Abstract
Bioactive natural products and derivatives remain an enduring starting point for the discovery of new cellular targets for disease intervention and lead compounds for the development of new therapeutic agents. The former goal is accomplished through the synthesis of bioactive cellular probes from natural products enabling insights into the mechanism of action of these natural products by classical affinity chromatography or more recent proteome profiling methods. However, the direct and selective modification of native natural products for these purposes remains a challenge due to the structural complexity and the wide functional group diversity found in these natural substances. The lack of selective synthetic methods available to directly manipulate unprotected complex small molecules, in particular to perform structure-activity relationship studies and prepare appropriate cellular probes, has recently begun to be addressed benefitting from the broader emerging area of chemoselective synthetic methodology. Thus, new reagents, catalysts and reaction processes are enabling both chemo- and site-selective modifications of complex, native natural products. In this review, we describe selected recent examples of these functionalization strategies in this emerging area.
1 Introduction
“By the river on its bank…will grow all kinds of trees…and their fruit will be for food, and their leaves for healing.”
Ezekiel 47:12 (NASB, 6th century B.C.)
Throughout history, natural products and derivatives have been one of the most productive sources of small molecules for the treatment of human ailments, for probe synthesis enabling the study of biological processes, and for discovery of leads for drug discovery.1–3 These ‘privileged structures’ display high structural diversity,4 cell permeability and specificity in interactions with their cellular targets5 having been optimized to their cellular targets through >3 billion years of microevolutionary pressure in the case of natural products with bacterial origins. Importantly, the homology between cellular proteins found in niche-competing bacteria, and other natural product-producing higher organisms, and the protein targets important for human disease intervention is fortuitously sufficient to enable high intrinsic value to natural products for drug discovery. In this post-genomic era, it is estimated that only 500 of about 3,000–10,000 potential therapeutic targets have been exploited,6 offering tremendous opportunities for the discovery of new cellular targets for disease intervention and the development of new therapeutic compounds.7–9 However, to fully exploit the potential of natural products, chemical modifications are often required in order to tailor their physicochemical properties, generate derivatives for structure-activity relationship (SAR) studies, and enable synthesis of molecular probes through conjugation of reporter tags (i.e. fluorophores, biotin) which retain bioactivity. While total, semi-, or diverted total synthesis10–12 of natural products provides access to analogues and probes for cellular studies especially those not readily available from the natural product itself,13–14 the development of scaleable, efficient syntheses of natural products is often a labor, cost, and time intensive endeavor that becomes more readily justified once a promising bioactivity for a natural product has been established. However, an alternative strategy involving direct and selective modification of native natural products remains a challenge due to the structural complexity, often limited availability, and wide functional group diversity found in these natural substances. Recognizing these opportunities, chemists have begun to develop creative approaches for the direct, microscale, functionalization of complex natural products in a chemo- and site-selective manner. Accordingly, new reagents, catalysts and reaction processes are being applied to enable both chemo- and site-selective modifications of native natural products to capitalize on the inherent reactivity of functional groups or to override this reactivity by the judicious choice of reagents and reaction conditions. The interest in performing chemo- and site-selective reactions with native natural products coincides with an emerging area in synthetic methodology, namely chemoselectivity inspired by a desire to improve efficiency by minimizing the use of protecting groups and relying on the inherent reactivity of functional groups in complex molecules led recently by Baran and coworkers.15–19 Indeed, much basic information regarding chemo- and site-selectivity is being gathered through the described studies with native natural products that can be applied in other settings including total synthesis endeavors.
Recently developed chemo- and site-selective methodologies for natural products are being utilized for SAR studies and the rapid synthesis of novel natural product-derived tools for the study of basic cellular processes providing insights into their mechanism of action at the molecular level. In this Review, we describe recently developed chemo- and site-selective approaches for the direct functionalization of complex bioactive natural products which are propelling the study of these information rich small molecules for basic discoveries in cell biology, enabling full exploitation of their potential for drug discovery, and contributing to a greater understanding of the inherent reactivity of functional groups in complex settings.
2 Systematic Chemo- and Site-Selective Derivatizations of Natural Products
2.1 Site-Selective Acylations of Alcohol-Containing Natural Products
Alcohols are the most common functional group found in natural products,4 and these metabolites display a vast array of pharmacologically important activities including immunosuppressive, anticancer, antibiotic, antifungal, and antiparasitic.3 The prevalence of hydroxyl groups in natural products, likely present to improve water solubility, offer many sites for functionalization and subsequent attachment of reporter tags. However, the direct, site-selective derivatization of polyol natural products is challenging given that differentiation must typically rely on steric differences and electronic differences between two secondary alcohols, for example, are subtle. To tackle this problem, new reagents, catalysts and reaction processes are being developed to achieve chemo- and site-selectivity in this context.
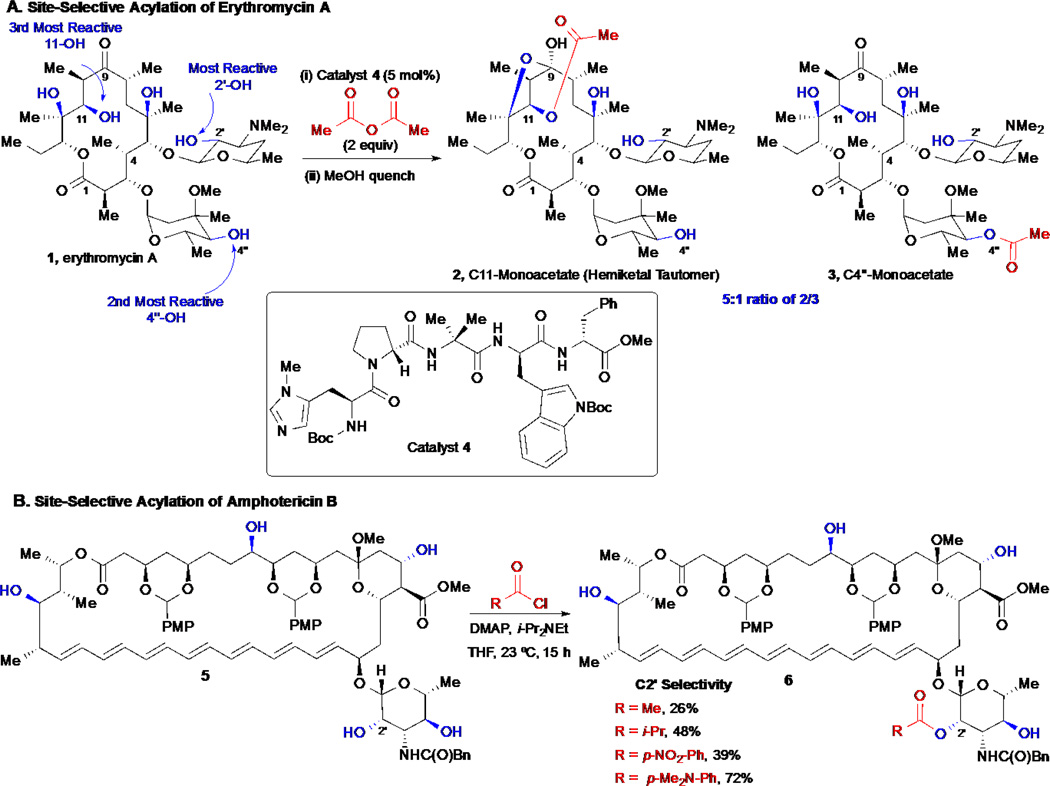
In 2006, an elegant early example of achieving chemo- and site-selectivity with complex natural products was described by Miller and co-workers, wherein a series of small peptides identified through library screening,20–22 were discovered to alter site selectivity during acylations of the macrolide antibiotic erythromycin A 1 (Scheme 1A).23 Peptide catalyst 4, enabled alteration of the intrinsic preference of the substrate towards acylation since the most reactive site was determined to be the C2’-alcohol upon acylation with an achiral promoter (i.e. 1.0 equiv Ac2O, N-methylimidazole). Employing catalyst 4 (2.0 equiv Ac2O) bearing a N-methyl histidine residue led to differential acylation of alcohols C11:C4” in a 4:1 ratio in addition to complete acylation of the most reactive C2’-alcohol. Following methanolysis of the reactive C2’ acetate, this enabled access to the C11 monoacetate 2 not readily accessible by other means. This initial report demonstrated the potential of altering site selectivity using peptide catalysts and was subsequently applied to other alcohol-containing natural products, including apoptolidin (vide infra).24
Scheme 1.
Site-selective acylation of erythromycin by S. Miller and amphotericin B by Burke.
In a different approach, Burke and co-workers developed a site-selective acylation of polyols by tuning the electronic nature of the acylating reagents and thus converting a minimally selective reaction when acetyl chloride is used, into a highly site-selective process when p-dimethylaminobenzoyl chloride was employed (Scheme 1B).25 Their hypothesis for this site selectivity was based on Hammond’s postulate which suggests that less reactive reagents should provide the best site selectivity, since the reactions proceed through more “product-like” transition states. They applied the optimized reaction conditions to the site-selective acylation of amphotericin B providing the C2’ acetylated derivative 6 (R= p-dimethylaminobenzoyl), which after subsequent global silylation of the remaining alcohols, deacetylation at the C2’-alcohol, conjugation to ergosterol and finally global desilylation, delivered an amphotericin-ergosterol conjugate. In addition, this strategy enabled deoxygenation and stereochemical inversion at the C2’-alcohol.
In 2012, Herrmann and co-workers employed small oligonucleotides as non-covalent, aptameric protecting groups for the site-selective acylation of the polyaminoglycoside antibiotic neomycin B. In this strategy, they took advantage of the inherent affinity of neomycin B to RNA aptamers to enable chemo- and site-selective monoacylation at either one or two of the six potentially reactive amines including two primary amines.26
2.2 Simultaneous Arming/SAR Studies via Rh-catalyzed O–H Insertions of Alcohol-Containing Natural Products
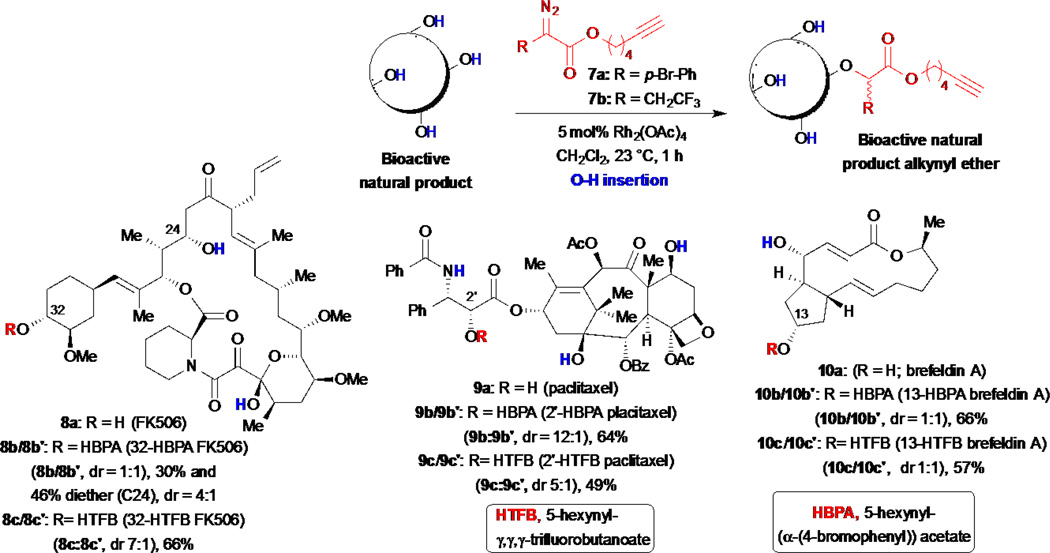
The Romo group has reported several approaches toward developing a ‘toolbox’ of synthetic strategies for the microscale derivatization of native natural products to enable simultaneous SAR studies and ‘arming’. The latter is achieved through simultaneous attachment of functional handles during the derivatization process, such as alkynes, for subsequent Sharpless-Hüisgen cycloaddition to prepare cellular probes once a tolerable site for modification has been identified. In the first report of this strategy in 2007, Romo and co-workers developed novel alkynyl diazo esters 7 for mild, direct, and tunable functionalization of alcohol containing compounds (Scheme 2).27 The strategy enables a two-step synthesis of natural product cellular probes through chemo- and site-selective Rh-catalyzed O–H insertions with alkynyl diazo esters followed by a Sharpless-Hüisgen cycloaddition with various reporter tags employing the pendant alkyne installed during the derivatization step. In their initial report, they demonstrated the ability of the alkynyl diazo ester 7a to selectively functionalize several complex natural products under mild neutral conditions, including FK506, taxol and brefeldin A with serviceable yields and chemo- and site-selectivities. It should be noted that mixtures of diastereomers are obtained ranging from 1:1 to 12:1 during O–H insertion dependent on substrate. However given that initial derivatizations are meant to establish gross structure-activity relationships, these mixtures can initially be assayed to determine if the particular site generally tolerates modification. It should be noted that generally speaking, an initial lack of site and even stereoselectivity can be desireable given that one cannot often predict which derivative will retain bioactivity. Subsequent conjugation to reporter tags for the synthesis of cellular probes was also demonstrated (vide infra). However, not surprisingly, N-H bonds also participate in these insertion reactions. Thus, for compounds that contain multiple alcohols and amines, the following order of reactivity was reported based on examples studied: 1° ROH ≈ 2° alkyl NH > 2° alkyl OH > 2° allylic OH ≥ aryl NH > phenolic OH > 3° alkyl OH > indole NH. Furthermore, as anticipated, the use of various rhodium catalysts altered site-selectivity with gibberelic acid methyl ester, however surprisingly even various achiral ligands led to differential site selectivity presumably due to the differential topology and reactivity of the derived Rh-metallocarbenoids. In a subsequent report, the same group introduced alkynyl diazo ester 7b that features a smaller steric footprint, an α-trifluoroethyl versus p-bromophenyl substituted ester, and demonstrated that otherwise identical FK506 derivatives (8b and 8c) exhibited markedly different interactions with known protein targets validating the large steric differences between these seemingly similar derivatives (vide infra).28
Scheme 2.
Romo and Liu’s simultaneous arming/SAR studies of alcohol-containing natural products by Rh(II)-catalyzed O–H insertions with diazo esters.
2.3 Simultaneous Arming/SAR Studies via Halogenation of Arene-Containing Natural Products
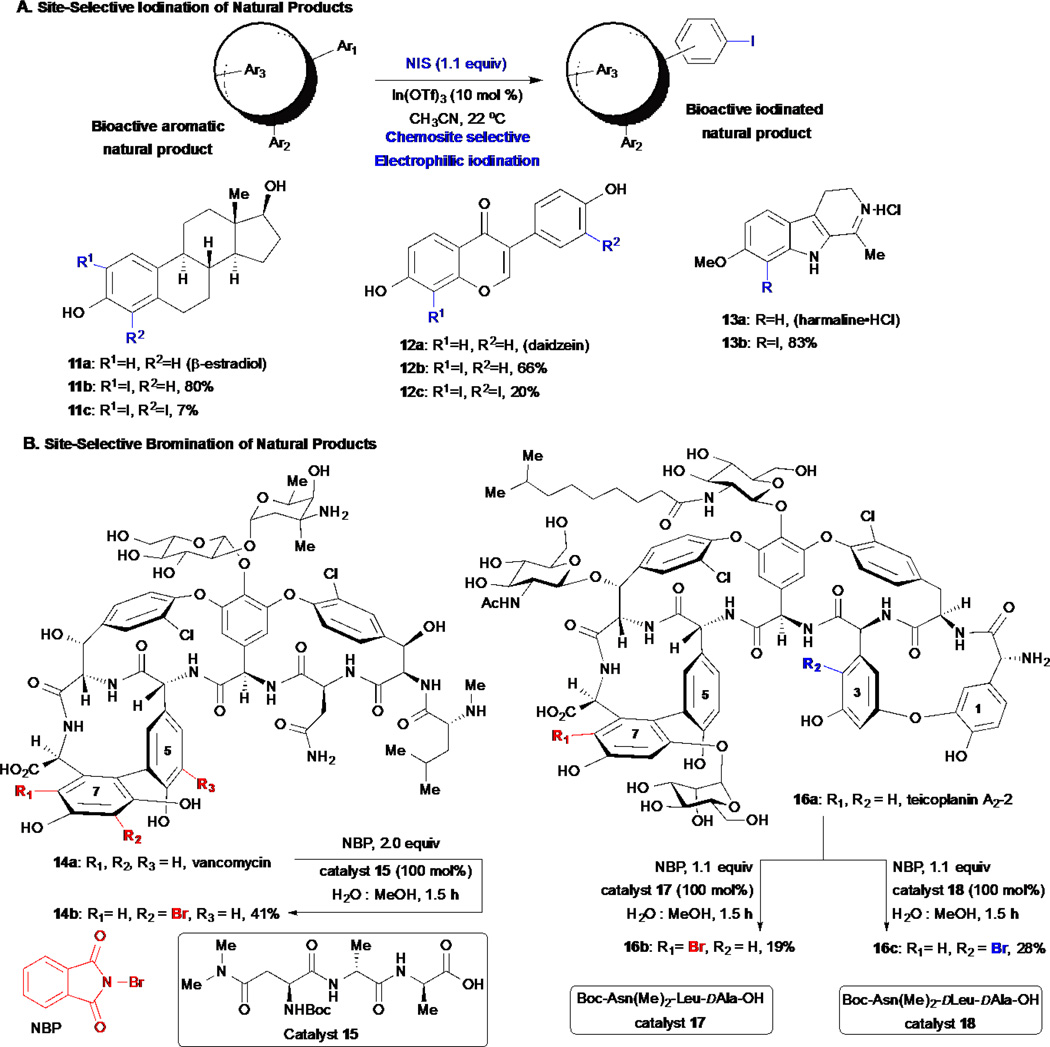
Halogenation of arene-containing natural products is an attractive option for the functionalization of natural products since the introduction of halogens offers derivatives with electronic and steric properties often markedly distinct from the parent compound e.g. fluorinated derivatives. In the case of bromo- and iodo- derivatives, and to a lesser extent chloro- derivatives, chemical handles are introduced that can be used for conjugation to reporter tags through transition metal-catalyzed cross couplings. Given that existing methods for arene iodination were typically harsh requiring highly acidic conditions, Romo and co-workers set out to develop a mild iodination procedure for arene-containing natural products. In 2010, they reported another installment of their simultaneous SAR/arming strategy by way of a chemo- and site-selective, In(OTf)3-catalyzed, electrophilic arene iodination with N-iodosuccinimide (Scheme 3A).29 This approach takes advantage of the inherent reactivity of arenes toward electrophilic iodination including control of site selectivity through electronic control. The strategy was applied to the chemo- and site-selective iodination of a diverse set of arene- and heterocyclic-containing compounds including the steroid β-estradiol 11a, the isoflavone daidzein 12a, and the psychoactive indole alkaloid harmaline 13a. They also demonstrated the ability of the resulting iodinated derivatives to participate in several Pd(0)-catalyzed cross coupling reactions, that provided access to several derivatives including biotin conjugates.
Scheme 3.
Mild halogenations by Romo and site-selective halogenations by S. Miller of arene containing-natural products.
In 2012, Miller and co-workers reported a chemo- and site-selective approach for the bromination of arene-containing molecules and its application to the generation of novel derivatives of the potent glycopeptide antibiotics vancomycin 14a and teicoplanin A2-2 16a (Scheme 3B).30–31 Guided by the known natural tendency of these glycopeptides to bind to D-Ala-D-Ala residues, the Miller group designed a family of small peptides that selectively bind to these compounds in defined ways, enabling site-selective bromination of this polyaromatic natural product with N-bromopthalimide (NBP). With catalyst 15, monobromination of phenyl ring 7 of vancomycin was achieved in a site selective manner. The control reaction with no catalyst leads to lower conversions and complex mixtures of brominated products that are difficult to separate. In a more impressive display of site selectivity, the use of peptide 17 enabled the monobromination of teicoplanin A2-2 16a at phenyl ring 7, while the use of catalyst 18 delivered monobromination at phenyl ring 3, albeit in modest yields. This elegant methodology provides direct access to novel analogs of vancomycin and derivatives that may pave the way for novel antibiotics to combat emerging vancomycin-resistant bacteria.
2.4 Simultaneous Arming/SAR Studies through Cyclopropanations of Olefin-Containing Natural Products
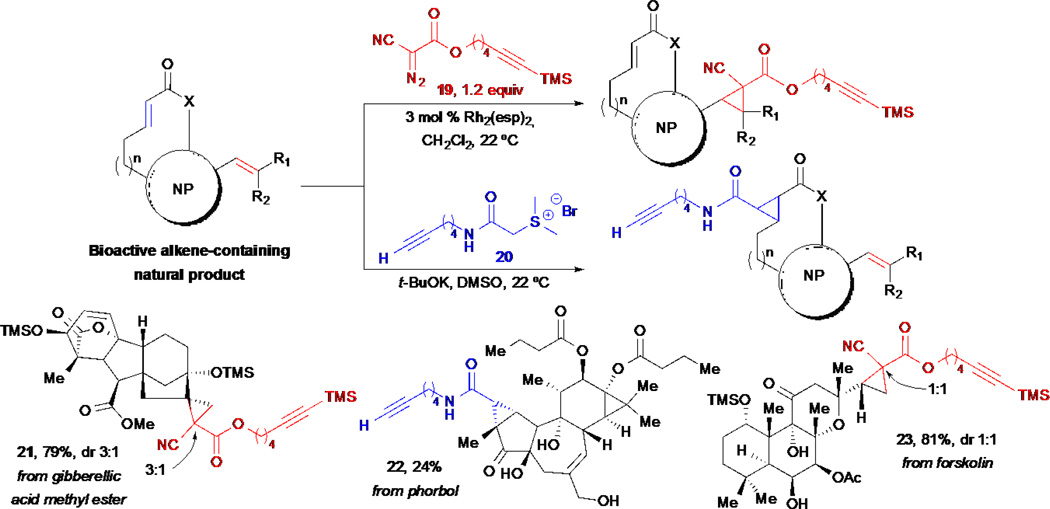
Alkenes are prevalent functional groups in bioactive natural products.4 While numerous reactions of alkenes are known, direct applications of these transformations to complex, unprotected natural products are limited. In order to exploit olefins as possible sites for simultaneous SAR/arming of alkene-containing natural products, Romo and co-workers developed alkynyl-bearing diazo reagents 19 and 20 for mild cyclopropanations of both electron-rich and electron-poor olefins, respectively (Scheme 4).32 Electron-rich, alkene-containing natural products were found to undergo chemo- and site-selective cyclopropanation reactions with TMS-alkynyl diazo ester 19. The TMS-alkyne derivative was required to avoid competing inter- and intramolecular cyclopropenations with the alkynyl group. Furthermore, for substrates containing reactive alcohol groups, temporary global silylation of reactive alcohols prior to cyclopropanation was required to prevent competitive OH insertions.
Scheme 4.
Romo’s simultaneous SAR/arming studies through cyclopropanation of olefin-containing natural products.
These alkynylated derivatives were then conjugated with reporter tags in one step, with concomitant in situ desilylation of the TMS-alkynes and O-silylated groups (vide infra).
For the cyclopropanation of electron-deficient alkenes, alkynyl amido sulfonium salt 20 was used, which upon treatment with t-BuOK in DMSO generated the corresponding sulfur ylide that reacted chemoselectively with electron-poor olefins. Several natural products were derivatized using this cyclopropanation reaction including derivatives of gibberellic acid, phorbol, and forskolin.
2.5 Site-Selective Epoxidation of Polyene-Containing Natural Products
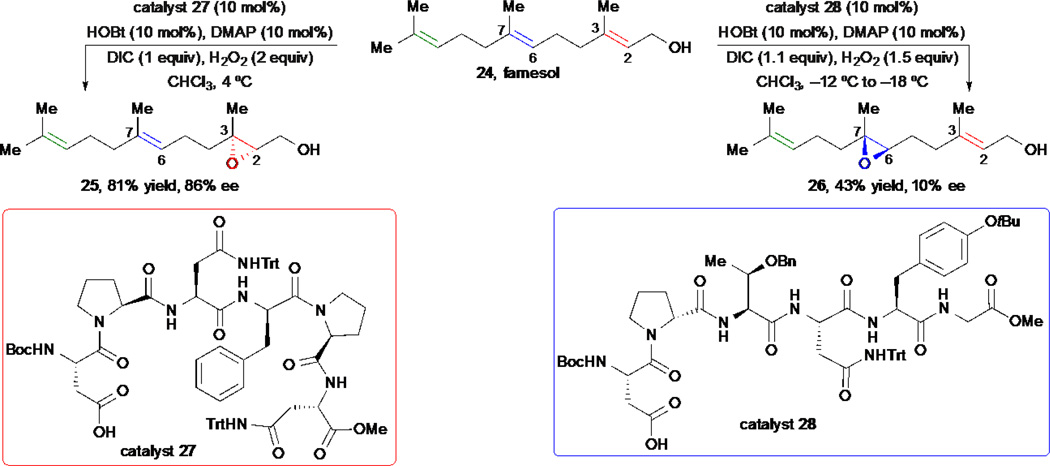
The epoxidation of simple olefins is one of the most well studied reactions in organic chemistry.33 While exquisite selectivity can be achieved for alkenes that possess a directing group (i.e. Sharpless epoxidation) or those with well-defined electronic differences, the site- and stereoselective epoxidation of molecules containing multiple alkenes with similar electronic and/or steric environments still remains a challenge. Until recently it could only be achieved to some extent by enzymatic transformations. By employing a combinatorial synthesis and library screening approach, Miller and co-workers identified a series of small peptides capable of catalyzing site-selective epoxidations of farnesol 24 and related polyenes (Scheme 5).34
Scheme 5.
S. Miller’s site-selective epoxidation of polyene-containing natural products.
Thus, when catalyst 27 was used, the C2-C3 epoxide 25 was obtained exclusively with excellent yield and good enantioselectivity. If catalyst 28 was employed, the site-selectivity changed completely to afford the C6-C7 epoxide 26, albeit in lower yield and low but measurable enantioselectivity.
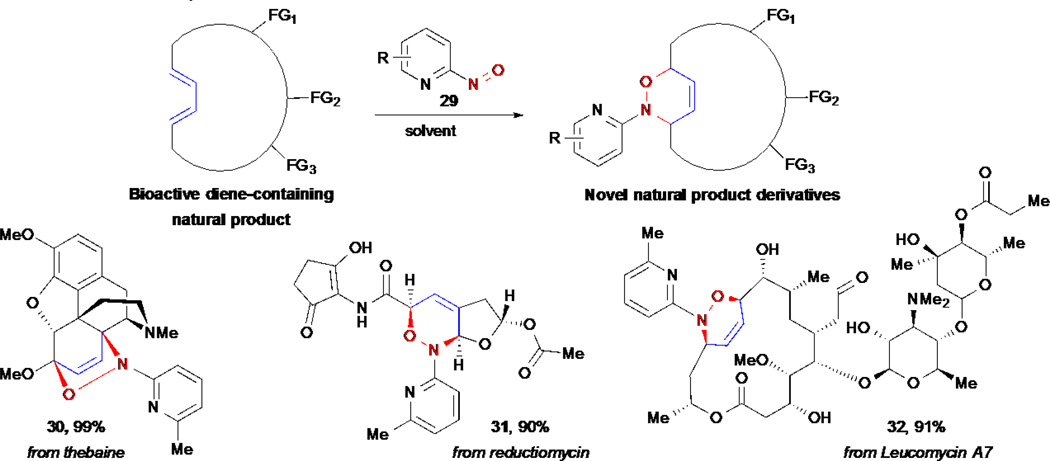
2.6 Nitroso Diels-Alder Reactions for the Derivatization of Diene-Containing Natural Products
Many natural products contain conjugated diene moieties that, in principle, can be useful handles for functionalization of these compounds through cycloaddition reactions. Toward this approach, Miller and co-workers applied a series of hetero-Diels-Alder reactions with nitroso derivatives that are remarkably chemoselective for dienes and tolerate several other functionalities present in these molecules (Scheme 6).35–37 Some of the advantages that this approach offers include the introduction of new heteroatoms and a six-membered ring that provides more rigidity and thus significantly alters the topology of derivatives. In addition, the possibility of performing further transformations of the derived oxazines, enabling access to several other derivatives, makes this strategy attractive for natural product derivatization. The use of 2-nitrosopyridine derivatives 29 was crucial in order to obtain reliably high yields and selectivities. These types of stabilized iminonitroso reagents offer the best combination of reactivity and stability for nitroso Diels-Alder reactions under mild conditions, since the use of more reactive nitroso species (e.g. acylnitroso) are often too reactive to be compatible with other fuctional groups present in complex natural products, and the use of less reactive ones (e.g. nitrosobenzene) require harsher reaction conditions and provide lower yields.
Scheme 6.
M. Miller’s nitroso Diels-Alder reactions for the selective derivatization of diene-containing natural products.
This approach was applied to the derivatization of several diene-containing natural products including the opiate alkaliod thebaine, the antitumor antibiotic reductiomycin and the macrolide antibiotic leucomycin A7. Furthermore, Miller and co-workers developed poymer-bound arylnitroso dienophiles for solid-phase chemistry that facilitate the synthesis of natural product-derived libraries for SAR studies.38–40
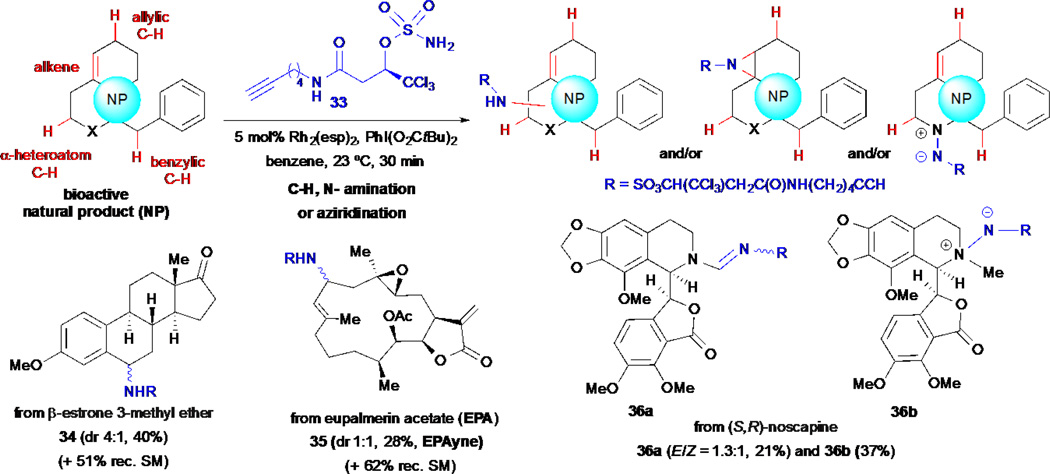
2.7 Simultaneous Arming/SAR Studies via C- and N-Amination and Alkene Aziridination of Natural Products
Recent advances in transition metal catalysis have enabled the direct and selective functionalization of C-H bonds through insertions of metallo carbenoid and nitrenoid reagents.41–42 The Rh-catalyzed C-H amination reactions pioneered by Du Bois,43–44 Lebel45 and others, offer a very attractive approach for the introduction of nitrogen substituents into unfunctionalized positions to deliver C-H aminated products and also aziridination of electron-rich alkenes under certain conditions. The application of this unique approach to the functionalization of native natural products offers an increased number of potential sites for derivatization while maintaining the desired bioactivity, since amination of unactivated sites does not directly perturb existing functional groups that are often required for bioactivity. Romo and coworkers developed an alkynyl sulfamate reagent 33 that enabled intermolecular C-H aminations and alkene aziridinations of native natural products for simultaneous arming/SAR studies (Scheme 7).46 The pendant alkyne offers a site for subsequent orthogonal conjugation to reporter tags toward cellular probes. They applied this methodology to the derivatization of several natural products and drugs and demonstrated the ability of these reaction conditions to deliver benzylic (e.g. 34) and allylic (e.g. 35) C-H aminated products, in addition to amidines (e.g. 36a) derived from tertiary amine-containing natural products through a C-H amination/oxidation sequence. Through unusual N-aminations, hydrazine sulfamate zwitterions (e.g 36b) were also obtained.
Scheme 7.
Romo and Cravatt’s simultaneous arming/SAR studies via C- and N- amination and alkene aziridination of natural products.
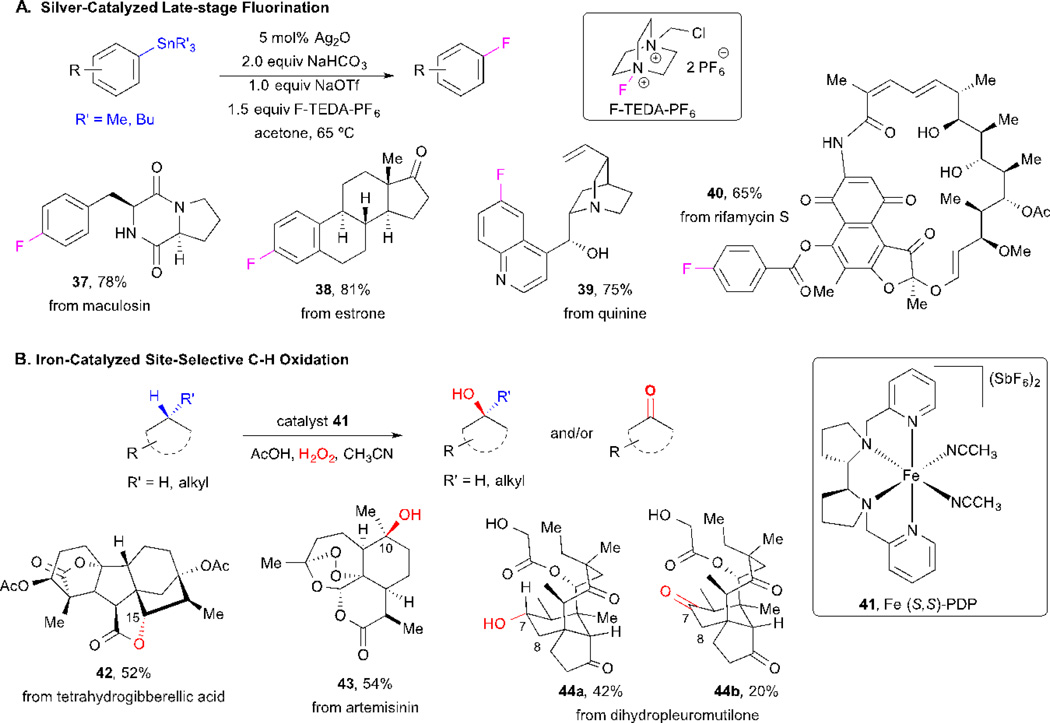
2.8 Late-Stage Fluorination and Selective C–H Oxidation of Natural Products
Fluorinated aromatic compounds are common motifs in medicinal and materials chemistry and are routinely used as tracers in medical applications.47–48 Late stage selective C–F bond formation is challenging due to the lack of available methods and the incompatibility with several functional groups. Recently, Ritter and coworkers developed a mild and selective silver-catalyzed flourination of aryl stannane substrates that has wide scope and functional group tolerance. They demonstrated the utility of this process for the fluorination of several complex natural product derivatives that included peptide, steroid, alkaloid and polyketide natural products (Scheme 8A).49 However, one of the drawbacks of this methodology is the synthesis of the starting arylstannanes, which might require several steps, but nonetheless, this approach enables the high yielding introduction of C–F bonds in complex settings which was previously not possible.
Scheme 8.
Ritter’s late-stage fluorination and White’s C-H oxidation of natural products.
The selective functionalization of aliphatic C–H bonds is one of the greatest challenges in organic chemistry due to their abundance yet lack of reactivity.42 White and coworkers recently developed Fe-catalyzed C–H oxidations that demonstrate good selectivity and are useful in complex settings by the functionalization of native natural products and derivatives (Scheme 8B).50–51 Under the optimized conditions using catalyst 41, C–H oxidation of a tetrahydrogibberellic acid derivative occurred at C-15 to selectively provide lactone 42 in 52% yield. Artemisinin underwent site-selective C-H oxidation at the tertiary C–H bond at the C-10 position, to provide derivative 43 exclusively. Mixtures of C–H oxidation products are often obtained as in the case of dihydropleuromutilone, which provided a mixture of the alcohol 44a and ketone 44b, however the oxidation proceeded with high site selectivity at C-7.
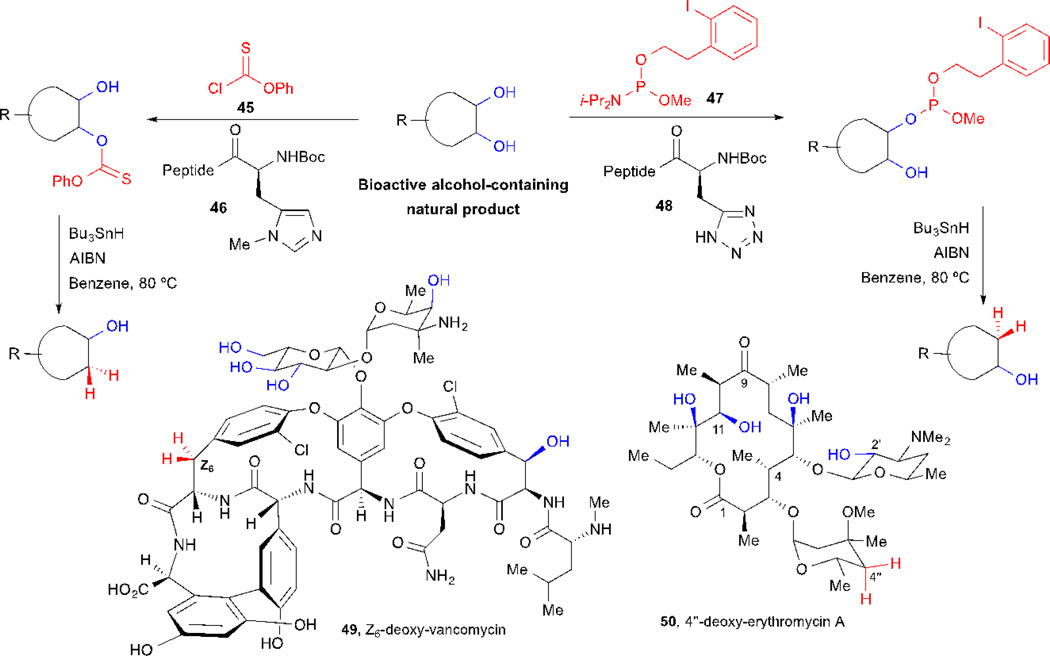
2.9 Deoxygenation of Hydroxy Groups based on Site-Selective Thiocarbonylations and Phosphoramidite Transfer
The ability to selectively remove a hydroxy group from a native polyol-containing natural product is an attractive goal in organic and medicinal chemistry, since it provides access to deoxy-derivatives in a concise manner for SAR studies. Although radical-initiated deoxygenation reactions of thiocarbonyl esters and phosphites are very well established,52–54 the direct synthesis of the requisite starting materials in complex natural products containing multiple funtional groups is still a difficult undertaking. Miller and co-workers recently further exploited their small peptide strategies with embeded imidazoles (e.g. 46, Scheme 9) as catalysts for the site-selective transfer of thionoformates to deliver thiocarbonyl esters enabling subsequent deoxygenation reactions in complex settings.55–58 This approach was applied to the deoxygenation of several polyol-containing molecules, including inositol and carbohydrates, as well as to a minimally protected form of the glycopeptide antibiotic vancomycin. In the latter example, the authors identified two peptide-based catalysts capable of delivering mono-thionocarbonates with remarkable site-selectivity, which after deoxygenation and deprotection provided novel deoxy-vancomycin analogues. One of these new derivatives (49),58 revealed that the removal of the hydroxy group at the Z-6 position had a profound effect on the conformation, since Z-6-deoxy-vancomycin 49 exists in at least two discrete conformations that interconvert at room temperature. This conformational flexibility had an effect on its biological activity as well, since Z-6-deoxy-vancomycin 49 was less potent than vancomycin against several bacterial strains. The optimized reaction conditions for site-selective thiocarbonylations also had some limitations, when it was applied to erythromycin A,57 side reactions were observed, which produced lower yields. Futhermore, when it was applied to 2’-acetyl-erythromycin A, only dealkylation of the amino group was observed.
Scheme 9.
S. Miller’s deoxygenation of alcohols based on peptide-catalyzed, site-selective thiocarbonylations and phosphoramidite transfers.
To overcome these problems, in a second iteration of this strategy, Miller and co-workers developed the phosphoramidite 47 that enables site-selective transfer by peptide catalysts with embedded tetrazoles (e.g. 48). The resulting phosphites undergo efficient deoxygenation of hydroxy groups under mild deoxygenation conditions similar to the Barton-McCombie reaction. When these reaction conditions were applied to erythromycin A, using phosphoramidite 47, 4”-deoxy-erythromycin A 50 could be obtained selectively following deoxygenation in good overall yield.
3 Applications to Cellular Probe Synthesis and Biological Studies
3.1 Approaches to the Direct Synthesis of Various Probes (i.e. fluorescent) from Natural Products
Fluorescently labeled small molecules are invaluable tools in chemical biology and are routinely used as indicators in enzymatic assays, reporter tags and cellular localization studies.59 While there are an increasing number of commercially available fluorophores with various linkers and reactive end groups,60 the synthesis of natural product probes containing a covalently attached reporter tag still presents significant challenges due to the limited number of “handles” for attachment as well as the structural complexity and wide functional group diversity found in natural products. To facilitate the attachment of fluorescent tags to a diverse set of natural products, La Clair and co-workers developed a family of functionalized labels, that are derivatives of a simple coumarin, which was chosen based on its lack of biological activity, excellent cell permeability, and both physicochemical and photophysical properties.61 Each derivative has a discrete, reactive end group used as the attachment point to a specific functional group. The utility of these labels was demonstrated by the synthesis of a diverse set of tagged natural products and comparative subcellular localization studies using LED fluorescence microscopy.
3.2 Arylpalladium(II) Reagents for Alkene Labeling in Water
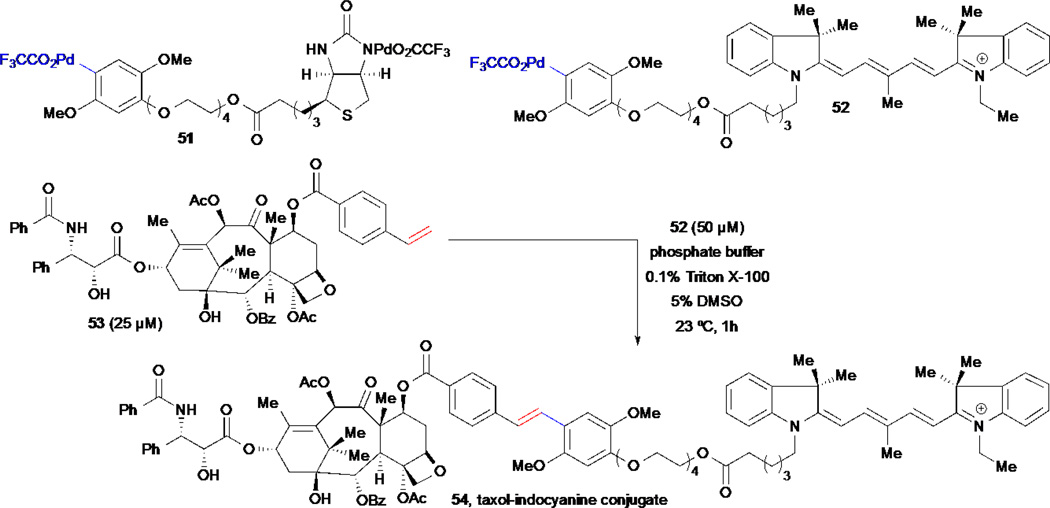
The development of methods for the selective covalent attachment of reporter tags in water poses a challenging prospect for natural product derivatization but would prove highly useful for highly polar natural products. Myers and co-workers recently described a set of air- and water-stable arylpalladium(II) reagents containing biotin 51 and indocyanine 52 labels that can be used for the tagging of alkene-containing compounds through Heck-coupling reactions in aqueous media, enabling the synthesis of complex natural product probes on small scale (Scheme 10).62 These organometallic compounds were prepared from the corresponding benzoic acid derivatives through decarboxylative palladation reactions and can be stored for several months. The arylpalladium (II) reagents were shown to participate in Heck couplings with styrenyl-substrates in aqueous media at 23 °C with good overall conversion. Substrates studied include a styrenyl-modified protein (lysozyme), FK506, and taxol derivatives. In the case of the taxol derivative, the authors demonstrated the feasibility of labeling the substrate on a microscale (25 µM) in just 1 hour under mild conditions using the organopalladium complex 52, to provide the targeted taxol-indocyanine conjugate 54 (Scheme 10). Reaction of the arylpalladium 51 containing biotin with FK506 derivative provided a biotinylated derivative that was used for affinity-enrichment experiment with cell lysates. The conjugate was shown to be an effective probe for capture of its corresponding protein receptors by affinity chromatography.
Scheme 10.
Myer’s aryl carboxylate Pd(II) reagents for alkene labelling in aqueous media.
3.3 Synthesis and Bioactivity of FK506 Probes
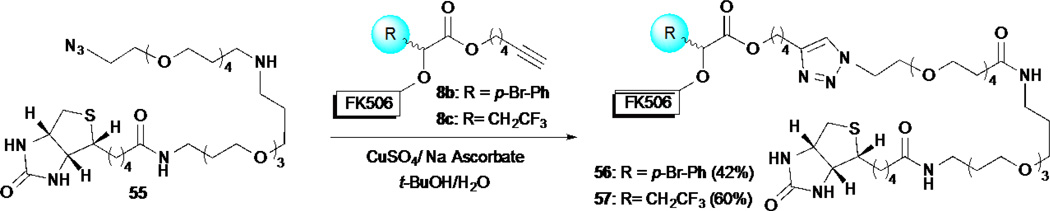
The development of site-selective rhodium-catalyzed O–H insertions by Romo and co-workers enabled the synthesis of several natural product derivatives for SAR studies as well as the expeditious synthesis of cellular probes for target identification.27–28 Toward studying the effect on biological activity that different α-substituents on the derived ethers generated through O–H insertions might have (i.e. α-trifluoroethyl versus p-bromophenyl, see Scheme 3 for structures), a set of diastereomeric FK506-derived ethers 8b–c, were separated and used in cellular assays and affinity chromatography experiments with biotin derived conjugates. Conjugation to biotin was carried out through Sharpless-Hüisgen cycloaddition with biotin azide 55 (Scheme 11). Using the IL-2 reporter assay, the FK506-HTFB derivatives 8c and 8c’ displayed a diminished inhibition of IL-2 production (α-trifluorethyl, ~2 fold, IC50 = 15.9 ± 2.8 and 19.2 ± 14.5 nM respectively) as compared to the FK506-HBPA derivatives 8b and 8b’ (α-p-bromophenyl, IC50 = 10.2 ± 3.8 and 7.3 ± 0.6 nM, respectively). A similar trend was observed with the biotin conjugates 56 and 57 (~4 fold, IC50 = 97.4 ± 17.5 and 27.9 ± 10.6 nM, respectively). Pulldown experiments were then performed using the FK506-biotin probes 56 and 57 which led to distinctive differences in the cellular proteins captured. Whereas the use of the α-p-bromophenyl probe 56 led to pull-down of the entire immunosuppressive complex including FKBP12, calcineurin A/B and calmodulin, the smaller α-trifluoroethyl conjugate 57 captured only FKBP12, demonstrating the significant effect that small changes in probes can have for pulldown experiments.
Scheme 11.
Romo’s synthesis of an FK506-biotin conjugate from O–H insertion derivatization.
3.4 Synthesis of forskolin and bexarotene-biotin Probes Through Cylopropanation/Conjugation Reactions
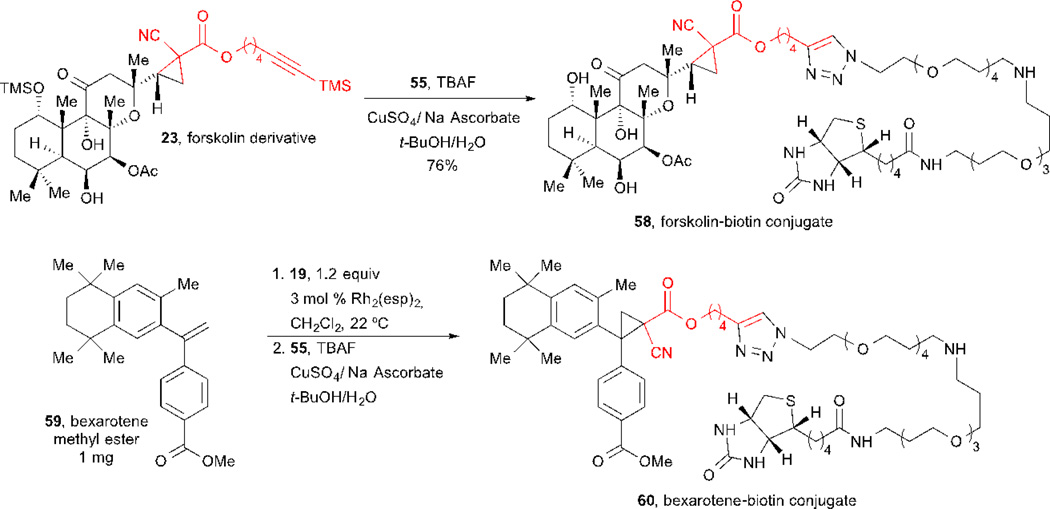
During the course of studying cyclopropanation conditions for simultaneous arming/SAR studies of natural products and drugs, Romo and co-workers demonstrated the feasibility of the derivatized products to undergo simultaneous desilylation and Sharpless-Hüisgen cycloaddition for the attachement of reporter tags.32 Using optimized reaction conditions (Scheme 12),63 the forskolin derivative 23 underwent desilylation and conjugation to biotin azide 55 to deliver conjugate 58 in 76% yield. Furthermore, the authors successfully applied the cyclopropanation/conjugation sequence on microscale to the synthesis of a bexarotene-biotin conjugate 60.
Scheme 12.
Romo’s synthesis of forskolin and bexarotene-biotin conjugates through cyclopropanation derivatization.
3.5 Site-Selective Acylations of Apoptolidin
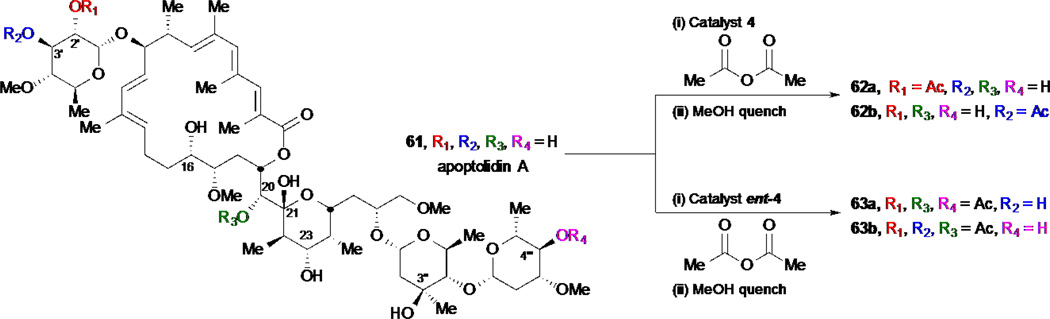
In a joint venture, Miller and Wender explored the site-selective derivatization of apoptolidin 61 using catalytic peptide-based acylations (Scheme 13).24 Apoptolidin is a complex polyketide natural product that possesses potent activity for induction of cell death in cells transformed with an oncogene but not normal cells.64–65 This remarkable selectivity has stirred tremendous interest in the synthetic and medicinal community to explore the chemistry and biology of this natural product; and although several derivatives have been produced through synthetic efforts, it has been a laborious undertaking.66 Treatment of apoptolidin under the reaction conditions previously developed in the Miller group with catalyst 4, provided a ~4:1 separable mixture of monoacetates 62a/62b with improved conversions in comparison to use of DMAP as an acylation catalyst which gave an ~1:1 ratio of these products. Interestingly, when the enantiomer of catalyst 4 was used (ent-4) a mixture of two discrete triacetate compounds 63a/63b were obtained and fully characterized. The new apoptolidin derivatives were then assayed and found to be quite similar with regard to potency, as compared to the parent compound, suggesting that small modifications are tolerated or alternatively that the acetates are cleaved during the cellular assays leading back to apoptolidin.
Scheme 13.
Site-selective acylations of apoptodilin A by Miller and Wender.
3.6 Synthesis, Bioactivity, and Cellular Target Identification of Eupalmerin Acetate Through CH Amination
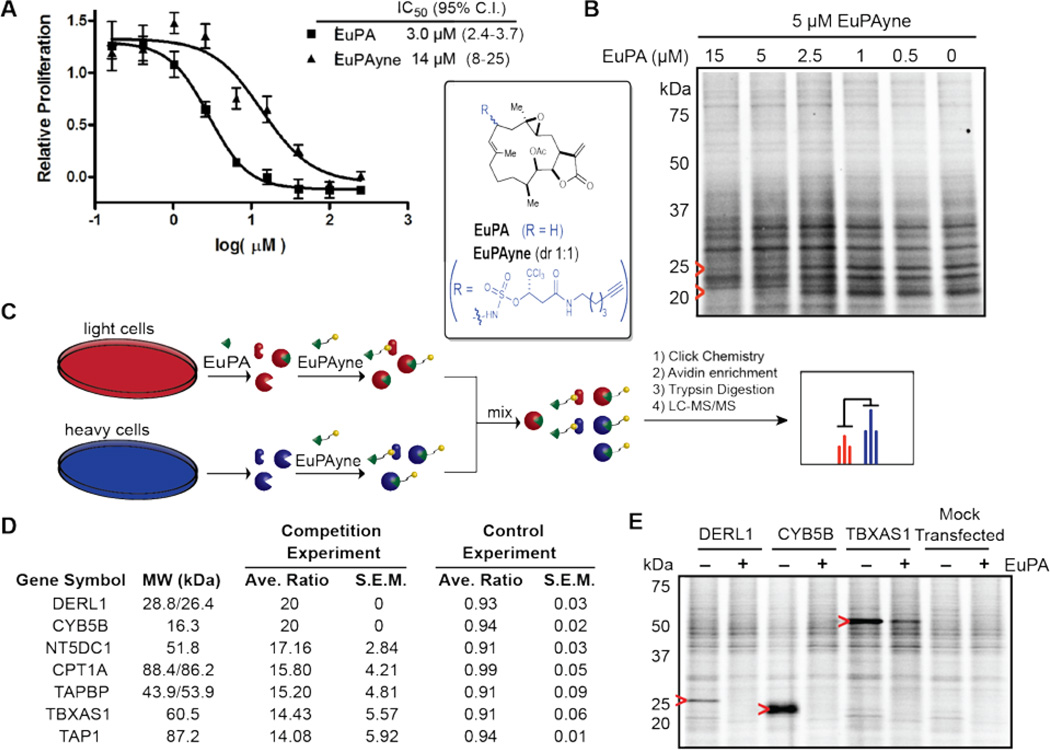
Eupalmerin acetate (EuPA) is a cembranolide diterpene natural product with promising anticancer activity, that has been shown to inhibit the growth of several cancer cell lines in vitro and in vivo including malignant glioma xenografts in a mouse model.67 However, the specific cellular target(s) of this natural product were unknown until recently. To demonstrate the utility of the C-H amination strategy for simultaneous arming/SAR studies of natural products, Romo and co-workers applied this strategy to EuPA. This natural product underwent site-selective allylic C-H amination to afford EPAyne 35 as a 1:1 mixture of diastereomers (see inset, Figure 1).46 The antiproliferative properties of EuPA-yne 35 was then measured and compared to that of the parent compound in HL-60 cells and EuPAyne was found to be only ~5X less potent (IC50 = 3.0 µM for EuPA versus 15 µM for EuPAyne, Figure 1A). This result suggested that EPAyne could be used for quantitative proteomic profiling to identify the cellular targets of EuPA, which presumably forms covalent adducts with cellular targets due to the presence of the exocylic double bond serving as a likely Michael acceptor toward nucleophilic residues. Initial proteome profiling with HL-60 cell lysates followed by conjugation to an azide-rhodamine reporter tag, SDS-PAGE, and fluorescent scanning revealed several protein bands that were specifically competed out with EuPA (Figure 7B, red arrows). To identify these targets, a competitive ABPP-SILAC68–70 experiment was performed, which uses a mixture of proteomes with both heavy and light isotopic tags. A click reaction to provide a biotin conjugate, enrichment, and analysis by MudPIT,71 led to the identification of three high-affinity targets (Figure 7C). The first was derlin-1 (DERL1) which is associated with cancer cell proliferation and both cytochrome b5 type B (CYB5B) and thromboxane A synthase (TBXAS1) which are known to be overexpressed in cancer (Figure 7E). These cellular targets were validated by labeling of transiently transfected 293T cells with the proposed targets and labelling with EPAyne (5 µM) ± EPA (15 µM) in competition experiments (Figure 7E, red arrows indicate the target’s expected molecular weight). This study demonstrated the utility of the simultaneous arming/SAR strategy for target indentification employing novel natural products.
Figure 1.
Bioactivity and cellular target identification of eupalmerin acetate through CH amination by Romo and Cravatt.
4 Conclusions
Over the past decade, synthetic chemists have developed new reagents, catalysts and reaction conditions for the direct functionalization of native bioactive natural products. These methods have enabled both the chemo- and site-selective synthesis of novel natural product derivatives for SAR studies and the synthesis of natural product-based probes for cellular target identification. This field is still in its infancy since the challenges associated with derivatization of each unique natural product displaying broad scales of reactivity and unique arrays of functionality are great. Further evidence for this growing field stems from the number of papers in this area appearing very recently with increasing frequency.72–74
5 Future Outlook
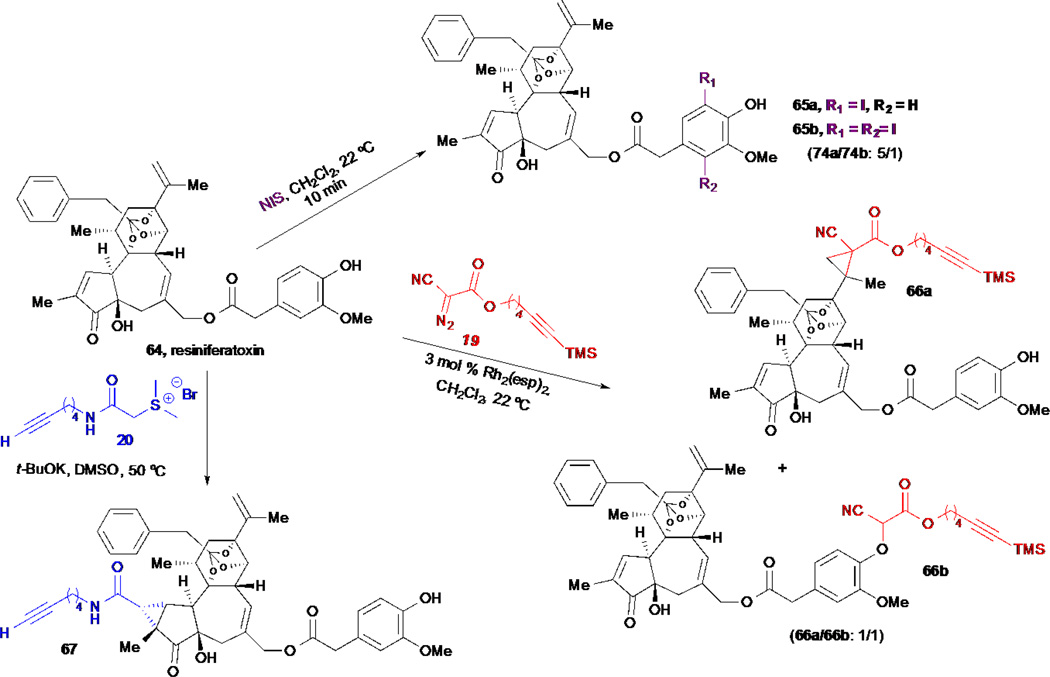
We anticipate that the application of the described strategies for the direct modification of unprotected natural products with both new and old bioactive natural products will facilitate a more rapid and thorough exploration of chemical space presented by these privledeged structures. These strategies will provide access to numerous probes useful for the study of cellular processes and the identification of novel targets for therapeutic intervention for drug discovery. As additional mild, microscale methods for natural product derivatization are developed, one can envision the ability to modify increasingly diverse sites of native natural products for full SAR exploration. The difficulty of performing SAR studies with natural products will no longer be a bottleneck in evaluating the therapeutic potential of bioactive natural products as leads for drug discovery. Toward this ideal goal, we present the application of several recently developed methods in our group for simultaneous arming/SAR studies to the microscale chemo- and site-selective derivatization of the complex natural product, resiniferatoxin (Scheme 14).75 Resiniferatoxin is an ultrapotent analog of capsaicin isolated from the cactus Euphorbia resinifera and is an agonist of the vanilloid receptor.76–77 Treatment of resiniferatoxin 64 with NIS in dichloromethane provided the known 5-iodo resiniferatoxin 65a,78 along with some of the bis-iodo derivative 65b. When the alkynyl diazoester reagent 19 was used in combination with catalytic amounts of Rh2(esp)2, a mixture of cyclopropanated resiniferatoxin 66a and O–H insertion derived ether 66b was obtained. Finally, cyclopropanation of the electron-deficient enone with alkynyl amide-containing sulfonium salt 20 provided the derivative 67. This example demonstrates the potential for accessing derivatives of a complex, native natural product at very different positions that are readied for conjugation to access cellular probes. We anticipate that the methods described herein and those to be developed in the future will enable the full potential of natural products to be realized in the future.
Scheme 14.
Romo/Robles’ microscale derivatization of resiniferatoxin.
6 Addendum
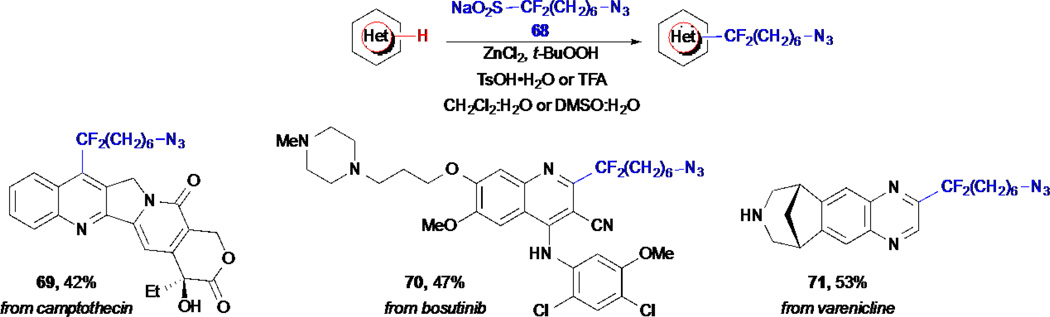
While this manuscript was under revision, an elegant approach for the C–H fucntionalization of heterocycle-containing natural products and pharmaceuticals with azide-terminated linkers was reported by Baran and co-workers (Scheme 15).73 This strategy is based on (flouro)alkanesulfinate reagents and their ability to form radicals in situ under oxidative conditions, that then react with heteroaromatics through a formal C–H functionalization.79–81 The azide-containing alkylsulfinate 68 allows the direct derivatization of various heterocycles and the resulting azidoalkyl chain can then be used for conjugation to reporter tags, as demostrated by the attachment of the derivatized products to an alkyne-containing monoclonal antibody through a Hüisgen cycloaddition. They applied this strategy to the derivatization of several natural products and pharmaceuticals and included the topoisomerase inhibitor camptothecin, the anticancer drug bosutinib, as well as varenicline (Chantix, used to treat nicotine addiction) in generally good yields and selectivities.
Scheme 15.
Baran’s C–H functionalization of natural products and pharmaceuticals with azide-terminated linkers.
Acknowledgements
We gratefully acknowledge National Institutes of Health (NIGMS, GM086307) and the Welch Foundation (A-1280) for generous support of the Romo Group’s contributions to this area of research.
Notes and References
- 1.Evans FJ. J. Ethnopharmacol. 1991;32:91–101. doi: 10.1016/0378-8741(91)90107-o. [DOI] [PubMed] [Google Scholar]
- 2.Carlson EE. ACS Chem. Biol. 2010;5:639–653. doi: 10.1021/cb100105c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henkel T, Brunne RM, Müller H, Reichel F. Angew. Chem., Int. Ed. 1999;38:643–647. doi: 10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv. Drug Delivery Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins AL, Groom CR. Nature Rev. Drug Discovery. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 7.Simon GM, Cravatt BF. Nature Chem. Biol. 2008;4:639–642. doi: 10.1038/nchembio1108-639. [DOI] [PubMed] [Google Scholar]
- 8.Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, Wang J, Wu RP, Gomez F, Loo JA, Wohlschlegel JA, Vondriska TM, Pelletier J, Herschman HR, Clardy J, Clarke CF, Huang J. Proc. Natl. Acad. Sci. USA. 2009;106:21984–21989. doi: 10.1073/pnas.0910040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenone M, Dancik V, Wagner BK, Clemons PA. Nature Chem. Biol. 2013;9:232–240. doi: 10.1038/nchembio.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Njardarson JT, Gaul C, Shan D, Huang X-Y, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:1038–1040. doi: 10.1021/ja039714a. [DOI] [PubMed] [Google Scholar]
- 11.Fürstner A, Kirk D, Fenster MDB, Aïssa C, De Souza D, Müller O. Proc. Natl. Acad. Sci. USA. 2005;102:8103–8108. doi: 10.1073/pnas.0501441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danishefsky S. Nat. Prod. Rep. 2010;27:1114–1116. doi: 10.1039/c003211p. [DOI] [PubMed] [Google Scholar]
- 13.La Clair JJ. Nat. Prod. Rep. 2010;27:969–995. doi: 10.1039/b909989c. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber SL. Proc. Natl. Acad. Sci. USA. 2011;108:6699–6702. doi: 10.1073/pnas.1103205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shenvi RA, O’Malley DP, Baran PS. Acc. Chem. Res. 2009;42:530–541. doi: 10.1021/ar800182r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns NZ, Baran PS, Hoffmann RW. Angew. Chem., Int. Ed. 2009;48:2854–2867. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]
- 17.Young IS, Baran PS. Nature Chem. 2009;1:193–205. doi: 10.1038/nchem.216. [DOI] [PubMed] [Google Scholar]
- 18.Newhouse T, Baran PS, Hoffmann RW. Chem. Soc. Rev. 2009;38:3010–3021. doi: 10.1039/b821200g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaich T, Baran PS. J. Org. Chem. 2010;75:4657–4673. doi: 10.1021/jo1006812. [DOI] [PubMed] [Google Scholar]
- 20.Sculimbrene BR, Miller SJ. J. Am. Chem. Soc. 2001;123:10125–10126. doi: 10.1021/ja016779+. [DOI] [PubMed] [Google Scholar]
- 21.Sculimbrene BR, Morgan AJ, Miller SJ. J. Am. Chem. Soc. 2002;124:11653–11656. doi: 10.1021/ja027402m. [DOI] [PubMed] [Google Scholar]
- 22.Sculimbrene BR, Morgan AJ, Miller SJ. Chem. Commun. 2003:1781–1785. doi: 10.1039/b304015c. [DOI] [PubMed] [Google Scholar]
- 23.Lewis CA, Miller SJ. Angew. Chem., Int. Ed. 2006;45:5616–5619. doi: 10.1002/anie.200601490. [DOI] [PubMed] [Google Scholar]
- 24.Lewis CA, Longcore KE, Miller SJ, Wender PA. J. Nat. Prod. 2009;72:1864–1869. doi: 10.1021/np9004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcock BC, Uno BE, Bromann GL, Clark MJ, Anderson TM, Burke MD. Nature Chem. 2012;4:996–1003. doi: 10.1038/nchem.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastian AA, Marcozzi A, Herrmann A. Nature Chem. 2012;4:789–793. doi: 10.1038/nchem.1402. [DOI] [PubMed] [Google Scholar]
- 27.Peddibhotla S, Dang Y, Liu JO, Romo D. J. Am. Chem. Soc. 2007;129:12222–12231. doi: 10.1021/ja0733686. [DOI] [PubMed] [Google Scholar]
- 28.Chamni S, He Q-L, Dang Y, Bhat S, Liu JO, Romo D. ACS Chem. Biol. 2011;6:1175–1181. doi: 10.1021/cb2002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou C-Y, Li J, Peddibhotla S, Romo D. Org. Lett. 2010;12:2104–2107. doi: 10.1021/ol100587j. [DOI] [PubMed] [Google Scholar]
- 30.Pathak TP, Miller SJ. J. Am. Chem. Soc. 2012;134:6120–6123. doi: 10.1021/ja301566t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathak TP, Miller SJ. J. Am. Chem. Soc. 2013;135:8415–8422. doi: 10.1021/ja4038998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robles O, Serna-Saldívar SO, Gutiérrez-Uribe JA, Romo D. Org. Lett. 2012;14:1394–1397. doi: 10.1021/ol300105q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia QH, Ge HQ, Ye CP, Liu ZM, Su KX. Chem. Rev. 2005;105:1603–1662. doi: 10.1021/cr0406458. [DOI] [PubMed] [Google Scholar]
- 34.Lichtor PA, Miller SJ. Nature Chem. 2012;4:990–995. doi: 10.1038/nchem.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li F, Yang B, Miller MJ, Zajicek J, Noll BC, Möllmann U, Dahse H-M, Miller PA. Org. Lett. 2007;9:2923–2926. doi: 10.1021/ol071322b. [DOI] [PubMed] [Google Scholar]
- 36.Yang B, Zollner T, Gebhardt P, Möllmann U, Miller MJ. Org. Biomol. Chem. 2010;8:691–697. doi: 10.1039/b922450e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang B, Miller PA, Möllmann U, Miller MJ. Org. Lett. 2009;11:2828–2831. doi: 10.1021/ol900997t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krchňák V, Moellmann U, Dahse H-M, Miller MJ. J. Comb. Chem. 2008;10:104–111. doi: 10.1021/cc7001414. [DOI] [PubMed] [Google Scholar]
- 39.Krchňák V, Waring KR, Noll BC, Moellmann U, Dahse H-M, Miller MJ. J. Org. Chem. 2008;73:4559–4567. doi: 10.1021/jo8004827. [DOI] [PubMed] [Google Scholar]
- 40.Krchňák V, Zajíček J, Miller PA, Miller MJ. J. Org. Chem. 2011;76:10249–10253. doi: 10.1021/jo201361s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies HML, Manning JR. Nature. 2008;451:417–424. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White MC. Science. 2012;335:807–809. doi: 10.1126/science.1207661. [DOI] [PubMed] [Google Scholar]
- 43.Fiori KW, Du Bois J. J. Am. Chem. Soc. 2007;129:562–568. doi: 10.1021/ja0650450. [DOI] [PubMed] [Google Scholar]
- 44.Zalatan DN, Du Bois J. J. Am. Chem. Soc. 2009;131:7558–7559. doi: 10.1021/ja902893u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lebel H, Huard K. Org. Lett. 2007;9:639–642. doi: 10.1021/ol062953t. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Cisar JS, Zhou C-Y, Vera B, Williams H, Rodríguez AD, Cravatt BF, Romo D. Nature Chem. 2013;5:510–517. doi: 10.1038/nchem.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki T, Taguchi T, Ojima I. Fluorine in Medicinal Chemistry and Chemical Biology. John Wiley & Sons, Ltd; 2009. pp. 1–46. [Google Scholar]
- 48.Babudri F, Farinola GM, Naso F, Ragni R. Chem. Commun. 2007:1003–1022. doi: 10.1039/b611336b. [DOI] [PubMed] [Google Scholar]
- 49.Tang P, Furuya T, Ritter T. J. Am. Chem. Soc. 2010;132:12150–12154. doi: 10.1021/ja105834t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen MS, White MC. Science. 2007;318:783–787. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 51.Chen MS, White MC. Science. 2010;327:566–571. doi: 10.1126/science.1183602. [DOI] [PubMed] [Google Scholar]
- 52.Barton DHR, McCombie SW. J. Chem. Soc., Perkin Trans. 1. 1975:1574–1585. [Google Scholar]
- 53.Crich D, Quintero L. Chem. Rev. 1989;89:1413–1432. [Google Scholar]
- 54.Zhang L, Koreeda M. J. Am. Chem. Soc. 2004;126:13190–13191. doi: 10.1021/ja0462777. [DOI] [PubMed] [Google Scholar]
- 55.Morgan AJ, Wang YK, Roberts MF, Miller SJ. J. Am. Chem. Soc. 2004;126:15370–15371. doi: 10.1021/ja047360x. [DOI] [PubMed] [Google Scholar]
- 56.Sánchez-Roselló M, Puchlopek ALA, Morgan AJ, Miller SJ. J. Org. Chem. 2008;73:1774–1782. doi: 10.1021/jo702334z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jordan PA, Miller SJ. Angew. Chem., Int. Ed. 2012;51:2907–2911. doi: 10.1002/anie.201109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fowler BS, Laemmerhold KM, Miller SJ. J. Am. Chem. Soc. 2012;134:9755–9761. doi: 10.1021/ja302692j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavis LD, Raines RT. ACS Chem. Biol. 2008;3:142–155. doi: 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson ID, Spence MTZ. The Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies. 2010 Life Technologies. [Google Scholar]
- 61.Alexander MD, Burkart MD, Leonard MS, Portonovo P, Liang B, Ding X, Joullié MM, Gulledge BM, Aggen JB, Chamberlin AR, Sandler J, Fenical W, Cui J, Gharpure SJ, Polosukhin A, Zhang H-R, Evans PA, Richardson AD, Harper MK, Ireland CM, Vong BG, Brady TP, Theodorakis EA, La Clair JJ. ChemBioChem. 2006;7:409–416. doi: 10.1002/cbic.200500466. [DOI] [PubMed] [Google Scholar]
- 62.Simmons RL, Yu RT, Myers AG. J. Am. Chem. Soc. 2011;133:15870–15873. doi: 10.1021/ja206339s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maisonneuve S, Fang Q, Xie J. Tetrahedron. 2008;64:8716–8720. [Google Scholar]
- 64.Kim JW, Adachi H, Shin-ya K, Hayakawa Y, Seto H. J. Antibiot. 1997;50:628–630. doi: 10.7164/antibiotics.50.628. [DOI] [PubMed] [Google Scholar]
- 65.Hayakawa Y, Kim JW, Adachi H, Shin-ya K, Fujita K-i, Seto H. J. Am. Chem. Soc. 1998;120:3524–3525. [Google Scholar]
- 66.Daniel PT, Koert U, Schuppan J. Angew. Chem., Int. Ed. 2006;45:872–893. doi: 10.1002/anie.200502698. [DOI] [PubMed] [Google Scholar]
- 67.Iwamaru A, Iwado E, Kondo S, Newman RA, Vera B, Rodríguez AD, Kondo Y. Mol. Cancer Ther. 2007;6:184–192. doi: 10.1158/1535-7163.MCT-06-0422. [DOI] [PubMed] [Google Scholar]
- 68.Everley PA, Gartner CA, Haas W, Saghatelian A, Elias JE, Cravatt BF, Zetter BR, Gygi SP. Mol. Cell. Proteom. 2007;6:1771–1777. doi: 10.1074/mcp.M700057-MCP200. [DOI] [PubMed] [Google Scholar]
- 69.Weerapana E, Wang C, Simon GM, Richter F, Khare S, Dillon MBD, Bachovchin DA, Mowen K, Baker D, Cravatt BF. Nature. 2010;468:790–795. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cisar JS, Cravatt BF. J. Am. Chem. Soc. 2012;134:10385–10388. doi: 10.1021/ja304213w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aebersold R, Mann M. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 72.Han S, Miller SJ. J. Am. Chem. Soc. 2013;135:12414–12421. doi: 10.1021/ja406067v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Q, Gui J, Pan C-M, Albone E, Cheng X, Suh EM, Grasso L, Ishihara Y, Baran PS. J. Am. Chem. Soc. 2013;135:12994–12997. doi: 10.1021/ja407739y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun X, Lee H, Lee S, Tan KL. Nature Chem. 2013;5:790–795. doi: 10.1038/nchem.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robles O, Romo D. Unpublished Results [Google Scholar]
- 76.Hergenhahn M, Adolf W, Hecker E. Tetrahedron Lett. 1975;19:1595–1598. [Google Scholar]
- 77.Walpole CSJ, Bevan S, Bloomfield G, Breckenridge R, James IF, Ritchie T, Szallasi A, Winter J, Wrigglesworth R. J. Med. Chem. 1996;39:2939–2952. doi: 10.1021/jm960139d. [DOI] [PubMed] [Google Scholar]
- 78.Wahl P, Foged C, Tullin S, Thomsen C. Mol. Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- 79.Fujiwara Y, Dixon JA, Rodriguez RA, Baxter RD, Dixon DD, Collins MR, Blackmond DG, Baran PS. J. Am. Chem. Soc. 2012;134:1494–1497. doi: 10.1021/ja211422g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujiwara Y, Dixon JA, O/'Hara F, Funder ED, Dixon DD, Rodriguez RA, Baxter RD, Herle B, Sach N, Collins MR, Ishihara Y, Baran PS. Nature. 2012;492:95–99. doi: 10.1038/nature11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Q, Ruffoni A, Gianatassio R, Fujiwara Y, Sella E, Shabat D, Baran PS. Angew. Chem., Int. Ed. 2013;52:3949–3952. doi: 10.1002/anie.201300763. [DOI] [PMC free article] [PubMed] [Google Scholar]