Abstract
IMPORTANCE
Hypoglycemia commonly occurs in patients with diabetes mellitus (DM) and may negatively influence cognitive performance. Cognitive impairment in turn can compromise DM management and lead to hypoglycemia.
OBJECTIVE
To prospectively evaluate the association between hypoglycemia and dementia in a biracial cohort of older adults with DM.
DESIGN AND SETTING
Prospective population-based study.
PARTICIPANTS
We studied 783 older adults with DM (mean age, 74.0 years; 47.0% of black race/ethnicity; and 47.6% female) who were participating in the prospective population-based Health, Aging, and Body Composition Study beginning in 1997 and who had baseline Modified Mini-Mental State Examination scores of 80 or higher.
MAIN OUTCOME MEASURES
Dementia diagnosis was determined during the follow-up period from hospital records indicating an admission associated with dementia or the use of prescribed dementia medications. Hypoglycemic events were determined during the follow-up period by hospital records.
RESULTS
During the 12-year follow-up period, 61 participants (7.8%) had a reported hypoglycemic event, and 148 (18.9%) developed dementia. Those who experienced a hypoglycemic event had a 2-fold increased risk for developing dementia compared with those who did not have a hypoglycemic event (34.4% vs 17.6%, P < .001; multivariate-adjusted hazard ratio, 2.1; 95% CI, 1.0–4.4). Similarly, older adults with DM who developed dementia had a greater risk for having a subsequent hypoglycemic event compared with participants who did not develop dementia (14.2% vs 6.3%, P < .001; multivariate-adjusted hazard ratio, 3.1; 95% CI, 1.5–6.6). Further adjustment for stroke, hypertension, myocardial infarction, and cognitive change scores produced similar results.
CONCLUSION AND RELEVANCE
Among older adults with DM, there seems to be a bidirectional association between hypoglycemia and dementia.
The growing body of evidence that diabetes mellitus (DM) may increase risk for developing cognitive impairment, including Alzheimer disease and vascular dementia,1,2 has prompted interest in whether DM treatment can prevent cognitive decline. Glycemic control has been proposed as a potential mechanism to improve cognitive outcomes among those with DM.1,3 However, trials evaluating the benefit of glycemic control have also highlighted the risk for hypoglycemia with intensive therapy.4–6
The brain uses glucose as a primary source of energy. Cognitive function becomes impaired when blood glucose drops to low levels,7 and severe hypoglycemia may cause neuronal damage.7–9 Although the acute effects of hypoglycemia have been well documented, it is unclear if the cognitive effects of hypoglycemia are persistent. Results from the few investigations of hypoglycemia and cognitive impairment have been conflicting, with some studies10,11 reporting an association but not other studies.12,13 In addition, little is known about the cognitive effects of hypoglycemia occurring in late life, when the brain may be more vulnerable.14
The direction of the association between hypoglycemia and cognitive impairment is also debated. Those with dementia or even milder forms of cognitive impairment may be less able to effectively manage complex DM behavioral and treatment regimens and recognize the symptoms of hypoglycemia and respond appropriately, increasing the risk for severe hypoglycemia.13,15–19 Whether a bidirectional association exists, in which hypoglycemia increases the risk for dementia and dementia increases the risk for hypoglycemia, has not been determined to our knowledge. Because the incidence of both hypoglycemia and dementia increases with age and duration of DM,20,21 a better understanding of the association between hypoglycemia and dementia is needed.
We prospectively examined the association between severe hypoglycemia and dementia in a biracial cohort of community-dwelling older adults with DM and without dementia at baseline. We hypothesized that severe hypoglycemia would be associated with an increased risk for dementia and that those diagnosed as having dementia would be at an increased risk for severe hypoglycemia.
Methods
Study Population
This study was approved by the institutional review boards of the University of Pittsburgh and the University of Tennessee, Memphis, and by that of the coordinating center at the University of California, San Francisco. All participants signed an informed written consent approved by the institutional review boards at the clinical and coordinating center sites.
We studied participants with DM enrolled in the Health, Aging, and Body Composition (Health ABC) Study, a prospective cohort study beginning in 1997 of 3075 community-dwelling older adults of white and black race/ethnicity then aged 70 to 79 years living in Memphis, Tennessee, or Pittsburgh, Pennsylvania. Among Medicare-eligible older adults within designated zip code areas, the Health ABC Study contacted potential participants from a random sample of white race/ethnicity and all of those of black race/ethnicity. Exclusion criteria included reported difficulties performing activities of daily living, walking a quarter of a mile, or climbing 10 steps without resting. Participants also had to be free of life-threatening cancers and plan to remain within the study area for at least 3 years. For our analytic cohort, we included those who had prevalent DM at baseline or who developed DM during the follow-up period and excluded those with evidence of possible cognitive impairment at study baseline, determined by a score of less than 80 on the Modified Mini-Mental State Examination (3MS).22,23 The final analytic cohort consisted of 783 Health ABC Study participants (25.4.% [783 of 3075]).
Measurements
Diabetes mellitus was determined at study baseline by self-report, the use of hypoglycemia medication, a fasting glucose level of 126 mg/dL or higher, or a 2-hour glucose tolerance test exceeding 200 mg/dL, in accord with the American Diabetes Association criteria in place near the start of the Health ABC Study (in 2002), as well as during annual follow-up assessments by self-report, the use of hypoglycemia medication, or a fasting glucose level of 126 mg/dL or higher (to convert glucose level to millimoles per liter, multiply by 0.0555). To determine insulin use, participants were asked to bring in all medications used in the last 2 weeks for a medication inventory at the annual visit. Glycated hemoglobin level was measured with a fasting whole blood sample using fully automated analyzers that utilize nonporous ion-exchange high-performance liquid chromatography.
Severe hypoglycemia was determined from review of hospital records, identifying a hypoglycemic event as the primary or secondary diagnosis related to any overnight hospitalization in an acute care hospital. Information was unavailable on episodes of mild hypoglycemia that did not result in a hospitalization.
Dementia onset was determined by the date of the first available record of a dementia diagnosis. This was identified through review of hospital records indicating a dementia-related hospital event (based on International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes) as the primary or secondary diagnosis related to the hospitalization or by a prescribed dementia medication (ie, galantamine, rivastigmine, memantine hydrochloride, donepezil hydrochloride, or tacrine hydrochloride) as part of the medication inventory at the annual visit.
In addition, at baseline and approximately every 2 years, the 3MS was administered at clinic visits. The 3MS is a brief, general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory.22 Scores range from 0 to 100 points, with lower scores indicating poorer performance.
Covariates
Possible covariates included the baseline self-reported age, sex, race/ethnicity, educational level (categorized as less than high school vs high school education or higher), and the number of alcoholic drinks per day (categorized as <1 vs ≥1). Depressive symptoms were assessed using the 20-item Center for Epidemiological Studies Depression Scale.24 Body mass index (calculated as weight in kilograms divided by height in meters squared) was determined from direct height and weight measurements at baseline. Hypertension was determined using self-report, medication use, and clinical measurements obtained at the baseline examination. History of stroke and myocardial infarction (MI) were based on self-report, clinic data, and medication use. Apolipoprotein E (APOE) genotype was determined by the 53-nuclease assay25 in the Human Genetics Laboratory at the University of Pittsburgh, and participants were coded as ε4 carriers or noncarriers.
Statistical Analysis
Bivariate analyses were used to test for associations between baseline characteristics and incident severe hypoglycemia or incident dementia. χ2 Test was used for categorical variables, and F test was used for continuous variables.
The baseline for the DM cohort was calculated as the beginning of the Health ABC Study for prevalent DM cases (398 [50.8%]) and as the time of first-recorded DM for incident cases (385 [49.2%]). For a given event type, either a hypoglycemia-associated hospitalization or a dementia diagnosis, the time to event was calculated as the time a participant first enrolled in the DM cohort until the occurrence of the first qualifying event. If the event did not occur, an outcome-specific censoring date was recorded as the date of the last available contact. Follow-up data for both dementia and hypoglycemia were available through May 1, 2011; the mean follow-up period before censoring by dementia, hypoglycemia, death, or follow-up loss was 9.4 years.
We used Kaplan-Meier survival curves to plot the survival curve for each outcome (hypoglycemia and dementia). To estimate the association between the timing of a severe hypoglycemic event and diagnosis of dementia among patients with DM, we used Cox proportional hazards regression models. The first model estimated the change in risk for a dementia diagnosis associated with a preceding hypoglycemic event, and the second model estimated the change in risk for a hypoglycemic event that is associated with a preceding dementia diagnosis. Both models used time-varying representations of the primary predictor (dementia was allowed to be time varying for the model of hypoglycemia, and hypoglycemia was allowed to be time varying for the model of dementia), allowing participants to change risk groups during the follow-up period before the event of interest. Predictor events that happened after the outcome of interest for a given model were not included in that model; an individual who had his or her first recorded hypoglycemic incident after being diagnosed as having dementia would not be considered hypoglycemic in the model estimating dementia risk. Analyses were adjusted for age, educational level, race/ethnicity, and any other covariates significantly associated (P < .05) with severe hypoglycemia or dementia in bivariate analysis. In addition, to evaluate if subclinical cognitive impairment was underlying the association between severe hypoglycemia and risk for dementia, we conducted a sensitivity analysis that adjusted for the change in 3MS score over time before the hypoglycemic event.
All analyses were conducted using statistical software (SAS, version 9.2; SAS Institute, Inc). Values were 2-tailed, with the statistical significance level set at P < .05.
Results
Of 3075 participants enrolled in the Health ABC Study, 783 (25.5%) had prevalent or incident DM and were without baseline cognitive impairment. The mean (SD) age of our analytic cohort was 74.0 (2.8) years at baseline, 368 (47.0%) were of black race/ethnicity, and 373 (47.6%) were female. Of 783 participants, 455 died during the follow-up period.
During the 12-year follow-up period, 61 participants with DM (7.8%) had a reported hypoglycemic event associated with a hospital visit (21 [2.7%] had >1 severe hypoglycemic event), and 148 participants (18.9%) developed dementia. Compared with those who did not experience a severe hypoglycemic event, participants who had a severe hypoglycemic event were less likely to have a high school education (P = .04) and were more likely to use insulin, have prevalent DM, be of black race/ethnicity, have lower baseline 3MS scores, and have higher glycated hemoglobin levels (P < .01 for all). There was no group difference by age, sex, comorbidities, or APOE ε4 status (Table 1). Compared with those who did not develop dementia, participants who developed dementia were less likely to have a history of MI (9.8% vs 20.5%, P = .007) and were more likely to have prevalent DM (58.8% vs 49.0%, P < .03), an APOE ε4 allele (28.6% vs 14.5%, P < .001), and a lower mean (SD) baseline 3MS score (90.4 [5.5] vs 91.6 [5.2], P < .02) but did not differ on other characteristics (P > .05).
Table 1.
Baseline Characteristics of 783 Participants Having Diabetes Mellitus With and Without Hypoglycemia
| Baseline Characteristic | Hypoglycemia (n = 61) | No Hypoglycemia (n = 722) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 74.6 (2.7) | 74.0 (2.8) | .10 |
| Black race/ethnicity, No. (%) | 44 (72.1) | 324 (44.9) | <.01 |
| Female sex, No. (%) | 31 (50.8) | 342 (47.4) | .60 |
| <High school education, No. (%) | 22 (36.1) | 173 (24.0) | .04 |
| Stroke, No. (%) | 9 (14.8) | 70 (9.7) | .22 |
| Myocardial infarction, No. (%) | 8 (13.1) | 105 (14.5) | .76 |
| Hypertension, No. (%) | 46 (75.4) | 511 (70.8) | .44 |
| Body mass index, mean (SD)a | 29.3 (5.3) | 29.1 (4.8) | .72 |
| Depression score ≥16, No. (%) | 3 (4.9) | 23 (3.2) | .47 |
| ≥1 Alcoholic drinks per day, No. (%) | 1 (1.6) | 51 (7.1) | .10 |
| Current smoker, No. (%) | 6 (9.8) | 58 (8.0) | .63 |
| APOE ε4 carrier, No./total No. (%) | 15/55 (27.3) | 191/681 (28.1) | .90 |
| Prevalent diabetes mellitus, No. (%) | 52 (85.2) | 346 (47.9) | <.01 |
| Insulin use, No. (%) | 58 (95.1) | 445 (61.6) | <.01 |
| Glycated hemoglobin level, mean (SD), % | 8.0 (1.2) | 7.2 (1.3) | <.01 |
| Baseline Mini-Mental State Examination score, mean (SD) | 89.6 (5.7) | 91.5 (5.2) | <.01 |
| Dementia medication use, No. (%) | 8 (13.1) | 52 (7.2) | .10 |
Abbreviation: APOE, apolipoprotein E.
SI conversion factor: To convert glycated hemoglobin level to proportion of total hemoglobin, multiply by 0.01.
Calculated as weight in kilograms divided by height in meters squared.
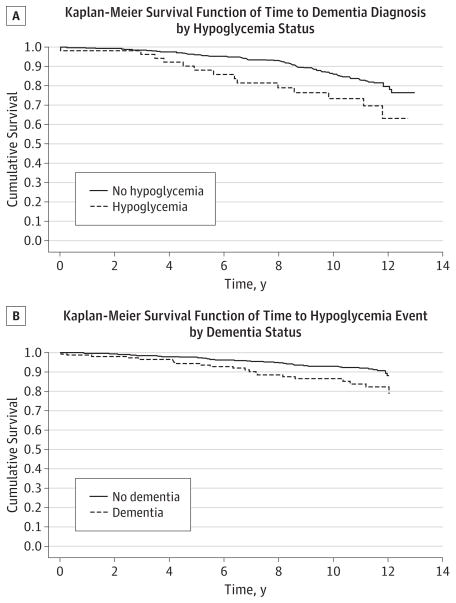
Older adults with DM who had a hypoglycemic event resulting in hospitalization were more likely to develop dementia (21 of 61 [34.4%]) compared with 127 of 722 [17.6%] among those who did not have a hypoglycemic event (P < .001) (Figure). Unadjusted results from Cox proportional hazards regression models (n = 783) revealed a 2-fold increased risk for dementia among those who experienced a preceding severe hypoglycemic event compared with those who did not have a hypoglycemic event. Adjustment for age, sex, education, insulin use, race/ethnicity, prevalent DM, APOE ε4 status, baseline 3MS score, and glycated hemoglobin level produced similar results (hazard ratio, 2.1; 95% CI, 1.0–4.4). In addition to severe hypoglycemia, other predictors of dementia were age, prevalent DM, APOE ε4 status, and baseline 3MS score, while sex, educational level, insulin use, race/ethnicity, and glycated hemoglobin level did not have a significant effect on dementia risk (Table 2). Additional adjustment for MI, stroke, and hypertension did not appreciably alter the results.
Figure. Survival Probability Curves From Kaplan-Meier Analysis Among Older Adults With Diabetes Mellitus.
A, Time to dementia associated with a preceding hypoglycemic event. B, Time to a hypoglycemic event associated with a preceding dementia diagnosis.
Table 2.
Multivariate-Adjusted Cox Proportional Hazards Regression Estimates for Time to Dementia Associated With a Hypoglycemic Event
| Variable | Hazard Ratio (95% CI) |
|---|---|
| Hypoglycemic event | 2.09 (1.00–4.35) |
| Age | 1.15 (1.08–1.22) |
| Black race/ethnicity | 0.77 (0.51–1.16) |
| Female sex | 0.78 (0.54–1.12) |
| Education | 1.30 (0.85–1.96) |
| APOE ε4 status | 2.17 (1.53–3.08) |
| Prevalent diabetes mellitus | 1.70 (1.12–2.58) |
| Insulin use | 1.04 (0.64–1.67) |
| Glycated hemoglobin level | 1.00 (0.85–1.17) |
| Baseline Mini-Mental State Examination score | 0.96 (0.93–0.99) |
Abbreviation: APOE, apolipoprotein E.
Similarly, the percentage of participants with a hypoglycemic event resulting in a hospitalization was higher among those having dementia compared with those not having dementia (21 of 148 [14.2%] vs 40 of 635 [6.3%], P < .001) (Figure). Unadjusted hazard ratios from Cox proportional hazards regression models showed a greater risk for having a subsequent severe hypoglycemic event among those with a preceding dementia diagnosis than among those without dementia. Adjustment for age, sex, education, insulin use, race/ethnicity prevalent DM, and baseline 3MS score produced similar results (hazard ratio, 3.1; 95% CI, 1.5–6.6) (Table 3). Additional adjustment for MI, stroke, and hypertension did not significantly affect the results.
Table 3.
Multivariate-Adjusted Cox Proportional Hazards Regression Estimates for Time to a Hypoglycemic Event Associated With Dementia
| Variable | Hazard Ratio (95% CI) |
|---|---|
| Dementia | 3.10 (1.46–6.58) |
| Age | 1.11 (1.02–1.22) |
| Black race/ethnicity | 2.47 (1.32–4.62) |
| Female sex | 0.95 (0.56–1.61) |
| Education | 0.96 (0.54–1.72) |
| Prevalent diabetes mellitus | 3.21 (1.48–6.97) |
| Insulin use | 4.71 (1.33–16.69) |
| Baseline Mini-Mental State Examination score | 0.97 (0.92–1.02) |
As a sensitivity analysis, we further adjusted for the slope of 3MS scores (change in 3MS score over time) before a severe hypoglycemic event. We found that the association between hypoglycemia and risk for dementia remained significant (hazard ratio, 2.2; 95% CI, 1.1–4.6).
Discussion
Among older adults with DM who were without evidence of cognitive impairment at study baseline, we found that clinically significant hypoglycemia was associated with a 2-fold increased risk for developing dementia and a number needed to harm of 5.9. Similarly, participants with dementia were more likely to experience a severe hypoglycemic event, with a number needed to harm of 12.7. The association remained even after adjustment for age, sex, educational level, race/ethnicity, comorbidities, and other covariates. These results provide evidence for a reciprocal association between hypoglycemia and dementia among older adults with DM.
Previous studies of hypoglycemia and cognitive impairment in older adults have had mixed results. Findings from a retrospective study10 conducted using the Kaiser Permanente Northern California Diabetes Registry demonstrated an almost 2-fold increased risk for dementia among older adults with DM who had a history of 1 or more hypoglycemic episodes. Risk was even greater for those with multiple episodes. Similarly, a population-based cross-sectional study11 found that a lifetime history of severe hypoglycemia was associated with poor late-life cognition, independent of premorbid cognition. Our investigation expands on these studies by prospectively investigating severe hypoglycemia and dementia among older adults who were initially free of cognitive impairment, allowing for a better evaluation of temporality. We were also able to assess the possible scenario that preclinical cognitive impairment is associated with greater risk for severe hypoglycemia and in turn with risk for dementia. Most importantly, after adjustment for change in global cognitive function, severe hypoglycemia remained associated with risk for developing dementia.
In contrast, the Fremantle Diabetes Study13 found no evidence that a history of hypoglycemia was associated with cognitive decline among older adults with normal cognition at baseline, although the short follow-up duration may have limited the ability to detect differences. The Diabetes Control and Complications Trial also did not find an association between hypoglycemia and cognitive impairment among younger adults with type 1 DM.12 Varied results may possibly be due to differences in cohort demographics, frequency or severity of hypoglycemia, or age at which the hypoglycemic event occurred. Older individuals have a higher incidence of hypoglycemia20 and may be more susceptible to adverse cognitive effects than younger age groups.14 Hypoglycemia may also differentially influence cognition depending on overall brain health, perhaps aggravating underlying pathologic conditions or triggering the onset of neurodegenerative processes.
Hypoglycemia may contribute to the pathogenesis of dementia through several possible mechanisms. Recurrent severe hypoglycemia has been shown to result in brain damage,7 with preferential vulnerability found in the cerebral cortex and hippocampus.7–9 Some evidence suggests that neuronal damage resulting from hypoglycemia may be enhanced in DM compared with non-DM brains, perhaps due to altered glucose metabolism or through insulin deficiency.8 Hypoglycemia may cause a loss of ionic homeostasis or increases in reactive oxygen species that can lead to neuronal death.9 Amyloid precursor protein may also be produced as a result of damage.26 In addition, insulin resistance and hyperinsulinemia may have a role in neurodegeneration through disrupting the normal function of insulin within the brain.27 Insulin may affect the metabolism of amyloid beta and tau proteins, and chronic hyperinsulinemia may exacerbate inflammatory markers and oxidative stress.28 Cerebrovascular disease is another potential mechanism. Pathologic microinfarcts have been shown to contribute to brain atrophy and cognitive impairment.29 Although we adjusted for stroke and MI and results were robust, we cannot rule out residual confounding, and we had no information on microinfarcts.
Our finding of a higher risk for hypoglycemia with dementia adds to a small body of literature demonstrating a greater incidence of hypoglycemia with impaired cognitive function.13,18,19,30 However, to our knowledge, no known prior study has prospectively evaluated the association between dementia and severe hypoglycemia among a diverse group of older individuals initially free of dementia. The heightened risk for hypoglycemia among those with dementia may reflect difficulties in managing DM. Insulin therapy13,30 and reported inability to self-manage DM medications13 have been previously identified as additional risk factors for hypoglycemia among those with cognitive impairment. Cognitive dysfunction, including dementia and milder forms of impairment, may also delay recognition of the symptoms of hypoglycemia and interfere with a patient’s ability to respond with corrective measures. Older adults have been shown to be less aware of the symptoms of hypoglycemia than younger persons31; however, it is unknown if this is more pronounced among those with cognitive impairment. More studies are needed to identify risk factors and effective management strategies to reduce the incidence of hypoglycemia among older persons with dementia, as well as in those with less severe forms of cognitive impairment.
Our findings emphasize the importance of cognitive function in the clinical management of older adults with DM. Certain medications known to carry a higher risk for hypoglycemia, such as insulin secretagogues and some sulfonylureas,20 may be inappropriate for older patients with or at risk for cognitive impairment. While physicians should be aware of the higher risk for hypoglycemia in patients with dementia because symptoms of hypoglycemia in older adults could be misinterpreted,20,32 delaying treatment, they should also consider the implications for the management and care of patients with lesser, subclinical levels of cognitive dysfunction. In addition, caretakers may not know the symptoms and treatment of hypoglycemia, and educational efforts may be needed.
This study has several strengths, including a prospective design with a long follow-up period and a large, diverse sample of community-dwelling older adults. Participants were free of dementia at study baseline, and we were able to assess temporality of hypoglycemia and dementia. Hypoglycemia was based on hospital records, which is a more objective measure than self-report. We were also able to adjust for multiple confounders. However, several study limitations also warrant consideration. While we tried to highlight the association of hypoglycemia and dementia defined by clinically important measures (hospitalization diagnostic codes or medication use), we were unable to investigate the effects of subclinical outcomes, such as moderate hypoglycemia or milder forms of cognitive impairment, nor were we able to include history of hypoglycemia before baseline. Although our case definition for dementia was likely very specific, it was probably insensitive to mild cases of cognitive impairment. In this context, power would most likely be affected, but the point estimate for the effect of hypoglycemia on dementia would not be biased.33 In addition, we had no brain magnetic resonance imaging measures, which may have helped to ascertain the subtype of dementia.
In summary, our results provide evidence for a bidirectional association between severe hypoglycemia and dementia. Hypoglycemia may impair cognitive health, and reduced cognitive function may increase the risk for a hypoglycemic event that could further compromise cognition, resulting in a detrimental cycle. Cognitive function should be considered in the clinical management of older individuals with DM.
Acknowledgments
Funding/Support: This study was supported by grants N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106 from the National Institute on Aging and by grants R01-AG028050 and R01-NR012459 from the National Institute of Nursing Research. This research was supported in part by the Intramural Research Program of the National Institutes of Health, by the National Institute on Aging, and by grant A201-0029 from the American Health Assistance Foundation. Dr Yaffe is supported in part by grant K24AG031155 from the National Institute on Aging.
Footnotes
Health ABC Study Principal Investigators and Staff: Clinical sites: University of Pittsburgh, Pittsburgh, Pennsylvania: Anne B. Newman, MD, MPH, principal investigator, and Diane Ives, study coordinator; University of Tennessee, Memphis: Suzanne Satterfield, MD, DrPH, principal investigator, and Jan Elam, study coordinator; Health ABC Coordinating Center: Steven R. Cummings, MD, and Michael C. Nevitt, PhD, principal investigators, Susan M. Rubin, MPH, project director; Sponsor: Tamara B. Harris, MD, and Melissa E. Garcia, MPH, National Institute on Aging (project office).
Conflict of Interest Disclosures: Dr Yaffe has served on data safety monitoring boards for Takeda Inc, Pfizer Inc, and Medivation Inc and served as a consultant for Novartis Inc.
Author Contributions: Dr Yaffe had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept or design: Yaffe.
Acquisition of data: Harris, Simonsick, Schwartz.
Analysis and interpretation of data: Yaffe, Hamilton, Simonsick, Schwartz.
Drafting of the manuscript: All authors. Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Hamilton.
Obtaining funding: Yaffe.
Study supervision: Yaffe, Schwartz.
References
- 1.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes: systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 2.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67(6):505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. [Accessed April 11, 2013];BMJ. 2010 340:b4909. doi: 10.1136/bmj.b4909. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2803744/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 7.Warren RE, Frier BM. Hypoglycaemia and cognitive function. Diabetes Obes Metab. 2005;7(5):493–503. doi: 10.1111/j.1463-1326.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 8.Bree AJ, Puente EC, Daphna-Iken D, Fisher SJ. Diabetes increases brain damage caused by severe hypoglycemia. [Accessed April 13, 2013];Am J Physiol Endocrinol Metab. 2009 297(1):E194–E201. doi: 10.1152/ajpendo.91041.2008. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2711670/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh SW, Hamby AM, Swanson RA. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia. 2007;55(12):1280–1286. doi: 10.1002/glia.20440. [DOI] [PubMed] [Google Scholar]
- 10.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301(15):1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aung PP, Strachan MW, Frier BM, Butcher I, Deary IJ, Price JF Edinburgh Type 2 Diabetes Study Investigators. Severe hypoglycaemia and late-life cognitive ability in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabet Med. 2012;29(3):328–336. doi: 10.1111/j.1464-5491.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson AM, Musen G, Ryan CM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-term effect of diabetes and its treatment on cognitive function [published correction appears in N Engl J Med. 2009;361(19):1914] N Engl J Med. 2007;356(18):1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce DG, Davis WA, Casey GP, et al. Severe hypoglycemia and cognitive impairment in older patients with diabetes: the Fremantle Diabetes Study. Diabetologia. 2009;52(9):1808–1815. doi: 10.1007/s00125-009-1437-1. [DOI] [PubMed] [Google Scholar]
- 14.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7(2):184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 15.Bauduceau B, Doucet J, Bordier L, Garcia C, Dupuy O, Mayaudon H. Hypoglycaemia and dementia in diabetic patients. Diabetes Metab. 2010;36(suppl 3):S106–S111. doi: 10.1016/S1262-3636(10)70476-6. [DOI] [PubMed] [Google Scholar]
- 16.Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25(3):245–254. doi: 10.1111/j.1464-5491.2007.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services: All Wales Research Into Elderly (AWARE) Study. Diabetes Res Clin Pract. 2000;50(3):203–212. doi: 10.1016/s0168-8227(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 18.de Galan BE, Zoungas S, Chalmers J, et al. ADVANCE Collaborative Group. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia. 2009;52(11):2328–2336. doi: 10.1007/s00125-009-1484-7. [DOI] [PubMed] [Google Scholar]
- 19.Punthakee Z, Miller ME, Launer LJ, et al. ACCORD Group of Investigators; ACCORD-MIND Investigators. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012;35(4):787–793. doi: 10.2337/dc11-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28(12):2948–2961. doi: 10.2337/diacare.28.12.2948. [DOI] [PubMed] [Google Scholar]
- 21.Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26(7):507–519. doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 23.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22(1):13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 24.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 25.Livak KJ. SNP genotyping by the 53-nuclease reaction. Methods Mol Biol. 2003;212(212):129–147. doi: 10.1385/1-59259-327-5:129. [DOI] [PubMed] [Google Scholar]
- 26.Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110(4):1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 27.Umegaki H. Neurodegeneration in diabetes mellitus. In: Ahmad SI, editor. Neurodegenerative Diseases. New York, NY: Springer; 2012. pp. 258–265. [Google Scholar]
- 28.Bosco DFA, Fava A, Plastino M, Montalcini T, Pujia A. Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J Cell Mol Med. 2011;15(9):1807–1821. doi: 10.1111/j.1582-4934.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol. 2011;70(5):774–780. doi: 10.1002/ana.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263–2272. doi: 10.1111/j.1532-5415.2011.03726.x. [DOI] [PubMed] [Google Scholar]
- 31.Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32(8):1513–1517. doi: 10.2337/dc09-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt P. Taking hypoglycaemia seriously: diabetes, dementia and heart disease. Br J Community Nurs. 2011;16(5):246–249. doi: 10.12968/bjcn.2011.16.5.246. [DOI] [PubMed] [Google Scholar]
- 33.White E. The effect of misclassification of disease status in follow-up studies: implications for selecting disease classification criteria. Am J Epidemiol. 1986;124(5):816–825. doi: 10.1093/oxfordjournals.aje.a114458. [DOI] [PubMed] [Google Scholar]