142. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. American Journal of Physiology--Legacy Content. 1963;205(5):807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Daan S, Damassa D, et al. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus) Proceedings of the National Academy of Sciences. 1975;72(9):3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Engeland WC, et al. Nycthemeral rhythm in adrenal responsiveness to ACTH. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1978;235(5):R210–R218. doi: 10.1152/ajpregu.1978.235.5.R210. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress update: Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends in Endocrinology & Metabolism. 1993;4(2):62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- Dallmann R, Touma C, Palme R, Albrecht U, Steinlechner S. Impaired daily glucocorticoid rhythm in Per1 Brd mice. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2006;192(7):769–775. doi: 10.1007/s00359-006-0114-9. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, et al. Chronic jet-lag increases mortality in aged mice. Current biology : CB. 2006;16(21):R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne J. Oscillating perceptions: the ups and downs of the CLOCK protein in the mouse circadian system. Journal of Genetics. 2008;87(5):437–446. doi: 10.1007/s12041-008-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la lglesia HO, Blaustein JD, et al. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport. 1995;6(13):1715–1722. doi: 10.1097/00001756-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Burns J, et al. Indirect projections from the suprachiasmatic nucleus to the ventrolateral preoptic nucleus: a dual tract-tracing study in rat. European Journal of Neuroscience. 2002;16(7):1195–1213. doi: 10.1046/j.1460-9568.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to the median preoptic nucleus in rat. Brain Research. 2003;987(1):100–106. doi: 10.1016/s0006-8993(03)03295-5. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: Implications for the circadian control of behavioural state. Neuroscience. 2005;130(1):165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- DeVries GJ, Buijs RM, Van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. Journal of comparative neurology. 1985;233(2):236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Jardon M, Reza M, Rosen GJ, Immerman E, Forger NG. Sexual differentiation of vasopressin innervation of the brain: cell death versus phenotypic differentiation. Endocrinology. 2008;149(9):4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annual review of physiology. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Dickmeis T. Glucocorticoids and the circadian clock. Journal of Endocrinology. 2009;200(1):3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, et al. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 1989;12(6):500–507. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- Doherty CJ, Kay SA. Circadian Control of Global Gene Expression Patterns. Annual Review of Genetics. 2010;44(1):419–444. doi: 10.1146/annurev-genet-102209-163432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2- deficient mice. Science Signaling. 2003;301(5631):379. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Edgar D, Dement W, et al. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. The Journal of Neuroscience. 1993;13(3):1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Reddy AB. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485(7399):459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers C, Kupfer D. Slow-wave sleep: do young adult men and women age differently? Journal of Sleep Research. 1997;6(3):211–215. doi: 10.1046/j.1365-2869.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- Engeland W, Arnhold M. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine. 2005;28(3):325–331. doi: 10.1385/ENDO:28:3:325. [DOI] [PubMed] [Google Scholar]
- España RA, Valentino RJ, et al. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and 84 nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121(1):201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, et al. Fos Expression in Orexin Neurons Varies with Behavioral State. The Journal of Neuroscience. 2001;21(5):1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH. A 24-hour periodicity in the"LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47(3):198–218. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Kruijver FPM, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. The Journal of Comparative Neurology. 2000;425(3):422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Foley NC, Tong TY, et al. Characterization of orderly spatiotemporal patterns of clock gene activation in mammalian suprachiasmatic nucleus. European Journal of Neuroscience. 2011;33(10):1851–1865. doi: 10.1111/j.1460-9568.2011.07682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, De Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(37):13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG. Control of Cell Number in the Sexually Dimorphic Brain and Spinal Cord. Journal of Neuroendocrinology. 2009;21(4):393–399. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornal C, Auerbach S, et al. Activity of serotonin-containing neurons in nucleus raphe magnus in freely moving cats. Experimental Neurology. 1985;88(3):590–608. doi: 10.1016/0014-4886(85)90074-3. [DOI] [PubMed] [Google Scholar]
- Franken P, Lopez-Molina L, Marcacci L, Schibler U, Tafti M. The transcription factor DBP affects circadian sleep consolidation and rhythmic EEG activity. The Journal of Neuroscience. 2000;20(2):617–625. doi: 10.1523/JNEUROSCI.20-02-00617.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. European Journal of Neuroscience. 2009;29(9):1820–1829. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- Franken P, Dudley CA, Estill SJ, Barakat M, Thomason R, O'Hara BF, McKnight SL. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proceedings of the National Academy of Sciences. 2006;103(18):7118–7123. doi: 10.1073/pnas.0602006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Shinohara K, et al. Gonadotropin-Releasing Hormone Exhibits Circadian Rhythm in Phase with Arginine-Vasopressin in Co-Cultures of the Female Rat Preoptic Area and Suprachiasmatic Nucleus. Journal of Neuroendocrinology. 2000;12(6):521–528. doi: 10.1046/j.1365-2826.2000.00481.x. [DOI] [PubMed] [Google Scholar]
- Furman S, Hill JM, et al. Sexual dimorphism of activity-dependent neuroprotective protein in the mouse arcuate nucleus. Neuroscience Letters. 2004;373(1):73–78. doi: 10.1016/j.neulet.2004.09.077. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Hubbard KE, Hotta CT, Dodd AN, Webb AA. How plants tell the time. Biochemical Journal. 2006;397(Pt 1):15. doi: 10.1042/BJ20060484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin JH, Kitay JI. Hypothalamic and Pituitary Regulation of Adrenocortical Function in the Hamster: Effects of Gonadectomy and Gonadal Hormone Replacement. Endocrinology. 1971;89(4):1047–1053. doi: 10.1210/endo-89-4-1047. [DOI] [PubMed] [Google Scholar]
- Gaus SE, Strecker RE, et al. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115(1):285–294. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Rosewell KL, et al. Suppression of Vasoactive Intestinal Polypeptide in the Suprachiasmatic Nucleus Leads to Aging-Like Alterations in cAMP Rhythms and Activation of Gonadotropin-Releasing Hormone Neurons. The Journal of Neuroscience. 2005;25(1):62–67. doi: 10.1523/JNEUROSCI.3598-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, et al. Alterations in RFamide-Related Peptide Expression Are Coordinated with the Preovulatory Luteinizing Hormone Surge. Endocrinology. 2008;149(10):4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental 'Jet Lag' Inhibits Adult Neurogenesis and Produces Long-Term Cognitive Deficits in Female Hamsters. PLoS ONE. 2010;5(12):1–12. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Blanco-Centurion C, et al. Effects of lateral hypothalamic lesion with the neurotoxin hypocretin-–saporin on sleep in Long–Evans rats. Neuroscience. 2003;116(1):223–235. doi: 10.1016/s0306-4522(02)00575-4. [DOI] [PubMed] [Google Scholar]
- Gogan F, Rotsztejn WH, et al. Interaction of the anti-estrogen CI-28 and estradiol on plasma LH and hypothalamic LH-RH in the female rat. Brain Research. 1980;184(1):109–118. doi: 10.1016/0006-8993(80)90590-9. [DOI] [PubMed] [Google Scholar]
- Golub MS, Takeuchi PT, Hoban-Higgins TM. Nutrition and circadian activity offset in adolescent rhesus monkeys. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 50–68. [Google Scholar]
- Gooley JJ, Chamberlain K, et al. Exposure to Room Light before Bedtime Suppresses Melatonin Onset and Shortens Melatonin Duration in Humans. Journal of Clinical Endocrinology & Metabolism. 2011;96(3):E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Research. 1982;245(1):198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Groeger JA, Viola AU, Lo JC, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neuroscience Letters. 1982;34(3):283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Güldner FH. Sexual dimorphisms of axo-spine synapses and postsynaptic density material in the suprachiasmatic nucleus of the rat. Neuroscience Letters. 1982;28(2):145–150. doi: 10.1016/0304-3940(82)90143-4. [DOI] [PubMed] [Google Scholar]
- Güldner FH. Numbers of neurons and astroglial cells in the suprachiasmatic nucleus of male and female rats. Experimental Brain Research. 1983;50(2–3):373–376. doi: 10.1007/BF00239203. [DOI] [PubMed] [Google Scholar]
- Güldner FH. Suprachiasmatic nucleus: Numbers of synaptic appositions and various types of synapses. Cell and Tissue Research. 1984;235(2):449–452. doi: 10.1007/BF00217872. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer JM, et al. Suprachiasmatic Regulation of Circadian Rhythms of Gene Expression in Hamster Peripheral Organs: Effects of Transplanting the Pacemaker. The Journal of Neuroscience. 2006;26(24):6406–6412. doi: 10.1523/JNEUROSCI.4676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proceedings of the National Academy of Sciences. 1999;96(19):10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, King AF, Possidente B, McGinnis MY, Lumia AR, Peckham EM, Lee TM. Changes in circadian rhythms during puberty in< i>Rattus norvegicus</i>: Developmental time course and gonadal dependency. Hormones and behavior. 2011;60(1):46–57. doi: 10.1016/j.yhbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Lee TM. The neuroendocrine control of the circadian system: Adolescent chronotype. Frontiers in Neuroendocrinology. 2012;33(3):211–229. doi: 10.1016/j.yfrne.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JD, Coen CW. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. The Journal of Comparative Neurology. 2006;494(1):190–214. doi: 10.1002/cne.20803. [DOI] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period Genes: Rhythmic and Nonrhythmic Compartments of the Suprachiasmatic Nucleus Pacemaker. The Journal of Neuroscience. 2001;21(19):7742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, et al. Gonadal Steroid Hormone Receptors and Sex Differences in the Hypothalamo-Pituitary-Adrenal Axis. Hormones and Behavior. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, McGivern RF. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. Stress Response: Sex Differences; pp. 511–517. R. S. Editor-in-Chief: Larry. [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Hastings MH. The VPAC2 Receptor Is Essential for Circadian Function in the Mouse Suprachiasmatic Nuclei. Cell. 2002;109(4):497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, et al. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137(9):3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- Hartmann E. Dreaming sleep (the D-state) and the menstrual cycle. Journal of Nervous and Mental Disease. 1966 doi: 10.1097/00005053-196611000-00003. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Herbert J. Neurotoxic lesions of the paraventriculo-spinal projection block the nocturnal rise in pineal melatonin synthesis in the Syrian hamster. Neuroscience Letters. 1986;69(1):1–6. doi: 10.1016/0304-3940(86)90404-0. [DOI] [PubMed] [Google Scholar]
- He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Fu YH. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325(5942):866–870. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Sexually dimorphic expression of androgen receptor immunoreactivity by somatostatin neurones in rat hypothalamic periventricular nucleus and bed nucleus of the stria terminalis. Journal of neuroendocrinology. 1995;7(7):543–553. doi: 10.1111/j.1365-2826.1995.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain research reviews. 2008;57(2):277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes MLHJ, Ruijter JM, et al. Vasopressin Increases GABAergic Inhibition of Rat Hypothalamic Paraventricular Nucleus Neurons In Vitro. Journal of Neurophysiology. 2000;83(2):705–711. doi: 10.1152/jn.2000.83.2.705. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain research. 1992;579(2):321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Hofman MA. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: sex differences and age-dependent changes. Journal of anatomy. 1988;160:127–143. [PMC free article] [PubMed] [Google Scholar]
- Hofman MA, Zhou JN, Swaab DF. Suprachiasmatic nucleus of the human brain: An immunocytochemical and morphometric analysis. The Anatomical Record. 1996;244(4):552–562. doi: 10.1002/(SICI)1097-0185(199604)244:4<552::AID-AR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Homma T, Sakakibara M, et al. Significance of Neonatal Testicular Sex Steroids to Defeminize Anteroventral Periventricular Kisspeptin Neurons and the GnRH/LH Surge System in Male Rats. Biology of Reproduction. 2009;81(6):1216–1225. doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- Hoorneman EMD, Buijs RM. Vasopressin fiber pathways in the rat brain following suprachiasmatic nucleus lesioning. Brain Research. 1982;243(2):235–241. doi: 10.1016/0006-8993(82)90246-3. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Cela V, et al. Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Research. 1998;795(1–2):277–281. doi: 10.1016/s0006-8993(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Hu WP, Li JD, Zhang C, Boehmer L, Siegel JM, Zhou QY. Altered circadian and homeostatic sleep regulation in prokineticin 2-deficient mice. Sleep. 2007;30(3):247. [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Hogenesch JB. Harmonics of circadian gene transcription in mammals. PLoS genetics. 2009;5(4):e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton LA, Gu G, Simerly RB. Development of a sexually dimorphic projection from the bed nuclei of the stria terminalis to the anteroventral periventricular nucleus in the rat. The Journal of neuroscience. 1998;18(8):3003–3013. doi: 10.1523/JNEUROSCI.18-08-03003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innominato PF, Lévi FA, Bjarnason GA. Chronotherapy and the molecular clock: Clinical implications in oncology. Advanced Drug Delivery Reviews. 2010;62(9–10):979–1001. doi: 10.1016/j.addr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic"island" containing the suprachiasmatic nucleus. Proceedings of the National Academy of Sciences. 1979;76(11):5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Sex differences and implications for translational neuroscience research. Workshop summary. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metabolism. 2005;2(5):297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Karatsoreos I, et al. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Hormones and Behavior. 2008;53(3):422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropinreleasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34(2):226. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Jin X, Shearman LP, et al. A Molecular Mechanism Regulating Rhythmic Output from the Suprachiasmatic Circadian Clock. Cell. 1999;96(1):57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Jobst EE, Allen CN. Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. European Journal of Neuroscience. 2002;16(12):2469–2474. doi: 10.1046/j.1460-9568.2002.02309.x. [DOI] [PubMed] [Google Scholar]
- Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ, Ptc LJ. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nature medicine. 1999;5(9):1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM, et al. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Research. 1992;580(1–2):62–67. doi: 10.1016/0006-8993(92)90927-2. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM, et al. In vivo measurement of a diurnal variation in vasopressin release in the rat suprachiasmatic nucleus. Brain Research. 1995;682(1–2):75–82. doi: 10.1016/0006-8993(95)00324-j. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, et al. Vasopressin and the Output of the Hypothalamic Biological Clock. Journal of Neuroendocrinology. 2010;22(5):362–372. doi: 10.1111/j.1365-2826.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo–pituitary–adrenal (HPA) axis. Molecular and cellular endocrinology. 2012;349(1):20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Kaneko K, et al. Adrenal Sensitivity to Adrenocorticotropin Varies Diurnally. Endocrinology. 1981;109(1):70–75. doi: 10.1210/endo-109-1-70. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Wang A, et al. A Role for Androgens in Regulating Circadian Behavior and the Suprachiasmatic Nucleus. Endocrinology. 2007a;148(11):5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Silver R. Minireview: The neuroendocrinology of the Suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology. 2007b;148(12):5640–5647. doi: 10.1210/en.2007-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Butler MP, LeSauter J, Silver R. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology. 2011;152(5):1970–1978. doi: 10.1210/en.2010-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karman BN, Tischkau SA. Circadian Clock Gene Expression in the Ovary: Effects of Luteinizing Hormone. Biology of Reproduction. 2006;75(4):624–632. doi: 10.1095/biolreprod.106.050732. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kauffman AS. Coming of age in the kisspeptin era: sex differences, development, and puberty. Molecular and cellular endocrinology. 2010;324(1):51–63. doi: 10.1016/j.mce.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway DJ, Boden MJ, Voultsios A. Reproductive performance in female ClockΔ19 mutant mice. Reproduction, Fertility and Development. 2005;16(8):801–810. doi: 10.1071/rd04023. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Boden MJ, et al. Circadian rhythms and fertility. Molecular and Cellular Endocrinology. 2012;349(1):56–61. doi: 10.1016/j.mce.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Kerdelhué B. Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology. 2002;75(3):158–163. doi: 10.1159/000048233. [DOI] [PubMed] [Google Scholar]
- Kiessling S, Eichele G, et al. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. Journal of Clinical Investigation. 2010;120(7):2600–2609. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature. 2010;465(7299):688–689. doi: 10.1038/465688a. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, et al. Involvement of Central Metastin in the Regulation of Preovulatory Luteinizing Hormone Surge and Estrous Cyclicity in Female Rats. Endocrinology. 2005;146(10):4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Fau - Virtanen M, Virtanen M, Fau - Elovainio M, et al. Prevalent cardiovascular disease, risk factors and selection out of shift work. Scand. J. Work Environ. Health. 322006:204–208. doi: 10.5271/sjweh.1000. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, et al. Release of Hypocretin (Orexin) during Waking and Sleep States. The Journal of Neuroscience. 2002;22(13):5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic nucleus: the mind's clock. Oxford University Press; 1991. [Google Scholar]
- Kleitman N. Sleep characteristics of infants. Journal of applied physiology (1948) 1953;6(5):269–282. doi: 10.1152/jappl.1953.6.5.269. [DOI] [PubMed] [Google Scholar]
- Koda S, Yasuda N, et al. Analyses of work-relatedness of health problems among truck drivers by questionnaire survey. Journal of occupational health. 2000;42(1):6–16. doi: 10.1539/sangyoeisei.kj00002552185. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock Mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences. 1971;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Sex Differences in the Daily Rhythm of Vasoactive Intestinal Polypeptide But Not Arginine Vasopressin Messenger Ribonucleic Acid in the Suprachiasmatic Nuclei. Endocrinology. 1998;139(10):4189–4196. doi: 10.1210/endo.139.10.6259. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, LeSauter J, Hamada T, Pitts S, Silver R. Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R. Hormones, brain and behavior. 18. Vol. 2. New York: Academic Press; 2002. Circadian rhythms in the endocrine system; pp. 33–91. [Google Scholar]
- Kriegsfeld LJ, LeSauter J, et al. Targeted Microlesions Reveal Novel Organization of the Hamster Suprachiasmatic Nucleus. Journal of Neuroscience. 2004a;24(10):2449–2457. doi: 10.1523/JNEUROSCI.5323-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. Journal of Comparative Neurology. 2004b;468(3):361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Gibson EM, et al. The Roles of RFamide-Related Peptide-3 in Mammalian Reproductive Function and Behaviour. Journal of Neuroendocrinology. 2010;22(7):692–700. doi: 10.1111/j.1365-2826.2010.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver FPM, Swaab DF. Sex Hormone Receptors Are Present in the Human Suprachiasmatic Nucleus. Neuroendocrinology. 2002;75(5):296–305. doi: 10.1159/000057339. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Sex differences in human stress response. Encyclopedia of stress. 2000a;3:424–429. [Google Scholar]
- Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Schürmeyer T, Kirschbaum C. Psychosocial stress and HPA functioning: no evidence for a reduced resilience in healthy elderly men. Stress: The International Journal on the Biology of Stress. 2000b;3(3):229–240. doi: 10.3109/10253890009001127. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors α and β in differentiation of mouse sexual behavior. Neuroscience. 2006;138(3):921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Kuljis DA, Loh DH, et al. Gonadal- and Sex-Chromosome-Dependent Sex Differences in the Circadian System. Endocrinology. 2013;154(4):1501–1512. doi: 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Shostak A, et al. Clock genes and sleep. Pflügers Archiv - European Journal of Physiology. 2012;463(1):3–14. doi: 10.1007/s00424-011-1003-9. [DOI] [PubMed] [Google Scholar]
- Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28(4):395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. Journal of Comparative Neurology. 2001;433(3):312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, et al. Discharge of Identified Orexin/Hypocretin Neurons across the Sleep-Waking Cycle. The Journal of Neuroscience. 2005;25(28):6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman M, Silver R, et al. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. The Journal of Neuroscience. 1987;7(6):1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F, Schibler U. Circadian Rhythms: Mechanisms and Therapeutic Implications. Annual Review of Pharmacology and Toxicology. 2007;47(1):593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- Lévi F, Okyar A, et al. Circadian Timing in Cancer Treatments. Annual Review of Pharmacology and Toxicology. 2010;50(1):377–421. doi: 10.1146/annurev.pharmtox.48.113006.094626. [DOI] [PubMed] [Google Scholar]
- Li H, Satinoff EVELYN. Changes in circadian rhythms of body temperature and sleep in old rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1995;269(1):R208–R214. doi: 10.1152/ajpregu.1995.269.1.R208. [DOI] [PubMed] [Google Scholar]
- Li XM, Delaunay F, Dulong S, Claustrat B, Zampera S, Fujii Y, Lévi F. Cancer inhibition through circadian reprogramming of tumor transcriptome with meal timing. Cancer research. 2010;70(8):3351–3360. doi: 10.1158/0008-5472.CAN-09-4235. [DOI] [PubMed] [Google Scholar]
- Loudon Andrew SI. Circadian Biology: A 2.5 Billion Year Old Clock. Current Biology. 2012;22(14):R570–R571. doi: 10.1016/j.cub.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, et al. Effect of Lesions of the Ventrolateral Preoptic Nucleus on NREM and REM Sleep. The Journal of Neuroscience. 2000;20(10):3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang Y-H, et al. Contrasting Effects of Ibotenate Lesions of the Paraventricular Nucleus and Subparaventricular Zone on Sleep–Wake Cycle and Temperature Regulation. The Journal of Neuroscience. 2001;21(13):4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bjorkum AA, et al. Selective Activation of the Extended Ventrolateral Preoptic Nucleus during Rapid Eye Movement Sleep. The Journal of Neuroscience. 2002;22(11):4568–4576. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luboshitzky R, et al. Relationship between rapid eye movement sleep and testosterone secretion in normal men. Journal of andrology. 1999;20(6):731. [PubMed] [Google Scholar]
- Lucassen EA, Rother KI, Cizza G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Annals of the New York Academy of Sciences. 2012;1264(1):110–134. doi: 10.1111/j.1749-6632.2012.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MM. Arginine vasopressin and vasoactive intestinal polypeptide fibers make appositions with gonadotropin-releasing hormone and estrogen receptor cells in the diurnal rodent Arvicanthis niloticus. Brain Research. 2005;1049(2):156–164. doi: 10.1016/j.brainres.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Ramanathan C, et al. Daily rhythms and sex differences in vasoactive intestinal polypeptide, VIPR2 receptor and arginine vasopressin mRNA in the suprachiasmatic nucleus of a diurnal rodent, Arvicanthis niloticus. European Journal of Neuroscience. 2009;30(8):1537–1543. doi: 10.1111/j.1460-9568.2009.06936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Genetic variation in the stress response: susceptibility to experimental allergic encephalomyelitis and implications for human inflammatory disease. Immunology today. 1991;12(2):57–60. doi: 10.1016/0167-5699(91)90158-P. [DOI] [PubMed] [Google Scholar]
- Manber R, et al. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–555. [PubMed] [Google Scholar]
- Manber R, Baker FC, Gress JL. Sex differences in sleep and sleep disorders: a focus on women's sleep. Int J Sleep Disorders. 2006;1:7–15. [Google Scholar]
- Maywood ES, Chesham JE, et al. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proceedings of the National Academy of Sciences. 2011;108(34):14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAbee MD, DonCarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in the developing male rat forebrain. Endocrinology. 1999;140(8):3674–3681. doi: 10.1210/endo.140.8.6901. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, et al. Sex Differences in the Brain: The Not So Inconvenient Truth. The Journal of Neuroscience. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989;12:215–220. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Meier AH. Daily Variation in Concentration of Plasma Corticosteroid in Hypophysectomized Rats. Endocrinology. 1976;98(6):1475–1479. doi: 10.1210/endo-98-6-1475. [DOI] [PubMed] [Google Scholar]
- Menna-Barreto L, Fau - Montagner H, Montagner R, Fau - Soussignan H, et al. The sleep/wake cycle in 4- to 14-month old children: general aspects and sex differences. Braz J Med Biol Res. 1989;22:103–106. 1989; [PubMed] [Google Scholar]
- Miller BH, Olson SL, et al. Circadian Clock Mutation Disrupts Estrous Cyclicity and Maintenance of Pregnancy. Current Biology. 2004;14(15):1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Olson SL, et al. Vasopressin Regulation of the Proestrous Luteinizing Hormone Surge in Wild-Type and Clock Mutant Mice. Biology of Reproduction. 2006;75(5):778–784. doi: 10.1095/biolreprod.106.052845. [DOI] [PubMed] [Google Scholar]
- Mintz EM, van den Pol AN, Casano AA, Albers HE. Distribution of hypocretin-(orexin) immunoreactivity in the central nervous system of Syrian hamsters (Mesocricetus auratus) Journal of Chemical Neuroanatomy. 2001;21(3):225–238. doi: 10.1016/s0891-0618(01)00111-9. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Maas YGH, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Medicine Reviews. 2003;7(4):321–334. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain research reviews. 2005;49(3):429–454. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annual review of neuroscience. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár CS, Kalló I, et al. Estradiol Down-Regulates RF-Amide-Related Peptide (RFRP) Expression in the Mouse Hypothalamus. Endocrinology. 2011;152(4):1684–1690. doi: 10.1210/en.2010-1418. [DOI] [PubMed] [Google Scholar]
- Mong JA, Baker FC, et al. Sleep, Rhythms, and the Endocrine Brain: Influence of Sex and Gonadal Hormones. Journal of Neuroscience. 2011;31(45):16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Ede MC, Czeisler CA. Mathematical modeling of circadian timing systems. New York: Raven Press; 1982. [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research. 1972;42(1):201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. The Journal of Comparative Neurology. 1972;146(1):1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- Moore RY, Danchenko RL. Paraventricular- subparaventricular hypothalamic lesions selectively affect circadian function. Chronobiology International: The Journal of Biological & Medical Rhythm Research. 2002;19(2):345. doi: 10.1081/cbi-120002876. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Nakagawa H, et al. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scandinavian journal of work, environment & health. 2005;31(3):179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- Morin LP, Cummings LA. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiology & Behavior. 1981;26(5):825–838. doi: 10.1016/0031-9384(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Morin LP, Shivers KY, et al. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience. 2006;137(4):1285–1297. doi: 10.1016/j.neuroscience.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Morin LP. Neuroanatomy of the extended circadian rhythm system. Experimental Neurology. 2012 doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Aeschbach D, et al. Circadian system, sleep and endocrinology. Molecular and Cellular Endocrinology. 2012;349(1):91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munaut C, Lambert V, et al. Presence of oestrogen receptor type beta in human retina. British Journal of Ophthalmology. 2001;85(7):877–882. doi: 10.1136/bjo.85.7.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends in Endocrinology & Metabolism. 2010;21(5):277–286. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E, Loveland KA, et al. Gender-related physiologic differences in human neonates and the greater vulnerability of males to developmental brain disorders. The journal of gender-specific medicine : JGSM : the official journal of the Partnership for Women's Health at Columbia. 2001;4(1):41–49. [PubMed] [Google Scholar]
- Nakamura TJ, Sellix MT, Menaker M, Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. American Journal of Physiology -Endocrinology And Metabolism. 2008;295(5):E1025–E1031. doi: 10.1152/ajpendo.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW. The circadian clock mutation alters sleep homeostasis in the mouse. The Journal of Neuroscience. 2000;20(21):8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niijima A, Nagai K, et al. Effects of light stimulation on the activity of the autonomic nerves in anesthetized rats. Physiology & Behavior. 1993;54(3):555–561. doi: 10.1016/0031-9384(93)90249-f. [DOI] [PubMed] [Google Scholar]
- Novak CM, Smale L, et al. Fos expression in the sleep-active cell group of the ventrolateral preoptic area in the diurnal murid rodent, Arvicanthis niloticus. Brain Research. 1999;818(2):375–382. doi: 10.1016/s0006-8993(98)01319-5. [DOI] [PubMed] [Google Scholar]
- Novak CM, Nunez AA. A sparse projection from the suprachiasmatic nucleus to the sleep active ventrolateral preoptic area in the rat. Neuroreport. 2000;11(1):93–94. doi: 10.1097/00001756-200001170-00019. [DOI] [PubMed] [Google Scholar]
- O'Hare MJ, Hornsby PJ. Absence of a circadian rhythm of corticosterone secretion in monolayer cultures of adult rat adrenocortical cells. Experientia. 1975;31(3):378–380. doi: 10.1007/BF01922597. [DOI] [PubMed] [Google Scholar]
- Oishi K, Amagai N, et al. Genome-wide Expression Analysis Reveals 100 Adrenal Gland-dependent Circadian Genes in the Mouse Liver. DNA Research. 2005;12(3):191–202. doi: 10.1093/dnares/dsi003. [DOI] [PubMed] [Google Scholar]
- O'Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget F-Y, Millar AJ. Circadian rhythms persist without transcription in a eukaryote. Nature. 2011a;469(7331):554–558. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011b;469(7331):498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orikasa C, Kondo Y, et al. Sexually dimorphic expression of estrogen receptor β in the anteroventral periventricular nucleus of the rat preoptic area: Implication in luteinizing hormone surge. Proceedings of the National Academy of Sciences. 2002;99(5):3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metabolism. 2006;4(2):163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ostrowski NL, Lolait SJ, et al. Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135(4):1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. The Journal of neuroscience. 2004;24(37):8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenweller JE, Meier AH. Adrenal Innervation May Be an Extrapituitary Mechanism Able to Regulate Adrenocortical Rhythmicity in Rats. Endocrinology. 1982;111(4):1334–1338. doi: 10.1210/endo-111-4-1334. [DOI] [PubMed] [Google Scholar]
- Palm C, Gross P. V2-vasopressin receptor antagonists—mechanism of effect and clinical implications in hyponatraemia. Nephrology Dialysis Transplantation. 1999;14(11):2559–2562. doi: 10.1093/ndt/14.11.2559. [DOI] [PubMed] [Google Scholar]
- Pan J-J, Qu H-Q, Rentfro A, McCormick JB, Fisher-Hoch SP, Fallon MB. Prevalence of Metabolic Syndrome and Risks of Abnormal Serum Alanine Aminotransferase in Hispanics: A Population-Based Study. PLoS ONE. 2011;6(6):e21515. doi: 10.1371/journal.pone.0021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Paul KN, Turek FW, et al. Influence of sex on sleep regulatory mechanisms. Journal of women's health (2002) 2008;17(7):1201–1208. doi: 10.1089/jwh.2008.0841. [DOI] [PubMed] [Google Scholar]
- Paul KN, Dugovic C, et al. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29(9):1211–1223. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, et al. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. The Journal of Neuroscience. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW. Uptake of 3H-Estradiol by the Female Rat Brain. An Autoradiographic Study. Endocrinology. 1968;82(6):1149–1155. doi: 10.1210/endo-82-6-1149. [DOI] [PubMed] [Google Scholar]
- Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction. 2008;135(4):559–568. doi: 10.1530/REP-07-0434. [DOI] [PubMed] [Google Scholar]
- Pilorz V, Steinlechner S, Oster H. Age and oestrus cycle-related changes in glucocorticoid excretion and wheel-running activity in female mice carrying mutations in the circadian clock genes< i>Per1</i>and< i>Per2</i>. Physiology & Behavior. 2009;96(1):57–63. doi: 10.1016/j.physbeh.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Poling MC, Kim J, et al. Development, Sex Steroid Regulation, and Phenotypic Characterization of RFamide-Related Peptide (Rfrp) Gene Expression and RFamide Receptors in the Mouse Hypothalamus. Endocrinology. 2012;153(4):1827–1840. doi: 10.1210/en.2011-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston EK, Gu G, Simerly RB. Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience. 2004;123(3):793–803. doi: 10.1016/j.neuroscience.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Ralph M, Foster R, et al. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Raps D, Barthe PL, et al. Plasma and adrenal corticosterone levels during the different phases of the sexual cycle in normal female rats. Experientia. 1971;27(3):339–340. doi: 10.1007/BF02138184. [DOI] [PubMed] [Google Scholar]
- Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O'Neill JS, Hastings MH. Circadian orchestration of the hepatic proteome. Current Biology. 2006;16(11):1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Artman HG, et al. Vasopressin exhibits a rhythmic daily pattern in cerebrospinal fluid but not in blood. Science. 1981;213(4513):1256–1257. doi: 10.1126/science.7268432. [DOI] [PubMed] [Google Scholar]
- Rihel J, Schier AF. Sites of action of sleep and wake drugs: insights from model organisms. Current Opinion in Neurobiology. doi: 10.1016/j.conb.2013.04.010. (0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JL, Clifton DK, et al. Circadian Regulation of Kiss1 Neurons: Implications for Timing the Preovulatory Gonadotropin-Releasing Hormone/Luteinizing Hormone Surge. Endocrinology. 2009;150(8):3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Fox TO, et al. Sex differences in the shape of the sexually dimorphic nucleus of the preoptic area and suprachiasmatic nucleus of the rat: 3-D computer reconstructions and morphometrics. Brain Research. 1986;371(2):380–384. doi: 10.1016/0006-8993(86)90380-x. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, et al. A marker for the end of adolescence. Current Biology. 2004;14(24):R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. Epidemiology of the human circadian clock. Sleep medicine reviews. 2007;11(6):429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Rood BD, Stott RT, et al. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. Journal of Comparative Neurology. 2013;521(10):2321–2358. doi: 10.1002/cne.23288. [DOI] [PubMed] [Google Scholar]
- Roselli CE. Sex differences in androgen receptors and aromatase activity in micro dissected regions of the rat brain. Endocrinology. 1991;128(3):1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhythms. Brain research reviews. 2009;61(2):281–306. doi: 10.1016/j.brainresrev.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM. Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neuroscience & Biobehavioral Reviews. 2010;34(8):1249–1255. doi: 10.1016/j.neubiorev.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Ruggiero L, Silver R. Encyclopedia of Animal Behavior. Oxford: Academic Press; 2010. Circadian and Circannual Rhythms and Hormones; pp. 274–280. In D. B. Editors-in-Chief: Michael & M. Janice (Eds.), [Google Scholar]
- Sack RL, Lewy AJ. Melatonin as a Chronobiotic: Treatment of Circadian Desynchrony in Night Workers and the Blind. Journal of Biological Rhythms. 1997;12(6):595–603. doi: 10.1177/074873049701200615. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32(12):1602. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage D, Maurel D, Bosler O. Corticosterone-dependent driving influence of the suprachiasmatic nucleus on adrenal sensitivity to ACTH. American Journal of Physiology-Endocrinology And Metabolism. 2002;282(2):E458–E465. doi: 10.1152/ajpendo.00287.2001. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, et al. The sleep switch: hypothalamic control of sleep and wakefulness. Trends in Neurosciences. 2001;24(12):726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, et al. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Saper CB. The neurobiology of sleep. Continuum (Minneapolis, Minn.) 2013;19(1 sleep disorders):19–31. doi: 10.1212/01.CON.0000427215.07715.73. [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Valatx J-L, et al. Role of the Lateral Preoptic Area in Sleep-Related Erectile Mechanisms and Sleep Generation in the Rat. The Journal of Neuroscience. 2000;20(17):6640–6647. doi: 10.1523/JNEUROSCI.20-17-06640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff I, Regestein Q, Tulchinsky D, Ryan KJ. Effects of estrogens on sleep and psychological state of hypogonadal women. JAMA: the journal of the American Medical Association. 1979;242(22):2405–2407. [PubMed] [Google Scholar]
- Schwartz W, Reppert S. Neural regulation of the circadian vasopressin rhythm in cerebrospinal fluid: a pre-eminent role for the suprachiasmatic nuclei. The Journal of Neuroscience. 1985;5(10):2771–2778. doi: 10.1523/JNEUROSCI.05-10-02771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Robbins RJ. Aging and Hypothalamic-Pituitary-Adrenal Response to Challenge in Humans. Endocrine Reviews. 1994;15(2):233–260. doi: 10.1210/edrv-15-2-233. [DOI] [PubMed] [Google Scholar]
- Sellix MT, Menaker M. Circadian clocks in the ovary. Trends in Endocrinology & Metabolism. 2010;21(10):628–636. doi: 10.1016/j.tem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43(3):313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Komm B, et al. The distribution of estrogen receptor-β mRNA in the rat hypothalamus. Steroids. 1996;61(12):678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, Shinoda K. Expression of estrogen receptors (α, β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neuroscience research. 2004;49(2):185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Kawano J, et al. Expression of estrogen receptors (α,β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neuroscience Research. 2004;49(2):185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, et al. Activation of Ventrolateral Preoptic Neurons During Sleep. Science. 1996;271(5246):216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, et al. Innervation of Histaminergic Tuberomammillary Neurons by GABAergic and Galaninergic Neurons in the Ventrolateral Preoptic Nucleus of the Rat. The Journal of Neuroscience. 1998;18(12):4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheward WJ, Naylor E, Knowles-Barley S, Armstrong JD, Brooker GA, Seckl JR, Harmar AJ. Circadian control of mouse heart rate and blood pressure by the suprachiasmatic nuclei: behavioral effects are more significant than direct outputs. PLoS One. 2010;5(3):e9783. doi: 10.1371/journal.pone.0009783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Honma S, et al. Circadian rhythms in the release of vasoactive intestinal polypeptide and arginine-vasopressin in organotypic slice culture of rat suprachiasmatic nucleus. Neuroscience Letters. 1994;170(1):183–186. doi: 10.1016/0304-3940(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Xu M, Winston EM, Shiromani SN, Gerashchenko D, Weaver DR. Sleep rhythmicity and homeostasis in mice with targeted disruption of mPeriod genes. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2004;287(1):R47–R57. doi: 10.1152/ajpregu.00138.2004. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Bushnell CD, et al. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 1992;131(1):381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, et al. Comparative distribution of estrogen receptor- α and -β mRNA in the rat central nervous system. The Journal of Comparative Neurology. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. The Journal of Comparative Neurology. 2001;436(1):64–81. [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annual Review of Psychology. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382(6594):810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Silver R, et al. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in mouse SCN. Neuroreport. 1999;10(15):3165–3174. doi: 10.1097/00001756-199910190-00008. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, et al. Influence of Perinatal Androgen on the Sexually Dimorphic Distribution of Tyrosine Hydroxylase-Immunoreactive Cells and Fibers in the Anteroventral Periventricular Nucleus of the Rat. Neuroendocrinology. 1985;40(6):501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Chang C, Muramatsu M. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. Journal of Comparative Neurology. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proceedings of the National Academy of Sciences. 1997;94(25):14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behavioural brain research. 1998;92(2):195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual review of neuroscience. 2002;25(1):507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired on hormones: endocrine regulation of hypothalamic development. Current Opinion in Neurobiology. 2005;15(1):81–85. doi: 10.1016/j.conb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 2008;40(6):501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Smarr BL, Morris E, et al. The Dorsomedial Suprachiasmatic Nucleus Times Circadian Expression of Kiss1 and the Luteinizing Hormone Surge. Endocrinology. 2012;153(6):2839–2850. doi: 10.1210/en.2011-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Jennes L, et al. Localization of the VIP2 Receptor Protein on GnRH Neurons in the Female Rat. Endocrinology. 2000;141(11):4317–4320. doi: 10.1210/endo.141.11.7876. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, et al. Kiss1 Neurons in the Forebrain as Central Processors for Generating the Preovulatory Luteinizing Hormone Surge. The Journal of Neuroscience. 2006;26(25):6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian Rhythms in Drinking Behavior and Locomotor Activity of Rats Are Eliminated by Hypothalamic Lesions. Proceedings of the National Academy of Sciences. 1972;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker RE, Morairty S, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behavioural Brain Research. 2000;115(2):183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- Sulzman F, Ellman D, et al. Neurospora circadian rhythms in space: a reexamination of the endogenous-exogenous question. Science. 1984;225(4658):232–234. doi: 10.1126/science.11540800. [DOI] [PubMed] [Google Scholar]
- Sumida H, Nishizuka M, Kano Y, Arai Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neuroscience letters. 1993;151(1):41–44. doi: 10.1016/0304-3940(93)90040-r. [DOI] [PubMed] [Google Scholar]
- Sun ZS, Albrecht U, et al. RIGUI, a Putative Mammalian Ortholog of the Drosophila period Gene. Cell. 1997;90(6):1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- Sun X, Whitefield S, et al. Electrophysiological analysis of suprachiasmatic nucleus projections to the ventrolateral preoptic area in the rat. European Journal of Neuroscience. 2001;14(8):1257–1274. doi: 10.1046/j.0953-816x.2001.0001755.x. [DOI] [PubMed] [Google Scholar]
- Suntsova N, Szymusiak R, et al. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. The Journal of Physiology. 2002;543(2):665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymusiak R, Alam N, et al. Sleep–waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Research. 1998;803(1–2):178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature Reviews Genetics. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei H, Okamura H, et al. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389(6650):512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- Thordstein M, Löfgren N, et al. Sex differences in electrocortical activity in human neonates. Neuroreport. 2006;17(11):1165–1168. doi: 10.1097/01.wnr.0000227978.98389.43. [DOI] [PubMed] [Google Scholar]
- Tonsfeldt KJ, Chappell PE. Clocks on top: the role of the circadian clock in the hypothalamic and pituitary regulation of endocrine physiology. Molecular and cellular endocrinology. 2012;349(1):3–12. doi: 10.1016/j.mce.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousson E, Meissl H. Suprachiasmatic Nuclei Grafts Restore the Circadian Rhythm in the Paraventricular Nucleus of the Hypothalamus. The Journal of Neuroscience. 2004;24(12):2983–2988. doi: 10.1523/JNEUROSCI.5044-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinology and metabolism clinics of North America. 1994;23(3):451. [PubMed] [Google Scholar]
- Turek FW, Dugovic C, Zee PC. Current understanding of the circadian clock and the clinical implications for neurological disorders. Archives of neurology. 2001;58(11):1781. doi: 10.1001/archneur.58.11.1781. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2006;290(4):R1128–R1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, et al. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. Journal of Clinical Endocrinology & Metabolism. 1996;81(7):2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel CJ, Reid KJ, et al. Effect of Atenolol on Nocturnal Sleep and Temperature in Young Men: Reversal by Pharmacological Doses of Melatonin. Physiology & Behavior. 1997;61(6):795–802. doi: 10.1016/s0031-9384(96)00534-3. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, et al. Lesions of the Suprachiasmatic Nucleus Indicate the Presence of a Direct Vasoactive Intestinal Polypeptide-Containing Projection to Gonadotrophin-Releasing Hormone Neurons in the Female Rat. Journal of Neuroendocrinology. 1993;5(2):137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, van Oudheusden HJ, et al. Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology. 1994;134(6):2636–2644. doi: 10.1210/endo.134.6.8194489. [DOI] [PubMed] [Google Scholar]
- van Esseveldt LE, Lehman MN, et al. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain research reviews. 2000;33(1):34–77. doi: 10.1016/s0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Ballance H, et al. Handbook of Systems Biology. San Diego: Academic Press; 2013. Chapter 21 - The Role of the Circadian System in Homeostasis; pp. 407–426. [Google Scholar]
- van Leeuwen FW, Caffe AR, et al. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Research. 1985;325(1–2):391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Verma P, Hellemans KG, Choi FY, Yu W, Weinberg J. Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiology & behavior. 2010;99(3):276–285. doi: 10.1016/j.physbeh.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129(5):2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kallo I. Oestrogen receptor α and β immunoreactive cells in the suprachiasmatic nucleus of mice: Distribution, sex differences and regulation by gonadal hormones. Journal of neuroendocrinology. 2008;20(11):1270–1277. doi: 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- Vida B, Deli L, et al. Evidence for Suprachiasmatic Vasopressin Neurones Innervating Kisspeptin Neurones in the Rostral Periventricular Area of the Mouse Brain: Regulation by Oestrogen. Journal of Neuroendocrinology. 2010;22(9):1032–1039. doi: 10.1111/j.1365-2826.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- Viola AU, Archer SN, James LM, Groeger JA, Lo JCY, Skene DJ, Schantz Mv, Dijk DJ. PER3 polymorphism predicts sleep structure and waking performance. Curr. Biol. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Langub MC, Jr, et al. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Research. 1995;689(2):254–264. doi: 10.1016/0006-8993(95)00548-5. [DOI] [PubMed] [Google Scholar]
- Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: A complexity beyond negative feedback. Frontiers in Neuroendocrinology. 2005;26(3–4):109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Webb IC, Patton DF, Hamson DK, Mistlberger RE. Neural correlates of arousal-induced circadian clock resetting: hypocretin/orexin and the intergeniculate leaflet. European Journal of Neuroscience. 2008;27(4):828–835. doi: 10.1111/j.1460-9568.2008.06074.x. [DOI] [PubMed] [Google Scholar]
- Weich S, Sloggett A, Lewis G. Social roles and the gender difference in rates of the common mental disorders in Britain: a 7-year, population-based cohort study. Psychological medicine. 2001;31(6):1055–1064. doi: 10.1017/s0033291701004263. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, et al. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14(4):697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annual review of physiology. 2010;72(1):551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. Social and psychosocial factors in autoimmune disease. In: Ader A, Felten DL, Cohen N, editors. Psychoneuroimmunology. San Diego: Academic Press; 1991. pp. 955–1012. [Google Scholar]
- Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J. Cortisol secretion is inhibited during sleep in normal man. Journal of Clinical Endocrinology & Metabolism. 1983;56(2):352–358. doi: 10.1210/jcem-56-2-352. [DOI] [PubMed] [Google Scholar]
- Wickham LA, Gao JP, et al. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmologica Scandinavica. 2000;78(2):146–153. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- Williams WP, Jarjisian SG, et al. Circadian Control of Kisspeptin and a Gated GnRH Response Mediate the Preovulatory Luteinizing Hormone Surge. Endocrinology. 2011;152(2):595–606. doi: 10.1210/en.2010-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WP, III, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Frontiers in endocrinology. 2012;3 doi: 10.3389/fendo.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A. Chronobiology and mood disorders. Dialogues in clinical neuroscience. 2003;5(4):315–325. doi: 10.31887/DCNS.2003.5.4/awirzjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor J, O’Hara B, Terao A, Selby C, Kilduff T, Sancar A, Edgar D, Franken P. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Fu YH. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature. 2005;434(7033):640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Kerbeshian MC, Hocker CG, Block GD, Menaker M. Rhythmic Properties of the Hamster Suprachiasmatic NucleusIn Vivo. The Journal of Neuroscience. 1998;18(24):10709–10723. doi: 10.1523/JNEUROSCI.18-24-10709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, et al. Effects of aging on central and peripheral mammalian clocks. Proceedings of the National Academy of Sciences. 2002;99(16):10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. European Journal of Neuroscience. 2002;16(8):1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R. Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. European Journal of Neuroscience. 2004;19(4):1105–1109. doi: 10.1111/j.1460-9568.2004.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lamia KA, Evans RM. Nuclear Receptors, Metabolism, and the Circadian Clock. Cold Spring Harbor Symposia on Quantitative Biology. 2007;72:387–394. doi: 10.1101/sqb.2007.72.058. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu A, et al. The Role of mPer2 Clock Gene in Glucocorticoid and Feeding Rhythms. Endocrinology. 2009;150(5):2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-α immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. The Journal of Comparative Neurology. 1997;389(1):81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Yoo S-H, Yamazaki S, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(15):5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA. Glucocorticoid cascade hypothesis revisited: role of gonadal steroids. Depression. 1995a;3(1–2):20–27. [Google Scholar]
- Young EA. The role of gonadal steroids in hypothalamic-pituitary-adrenal axis regulation. Critical reviews in neurobiology. 1995b;9(4):371. [PubMed] [Google Scholar]
- Zeitzer JM, Ayas NT, et al. Absence of Detectable Melatonin and Preservation of Cortisol and Thyrotropin Rhythms in Tetraplegia. Journal of Clinical Endocrinology & Metabolism. 2000;85(6):2189–2196. doi: 10.1210/jcem.85.6.6647. [DOI] [PubMed] [Google Scholar]
- Zhang J-Q, Cai W-Q, et al. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Research. 2002;935(1– 2):73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wing Y. Sex differences in insomnia: a meta-analysis. SLEEP-NEW YORK THEN WESTCHESTER- 2006;29(1):85. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nature Reviews Molecular Cell Biology. 2010;11(11):764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- Zheng B, Larkin DW, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400(6740):169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105(5):683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134(6):2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]
- Zhou J-N, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer's disease. Neurobiology of Aging. 1995;16(4):571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465(7299):690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]