Abstract
Many antidepressant drugs interact with σ receptors and accumulating evidence suggests that these proteins mediate antidepressant-like effects in animals and humans. σ Receptors are localized in brain regions affected in depression, further strengthening the hypothesis that they represent logical drug development targets. In this study, two novel σ receptor agonists (UMB23, UMB82) were evaluated for antidepressant-like activity in mice. First, radioligand binding studies confirmed that the novel compounds had preferential affinity for σ receptors. Second, the forced swim test, a well established animal model for screening potential antidepressant drugs, showed that both compounds dose-dependently reduced immobility time. The σ receptor antagonist BD1047 attenuated the antidepressant-like effects of UMB23 and UMB82. Third, locomotor activity suggested that the effects of UMB23 and UMB82 in the forced swim test were not due to non-specific motor activating effects. Together, the data provide further evidence that σ receptor agonists represent a possible new class of antidepressant medication.
Keywords: Forced swim test, Depression, Sigma receptor, UMB23, UMB82, Mouse
1. Introduction
Depression affects about 20% of Americans and it is the leading cause of disability in the United States (Kessler et al., 1994; Costello et al., 2002; Nestler et al., 2002). Despite recent advances in antidepressant therapy, it still takes several weeks before existing medications are effective and about 30% of individuals do not respond (Stahl, 2000; Nestler et al., 2002). Given that depression is one of the most common and costly brain diseases, there is a need to develop more effective medications to treat this devastating disorder. Among the novel medication development options being pursued, σ receptor agonists are a rational and viable avenue for further investigation.
In 1976, the existence of σ receptors was postulated by the group of Martin, and these receptors were recognized as the site through which the psychotomimetic effects of SKF-10,047 (N-allylnormetazocine) were mediated (Martin et al., 1976). σ Receptors were originally thought to be a type of opiate receptor. However, this was soon refuted because many effects of the prototypical σ ligand SKF-10,047 could not be reversed by opiate antagonists (Iwamoto, 1981; Vaupel, 1983; Young and Khazan, 1984). In the1980s, binding studies by Tsung-Ping Su indicated that σ receptors were new and previously uncharacterized receptors (Su, 1981; Su, 1982). When the receptor was cloned in the 1990s, the results confirmed that its sequence differed from all other known proteins (Hanner et al., 1996). The endogenous ligand for σ receptors has yet to be conclusively determined, although the identity of the receptor protein is now well established.
Biochemical and pharmacological studies indicate the existence of multiple σ receptor subtypes, and the best characterized are the σ-1 and σ-2 sites. The σ-1 subtype has been cloned from various species, such as rat, mouse, and human (Hanner et al., 1996; Seth et al., 1997; Pan et al., 1998; Seth et al., 1998; Mei and Pasternak, 2001). It is a 233 amino acid protein with two putative transmembrane spanning regions (Jbilo et al., 1997; Aydar et al., 2002). In contrast to σ-1 receptors, the σ-2 receptor has not yet been cloned. It is thought to be a 25-29 kDa protein that is enriched in lipid rafts, whereby it affects calcium signaling via sphingolipid products (Crawford et al., 2002; Gebreselassie and Bowen, 2004).
σ Receptors distribute extensively in the nervous system. Within the brain, σ receptors concentrate in the hippocampus, frontal cortex, and olfactory bulb (Gundlach et al., 1986; Mclean and Weber, 1988; Bouchard and Quirion, 1997; Alonso et al., 2000), which is consistent with a role for these receptors in depression.
In 1996, Narita and co-workers demonstrated that many antidepressant drugs have significant affinity for σ receptors, particularly the σ-1 subtype (Narita et al., 1996). Prior to then, many investigators confirmed the ability of select antidepressant drugs, including selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors, and tricyclic antidepressants (TCAs), to interact with σ receptors (Schmidt et al., 1989; Itzhak and Kassim, 1990; Weber et al., 1986).
The ability of antidepressant drugs to interact with σ receptors appears functionally relevant because several studies have shown that activation of σ receptors can produce antidepressant-like effects in animals and humans. Using the forced swim and tail suspension tests, earlier investigators have demonstrated that σ receptors agonists such as igmesine, SA4503 (1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine), (+)-SKF-10,047, di-otolylguanidine (DTG) and (+)-pentazocine produce antidepressant-like actions in rodents (Matsuno et al., 1996; Skuza and Rogoz, 1997, 2002; Ukai et la., 1998; Urani et al., 2001). A role for σ receptors in these effects was further confirmed by the ability of σ receptor antagonists such as BD1047 (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine) and NE-100 (N,N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]ethylamine) to attenuate the antidepressant-like actions of the agonists (Okuyama et al., 1993; Matsumoto et al., 1995; Kobayashi et al., 1996; Urani et al., 2001). In humans, the high affinity σ receptor agonist igmesine hydrochloride yielded promising results in Phase II clinical trials. In a six week, multi-center, double-blind, placebo-controlled study in 348 patients with major depression (DSM-IV), igmesine was as effective as the SSRI fluoxetine (Pharmaprojects, 2004). The available clinical and preclinical data therefore indicate that σ receptor agonists possess antidepressant potential.
In the present study, we examined the antidepressant-like effects of two novel σ receptor agonists (UMB23 and UMB82) in the forced swim test, the most widely used animal model for screening potential antidepressant drugs (Cryan et al., 2002; Nestler et al., 2002; Cryan and Mombereau, 2004). The novel compounds represent analogs of BD1008 (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine) and AC927 (N-phenethylpiperidine oxalate), highly selective σ receptor ligands (de Costa et al., 1992; Maeda et al., 2002). From these two lead compounds, a number of selective σ receptor antagonists were developed (Maeda et al., 2002; Matsumoto et al., 2003, 2004). However, initial functional characterizations suggested that the compounds, UMB23 (1-(3-phenylpropyl)piperidine oxalate) and UMB82 (2-(3,4-dichlorophenyl)-N-ethyl-N-(2-piperidin-1-ylethyl)ethanamine oxalate) acted as agonists, instead of antagonists, at σ receptors. To further characterize these putative agonists, the binding affinities of the compounds for σ receptors and monoamine transporters were measured to determine whether their antidepressant-like actions were likely mediated through σ receptors. In addition, the effects of these compounds on locomotor activity were measured to determine whether stimulant effects could account for their apparent antidepressant-like actions. The antidepressant drugs desipramine (TCA) and fluvoxamine (SSRI) were used as positive controls for antidepressant-like actions. The selective, well established σ receptor agonist DTG was also used as a positive control.
2. Experimental procedures
2.1. Animals
Male, Swiss Webster mice (24-28 g; Harlan, Dublin, VA; Indianapolis, IN) and Sprague Dawley rats (200-250g; Harlan, Indianapolis, IN) were group housed with food and water available ad libitum. All procedures involving animals were performed as approved by the Institutional Animal Care and Use Committee at the university where the studies were conducted.
2.2. Drugs and chemicals
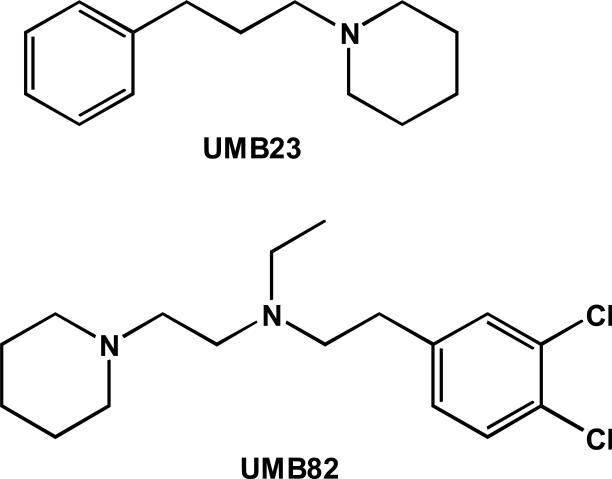
UMB23 and UMB82 (Fig. 1) were synthesized by Dr. Andrew Coop (University of Maryland, Baltimore, MD). UMB23 was prepared as previously described (Maeda et al., 2002). UMB82 was synthesized following similar procedures to BD1008 and its analogs (de Costa et al., 1992; Matsumoto et al., 2004, 2006). The compounds were converted to oxalate salts, and spectral analysis was consistent with their assigned structures. CHN analysis was also satisfactory (±0.4%).
Figure 1.
Structures of the novel σ receptor ligands: UMB23 (1-(3-phenylpropyl)piperidine oxalate) and UMB82 (2-(3,4-dichlorophenyl)-N-ethyl-N-(2-piperidin-1-ylethyl)ethanamine oxalate).
DTG acetate was crystallized from the free base (Aldrich, Milwaukee, WI) by Dr. Christopher McCurdy (University of Mississippi, University, MS). Fluvoxamine and BD1047 were purchased from Tocris (Ballwin, MO). Desipramine was obtained from Sigma (St. Louis, MO). Radioligands were purchased from Perkin Elmer (Boston, MA). All other chemicals and reagents were procured from standard commercial suppliers (Sigma, St. Louis, MO).
2.3. Radioligand binding assays
The affinities of the compounds for σ receptors and monoamine transporters were measured in rat brain homogenates using procedures previously described (Matsumoto et al., 2004). Competition binding assays were run for the two established σ receptor subtypes, σ-1 and σ-2. Briefly, whole brain homogenates (400-500 μg protein) were incubated with 5 nM [3H](+)-pentazocine to label σ-1 receptors, or 3 nM [3H]DTG in the presence of 300 nM (+)-pentazocine to label σ-2 receptors. Non-specific binding was determined in the presence of 10 μM haloperidol. Twelve concentrations of test compound (0.05-10,000 nM) were incubated for 120 min at 25° C to evaluate their ability to displace the binding of each radioligand.
Since antidepressant drugs interact with monoamine transporters, the affinities of the novel compounds for serotonin, norepinephrine, and dopamine transporters were also measured (Matsumoto et al., 2004). Briefly, serotonin transporters were assayed in 1.5 mg wet weight brainstem homogenates using 0.2 nM [3H]paroxetine; non-specific binding was determined in the presence of 1.5 μM imipramine. Norepinephrine transporters were assayed in 8 mg wet weight cerebral cortical homogenates using 0.5 nM [3H]nisoxetine; non-specific binding was determined with 4 μM desipramine. Dopamine transporters were assayed in 2 mg wet weight striatal homogenates using 0.5 nM [3H]WIN35,428 [(-)-3β-(4-fluorophenyl)tropane-2β-carboxylic acid methyl ester]; non-specific binding was determined in the presence of 50 μM cocaine. Twelve concentrations of test compound were incubated for 90 min at 25° C for the serotonin transporter assays, 60 min at 4° C for the norepinephrine transporter assays, or 120 min at 4° C for the dopamine transporter assays to evaluate their ability to displace the binding of each radioligand.
In addition, the affinities of the compounds for a select group of neurotransmitter receptors were examined (Matsumoto et al., 2004). These receptors were chosen for testing because historic σ receptor compounds, which were non-selective, tended to have affinities for these sites. Briefly, dopamine (D2) receptors were labeled with 5 nM [3H](-)-sulpiride; non-specific binding was determined with 1 μM haloperidol. Opioid receptors were labeled with 2 nM [3H]bremazocine; non-specific binding was determined with 10 μM levollorphan. N-methyl-D-aspartate (NMDA) receptors were labeled with 5 nM [3H]TCP (1-[1-(2-thienyl)cyclohexyl]piperidine); non-specific binding was determined with 10 μM cyclazocine. Serotonin (5-HT2) receptors were labeled with 2 nM [3H]ketanserin; non-specific binding was determined with 1 μM mianserin. The incubations were performed for 60 min at 25° C for the dopamine and opioid receptor assays, 30 min at 37° C for the serotonin receptor assays, and for 60 min at 4° C for the NMDA receptor assays.
The assays were terminated by adding ice-cold buffer and vacuum filtration through glass fiber filters. Counts were extracted from the filters using Ecoscint (National Diagnostics, Manville, NJ) for at least 8 h prior to counting.
2.4. Forced swim test
Mice were acclimated to the testing room for at least 30 min. To evaluate whether the compounds produced antidepressant-like actions, the mice were injected (i.p.) with one of the following treatments: desipramine (10-20 mg/kg, N=30), fluvoxamine (0.1-10 mg/kg, N=30), DTG (5-10 mg/kg, N=26), UMB23 (2.5-20 mg/kg, N=45), UMB82 (10-40 mg/kg, N=40), or saline (N=10). Thirty min after receiving their treatments, the mice were placed in individual cylinders of water (10 cm deep) for 6 min and videotaped. Swimming, climbing, and immobility time during minutes 2-6 were quantified as dependent measures. Swimming was operationally defined as horizontal movements, with paw treading, across the swim chamber that included crossing into another quadrant. Climbing was characterized by upward directed movements of the forepaws along the sides of the swim chamber. Immobility was defined as no activity other than what was required to keep the animal's head above the water.
To confirm that the antidepressant-like effects of the novel compounds were mediated through σ receptors, another two groups of animals were pretreated (i.p.) with the well established σ receptor antagonist BD1047 (5 mg/kg, N=30). After 15 min, the mice were treated with saline (N=10), UMB23 (20 mg/kg, N=10) or UMB82 (40 mg/kg, N=10). Following the treatments, the animals were evaluated in the forced swim test as described above.
2.5. Locomotor activity
Mice were acclimated to the chambers of an automated activity monitoring system (San Diego Instruments, San Diego, CA) for 30 min. The animals were injected (i.p.) with saline (N=8) or a compound shown to be effective in the forced swim test: despiramine (10, 20 mg/kg, N=16), fluvoxamine (1, 10 mg/kg, N=16), DTG (5, 10 mg/kg, N=16), UMB23 (5, 10, 20 mg/kg, N=24), UMB82 (20, 40 mg/kg, N=15). Locomotor activity was quantified for the next 40 min as the number of disruptions made by the animals in the 16 × 16 photobeam grid surrounding each testing enclosure. The 40 min testing period takes into account the 30 min pretreatment and subsequent 6 min testing time during which the forced swim test was conducted, relative to the time of the injection. Data were evaluated during the 4 min corresponding to the data collection period of the forced swim tests.
2.6. Data analysis
Data from the receptor binding studies were evaluated using GraphPad Prism (San Diego, CA) to calculate IC50 values. Apparent Ki values were then calculated using the Cheng-Prusoff equation and Kd values that were determined in earlier saturation assays. Data from the behavioral studies were evaluated using one-way analysis of variance (ANOVA), followed when applicable by Dunnett's tests for post-hoc assessments (GraphPad Prism, San Diego, CA). P<0.05 was considered statistically significant.
3. Results
3.1. Binding assays
The novel compounds tested herein had nanomolar affinities for σ receptors (Table 1). In contrast, they had micromolar to negligible affinities for monoamine transporters and a select group of neurotransmitter receptors (Table 1).
Table 1.
Binding affinities (in nM) for novel σ receptor compounds.
| UMB23 | UMB82 | |
|---|---|---|
| Sigma receptors | ||
| Sigma-1 | 41 ± 4 | 3 ± 0.2 |
| Sigma-2 | 32 ± 1 | 29 ± 4 |
| Monoamine transporters | ||
| Serotonin transporter | >10,000 | 965 ±53 |
| Norepinephrine transporter | >10,000 | 1147± 87 |
| Dopamine transporter | >10,000 | 844 ± 57 |
| Other receptors | ||
| Opiate | >10,000 | >10,000 |
| NMDA | >10,000 | >10,000 |
| Serotonin | >10,000 | 7132 ± 717 |
| Dopamine | >10,000 | 924 ±110 |
The numbers in the table represent Ki values in nM.
3.2. Forced swim test
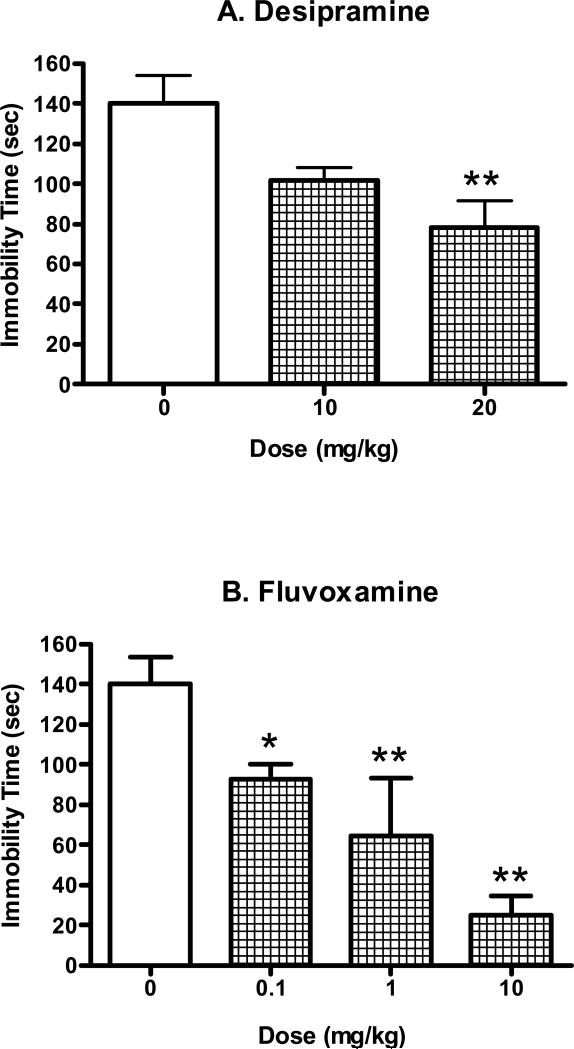
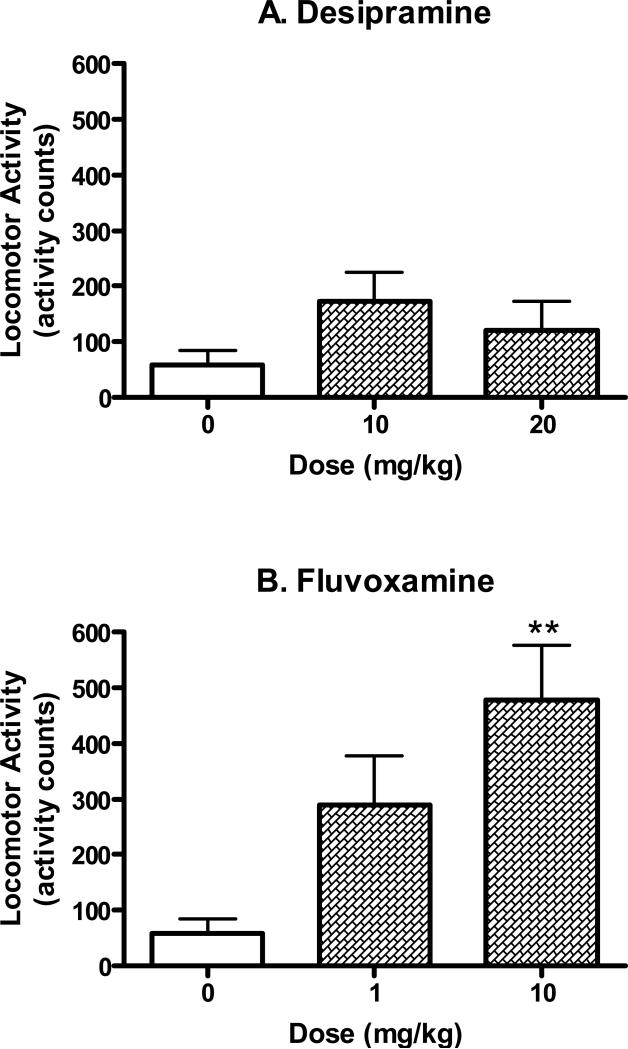
Desipramine and fluvoxamine, clinically used antidepressants, served as positive controls and were shown to dose-dependently reduce immobility time in the forced swim test (Fig. 2). Analysis of variance confirmed that there was a significant difference between the animals treated with various doses of desipramine (F(2,27)=7.06, P<0.005). Post-hoc testing using Dunnett's tests revealed that the 20 mg/kg dose of desipramine differed significantly from the saline control (q=3.72, P<0.01). Similarly, fluvoxamine produced a dose-dependent change in immobility time (F(3,26)=10.57, P<0.001). Subsequent analysis using post-hoc Dunnett's tests indicated that the following doses of fluvoxamine significantly reduced immobility time relative to the saline control: 1 mg/kg (q=3.49, P<0.01) and 10 mg/kg (q=5.33, P<0.01).
Figure 2.
Antidepressant-like effects of desipramine (10-20 mg/kg, i.p.) and fluvoxamine (0.1-10 mg/kg, i.p.) in the mouse forced swim test. The tests started 30 min after the injection of saline or drugs. Data are expressed as mean ± S.E.M. (N=5-10/group). *P<0.05, **P<0.01.
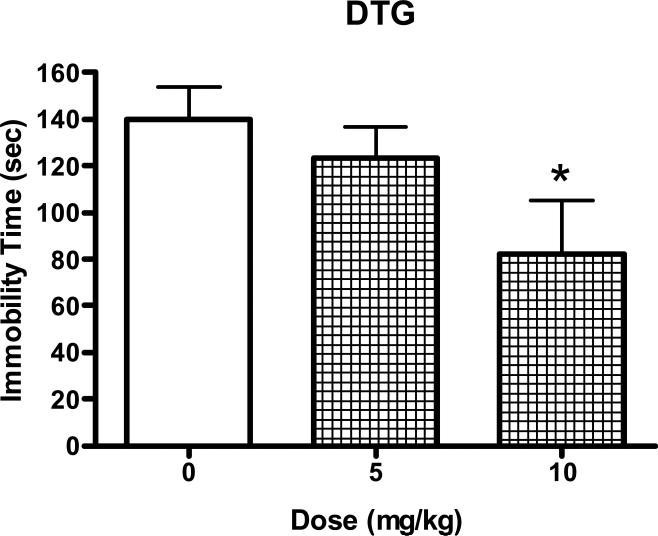
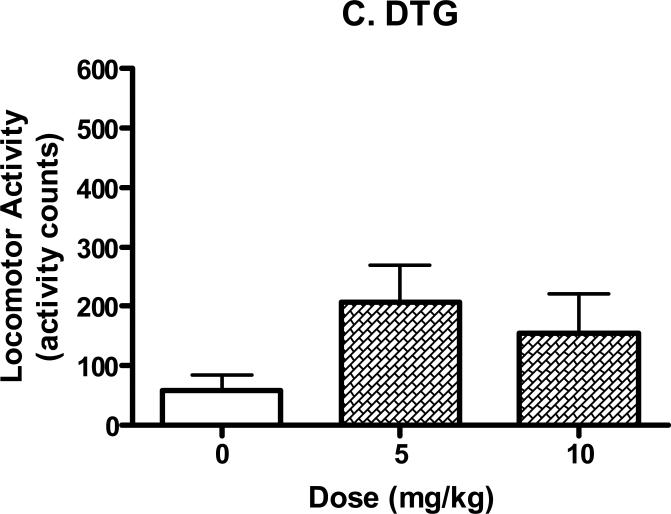
The well studied σ receptor agonist DTG also significantly reduced immobility time in the forced swim test (Fig. 3; F(2,23)=4.25, P<0.05). Post-hoc Dunnett's tests confirmed that the 10 mg/kg dose of DTG produced effects that differed significantly from the controls (q=2.91, P<0.05).
Figure 3.
Antidepressant-like effects of DTG (5-10 mg/kg, i.p) in the mouse forced swim test. The tests started 30 min after the injection of saline or drug. Data are expressed as mean ± S.E.M. (N=7-10/group). *P<0.05.
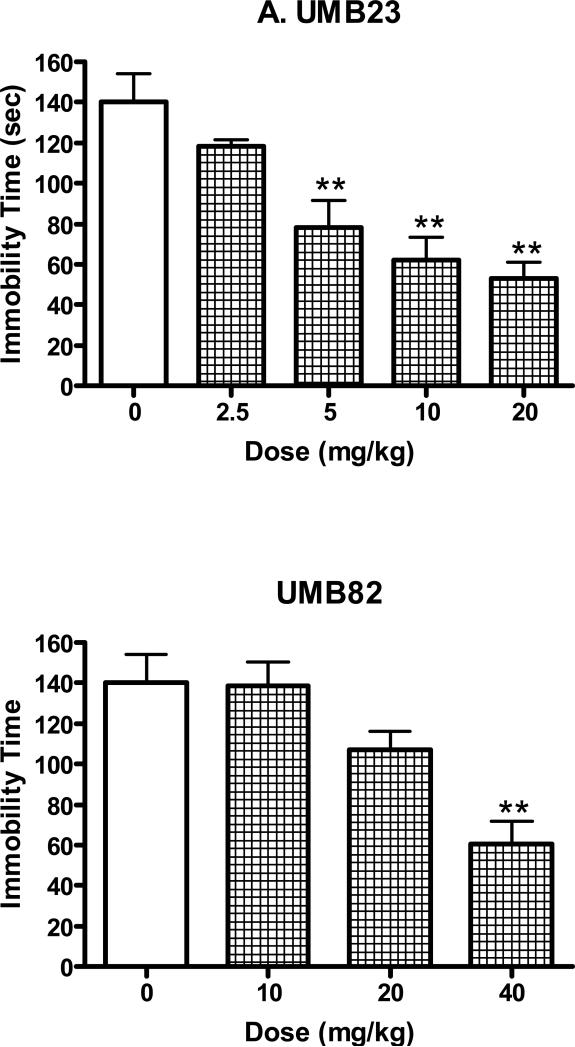
Similar to DTG, the novel σ receptor agonists UMB23 and UMB82 produced antidepressant-like actions by reducing immobility time in the forced swim test (Fig. 4). Analysis of variance demonstrated that the dose-dependent changes produced by UMB23 were statistically significant (F(4,40)=14.22, P<0.0001). Post-hoc analysis using Dunnett's tests revealed that the following doses of UMB23 differed significantly from the saline control group: 5 mg/kg (q=3.64, P<0.01), 10 mg/kg (q=5.59, P<0.01), and 20 mg/kg (q=6.26, P<0.01). Analysis of variance also showed that the changes in immobility time produced by UMB82 were statistically significant (F(3,36)=10.28, P<0.0001). Post-hoc Dunnett’s tests confirmed that the reductions produced by the 40 mg/kg dose of UMB82 were significantly different from controls (q=4.85, P<0.01).
Figure 4.
Antidepressant-like effects of UMB23 (2.5-20 mg/kg, i.p.) and UMB82 (10-40 mg/kg, i.p.) in the mouse forced swim test. The tests started 30 min after the injection of saline or drugs. Data are expressed as mean ± S.E.M. (N=5-10/group). **P<0.01.
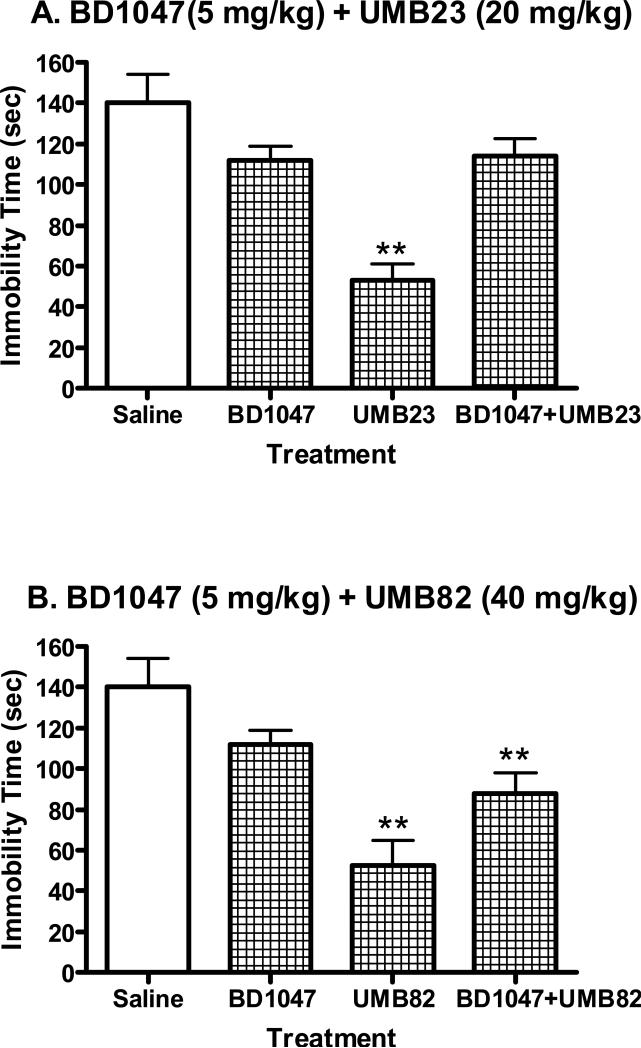
Pretreatment with the σ receptor antagonist BD1047 attenuated the antidepressant-like effects of UMB23 and UMB82 (Fig. 5). Alone, BD1047 had no significant effects on immobility time (n.s.). Analysis of variance confirmed a significant difference between the various treatment groups in the antagonism study for UMB23 (F(3,36)=14.11, P<0.0001). Post- hoc tests confirmed that only the UMB23 treatment group differed significantly from saline (q=6.29, P<0.01). Similarly, there was a significant difference between the treatment groups in the antagonism study for UMB82 (F(3,36)=9.70, P<0.0001). Post-hoc tests indicated that both the UMB82 treatment group (q=5.22, P<0.01) and the BD1047+UMB82 group (q=3.20, P<0.01) differed significantly from saline, with the reduction in immobility time induced by UMB82 noticeably attenuated by pretreatment with BD1047 (Fig. 5).
Figure 5.
Antidepressant-like effects of BD1047 (5 mg/kg, i.p.) + UMB23 (20 mg/kg, i.p.) and BD1047 (5 mg/kg, i.p.) + UMB82 (40 mg/kg, i.p.) in the mouse forced swim test. The mice were injected with saline or drugs 15 min after the BD1047 pretreatment. The tests started 15 min after the injection of saline or drugs. Data are expressed as mean ± S.E.M. (N=10/group). *P<0.05, **P<0.01.
3.3. Locomotor activity
Fig. 6 and 7 summarize the effects of the antidepressant drugs and σ receptor agonists on locomotor activity. There was a significant increase in locomotor activity produced by fluvoxamine (Fig. 6; F(2,21)=7.43, P<0.005). Post-hoc Dunnett's tests confirmed that the 10 mg/kg dose of fluvoxamine produced effects that differed significantly from the saline controls (q=3.85, P<0.01). However, the lower dose (1 mg/kg) of fluvoxamine which produced antidepressant-like effects in the forced swim test did not significantly alter locomotor activity (q=2.13, n.s.).
Figure 6.
Locomotor activity of desipramine (10-20 mg/kg, i.p.), fluvoxamine (1-10 mg/kg, i.p.) and DTG (5-10 mg/kg, i.p.). Data was evaluated during the 4 min corresponding to the data collection period of the forced swim tests. Data are expressed as mean ± S.E.M. (N=8/group). **P<0.01.
Figure 7.
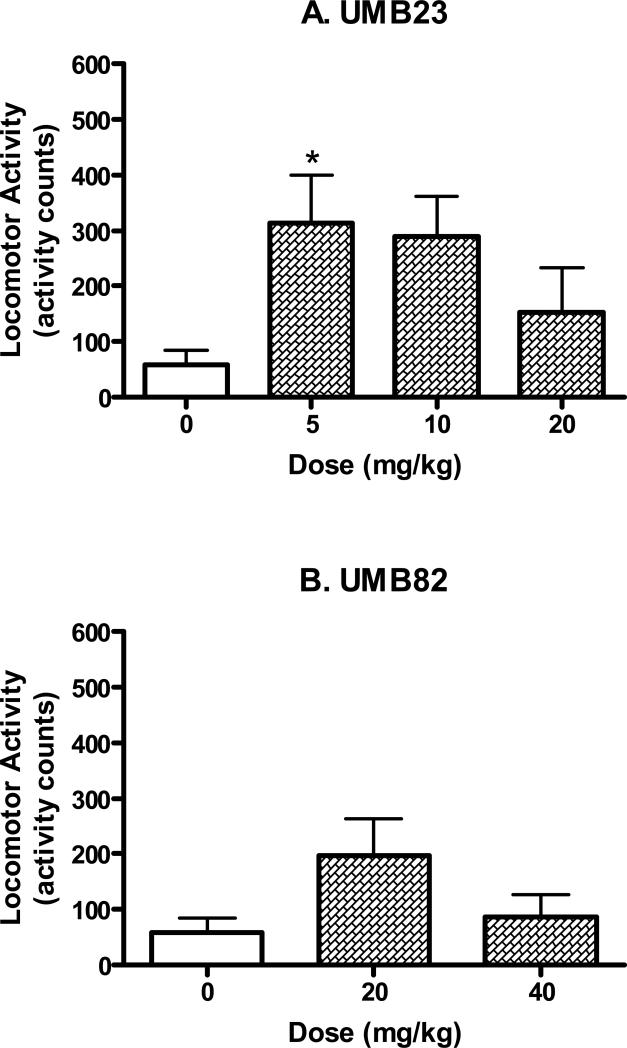
Locomotor activity of UMB23 (5-20 mg/kg, i.p.) and UMB82 (20-40 mg/kg, i.p.). Data was evaluated during the 4 min corresponding to the data collection period of the forced swim tests. Data are expressed as mean ± S.E.M. (N=7-8/group). *P<0.05, **P<0.01.
None of the other tested compounds had significant effects on locomotor activity at the doses and time points studied (Fig. 7). Analysis of variance confirmed no significant effects for desipramine (F(2,21)=1.58, n.s.), DTG (F(2,21)=1.90, n.s.), UMB23 (F(3,28)=2.85, n.s.), and UMB82 (F(2,20)=2.44, n.s.).
4. Discussion
In the present study, we introduced two new putative σ receptor agonists, UMB23 and UMB82. Both novel compounds had preferential affinity for σ receptors compared to monoamine transporters and a select group of neurotransmitter receptors. UMB23 in particular displayed a high degree of selectivity, exhibiting nanomolar affinities for each of the σ receptor subtypes, and no measurable affinity for all of the other radioligand binding sites tested in this study. The poor affinities of the compounds for serotonin, norepinephrine, and dopamine transporters, relative to σ receptors, thus increased the likelihood that the observed antidepressant-like effects were mediated through σ receptors. Among the other binding sites that were studied (opioid, NMDA, 5-HT2, D2 receptors), all were potential targets or modulators of antidepressant drugs (Jutkiewicz, 2006; Nestler and Carlezon, 2005; Schechter et al., 2005). Thus, it was significant that the two novel compounds (UMB23, UMB82) displayed little to no binding to these sites. The radioligand binding profiles of UMB23 and UMB82 are thus highly favorable for selectively probing actions that are mediated through σ receptors.
In the forced swim test, the novel σ receptor agonists significantly attenuated immobility time, thus exhibiting a pattern similar to well established σ receptor agonists and known antidepressant medications. The well established σ receptor antagonist BD1047 attenuated the actions of the novel compounds, strengthening the likelihood that the antidepressant-like actions of UMB23 and UMB82 were mediated through σ receptors. The ability of BD1047 to attenuate the antidepressant-like actions of UMB23 in the forced swim test was statistically significant, while its effects against UMB82 were noticeable, but not quite significant. One possible explanation for this subtle difference is that UMB23 is a highly selective compound for σ receptors, and all of its observed antidepressant-like actions were most likely mediated through these proteins. By contrast, UMB82 had weak affinity for a number of non-σ binding sites which could have contributed a little to its antidepressant-like actions. Therefore, BD1047 was able to attenuate most, but not all, of the antidepressant-like effects of UMB82 in the forced swim test. This probably contributed to the increased variability in the response of UMB82, as compared to UMB23, in the antagonism portions of the study.
Since reductions in immobility time were accompanied by increases in swimming or climbing behaviors, it was important to confirm that the effects of UMB23 and UMB82 in the forced swim test could not be explained by non-specific stimulant effects of the test compounds; such an adverse interpretation would be reflected in the locomotor studies by a concomitant increase in locomotor activity at doses that produced antidepressant-like responses. Similar to the well established TCA despiramine and the σ receptor agonist DTG, the novel compound UMB82 had no significant effects on locomotor activity at doses that produced antidepressant-like actions, thus confirming that its effects in the forced swim test could not be explained by non-specific stimulant effects. UMB23, the other novel compound, produced an increase in locomotor activity at some doses, but there was no consistent relationship between the doses that produced antidepressant-like effects and locomotor stimulant effects, indicating that changes in locomotor behavior were not responsible for apparent antidepressant-like actions. For the clinically used SSRI fluvoxamine, there was a trend for locomotor stimulatory effects to accompany antidepressant-like actions, although a mismatch between the two effects could be detected at lower doses. Therefore, for all of the tested compounds, including UMB23 and UMB82, reductions in immobility time in the forced swim test were not accompanied by parallel increases in locomotor activity, providing further evidence of antidepressant-like actions of the compounds.
Although accumulated preclinical and clinical data suggest that targeting σ receptors alone is sufficient for producing antidepressant-like actions (Matsuno et al., 1996; Pharmaprojects, 2004; Urani et al., 2001), it should be noted that activation of σ receptors is not required for producing antidepressant-like actions. Desipramine and paroxetine are two antidepressant drugs that have weak affinity for σ receptors (Narita et al., 1996) and still produce therapeutic effects in humans. Therefore, σ receptors most likely represent an initial target, similarly to monoamine transporters, in a cascade of events that ultimately convey antidepressant actions.
Although the downstream mechanisms underlying the ability of σ receptor agonists to produce antidepressant-like actions have yet to be fully elucidated, evidence has accumulated to show that σ receptor agonists stimulate a variety of neural adaptations in the central nervous system that are relevant to antidepressant actions. For example, antidepressant medications produce an enhancement of serotonin neurotransmission following chronic administration (Blier et al., 1994; Owens, 1996). Antidepressant drugs, such as SSRIs and monoamine oxidase inhibitors, cause initial reductions in the firing of serotonergic neurons with acute administration, which recover with long term administration of these drugs (Aghajanian, 1978; Nicholson et al., 1982; Chaput et al., 1986; Beique et al., 2000). The recovery in firing of serotonergic neurons is thought to develop after desensitization of autoreceptors, and has been proposed as a mechanism that explains the three to four week delay in clinical efficacy of antidepressant medications (Blier et al., 1994). Following three weeks of treatment, the σ–1 receptor agonist (+)-pentazocine actually increased the firing of serotonergic neurons, leading to an enhancement of serotonergic neurotransmission (Bermack and Debonnel, 2001). This enhancement was observed as early as after two days of treatment (Bermack and Debonnel, 2001), as compared to SSRI- and monoamine oxidase inhibitor-induced changes that took several weeks to emerge. The (+)-pentazocine-induced effects appeared to be mediated through σ receptors because they could be prevented by co-administration of the σ receptor antagonist, NE-100 (Bermack and Debonnel, 2001). This σ receptor-induced enhancement in serotonin neuronal function has been proposed to predict a more rapid onset of antidepressant efficacy as compared to existing medications, and may also contribute to added efficacy (Bermack and Debonnel, 2001).
In recent years, the importance of antidepressant-induced structural changes in the central nervous system, such as neuronal sprouting, has also gained increasing attention (Manji et al., 2003) and recent evidence confirms that σ receptor agonists possess this property. Su and coworkers reported that the σ-active antidepressant drugs, fluvoxamine and imipramine, enhance nerve growth factor (NGF)-induced neurite sprouting in PC12 cells (Takebayashi et al., 2002). This effect was reproduced by the selective σ-1 receptor agonist (+)-pentazocine (Takebayashi et al., 2002), suggesting that activation of σ receptors, particularly the σ-1 subtype, can produce critical structural modifications that are thought to impart antidepressant efficacy. The role of σ receptors, particularly the σ-1 subtype in these effects was validated by the ability of the selective antagonist NE-100 to attenuate the actions of (+)-pentazocine, imipramine and fluvoxamine (Takebayashi et al., 2002). Moreover, overexpression of σ-1 receptors in PC12 cells enhanced NGF-induced neurite sprouting similar to treatments with σ-active agonists and antidepressant drugs, while treatment with an antisense oligonucleotide against σ-1 receptors reduced the NGF-induced effects (Takebayashi et al., 2002).
There is also abundant evidence that brain-derived neurotrophic factor (BDNF) contributes to the actions of antidepressant drugs (Malberg and Blendy, 2005; Tardito et al., 2006). The significance of σ receptors in these effects was recently demonstrated, particularly with regard to the role of BDNF signaling in glutamate release (Yagasaki et al., 2006). In these studies, chronic treatment with the σ-active antidepressant drugs, imipramine and fluvoxamine, potentiated BDNF signaling and BDNF-induced glutamate release (Yagasaki et al., 2006). The effects of BDNF on signaling and the subsequent release of glutamate were both significantly attenuated by the σ receptor antagonist BD1047 (Yagasaki et al., 2006). Overexpression of σ-1 receptors also enhanced the BDNF-induced effects (Yagasaki et al., 2006), providing further evidence for the involvement of σ receptors in these effects.
Together, the body of literature demonstrates that σ receptor agonists produce antidepressant-like effects under a variety of preclinical and clinical conditions. Future studies to identify σ receptor agonist-induced downstream events, particularly those that converge with classical antidepressant drugs, will be important for fully understanding the mechanisms through which σ receptor agonists impart their antidepressant-like actions.
Acknowledgements
We appreciate the expert technical assistance of Dr. Buddy Pouw (University of Oklahoma Health Sciences Center, Oklahoma City, OK) in performing the radioligand binding studies. Ms. Tara White (University of Oklahoma Health Sciences Center, Oklahoma City, OK) assisted with the forced swim tests. We also thank Dr. Christopher McCurdy (University of Mississippi, University, MS) for providing the DTG acetate. This work was supported in part by the following grants: DA11979, DA13978. AC is the recipient of a K02 award (DA19634).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK. In: Essays in neurochemistry and neuropharmacology. Youdim MBH, Lovenberg W, Sharman DF, Lagnado JR, editors. Vol. 3. John Wiley and Sons; New York: 1978. pp. 1–32. [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma-1 receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The σ receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Beique JC, Blier P, de Montigny C, Debonnel G. Potentiation by (−)pindolol of the activation of postsynaptic 5-HT1A receptors induced by venlafaxine. Neuropsychopharmacology. 2000;23:294–306. doi: 10.1016/S0893-133X(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Bermack JE, Debonnel G. Modulation of serotonergic neurotransmission by short- and long-term treatments with sigma ligands. Br. J. Pharmacol. 2001;134:691–699. doi: 10.1038/sj.bjp.0704294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Quirion R. [3H] 1,3-Di(2-tolyl) guanidine and [3H] (+) pentazocine binding sites in the rat brain: Autoradiographic visualization of the putative sigma-1 and sigma-2 receptor subtypes. Neuroscience. 1997;76:467–477. doi: 10.1016/s0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: Electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, Biederman J, Goldsmith HH, Kaufman J, Lewinsohn PM, Hellander M, Hoagwood K, Koretz DS, Nelson CA, Leckman JF. Development and natural history of mood disorders. Biol. Psychiatry. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- Crawford KW, Coop A, Bowen WD. σ2 Receptors regulate changes in sphingolipid levels in breast tumor cells. Eur. J. Pharmacol. 2002;443:207–209. doi: 10.1016/s0014-2999(02)01581-9. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol. Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- De Costa BR, Radesca L, Di Paolo L, Bowen WD. Synthesis, characterization, and biological evaluation of a novel class of N-(arylethyl)-N-alkyl-2-(1-pyrrolidinyl)ethylamines: structural requirements and binding affinity at the sigma receptor. J. Med. Chem. 1992;35:38–47. doi: 10.1021/jm00079a004. [DOI] [PubMed] [Google Scholar]
- Gebreselassie D, Bowen WD. σ2 Receptors are specially localized to lipid rafts in rat liver membranes. Eur. J. Pharmacol. 2004;493:19–28. doi: 10.1016/j.ejphar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J. Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian σ1-binding site. Proc. Natl. Acads Sci. USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su T-P. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. USA. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Kassim CO. Clorgyline displays high affinity for σ-binding sites in C57BL/6 mouse brain. Eur. J. Pharmacol. 1990;176:107–108. doi: 10.1016/0014-2999(90)90139-w. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET. Locomotor activity and antinociception after putative mu, kappa and sigma opioid receptor agonists in the rat : influence of dopaminergic agonists and antagonists. J. Pharmacol. Exp. Ther. 1981;217:451–460. [PubMed] [Google Scholar]
- Jbilo O, Vidal H, Paul R, De Nys N, Bensaid M, Silve S, Carayon P, Davi D, Galiegue S, Bourrie B, Guillemot J-C, Ferrara P, Loison G, Maffrand J-P, Le Fur G, Casellas P. Purification and characterization of the human SR 31747A-binding protein. A nuclear membrane protein related to yeast sterol isomerase. J. Biol. Chem. 1997;272:27107–27115. doi: 10.1074/jbc.272.43.27107. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM. The antidepressant-like effects of delta-opioid receptor agonists. Mol. Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H, Kendler KS. Lifetime and12-month prevalence of DMS-III-R psychiatric disorders in the United States. Results from the national cormorbidity survey. Arch. Gen. Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Matsuno K, Nakata K, Mita S. Enhancement of acetylcholine release by SA4503, a novel σ1 receptor agonist, in the rat brain. J. Pharmacol. Exp. Ther. 1996;279:106–113. [PubMed] [Google Scholar]
- Maeda DY, Williams W, Kin WE, Thatcher LN, Bowen WD, Coop A. N-Arylalkylpiperidines as high affinity sigma-1 and sigma-2 receptor ligands: phenylpropylamines as potential leads for sigma-2 agents. Bioorg. Med. Chem. Lett. 2002;12:497–500. doi: 10.1016/s0960-894x(01)00788-0. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. Trends Pharmacol. Sci. 2005;26:631–638. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payan JL, Denicoff K, Gray NA, Zarate CA, Jr., Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol. Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel σ receptor ligands: Antidystonic effects in rats suggest σ receptor antagonism. Eur. J. Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Gilmore DL, Pouw B, Bowen WD, Williams W, Kausar A, Coop A. Novel analogs of the σ receptor ligand BD1008 attenuate cocaine-induced toxicity in mice. Eur. J. Pharmacol. 2004;492:21–26. doi: 10.1016/j.ejphar.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. σ Receptors: potential medications development target for anti-cocaine agents. Eur. J. Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Nakazawa M, Okamoto K, Kawashima Y, Mita S. Binding properties of SA4503, a novel and selective σ1 receptor agonist. Eur. J. Pharmacol. 1996;306:271–279. doi: 10.1016/0014-2999(96)00201-4. [DOI] [PubMed] [Google Scholar]
- Mclean S, Weber E. Autoradiographic visualization of haloperidol-sensitive sigma receptors in guinea-pig brain. Neuroscience. 1988;25:259–269. doi: 10.1016/0306-4522(88)90024-3. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. Molecular cloning and pharmacological characterization of the rat σ1 receptor. Biochem. Pharmacol. 2001;62:349–355. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Narita N, Hashimoto K, Tomitaka S, Minabe Y. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur. J. Pharmacol. 1996;307:117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol. Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Nicholson VJ, Wieringa JH, Van Delft AM. Comparative pharmacology of mianserin, its main metabolites and 6-azamianserin. Naunyn Schmiedeberg Arch. Pharmacol. 1982;319:48–53. doi: 10.1007/BF00491478. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Imagawa Y, Ogawa S, Araki H, Ajima A, Tanaka M, Muramatsu M, Nakazato A, Yamaguchi K, Yoshida M, Otomo S. NE-100, a novel σ receptor ligand: In vivo tests. Life Sci. 1993;53:PL285–PL290. doi: 10.1016/0024-3205(93)90588-t. [DOI] [PubMed] [Google Scholar]
- Owens MJ. Molecular and cellular mechanisms of antidepressant drugs. Depress. Anxiety. 1996;4:153–159. doi: 10.1002/(SICI)1520-6394(1996)4:4<153::AID-DA1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Pan Y-X, Mei J, Xu J, Wan B-L, Zuckerman A, Pasternak GW. Cloning and characterization of a mouse σ1 receptor. J. Neurochem. 1998;70:2279–2285. doi: 10.1046/j.1471-4159.1998.70062279.x. [DOI] [PubMed] [Google Scholar]
- Pharmaprojects . Igmesine hydrochloride, accession number 15962. PJB Pulications, Ltd.; 2004. [Google Scholar]
- Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, Malberg JE, Rosenzweig-Lipson S. Innovative approaches for the development of antidepressant drugs: Current and future strategies. NeuroRx. 2005;2:590–611. doi: 10.1602/neurorx.2.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Lebel L, Koe BK, Seeger T, Heym J. Sertraline potently displaces (+)-[3H]3-PPP binding to σ-sites in rat brain. Eur. J. Pharmacol. 1989;165:335–336. doi: 10.1016/0014-2999(89)90734-6. [DOI] [PubMed] [Google Scholar]
- Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 σ receptor. Biochem. Biophys. Res. Commun. 1997;241:535–40. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a σ receptor from rat brain. J. Neurochem. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- Skuza G, Rogoz Z. Effect of 1,3-di-o-tolylguanidine (DTG), rimcazole and EMD 57445, the σ receptor ligands, in the forced swimming test. Pol. J. Pharmacol. 1997;49:329–335. [PubMed] [Google Scholar]
- Skuza G, Rogoz Z. A potential antidepressant activity of SA4503, a selective sigma receptor agonist. Behav. Pharmacol. 2002;13:537–543. doi: 10.1097/00008877-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Essential psychopharmacology of depression and bipolar disorder. Cambridge University Press; New York: 2000. [Google Scholar]
- Su T-P. Psychotomimetic opioid binding: Specific binding of [3H]-SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. Eur. J. Pharmacol. 1981;75:81–82. doi: 10.1016/0014-2999(81)90352-6. [DOI] [PubMed] [Google Scholar]
- Su T-P. Evidence for sigma opioid receptor: Binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su T-P. Nerve growth factor-induced neurite sprouting in PC12cells involves sigma-1 receptors: Implications for antidepressants. J. Pharmacol. Exp. Ther. 2002;303:1227–1237. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: A critical overview. Pharmacol. Rev. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- Ukai M, Maeda H, Nanya Y, Kameyama T, Matsuno K. Beneficial effects of acute and repeated administrations of σ receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol. Biochem. Behav. 1998;61:247–252. doi: 10.1016/s0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Urani A, Roman FJ, Phan VL, Su TP, Maurice T. The antidepressant-like effect induced by σ1-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J. Pharmacol. Exp. Ther. 2001;298:1269–1279. [PubMed] [Google Scholar]
- Vaupel DB. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur. J. Pharmacol. 1983;92:269–274. doi: 10.1016/0014-2999(83)90297-2. [DOI] [PubMed] [Google Scholar]
- Weber E, Sonders M, Quarum M, McLean S, Pou S, Keana JFW. 1,3-Di(2-[5-3H]tolyl)guanidine: A selective ligand that labels σ-type receptors for psychotomimetic opiates and antipsychotic drugs. Proc. Natl. Acad. Sci. USA. 1986;83:8784–8788. doi: 10.1073/pnas.83.22.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su T-P, Kunugi H. Chronic antidepressants protentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J. Biol. Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- Young GA, Khazan N. Differential neuropharmacological effects of mu, kappa and sigma opioid agonists on cortical EEG power spectra in the rat. Stereospecificity and naloxone antagonism. Neuropharmacology. 1984;23:1161–1165. doi: 10.1016/0028-3908(84)90233-8. [DOI] [PubMed] [Google Scholar]