Abstract
Objective
To assess autism spectrum disorder (ASD) behaviors in children with mucopolysaccharidosis Type IIIA (MPS IIIA), using a standard measure, understand the behavioral evolution of the disease, and provide specific guidelines for diagnosis.
Study design
Children (n=21) with documented enzyme deficiency and SGSH gene mutations, cognitive age-equivalent over 12 months, and early onset were administered the Autism Diagnostic Observation Schedule (ADOS) (Module 1) and Bayley Scales of Infant Development–III (BSID-III). ADOS Social Affect and Restricted Repetitive Behavior total scores are reported as well as BSID-III cognitive age-equivalent using descriptive statistics and graphic presentations.
Results
Thirteen of 21 children met ADOS criteria for ASD/autism. ADOS score was strongly associated with age; all 11 children over 46 months met criteria, and 8 of 10 under 46 months did not. Social and affective abnormalities were most frequent; restricted interests and repetitive behaviors were largely absent. Lack of cognitive growth paralleled ADOS score.
Conclusions
An increased incidence of autistic-like social behaviors occurred between ages 3 and 4 in children with early onset MPS IIIA. Although more frequent in the severely impaired, ASD behaviors were observed across the entire range of cognitive impairment. Clinicians must be aware that when a child acquires autistic-like behaviors, MPS IIIA should be included in the differential diagnosis.
Keywords: autism spectrum disorder, ASD, Sanfilippo syndrome, Autism Diagnostic Observation Schedule, ADOS
Mucopolysaccharidosis type III (MPS IIIA), is a lysosomal disorder associated with progressive dementia and severe behavioral disruption. It is a rare (about 1 in 100,000 births),6–9 autosomal recessive disease caused by decrease in heparan-N-sulfatase (sulfamidase) catalytic activity, a necessary metabolic step in degradation of the glycosaminoglycan (GAG) heparan sulfate. Undegraded heparin sulfate is evident in many cells of the central nervous system. Although MPS IIIA is a somewhat heterogeneous disorder, it is characterized by progressive neurodegeneration, dementia, and physical disability, with death typically occurring in the second decade of life.1 In the classic form of MPS IIIA, symptoms become apparent between 2 and 6 years of age, although diagnosis often lags behind the earliest symptoms.10 Some patients with MPS IIIA who have onset and diagnosis after 6 years of age, have a slower decline1,11.
Clinical observation and parent report have indicated that many children with MPS III have behaviors that are often associated with autism spectrum disorder (ASD),1–3 a pervasive developmental disorder characterized by impairment in social communication, restricted interests and repetitive behaviors. Declines in social connectivity and functional communication have been described in MPS III, but never directly measured1,3,4. Restricted interests, behavioral rigidity and repetitive behaviors have not been reported. A group of children with Sanfilippo syndrome type A was evaluated for ASD behaviors using a standard assessment method, the Autism Diagnostic Observation Schedule (ADOS),5 in order to understand the behavioral evolution of the disease and provide guidelines for identification and intervention.
We hypothesized that those children with MPS IIIA who meet ADOS criteria for ASD or autism will be older and consequently will be at a more advanced stage of disease than those who do not. Additionally, poor eye contact, social reciprocity, and communication skills, rather than rigid and repetitive interests and behaviors, will characterize children with MPS IIIA.
Methods
A total of 30 children with Sanfilippo syndrome type A were enrolled. Twenty-five children with MPS IIIA, age 2 to 18 years, were recruited into this neurobehavioral study from a natural history study. Patients in the NH study met the following criteria: (1) confirmed diagnosis of MPS IIIA by enzyme or mutation analysis; (2) minimum chronological age of one year; and (3) developmental age of at least 12 months on the Vineland Adaptive Behavior Scales.12 We also enrolled 5 patients with MPS IIIA who were seen clinically and who met the same criteria but were not in the NH study.
The University of Minnesota Institutional Review Board approved this neurobehavioral study and also the natural history longitudinal study. Written informed consent was obtained from the parents or guardians of the children who served as subjects of the investigation.
Children in the study were classified as having either the classic, early form of Sanfilippo syndrome type A if they were diagnosed before 6 years of age, or the late-onset form with slower decline if diagnosed after age 6.1,8 We found that diagnosis under age 6 was associated with severe genotypes, and those diagnosed later had at least one mutation associated with late-onset MPS IIIA.13 One child who was diagnosed with Sanfilippo syndrome after age 6 had an autism diagnosis until he was noted to be declining; he had a known severe genotype and was retained in this sample.
To increase homogeneity and to use only one ADOS module, we excluded the six slow progressing, late-diagnosed children from this neurobehavioral study, as well as one of their siblings, yielding a sample of 23 children. One child who was diagnosed before age 6 had phrase speech and thus was given ADOS module 2. One child was not administered the ADOS. The final number for analysis was 21.
ADOS
The ADOS is a semi-structured observation designed to observe and judge the quality of a child’s social communication and play and assess the presence of any intense interests or repetitive behaviors. The ADOS yields total scores for Social Affect (SA) and Restricted and Repetitive Behavior (RRB) yielding an overall classification indicating behaviors and symptoms consistent with autism, consistent with milder indications of ASD, or not consistent with ASD (‘nonspectrum’). In order to evaluate behaviors compatible with ASD using this measure most effectively, a cognitive age-equivalent of at least 18 months is recommended. The ADOS has 4 different modules and the tasks administered on each module are tailored to the individual’s language level: (1) children who are nonverbal or who communicate in primarily single words; (2) children who regularly use phrase speech; (3) children who speak in full and complex sentences; and (4) older adolescents and adults who have fluent language. In the current sample, only children were included who were administered Module 1. The revised algorithms of the ADOS were used.14
In addition to comparison of total scores in the areas of social affect and restricted/repetitive behaviors, individual behaviors that are observed and coded on the ADOS were also analyzed. The two examiners who performed the ADOS are both “research reliable,” a credential that indicates a high level of interrater reliability in coding.
The NH study included a comprehensive evaluation of neurodevelopment (Bayley Scales of Infant Development-Third Edition; BSID-III)15 and those data are included in this neurobehavioral study. The clinical patients were also given the BSID-III. Two experienced examiners performed the BSID on these children. Only cognitive age-equivalents are reported here as many of the patients were over the age where standardized scores could be calculated.
Results
In the total sample of 21 children, 7 were female; the mean age for this sample was 54 months (range 22 to 106 months). Of the 21 children, 62% met criteria for ASD using ADOS Module 1. Table I indicates patient characteristics. In this sample, the average age of MPS III diagnosis was 46 months±19 (range 21–98); notably, this does not include the siblings diagnosed because of the proband’s diagnosis. As there were 5 pairs of siblings, the average age is thus based on 16 diagnoses.
Table I.
Patient characteristics.
| Total (N=21) | ADOS Non-ASD (N=8) | ADOS ASD (N=13) | Cog Age Equiv <18 mos (N=8) | Cog Age Equiv >18 mos (N=13) | |
|---|---|---|---|---|---|
| Female | 7 (33.3%) | 2 (25.0%) | 5 (38.5%) | 2 (25.0%) | 5 (38.5%) |
| Male | 14 (66.7%) | 6 (75.0%) | 8 (61.5%) | 6 (75.0%) | 8 (61.5%) |
| Age (mo) | 54.1 (24.6) range 22–106 |
33.5 (7.7) range 22–45 |
66.8 (22.6) range 26–105 |
42.8 (15.5) range 26–105 |
72.6 (26.1) range 22–76 |
| Cognitive Age Equivalent | 19.5 (6.5) range 8–27 |
24.1 (3.3) range 21–30 |
16.6 (6.3) range 8–27 |
12.5 (3.3) range 8–17 |
23.8 (3.3) range 19–30 |
| Developmental Quotient | 46.5 (27.2) range 8–91 |
74.1 (12.1) range 55–91 |
29.5 (18.2) range 8–62 |
22.6 (17.9) range 8–62 |
61.2 (20.9) range 26–91 |
| Module 1, No Words | 10 (47.6%) | 2 (25.0%) | 8 (61.5%) | 8 (100%) | 4 (31%) |
| Module 1, Some Words | 11 (52.4%) | 6 (75.0%) | 5 (38.5%) | 0 (0.0%) | 9 (69%) |
| Meets ADOS criteria for autism | 12 (57.1%) | 0 (0.0%) | 12 (92.3%) | 7 (87.5%) | 5 (38.5%) |
| Meets ADOS criteria for ASD | 1 (4.8%) | 0 (0.0%) | 1 (7.7%) | 1 (12.5%) | 0 (0.0%) |
| ADOS Non-spectrum | 8 (38.1%) | 8 (100%) | 0 (0.0%) | 0 (0.0%) | 8 (61.5%) |
| Social Affect (SA) | 10.5 (7.0) range 0–20 |
2.5 (1.5) range 0–5 |
15.5 (3.2) range 10–20 |
16.00 (2.6) range 12–19 |
7.15 (6.7) range 0–20 |
| Restricted Repetitive Behavior (RRB) | 2.2 (1.6) range 0–6 |
0.9 (0.6) range 0–2 |
3.0 (1.4) range 1–6 |
3.25 (1.0) range 2–5 |
1.54 (1.5) range 0–6 |
| Total SA + RRB | 12.7 (8.1) range 0–22 |
3.2 (2.1) range 0–7 |
18.5 (3.3) range 12–22 |
19.25 (2.31) range 15–22 |
8.62 (7.7) range 0–22 |
Values presented are mean (s.d.) or N (%) where indicated.
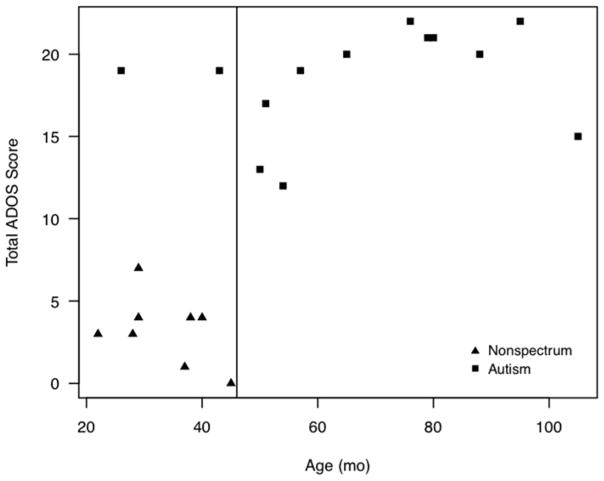
A strong association of ADOS score with age was found such that all 11 children over the age of 46 months met the ADOS cutoffs for ASD or autism. Only two children (20%) under the age of 46 months also met criteria. Those who did not meet criteria for autism were all under 46 months (Figure 1).
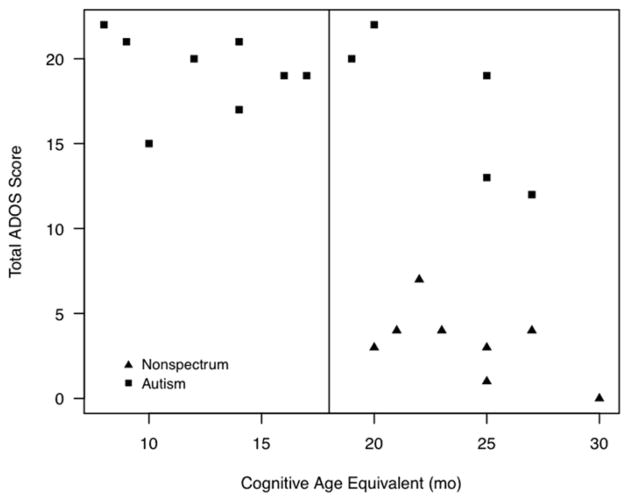
Figure 1.
Scatterplot of chronological age (a) and cognitive age-equivalent (b) against total ADOS score. Higher ADOS scores indicate more behaviors compatible with ASD.
Of those children meeting the ADOS criteria for ASD, the most frequent items that differentiated them from those who did not meet ASD criteria were items reflecting social and affective abnormalities. Items indicating restricted or repetitive behaviors were largely absent, with the exception of “unusual sensory interests” typically characterized by participants biting the test materials. Behaviors that most differentiated children scoring in the ASD or autism range on the ADOS from those who did not included use of facial expressions to communicate, frequency of vocalizations directed towards others, use of gestures to communicate, eye contact, activity level, requesting (quality and frequency), response to joint attention, response to name, responsive social smile, showing, shared enjoyment when interacting, and unusual sensory interests (Table II; available at www.jpeds.com).
Table II.
Item characteristics; ADOS responses ordered by difference between autism and non-spectrum.
| ADOS Item and Score | Overall (N=21) | Nonspectrum (N=8) | ASD/Autism (N=13) | Autism Cog AE <=18 (N=8) | Autism Cog AE >18 (N=5) |
|---|---|---|---|---|---|
| Directed facial expressions 0 | 8 (38.1%) | 8 (100%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Directed facial expressions ≥1 | 13 (61.9%) | 0 (0.0%) | 13 (100%) | 8 (100%) | 5 (100%) |
| Unusual eye contact 0 | 9 (42.9%) | 8 (100%) | 1 (7.7%) | 0 (0.0%) | 1 (20.0%) |
| Unusual eye contact ≥1 | 12 (57.1%) | 0 (0.0%) | 12 (92.3%) | 8 (100%) | 4 (80.0%) |
| Quality of social overtures 0 | 7 (33.3%) | 7 (87.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Quality of social overtures ≥1 | 14 (66.7%) | 1 (12.5%) | 13 (100%) | 8 (100%) | 5 (100%) |
| Directed vocalization 0 | 8 (38.1%) | 7 (87.5%) | 1 (7.7%) | 1 (12.5%) | 0 (0.0%) |
| Directed vocalization ≥1 | 13 (61.9%) | 1 (12.5%) | 12 (92.3%) | 7 (87.5%) | 5 (100%) |
| Gestures 0 | 8 (38.1%) | 7 (87.5%) | 1 (7.7%) | 1 (12.5%) | 0 (0.0%) |
| Gestures ≥1 | 13 (61.9%) | 1 (12.5%) | 12 (92.3%) | 7 (87.5%) | 5 (100%) |
| Requesting 0 | 11 (52.4%) | 8 (100%) | 3 (23.1%) | 3 (37.5%) | 0 (0.0%) |
| Requesting ≥1 | 10 (47.6%) | 0 (0.0%) | 10 (76.9%) | 5 (62.5%) | 5 (100%) |
| Response to name 0 | 11 (52.4%) | 8 (100%) | 3 (23.1%) | 2 (25.0%) | 1 (20.0%) |
| Response to name ≥1 | 10 (47.6%) | 0 (0.0%) | 10 (76.9%) | 6 (75.0%) | 4 (80.0%) |
| Shared enjoyment 0 | 11 (52.4%) | 8 (100%) | 3 (23.1%) | 2 (25.0%) | 1 (20.0%) |
| Shared enjoyment ≥1 | 10 (47.6%) | 0 (0.0%) | 10 (76.9%) | 6 (75.0%) | 4 (80.0%) |
| Showing 0 | 6 (28.6%) | 6 (75.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Showing ≥1 | 15 (71.4%) | 2 (25.0%) | 13 (100%) | 8 (100%) | 5 (100%) |
| Integration gaze behaviors 0 | 12 (57.1%) | 8 (100%) | 4 (30.8%) | 3 (37.5%) | 1 (20.0%) |
| Integration gaze behaviors ≥1 | 9 (42.9%) | 0 (0.0%) | 9 (69.2%) | 5 (62.5%) | 4 (80.0%) |
| Overactivity 0 | 10 (47.6%) | 7 (87.5%) | 3 (23.1%) | 2 (25.0%) | 1 (20.0%) |
| Overactivity ≥1 | 11 (52.4%) | 1 (12.5%) | 10 (76.9%) | 6 (75.0%) | 4 (80.0%) |
| Response to joint attention 0 | 10 (47.6%) | 7 (87.5%) | 3 (23.1%) | 3 (37.5%) | 0 (0.0%) |
| Response to joint attention ≥1 | 11 (52.4%) | 1 (12.5%) | 10 (76.9%) | 5 (62.5%) | 5 (100%) |
| Responsive social smile 0 | 11 (52.4%) | 7 (87.5%) | 4 (30.8%) | 3 (37.5%) | 1 (20.0%) |
| Responsive social smile ≥1 | 10 (47.6%) | 1 (12.5%) | 9 (69.2%) | 5 (62.5%) | 4 (80.0%) |
| Initiation of joint attention 0 | 6 (28.6%) | 5 (62.5%) | 1 (7.7%) | 1 (12.5%) | 0 (0.0%) |
| Initiation of joint attention ≥1 | 15 (71.4%) | 3 (37.5%) | 12 (92.3%) | 7 (87.5%) | 5 (100%) |
| Unusual sensory interest 0 | 9 (42.9%) | 6 (75.0%) | 3 (23.1%) | 2 (25.0%) | 1 (20.0%) |
| Unusual sensory interest ≥1 | 12 (57.1%) | 2 (25.0%) | 10 (76.9%) | 6 (75.0%) | 4 (80.0%) |
| Pointing 0 | 4 (19.0%) | 4 (50.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Pointing ≥1 | 17 (81.0%) | 4 (50.0%) | 13 (100%) | 8 (100%) | 5 (100%) |
| Giving 0 | 7 (33.3%) | 5 (62.5%) | 2 (15.4%) | 2 (25.0%) | 0 (0.0%) |
| Giving ≥1 | 14 (66.7%) | 3 (37.5%) | 11 (84.6%) | 6 (75.0%) | 5 (100%) |
| Intonation of vocalization 0 | 7 (33.3%) | 5 (62.5%) | 2 (15.4%) | 1 (12.5%) | 1 (20.0%) |
| Intonation of vocalizations ≥1 | 13 (61.9%) | 3 (37.5%) | 10 (76.9%) | 6 (75.0%) | 4 (80.0%) |
| Intonation of vocalizations 8 | 1 (4.8%) | 0 (0.0%) | 1 (7.7%) | 1 (12.5%) | 0 (0.0%) |
| Functional play with objects 0 | 5 (23.8%) | 4 (50.0%) | 1 (7.7%) | 1 (12.5%) | 0 (0.0%) |
| Functional play with objects ≥1 | 16 (76.2%) | 4 (50.0%) | 12 (92.3%) | 7 (87.5%) | 5 (100%) |
| Anxiety 0 | 18 (85.7%) | 5 (62.5%) | 13 (100%) | 8 (100%) | 5 (100%) |
| Anxiety ≥1 | 3 (14.3%) | 3 (37.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Repetitive interest 0 | 7 (33.3%) | 4 (50.0%) | 3 (23.1%) | 1 (12.5%) | 2 (40.0%) |
| Repetitive interest ≥1 | 14 (66.7%) | 4 (50.0%) | 10 (76.9%) | 7 (87.5%) | 3 (60.0%) |
| Hand as tool 0 | 15 (71.4%) | 7 (87.5%) | 8 (61.5%) | 5 (62.5%) | 3 (60.0%) |
| Hand as tool ≥1 | 6 (28.6%) | 1 (12.5%) | 5 (38.5%) | 3 (37.5%) | 2 (40.0%) |
| Imagination creativity 0 | 2 (9.5%) | 2 (25.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Imagination creativity ≥1 | 19 (90.5%) | 6 (75.0%) | 13 (100%) | 8 (100%) | 5 (100%) |
| Tantrums negative 0 | 16 (76.2%) | 7 (87.5%) | 9 (69.2%) | 5 (62.5%) | 4 (80.0%) |
| Tantrums negative≥1 | 5 (23.8%) | 1 (12.5%) | 4 (30.8%) | 3 (37.5%) | 1 (20.0%) |
| Hand/finger movements 0 | 19 (90.5%) | 8 (100%) | 11 (84.6%) | 6 (75.0%) | 5 (100%) |
| Hand/finger movements ≥1 | 2 (9.5%) | 0 (0.0%) | 2 (15.4%) | 2 (25.0%) | 0 (0.0%) |
| Immediate echolalia 0 | 12 (57.1%) | 7 (87.5%) | 5 (38.5%) | 4 (50.0%) | 1 (20.0%) |
| Immediate echolalia missing 8 | 9 (42.9%) | 1 (12.5%) | 8 (61.5%) | 4 (50.0%) | 4 (80.0%) |
| Language level ≥1 | 21 (100%) | 8 (100%) | 13 (100%) | 8 (100%) | 5 (100%) |
| Self injurious behavior 0 | 21 (100%) | 8 (100%) | 13 (100%) | 8 (100%) | 5 (100%) |
Cognitive impairment paralleled their ADOS scores (Figure 2). Because a cognitive age-equivalent of 18 months or higher is recommended when using the ADOS and the majority of participants in this study were functioning below that level, comparisons between higher and lower functioning participants were made to ensure the ADOS was capturing valid behaviors. Children scoring above 18 months in cognitive age equivalent had fewer but not different autistic-like behaviors compared with those scoring below 18 months (Table II). For those interested, Table III (available at www.jpeds.com) summarizes the ADOS results of the children with the later onset form.
Table III.
Characteristics of excluded patients diagnosed after age 6
| ID | Sex | Age at DX in years | Age at Testing (months) | Cog AE (months) | ADOS Module | ADOS SA Total | ADOS RRB Total | ADOS SA+RRB Total | ADOS range |
|---|---|---|---|---|---|---|---|---|---|
| MPS3005 | M | 6.42 | 80 | 46 | 2 | 1 | 1 | 2 | Nonspectrum |
| MPS3014 | F | 10.33 | 201 | 45 | 2 | 12 | 5 | 17 | Autism |
| MPS3017 | M | 10.50 | 132 | 28 | 2 | 7 | 5 | 12 | Autism |
| MPS3018 | F | 12.42 | 155 | 61 | 2 | 4 | 0 | 4 | Nonspectrum |
| MPS3020 | F | 15.58 | 220 | 6 | 1 | 17 | 2 | 19 | Autism |
| MPS3026 | M | 10.17 | 133 | 51 | Not tested |
Discussion
This study used the ADOS, the “gold standard” tool for assessing for behaviors compatible with ASD, to systematically evaluate for a wide range of ASD behaviors in children with MPS IIIA. Previous studies have employed behavior checklists and clinical observations1. Because all but one of the children with the severe phenotype did not have more than single word language, we were able to only use children who had been administered ADOS Module 1 (nonverbal or speaking in primarily single words). Use of the same module allowed comparison across a homogeneous subject group, as well as across individual ADOS items.
We found a strong association of ADOS score with age, with an increased incidence of autistic-like social behaviors emerging between ages 3 and 4 in those children with the early onset form of the disease (diagnosed before the age of six). The most common changes occurring between the ages of 3 and 4 included a decrease in social communicative behaviors, including use of nonverbal behaviors like eye contact, facial expressions and gestures, directed vocalizations, responsiveness to their name, and shared enjoyment in interacting. Restricted interests, rigidity, and repetitive behaviors, like hand flapping and finger flicking, were largely absent. The repetitive behaviors observed were primarily restricted to oral behaviors and did not include other behaviors usually associated ASD.
Several studies have found a smaller percentage of children with MPS IIIA having autistic behaviors than was found in this study. For example, Heron et al1 found that of the patients with MPS IIIA diagnosed before the age of 5 years, 67% had abnormal behavior and 20% had autism-related symptoms. However, because we found that age at assessment is associated with the presence or absence of autistic symptoms, it may be that the patients in their study were not old enough to have developed such symptoms. Similarly, the distinction between early onset and later onset needs to be made when discussing phenotypes and autistic behaviors. Children with later onset MPS IIIA were omitted from this study in order to keep our sample more homogenous because they have a different trajectory of development than the early onset form. Of the children omitted (diagnosed between 6 and 15 years), 4 of the 5 children who were administered the ADOS had phrase speech, whereas 21 of the 22 children with the early onset form spoke in single words or were nonverbal (Table III).
A limitation of this study was administration of the ADOS to some children who were severely impaired cognitively (cognitive age-equivalent below 18 months). Although we found a high correlation between cognitive age-equivalent and autistic behaviors, low cognitive age-equivalent alone cannot account for autistic behaviors. Comparison of those with cognitive age-equivalent above 18 months to those below 18 months indicates that, and the lower functioning children had more symptoms, the pattern was the same as for the higher functioning children. It is likely that onset of autistic symptoms and declining cognitive age go hand-in-hand with disease progression.
The emergence of new autistic-like symptoms in children with early onset Sanfilippo syndrome provides a model of ‘acquired’ autistic behaviors. Although identifiable genetic etiologies are found in 10–20% of individuals with ASD16 we are unaware of any other genetic condition in which autistic-like behaviors emerge from an otherwise normal developmental and behavioral background. Children with MPS IIIA would not qualify for a formal diagnosis of autism, as they do not have social difficulties prior to age 3, nor do they consistently have restricted/repetitive behaviors, other than hyper-orality; however, characterizing the behavioral phenotype by age enables us to view MPS IIIA as a model for acquisition of autistic-like social behaviors. This perspective may lead us to better understand the overlapping neural characteristics of both disorders.
The neural link is supported by the finding that the behavioral abnormalities in MPS IIIA can be described as a Klüver-Bucy-like presentation that appears after the age of 2 or 3 years, with diminished fear, startle, poor social interaction, orality, and reduced emotional attachment and compliance with parents associated change in amygdala volume.17 Also, damage to the medial prefrontal cortex can result in personality traits similar to ASD.18
Another neural link is demonstrated by the association of autistic behavior (social and behavioral) with abnormalities in heparan sulfate metabolism in murine models; given the central role of heparan sulfate in MPS III, this specific chemical link needs further exploration19. Although we have not administered the ADOS to other patients with MPS I (Hurler syndrome) and MPS II (Hunter syndrome), from both our clinical observations of a large number of such patients and our review of the literature, no parallel acquisition of autism has been documented, even though they share biochemically similar pathology (accumulation of heparan sulfate).
Because there is no treatment for Sanfilippo syndrome at present, interventions designed for children with ASD may provide a means of alleviating some of the autism-like symptoms in affected children. A study of the value of applied behavior analysis (ABA) for children with MPS IIIA is warranted.
We conclude that an increase of autistic-like social behaviors in children with the early onset form of MPS IIIA occurs between 3 and 4 years of age. The onset of autistic-like symptoms around 46 months (and in a few cases before that), suggests that such symptoms are characteristic of disease progression in MPS IIIA. All children after 46 months had sufficient symptoms to meet ADOS criteria for autism or ASD. Lack of developmental gains or decreasing cognition together with acquired autistic social behaviors should be a red flag for pediatricians; MPS IIIA should be included in their differential diagnosis.
Acknowledgments
The Lysosomal Disease Network (U54NS065768) is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science, the National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Supported by Shire (E.S. and C.W.), the National Center for Advancing Translational Sciences (UL1TR000114), and the University of Minnesota Foundation Autism Initiative.
We would like to acknowledge the University of Minnesota Autism Spectrum and Neurodevelopmental Disorders Clinic, the Center for Neurobehavioral Development, Amy Esler, PhD (University of Minnesota Autism Spectrum and Neurodevelopmental Disorders Clinic) who conducted several of the ADOS assessments, Brianna Yund (funded by National Institute of Neurological Disorders and Stroke [U54NS065768]), who entered the data, and the families who helped advance research by participating in this study. We thank Patrick Haslett, MD (employee of Shire Pharmaceuticals), for his role in designing the overall trial and assistance in reviewing the initial manuscript.
Abbreviations and Acronyms
- MPS IIIA
Mucopolysaccharidosis Type IIIA
- ASD
Autism Spectrum Disorder
- ADOS
Autism Diagnostic Observation Schedule
- SCSH
Ser298Pro in the sulphamidase gene
- NH
Natural History
- BSID-III
Bayley Scales of Infant Development – Third Edition
- SA
Social Affect
- RRB
Restricted and Repetitive Behavior
Footnotes
Registered with ClinicalTrials.gov: NCT 01047306
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. E.S., C.W., and K.D. are consultants for Shire Pharmaceuticals.
The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heron B, Mikaeloff Y, Froissart R, Caridade G, Maire I, Caillaud C, et al. Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. Am J Med Genet. 2011;155A:58–68. doi: 10.1002/ajmg.a.33779. [DOI] [PubMed] [Google Scholar]
- 2.Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, et al. Specific Genetic disorders and autism: Clinical contribution towards their identification. J Autism Dev Dis. 2005;35:103–16. doi: 10.1007/s10803-004-1038-2. [DOI] [PubMed] [Google Scholar]
- 3.Jabourian A, Turpin JC, Maire I, Baumann N. Autistic-like disorders and Sanfilippo syndrome. Ann Med Psychol. 2011;160:421–26. [Google Scholar]
- 4.Wijburg FA, Węgrzyn G, Burton BK, Tylki-Szymańska A. Mucopolysaccharidosis type III (Sanfilippo Syndrome) and misdiagnosis of idiopathic developmental delay, attention deficit/hyperactivity disorder or autism spectrum disorder. Acta pædiatr. 2013 doi: 10.1111/apa.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lord C, Rutter M, DiLavore P, Risi S. Autism diagnostic observation schedule. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- 6.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–54. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 7.Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–56. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 8.Baehner F, Schmiedeskamp C, Krummenauer F, Miebach E, Bajbouj M, Whybra C, et al. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–17. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- 9.Malm G, Lund AM, Månsson JE, Heiberg A. Mucopolysaccharidoses in the Scandinavian countries: incidence and prevalence. Acta Paediatr. 2008;97:1577–1581. doi: 10.1111/j.1651-2227.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 10.Cleary MA, Wraith JE. Management of mucopolysaccharidosis type III. Arch Dis Child. 1993;69:403–6. doi: 10.1136/adc.69.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopwood J. Sanfilippo Syndrome: Clinical genetic diagnosis and therapies. In: Barranger J, Cabrera-Salazar M, editors. Lysosomal storage disorders. Springer; 2007. pp. 415–32. [Google Scholar]
- 12.Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales. 2. San Antonio, TX: Psychological Corporation; 2005. [Google Scholar]
- 13.Whitley CB, Cooksley R, Diethelm-Okita B, Delaney K, Haslett P, Richard C, Shapiro E. Genotype, Disease Onset, and Neurocognitive Phenotype of Sanfilippo Syndrome Type A. Mol Gen Metab. 2012;105:S58. [Google Scholar]
- 14.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: Revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37:613–27. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 15.Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio, TX: Psychological Corporation; 2006. [Google Scholar]
- 16.Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nature Rev Gen. 2008;9:341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potegal M, Yund B, Rudser K, Delaney K, Nestrasil I, Whitley C, Shapiro E. Mucopolysaccharidosis Type IIIA as a variant of Klüver-Bucy syndrome: A comparison of social/emotional characteristics of children with MPS IIIA to those with MPS IH. Mol Gen Metab. 2012;105:S53. [Google Scholar]
- 18.Umeda S, Mimura M, Kato M. Acquired personality traits of autism following damage to the medial prefrontal cortex. Soc Neurosci. 2010;5:19–29. doi: 10.1080/17470910902990584. [DOI] [PubMed] [Google Scholar]
- 19.Irie F, Badie-Mahdavi H, Yamaguchi Y. Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. PNAS. 2012;109:5052–56. doi: 10.1073/pnas.1117881109. [DOI] [PMC free article] [PubMed] [Google Scholar]