The present study provides data about outcomes of consecutive metastatic breast cancer patients treated at an academic hospital. The findings support the importance of considering breast cancer in distinct subgroups with the aim of obtaining more precise information about prognosis and expected benefit from treatment. The study also provides insights for future clinical trial design.
Keywords: Outcome measure, Surrogate endpoint, Survival analysis, Breast neoplasms
Abstract
No gold standard treatment exists for metastatic breast cancer (MBC). Clinical decision making is based on knowledge of prognostic and predictive factors that are extrapolated from clinical trials and, sometimes, are not reliably transferable to a real-world scenario. Moreover, misalignment between endpoints used in drug development and measures of outcome in clinical practice has been noted. The roles of overall survival (OS) and progression-free survival (PFS) as primary endpoints in the context of clinical trials are the subjects of lively debate. Information about these parameters in routine clinical practice is potentially useful to design new studies and/or to interpret the results of clinical research. This study analyzed the impact of patient and tumor characteristics on the major measures of outcome across different lines of treatment in a cohort of 472 patients treated for MBC. OS, PFS, and postprogression survival (PPS) were analyzed. The study showed how biological and clinical characteristics may have different prognostic value across different lines of therapy for MBC. After first-line treatment, the median PPS of luminal A, luminal B, and human epidermal growth factor receptor 2 (HER2)-positive groups was longer than 12 months. The choice of OS as a primary endpoint for clinical trials could not be appropriate with these subtypes. In contrast, OS could be an appropriate endpoint when PPS is expected to be low (e.g., triple-negative subtype after the first line; other subtypes after the third line). The potential implications of these findings are clinical and methodological.
Implications for Practice:
Although randomized clinical trials are recognized as the highest level of scientific evidence to demonstrate the efficacy of a treatment, sometimes they do not reflect the clinical circumstances faced in a real-world scenario. The present study provides data about outcomes of consecutive metastatic breast cancer patients treated at an academic hospital. The findings support the importance of considering breast cancer in distinct subgroups with the aim of obtaining more precise information about prognosis and expected benefit from treatment. The study also provides insights for future clinical trial design.
Introduction
Breast cancer (BC) is the leading malignancy among women in both Europe and the U.S. and is becoming an emerging oncologic disease in developing countries [1, 2]. Approximately 30% of women with early stage BC will develop metastases, whereas metastatic breast cancer (MBC) occurs in approximately 6%–7% of newly diagnosed cases [3]. The 5-year relative survival for women with MBC is ∼25%, and the median overall survival (OS) is usually reported to be ∼24 months [4–6]. In contrast, single clinical trials tend to report different results in terms of outcome depending on the specific characteristics of the enrolled population. Some recent trials on first-line treatment of MBC report median values of OS that are notably longer than those observed in previous studies [7–12]. Although comparison across different trials needs to be performed with extreme caution, this source of information could be useful if the trial population is homogeneous enough and well defined at molecular, pathological, and clinical levels. Among the different disease- and patient-related variables that may influence the prognosis of patients with MBC, tumor subtypes, sites of metastatic involvement, burden of disease, and patient comorbidities are considered to be of value [13].
Despite great improvements in early diagnosis and the strict standardization of the adjuvant setting, no gold standard therapy has been defined or validated for MBC. Multiple effective options for treatment are available, and the choice should be tailored to the individual patient on the basis of specific factors that are key points of the therapeutic algorithm: hormone receptor status, human epidermal growth factor receptor 2 (HER2) status, tumor burden, and prior treatments. Only a percentage of patients who receive a specific treatment really benefit from it [14]. Accordingly, there is increasing scientific interest in the identification of prognostic and predictive factors that maximize survival, that save patients from unnecessary toxicity, and that maintain a good standard of quality of life.
In recent years, several new drugs have been proposed for MBC, but too often, they do not show OS benefit. Some of the negative results appear to be related to the design of the randomized clinical trial (RcT) [15]. The primary measure of clinical benefit in MBC has traditionally been OS [16]. OS, defined as the time from randomization to death from any cause, addresses both safety and efficacy. Nonetheless, confounded by subsequent therapy and crossover, the value of OS as a primary endpoint in trials that evaluate new therapeutic agents for MBC has been questioned, especially in the context of first-line treatment. In fact, because of requirements for large sample sizes and long durations of follow-up, many RcTs cannot achieve the statistical power to detect a plausible increase in OS [17–19]. For these reasons, alternative event-driven endpoints that could act as predictive surrogates of OS have been proposed. Progression-free survival (PFS), defined as the time from randomization to tumor progression or death from any cause, is commonly adopted as an alternative endpoint to OS in first-line setting [19]. It requires a shorter follow-up period and a smaller sample size. PFS was informally accepted as a surrogate endpoint for OS in clinical cancer research [20, 21]. However, in several studies, treatment that demonstrated advantage in terms of PFS did not determine the same effect on OS [19]. The fact that PFS benefit was not translated into a statistically significant benefit in terms of OS, however, could not be interpreted as lack of improvement in OS as a whole. In particular, one possible explanation for the low correlation between PFS and OS is the observation of long postprogression survival (PPS). PPS is defined as the time from tumor progression to death from any cause. This relatively new measure of outcome has gained interest for understanding treatment effects. In fact, when examining the results of RcTs, the probability of detecting a statistically significant difference in OS depends on the length of the median PPS interval. In other words, the longer PPS is, there is less chance of detecting a statistically significant difference in OS between the treatment arms of an RcT. By using simulation methods to generate clinical trials, it has been shown that the sample size required for detecting a statistically significant difference in OS is directly correlated to the duration of PPS. When median PPS is short, the correlation between the hazard ratios (HRs) for PFS and OS is high, but when median PPS is longer than 12 months, the correlation between the two HR estimates decreases significantly. Accordingly, the notion that drugs that significantly prolong PFS should necessarily prolong OS is questionable [22]. To date, no data are available about PPS in patients treated outside of RcTs.
The aim of this study is to analyze the impact of patient and tumor characteristics on the outcome measures of a cohort of 472 patients with MBC. Results of this study are estimated to be potentially useful as a basis for the design of future clinical trials.
Materials and Methods
Study Design
We retrospectively reviewed a total of 472 consecutive MBC patients, treated at the Department of Oncology of the University Hospital of Udine, Udine, Italy, from January 2004 through July 2012.
For each patient, individual data and information on primary and advanced disease were collected from electronic health records. Based on this data set, the following BC subgroups were defined: “luminal A” (positive for estrogen receptor [ER] or progesterone receptor [PR], HER2 negative, Ki-67 ≤14%), “luminal B” (ER or PR positive, HER2 negative, Ki-67 >14%), “HER2 positive” (HER2 positive and any ER or PR status), “triple negative” (ER and PR negative, HER2 negative) [23, 24]. HER2-positive disease was further categorized according to the concomitant expression of hormone receptors (HER2 positive and ER or PR positive vs. HER2 positive and ER and PR negative). The cutoff point of 1% was used to define ER and/or PR positivity [25]. Time to development of MBC was defined as the interval between diagnosis of primary BC and diagnosis of MBC. OS was defined as the time elapsed between the start of treatment for metastatic disease and death or last follow-up. PFS was defined as the interval between the start of treatment for MBC and the occurrence of disease progression or death for any cause, whichever occurred first. PFS was calculated for the first four lines of treatment and were defined accordingly as PFS1, PFS2, PFS3, and PFS4. PPS was defined as the interval between progression and death or last follow-up. According to the starting point (evidence of progression after specific lines of treatment), PPS was defined as PPS1, PPS2, PPS3, and PPS4. The date of progression was defined as the date at which progression was first evident (e.g., imaging, biochemical examination, clinical visit), according to clinical practice.
The following variables were studied as possible prognostic factors for specific clinical outcome: ER status, PR status, HER2 status, Ki-67 status, previous adjuvant endocrine therapy (ET) and/or chemotherapy (CT) (yes vs. no), visceral metastatic site involved (yes vs. no), pulmonary sites involved (yes vs. no), brain (yes vs. no), and liver metastasis (yes vs. no), bone-only localizations (yes vs. no), age at diagnosis (<35 years, 65–70 years, or >70 years vs. 35–65 years), performance status according to the Eastern Cooperative Oncology Group (ECOG) scale (ECOG ≥2 vs. ECOG 1 and ECOG ≥2 vs. ECOG 0) [26–28].
Statistical Analysis
Descriptive analysis of clinical and pathological characteristics was performed. For categorical variables, such as age, histotype, grade, ER, PR, HER2 status, Ki-67, the frequency distribution was calculated. For the continuous variables, 25th, 50th, and 75th percentiles were calculated.
Outcome measures analyzed were OS, PFS, and PPS. In particular, the analyses were performed considering every type of therapy (OS_tot, PFS_tot, PPS_tot), ET lines only (OS_ET, PFS_ET, PPS_ET) and CT lines only (OS_CT, PFS_CT, PPS_CT).
We estimated HR, with a 95% confidence interval (CI), using uni- and multivariate Cox’s proportional hazards regression model. The selection of covariates in the final model was based on both clinical relevance and statistical significance. The significance level was set at p = .05. All variables that showed statistical significance in univariate analysis were included in multivariate analysis. Kaplan-Meier analysis and the log-rank test were performed to compare survival curves among different population subgroups.
Results
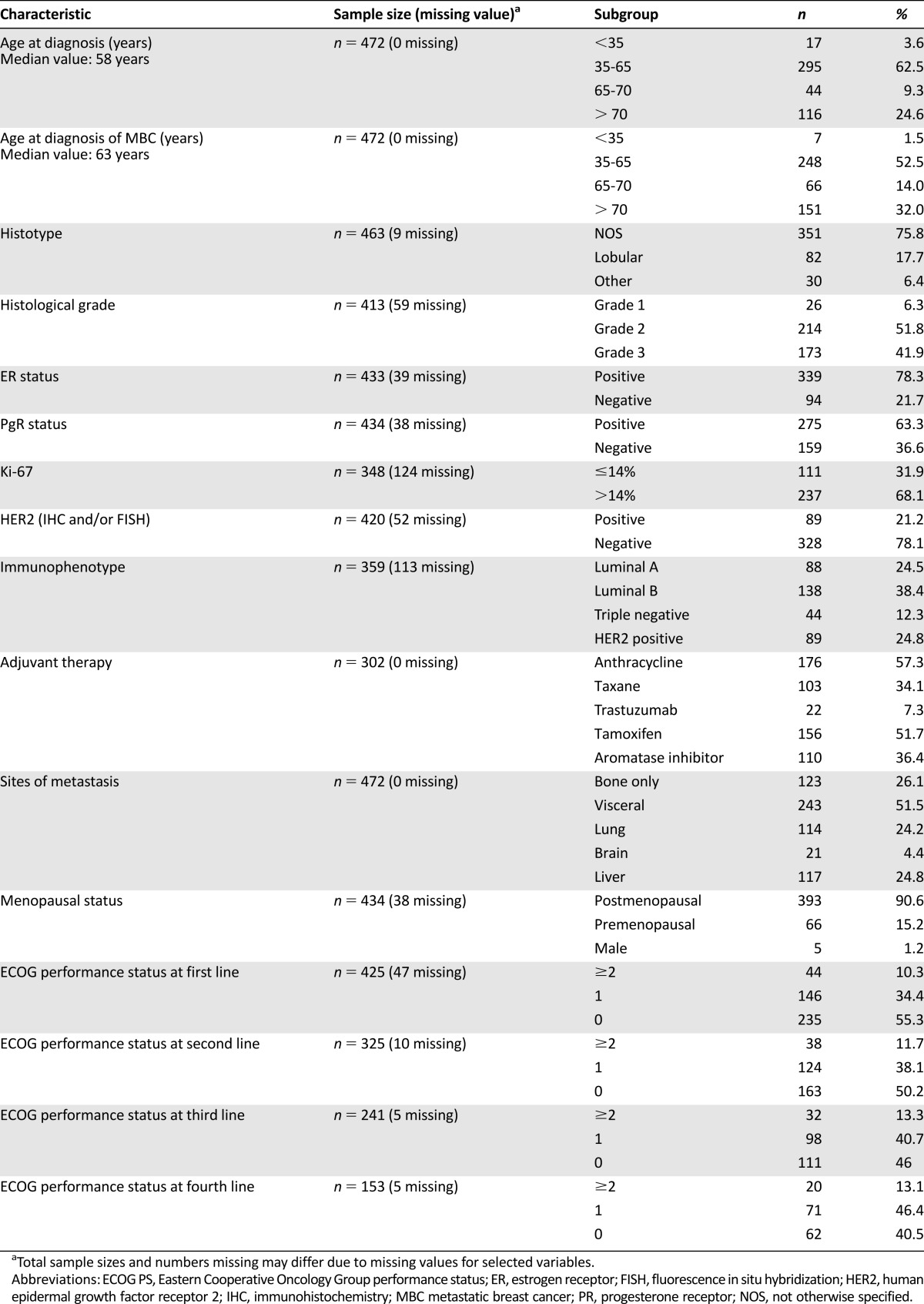
Median age at diagnosis was 58 years, and median age at diagnosis of MBC was 63 years; 147 patients presented de novo metastatic disease.
Most patients (78.3%) had ER-positive disease. Eighty-eight patients (24.5%) had a luminal A disease, 138 (38.4%) had a luminal B disease, 89 (24.8%) had a HER2-positive disease, and 44 (12.3%) had a triple-negative phenotype. Distributions of patient personal data and primary and advanced disease characteristics are shown in Table 1.
Table 1.
Patient and disease characteristics
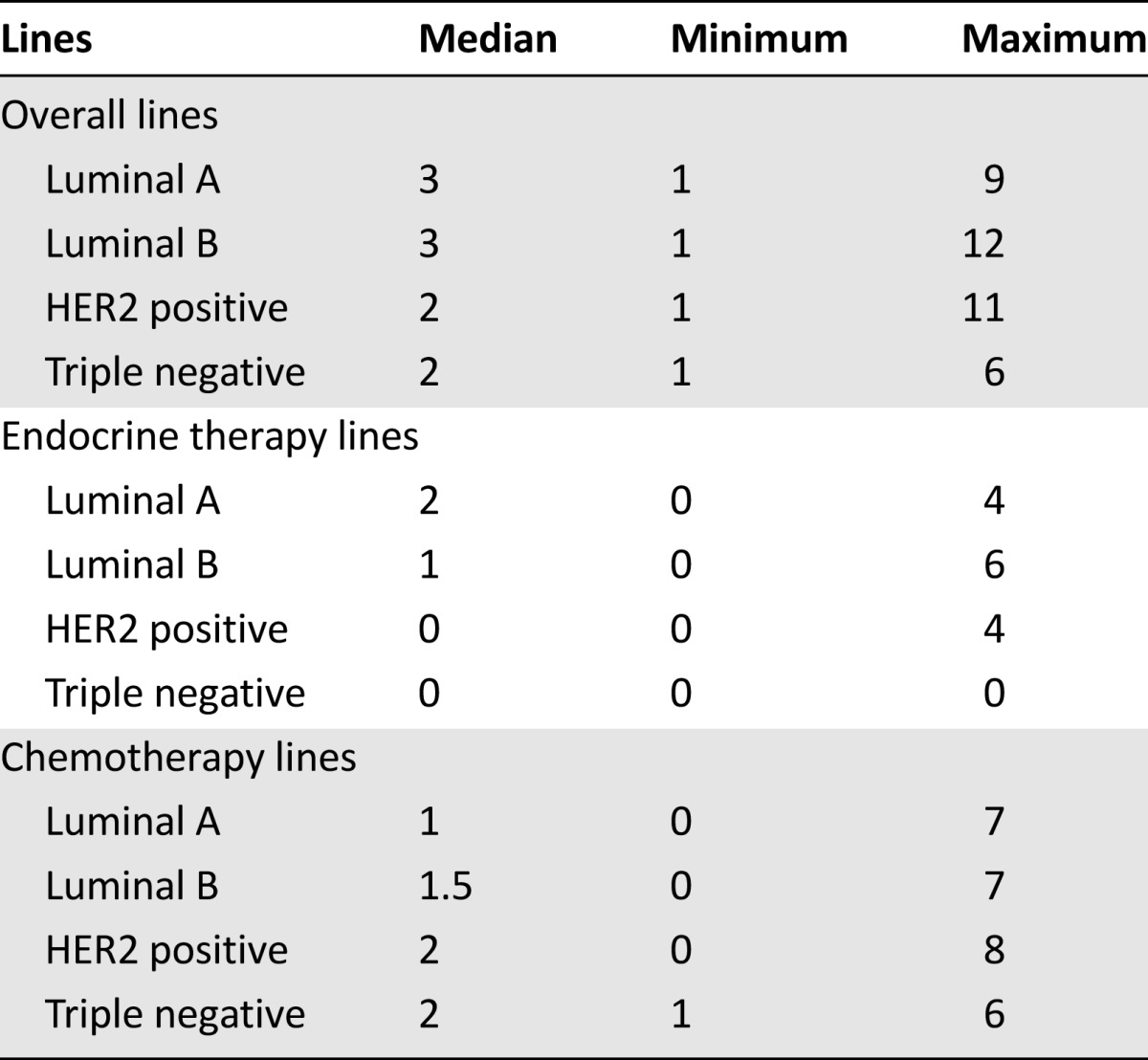
The median number of therapeutic lines for metastatic disease was 3 (range: 1–12 therapeutic lines). Specifically, the median number of CT lines was 1 (range: 0–9 CT lines), and the median number of ET lines was 1 (range: 0–6 ET lines). The number of lines among different breast cancer subtypes is presented in Table 2.
Table 2.
Number of lines received across different immunophenotypes
Median time to development of MBC from initial diagnosis of BC was 21.7 months (range: 0–175 months); median OS was 34 months (25th–75th percentile: 13.7–58.5). In patients with hormone receptor-positive disease, no statistically significant difference in OS was observed according to the type of first-line treatment (CT vs. ET, log-rank test p = .83; median, 38 months and 36.7 months, respectively).
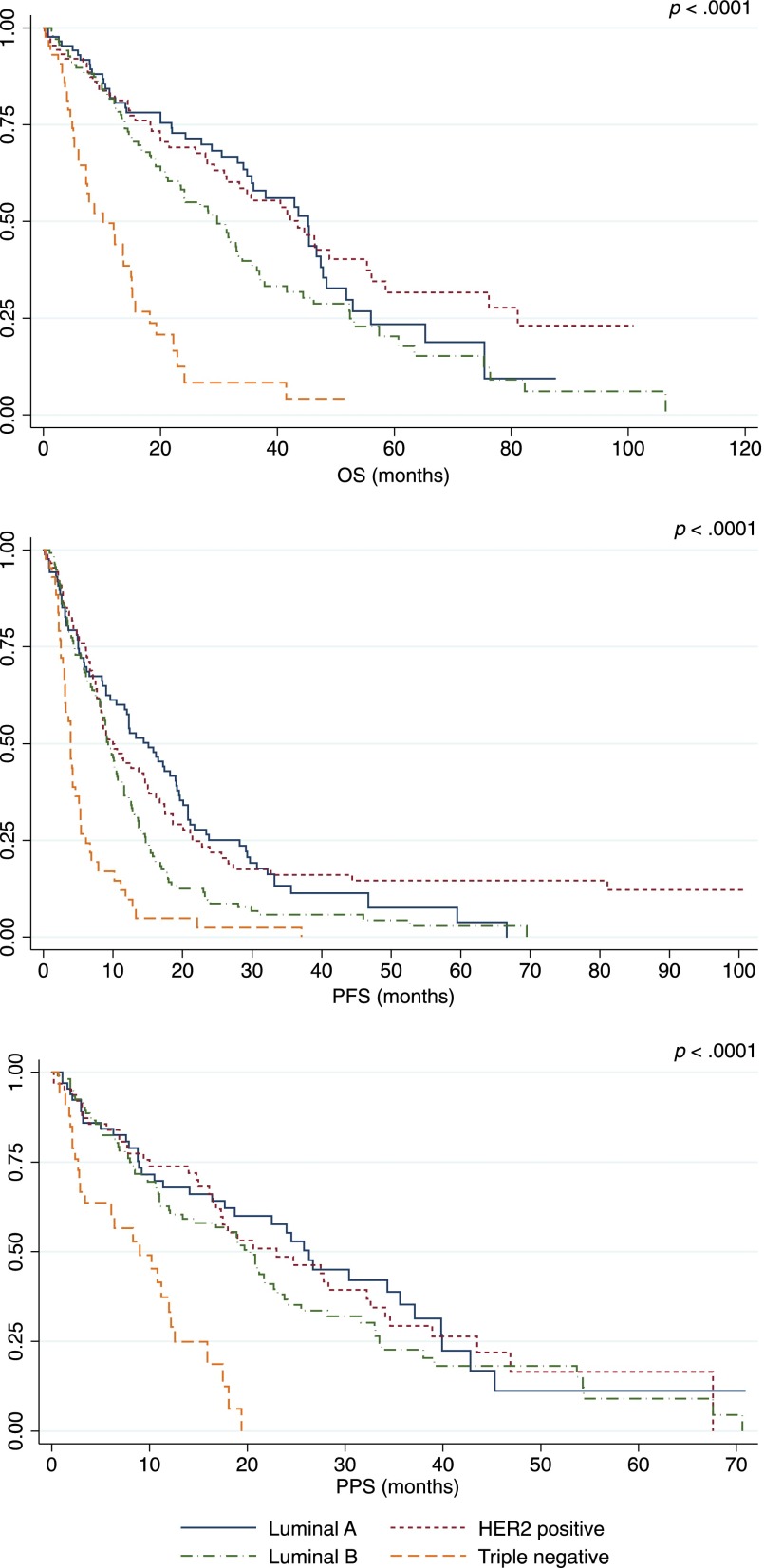
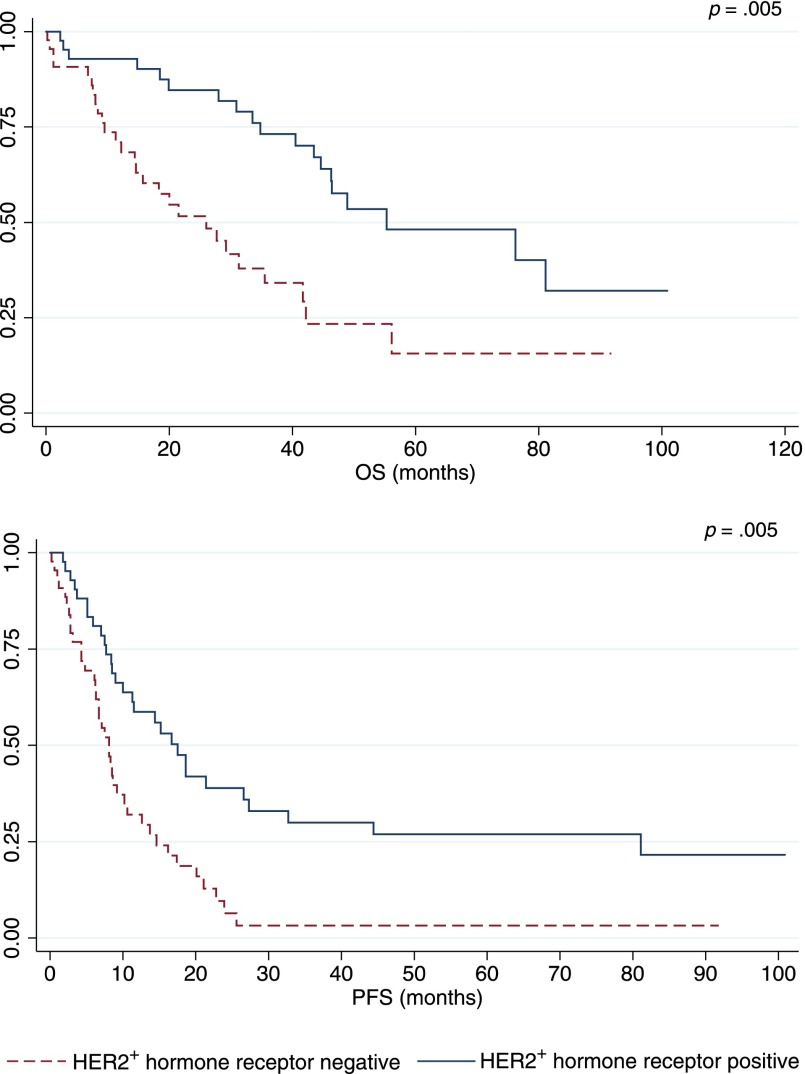
Table 3 summarizes outcome estimates for each line of therapy. Distinct analysis of chemotherapy and endocrine therapy lines is also shown. Median PFS and PPS progressively decreased in the different lines of therapy beyond the first. Different outcomes were observed among BC subtypes (Table 4, Fig. 1). Patients with luminal A and HER2-positive disease experienced the best prognoses. In the HER2-positive group, outcomes varied on the basis of concomitant expression of hormone receptors (Fig. 2). In particular, among HER2-positive cases, a better outcome was noted for hormone receptor positive disease versus hormone receptor negative disease (OS: 55.3 months vs. 26.0 months; PFS: 17.5 months vs. 8.1 months; PPS: 27.8 months vs. 14.0 months).
Table 3.
Outcome estimates according to the line of therapy
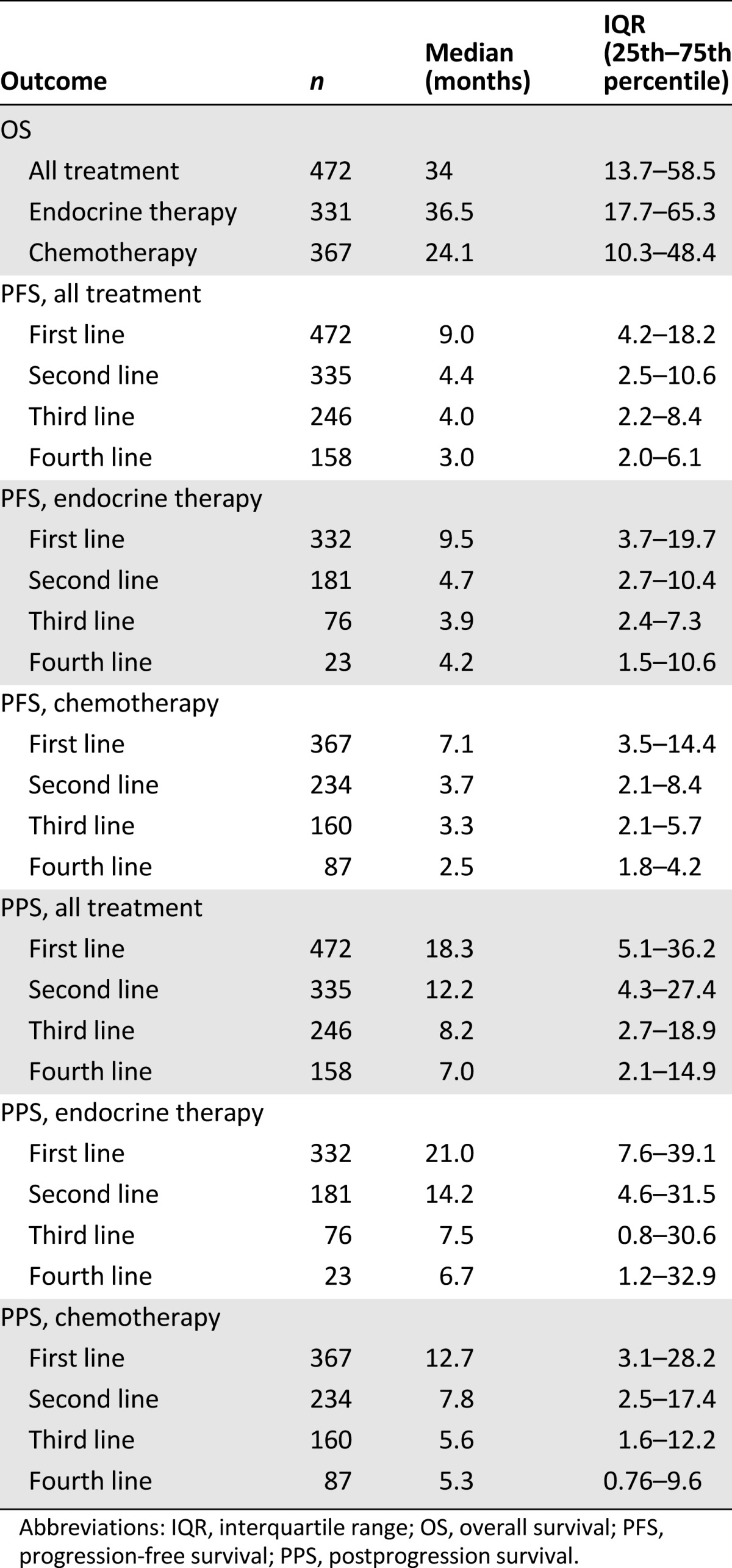
Table 4.
Outcome estimates according to breast cancer subtypes
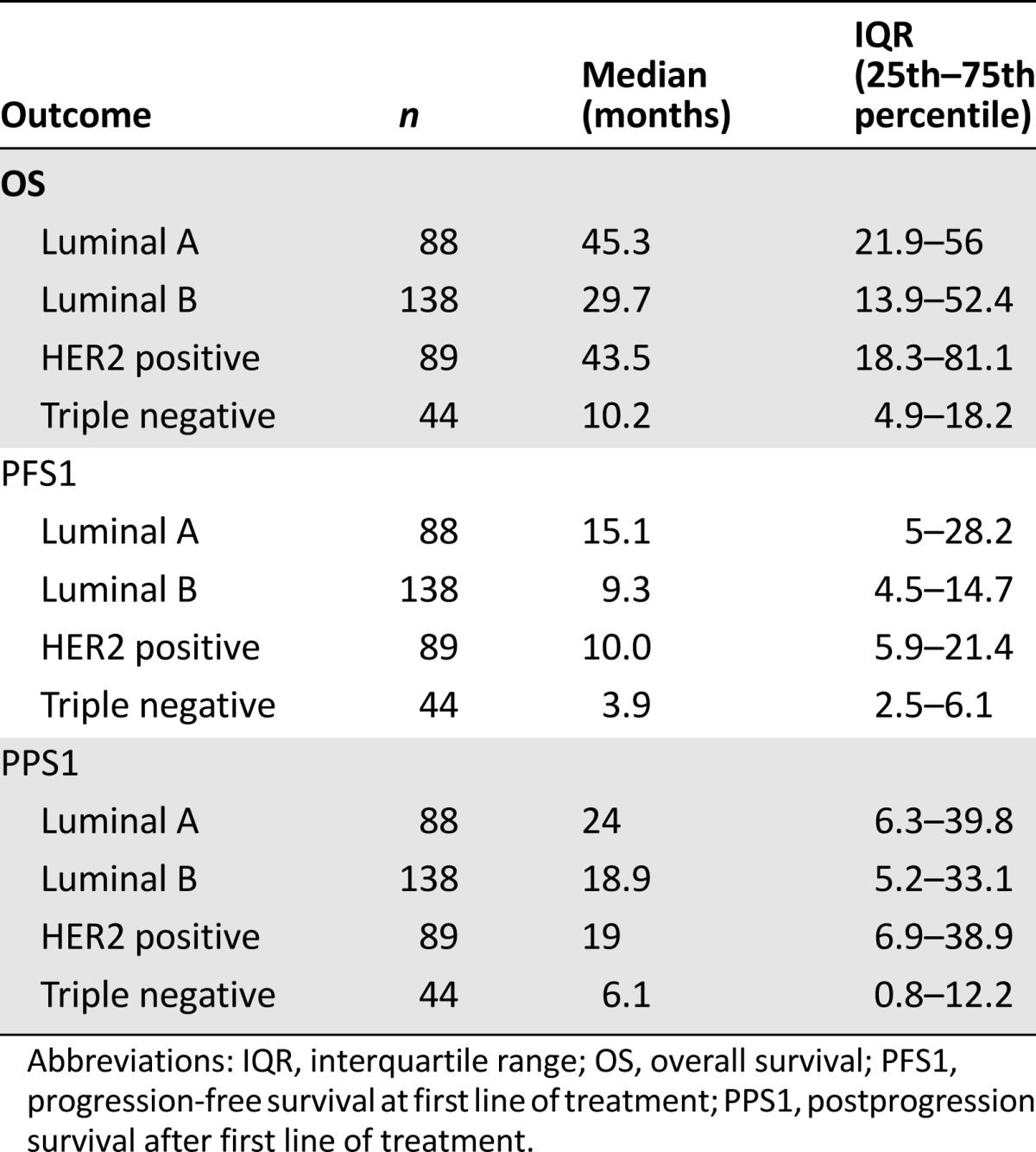
Figure 1.
Comparison of OS, PFS at first line of treatment, and PPS after the first line of treatment between different immunophenotypes.
Abbreviations: OS, overall survival; PFS, progression-free survival; PPS, postprogression survival.
Figure 2.
HER2+ population, comparison between hormone receptor-positive and -negative subgroups.
Abbreviations: OS, overall survival; PFS, progression-free survival.
Multivariate analysis was performed to test the independent association between variables and measures of outcome overall and for each line of treatment (Table 5). Better prognosis in terms of OS was observed in patients with ER-positive and HER2-positive disease. In contrast, having pulmonary or hepatic localizations at diagnosis of metastatic disease was associated with an unfavorable outcome.
Table 5.
Multivariate Cox proportional hazards regression model
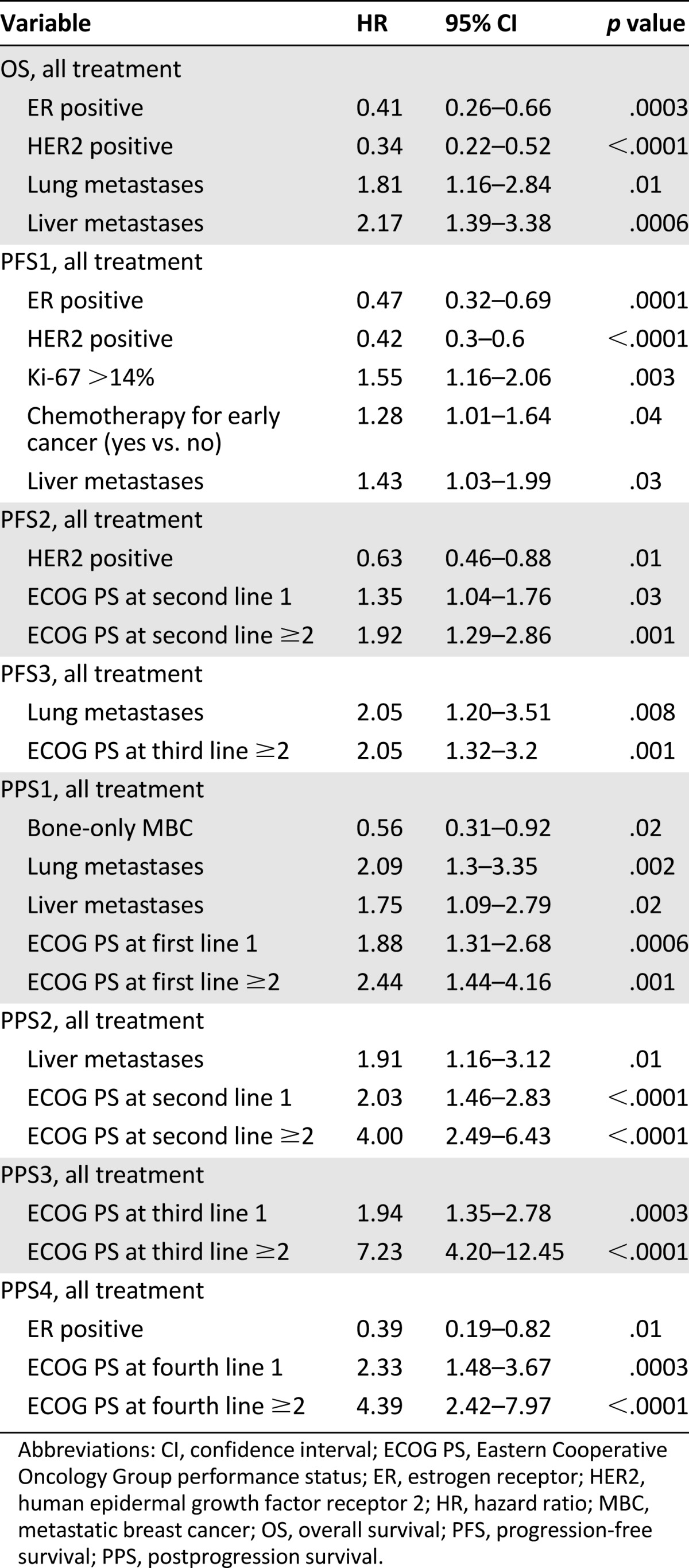
HER2-positive disease was associated with longer PFS1_tot (HR: 0.42 [95% CI: 0.3–0.6], p < .0001) and PFS2_tot (HR: 0.63 [95% CI: 0.46–0.88], p = .01). Bone-only disease was associated with longer PPS1_tot (HR: 0.56 [95% CI: 0.31–0.92], p = .02), whereas liver metastasis (HR: 1.75 [95% CI: 1.09–2.79], p = .02) and lung metastasis (HR: 2.09 [95% CI: 1.3–3.35], p = .002) at the diagnosis of metastatic disease had an unfavorable impact on PPS1_tot. Worse performance status (ECOG ≥2) remained a significant unfavorable prognostic factor in terms of PFS2_tot (HR: 1.92 [95% CI: 1.29–2.86], p = .001) and PFS3_tot (HR: 2.05 [95% CI: 1.32–3.2], p = .001). It was also independently associated with PPS1_tot (HR: 2.44 [95% CI: 1.44–4.16], p = .001), and it maintained the association with PPS after subsequent therapeutic lines.
Regression analysis was conducted to evaluate the prognostic value of different immunophenotypes (the luminal A phenotype was used as a reference). On multivariate analysis, the triple-negative phenotype conferred a significantly poorer outcome in terms of OS_tot (HR: 3.1 [95% CI: 1.9–5.04], p = .0001), OS_CT (HR: 1.8 [95% CI: 1.04–3.16], p = .0353), PFS1_tot (HR: 3.6 [95% CI: 2.35–5.48], p < .0001), PFS2_tot (HR: 2.77 [95% CI: 1.68–4.57], p < .0001), PFS1_CT (HR: 2.17 [95% CI: 1.36–3.46], p = .0011), and PPS1_tot (HR: 2.09 [95% CI: 1.2–3.65], p = .009). HER2-positive disease was associated with better prognosis in terms of OS_tot (HR: 0.65 [95% CI: 0.42–0.99], p = .0488), OS_CT (HR: 0.51 [95% CI: 0.31–0.83], p = .0065), and PFS1_ET (HR: 0.48 [95% CI: 0.3–0.79], p = .0035). The luminal B subgroup showed an unfavorable outcome in terms of PFS1_tot (HR: 1.41 [95% CI: 1.03–1.93], p = .0312) and PFS1_ET (HR: 1.52 [95% CI: 1.1–2.09], p = .0109).
Discussion
There is a growing interest in the role and interpretation of the main measures of outcome used as endpoints in clinical cancer research. Traditionally, OS has been considered the most objective measure of efficacy in trials that test the value of anticancer agents. PFS has been proposed as a potential surrogate for OS that could allow faster evaluation of a new drug. Pros and cons have been raised about the use of PFS and OS [19, 29–31], the latter being considered the most objective; however, the use of OS as a measure of efficacy could be confounded by treatment beyond the first line. In fact, in clinical trials, the probability of pointing out differences in OS between two or more treatment arms is inversely related to the duration of PPS, a recently introduced measure of outcome that may offer insights into the interpretation of study results [22].
The recent literature has several examples of clinical trials in which, although a PFS benefit was observed, no improvement in OS was documented. In such cases, a long median PPS may explain the lack of statistical difference in OS [32]. This is a potential confounding factor in interpreting the results of first-line trials and could be occurred in studies that tested the combination of chemotherapy with the antiangiogenic agent bevacizumab [7–9]. In contrast, the EMBRACE study, which analyzed the role of eribulin in heavily treated patients with MBC, had more chances to observe benefit for OS because of the short PPS [33].
In patients with MBC, outcome is dependent on the presence of several prognostic factors and benefit of treatment, with the latter ultimately related to predictive factors. Because earlier clinical trials were conducted in heterogeneous populations that were not differentiated for prognostic and predictive factors, information about estimate of outcome is not reliably transferable to real clinical practice [34].
The present study investigated the different measures of outcome (OS, PFS across subsequent lines of therapy, PPS) in a real-world scenario, including both patients with MBC enrolled in clinical trials and patients with MBC treated in the context of routine clinical practice.
Interestingly, median OS was 34 months for the whole population, suggesting that prognosis of patients with MBC is better than expected based on the results of the earliest clinical trials testing chemotherapy agents [6]. The observation of incremental improvement of survival over time has been documented by several analyses of retrospective series [35–37]. Although only inferred, the advances in OS documented in more recent years have been attributed to the introduction of active anticancer agents administered sequentially across different lines of treatment [38].
In the present series, the highest median OS has been observed for patients with luminal A or HER2-positive phenotype confirming the best prognosis for these subgroups, with the outcome of the HER2-positive group being favorably influenced by anti-HER2 therapy.
The present analysis also confirmed the observation that median PFS usually decreases across subsequent lines. This clinical finding probably reflects the occurrence of resistance to anticancer agents but also could be related to clinical conditions that may preclude optimal access to therapy (e.g., deterioration of performance status, cumulative side effects of therapy). The observation that, in HER2-positive disease, the therapeutic benefit is maintained longer than in the other subgroups supports the role of anti-HER2 agents beyond first-line treatment.
Interestingly, similar results were recently reported by Seah et al., who analyzed a series of 199 consecutive patients with MBC at the Dana-Farber Cancer Institute in Boston, Massachusetts [34]. The authors observed that tumor subtypes were differently associated with number of therapeutic lines, duration of chemotherapy, and OS. Patients with HER2-positive disease experienced the longest duration of chemotherapy and received the highest number of lines. In contrast, in patients with triple-negative BC, the duration of treatment was always the shortest, regardless of the line examined.
The analysis of PPS in a real-world scenario showed interesting findings. From a methodological point of view, it is important to note that although PPS can be defined simply as the difference between OS and PFS (i.e., PPS = OS − PFS), it is a time-to-event measure. Interestingly, the PPS value observed in our series has been evaluated by using standard methods of survival analysis. After the first and second lines of treatment, PPS was 18.3 months and 12.2 months, respectively. In both cases, the figure is over the 12-month threshold that could decrease the chance to point out OS differences in the context of a hypothetical clinical trial [22]. Accordingly, in similar scenarios, PFS should be favored over OS as a primary endpoint in trials testing anticancer agents in first- or second-line setting.
On the other hand, PPS after first line treatment was particularly short in patients with the absence of ER, PR and HER2 receptors, suggesting that a clinical trial conducted on a population selected for a triple-negative phenotype could adopt OS as a valid primary endpoint.
Although this study is affected by the limits that are typical of a retrospective design (e.g., heterogeneity in the method and intervals of disease assessment, variation in the choice of treatment lines over time), it analyzed a consecutive series of patients with MBC treated in a real-world scenario. In addition, a detailed review of the electronic health records guaranteed the accuracy of information about clinical and pathological findings. Results confirmed the prognostic role of specific disease and patient characteristics and were in line with data in the literature, suggesting that outcome is largely dependent on immunophenotype as defined in a sample of the primary tumor [39]. Furthermore, the study analyzed outcome through different measures and across sequential lines of treatment.
Conclusion
The observation of distinct values for PFS and PPS according to disease and patient variables underscores the importance of defining endpoints for clinical trials on the basis of characteristics of the study population. In other words, in the era of tailored treatment, the new concept of “tailored endpoints” should be taken into account when designing clinical trials. For this purpose, data acquired from consecutive series of patients treated in clinical practice may represent an added value to the development of novel anticancer drugs.
Acknowledgments
This work and the paper were supported by the Monica Boscolo research grant. M.B. and L.G. contributed equally to this work.
Author Contributions
Conception/Design: Fabio Puglisi, Marta Bonotto, Lorenzo Gerratana
Provision of study material or patients: Fabio Puglisi, Marta Bonotto, Lorenzo Gerratana, Elena Poletto, Pamela Driol, Manuela Giangreco, Stefania Russo, Alessandro M. Minisini, Claudia Andreetta, Mauro Mansutti, Federica E. Pisa, Gianpiero Fasola
Collection and/or assembly of data: Marta Bonotto, Lorenzo Gerratana, Elena Poletto, Pamela Driol
Data analysis and interpretation: Fabio Puglisi, Marta Bonotto, Lorenzo Gerratana, Manuela Giangreco, Federica E. Pisa
Manuscript writing: Fabio Puglisi, Marta Bonotto, Lorenzo Gerratana, Alessandro M. Minisini
Final approval of manuscript: Fabio Puglisi, Marta Bonotto, Lorenzo Gerratana, Elena Poletto, Pamela Driol, Manuela Giangreco, Stefania Russo, Alessandro M. Minisini, Claudia Andreetta, Mauro Mansutti, Federica E. Pisa, Gianpiero Fasola
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. The Oncologist. 2005;10(suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg PA, Hortobagyi GN, Smith TL, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol. 1996;14:2197–2205. doi: 10.1200/JCO.1996.14.8.2197. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Clemons M. First-line treatment options for patients with HER-2 negative metastatic breast cancer: The impact of modern adjuvant chemotherapy. The Oncologist. 2007;12:785–797. doi: 10.1634/theoncologist.12-7-785. [DOI] [PubMed] [Google Scholar]
- 6.Kiely BE, Soon YY, Tattersall MHN, et al. How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: A systematic review of recent randomized trials. J Clin Oncol. 2011;29:456–463. doi: 10.1200/JCO.2010.30.2174. [DOI] [PubMed] [Google Scholar]
- 7.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 8.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 9.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 10.Baselga J, Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swain SM, Kim S-B, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardoso F, Harbeck N, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii11–vii19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 14.Bast RC, Jr, Hortobagyi GN. Individualized care for patients with cancer - a work in progress. N Engl J Med. 2004;351:2865–2867. doi: 10.1056/NEJMe048300. [DOI] [PubMed] [Google Scholar]
- 15.Vriens BEPJ, Lobbezoo DJA, de Hoon JPJ, et al. If there is no overall survival benefit in metastatic breast cancer: Does it imply lack of efficacy? Taxanes as an example. Cancer Treat Rev. 2013;39:189–198. doi: 10.1016/j.ctrv.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Pazdur R. Endpoints for assessing drug activity in clinical trials. The Oncologist. 2008;13(suppl 2):19–21. doi: 10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 17.Buyse M, Sargent DJ, Saad ED. Survival is not a good outcome for randomized trials with effective subsequent therapies. J Clin Oncol. 2011;29:4719–4720; author reply 4720–4721. doi: 10.1200/JCO.2011.38.4206. [DOI] [PubMed] [Google Scholar]
- 18.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26:1987–1992. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, McLeod D, Batist G, et al. In the end what matters most? A review of clinical endpoints in advanced breast cancer. The Oncologist. 2011;16:25–35. doi: 10.1634/theoncologist.2010-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 21.Miksad RA, Zietemann V, Gothe R, et al. Progression-free survival as a surrogate endpoint in advanced breast cancer. Int J Technol Assess Health Care. 2008;24:371–383. doi: 10.1017/S0266462308080495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 24.Rakha EA, Ellis IO. Modern classification of breast cancer: Should we stick with morphology or convert to molecular profile characteristics. Adv Anat Pathol. 2011;18:255–267. doi: 10.1097/PAP.0b013e318220f5d1. [DOI] [PubMed] [Google Scholar]
- 25.Harbeck N, Rody A. Lost in translation? Estrogen receptor status and endocrine responsiveness in breast cancer. J Clin Oncol. 2012;30:686–689. doi: 10.1200/JCO.2011.38.9619. [DOI] [PubMed] [Google Scholar]
- 26.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niikura N, Liu J, Hayashi N, et al. Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: A single-institution retrospective analysis. The Oncologist. 2011;16:155–164. doi: 10.1634/theoncologist.2010-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 29.Booth CM, Eisenhauer EA. Progression-free survival: Meaningful or simply measurable? J Clin Oncol. 2012;30:1030–1033. doi: 10.1200/JCO.2011.38.7571. [DOI] [PubMed] [Google Scholar]
- 30.Oxnard GR, Morris MJ, Hodi FS, et al. When progressive disease does not mean treatment failure: Reconsidering the criteria for progression. J Natl Cancer Inst. 2012;104:1534–1541. doi: 10.1093/jnci/djs353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dancey JE, Dodd LE, Ford R, et al. Recommendations for the assessment of progression in randomised cancer treatment trials. Eur J Cancer. 2009;45:281–289. doi: 10.1016/j.ejca.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Cortés J, Calvo E, González-Martín A, et al. Progress against solid tumors in danger: The metastatic breast cancer example. J Clin Oncol. 2012;30:3444–3447. doi: 10.1200/JCO.2012.41.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 34.Seah DSE, Luis IV, Macrae E, et al. Use and duration of chemotherapy in patients with metastatic breast cancer according to tumor subtype and line of therapy. J Natl Compr Canc Netw. 2014;12:71–80. doi: 10.6004/jnccn.2014.0008. [DOI] [PubMed] [Google Scholar]
- 35.Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer. 2004;100:44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 36.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawood S, Haaland B, Albarracin CT, et al. Is the proportion of patients with synchronous stage IV breast cancer surviving >2 years increasing over time? J Clin Oncol. 2013;31(suppl):524a. doi: 10.1159/000371746. [DOI] [PubMed] [Google Scholar]
- 38.Mauri D, Polyzos NP, Salanti G, et al. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008;100:1780–1791. doi: 10.1093/jnci/djn414. [DOI] [PubMed] [Google Scholar]
- 39.Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]