This study from two large centers describes the profile and predictors of costs of reduced intensity conditioning hematopoietic cell transplantation. Results indicate comparable costs between two centers with different practice patterns. Identification of optimum donors with favorable cost-benefit ratio and interventions to prevent complications such as graft-versus-host disease may yield clinical and financial benefits. The study also underscores the need for collection of resource utilization data in a prospective fashion with clinical studies.
Keywords: Economics, Hematopoietic stem cell transplantation, Costs, Allogeneic transplantation
Abstract
Reduced intensity conditioning (RIC) regimens have allowed older patients and those with comorbidities to receive hematopoietic cell transplantation (HCT). We analyzed medical costs from the beginning of conditioning to 100 days after HCT for 484 patients and up to 2 years for 311 patients who underwent a RIC HCT at two institutions from January 2008 to December 2010. Multiple linear regression was used to analyze the association between clinical variables, center effect, and costs. Patient and transplant characteristics were comparable between the sites, although differences were seen in pretransplant performance scores. Significant predictors for lower costs for the first 100 days included a diagnosis of lymphoma/myeloma and use of human leukocyte antigen-matched related donors. Grade II-IV acute graft-versus-host disease (GVHD) was associated with higher costs. The overall short-term costs between the two institutions were comparable when adjusted for clinical variables (p = .43). Late costs between 100 days and 2 years after HCT were available for one cohort (n = 311); median costs during this period were $39,000 and accounted for 39% of costs during the first 2 years. Late costs were not associated with any pretransplant variables, but were higher with extensive chronic GVHD and death. After adjustment for clinical characteristics, the overall costs of the RIC transplants were similar between the two institutions despite different management approaches (inpatient vs. outpatient conditioning) and accounting methodologies. Use of unrelated/alternative donors, transplant for diseases other than lymphoma or myeloma, and acute GVHD were predictors for higher early costs, and extensive chronic GVHD and death were associated with higher late costs.
Implications for Practice:
This study from two large centers describes the profile and predictors of costs of reduced intensity conditioning (RIC) hematopoietic cell transplantation. Results indicate comparable costs between two centers with different practice patterns. Identification of optimum donors with favorable cost-benefit ratio and interventions to prevent complications such as graft-versus-host disease may yield clinical and financial benefits. A commitment to lowering costs while preserving quality of care is essential in the current environment, especially for RIC transplants. These transplants are done mainly for the older population, a majority of whom may be prone to insufficient coverage and declining reimbursements by Medicare. The study also underscores the need for collection of resource utilization data in a prospective fashion with clinical studies.
Introduction
A major medical milestone was reached in January 2013 with the performance of the 1 millionth stem cell transplant. Improved supportive care including practices to decrease organ toxicity and better infection control has increased survival after this complicated medical procedure [1–3]. Availability of alternative donors such as haploidentical donors or cord blood and the advent of nonmyeloablative (NMA)/reduced-intensity conditioning (RIC) regimens for allogeneic transplants have made this treatment available to many more patients irrespective of age, race/ethnicity, and comorbidities [4, 5]. Although there are some differences in the intensity of NMA (lowest) and RIC regimens (intermediate between myeloablative and NMA), NMA regimens were included with the RIC regimens in this analysis and hereafter are referred to collectively as RIC for this article. The ability to use these regimens to successfully transplant patients has likely improved the dismal outcomes of hematological malignancies in older patients treated with conventional chemotherapy [4, 6]. However, the newer transplant approaches have also raised questions about whether resource utilization would be different from conventional high-dose transplants because of the underlying differences in age, comorbidities, and occurrence of post-transplant complications in patients who undergo RIC transplants [7].
Previous single-institution studies have compared the costs of RIC transplants with those of myeloablative transplants in patients transplanted before the year 2007, and only a few have assessed long-term costs [8–11]. Our goal was to use the clinical and financial data from two large academic transplant centers performing hematopoietic cell transplantation (HCT) to characterize the total costs and predictors of costs of RIC transplants in the setting of different practice patterns in a relatively contemporary cohort of patients. For one cohort, we were also able to evaluate long-term costs to assess the impact of the downstream effects of HCT.
Patients and Methods
Patients
Patients (n = 484) who underwent a first allogeneic HCT using RIC from January 1, 2008, through June 30, 2010, at the Fred Hutchinson Cancer Research Center (FHCRC; n = 147) and the Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC; n = 337) were included. All patients who had a prior HCT (autologous or allogeneic) were excluded because we have recently reported in a separate paper information about cost profiles of second allogeneic HCT following a prior autologous or allogeneic transplant in which 70% of the study population received RIC regimens [12]. Patients who had a subsequent HCT in the study time frame of 100 days for FHCRC (n = 4) or 2 years for DF/BWCC (n = 24) had associated costs included in the analysis. The study protocol was approved by the institutional review boards of FHCRC and DF/BWCC.
Conditioning, Graft-Versus-Host Disease Prophylaxis, and Supportive Care
The majority of patients at FHCRC received NMA doses of total body irradiation (TBI; 200–300 cGy) along with fludarabine for their HCT. At DF/BWCC, 88% patients received fludarabine and busulfan. Graft-versus-host disease (GVHD) prophylaxis was primarily a combination of mycophenolate mofetil and calcineurin inhibitor (tacrolimus or cyclosporine) at FHCRC; tacrolimus and sirolimus with or without low-dose methotrexate (5 mg/m2 on days 1, 3, and 6) were used at DF/BWCC. Antimicrobial prophylaxis and blood product and nutritional support were provided per institutional guidelines.
Most RIC transplants at FHCRC were performed as outpatients with hospital admission only for cell infusion (if mandated by insurance or for stem cell products arriving when the outpatient clinic was closed). At DF/BWCC, most patients were admitted for the conditioning and cell infusion and were discharged 48 hours after the infusion. At both sites, patients were subsequently admitted to the hospital primarily for management of febrile neutropenia, severe GVHD requiring parenteral nutrition and intravenous medications, severe pain requiring intravenous narcotics, inability to maintain oral intake, or other complications. Approximately 80% patients at DF/BWCC continued to receive all of their post-transplant care at the institution [13].
Costs
Actual medical costs and total hospital days from 7 days before graft infusion (day −7) of the transplant to day 100 were retrieved from the administrative database, reflecting the perspective of the health care system. For FHCRC, costs were calculated from medical charges and departmental ratio of costs to charges. DF/BWCC costs were calculated using an internal system that allocates costs to services based on departmental input for resource use. Costs incurred before day −7, including the pretransplant evaluation of the patient and those of stem cell procurement, were not included. Similar costs were captured for both the centers. For the subgroup of patients at DF/BWCC that continued to be followed by providers at the study site beyond the first 3 months, long-term cost data for up to 2 years after HCT were obtained and analyzed. Long-term costs were not analyzed from FHCRC because most patients relied on local providers for monitoring and treatment and returned to FHCRC mainly for consultative care for chronic GVHD, and this would have biased the results. Certain costs such as those for prescription medications, direct nonmedical costs (e.g., transportation, lodging), indirect costs (e.g., time off work, caregiving time), and professional fees were excluded. All costs were adjusted to 2010 U.S. dollars using the medical care component of the consumer price index [14].
Statistical Analysis
A descriptive analysis of the patient characteristics as well as inpatient and outpatient costs for the first 100 days and beyond (for DF/BWCC patients) was performed. Pretransplant and post-transplant predictors of costs were identified using multiple linear regression considering patient characteristics (demographics, disease variables, cytomegalovirus [CMV] status, Karnofsky performance score), transplant characteristics (stem cell source, human leukocyte antigen [HLA] matching, donor type, conditioning regimen), and post-transplant complications (death, relapse, and acute grade II-IV or chronic GVHD). Year of transplant and transplant center were also included in the model for the short-term costs.
Given that the distribution of costs is highly skewed, the logarithm of costs was used for multivariate analysis. Results are presented as “cost multipliers,” which are the ratios of costs for patients with specific characteristics or complications compared with those without. For example, a cost multiplier of 1.5 for unrelated donor (URD) corresponds to a 50% increase in costs of patients who received URD HCT as compared with other donor sources.
Results
Clinical Characteristics
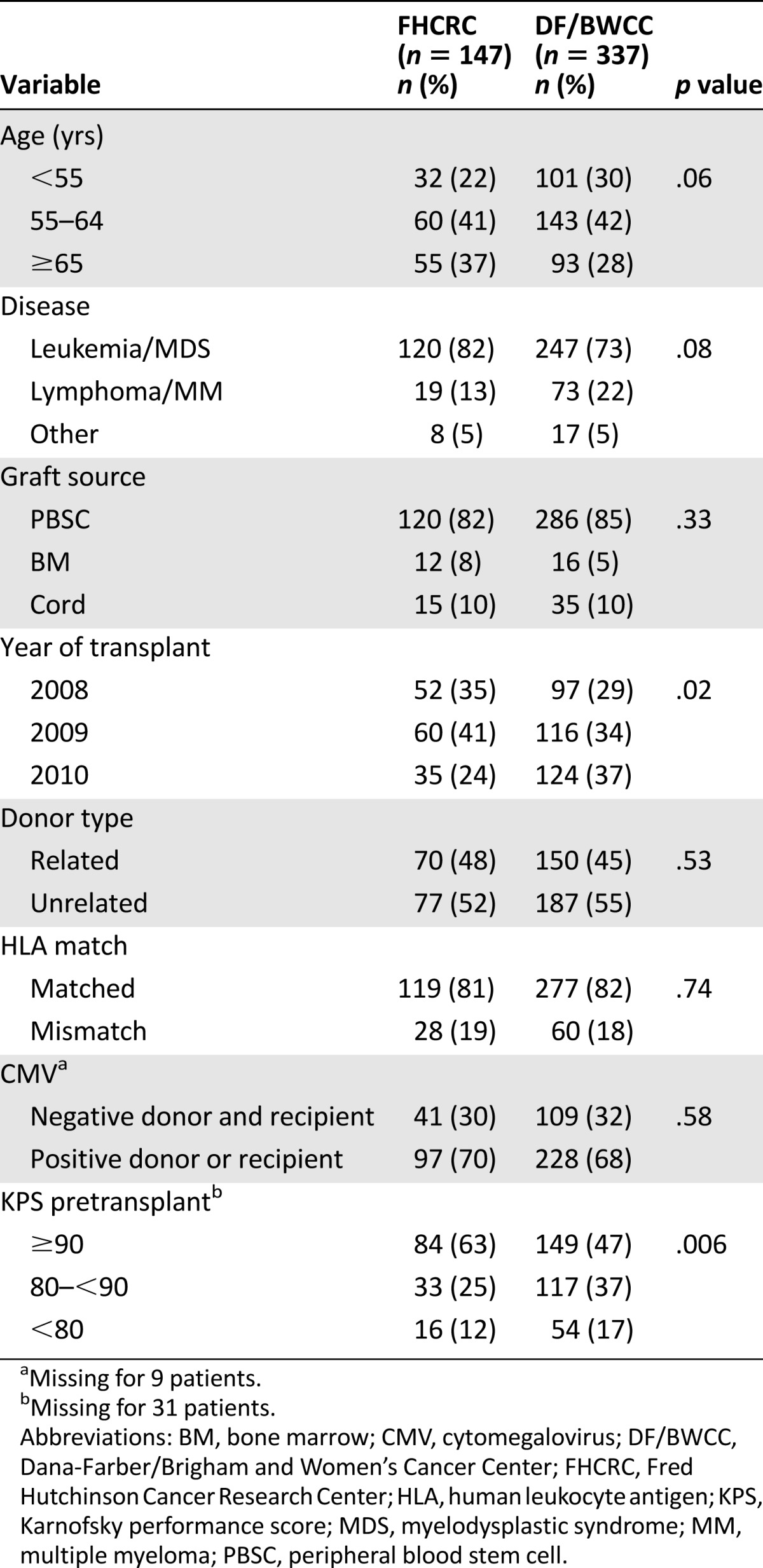
Patient characteristics are listed in Table 1. Although there were no significant differences between the FHCRC and DF/BWCC cohort in terms of age or disease distribution, graft source, donor type, HLA matching, and CMV serostatus, the pretransplant performance scores were higher at FHCRC, with 63% versus 47% patients at DF/BWCC with a Karnofsky performance score ≥90 (p = .006).
Table 1.
Baseline characteristics
Short-Term Costs
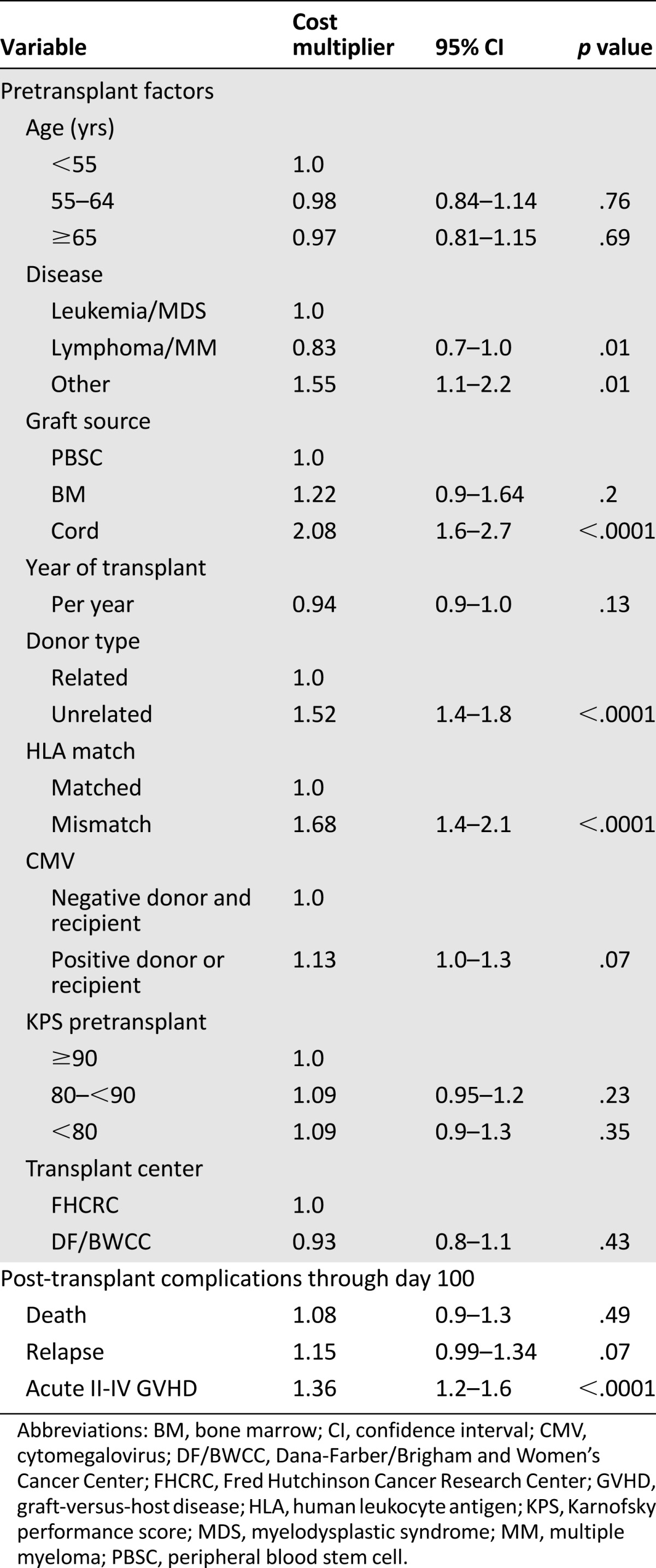
Median costs for the first 100 days in 2010 dollars at FHCRC and DF/BWCC were $129,000 (interquartile range [IQR] $84,000–$171,000) and $96,000 (IQR $74,000–$152,000), respectively (p = .0002; Table 2). The proportion of cost attributable to hospitalization was 42% for FHCRC and 87% for DF/BWCC. Costs for the initial transplant days (day −7 to day 2), especially the inpatient costs, were higher for DF/BWCC because of the hospital admission for conditioning (supplemental online Table 1) Significant pretransplant predictors for costs included use of mismatched donors (68% increase in costs; p < .0001), unrelated donors (52% increase in costs; p < .0001), or cord blood as a source of stem cells (100% increase in costs; p < .0001) compared with matched related donors and transplantation for nonmalignant hematological disorders (55% increase in costs; p = .01). Among the post-transplant complications, grade II–IV acute GVHD (37% increase in costs; p < .0001) was associated with higher costs. The overall short-term costs between the two institutions were comparable when adjusted for pre- and post-transplant variables (p = .43). Table 3 summarizes the predictors of short-term costs.
Table 2.
Costs from days −7 to 100 and days 101 to 730
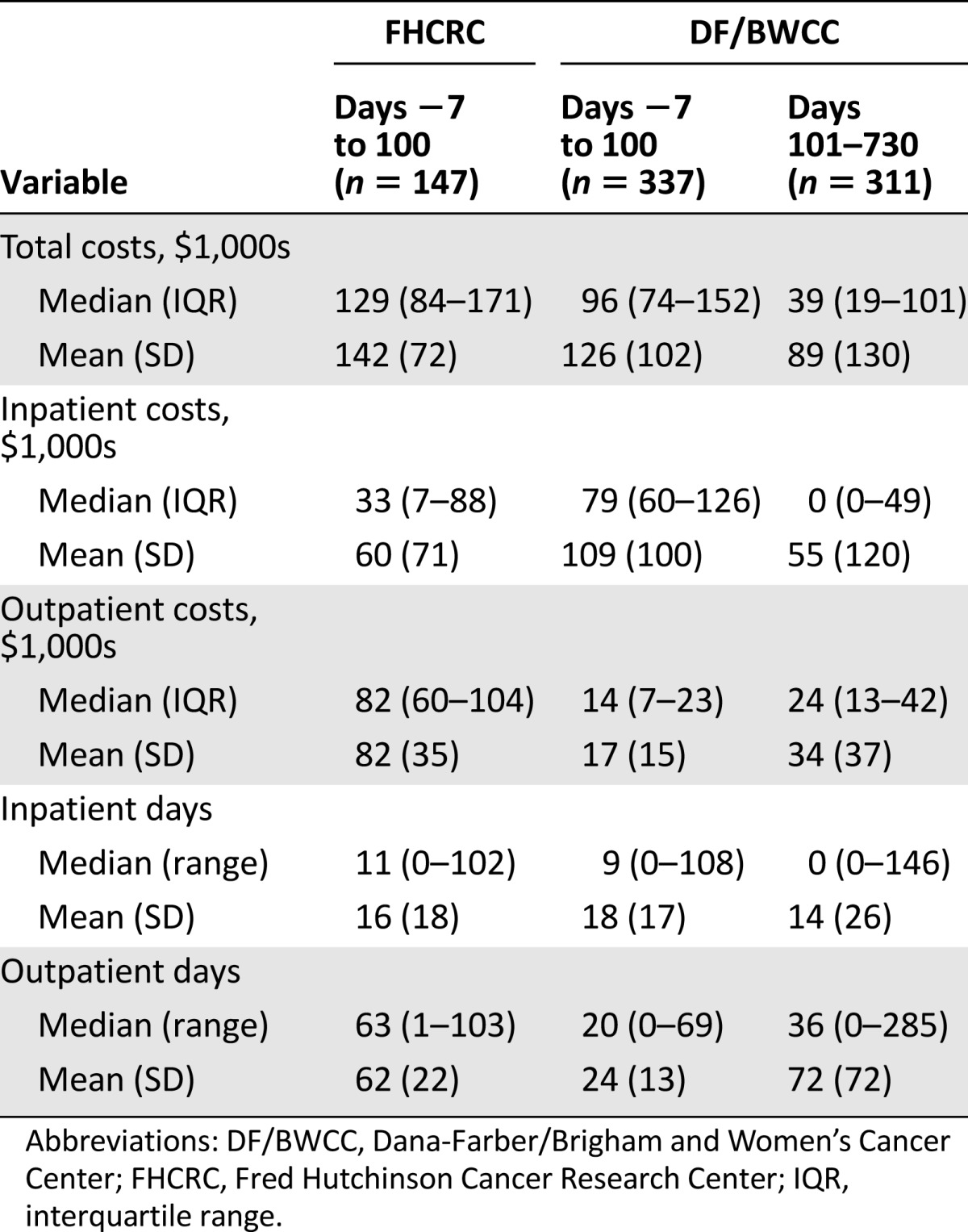
Table 3.
Predictors for short-term costs (days −7 to 100)
Long-Term Costs
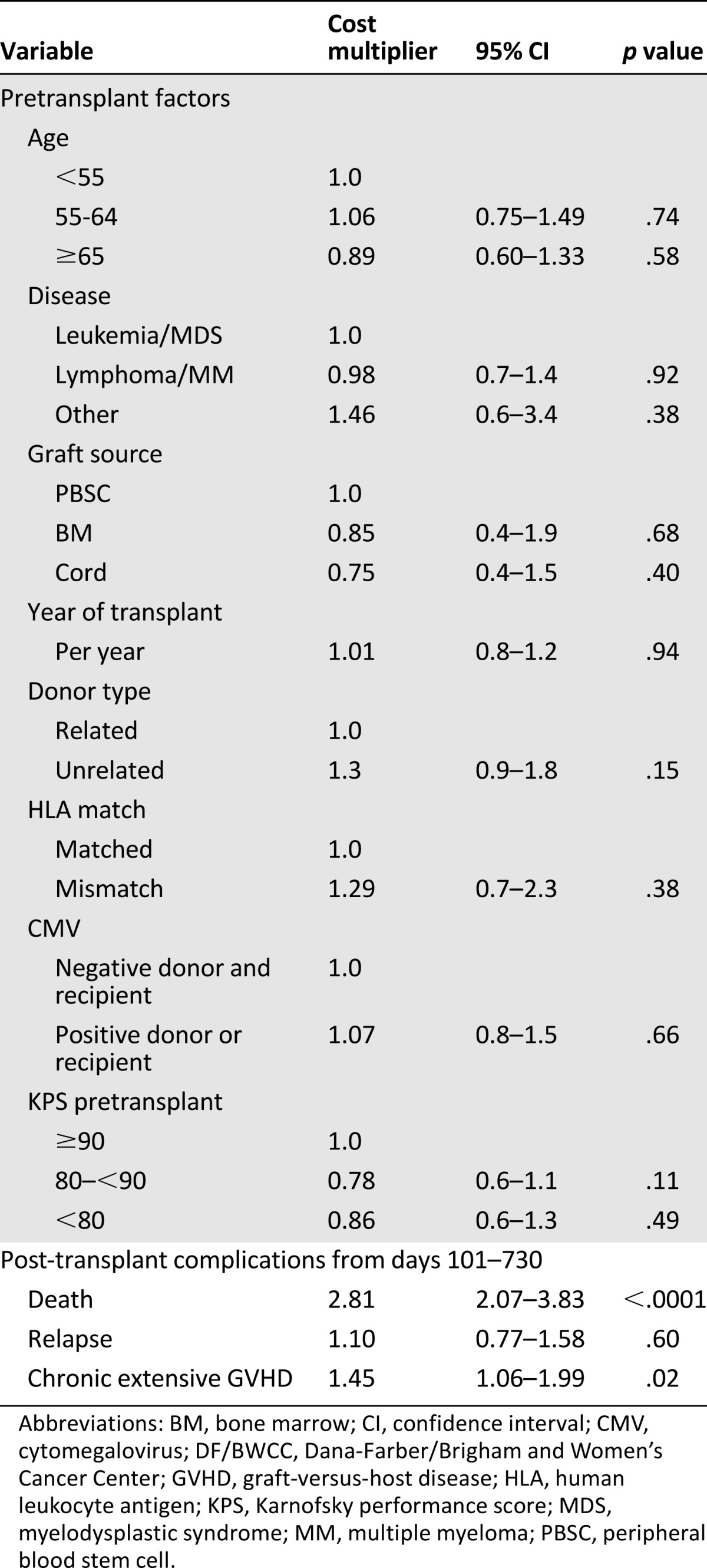
This analysis included 311 patients from DF/BWCC who continued to receive the majority of their care at the transplant center itself. Median costs between day +100 and 2 years were $39,000 (IQR $19,000–$101,000). Most patients remained outpatient, with a median number of hospital days after the first 100 days of 0 (range 0–146 days). Median outpatient days were similar in patients with or without chronic GVHD (34 vs. 37; p = .19). Thirty-nine percent of the total costs accrued through 2 years occurred after day +100. Unlike with short-term costs, none of the pretransplant variables showed a significant association with long-term costs. Chronic extensive GVHD (45% increase in costs; p = .02) and death after day 100 (181% increase in costs; p < .0001) were associated with higher costs in this time frame (Table 4).
Table 4.
Predictors for long-term costs (days 101–730); DF/BWCC only
Discussion
In the current health care environment, it is important to maximize health outcomes while trying to contain costs. The first step in decreasing costs is to understand the drivers of costs, especially the modifiable ones. Our results show that for the RIC transplants, patient factors such as age, performance score, or CMV status were not associated with costs, similar to myeloablative transplants. Instead, underlying disease emerged as a significant predictor of costs, with transplants done for lymphoma/myeloma being less expensive than those for leukemia/myelodysplastic syndrome or nonmalignant conditions [15–18]. Similarities were again noted with myeloablative cost analyses in that use of donors other than matched related donors and post-transplant complications such as GVHD were associated with higher short-term costs [16, 19].
Although donor type may not appear to be as modifiable a factor, a recent Center for International Blood and Marrow Transplant Research (CIBMTR) analysis found comparable to superior outcomes with the use of an older matched related donor over a younger matched unrelated donor for older HCT recipients [20]. Use of unrelated donors in our study was associated with 50% higher costs than matched related donors, indicating that the approach to selecting donors suggested by the CIBMTR study may result in cost savings too. An ongoing cost-effectiveness analysis accompanying Blood and Marrow Transplant Clinical Trial Network study (BMT CTN) 1101 may also help to discern whether haploidentical or cord blood donors are more cost-effective.
Another interesting finding in our study was that after 100 days, there was no association between the pretransplant variables with costs. Instead, the post-transplant complication of chronic GVHD emerged as a predictor for higher costs unlike in prior studies. This is likely due to the need for increased follow-up, use of expensive therapies such as extracorporeal photopheresis, and associated late complications, although we did not have the financial details to evaluate these hypotheses. The occurrence of death being associated with higher costs has been reported by other investigators [17, 21].
Our study also describes the pattern of costs over time. In the myeloablative setting, 84% of 1-year costs were sustained within the first 100 days as opposed to 68% for the RIC transplants in this study [17]. The shift of costs beyond 100 days may be due to complications such as GVHD occurring later in the NMA/RIC transplants [22] and higher comorbidities in this population, although we could not substantiate these hypotheses in our study. Petersen et al. have reported previously that even though the NMA transplant itself was done as an outpatient procedure, complications accounted for a median of 44 days of inpatient stay in the first year post-HCT in their cohort [23].
Most important, we found that the inpatient costs at FHCRC for comparable hospital days were higher than those at DF/BWCC in univariate analysis likely because of different accounting methodologies. However, the overall short-term costs were comparable between two large academic centers after adjustment for patient-, transplant-, and disease-related variables despite the different practices of outpatient (FHCRC) versus inpatient (DF/BWCC) conditioning for HCT. Rizzo et al. have shown cost savings with an outpatient transplant program, but this was prior to the common use of RIC regimens [24]. Myeloablative and RIC transplants have inherent differences in population and treatment characteristics that could explain our inability to detect differences in inpatient versus outpatient RIC approaches. A possible reason for the costs of outpatient transplants at FHCRC being comparable to inpatient chemotherapy-only conditioning regimens at DF/BWCC could be the use of TBI even as a single fraction that may increase costs of the nonmyeloablative regimens used at FHCRC. Our results do indicate that for individual centers, cost-cutting efforts should be directed toward identifying site-specific cost drivers and addressing those rather than trying to shift from one practice pattern to another to help save costs.
A few caveats to our conclusions should be noted. The long-term cost data were available only from the DF/BWCC cohort and may still be an underestimate for the total costs as some medical care may have been received outside the institution. Previous HCT cost studies from DF/BWCC found that the costs sustained outside the institution were <4% of total health care utilization [9]. Although complete long-term data could be captured using a claims-based database for analysis, data collection is expensive and the perspective is that of the insurer rather than the health care system [18]. Institution-level data, as used in our study, provide a more detailed assessment of the patient- and transplant-related variables as predictors for costs than can be discerned through a claims database. We did not have uniform data on comorbidities from both sites; hence, this variable was not included in the models, and Karnofsky performance score was used as a surrogate for pretransplant clinical status. Although the inclusion of two large academic transplant centers makes the results somewhat generalizable, they are still applicable mainly to low-dose TBI with or without fludarabine and fludarabine plus busulfan regimens reflecting the practice patterns at the two sites.
Conclusion
Despite these limitations, our study, which used detailed clinical and cost data from two different institutions to evaluate a recent cohort of allogeneic transplant patients, demonstrates that the predictors of short-term costs of RIC transplants are similar to those of myeloablative transplants, although the costs may be shifted disproportionately after 100 days of HCT. These results suggest the need for a formal cost-effectiveness analysis between myeloablative and RIC regimens if it is found that the ultimate outcomes of the two approaches are equivalent. This question of clinical effectiveness is being evaluated in a randomized prospective fashion by the BMT CTN 0901 study. Our results also suggest that comparable costs can occur between two centers with very different practice patterns, indicating that the success of interventions to reduce costs depends very much on the local environment and how much it has already evolved to minimize costs. On a broader level, identification of preventive strategies for transplant-related complications can yield favorable clinical and financial benefits. A commitment to lowering costs while preserving quality of care is essential in the current health care environment, especially for RIC transplants that are done mainly in the older population. A majority of these patients are Medicare beneficiaries and thus vulnerable to various federal budget cuts that can directly impact the reimbursements to the transplant center, hence the need to optimize costs at the institutional level. Finally, as has been mentioned by previous investigators, collection of costs/resource utilization data in a prospective fashion with clinical studies has the best chance of putting the results into perspective, balancing costs and clinical efficacy to help guide decision making for these interventions.
This article is available for continuing medical education credit at CME.TheOncologist.com.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants CA18029, HL36444, and CA78902.
Footnotes
Part of this research was presented at the American Society of Clinical Oncology Annual Meeting 2013, Chicago, Illinois, May 31–June 4, 2013.
Author Contributions
Conception/design: Nandita Khera, Stephanie J. Lee
Provision of study material or patients: Amy Emmert, Brenda M. Sandmaier, Edwin P. Alyea
Collection and/or assembly of data: Nandita Khera, Amy Emmert, Barry E. Storer, Edwin P. Alyea, Stephanie J. Lee
Data analysis and interpretation: Nandita Khera, Amy Emmert, Barry E. Storer, Brenda M. Sandmaier, Edwin P. Alyea, Stephanie J. Lee
Manuscript writing: Nandita Khera, Amy Emmert, Barry E. Storer, Brenda M. Sandmaier, Edwin P. Alyea, Stephanie J. Lee
Final approval of manuscript: Nandita Khera, Amy Emmert, Barry E. Storer, Brenda M. Sandmaier, Edwin P. Alyea, Stephanie J. Lee
Disclosures
The authors indicated no financial relationships.
References
- 1.Ruutu T, Eriksson B, Remes K et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood 2002;100:1977–1983. [DOI] [PubMed]
- 2.McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 3.Yokoe D, Casper C, Dubberke E et al. Infection prevention and control in health-care facilities in which hematopoietic cell transplant recipients are treated. Bone Marrow Transplant 2009;44:495–507. [DOI] [PubMed]
- 4.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunstein CG, Fuchs EJ, Carter SL et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011;118:282–288. [DOI] [PMC free article] [PubMed]
- 6.McClune BL, Weisdorf DJ, Pedersen TL et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol 2010;28:1878–1887. [DOI] [PMC free article] [PubMed]
- 7.Mineishi S. Overcoming the age barrier in hematopoietic stem cell transplantation: Progress, but still a long way to go. JAMA. 2011;306:1918–1920. doi: 10.1001/jama.2011.1612. [DOI] [PubMed] [Google Scholar]
- 8.Cordonnier C, Maury S, Esperou H, et al. Do minitransplants have minicosts? A cost comparison between myeloablative and nonmyeloablative allogeneic stem cell transplant in patients with acute myeloid leukemia. Bone Marrow Transplant. 2005;36:649–654. doi: 10.1038/sj.bmt.1705109. [DOI] [PubMed] [Google Scholar]
- 9.Saito AM, Zahrieh D, Cutler C, et al. Lower costs associated with hematopoietic cell transplantation using reduced intensity vs high-dose regimens for hematological malignancy. Bone Marrow Transplant. 2007;40:209–217. doi: 10.1038/sj.bmt.1705733. [DOI] [PubMed] [Google Scholar]
- 10.Svahn BM, Alvin O, Ringdén O, et al. Costs of allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82:147–153. doi: 10.1097/01.tp.0000226171.43943.d3. [DOI] [PubMed] [Google Scholar]
- 11.Svahn BM, Remberger M, Alvin O, et al. Increased costs after allogeneic haematopoietic SCT are associated with major complications and re-transplantation. Bone Marrow Transplant. 2012;47:706–715. doi: 10.1038/bmt.2011.162. [DOI] [PubMed] [Google Scholar]
- 12.Khera NSB, Storer B, Sandmaier BM, et al. Costs of second allogeneic hematopoietic cell transplantation. Transplantation. 2013;96:108–115. doi: 10.1097/TP.0b013e318294caf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abou-Nassar KE, Kim HT, Blossom J et al. The impact of geographic proximity to transplant center on outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012;708–715. [DOI] [PMC free article] [PubMed]
- 14.Overview of BLS Statistics on Inflation and Prices. Available from http://www.bls.gov/bls/inflation.htm
- 15.Griffiths RI, Bass EB, Powe NR, et al. Factors influencing third party payer costs for allogeneic BMT. Bone Marrow Transplant. 1993;12:43–48. [PubMed] [Google Scholar]
- 16.Lee SJ, Klar N, Weeks JC, et al. Predicting costs of stem-cell transplantation. J Clin Oncol. 2000;18:64–71. doi: 10.1200/JCO.2000.18.1.64. [DOI] [PubMed] [Google Scholar]
- 17.Saito AM, Cutler C, Zahrieh D, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14:197–207. doi: 10.1016/j.bbmt.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majhail NS, Mau LW, Denzen EM, et al. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: A study using a large national private claims database. Bone Marrow Transplant. 2013;48:294–300. doi: 10.1038/bmt.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espérou H, Brunot A, Roudot-Thoraval F, et al. Predicting the costs of allogeneic sibling stem-cell transplantation: Results from a prospective, multicenter, French study. Transplantation. 2004;77:1854–1858. doi: 10.1097/01.tp.0000129409.84087.62. [DOI] [PubMed] [Google Scholar]
- 20.Alousi AM, Le-Rademacher J, Saliba RM et al. Who is the better donor for older hematopoietic transplant recipients: An older-aged sibling or a young, matched unrelated volunteer? Blood 2013;121:2567–2573. [DOI] [PMC free article] [PubMed]
- 21.Welch HG, Larson EB. Cost effectiveness of bone marrow transplantation in acute nonlymphocytic leukemia. N Engl J Med. 1989;321:807–812. doi: 10.1056/NEJM198909213211207. [DOI] [PubMed] [Google Scholar]
- 22.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 23.Petersen SL, Madsen HO, Ryder LP, et al. Haematopoietic stem cell transplantation with non-myeloablative conditioning in the outpatient setting: Results, complications and admission requirements in a single institution. Br J Haematol. 2004;125:225–231. doi: 10.1111/j.1365-2141.2004.04897.x. [DOI] [PubMed] [Google Scholar]
- 24.Rizzo JD, Vogelsang GB, Krumm S, et al. Outpatient-based bone marrow transplantation for hematologic malignancies: cost saving or cost shifting? J Clin Oncol. 1999;17:2811–2818. doi: 10.1200/JCO.1999.17.9.2811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


