This study assessed the impact of misclassification in receptor assays on discordance in hormone receptor status and estimated the accuracy of hormone receptor assays. A considerable proportion of discordance in hormone receptor status can be attributed to misclassification in receptor assessment, although the accuracy of receptor assays was excellent. Biopsy of recurrent tumors for receptor retesting should be conducted after considering feasibility, cost, and previous hormone receptor status.
Keywords: Estrogen receptor, Discordance, Metastasis biopsy, Breast neoplasms, Second primary neoplasm, Metastatic breast carcinoma
Abstract
Introduction.
Discordance in hormone receptor status has been observed between two breast tumors of the same patients; however, the degree of heterogeneity is debatable with regard to whether it reflects true biological difference or the limited accuracy of receptor assays.
Methods.
A Bayesian misclassification correction method was applied to data on hormone receptor status of two primary breast cancers from the Surveillance, Epidemiology, and End Results database between 1990 and 2010 and to data on primary breast cancer and paired recurrent/metastatic disease assembled from a meta-analysis of the literature published between 1979 and 2014.
Results.
The sensitivity and specificity of the estrogen receptor (ER) assay were estimated to be 0.971 and 0.920, respectively. After correcting for misclassification, the discordance in ER between two primary breast cancers was estimated to be 1.2% for synchronous ipsilateral pairs, 5.0% for synchronous contralateral pairs, 14.6% for metachronous ipsilateral pairs, and 25.0% for metachronous contralateral pairs. Technical misclassification accounted for 53%–83% of the ER discordance between synchronous primary cancers and 11%–25% of the ER discordance between metachronous cancers. The corrected discordance in ER between primary tumors and recurrent or metastatic lesions was 12.4%, and there were more positive-to-negative changes (10.1%) than negative-to-positive changes (2.3%). Similar patterns were observed for progesterone receptor (PR), although the overall discordance in PR was higher.
Conclusion.
A considerable proportion of discordance in hormone receptor status can be attributed to misclassification in receptor assessment, although the accuracy of receptor assays was excellent. Biopsy of recurrent tumors for receptor retesting should be conducted after considering feasibility, cost, and previous ER/PR status.
Abstract
摘要
引言. 现已发现同一患者的两种乳腺肿瘤之间存在激素受体状态的不一致性;然而,这是否反映了真正的生物学差异或受体检测精确性不够高,就此而言,异质性程度仍无定论。
方法. 检索监测、流行病学以及最终结果数据库1990 ∼ 2010年数据,利用Bayesian错误分类校正方法分析两种原发性乳腺癌的激素受体状态,同时从1979 ∼ 2014年发表的meta分析文献中收集原发乳腺癌及其配对的复发/转移性疾病数据并进行分析。
结果. 雌激素受体(ER)检测的敏感性和特异性分别为0.971、0.920。对错误分类校正后,分析两种原发性乳腺癌在ER方面的不一致性,对于同时性同侧配对肿瘤估计为1.2%,对于同时性对侧配对肿瘤估计为5.0%,对于异时性同侧配对肿瘤估计为14.6%,对于异时性对侧配对肿瘤估计为25.0%。就同时性原发性癌而言,技术上的错误分类导致了53%∼83%的ER不一致性;就异时性癌而言,技术上的错误分类导致了11%∼25%的ER不一致性。原发性肿瘤和复发或转移性病灶之间ER不一致性在改正后为12.4%,阳性到阴性的改变率(10.1%)比阴性到阳性的改变率(2.3%)更多。类似的模式也见于孕激素受体(PR),不过总体上PR中的不一致性更高。
结论. 尽管受体检测的精确性非常高,激素受体状态的不一致性在很大程度上归于受体评估的分类错误。肿瘤复发病灶活检时,在衡量了可行性、费用以及既往ER/PR状态后,应该重新检测受体状态。The Oncologist 2014;19:592–601
Implications for Practice:
Discordance in hormone receptor status between primary and metastatic breast cancer lesions cannot be ignored. However, the extent of discordance and direction of changes matter. This study found a considerable proportion of discordance in hormone receptor status can be attributed to misclassification in receptor assessment, even though the accuracy of receptor assays is excellent. After accounting for misclassification, biological discordance exists, and there are more positive-to-negative switches in receptor status than negative-to-positive switches. Rebiopsy of metastatic sites for receptor retesting may be recommended on a case-by-case basis after considering the feasibility, cost, and previous status of hormone receptor.
Introduction
The importance of hormone receptors in breast cancer biology has been recognized. Hormone receptor-positive versus -negative cancers have fundamentally different natural histories, leading to different prognoses and treatment strategies [1, 2]. The discordance in estrogen receptor (ER) and progesterone receptor (PR) status between two primary breast cancers of the same patient has been reported to be ∼20% and ∼40%, respectively [3–6]. Discordance in these biomarkers has also been reported between primary tumor and a lesion of regional recurrence or distant metastasis, with discordance rates ranging from 10% to 40% [7]. Although the National Comprehensive Cancer Network guidelines recently recommended a biopsy of metastatic deposits when feasible [8], clinical management of breast cancer metastasis has been based largely on the initial assessment of the primary tumor. This lack of concordance is of concern and might be an important reason for treatment failure.
The degree of dissimilarity is debatable with regard to whether it reflects true biological difference or the limited accuracy of receptor assays [7, 9, 10]. Originally, quantification of ER and PR was done by ligand-binding assays (LBAs), which have largely been replaced by easier and more inexpensive immunohistochemistry (IHC) methods since the early 1990s. IHC has rapidly become the predominant method for measuring ER and PR in clinical practice due to its many advantages, but its limitations in accuracy are widely recognized because it depends on multiple variables including specimen handling, tissue fixation, antibody type, antigen retrieval, staining, and scoring methods [11–13]. Some studies have reported differences in the accuracy of hormonal receptor scoring according to the method used [7]. However, it is unclear how much the limited accuracy of assays contributes to the overall discordance in hormone receptors between two primary breast cancers or between primary tumors and recurrent or metastatic lesions.
The purpose of the present study is to assess the impact of misclassification in receptor assays on discordance in hormone receptor status and to estimate the accuracy of hormone receptor assays. The discordance of hormone receptor status was evaluated between two primary breast cancers and between primary breast cancer and recurrent or metastatic sites. We applied a Bayesian misclassification correction method to data on hormone receptor status of two primary breast cancers from the Surveillance, Epidemiology, and End Results (SEER) database, 1990–2010 and to data on primary and recurrent or metastatic disease pairs assembled from a meta-analysis of 36 studies. The Bayesian approach combines prior information about assay accuracy and receptor prevalence in the observed data, resulting in a posterior distribution for these quantities and, in turn, a posterior distribution for the actual discordance rate. Because the cohort of two primary breast cancers and the cohort of primary and metastatic pairs represent two different clinical scenarios, we handled their data differently in the model, but we utilized all data to estimate common parameters of assay accuracy.
Methods
SEER: Two Primary Tumors
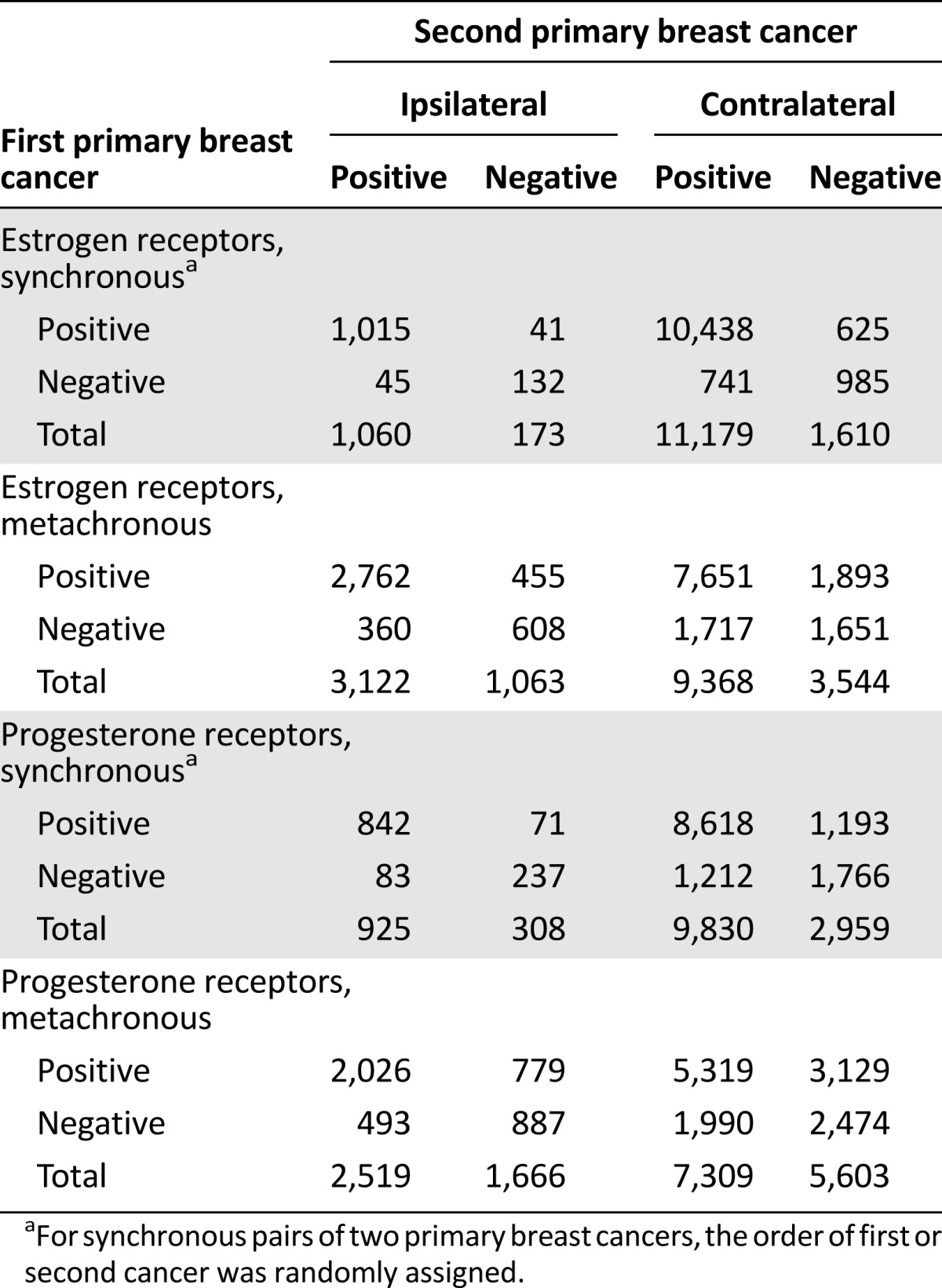
Using the large data set from the National Cancer Institute’s SEER program from 1990 to 2010 [14], we identified 31,119 female patients who had two primary breast cancers with available information on hormone receptors and laterality in both cancers. There were 14,022 patients with “synchronous” breast cancers (defined as two cancers diagnosed within 6 months) and 17,097 patients with metachronous cancers. In 5,418 patients, two cancers occurred in the same breast (“ipsilateral” breast cancer) and in 25,701 patients, cancers occurred in both breasts (“bilateral” or “contralateral” breast cancer). Table 1 cross-tabulates ER and PR status of the two cancers according to laterality and time interval between two cancers.
Table 1.
Discordance of hormone receptors of two primary breast cancers: data from Surveillance, Epidemiology, and End Results, 1990–2010
Meta-Analysis of Primary Tumors and Metastatic Lesions
Following the guidelines of the Meta-analysis of Observational Studies in Epidemiology Group [15], we searched the PubMed database from 1979 to 2014 using the following combination of key words: (“estrogen receptor” or “progesterone receptor” or “hormone receptor”) and “discordance” and “breast cancer.” Next, we manually checked the references of all identified articles, reviews, and editorials. Studies were considered eligible if they measured ER and/or PR status for both primary breast cancer and recurrent or metastatic lesion. Only full published papers were included because the study required detail on the number of changes in receptor status. Of multiple articles published from the same institution, only the article with the largest sample size was chosen (e.g., of the two studies from University of Texas MD Anderson Cancer Center [4, 16], we used the later study [4]). If necessary, we e-mailed the authors of studies for additional data needed for the meta-analysis. We also followed up relevant abstracts identified from the past 5 years of American Society of Clinical Oncology (ASCO) meetings and San Antonio Breast Cancer Symposiums for full publications.
Of the 352 papers identified through PubMed, we found 17 eligible papers. Through the references of these papers as well as other reviews and meeting abstracts, we found an additional 19 papers, so a total of 36 studies met our selection criteria and were included in the meta-analysis. The main characteristics from the selected studies were extracted, including publication details such as the authors’ names and the publication year, the methods of the receptor assay (IHC, biochemical methods [i.e., LBA], or the two methods mixed), the exact number of hormone receptor concordance or discordance between primary and recurrent or metastatic disease, the direction of changes, the site of recurrence or metastasis if available, and the study design (prospective or retrospective). Characteristics of the 36 studies are presented in Table 2.
Table 2.
Characteristics of studies included in the meta-analysis on hormonal receptor changes from primary breast cancer to recurrent or metastatic sites, 1979–2014

Using the random effects model described by DerSimonian and Laird [17], we summarized the discordant proportion of ER or PR status. The reason for heterogeneity across studies was examined with stratification by methods of receptor assays or location of recurrence (regional recurrence vs. distant metastasis). Mixed-effect meta-regression was used to test statistical significance of the stratification factors. We further estimated the pooled proportions of positive-to-negative change and negative-to-positive change using random effects models. The meta-analysis was performed using Stata 12.0 (StataCorp, College Station, TX, http://www.stata.com).
Statistical Analysis
A Bayesian misclassification correction method was used to estimate the discordance proportion in hormone receptor status between pairs of tumors as well as the sensitivity and specificity of the assays [18]. For the i-th cancer (i = 1, 2), let be the true hormone receptor status and be the reported hormone receptor status, which may not always be the same as the underlying true value. There are five strata, including synchronous ipsilateral primaries, synchronous contralateral primaries, metachronous ipsilateral primaries, metachronous contralateral primaries, and primary tumor to metastatic lesion. For the k-th stratum, we fit a logistic regression model that accounted for the association between the true hormone receptor statuses for the two cancers. Because the measurement procedure for hormone receptor status is routine, we assume that the misclassification parameters, that is, sensitivity (SN) and specificity (SP), are the same across all strata, and we assume the misclassifications for cancer pairs are independent of each other. As such, we are able to model the two-primary-breast-cancer cohort and the primary-to-metastasis cohort differently by allowing stratum-specific parameters of discordance, whereas we can use all data to estimate common sensitivity and specificity. The mathematical forms of the models are listed below. For the k-th (k = 1, …, 5) stratum:
| (1) |
| (2) |
| (3) |
The prior distribution (researchers’ beliefs) of estrogen receptor-positive prevalence of the first cancer (i.e., was set to follow a β distribution with 95% credible interval (CI) between 0.68 and 0.88 (i.e., β [49.07, 13.14]). We chose the prior distribution of progesterone receptor-positive prevalence of the first cancer to follow a β distribution with a 95% credible interval between 0.56 and 0.76 (i.e., β [55.8, 28.33]). We chose the prior distribution of the intercept (β0) to follow a normal distribution with a mean of −1 and variance of 1. As for log odds ratio (β1), the main parameter of interest, we assumed a weakly informative prior distribution: normal distribution with a mean of 1.4 and variance of 100. Two prior distributions of sensitivity and specificity were assumed. One prior distribution is less informative by assuming a β distribution with a 95% CI between 0.70 and 0.95. The other prior distribution is informative by assuming a β distribution with a 95% CI between 0.85 and 0.95.
The posterior distributions of the above parameters were estimated using the Markov Chain Monte Carlo method implemented in package JAGS (Just Another Gibbs Sampler). JAGS was called up within the R package “rjags.” We ran three chains, each of which has 3 million iterations with burn-in of 1 million iterations and a thinning rate of every 100. The convergence was checked by the Gelman-Rubin method, and the diagnostic results were satisfactory.
Results
Sensitivity and Specificity
Figure 1 shows the density distribution of sensitivity and specificity of hormone receptors. Under two prior briefs on sensitivity and specificity, the posterior distributions are very similar (the blue and red solid lines almost overlap), suggesting that the information on sensitivity and specificity contained in the large data set of cancer pairs is dominant and that we can robustly estimate the sensitivity and specificity of hormone receptors. In particular, the sensitivity of the ER assay was estimated to be 0.971 (95% CI: 0.958–0.983), with specificity of 0.920 (95% CI: 0.871–0.967). The sensitivity of the PR assay was 0.952 (95% CI: 0.933–0.973), with specificity of 0.905 (95% CI: 0.865–0.948).
Figure 1.

Probability density of sensitivity or specificity of hormone receptors. Short-dash lines show density distribution of less informative priors (K1, sensitivity or specificity follows β distribution with a 95% credible interval [CI] between 0.70 and 0.95); dash lines show density distribution of informative prior (K2, sensitivity or specificity follows β distribution with a 95% CI between 0.85 and 0.95); blue solid lines show posterior density, given the less informative priors and the data; and red solid lines show posterior density, given the informative priors and the data. In the mathematic notations, p stands for probability, D stands for data, and K stands for prior knowledge.
Two Primary Breast Cancers
The observed discordance in ER status between two primary breast cancers was the highest for metachronous contralateral pairs (28.0%) and the lowest in synchronous ipsilateral pairs (7.0%), with metachronous ipsilateral pairs (19.5%) and synchronous contralateral pairs (10.7%) in between (Table 3). It should be noted that these observed discordances assume 100% accuracy of the ER assay. After correcting for misclassification in ER assessment, we found that the estimates of discordance rates were smaller than those observed proportions in all four strata. Of note, the posterior distribution of discordance was almost the same, even assuming different prior distributions of sensitivity and specificity, so we presented only results under less informative prior briefs. The magnitude of a misclassification’s impact on discordance, as indicated by relative differences between observed and posterior-discordant proportions, varied across strata. In particular, the posterior estimates of discordant proportion for synchronous ipsilateral pairs and synchronous contralateral pairs were only 1.2% and 5.0%, respectively, representing 83% and 53% relative reductions. This finding suggests that technical misclassification accounted for a large amount of discordance. Concerning metachronous cancer pairs, the posterior estimates of discordant proportion were 14.6% and 24.8% for ipsilateral and contralateral cancers, respectively, representing 25% and 11% relative reductions. Interestingly, there was a higher positive-to-negative ER change (8.6%) than negative-to-positive ER change (6.0%) in metachronous ipsilateral pairs.
Table 3.
Posterior distribution of discordance in hormone receptors of two primary breast cancers

Similar patterns were found for PR, although discordant proportions, either observed or corrected, for PR status between two primary tumors were higher than those for ER status. Interestingly, the posterior proportions of positive-to-negative changes in PR status were almost twofold higher than the proportions of negative-to-positive change for metachronous breast cancer pairs.
We explored whether discordant proportions depended on the time interval between the first and second cancer for the metachronous cancer pairs (supplemental online Table 1). We found that discordant proportions for ipsilateral metachronous cancers increased as the time interval between the first and second cancer increased; however, there was no clear time-dependent trend for contralateral metachronous breast cancers.
Primary Breast Cancer and Recurrent or Metastatic Lesions
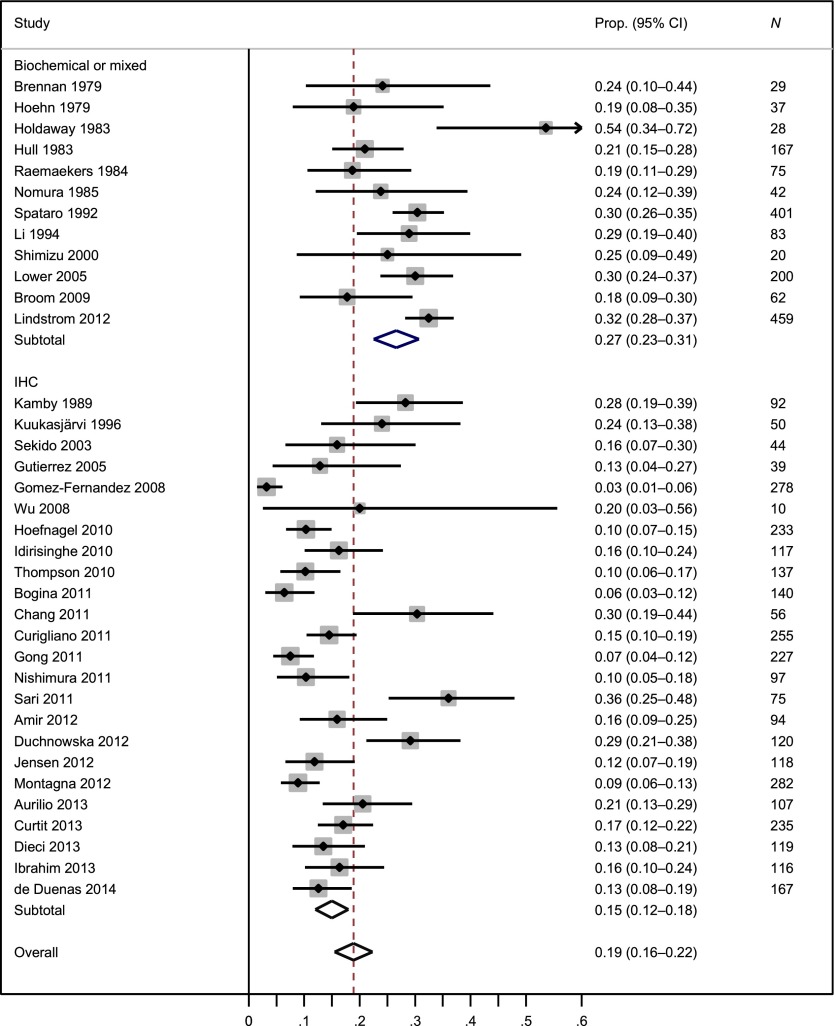
A total of 4,811 patients from 36 studies were included in the meta-analysis of ER changes from primary breast cancers to recurrent or metastatic lesions. The weighted average ER-discordant proportion was 19% (95% CI: 16%–22%), but there was considerable heterogeneity in ER discordance across studies, as indicated by I2 of 0.90 (Fig. 2) [19]. We found that the ER-discordant proportion was 15% in studies with the IHC assay and 27% in studies with biochemical or mixed assays (one tumor measured with the IHC method and another tumor measured with the biochemical method), and the difference was statistically significant (p = .001). Using studies with IHC assay, we found that the ER-discordant proportion was 16% in prospective studies and 15% in retrospective studies (p = .84). The ER-discordant proportion was 22% between primary tumors and distant metastatic lesions and was 13% between primary tumors and locoregional recurrences (p = .08). Supplemental online Figures 1 and 2 present the forest plots of ER change from positive to negative and from negative to positive, respectively.
Figure 2.
Meta-analysis of the proportion of estrogen receptor discordance by type of assay.
Abbreviations: CI, confidence interval; Prop., proportion.
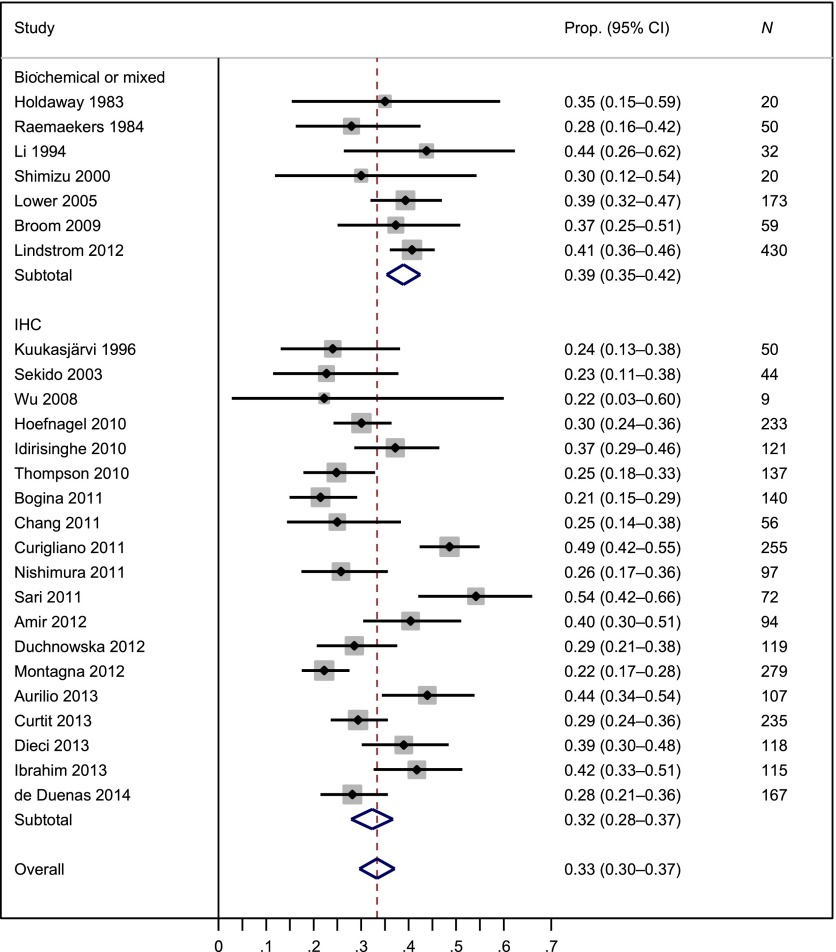
The meta-analysis of PR status included 3,232 patients from 26 studies. The weighted average PR-discordant proportion was 33% (95% CI: 30%–37%), but there was large heterogeneity in PR discordance across studies, as indicated by I2 of 0.77 (Fig. 3). The PR-discordant proportion between primary tumors and distant metastatic lesions (36%) was higher than that between primary tumors and locoregional recurrences (25%, p = .03). We did not find that PR discordance depended on whether assays were IHC or biochemical methods (p = .28) or whether study design was prospective or retrospective (p = .76). Supplemental online Figures 3 and 4 present the forest plots of PR change from positive to negative and from negative to positive, respectively.
Figure 3.
Meta-analysis of the proportion of progesterone receptor discordance by type of assay.
Abbreviations: CI, confidence interval; Prop., proportion.
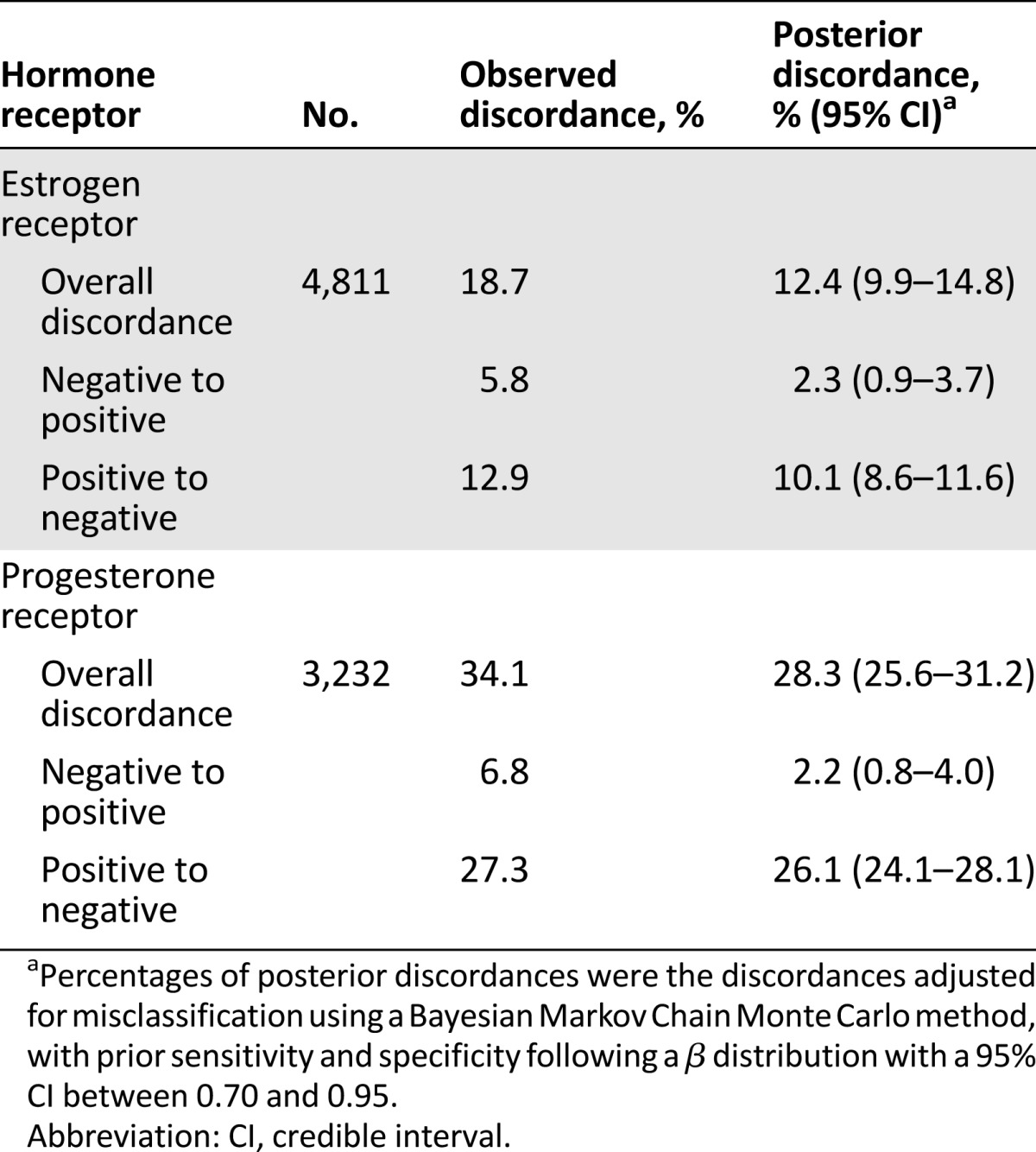
After accounting for misclassification in hormone receptor assays, we found that the posterior-discordant proportion was 12.4% for ER status, representing 34% relative reduction from observed discordance (Table 4). The corrected discordant proportion in PR status was 28.3%, representing 17% relative reduction from observed discordance. In particular, the corrected proportion of negative-to-positive change was 2.3% for ER status and 2.2% for PR status. The opposite change from positive to negative was 10.1% for ER status and 26.1% for PR status.
Table 4.
Posterior distribution of discordance in hormone receptors of primary breast tumors and recurrent or metastatic lesions
Discussion
In the present study, we used a large data set of more than 35,000 patients with two breast cancers or paired recurrent or metastatic diseases to examine the role of misclassification in the discordance of hormone receptors. We found that although misclassification accounted for a considerable proportion of discordance between two primary breast cancers or between primary cancer and metastatic lesions, a genuine change or difference in tumor biology existed. The study also gave estimates of biological changes between two tumors of the same patients after correcting for misclassification and the sensitivity and specificity of hormone receptor assessment.
Discordances in hormone receptors between primary and paired metastatic breast cancers or between two primary breast cancers have been well-documented. It has been soberly recognized that discordant receptor results can be caused by a true switch in tumor biology, by sampling of a heterogeneous tumor, and by limited accuracy of receptor assays [7]. The accuracy of immunohistochemistry-based receptor assays is not 100% because multiple factors, such as tissue fixation, staining methods, antigen retrieval, and subjective scoring, can affect the accuracy of receptor assays [11, 12, 20, 21]. A recent publication identified 15 preanalytical variables that were capable of affecting immunohistochemistry based on formalin-fixed, paraffin-embedded tissue [22]. The relative contribution of the limited accuracy of receptor assays to overall receptor discordance is unknown because there is no independent gold standard method to establish the accuracy of IHC-based receptor assays. Using a Bayesian misclassification correction method, we found that the accuracy of ER and PR assays was excellent but not perfect; the sensitivity and specificity of the ER assay were 0.971 and 0.920, respectively, whereas the sensitivity and specificity of the PR assay were 0.952 and 0.905, respectively. These findings have direct implications for hormonal therapy of breast cancer, both in patients with two breast cancers and in patients with a single breast cancer, because the extent of under- and overtreatment depends on the accuracy of evaluative testing that places a patient in a particular subgroup. In the early 1990s, IHC assays replaced LBAs. In 2010, ASCO and the College of American Pathologists issued new guidelines lowering the cutoff point for ER and PR positivity from 10% positive nuclei to 1% positive nuclei [23]. Because almost all data used in the present study were collected between 1990 and 2010, the sensitivity and specificity estimates mainly reflect the average accuracy of IHC-based receptor assays with 10% positively stained tumor cells as the positivity cutoff point. Although a study showed that the change in cutoff point did not significantly affect the number of ER-positive patients [24], we anticipate that the sensitivity of receptor assays may increase and the specificity may decrease after the 1% positive cutoff point has been used in pathologic practice.
After correcting for misclassification due to the imperfect accuracy of receptor assays, we provided the benchmark of biological heterogeneity between two primary breast cancers at a population level, thus it has important clinical implications for management of patients with two primary breast cancers. For the vast majority of the patients with two synchronous, especially unilateral synchronous, breast cancers, ER and PR status is concordant between two tumors. This does not necessarily deny the existence of intertumor heterogeneity. Instead, it suggests that the dominant clone of different foci of disease is essentially the same. For metachronous pairs, the overall discordance rates in both ER and PR are higher than those for synchronous cases. Further studies can help determine whether the hormone-receptor status of the previous cancer has value as a prognostic indicator for the current breast cancer among patients with metachronous breast cancers and whether inconsistent receptor status predicts worse clinical outcomes among patients with synchronous breast cancers.
Our study demonstrated that conversion in receptor status between primary cancer and metastatic lesion should be interpreted with caution. We found that 34% of ER conversion and 17% of PR conversion could be due to technical misclassification. This finding is in line with a recent prospective study showing that the conversion rate for ER was 21% in local laboratories and 13% in a central laboratory (38% relative reduction) and that the conversion rate for PR was 35% and 28% in local and central laboratories, respectively (i.e., 20% relative reduction) [25]. After correcting for misclassification, less than 3% of primary tumor and metastatic pairs showed a negative-to-positive receptor change. In contrast, 10.1% of patients had a positive-to-negative change in ER and 26.1% of patients had a positive-to-negative change in PR. These benchmark estimates have direct clinical implications. Rebiopsy of recurrent and metastatic lesions may render change in hormonal therapy and chemotherapy for these patients. Studies have shown that 8%–17.5% of patients with relapsed disease had changed their therapies based on discordance in hormone receptors and human epidermal growth factor receptor 2 (HER2) [25–27]. Although it is technically feasible to conduct biopsy at metastatic sites, approximately 20% of biopsies from metastases could not be analyzed because of insufficient cells, especially biopsies done with fine-needle aspiration and core biopsies from bone and bone marrow [27]. In the situation that a metastatic lesion cannot be evaluated, if the primary tumor is ER or PR negative, the metastatic lesion is more likely to be ER or PR negative as well, so chemotherapy rather than hormonal therapy can be considered; if the primary tumor is ER positive, there is still a possibility that the metastatic lesion is negative, so it is important to evaluate whether the clinical course of the disease (e.g., response to tamoxifen or aromatase inhibitors) is consistent with ER-positive cancers.
To our knowledge, this study is the first investigation that formally evaluated the impact of misclassification on discordance in hormone receptor status between two primary breast cancers or between primary cancers and recurrent or metastatic lesions. A comprehensive meta-analysis was also conducted to summarize discordance rates in ER and PR status between primary cancers and recurrent or metastatic lesions, using the literature published between 1979 and 2014. The study developed a Bayesian misclassification correction model that utilized paired tumor data to estimate the accuracy of receptor assays. This study has several limitations. First, the Bayesian misclassification model assumes that the distributions of sensitivity and specificity of the two tumors are the same; however, this may not be true in practice, especially for distant metastatic lesions, for which fine-needle aspiration is often used to biopsy metastasis, and biopsy methods may affect the accuracy of receptor assessment. The accuracy of receptor assays may also depend on pathological departments and calendar year (measurement of ER and PR may be improved over time). Second, the model we used can help gauge the contribution of misclassification to overall discordance at a population level, but we cannot pinpoint which tumor is false negative or false positive.
Conclusion
This large, comprehensive study showed that a considerable proportion of discordance in hormone receptor status can be attributed to misclassification in receptor assessment, although the accuracy of receptor assays was estimated to be excellent. After correcting for misclassification, the study provides a benchmark of breast cancer heterogeneity at a population level, which calls for understanding of the mechanism of tumor heterogeneity. In particular, we showed that the discordance rate in receptor status between two primary cancers was highest for metachronous contralateral pairs and lowest for synchronous ipsilateral pairs. After accounting for misclassification, biological discordance exists between primary breast cancer and recurrent or metastatic site, and there are more positive-to-negative switches in receptor status than negative-to-positive switches. The clinical implications of change from positive to negative could be substantial and demand novel therapeutic approaches to warrant rebiopsy. Moreover, misclassification in ER and PR assays cannot be ignored and suggests that biopsy of metastatic sites for ER and PR retesting may be recommended on a case-by-case basis after considering the difficulty, cost, and side effects associated with tissue biopsy, in addition to the previous status of ER and PR and response to endocrine therapy.
This article is available for continuing medical education credit at CME.TheOncologist.com.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This project is partially supported by the American Cancer Society (Grant MRSG-13-063-01 to D.H.). We thank Dr. Olufunmilayo I. Olopade for the helpful discussion of clinical implications.
Footnotes
For Further Reading: Elsa Curtit, Virginie Nerich, Laura Mansi et al. Discordances in Estrogen Receptor Status, Progesterone Receptor Status, and HER2 Status Between Primary Breast Cancer and Metastasis. The Oncologist 2013;18:667–674.
Implications for Practice: Discordance in estrogen receptor and progesterone receptor expression between the primary breast tumor and the corresponding metastatic lesion was high (17% and 29%, respectively), whereas HER2 status remained stable (4% of discordance). Previous chemotherapy, and specifically anthracycline-based chemotherapy, was associated with a switch in estrogen receptor status. Further studies are warranted to confirm these data and to determine the interest of systematic rebiopsy in the metastatic setting. In the era of a more personalized approach to medicine and of genomic investigations of tumors, reliable and recent evaluation of tumor prognostic and predictive factors remains a challenge.
Author Contributions
Conception/Design: Dezheng Huo
Collection and/or assembly of data: Dezheng Huo, Dominique Sighoko
Data analysis and interpretation: Dezheng Huo, Dominique Sighoko, Juxin Liu, Ningqi Hou, Paul Gustafson
Manuscript writing: Dezheng Huo, Dominique Sighoko, Juxin Liu, Ningqi Hou, Paul Gustafson
Final approval of manuscript: Dezheng Huo, Dominique Sighoko, Juxin Liu, Ningqi Hou, Paul Gustafson
Disclosures
The authors indicated no financial relationships.
References
- 1.Wolff AC, Dowsett M. Estrogen receptor: A never ending story? J Clin Oncol. 2011;29:2955–2958. doi: 10.1200/JCO.2011.35.4589. [DOI] [PubMed] [Google Scholar]
- 2.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coradini D, Oriana S, Mariani L, et al. Is steroid receptor profile in contralateral breast cancer a marker of independence of the corresponding primary tumour? Eur J Cancer. 1998;34:825–830. doi: 10.1016/s0959-8049(97)10121-6. [DOI] [PubMed] [Google Scholar]
- 4.Gong Y, Han EY, Guo M, et al. Stability of estrogen receptor status in breast carcinoma: A comparison between primary and metastatic tumors with regard to disease course and intervening systemic therapy. Cancer. 2011;117:705–713. doi: 10.1002/cncr.25506. [DOI] [PubMed] [Google Scholar]
- 5.Huo D, Melkonian S, Rathouz PJ, et al. Concordance in histological and biological parameters between first and second primary breast cancers. Cancer. 2011;117:907–915. doi: 10.1002/cncr.25587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swain SM, Wilson JW, Mamounas EP, et al. Estrogen receptor status of primary breast cancer is predictive of estrogen receptor status of contralateral breast cancer. J Natl Cancer Inst. 2004;96:516–523. doi: 10.1093/jnci/djh097. [DOI] [PubMed] [Google Scholar]
- 7.Pusztai L, Viale G, Kelly CM, et al. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. The Oncologist. 2010;15:1164–1168. doi: 10.1634/theoncologist.2010-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson RW, Allred DC, Anderson BO, et al. Metastatic breast cancer, version 1.2012: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:821–829. doi: 10.6004/jnccn.2012.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gown AM. Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol. 2008;21(suppl 2):S8–S15. doi: 10.1038/modpathol.2008.34. [DOI] [PubMed] [Google Scholar]
- 10.Allison KH. Estrogen receptor expression in breast cancer: We cannot ignore the shades of gray. Am J Clin Pathol. 2008;130:853–854. doi: 10.1309/AJCP3P3XHTCYGZIA. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes A, Jasani B, Barnes DM, et al. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: Interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol. 2000;53:125–130. doi: 10.1136/jcp.53.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rüdiger T, Höfler H, Kreipe HH, et al. Quality assurance in immunohistochemistry: Results of an interlaboratory trial involving 172 pathologists. Am J Surg Pathol. 2002;26:873–882. doi: 10.1097/00000478-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein NS, Ferkowicz M, Odish E, et al. Minimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinoma. Am J Clin Pathol. 2003;120:86–92. doi: 10.1309/QPHD-RB00-QXGM-UQ9N. [DOI] [PubMed] [Google Scholar]
- 14. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973-2010), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission. Available at www.seer.cancer.gov.
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20:1953–1958. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Gustafson P. Measurement Error and Misclassification in Statistics and Epidemiology: Impacts and Bayesian Adjustments. London, U.K.: Chapman and Hall/CRC; 2003. p. 200. [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury T, Sait S, Hwang H, et al. Delay to formalin fixation effect on breast biomarkers. Mod Pathol. 2009;22:1457–1467. doi: 10.1038/modpathol.2009.117. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes A, Jasani B, Balaton AJ, et al. Study of interlaboratory reliability and reproducibility of estrogen and progesterone receptor assays in Europe. Documentation of poor reliability and identification of insufficient microwave antigen retrieval time as a major contributory element of unreliable assays. Am J Clin Pathol. 2001;115:44–58. doi: 10.1309/H905-HYC1-6UQQ-981P. [DOI] [PubMed] [Google Scholar]
- 22.Engel KB, Moore HM. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2011;135:537–543. doi: 10.5858/2010-0702-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 23.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welsh AW, Moeder CB, Kumar S, et al. Standardization of estrogen receptor measurement in breast cancer suggests false-negative results are a function of threshold intensity rather than percentage of positive cells. J Clin Oncol. 2011;29:2978–2984. doi: 10.1200/JCO.2010.32.9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Dueñas EM, Hernández AL, Zotano AG, et al. Prospective evaluation of the conversion rate in the receptor status between primary breast cancer and metastasis: Results from the GEICAM 2009-03 ConvertHER study. Breast Cancer Res Treat. 2014;143:507–515. doi: 10.1007/s10549-013-2825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson AM, Jordan LB, Quinlan P, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: The Breast Recurrence In Tissues Study (BRITS) Breast Cancer Res. 2010;12:R92. doi: 10.1186/bcr2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30:587–592. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoehn JL, Plotka ED, Dickson KB. Comparison of estrogen receptor levels in primary and regional metastatic carcinoma of the breast. Ann Surg. 1979;190:69–71. doi: 10.1097/00000658-197907000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan MJ, Donegan WL, Appleby DE. The variability of estrogen receptors in metastatic breast cancer. Am J Surg. 1979;137:260–262. doi: 10.1016/0002-9610(79)90159-4. [DOI] [PubMed] [Google Scholar]
- 30.Hull DF, III, Clark GM, Osborne CK, et al. Multiple estrogen receptor assays in human breast cancer. Cancer Res. 1983;43:413–416. [PubMed] [Google Scholar]
- 31.Holdaway IM, Bowditch JV. Variation in receptor status between primary and metastatic breast cancer. Cancer. 1983;52:479–485. doi: 10.1002/1097-0142(19830801)52:3<479::aid-cncr2820520317>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Raemaekers JM, Beex LV, Koenders AJ, et al. Concordance and discordance of estrogen and progesterone receptor content in sequential biopsies of patients with advanced breast cancer: Relation to survival. Eur J Cancer Clin Oncol. 1984;20:1011–1018. doi: 10.1016/0277-5379(84)90102-0. [DOI] [PubMed] [Google Scholar]
- 33.Nomura Y, Tashiro H, Shinozuka K. Changes of steroid hormone receptor content by chemotherapy and/or endocrine therapy in advanced breast cancer. Cancer. 1985;55:546–551. doi: 10.1002/1097-0142(19850201)55:3<546::aid-cncr2820550313>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.Kamby C, Rasmussen BB, Kristensen B. Oestrogen receptor status of primary breast carcinomas and their metastases. Relation to pattern of spread and survival after recurrence. Br J Cancer. 1989;60:252–257. doi: 10.1038/bjc.1989.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spataro V, Price K, Goldhirsch A, et al. Sequential estrogen receptor determinations from primary breast cancer and at relapse: Prognostic and therapeutic relevance. The International Breast Cancer Study Group (formerly Ludwig Group) Ann Oncol. 1992;3:733–740. doi: 10.1093/oxfordjournals.annonc.a058330. [DOI] [PubMed] [Google Scholar]
- 36.Li BD, Byskosh A, Molteni A, et al. Estrogen and progesterone receptor concordance between primary and recurrent breast cancer. J Surg Oncol. 1994;57:71–77. doi: 10.1002/jso.2930570202. [DOI] [PubMed] [Google Scholar]
- 37.Kuukasjarvi T, Kononen J, Helin H, et al. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol. 1996;14:2584–2589. doi: 10.1200/JCO.1996.14.9.2584. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu C, Fukutomi T, Tsuda H, et al. c-erbB-2 protein overexpression and p53 immunoreaction in primary and recurrent breast cancer tissues. J Surg Oncol. 2000;73:17–20. doi: 10.1002/(sici)1096-9098(200001)73:1<17::aid-jso5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Sekido Y, Umemura S, Takekoshi S, et al. Heterogeneous gene alterations in primary breast cancer contribute to discordance between primary and asynchronous metastatic/recurrent sites: HER2 gene amplification and p53 mutation. Int J Oncol. 2003;22:1225–1232. [PubMed] [Google Scholar]
- 40.Lower EE, Glass EL, Bradley DA, et al. Impact of metastatic estrogen receptor and progesterone receptor status on survival. Breast Cancer Res Treat. 2005;90:65–70. doi: 10.1007/s10549-004-2756-z. [DOI] [PubMed] [Google Scholar]
- 41.Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: Relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Fernandez C, Daneshbod Y, Nassiri M, et al. Immunohistochemically determined estrogen receptor phenotype remains stable in recurrent and metastatic breast cancer. Am J Clin Pathol. 2008;130:879–882. doi: 10.1309/AJCPD1AO3YSYQYNW. [DOI] [PubMed] [Google Scholar]
- 43.Wu JM, Fackler MJ, Halushka MK, et al. Heterogeneity of breast cancer metastases: Comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14:1938–1946. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broom RJ, Tang PA, Simmons C, et al. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: Discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009;29:1557–1562. [PubMed] [Google Scholar]
- 45.Idirisinghe PK, Thike AA, Cheok PY, et al. Hormone receptor and c-ERBB2 status in distant metastatic and locally recurrent breast cancer. Pathologic correlations and clinical significance. Am J Clin Pathol. 2010;133:416–429. doi: 10.1309/AJCPJ57FLLJRXKPV. [DOI] [PubMed] [Google Scholar]
- 46.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogina G, Bortesi L, Marconi M, et al. Comparison of hormonal receptor and her-2 status between breast primary tumours and relapsing tumours: Clinical implications of progesterone receptor loss. Virchows Arch. 2011;459:1–10. doi: 10.1007/s00428-011-1097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curigliano G, Bagnardi V, Viale G, et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann Oncol. 2011;22:2227–2233. doi: 10.1093/annonc/mdq751. [DOI] [PubMed] [Google Scholar]
- 49.Sari E, Guler G, Hayran M, et al. Comparative study of the immunohistochemical detection of hormone receptor status and HER-2 expression in primary and paired recurrent/metastatic lesions of patients with breast cancer. Med Oncol. 2011;28:57–63. doi: 10.1007/s12032-010-9418-2. [DOI] [PubMed] [Google Scholar]
- 50.Nishimura R, Osako T, Okumura Y, et al. Changes in the ER, PgR, HER2, p53 and Ki-67 biological markers between primary and recurrent breast cancer: Discordance rates and prognosis. World J Surg Oncol. 2011;9:131. doi: 10.1186/1477-7819-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang HJ, Han SW, Oh DY, et al. Discordant human epidermal growth factor receptor 2 and hormone receptor status in primary and metastatic breast cancer and response to trastuzumab. Jpn J Clin Oncol. 2011;41:593–599. doi: 10.1093/jjco/hyr020. [DOI] [PubMed] [Google Scholar]
- 52.Jensen JD, Knoop A, Ewertz M, et al. ER, HER2, and TOP2A expression in primary tumor, synchronous axillary nodes, and asynchronous metastases in breast cancer. Breast Cancer Res Treat. 2012;132:511–521. doi: 10.1007/s10549-011-1610-3. [DOI] [PubMed] [Google Scholar]
- 53.Montagna E, Bagnardi V, Rotmensz N, et al. Breast cancer subtypes and outcome after local and regional relapse. Ann Oncol. 2012;23:324–331. doi: 10.1093/annonc/mdr129. [DOI] [PubMed] [Google Scholar]
- 54.Duchnowska R, Dziadziuszko R, Trojanowski T, et al. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012;14:R119. doi: 10.1186/bcr3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindstrom LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 56.Ibrahim T, Farolfi A, Scarpi E, et al. Hormonal receptor, human epidermal growth factor receptor-2, and Ki67 discordance between primary breast cancer and paired metastases: Clinical impact. Oncology. 2013;84:150–157. doi: 10.1159/000345795. [DOI] [PubMed] [Google Scholar]
- 57.Dieci MV, Barbieri E, Piacentini F, et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: A single-institution analysis. Ann Oncol. 2013;24:101–108. doi: 10.1093/annonc/mds248. [DOI] [PubMed] [Google Scholar]
- 58.Aurilio G, Monfardini L, Rizzo S, et al. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol. 2013;52:1649–1656. doi: 10.3109/0284186X.2012.754990. [DOI] [PubMed] [Google Scholar]
- 59.Curtit E, Nerich V, Mansi L, et al. Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. The Oncologist. 2013;18:667–674. doi: 10.1634/theoncologist.2012-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.