Abstract
Measles virus (MV) deficient in C protein (Cko) expression efficiently induces both stress granules (SG) and interferon (IFNβ), whereas isogenic wild-type (WT) and V mutant (Vko) viruses do not. We therefore examined the effect of IFNβ pretreatment on SG formation, and the roles played by the IFN-inducible double-stranded (ds) RNA-dependent protein kinase (PKR) and dsRNA adenosine deaminase (ADAR1). SG formation in ADAR1-sufficient cells infected with WT or Vko mutant virus was enhanced by IFN treatment and was PKR-dependent. SG formation in Cko virus-infected cells was already high without IFN treatment and was not further enhanced by IFN. IFN treatment alone, in the absence of infection, induced SG formation in ADAR1-deficient but not ADAR1-sufficient cells. Type I IFN-induced enhancement in SG formation occurred by a canonical IFN signaling response dependent upon STAT1 and STAT2. These results further establish ADAR1 as a suppressor of the interferon and SG innate immune responses.
Keywords: adenosine deaminase acting on RNA (ADAR), interferon (IFN), stress granules (SG), signal transducers and activators of transcription (STAT)
INTRODUCTION
Measles virus (MV), an enveloped virus that possesses a nonsegmented, negative-sense single-stranded RNA genome (−ssRNA), is a prototype Morbillivirus of the Paramyxoviridae family (Griffin et al., 2012; Knipe et al., 2007). The ~15.9-kb −ssRNA genome carries six genes that encode six virion proteins, the nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion envelope glycoprotein (F), hemagglutinin envelope glycoprotein (H), and the RNA-dependent RNA polymerase or large protein (L). The P/V/C gene, in addition to coding for the P protein that is a polymerase cofactor, also encodes two accessory nonstructural proteins, V and C. Studies of mutant viruses defective for expression of either V or C protein have revealed that V and C function to affect antiviral innate immune responses to MV, including both the induction of IFN and the subsequent actions of IFN (Caignard et al., 2009; Fontana et al., 2008; Ramachandran et al., 2008; Randall and Goodbourn, 2008; Schuhmann et al., 2011; Sparrer et al., 2012). MV remains an important human pathogen. Infection of the respiratory system and spread to the lymphatic system can result in immune suppression and febrile disease in children (Muhlebach et al., 2011). In spite of an effective vaccine, measles disease remains a leading cause of morbidity and mortality in developing countries (Griffin et al., 2012). The potential for use of attenuated MV as an engineered oncolytic agent, together with the need for improved vaccines and immunization regimens, have fueled efforts to better understand the molecular basis of host innate responses to MV infection.
The interferon (IFN) system is a cornerstone of host innate antiviral immunity. Among the cellular sensors of viral infection that trigger the transcriptional induction of IFNs are the cytoplasmic retinoic acid-inducible gene I (RIG-I) family of proteins. These sensors detect viral RNAs, including MV RNA (McAllister et al., 2010), as foreign in a manner that differentiates them from cellular RNAs, thereby activating the signaling responses leading to IFN production (Borden et al., 2007; Yoneyama and Fujita, 2010). The action of IFNs involves transcriptional induction of IFN-stimulated genes (ISGs) whose products are responsible for the biologic activities of IFNs, including their antiviral activity (Randall and Goodbourn, 2000; Samuel, 2001). Among the pathways through which type I IFNs signal is the canonical JAK-STAT pathway (Friedman and Stark, 1985; Borden et al., 2007). Binding of type I IFNs to their cognate receptor leads to activation of Jak1 and Tyk2 kinases and phosphorylation of cytoplasmic signal inducers and activators of transcription STAT1 and STAT2, which together with interferon regulatory factor IRF9 form a trimeric complex that translocates to the nucleus and drives the expression of genes under the control of an interferon-stimulated response element ISRE (Schindler et al., 2007). Two of the ISRE-containing ISGs induced by IFN through JAK-STAT signaling encode enzymes that bind double-stranded (ds) RNA: the protein kinase PKR; and, the RNA adenosine deaminase ADAR1.
PKR is regulated by dsRNA as an effector of activation (Sadler and Williams, 2007; Samuel, 1993), whereas ADAR1 utilizes dsRNA as its substrate (Bass, 2002; Samuel, 2011). The best-characterized substrate of PKR is translation initiation factor eIF2α, which when phosphorylated on serine 51, leads to the inhibition of translation. For several viruses, PKR is antiviral and proapoptotic (Pfaller et al., 2011; Sadler and Williams, 2007; Samuel, 2011). ADAR1 catalyzes the C6 deamination of adenosine in dsRNA structures, a process known as A-to-I editing that can lead to altered decoding during translation or altered stabilization of RNA structures. I-U mismatch bp are less stable than A-U bp (Bass, 2002; Samuel, 2011). ADAR1, in contrast to PKR, is typically proviral and antiapoptotic (Samuel, 2001, 2011).
The opposing activities of PKR and ADAR1 are illustrated with MV. PKR activation correlates with reduced virus yield (Toth et al., 2009a), enhanced IFN induction (McAllister et al., 2010), formation of stress granules (Okonski and Samuel, 2013), and increased cytotoxicity and apoptosis (Toth et al., 2009b). ADAR1 deficiency leads to increased PKR activation (Toth et al., 2009b), enhanced IRF3 activation and increased IFN induction (Li et al., 2012; Ward et al., 2011), increased cytotoxicity (Toth et al., 2009b; Ward et al., 2011), and increased stress granule formation (Okonski and Samuel, 2013) in MV-infected cells. In contrast, ADAR1 sufficiency or overexpression leads to increased virus yields (Gelinas et al., 2011; Toth et al., 2009b), suppression of stress granule formation, PKR activation and IFN induction (Li et al., 2012; Okonski and Samuel, 2013) and suppression of apoptosis (Toth et al., 2009b). As is seen with MV-infected ADAR1-deficient cells in culture (Li et al., 2012), the importance of ADAR1 to normal physiology including the regulation of IFN production is further illustrated by the phenotypes of ADAR1 null mice (Hartner et al., 2009) and human Aicardi-Goutieres syndrome patients with mutations in ADAR1 (Rice et al., 2012), both conditions of which reveal ADAR1 as a suppressor of type I IFN production and signaling.
Stress granule formation is a hallmark of virus infection and is PKR-dependent for MV and several additional viruses (Anderson and Kedersha, 2008, 2009; Beckham and Parker, 2008). Inhibition of translation initiation, including by eIF2α phosphorylation, leads to aggregates of stalled initiation complexes that contain translationally silent mRNAs, 40S ribosome subunits, eIF4E, eIF4G and eIF3 factors, and RNA binding proteins including G3BP1 (Ras GAP SH3-domain binding protein 1) and TIA-1 (T-cell restricted intracellular antigen 1) that are characteristic SG markers (Anderson and Kedersha, 2008, 2009; Beckham and Parker, 2008). Mutant MV unable to express C protein is an especially robust inducer of SG formation compared to either isogenic parental MV or a mutant unable to express V protein, both of which are poor SG inducers (Okonski and Samuel, 2013). MV-induced formation of SG is dependent upon catalytically active PKR protein (Okonski and Samuel, 2013; Taghavi and Samuel, 2013). The IFN inducible p150 form of ADAR1 impairs SG formation following infection, but the p110 constitutive form and the p150 catalytic mutant do not (Okonski and Samuel, 2013).
The Cko mutant of MV that is an efficient inducer of SG also is a potent inducer of IFNβ. We, therefore, wanted to test whether IFN treatment affected the SG response in either uninfected or MV-infected cells, and if so, to assess the roles played by PKR and ADAR1 in the process. We found SG formation in ADAR1-sufficient cells following WT or Vko mutant MV infection was enhanced by IFN pretreatment and was PKR-dependent. The robust SG formation seen following Cko mutant infection was not further enhanced by IFN treatment. Treatment alone with either IFNα or IFNβ, in the absence of subsequent infection, induced SG formation in ADAR1kd cells but not in ADAR1-sufficient cells. The IFN induced enhancement in SG formation occurred by canonical type 1 IFN signaling dependent upon both STAT1 and STAT2 transcription factors. These results further establish ADAR1 as a suppressor of host innate responses, including the interferon and SG responses.
METHODS
Cells and viruses
Vero cells and parental HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (D-MEM, Gibco) supplemented with 5 % (v/v) fetal bovine serum (Hyclone), penicillin (100 μg/mL) and streptomycin (100 units/mL) (Gibco). HeLa cells stably knocked-down for PKR (PKRkd) (Zhang et al., 2008; Zhang and Samuel, 2007) or ADAR1 (ADAR1kd) (Li et al., 2010; Toth et al., 2009b) expression by a short hairpin RNAi silencing strategy, and drug resistant control cells (CONkd), were as described previously. These cells were maintained under selection in the presence of puromycin (1 g/mL, Sigma-Aldrich). Parental 2fTGH cells (McKendry et al., 1991) and derived mutant lines U3A (STAT1 deficient) and U6A (STAT2 deficient) (Leung et al., 1995), generously provided by George Stark (Cleveland Clinic, Cleveland, OH), were maintained in D-MEM with 10% fetal bovine serum. Recombinant Moraten measles virus vaccine strain was used as described previously (Devaux et al., 2007; Toth et al., 2009a) and included wild-type (WT) parental virus and isogenic mutants deficient for either V protein (Vko) or C protein (Cko) expression. These recombinant viruses were engineered to express green fluorescent protein (GFP) downstream from the viral (H) hemagglutinin gene. Virus infections were carried out at a multiplicity of infection (MOI) of 0.1 or 1 50% tissue culture dose (TCID50) per cell as indicated (Karber, 1931). Interferon treatment was with 1000 units/mL for 18-24 h with IFNβ unless otherwise specified. IFNβ was from Toray Industries and IFNαA/D from PBL Interferon Source.
Immunofluorescence analysis
Immunofluorescence was carried out essentially as described (Okonski and Samuel, 2013). Briefly, cells seeded in 12-well plates and grown on 18-mm glass coverslips were either infected or not, and then were fixed at the indicated time after infection with 10% (vol/vol) neutral buffered formalin (Sigma). Permeablization and blocking were done using Tris-buffered saline (TBS) supplemented with 2% (vol/vol) normal donkey serum (Jackson ImmunoResearch) and 0.2% (vol/vol) Triton X-100 (CBP buffer). Permeablized cells were incubated overnight at 4° C with primary antibody diluted in CBP buffer. Primary antibodies used for immune fluorescence were mouse monoclonal anti-dsRNA antibody (clone J2, English & Scientific Consulting) (1:200 dilution); mouse monoclonal anti-G3BP1 antibody (Sigma-Aldrich) (1:500); rabbit anti-G3BP1 antibody (Sigma-Aldrich) (1:500); chicken anti-GFP antibody (Molecular Probes) (1:300); and, goat anti-TIA-1 antibody (Santa Cruz Biotechnology) (1:50). Coverslips with cells treated with primary antibodies then were washed with TBS supplemented with 0.7% fish gelatin and Triton X-100 (QW buffer) before incubation at 37 °C for 1 h with secondary antibody diluted in CBP. Secondary antibodies for immunofluorescence were Alexa Fluor 350 anti-rabbit, Alexa Fluor 594 anti-mouse, and Alexa Fluor 488 anti-chicken (all from Molecular Probes) (1:250). Coverslips were washed with QW buffer and then with TBS before mounting using ProLong Gold (Molecular Probes). Slides were analyzed and images were captured using an IX71 Fluorescence Microscope (Olympus) with a Retiga-2000R camera and Q-Capture PRO software (version 6.0, QImaging). Images were processed using IrfanView (version 4.33) and the individual channels were overlaid using GIMP (version 2.8.2).
Quantification of stress granules
To determine the number of SG-positive cells, three representative wide-field 40× images were selected per experiment and a minimum of 100 cells were counted. Cells that exhibited punctate immunofluorescent cytoplasmic foci with the SG marker G3BP1 were scored as SG-positive. Percentages were determined as the number of SG-containing cells divided by the total number of cells ×100.
Western immunoblot analysis
Western blotting was carried out as previously described (Okonski and Samuel, 2013; Toth et al., 2009a). Briefly, whole cell extracts were prepared at 24 h after infection unless otherwise specified. Protein concentrations were determined by the Bradford method using Bio-Rad Protein Assay reagent (Bio-Rad). Protein fractionation (25 to 30 μg total protein/lane) was by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. Proteins were transferred from gels to nitrocellulose membranes, blocked with 5 % (wt/vol) non-fat dry milk in PBS or with 5 % (wt/vol) bovine serum albumin in TBS (for phosphoproteins) for 60 min. Primary antibodies used for detection were rabbit antisera directed against MV proteins N(505) (1:5000), V(C-term) (1:5000), C(2) (1:5000) and H(cyt) (1:5000) generously provided by Roberto Cattaneo (Mayo, Rochester, MN); rabbit polyclonal anti-GFP (Molecular Probes) (1:1000); rabbit polyclonal anti-PKR (Cell Signaling) (1:1000); mouse anti-G3BP1 (Sigma-Aldrich) (1:5000); rabbit monoclonal phospho-anti-PKR(T446) (Epitomics) (1:1000); and, mouse monoclonal anti-tubulin (Sigma-Aldrich) (1:5000). Secondary antibodies were anti-rabbit IRDye800 and anti-mouse IRDye680 (both from LI-COR) (1:10000). Membranes were scanned using a LI-COR Odyssey FX imaging system at optimized intensities and quantified using the Odyssey image processing software (version 3.0, LI-COR). Images were further processed using GIMP (version 2.8.2).
Virus infections and growth assays
CONkd, PKRkd, and ADARkd cells were left untreated or were treated with IFNβ at 1000 units/mL for 18- 24 h before infection with 1 TCID50 /cell. After 2 h of virus adsorption the inoculum was removed, the monolayers rinsed twice and the cultures incubated in maintenance medium for 24 h. Total virus yields were determined by 50% TCID50 titration on Vero cells according to Spearman Karber method (Devaux et al., 2008; Karber, 1931).
RESULTS
Interferon treatment of cells deficient in ADAR1 leads to formation of stress granules in the absence of infection comparable to that seen in measles virus-infected cells
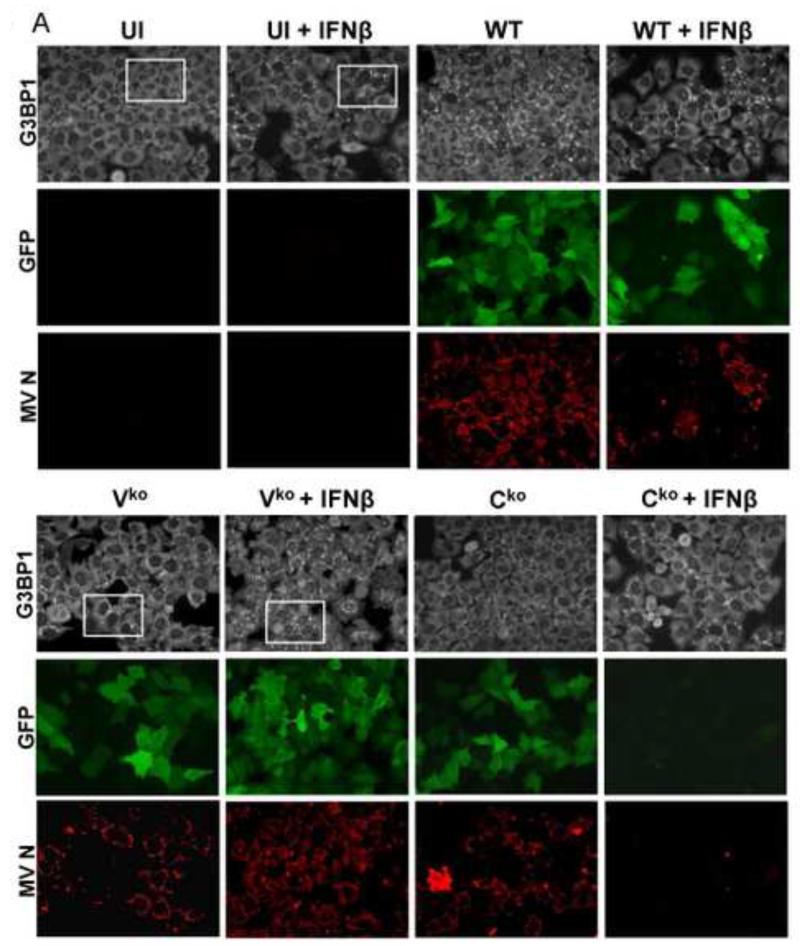
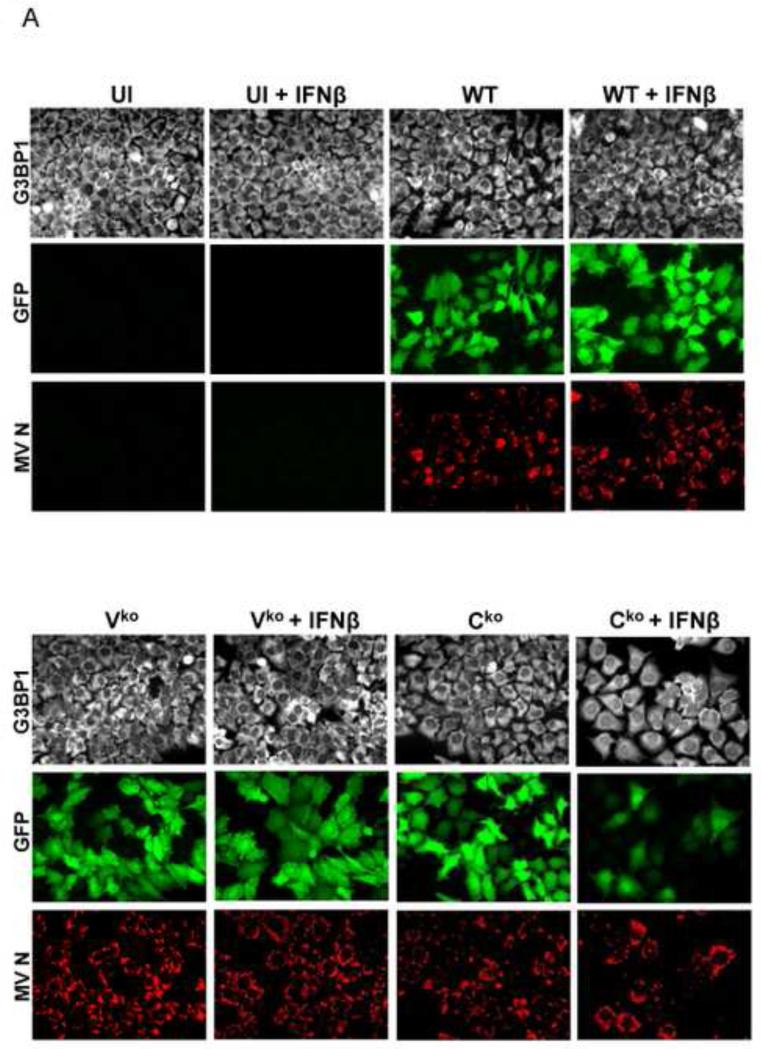
ADAR1 deficiency is characterized by increased activation of protein kinase PKR, induction of IFN and formation of stress granules following infection with WT or Vko measles virus (MV), both of which expresses C protein (Li et al., 2010, 2012; Okonski and Samuel, 2013). Because WT virus is a poor inducer of both IFN and SG in ADAR1-sufficient cells, but becomes a good inducer in ADAR1-deficient cells with a SG inducing capacity comparable to that of Cko MV, we examined the effect of IFN pretreatment on SG formation. We used the Moraten strain of MV because entry of the vaccine strain is by the CD46 receptor present on HeLa cells (Navaratnarajah et al., 2009). HeLa cells stably deficient in ADAR1 (ADAR1kd) express less than 15% of the p150 and p110 ADAR1 proteins compared to CONkd control or parental HeLa cells (Li et al., 2012; Toth et al., 2009b). To test whether IFN pretreatment affects SG formation, ADAR1kd cells were either left untreated or were treated with IFNβ for 18 h, and then left uninfected or infected for 24 h. The staining pattern of G3BP1, an established marker of SG (Kedersha et al., 1999; Tourriere et al., 2003), was assessed by confocal immunofluorescence microscopy (Fig. 1).
Figure 1. Stress granule formation is enhanced by interferon treatment of cells deficient in ADAR1.
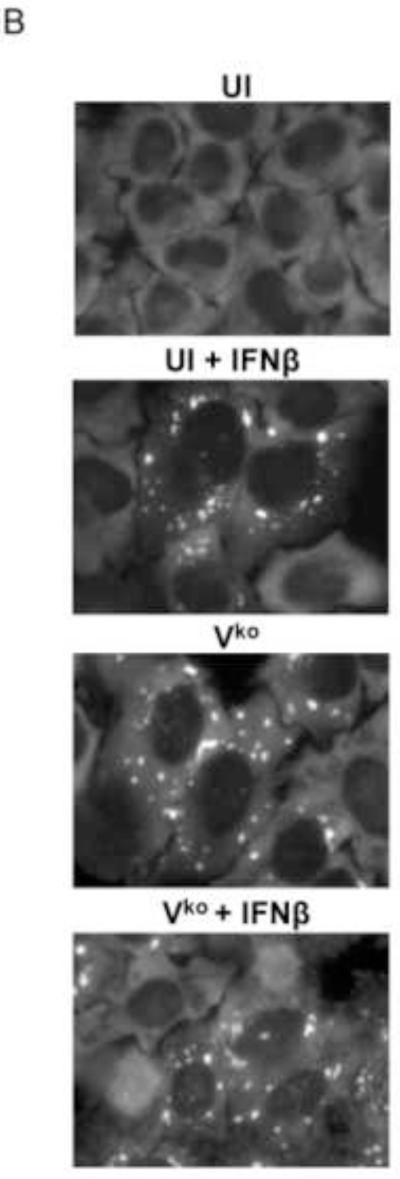
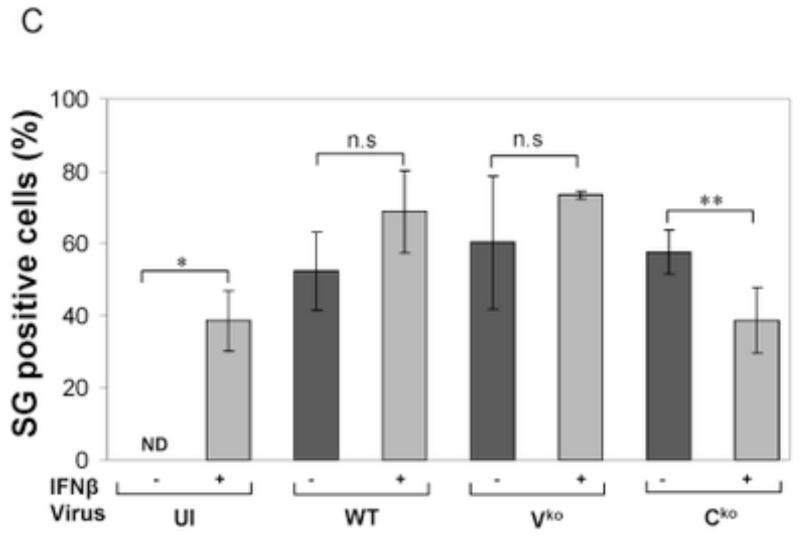
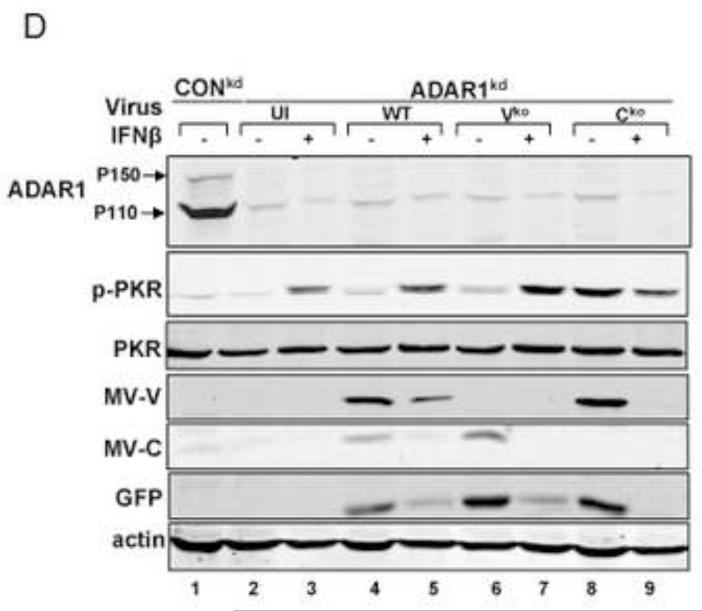
(A) HeLa ADAR1kd cells were either left untreated or were treated with IFNβ (1000 U/mL) for 18 h. Cells then were either left uninfected (UI) or were infected with WT, Vko or Cko measles virus (MV) at a multiplicity of infection of 1 TCID50/cell. At 24 h after infection cells were analyzed by immunofluorescence microscopy as described under Materials and Methods using antibodies to G3BP1 (white) as a marker for SG formation and N (red) and GFP (green) for viral infection. (B) Magnification of the framed sections in panel A. (C) Quantification of SG-positive cells. Three representative wide-field 40× images were selected and a minimum of 100 cells were examined for the presence or absence of SG. Results are expressed as the percentage of infected cells that are positive for SG as described under the Methods section. Results are mean values and standard errors from three independent experiments. N.D., not detectable. Statistical significance determined by Students t test (two tailed, two sample with equal variance) comparing untreated cells to IFN treated cells. * P < 0.001, ** P < 0.05, n.s, not significant. (D) At 24 h after infection whole-cell extracts were prepared and analyzed by Western immunoblot assay using antibodies against ADAR1, phospho-Thr446 PKR (p-PKR), PKR, MV C protein, GFP, and actin.
A punctate staining pattern of cytoplasmic foci was observed for G3BP1 in untreated ADAR1kd cells following infection with either WT, Vko or Cko virus (Fig. 1A). In untreated cells that were not infected, the G3BP1 signal was disperse and uniform in the cytoplasm (Fig. 1A), in agreement with recent observations (Okonski and Samuel, 2013). By contrast, when the ADAR1kd cells were pretreated with IFNβ, the punctate G3BP1 staining pattern was observed not only in infected cells but also in uninfected cells (Fig. 1A, inset1B). Quantification revealed that about 40% of the uninfected ADAR1kd cells were SG positive following treatment with IFNβ, whereas SG formation was not detectable in untreated ADAR1kd cells in the absence of infection (Fig. 1C). IFN pretreatment inhibited both WT and mutant MV protein production measured by immunofluoresence (Fig. 1A) and western analysis (Fig. 1D). IFN pretreatment modestly increased the SG-positive cells from about 50-60% to about 60-70% following infection with either WT or Vko virus (Fig. 1C). By contrast, IFN pretreatment decreased the number of SG-positive cells (Fig. 1C) and viral protein production (Fig. 1D) following Cko virus infection, possibly due in part to the enhanced cytotoxicity and apoptosis seen with the C mutant compared to WT or V mutant virus in ADAR1kd cells (Toth et al., 2009a).
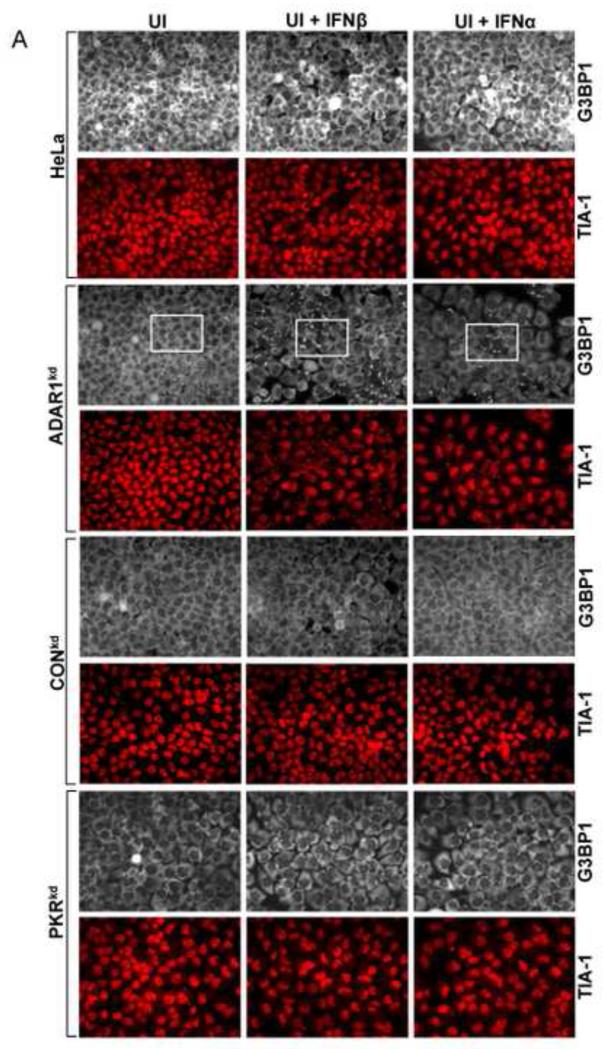
Interferon treatment does not detectably trigger stress granule formation in ADAR1 sufficient cells
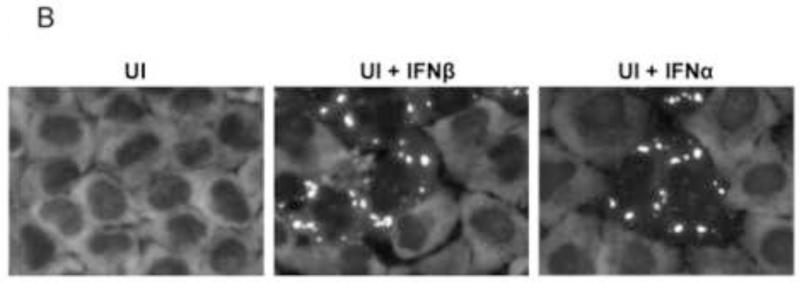
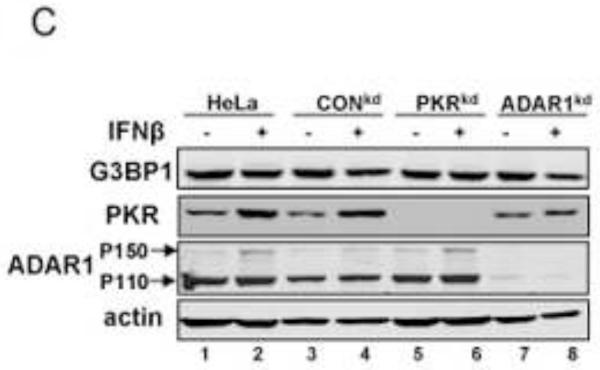
To test whether IFN treatment also affects SG formation in the ADAR1-sufficient cells similar to the enhancing effect observed in the ADAR1kd cells (Fig. 1), the responses of parental HeLa cells, the CONkd control, and HeLa cells stably deficient in PKR (PKRkd) were compared to the ADAR1kd line. Cells were treated with either alpha or beta type I IFN or were left untreated. The staining pattern of G3BP1 then was assessed by immunofluorescence microscopy (Fig. 2A). The G3BP1 signal in ADAR1-sufficient cells (parental HeLa cells, CONkd cells and PKRkd cells) was uniformly dispersed throughout the cytoplasm, both in untreated cells and cells treated with either IFNβ or IFNα for 18 h (Fig. 2A). By contrast, G3BP1 displayed a punctate cytoplasmic staining pattern characteristic of SG in the ADAR1kd cells when treated with either IFNβ or IFNα (Fig. 2A, inset 2B). ADAR1kd cells not treated with IFN showed a diffuse cytoplasmic staining comparable to untreated or IFN-treated parental, PKRkd or CONkd cells. The staining pattern of a second SG marker, the TIA-1 protein, was also assessed. The TIA-1 signal was largely nuclear in all four of the cell lines when left untreated. However, TIA-1 translocated to the cytoplasm and formed punctate foci in IFN-treated ADAR1kd cells but not in IFN-treated parental, PKRkd or CONkd cells (Fig. 2A), consistent with observations for G3BP1 (Fig. 1, 2A). Finally, the steady-state level of G3BP1 assessed by Western analysis was comparable in untreated and IFN-treated cells (Fig. 2C). PKR protein levels were increased by IFN treatment in parental and CONkd cells, whereas in PKRkd cells that did not show SG formation (Fig. 2A) following IFN treatment the level of PKR protein was still greatly reduced (Fig. 2C) consistent with prior observations (Okonski and Samuel, 2013).
Figure 2. Interferon treatment induces stress granule formation in the absence of viral infection in cells stably deficient for ADAR1 but not in cells stably deficient for PKR.
Parental HeLa cells and ADAR1kd, PKRkd and CONkd cells were either left untreated (UT) or were treated with either IFNβ or IFNα (1000 U/mL) for 24 h. (A) Cells then were analyzed by immunofluorescence microscopy as described under Materials and Methods using antibodies to G3BP1 (white) and TIA-1 (red) as markers for SG formation. (B) Magnification of the framed sections in panel A. (C) Extracts were prepared and analyzed by Western immunoblot assay using antibodies against G3BP1, PKR, ADAR1 and actin.
Interferon treatment enhances stress granule formation following infection of ADAR1 and PKR-sufficient cells
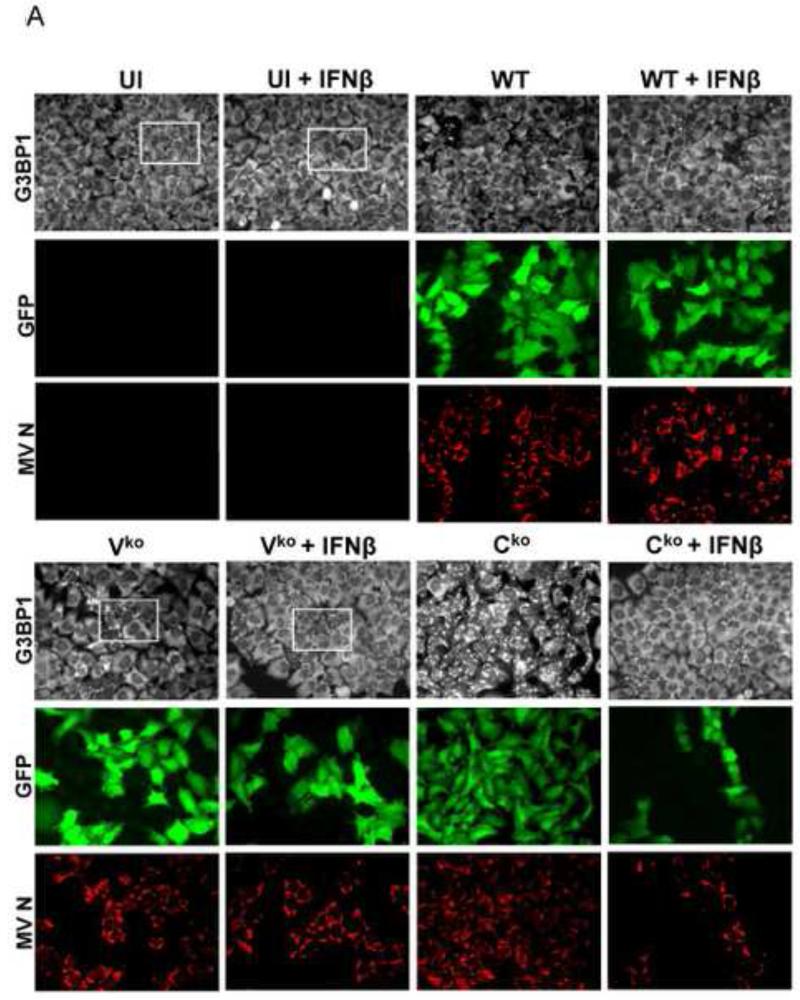
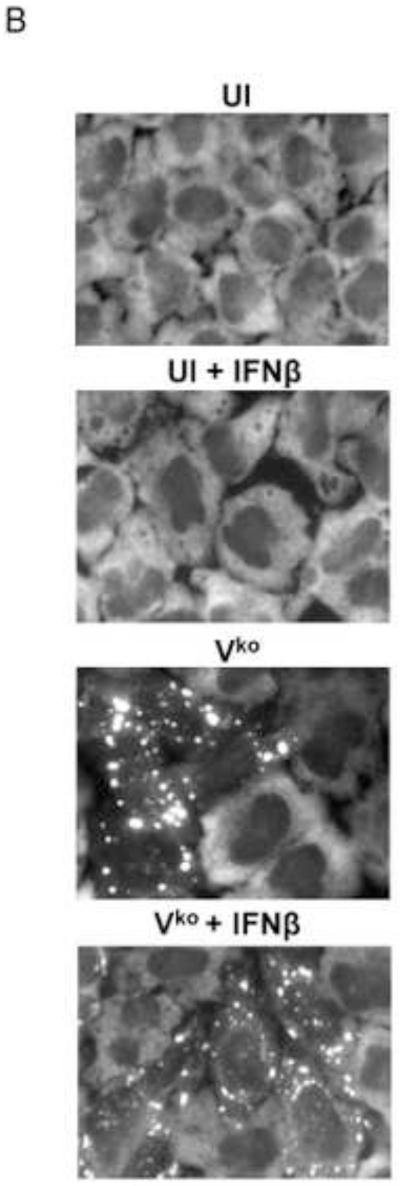
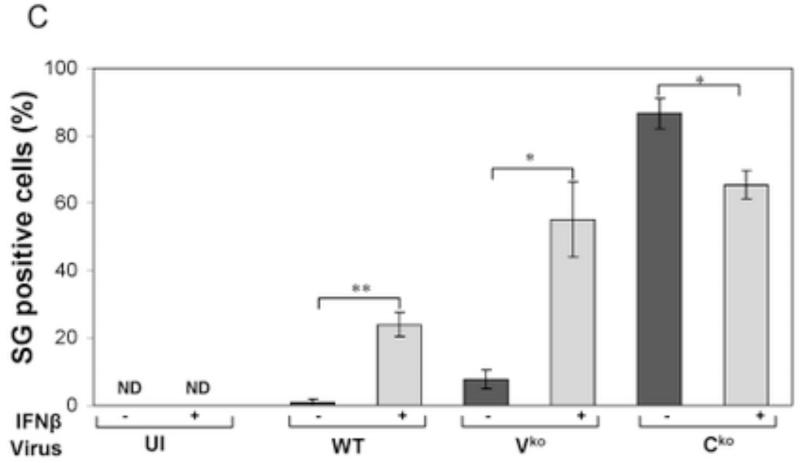
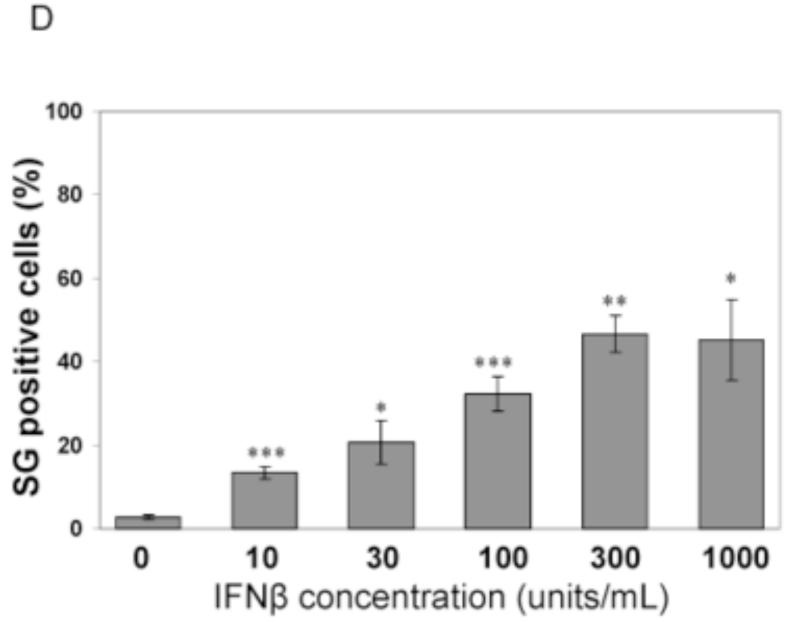
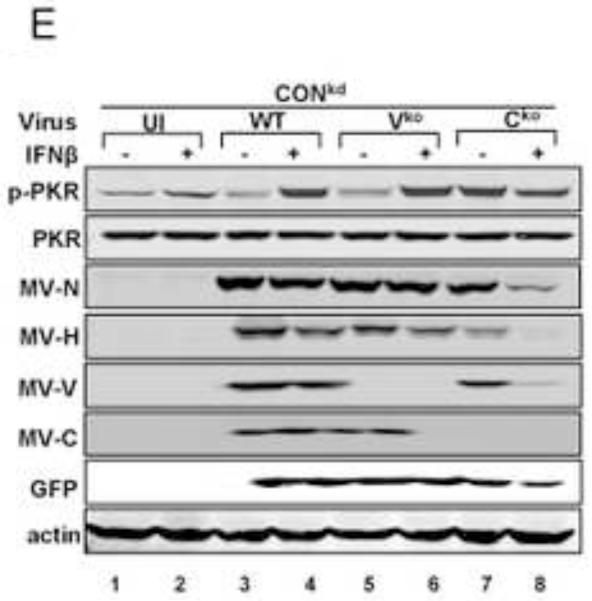
To test whether IFN treatment affected SG formation in cells sufficient for both ADAR1 and PKR when infected, HeLa CONkd cells were examined. Immunofluorescence microscopy revealed an increase in SG-positive cells in WT and Vko mutant infected CONkd cells that had been treated with 1000 u/mL IFNβ compared to untreated CONkd cells (Fig. 3A). SG formation was not detectable in uninfected CONkd cells, either untreated or when IFN-treated (Fig. 3A, inset 3B). Quantification showed that SG formation was most robust in Vko-infected CONkd cells that had been IFN-treated, increasing from a level of about 10% in untreated to about 55% in IFN-treated cells (Fig. 3C). The induction of SG formation in Vko infected CONkd cells was dependent upon the concentration of IFN used, with an increase observed with 10 u/mL and saturation achieved between 100 and 300 u/mL of IFN treatment for 24 h (Fig. 3D). IFN treatment also increased the SG formation in cells infected with WT virus, but under these conditions only reached a level of about 25% (Fig. 3C). By contrast, IFN treatment decreased rather than increased the number of SG-positive cells following infection with Cko virus (Fig. 3C), a virus that is a known potent inducer of SG formation (Okonski and Samuel, 2013). Western blot analysis showed that IFN treatment alone did not significantly increase the phosphorylation of PKR in uninfected CONkd cells, but that Thr446 phosphorylation of PKR was increased in WT and Vko infected cells that had been IFN-treated (Fig. 3E). Furthermore, IFN treatment nearly abolished the expression of N and H viral proteins in Cko-infected cells (Fig. 3E).
Figure 3. Interferon treatment enhances stress granule formation following infection with wild-type measles virus and mutant virus deficient for V-protein expression, but not with C-protein deficient mutant virus that already is an efficient inducer even in untreated cells.
(A) HeLa CONkd cells were either left untreated or treated with IFNβ (1000 U/mL) for 18 h. Cells then were either left uninfected (UI) or were infected with WT, Vko or Cko MV at a multiplicity of infection of 1 TICD50/cell. At 32 h after infection, cells were analyzed by immunofluorescence microscopy as described under Materials and Methods using antibodies to G3BP1 (white) as a marker for SG formation and N protein (red) and GFP (green) for viral infection. (B) Magnification of the framed sections in panel A. (C) Quantification of SG-positive cells as described for figure 1. Results are expressed as the percentage of infected cells that are positive for SG. Results are mean values and standard errors from three independent experiments. N.D., not detectable. Statistical significance determined by students t test (two tailed, two sample with equal variance) comparing untreated cells to IFN treated cells. * P < 0.005, ** P < 0.0005, n.s, not significant. (D) Quantification of SG positive cells when pretreated for 24 h with increasing concentrations of IFNβ prior to infection with Vko virus. Results are mean values and standard errors from three independent experiments. N.D.,not detectable. Statistical significance determined by Students t test (two tailed, two sample with equal variance) comparing untreated cells to IFN-treated cells * P<0.005, ** P<0.0005. (E) At 32 h after infection whole-cell extracts were prepared and analyzed by Western immunoblot assay using antibodies against phospho-Thr446 PKR (p-PKR), PKR, MV N, H, V and C proteins, GFP, and actin.
The Cko mutant grew poorly relative to WT and Vko viruses in both untreated and IFN-treated cells as measured by infectious yield (Table 1), consistent with immunofluorescence microscopy for viral proteins (Fig. 1, 3). Furthermore, the IFN treatment reduced the yield of infectious MV by more than 90% in the PKRkd cells, comparable to the reduction seen in CONkd cells for WT, Vko and Cko viruses (Table 1).
Table 1.
Antiviral Activity of Interferon Measured with Measles Virus in Cells Stably Deficient for either ADAR1 or PKR Compared to Control Cellsa
| Virus Yield | |||
|---|---|---|---|
| Cell | Virus | Untreated (TICD50/ml) |
IFN-treated (TICD50/ml) |
| CONkd | WT | 1.4 ± 0.3 × 106 | 1.3 ± 0.2 × 105 |
| Vko | 1.0 ± 0.1 × 106 | 6.6 ± 1.7 × 104 | |
| Cko | 2.8 ± 0.1 × 104 | 2.6 ± 0.6 × 103 | |
| PKRkd | WT | 1.0 ± 1.0 × 106 | 1.4 ± 0.1 × 105 |
| Vko | 1.5 ± 0.3 × 106 | 1.1 ± 0.1 × 105 | |
| Cko | 8.0 ± 0.8 × 104 | 3.5 ± 0.3 × 103 | |
| ADAR1kd | WT | 1.0 ± 0.1 × 106 | 1.4 ± 0.1 × 105 |
| Vko | 4.3 ± 0.1 × 105 | 2.8 ± 0.2 × 104 | |
| Cko | 2.0 ± 0.5 × 104 | 6.8 ± 0.2 × 102 | |
Virus yields were measured with the Moraten parental strain and mutants deficient in the expression of either V protien (Vko) or C protein (Cko) in puromycin-resistant control knockdown cells (CONKD), ADAR1 stable knockdown (ADAR1KD) and PKR stable knockdown (PKRKD) HeLa cell clones either untreated or pretreated with 1000 units/mL IFNβ prior to infection. Yields were determined by 50% TICD50 titation on Vero cells according to the Spearman-Karber method (Devaux 2008, Karber 1931). Results are means and standard errors (n=3).
Stress granule formation is impaired by PKR deficiency in interferon-treated cells
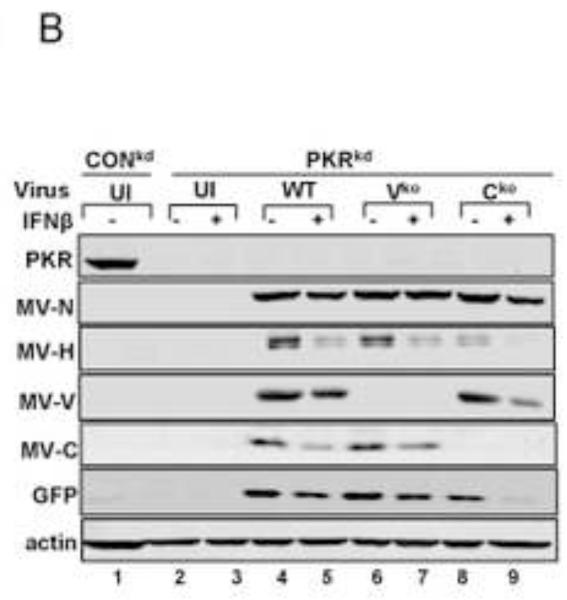
The dsRNA-dependent protein kinase is an established modulator of the host response to MV infection (McAllister et al., 2010; Okonski and Samuel, 2013; Toth et al., 2009a, 2009b). Because SG formation induced by MV is a known PKR-dependent process in cells that have not been treated with IFN (Okonski and Samuel, 2013), we tested the PKR requirement for SG formation in IFN-treated cells. SG formation assessed by the presence of punctate cytoplasmic G3BP1 foci was not detectable in PKRkd cells, either untreated as previously established (Okonski and Samuel, 2013) or IFNβ-treated following infection with WT, Vko or Cko virus (Fig. 4A). IFN treatment reduced viral protein production even in PKRkd cells (Fig. 4B) in the absence of SG formation (Fig. 4A).
Figure 4. Stress granule formation is impaired by PKR deficiency both in untreated and interferon-treated cells following measles virus infection.
(A) HeLa PKRkd cells deficient in PKR kinase were either left untreated or were treated with IFNβ (1000 U/mL) for 18 h. Cells then were either left uninfected (UI) or were infected with WT, Vko or Cko MV at a multiplicity of infection of 1 TICD50/cell. Cells were analyzed by immunofluorescence microscopy at 24 h after infection as described under Materials and Methods using antibodies to G3BP1 (white) as a marker for SG formation and N protein (red) and GFP (green) for viral infection. (B) At 24 h after infection whole-cell extracts were prepared from untreated (−) or IFN-treated (+) PKRkd cells and analyzed by Western immunoblot assay using antibodies against PKR, measles virus MV N, H, V and C proteins, GFP, and actin. CONkd extract from untreated uninfected cells is shown in lane 1 as a control.
Interferon treatment alone does not cause an increased production of dsRNA comparable to that observed following infection with MV deficient in C protein expression
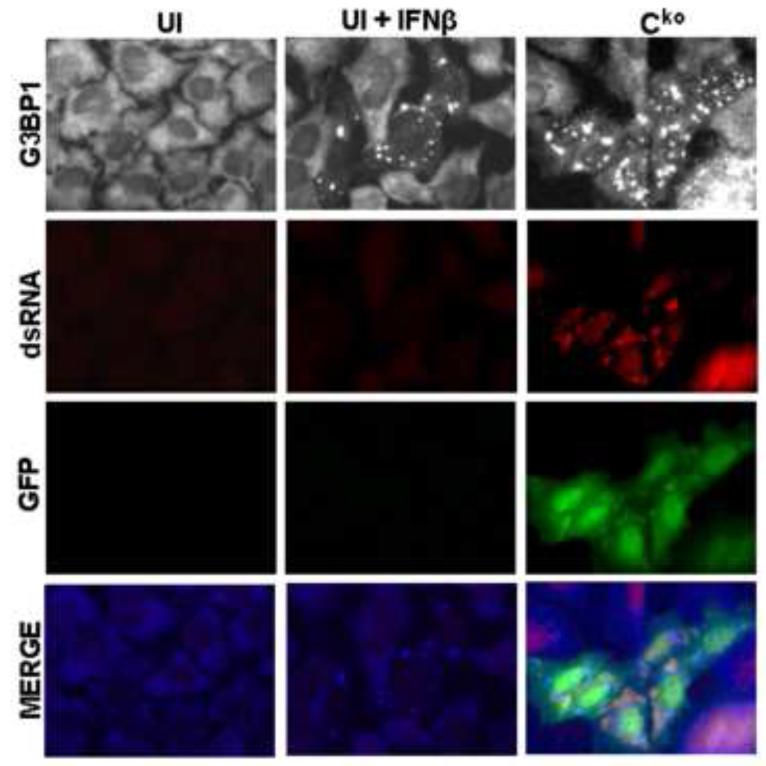
The Cko mutant of MV is a potent activator of PKR autophosphorylation in both CONkd and ADAR1kd cells in the absence of IFN treatment (Fig. 1D, 3E) (Li et al., 2012; Toth et al., 2009b). RNA with double-stranded character is the activator of PKR (Samuel, 1979; 2001). dsRNA formation was detected in untreated Cko-infected cells but not in uninfected cells by immunostaining with an antibody specific for dsRNA (Fig. 5), consistent with recent observations (Pfaller et al., 2014). Even though IFNβ treatment induced the formation of SG in uninfected ADAR1kd cells similar to the SG level seen in Cko-infected untreated cells, dsRNA-specific staining with the J2 antibody was not detectable above background level in the untreated, uninfected cells (Fig. 5). However, IFNβ treatment alone modestly increased PKR phosphorylation in CONkd cells, and even more so in ADAR1kd cells, in the absence of infection; Cko virus infection led to a greater increase in PKR phosphorylation even in untreated cells (Fig. 1D, 3E). These results suggest that the threshold for detection of dsRNA in response to IFN treatment measured by Western analysis for PKR Thr446 autophosphorylation and SG formation by immunofluorescence with G3BP1 antibody are comparable and more sensitive than the threshold for dsRNA detection by staining with the J2 antibody.
Figure 5. Interferon treatment alone does not cause increased production of dsRNA comparable to that observed following infection with measles virus deficient in C protein expression.
Immunofluorescence staining against dsRNA (red) and GFP (green) in ADAR1kd HeLa cells either left untreated and uninfected (UI), treated with IFNβ (1000 U/mL) for 24 h but left uninfected (UI+IFNβ), or left untreated but infected Cko MV at a multiplicity of infection of 1 TCID50/cell for 24 h (Cko). Images were taken at 40× magnification.
Both STAT1 and STAT2 are required for the interferon induced enhancement of stress granule formation following infection with measles virus
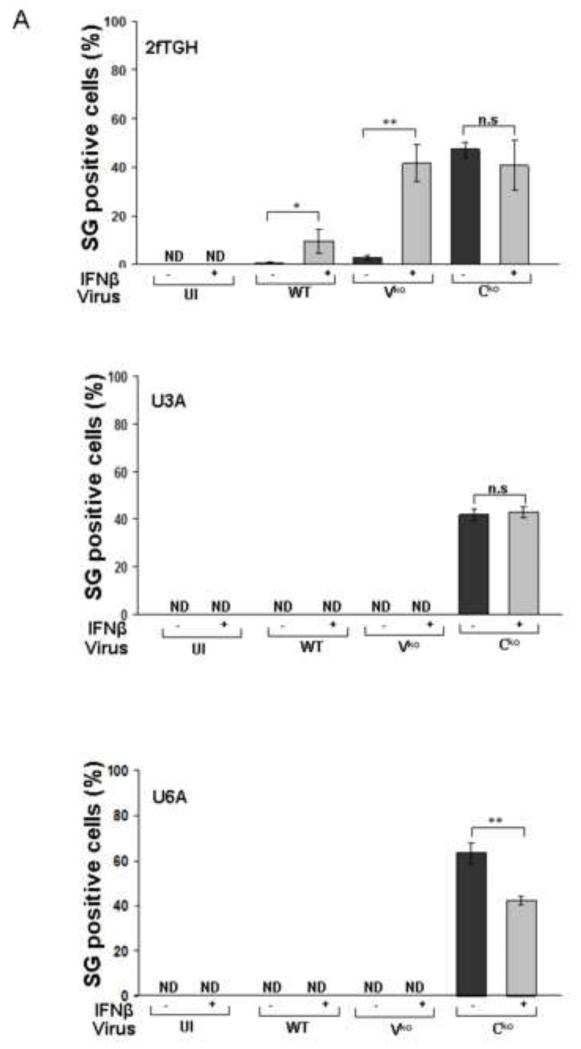
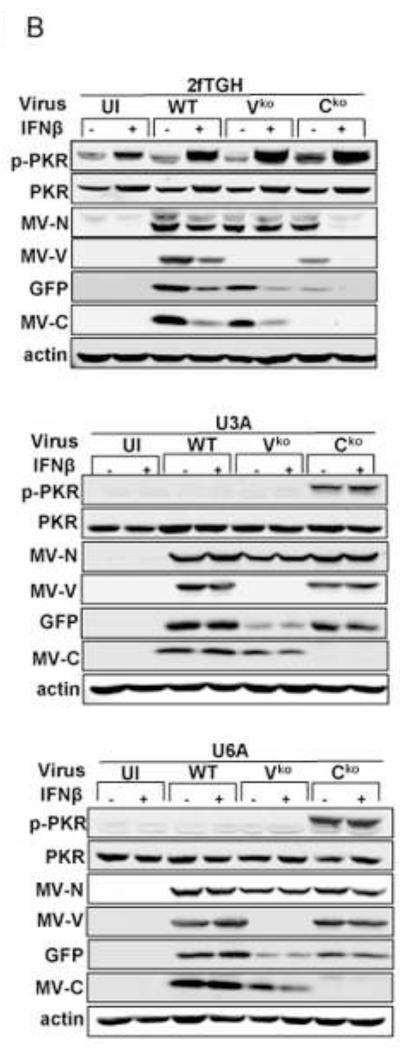
SG formation in ADAR1-sufficient CONkd cells following infection with WT and Vko mutant MV was enhanced by pretreatment of the cells with IFN (Fig. 3). The canonical JAK-STAT pathway for type I IFN signaling requires transcription factors STAT1 and STAT2 together with IRF9, although additional non-canonical signaling responses are known that are independent of STAT1 (Platanias, 2005; Wesoly et al., 2007). We used human 2fTGH parental fibrosarcoma cells, U3A mutant cells deficient in STAT1, and U6A mutant cells deficient in STAT2 to test the requirement for the STAT1 and STAT2 factors in mediating the IFN induced formation of SG. As shown in Figure 6A, both STAT1 and STAT2 were required for the IFN-induced enhancement of SG formation following infection with WT and Vko viruses. By contrast, SG formation in response to infection with Cko mutant virus that expresses high levels of dsRNA was not dependent upon either STAT1 or STAT2 (Fig. 6A). Phosphorylation of PKR was not observed in the IFN-treated U3A or U6A cells following infection with WT or Vko virus, whereas increased Thr446 phosphorylation of PKR was observed in the IFN-treated parental 2fTGH cells following infection but unexpectedly not in untreated cells (Fig. 6B). These results suggest that a canonical STAT1- and STAT2-dependent IFN signaling response occurs following IFN treatment that leads to enhanced SG formation following infection.
Figure 6. Both STAT1 and STAT2 are required for the interferon-induced enhancement of stress granule formation following infection with measles virus.
2fTGH parental cells, U3A mutant cells deficient in STAT1, and U6A mutant cells deficient in STAT2 cells were either left untreated (−) or were treated (+) with IFNβ (1000 U/mL) for 18 h. Cells then were either left uninfected (UI) or infected with WT, Vko or Cko MV at a multiplicity of infection of 1 TCID50/cell. (A) Quantification of SG-positive cells was carried out as described for figure 1. At 24 h after infection cells were analyzed by immunofluorescence microscopy as described under Materials and Methods using antibodies to G3BP1 as a marker for SG formation and N protein for viral infection. Results are mean values and standard errors from three independent experiments. N.D., not detectable. Statistical significance determined by students t test (two tailed, two sample with equal variance) comparing untreated cells to IFN treated cells. * P < 0.05, ** P < 0.005, n.s., not significant (B) At 24 h after infection whole-cell extracts were prepared and analyzed by Western immunoblot assay using antibodies against phospho-Thr446 PKR (p-PKR), PKR, MV N, V and C proteins, GFP, and actin.
DISCUSSION
Although stress granule formation has been implicated to play a role in the innate immune response following infection with RNA viruses (Ng et al., 2013; Okonski and Samuel, 2013; Onomoto et al., 2012; Reineke and Lloyd, 2012; Toth et al., 2006), little is known with regard to the possible effect of IFNβ treatment and hence IFN action on the stress granule response in the absence of infection. Our studies, therefore, were carried out with the aim of assessing the role of interferon in regulating the stress granule response, both in uninfected cells and in cells subsequently infected with measles virus. We used both the parental wildtype Moraten MV strain and isogenic mutant viruses defective in either C or V protein expression, as these two accessory viral proteins are known to antagonize IFN production and action (Randall and Goodbourne,, 2008). We also used cells stably deficient in the PKR kinase (PKRkd) or ADAR1 deaminase (ADAR1kd), as these two IFN inducible dsRNA-binding proteins are known to affect virus-induced stress responses in opposite ways: stress granule formation induced by MV is PKR-dependent but impaired by ADAR1 (Okonski and Samuel, 2013); and, PKR is generally antiviral and proapoptotic whereas ADAR1 is often proviral and antiapoptotic (Pfaller et al., 2011). We provide evidence herein that canonical type I IFN signaling amplifies the stress granule response, not only in cells infected with MV but also in uninfected cells, most strikingly when they are deficient for ADAR1 protein. The robustness of the SG response is modulated by interferon treatment, and by both PKR and ADAR1. Several important points emerge from these observations.
First, our results presented herein further establish that Cko mutant measles virus in contrast to WT or Vko mutant is an efficient inducer of SG formation not only in HeLa cells but also in 2fTGH fibrosarcoma cells in agreement with earlier observations (Okonski and Samuel, 2013; Pfaller et al., 2014). For two main reasons we then considered the possibility that either IFN induction or IFN signaling, or both, were necessary for a maximal virus-induced SG response: because the C mutant, which efficiently induces SG formation in a PKR-dependent manner (Okonski and Samuel, 2013), also is a potent inducer of IFNβ compared to WT and the Vko mutant virus (Li et al., 2012; McAllister et al., 2010); and, because PKR likewise enhances IFN induction in a manner that depends upon PKR catalytic activity (Taghavi and Samuel, 2013).
Our demonstration that IFN pretreatment enhanced stress granule formation upon infection with WT or Vko mutant virus, both in CONkd HeLa cells (Fig. 3) and in the parental 2fTGH cells (Fig. 6), suggests a role for IFN action in the stress granule response. Furthermore, the fact that the Vko mutant consistently showed a greater enhancement of SG formation following IFN treatment than did WT virus is consistent with the known function of V protein as an antagonist of IFN signaling (Randall and Goodbourn, 2008; Samuel, 2001). The link between SG enhancement and IFN action was independently established by using Stat1-deficient U3A and Stat2-deficient U6A mutant cells, neither of which displayed stress granules following infection with either WT or Vko mutant virus in contrast to parental 2fTGH cells which did. These results suggest that canonical Jak-Stat type I IFN signaling is required in order to achieve a maximal virus-induced SG response following infection with WT or Vko virus. Likewise, in hepatitis C virus infected cells, type I IFN treatment has been observed to affect the SG response. In combination with IFN, HCV induces the dynamic oscillation of cytoplasmic SG assembly and disassembly, with reduced production of IFN-induced proteins when SG are present even though IFN-induced mRNAs are expressed (Gariagorta et al., 2012; Ruggieri et al., 2012).
Among the ~300 to 400 known IFN inducible genes (Schoggins et al., 2011) are PKR (Kuhen and Samuel, 1997; Samuel, 1979) and ADAR1 (George and Samuel, 1999; Patterson et al., 1995). Stress granule formation in response to MV infection (Okonski and Samuel, 2013), as well as for several additional viruses (Lloyd, 2013), is a PKR-dependent process. In the 2fTGH cells, the level of phospho-Thr446 PKR was increased by both IFN treatment and infection with WT, Vko or Cko virus. However, with the U3A and U6A cells, the level of phospho-Thr446 PKR remained low following infection even in IFN-treated cells. While PKR induction and activation is one of the stress granule enhancing effects of the IFN treatment, we cannot exclude that other type I IFN inducible gene products also contribute to the enhanced stress granule formation seen following treatment with either beta or alpha IFN.
An antiviral effect of IFN was observed in PKR-deficient cells (PKRkd) in the absence of detectable SG formation. We found that the Cko mutant replicated poorly in untreated cells relative to WT or Vko mutant virus as was also seen by Toth et al., 2009b. However, the C and V mutants and WT virus were all sensitive to the antiviral activity of IFN in PKRkd cells as well as ADARkd and CONkd cells. These results suggest that SG formation is not an obligate requirement for the antiviral action of IFN as SG formation was not observed in PKRkd cells, either untreated or IFN-treated, following infection (Fig. 4) consistent with earlier observations (Okonski and Samuel, 2013).
By contrast to WT and Vko viruses, the Cko mutant induced stress granule formation to comparably high levels in both untreated and IFN-pretreated HeLa (Fig. 3) and 2fTGH (Fig. 6) cells. Furthermore, the Cko mutant induced stress granules in both U3A and U6A mutant cells as efficiently as in parental 2fTGH cells. This finding was unexpected. These results suggest that canonical IFN signaling is not an obligatory requirement for the enhanced stress granule formation seen with Cko compared to the Vko mutant and WT virus. Conceptually similar results have been recently described for leader L protein mutant of Theiler’s murine encephalomyelitis virus (TMEV). Stress granules are formed in Stat1-deficient U3A fibrosarcoma cells when infected with L mutant TMEV, but not when infected with WT TMEV (Borghese and Michiels, 2011). Thus, virus-induced stress granule formation with two mutant viruses, the measles virus Cko mutant and the TMEV L mutant, does not require canonical IFN signaling, suggestive of a different mechanism, at least quantitatively, for these mutant viruses compared to their wildtype parents. One possible alternative mechanism relates to the high level of viral dsRNA produced in cells infected with the Cko mutant MV compared to either WT or Vko viruses (Pfaller et al., 2014). Conceivably the high level of dsRNA in Cko infected cells compensates sufficiently for a low concentration of an otherwise IFN inducible gene product in order to achieve a near maximal stress granule response. Consistent with this notion, analyses of genetically altered cell lines have shown that the transcriptional induction of gene expression by dsRNA can be mediated by ISRE elements without activation of the ISGF3 factor and without the requirement of type I IFN signaling components including Jak1, Tyk2, IRF9 and Stat2 (Bandyopadhyay et al., 1995; Guo et al., 2000).
Our results further illustrate that efficient virus-induced stress granule formation correlates with reduced viral replication and the induction of IFN, consistent with earlier observations (Toth et al., 2006). Other studies also have shown that efficient virus-induced stress granule formation correlates with reduced virus growth and increased IFN production, for example in the case of leader L protein mutants of TMEV and mengo virus. In contrast to the L mutants, WT mengo and TMEV viruses replicate efficiently, suppress the induction of IFN, and fail to induce stress granules (Borghese and Michiels, 2011; Langereis et al., 2013). Likewise, poliovirus and encephalomyocarditis virus (EMCV) replication also are reduced by stress granule formation. Although WT poliovirus (White et al., 2007) and WT EMCV (Ng et al., 2013) impair stress granule formation through a protease 3C-mediated cleavage of G3BP1 protein, G3BP1 is not cleaved in TMEV-infected cells (Borghese and Michiels, 2011). These findings, together with our observations (Okonski and Samuel, 2013; herein), are consistent with the notion that stress granule formation impairs the growth of both negative-strand and positive-strand RNA viruses. Influenza A virus and vaccinia virus also suppress stress granule formation and are poor IFN inducers, but mutants of these viruses deleted for expression of their respective PKR antagonists NS1 (Khaperskyy et al., 2012) and E3L (Simpson-Holley et al., 2011), induce IFN, replicate poorly, and induce stress granules. Cytoplasmic stress granule induction by ΔNS1 mutant influenza and ΔE3L mutant vaccinia virus is PKR-dependent (Khaperskyy et al., 2012; Simpson-Holley et al., 2011).
Evidence has been presented that G3BP1, which is not IFN inducible at least in HeLa cells (Fig. 2B), is necessary for IFN induction triggered by EMCV infection, and that PKR is essential for both stress granule formation and IFN induction by EMCV (Ng et al., 2013). Studies also have shown that for ΔNS1 mutant influenza virus, both the formation of stress granules containing RIG-I and the activation of PKR correlates with increased IFN induction (Onomoto et al., 2012). By contrast, with L mutant mengovirus, the formation of stress granules and MDA5 localization to stress granules are dispensable for IFN induction (Langereis et al., 2013). The reasons for the differences between these studies (Langereis et al., 2013; Ng et al., 2013; Onomoto et al., 2012) with regard to the role for stress granules in virus sensing and IFN induction are unclear, but may relate to differences in the viruses studied and host cells utilized. For measles virus, the production of IFN correlates well with the formation of stress granules, and both processes show a dependence upon catalytically active PKR protein kinase (McAllister et al., 2012; Okonski and Samuel, 2013; Taghavi and Samuel, 2013). The results presented herein extend these findings and establish that IFN action enhances the formation of stress granules by a canonical type I IFN signaling mechanism that is dependent upon PKR and modulated by ADAR1. As suggested by Reineke and Llyod (2012), it is conceivable that RNA stress granule biology may be exploited as one component of the innate antiviral response, a possibility that is increasingly attractive given the crosstalk at multiple levels between stress responses and innate immunity.
Highlights.
We examined the effect of interferon on the induction of stress granules (SG]. 80
SG formation was PKR-dependent and impaired by ADAR1 dsRNA adenosine deaminase. 80
IFN α and β induced SG in the absence of infection in ADARl-deficient cells. 77
IFN-induced enhancement of SG was Stat1 and Stat2 dependent. 61
IFN enhanced SG formation in measles WT and Vko but not Cko infected cells. 76
ACKNOWLEDGMENTS
This work was supported in part by research grants AI-12520 and AI-20611 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Public Health Service. We thank Drs. C. George and C. Pfaller for insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33(3):141–50. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10(6):430–6. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay SK, Leonard GT, Jr., Bandyopadhyay T, Stark GR, Sen GC. Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J Biol Chem. 1995;270(33):19624–9. doi: 10.1074/jbc.270.33.19624. [DOI] [PubMed] [Google Scholar]
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–46. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham CJ, Parker R. P bodies, stress granules, and viral life cycles. Cell Host Microbe. 2008;3(4):206–12. doi: 10.1016/j.chom.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6(12):975–90. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese F, Michiels T. The leader protein of cardioviruses inhibits stress granule assembly. J Virol. 2011;85(18):9614–22. doi: 10.1128/JVI.00480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caignard G, Bourai M, Jacob Y, Tangy F, Vidalain P0. Inhibition of IFN-alpha/beta signaling by two discrete peptides within measles virus V protein that specifically bind STAT1 and STAT2. Virology. 2009;383(1):112–20. doi: 10.1016/j.virol.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Devaux P, Hodge G, McChesney MB, Cattaneo R. Attenuation of V- or C-defective measles viruses: infection control by the inflammatory and interferon responses of rhesus monkeys. J Virol. 2008;82(11):5359–67. doi: 10.1128/JVI.00169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P, von Messling V, Songsungthong W, Springfeld C, Cattaneo R. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology. 2007;360(1):72–83. doi: 10.1016/j.virol.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Fontana JM, Bankamp B, Bellini WJ, Rota PA. Regulation of interferon signaling by the C and V proteins from attenuated and wild-type strains of measles virus. Virology. 2008;374(1):71–81. doi: 10.1016/j.virol.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Friedman RL, Stark GR. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985;314(6012):637–9. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Garaigorta U, Heim MH, Boyd B, Wieland S, Chisari FV. Hepatitis C virus (HCV) induces formation of stress granules whose proteins regulate HCV RNA replication and virus assembly and egress. J. Virol. 2012;86(20):11043–56. doi: 10.1128/JVI.07101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JF, Clerzius G, Shaw E, Gatignol A. Enhancement of replication of RNA viruses by ADAR1 via RNA editing and inhibition of RNA-activated protein kinase. J Virol. 2011;85(17):8460–6. doi: 10.1128/JVI.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CX, Samuel CE. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc Natl Acad Sci USA. 1999;96(8):4621–6. doi: 10.1073/pnas.96.8.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012;36(3):649–62. doi: 10.1111/j.1574-6976.2012.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Peters KL, Sen GC. Induction of the human protein P56 by interferon, double-stranded RNA, or virus infection. Virology. 2000;267(2):209–19. doi: 10.1006/viro.1999.0135. [DOI] [PubMed] [Google Scholar]
- Hartner JC, Walkley CR, Lu J, Orkin SH. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat Immunol. 2009;10(1):109–15. doi: 10.1038/ni.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karber G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1931;162:480–416. [Google Scholar]
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147(7):1431–42. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaperskyy DA, Hatchette TF, McCormick C. Influenza A virus inhibits cytoplasmic stress granule formation. Faseb J. 2012;26(4):1629–39. doi: 10.1096/fj.11-196915. [DOI] [PubMed] [Google Scholar]
- Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. Fields virology. 5th ed. Lipppincott Williams & Wilkins; Philadelphia, PA: 2007. 5th ed. [Google Scholar]
- Kuhen KL, Samuel CE. Isolation of the interferon-inducible RNA-dependent protein kinase Pkr promoter and identification of a novel DNA element within the 5′-flanking region of human and mouse Pkr genes. Virology. 1997;227(1):119–30. doi: 10.1006/viro.1996.8306. [DOI] [PubMed] [Google Scholar]
- Langereis MA, Feng Q, van Kuppeveld FJ. MDA5 localizes to stress granules, but this localization is not required for the induction of type I interferon. J Virol. 2013;87(11):6314–25. doi: 10.1128/JVI.03213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S, Qureshi SA, Kerr IM, Darnell JE, Jr., Stark GR. Role of STAT2 in the alpha interferon signaling pathway. Mol Cell Biol. 1995;15(3):1312–7. doi: 10.1128/mcb.15.3.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okonski KM, Samuel CE. Adenosine deaminase acting on RNA 1 (ADAR1) suppresses the induction of interferon by measles virus. J Virol. 2012;86(7):3787–94. doi: 10.1128/JVI.06307-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wolff KC, Samuel CE. RNA adenosine deaminase ADAR1 deficiency leads to increased activation of protein kinase PKR and reduced vesicular stomatitis virus growth following interferon treatment. Virology. 2010;396(2):316–22. doi: 10.1016/j.virol.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd RE. Regulation of stress granules and P-bodies during RNA virus infection. Wiley Interdiscip Rev RNA. 2013;4(3):317–31. doi: 10.1002/wrna.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CS, Taghavi N, Samuel CE. Protein kinase PKR amplification of interferon beta induction occurs through initiation factor eIF-2alpha-mediated translational control. J Biol Chem. 2012;287(43):36384–92. doi: 10.1074/jbc.M112.390039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CS, Toth AM, Zhang P, Devaux P, Cattaneo R, Samuel CE. Mechanisms of protein kinase PKR-mediated amplification of beta interferon induction by C protein-deficient measles virus. J Virol. 2010;84(1):380–6. doi: 10.1128/JVI.02630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendry R, John J, Flavell D, Muller M, Kerr IM, Stark GR. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both alpha and gamma interferons. Proc Natl Acad Sci USA. 1991;88(24):11455–9. doi: 10.1073/pnas.88.24.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VH, Navaratnarajah CK, Frenzke M, Wong XX, Sawatsky B, Ramachandran S, McCray PB, Jr., Cichutek K, von Messling V, Lopez M, Cattaneo R. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480(7378):530–3. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnarajah CK, Leonard VH, Cattaneo R. Measles virus glycoprotein complex assembly, receptor attachment, and cell entry. Curr Top Microbiol Immunol. 2009;329:59–76. doi: 10.1007/978-3-540-70523-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CS, Jogi M, Yoo JS, Onomoto K, Koike S, Iwasaki T, Yoneyama M, Kato H, Fujita T. Encephalomyocarditis virus disrupts stress granules, the critical platform for triggering antiviral innate immune responses. J Virol. 2013;87(17):9511–22. doi: 10.1128/JVI.03248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonski KM, Samuel CE. Stress granule formation induced by measles virus is protein kinase PKR dependent and impaired by RNA adenosine deaminase ADAR1. J Virol. 2013;87(2):756–66. doi: 10.1128/JVI.02270-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onomoto K, Jogi M, Yoo JS, Narita R, Morimoto S, Takemura A, Sambhara S, Kawaguchi A, Osari S, Nagata K, Matsumiya T, Namiki H, Yoneyama M, Fujita T. Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity. PLoS One. 2012;7(8):e43031. doi: 10.1371/journal.pone.0043031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Thomis DC, Hans SL, Samuel CE. Mechanism of interferon action: double-stranded RNA-specific adenosine deaminase from human cells is inducible by alpha and gamma interferons. Virology. 1995;210(2):508–11. doi: 10.1006/viro.1995.1370. [DOI] [PubMed] [Google Scholar]
- Pfaller CK, Li Z, George CX, Samuel CE. Protein kinase PKR and RNA adenosine deaminase ADAR1: new roles for old players as modulators of the interferon response. Curr Opin Immunol. 2011;23(5):573–82. doi: 10.1016/j.coi.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller CK, Radeke MJ, Cattaneo R, Samuel CE. Measles Virus C Protein Impairs Production of Defective Copyback Double-stranded Viral RNA and Activation of Protein Kinase R. J Virol. 2014;88:456–68. doi: 10.1128/JVI.02572-13. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Parisien JP, Horvath CM. STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition. J Virol. 2008;82(17):8330–8. doi: 10.1128/JVI.00831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Reineke LC, Lloyd RE. Diversion of stress granules and P-bodies duringviral infection. Virology. 2012;436(2):255–67. doi: 10.1016/j.virol.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, Dickerson JE, Bhaskar SS, Zampini M, Briggs TA, Jenkinson EM, Bacino CA, Battini R, Bertini E, Brogan PA, Brueton LA, Carpanelli M, De Laet C, de Lonlay P, del Toro M, Desguerre I, Fazzi E, Garcia-Cazorla A, Heiberg A, Kawaguchi M, Kumar R, Lin JP, Lourenco CM, Male AM, Marques W, Jr., Mignot C, Olivieri I, Orcesi S, Prabhakar P, Rasmussen M, Robinson RA, Rozenberg F, Schmidt JL, Steindl K, Tan TY, van der Merwe WG, Vanderver A, Vassallo G, Wakeling EL, Wassmer E, Whittaker E, Livingston JH, Lebon P, Suzuki T, McLaughlin PJ, Keegan LP, O’Connell MA, Lovell SC, Crow YJ. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet. 2012;44(11):1243–8. doi: 10.1038/ng.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri A, Dazert E, Metz P, Hofmann S, Bergeest JP, Mazur J, Bankhead P, Hiet MS, Kallis S, Alvisi G, Samuel CE, Lohmann V, Kaderali L, Rohr K, Frese M, Stoecklin G, Bartenschlager R. Dynamic oscillation of translation and stress granule formation mark the cellular response to virus infection. Cell Host Microbe. 2012;12(1):7185. doi: 10.1016/j.chom.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. Structure and function of the protein kinase R. Curr Top Microbiol Immunol. 2007;316:253–92. doi: 10.1007/978-3-540-71329-6_13. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci USA. 1979;76(2):600–4. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993;268(11):7603–6. [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Adenosine deaminases acting on RNA (ADARs) are both antiviral and proviral. Virology. 2011;411(2):180–93. doi: 10.1016/j.virol.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059–63. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–5. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann KM, Pfaller CK, Conzelmann KK. The measles virus V protein binds to p65 (RelA) to suppress NF-kappaB activity. J Virol. 2011;85(7):3162–71. doi: 10.1128/JVI.02342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Holley M, Kedersha N, Dower K, Rubins KH, Anderson P, Hensley LE, Connor JH. Formation of antiviral cytoplasmic granules during orthopoxvirus infection. J Virol. 2011;85(4):1581–93. doi: 10.1128/JVI.02247-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrer KM, Pfaller CK, Conzelmann KK. Measles virus C protein interferes with Beta interferon transcription in the nucleus. J Virol. 2012;86(2):796–805. doi: 10.1128/JVI.05899-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi N, Samuel CE. RNA-dependent protein kinase PKR and the Z-DNA binding orthologue PKZ differ in their capacity to mediate initiation factor eIF2alpha-dependent inhibition of protein synthesis and virus-induced stress granule formation. Virology. 2013;443(1):48–58. doi: 10.1016/j.virol.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AM, Devaux P, Cattaneo R, Samuel CE. Protein kinase PKR mediates the apoptosis induction and growth restriction phenotypes of C protein-deficient measles virus. J Virol. 2009a;83(2):961–8. doi: 10.1128/JVI.01669-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AM, Li Z, Cattaneo R, Samuel CE. RNA-specific adenosine deaminase ADAR1 suppresses measles virus-induced apoptosis and activation of protein kinase PKR. J Biol Chem. 2009b;284(43):29350–6. doi: 10.1074/jbc.M109.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AM, Zhang P, Das S, George CX, Samuel CE. Interferon action and the double-stranded RNA-dependent enzymes ADAR1 adenosine deaminase and PKR protein kinase. Prog Nucleic Acid Res Mol Biol. 2006;81:369–434. doi: 10.1016/S0079-6603(06)81010-X. [DOI] [PubMed] [Google Scholar]
- Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160(6):823–31. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ward SV, George CX, Welch MJ, Liou LY, Hahm B, Lewicki H, de la Torre JC, Samuel CE, Oldstone MB. RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proc Natl Acad Sci USA. 2011;108(1):331–6. doi: 10.1073/pnas.1017241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesoly J, Szweykowska-Kulinska Z, Bluyssen HA. STAT activation and differential complex formation dictate selectivity of interferon responses. Acta Biochim Pol. 2007;54(1):27–38. [PubMed] [Google Scholar]
- White JP, Cardenas AM, Marissen WE, Lloyd RE. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe. 2007;2(5):295–305. doi: 10.1016/j.chom.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Recognition of viral nucleic acids in innate immunity. Rev Med Virol. 2010;20(1):4–22. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- Zhang P, Jacobs BL, Samuel CE. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J Virol. 2008;82(2):840–8. doi: 10.1128/JVI.01891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Samuel CE. Protein kinase PKR plays a stimulus- and virus-dependent role in apoptotic death and virus multiplication in human cells. J Virol. 2007;81(15):8192–200. doi: 10.1128/JVI.00426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]