The authors describe the case of one patient with hormone receptor-positive breast adenocarcinoma with a somatically acquired IDH1 (isocitrate dehydrogenase 1) p.R132L mutation. The patient’s serum and urine displayed marked elevations in the concentration of the oncometabolite 2-hydroxyglutarate (2-HG). These findings may suggest future avenues for disease monitoring through noninvasive measurement of 2-HG, as well as for the development and study of targeted therapies against the aberrant IDH1 enzyme.
Keywords: Isocitrate dehydrogenase 1, 2-Hydroxyglutarate, Breast adenocarcinoma, Targeted therapy
Abstract
Mutations in the IDH1 and IDH2 (isocitrate dehydrogenase) genes have been discovered across a range of solid-organ and hematologic malignancies, including acute myeloid leukemia, glioma, chondrosarcoma, and cholangiocarcinoma. An intriguing aspect of IDH-mutant tumors is the aberrant production and accumulation of the oncometabolite 2-hydroxyglutarate (2-HG), which may play a pivotal oncogenic role in these malignancies. We describe the first reported case of an IDH1 p.R132L mutation in a patient with hormone receptor-positive (HR+) breast adenocarcinoma. This patient was initially treated for locally advanced disease, but then suffered a relapse and metastasis, at which point an IDH1-R132 mutation was discovered in an affected lymph node. The mutation was subsequently found in the primary tumor tissue and all metastatic sites, but not in an uninvolved lymph node. In addition, the patient’s serum and urine displayed marked elevations in the concentration of 2-HG, significantly higher than that measured in six other patients with metastatic HR+ breast carcinoma whose tumors were found to harbor wild-type IDH1. In summary, IDH1 mutations may impact a rare subgroup of patients with breast adenocarcinoma. This may suggest future avenues for disease monitoring through noninvasive measurement of 2-HG, as well as for the development and study of targeted therapies against the aberrant IDH1 enzyme.
Implications for Practice:
Isocitrate dehydrogenase (IDH) mutations have been identified in various hematologic and solid-tumor malignancies. To date, there are no published reports of these mutations in breast cancer. With this article, we describe a case of hormone receptor-positive adenocarcinoma of the breast with an IDH1 (p.R132L) mutation. The impacted patient had markedly elevated levels of serum and urine 2-hydroxyglutarate, an oncometabolite that accumulates as a result of the neomorphic activity of the altered IDH enzyme. IDH1 mutations may impact a rare subgroup of patients with breast adenocarcinoma, and these findings may carry future therapeutic implications, given the emergence of targeted therapies against the altered IDH protein.
Introduction
The recent identification of isocitrate dehydrogenase (IDH) mutations in human malignancies has generated a significant amount of excitement, as it serves as a rare example of a cancer mutation in a metabolic enzyme that confers neomorphic activity and may be a target of therapy [1–3]. Normally, IDH1 and IDH2 are essential enzyme components of oxidative phosphorylation and catalyze the decarboxylation of isocitrate to α-ketoglutarate (α-KG) in the cytosol and peroxisomes (in the case of IDH1) and in mitochondria (in the case of IDH2), leading to the production of reduced nicotinamide adenine dinucleotide phosphate (NADPH). These enzymes are encoded by their respective genes (IDH1 and IDH2). In contrast to normal IDH enzymes, the mutant forms catalyze an NADPH-dependent reduction of α-KG to the metabolite 2-hydroxyglutarate (2-HG), in place of the normal process of isocitrate decarboxylation, leading to accumulation of 2-HG. High levels of 2-HG are thought to drive the tumorigenic process through the modulation of a number of α-KG-dependent dioxygenases that control DNA methylation, histone methylation, hypoxic response, and a number of other cellular processes [4–6].
Parsons et al. first identified IDH1 mutations in 12% of patient samples of glioblastoma multiforme (GBM) [3]. The mutations were more common in younger patients and in those whose disease had emerged from lower grade gliomas. Those with IDH1-mutant GBM displayed a significantly longer median survival compared with those displaying wild-type IDH1 [3]. Additional studies have confirmed that IDH1 mutations also occur commonly in low-grade gliomas [7, 8]. Mutations in IDH1 as well as IDH2 were subsequently discovered in various myeloid malignancies [9, 10], including acute myeloid leukemia (AML) [2, 11], in which case they have been associated with intermediate-risk cytogenetics and concurrent NPM1 mutations [12, 13]. Their prognostic impact in AML is a topic of significant debate, without clear resolution [12–17]. Intriguingly, patients with IDH-mutant AML display elevated levels of 2-HG, which are present at low baseline levels in normal human tissue. This observation has led to subsequent studies demonstrating that elevated serum and urine 2-HG may be used as a biomarker of disease activity [18–21].
In addition to gliomas, IDH mutations have been discovered in various other solid tumors, including cholangiocarcinoma [1], chondrosarcoma [22, 23], prostatic adenocarcinoma [24], and melanoma [25], among others [26–29], with codon R132 of IDH1 the most commonly mutated site. To date, IDH mutations have not been reported in breast carcinomas. With this report, we describe the case of a patient with metastatic hormone receptor-positive adenocarcinoma of the breast, whose tumor exhibited an IDH1 p.R132L mutation, and whose serum and urine displayed marked elevations of the oncometabolite 2-HG.
Methods
A total of 454 female patients diagnosed with breast cancer underwent mutational profiling as part of their clinical care received at the Massachusetts General Hospital Cancer Center, from May 2010 to December 2013. Relevant clinical information was gathered from review of the medical record. Retrospective genotyping on additional surgical specimens from a single patient with IDH1-mutant breast adenocarcinoma was performed with approval from the institutional review board.
The presence of common cancer gene mutations was evaluated in nucleic acids extracted from patient diagnostic tumor specimens using a custom and clinically validated Applied Biosystems (Foster City, CA, http://www.appliedbiosystems.com) Prism SNaPshot Multiplex system, as previously reported [1, 30]. This assay simultaneously queried for more than 130 mutational hotspots across more than 15 cancer genes. Mutational analysis of IDH1 at nucleotide positions c.394 and c.395 (amino acid position p.R132) and IDH2 at nucleotide positions c.418 and c.419 (amino acid position p.R140) and c.514 and c.515 (amino acid position p.R172) was incorporated into this clinical platform in May 2010 and November 2012, respectively.
Our mutational profiling of breast cancer patients clinically revealed a single case harboring an IDH1 c.395G>T (p.R132L) mutation. This finding was made in the patient’s diagnostic lymph node specimen containing metastatic, hormone receptor-positive (HR+), adenocarcinoma of the breast. Subsequently, all diagnostic tumor samples previously obtained during the patient’s course were retrospectively secured for mutational profiling using the same mutational profiling platform. The available samples containing tumor tissue included primary tissue from the left breast, central duct, and the sentinel lymph node obtained during initial lumpectomy. In addition, left breast tissue and an area of uninvolved lymph node obtained during a subsequent mastectomy were included in the analysis.
Serum and urine samples were obtained for 2-HG measurement, which was performed through collaboration with Agios Pharmaceuticals, Inc. (Cambridge, MA, http://www.agios.com). In brief, labeled 13C5-2HG was obtained from Agios Pharmaceuticals, and 2-HG was obtained from Toronto Research Chemicals (Toronto, ON, Canada, http://www.trc-canada.com). Liquid chromatography coupled to tandem mass spectrometry was performed using an AB SCIEX 4000 (Framingham, MA, http://www.absciex.com) operating in negative electrospray mode. Magnetic resonance mammography data were acquired for each compound using the following transitions: 2-HG (146.9/128.8 amu), 13C5-2HG (151.9/133.8 amu), and 3HMG (160.9/98.9 amu). Chromatographic separation was performed using an ion-exchange column (Bio-Rad Fast Acid Analysis, 9 μm, 7.8 mm × 100 mm; Bio-Rad, Hercules, CA, http://www.bio-rad.com). The flow rate was 1 mL per minute of 0.1% formic acid in water with a total run time of 4 minutes.
The study samples were prepared as follows: urine was diluted with 10× phosphate-buffered saline, and 30 μL of diluted urine was extracted by adding 200 μL of methanol with 200 ng/mL 13C5-2HG as internal standard; 10 μL of serum sample was extracted by adding 200 μL of methanol with 200 ng/mL 13C5-2HG. Both sample types were then vortexed, centrifuged at 4,000 rpm at 5°C, and 150 μL of supernatant was transferred to a clean 96-well plate. The samples were dried down and reconstituted in 200 μL of 0.1% formic acid in water, and 10 μL was injected on column.
Results
Case History
At the time of the study, the patient was a 61-year-old female, with a diagnosis of estrogen receptor-positive (ER+), progesterone receptor-positive (PR+), human epidermal growth factor receptor 2-negative (HER2−) breast adenocarcinoma, with metastasis to pleura, bone, mediastinal lymph nodes, and intracranial dura. Approximately 3 years prior, she was diagnosed with stage IIA, T1cN1M0, invasive ductal carcinoma of the left breast after presenting with bloody nipple discharge. A mammogram had initially revealed a hypoechoic nodule in the breast, which was followed by an ultrasound-guided core biopsy, revealing atypical ductal hyperplasia and fat necrosis. One month later, lumpectomy and sentinel lymph node biopsy established the diagnosis of invasive ductal carcinoma with lymph node involvement. Due to positive margins, she underwent a left-sided mastectomy and lymph node dissection, which revealed a 1.2-cm moderately differentiated, invasive ductal carcinoma (ER+/PR+, HER2−), with extensive lymphovascular invasion and metastatic lymph node involvement in 2 of 11 sampled lymph nodes. She underwent adjuvant chemotherapy with cyclophosphamide and docetaxel. She was then prescribed hormonal therapy with letrozole, which she discontinued after a few months because of adverse side effects.
Approximately 3 years after the initial diagnosis, the patient was admitted to the hospital with acute dyspnea and right-sided facial numbness. Imaging revealed diffusely metastatic disease to the intracranial dura, pleura, bones, as well as the mediastinal, hilar, and paracardiac lymph nodes. A mediastinoscopy with lymph node biopsy confirmed metastatic adenocarcinoma, ER+, PR+, and HER2−, consistent with a primary malignancy of the breast. At this time, an IDH1 c.395G>T (p.R132L) mutation was detected in a lymph node sample through clinical genotyping performed at our institution.
The patient was then treated with the aromatase inhibitor exemestane. A few months later, she developed worsening neurologic symptoms because of progressive dural-based lesions, and she received cranial base irradiation followed by initiation of salvage chemotherapy with vinorelbine. Over the course of the next 3 months, she was managed for worsening pleural effusions as well as neurologic and mental status deterioration. Unfortunately, she expired 14 months after her diagnosis of disease recurrence.
Analysis of IDH1 Mutational Status
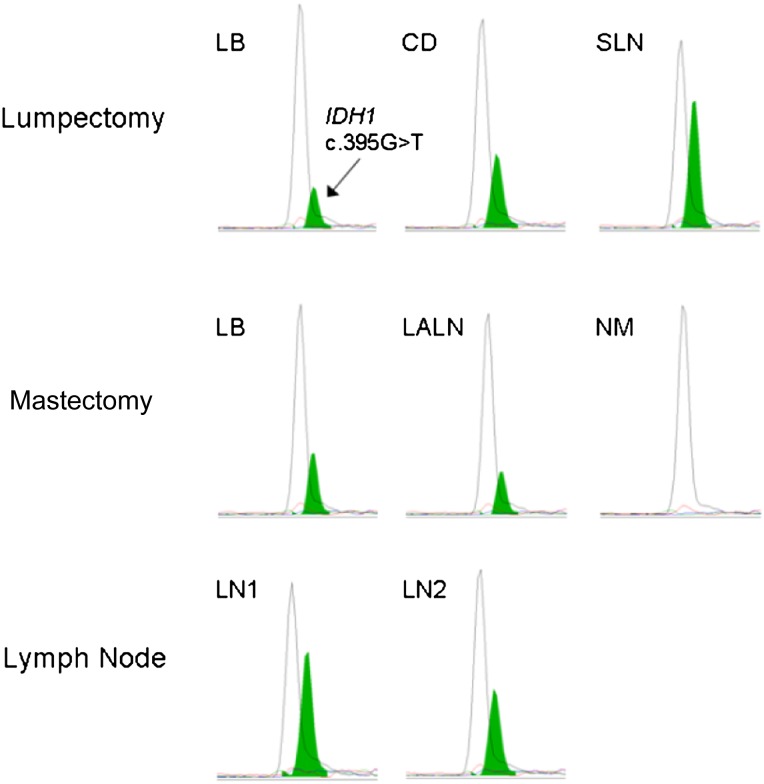
Following the initial identification of an IDH1 R132L mutation in the patient’s lymph node metastasis, additional diagnostic tumor samples, obtained during the patient’s initial lumpectomy, were retrospectively tested, including primary tissue from the left breast, central duct, and the sentinel lymph node. Mutational profiling using the same clinical genotyping platform revealed the presence of an IDH1 p.R132L mutation in all lumpectomy specimens containing tumor tissue. Samples from the subsequent mastectomy were also available, including tissue from the left breast and the lone involved left axillary lymph node, which both again harbored the IDH1 p.R132L mutation. Finally, lymph node tissue obtained nearly 1 year later to confirm metastatic disease was similarly evaluated, and again was found to harbor the IDH1 p.R132L mutation (Fig. 1). Of note, an area of uninvolved lymph node was evaluated and found to be IDH1-wild-type, indicating that the IDH1 mutation in the tumor tissue was somatically acquired.
Figure 1.
Identification of an IDH1 p.R132L mutation in tumor tissue from a breast cancer patient. A 61-year-old female patient diagnosed with estrogen receptor-positive, progesterone receptor-positive, human epidermal growth factor receptor 2-negative invasive ductal carcinoma underwent mutational profiling. An IDH1 c.395G>T (p.R132L) mutation (green peak) was identified in tissue obtained by lumpectomy from the left breast, central duct, and the sentinel lymph node (upper panels). Tissue from a subsequent left-sided mastectomy and lymph node dissection was evaluated and the same IDH1 mutation was identified in malignant breast tissue (LB) and the left axillary lymph node (middle panels). Conversely, no IDH1 p.R132 mutation was found in an uninvolved lymph node, where no malignancy was found during pathological review. Metastatic lesions obtained from a later surgery were also identified to harbor the IDH1 p.R132L mutation from two lymph nodes (LN1 and LN2) (lower panels).
Abbreviations: CD, central duct; IDH1, isocitrate dehydrogenase 1 gene; LALN, left axillary lymph node; LB, left breast; LN, lymph node; NM, no malignancy; SLN, sentinel lymph node.
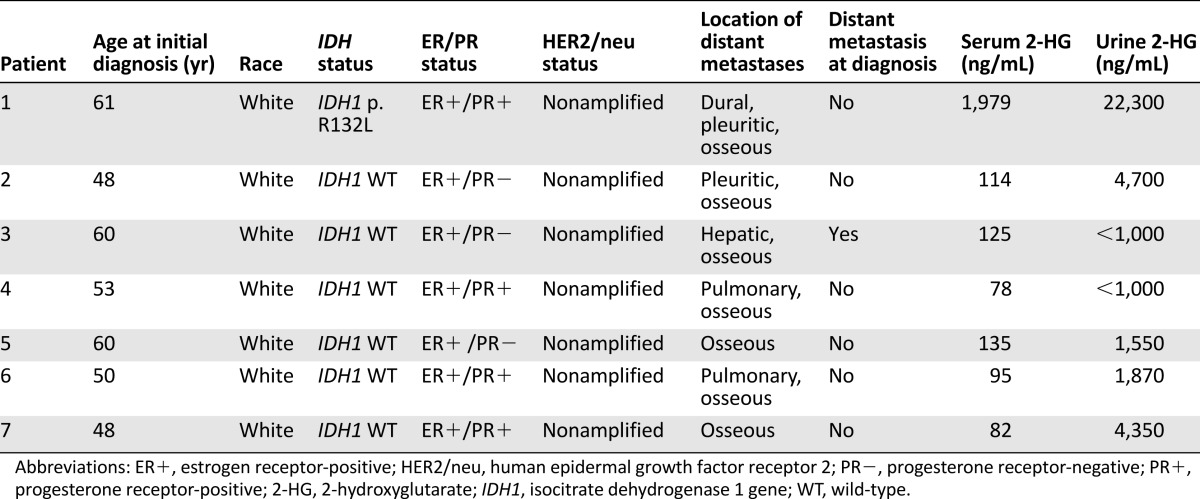
Primary tumor samples from an additional six patients with HR+, HER2 nonamplified, breast cancer were similarly tested and found to be wild-type for IDH1. The demographic and clinical features of these patients are described in Table 1.
Table 1.
Demographic and clinical characteristics of patients with metastatic hormone receptor-positive adenocarcinoma
Measurement of the Oncometabolite 2-HG
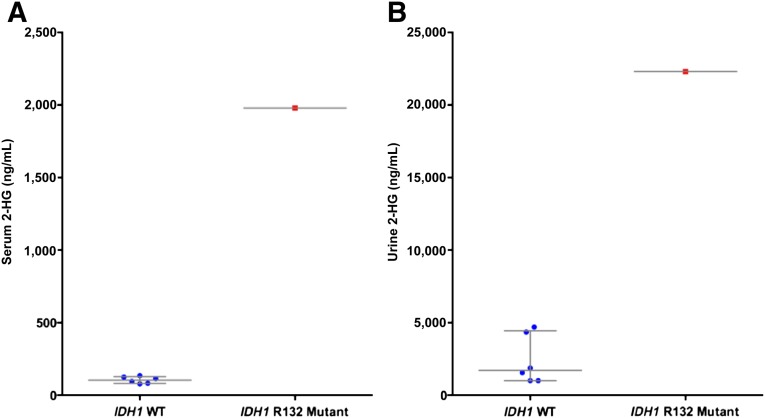
Serum and urine samples were obtained from the patient with IDH1-mutant malignancy, as well as the six patients with IDH-wild-type tumors, for 2-HG measurement. Serum and urine 2-HG levels were markedly elevated in the IDH1 p.R132L-mutant patient, with a serum concentration of 1,979 ng/mL and a urine concentration of 22,300 ng/mL. In contrast, the serum and urine concentrations of 2-HG in the IDH1-wild-type patients were significantly lower. The median serum concentration for wild-type patients was 104.5 ng/mL (range, 78–135 ng/mL) and the median urine concentration was 1,710 ng/mL (range <1,000–4,700 ng/mL) (Fig. 2; Table 1). Of note, in a previous study, serum 2-HG levels in normal, healthy individuals were at a similarly low baseline level (median 91 ng/mL, range 33–93 ng/mL) [20].
Figure 2.
2-HG measurements in samples from patients with a wild-type IDH1 (n = 6) versus the patient harboring the IDH1 p.R132L mutation in serum (A) and urine (B). Each point represents an individual patient sample, with blue dots depicting IDH1-wild-type samples and the red dots depicting IDH1-mutant samples. The left column in each figure represents the wild-type samples and the right column represents the mutant sample. Horizontal bars indicate the median, and vertical lines indicate the lower and upper quartiles. All figures describe a statistically significant difference in 2-HG levels between the IDH1-mutant sample relative to the wild-type samples (p < .05).
Abbreviations: 2-HG, 2-hydroxyglutarate; IDH1, isocitrate dehydrogenase 1 gene; WT, wild-type.
Discussion
To our knowledge, this is the first report of an IDH1 mutation detected in breast adenocarcinoma. In this case, the patient carried a diagnosis of hormone receptor-positive breast cancer, initially early-stage, but which subsequently recurred as widely metastatic disease. The IDH1 mutation was initially detected at the time of disease relapse, when serum and urine assays also revealed markedly high concentrations of the oncometabolite 2-hydroxyglutarate. Retrospective genotyping revealed that the same mutation was present in the malignant tissue at both initial diagnosis and relapse. The inability to detect the mutation in lymph node tissue unaffected by metastatic adenocarcinoma in this patient indicates that the IDH1 mutation was somatically acquired and a specific feature of the tumor.
Over the course of clinical genotyping performed at our institution, this has been the only case of an IDH1 mutation in breast cancer among 454 total cases tested. Furthermore, the Cancer Genome Atlas (http://cancergenome.nih.gov) reports only 2 cases of IDH1-mutant invasive breast carcinoma among 825 tested. One case was an ER+, PR+, HER2+ invasive ductal carcinoma that harbored a rare IDH1 Y235C mutation. The other case was a mixed infiltrating ductal and mucinous carcinoma carrying an IDH1 R132C mutation, but the ER, PR, and HER2 statuses were not indicated. We could therefore infer that mutations in IDH1 are a rare finding in breast cancer.
We, along with other groups, have previously demonstrated that elevated 2-HG levels are a specific feature of IDH-mutant AML [19, 20]. However, up to the time of preparation for this manuscript, elevations in serum and urine 2-HG have not been reported in IDH-mutant solid tumors. Levels of intratumoral 2-HG have been shown to be elevated in IDH1-mutant glioma tissue [18, 31] using both in vitro and in vivo techniques [32, 33]. Nevertheless, an earlier small study of IDH1-mutant gliomas failed to detect an elevation in circulating 2-HG levels in sera of affected patients [34]. In IDH-mutant AML, intracellular 2-HG levels are measured at approximately 50-fold higher levels in affected malignant cells compared with their IDH-wild-type counterparts [21]. Serum levels of 2-HG in IDH-mutant AML patients are above a concentration of 1,000 ng/mL in the vast majority of cases, decrease upon cytoreduction with antileukemic therapy, and again increase with relapse [20, 21].
Serum and urine samples, obtained from the above-described patient with IDH1-mutant breast carcinoma, revealed markedly elevated 2-HG levels, likely reflective of her widely disseminated, metastatic disease. These levels were significantly higher than those measured in six other patients with metastatic HR+ breast cancer whose tumors displayed wild-type IDH. This supports the hypothesis that the elevation in 2-HG was related to the marked neomorphic activity of the altered IDH1 enzyme, rather than being a feature of metastatic breast adenocarcinoma.
It has been reported in glioma that IDH1 mutations are early events in the carcinogenic process [35], with the pathogenesis and transformation of IDH1-mutant gliomas linked to suppressed cellular differentiation, with 2-HG implicated as a mediator in these processes [11, 18]. In AML, IDH mutations have been associated with the aberrant hypermethylation of promoter sites involved in myeloid differentiation [36], an underlying pathogenic process in forms of myelodysplasia and AML [36–39]. Emerging evidence suggests that 2-HG may be a key leukemogenic oncometabolite impacting such epigenetic modulation [21, 40, 41]. Although the tumorigenic role of the IDH1 mutation in this patient with breast carcinoma is not known, the presence of the mutation at all known metastatic sites and the dramatic accumulation of the 2-HG oncometabolite in the serum suggest that it was likely an important event in the underlying etiology of this malignancy.
Conclusion
We here describe an intriguing case of IDH1-mutant breast cancer. Apart from the novelty of this mutation in this malignancy, the concurrent marked elevation of the metabolite 2-HG in the serum and urine is similarly intriguing. As described in other tumors to date, it will be of interest to determine whether 2-HG may play an important role in the genesis and perpetuation of malignancy in breast cancer through its impact on epigenetic and metabolic modulation. In addition, 2-HG measurement may provide a noninvasive manner of diagnosing disease and following disease activity during treatment and remission. Our patient had hormone receptor-positive disease, and whether IDH1 mutations are a feature of a rare subset of this category of breast cancer requires further study. Finally, targeted therapies, in the form of IDH inhibitors, have shown great promise in preclinical models [42], and are currently entering early-phase clinical trials. The therapeutic impact of such agents in IDH-mutant disease, across the spectrum of solid-tumor and hematologic malignancies, is awaited with significant hope and anticipation.
Acknowledgments
This work was supported in part by a Massachusetts General Hospital Cancer Center Thematic Priority #3 Grant. Amir T. Fathi, Hossein Sadrzadeh, and Darrell R. Borger contributed equally to this article.
Author Contributions
Conception/design: Amir T. Fathi, Darrell R. Borger
Provision of study material or patients: Amir T. Fathi, Hossein Sadrzadeh, Amy H. Comander, Michaela J. Higgins, Aditya Bardia, Kimberly S. Straley, Katharine E. Yen, Sam Agresta, Hyeryun Kim, David P. Schenkein, Darrell R. Borger
Collection and/or assembly of data: Amir T. Fathi, Hossein Sadrzadeh, Ashley Perry, Meghan Burke, Regina Silver, Kimberly S. Straley, Katharine E. Yen, Sam Agresta, Hyeryun Kim, David P. Schenkein
Data analysis and interpretation: Amir T. Fathi, Darrell R. Borger
Manuscript writing: Amir T. Fathi, Amy H. Comander, Michaela J. Higgins, Aditya Bardia, Darrell R. Borger
Final approval of manuscript: Amir T. Fathi, Hossein Sadrzadeh, Amy H. Comander, Michaela J. Higgins, Aditya Bardia, Ashley Perry, Meghan Burke, Kimberly S. Straley, Katharine E. Yen, Sam Agresta, Hyeryun Kim, David P. Schenkein, Darrell R. Borger
Disclosures
Amir Fathi: Agios Pharmaceuticals (C/A); David Schenkein, Katharine E. Yen, Kimberly Straley: Agios Pharmaceuticals (E, OI); Sam Agresta: Agios Pharmaceuticals (E, OI); Darrell Borger: BioReference Laboratories, Inc. (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. The Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Losman JA, Looper RE, Koivunen P, et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–1625. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: A study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 8.Bleeker FE, Lamba S, Leenstra S, et al. IDH1 mutations at residue p.R132 (IDH1R132) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat. 2009;30:7–11. doi: 10.1002/humu.20937. [DOI] [PubMed] [Google Scholar]
- 9.Pardanani A, Lasho TL, Finke CM, et al. IDH1 and IDH2 mutation analysis in chronic- and blast-phase myeloproliferative neoplasms. Leukemia. 2010;24:1146–1151. doi: 10.1038/leu.2010.77. [DOI] [PubMed] [Google Scholar]
- 10.Thol F, Weissinger EM, Krauter J, et al. IDH1 mutations in patients with myelodysplastic syndromes are associated with an unfavorable prognosis. Haematologica. 2010;95:1668–1674. doi: 10.3324/haematol.2010.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcucci G, Maharry K, Wu YZ, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnittger S, Haferlach C, Ulke M, et al. IDH1 mutations are detected in 6.6% of 1414 AML patients and are associated with intermediate risk karyotype and unfavorable prognosis in adults younger than 60 years and unmutated NPM1 status. Blood. 2010;116:5486–5496. doi: 10.1182/blood-2010-02-267955. [DOI] [PubMed] [Google Scholar]
- 14.Abbas S, Lugthart S, Kavelaars FG, et al. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: Prevalence and prognostic value. Blood. 2010;116:2122–2126. doi: 10.1182/blood-2009-11-250878. [DOI] [PubMed] [Google Scholar]
- 15.Green CL, Evans CM, Hills RK, et al. The prognostic significance of IDH1 mutations in younger adult patients with acute myeloid leukemia is dependent on FLT3/ITD status. Blood. 2010;116:2779–2782. doi: 10.1182/blood-2010-02-270926. [DOI] [PubMed] [Google Scholar]
- 16.Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 17.Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiNardo CD, Propert KJ, Loren AW, et al. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood. 2013;121:4917–4924. doi: 10.1182/blood-2013-03-493197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fathi AT, Sadrzadeh H, Borger DR, et al. Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response. Blood. 2012;120:4649–4652. doi: 10.1182/blood-2012-06-438267. [DOI] [PubMed] [Google Scholar]
- 21.Gross S, Cairns RA, Minden MD, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amary MF, Bacsi K, Maggiani F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 23.Arai M, Nobusawa S, Ikota H, et al. Frequent IDH1/2 mutations in intracranial chondrosarcoma: A possible diagnostic clue for its differentiation from chordoma. Brain Tumor Pathol. 2012;29:201–206. doi: 10.1007/s10014-012-0085-1. [DOI] [PubMed] [Google Scholar]
- 24.Kang MR, Kim MS, Oh JE, et al. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer. 2009;125:353–355. doi: 10.1002/ijc.24379. [DOI] [PubMed] [Google Scholar]
- 25.Shibata T, Kokubu A, Miyamoto M, et al. Mutant IDH1 confers an in vivo growth in a melanoma cell line with BRAF mutation. Am J Pathol. 2011;178:1395–1402. doi: 10.1016/j.ajpath.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 27.Gaal J, Burnichon N, Korpershoek E, et al. Isocitrate dehydrogenase mutations are rare in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2010;95:1274–1278. doi: 10.1210/jc.2009-2170. [DOI] [PubMed] [Google Scholar]
- 28.Cairns RA, Iqbal J, Lemonnier F, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119:1901–1903. doi: 10.1182/blood-2011-11-391748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murugan AK, Bojdani E, Xing M. Identification and functional characterization of isocitrate dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys Res Commun. 2010;393:555–559. doi: 10.1016/j.bbrc.2010.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias-Santagata D, Akhavanfard S, David SS et al. Rapid targeted mutational analysis of human tumours: A clinical platform to guide personalized cancer medicine. EMBO Mol Med 2010;2:146–158. [DOI] [PMC free article] [PubMed]
- 31.Luchman HA, Stechishin OD, Dang NH, et al. An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro Oncol. 2012;14:184–191. doi: 10.1093/neuonc/nor207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andronesi OC, Kim GS, Gerstner E et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med 2012;4:116ra4. [DOI] [PMC free article] [PubMed]
- 33.Andronesi OC, Rapalino O, Gerstner E, et al. Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest. 2013;123:3659–3663. doi: 10.1172/JCI67229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capper D, Simon M, Langhans CD, et al. 2-Hydroxyglutarate concentration in serum from patients with gliomas does not correlate with IDH1/2 mutation status or tumor size. Int J Cancer. 2012;131:766–768. doi: 10.1002/ijc.26425. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 38.Khan R, Schmidt-Mende J, Karimi M, et al. Hypomethylation and apoptosis in 5-azacytidine-treated myeloid cells. Exp Hematol. 2008;36:149–157. doi: 10.1016/j.exphem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Lugthart S, Figueroa ME, Bindels E, et al. Aberrant DNA hypermethylation signature in acute myeloid leukemia directed by EVI1. Blood. 2011;117:234–241. doi: 10.1182/blood-2010-04-281337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reitman ZJ, Parsons DW, Yan H. IDH1 and IDH2: Not your typical oncogenes. Cancer Cell. 2010;17:215–216. doi: 10.1016/j.ccr.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: Alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102:932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Travins J, DeLaBarre B, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]