A rapid surge of young-female breast cancer (YFBC) has been observed in Taiwan and other East Asian countries. This study showed that emerging YFBC in Taiwan is uniquely associated with favorable pathological features and better outcomes and should not be regarded as the mirror image of its Western counterpart.
Keywords: Breast cancer, Estrogen, Young, Asia
Abstract
Background.
A rapid surge of young-female breast cancer (YFBC) has been observed in Taiwan and other East Asian countries. We recently reported that these cases of YFBC, in contrast to their Western counterparts, are predominantly luminal A subtype. YFBC in Asia may have distinct clinicopathological features and outcomes.
Methods.
Data collected prospectively by participating hospitals were retrieved from the Taiwan Cancer Database. A total of 15,881 women with newly diagnosed stage I–III breast cancer in 2002–2006 were included. The age at diagnosis was categorized into nine 5-year groups (from <30 years to ≥65 years). Clinicopathological variables and patient disease-free survival (DFS) were compared by age group.
Results.
The rates of stage I, estrogen receptor-positive (ER+), and progesterone receptor-positive breast cancer were higher in the younger patients (<50 years) than in the older patients (≥50 years). Univariate analysis showed that the 40–44 and 45–49 age groups were significantly associated with longer DFS than the other age groups. In the ER+ subgroup, multivariate analysis consistently showed that the 40–44 age group was significantly associated with longer DFS than the other age groups except for the 45–49 age group. In contrast, multivariate analysis of the ER-negative subgroup revealed no significant difference of DFS between the 40–44 age group and other age groups.
Conclusion.
Emerging YFBC in Taiwan is uniquely associated with favorable pathological features and better outcomes and should not be regarded as the mirror image of its Western counterpart.
Abstract
摘要
背景. 在台湾地区以及东亚其他地区已发现年轻女性乳腺癌(YFBC)迅速飙升。我们最近报告了与西方国家相比,东亚YFBC病例主要为luminal A亚型。亚洲YFBC可能具有独特的临床病理学特征以及疾病转归。
方法. 在台湾地区癌症数据库中进行检索,参与研究的医院前瞻性收集数据。共入组15 881例2002∼2006年新诊断的I∼III期乳腺癌女性。 确诊时年龄分成9组,每5岁为1个年龄组(<30岁∼ ≥65岁)。比较各年龄组的临床病理学变量与患者无疾病生存期(DFS)。
结果. 年轻患者(< 50岁)中I期、雌激素受体阳性(ER+)、孕激素受体阳性乳腺癌发生率高于年老患者(≥ 50岁)。单变量分析显示,40 ∼ 44岁与45 ∼ 49岁年龄组DFS显著长于其他年龄组。对于ER+亚型,多变量分析一致显示,除外45 ∼ 49岁年龄组,40 ∼ 44岁年龄组DFS显著长于其他年龄组。相反,对ER阴性亚型的多变量分析表明,40 ∼ 44岁年龄组DFS与其他年龄组之间差异无统计学意义。
结论. 台湾地区出现的YFBC病例具有良好的病理学特征以及更好的转归,不应与西方病例等同视之。The Oncologist 2014;19:583–591
Implications for Practice:
The emerging YFBCs in Taiwan are predominantly estrogen receptor-positive (ER+) and young patients (aged 40–49 years) with ER+ breast cancer are uniquely associated with better outcome. The findings imply that estrogen–related environmental changes play an important role in the carcinogenesis of YFBC in Taiwan and other East Asian countries. For clinical practice, our study suggests that clinicians may need to perform an adjustment when using Adjuvant! Online (www.adjuvantonline.com) to evaluate the relapse and mortality risks for young patients with ER+ breast cancer in East Asia.
Introduction
The incidence of invasive female breast cancer has been rapidly increasing in East Asian countries, including Singapore, Korea, Japan, and Taiwan [1–4]. Previous age-cohort analyses have revealed a much stronger birth-cohort effect on the incidence of breast cancer for Singaporean [1], Japanese [3], and Taiwanese [4] women compared with white American women, and this stronger birth-cohort effect correlated directly with a dramatic increase in the incidence of young-female breast cancer (YFBC) in these countries. Environmental changes are likely to have a substantial impact on this increased incidence, and the major environmental differences between the younger and older generations in these countries seems to be the increasing westernization of their lifestyles. Consequently, a westernized lifestyle is thought to be the major reason behind this trend and might be considered to suggest that emerging YFBC in East Asia mirrors the situation in Western countries [5].
In Western countries, breast cancer in young women is characterized by higher frequencies of estrogen receptor-negative (ER–), high-grade, and basal subtype and is associated with a poor prognosis [6–8]. However, our previous single-center study showed that young (aged <50 years) breast cancer patients in Taiwan are characterized by a high prevalence of the luminal A subtype (defined as estrogen receptor-positive [ER+] and/or progesterone receptor-positive [PR+]/human epidermal growth factor receptor type 2 [HER2] negative) and a low prevalence of the basal-like subtype [9]. This suggests that YFBC in Taiwan and other East Asian countries may be a new disease entity. We used a nationwide registry database to validate the unique clinicopathological features of YFBC in Taiwan and investigated patient outcomes.
Patients and Methods
Sources of Data
We obtained clinicopathological data and relapse and/or survival data of cases of newly diagnosed female breast cancer from computerized national Taiwanese population registries, including the Taiwan Cancer Database (TCDB) and the National Death Certification System, for analysis. The TCDB was initiated in 2002 by the Bureau of Health Promotion, Department of Health, Taiwan. The original data were prospectively collected by a breast cancer treatment committee at each of the 32 participating hospitals, covering 71.7% of all cases of invasive breast cancer in Taiwan between 2002 and 2006.
Disease status was followed up in all of the newly diagnosed breast cancer patients by means of computerized data linkage with national health profiles on the national cancer registry and death certification systems in Taiwan from January 2002 through December 2011. The study data were approved for release by the data release review board of the Bureau of Health Promotion, and the study protocol was approved by the research ethics committee of the College of Public Health, National Taiwan University.
Selection of the Study Subjects
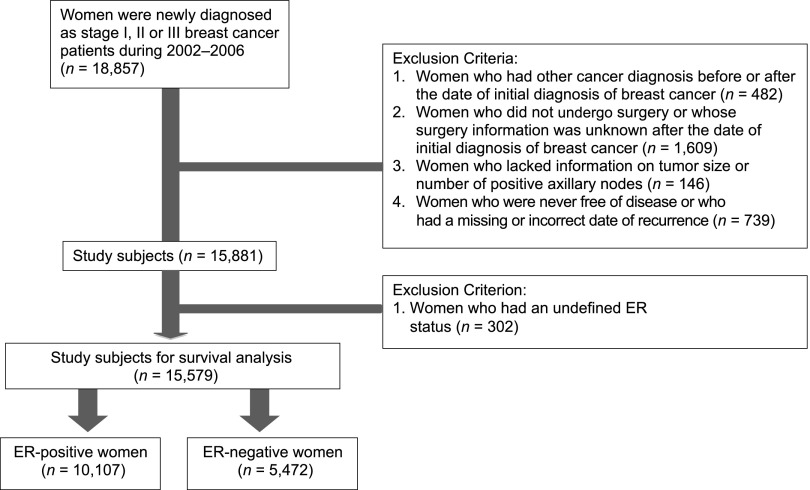
The TCDB database included 18,857 newly diagnosed patients from 2002 to 2006 with stage I, II, or III breast cancer according to the criteria of the American Joint Committee on Cancer (AJCC). We excluded 482 patients who were diagnosed with other cancers 2.5 years before or after the diagnosis of breast cancer, 1,609 patients who did not undergo curative surgery or who lacked information on the type of surgery, 146 patients who lacked information on tumor size or number of positive axillary lymph nodes, and 739 patients who were never free of disease or who had missing dates with regard to recurrence. A total of 15,881 patients were eligible for analysis for the association of age with clinicopathological features. To analyze patient outcomes, we further excluded 302 patients whose ER status was unknown. Finally, a total of 15,579 patients, including 10,107 with ER+ breast cancers and 5,472 with ER– breast cancers were included for analysis (Fig. 1).
Figure 1.
Process of selecting the study subjects.
Outcome Measurements and Variable Definitions
The outcome endpoint of this study was disease-free survival (DFS), which was defined as the duration from diagnosis to confirmation of locoregional or distant disease recurrence, contralateral new primary breast cancer, or date of death from breast cancer. The main factors of interest were age at diagnosis and ER status. The other controlled variables included in the model were AJCC stage, tumor size, lymph nodes, histological grade, PR, neoadjuvant and/or adjuvant chemotherapy, and hormonal therapy.
Statistical Analysis
The age at diagnosis was categorized into nine 5-year groups (from <30 years to ≥65 years). For further comparisons, we recategorized the age groups into four 10-year groups (≤39, 40–49, 50–59, and ≥60 years). Data on clinicopathological features among the age groups were compared by the chi-square test. The “linear-by-linear” association test was used to analyze the association of age groups with AJCC stage and histological grade. In survival analysis, the cumulative hazard by follow-up year was derived by the Kaplan-Meier method and compared by the log-rank test. Cox proportional hazard regression models were used to estimate the multivariate-adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the risk predictors of progression to the clinical outcomes of interest. Statistical significance levels were determined by two-tailed tests (p < .05). All analyses were performed using the SAS statistical software package (version 9.2; SAS Institute, Cary, NC, http://www.sas.com).
Results
Clinicopathological Characteristics by Age Groups
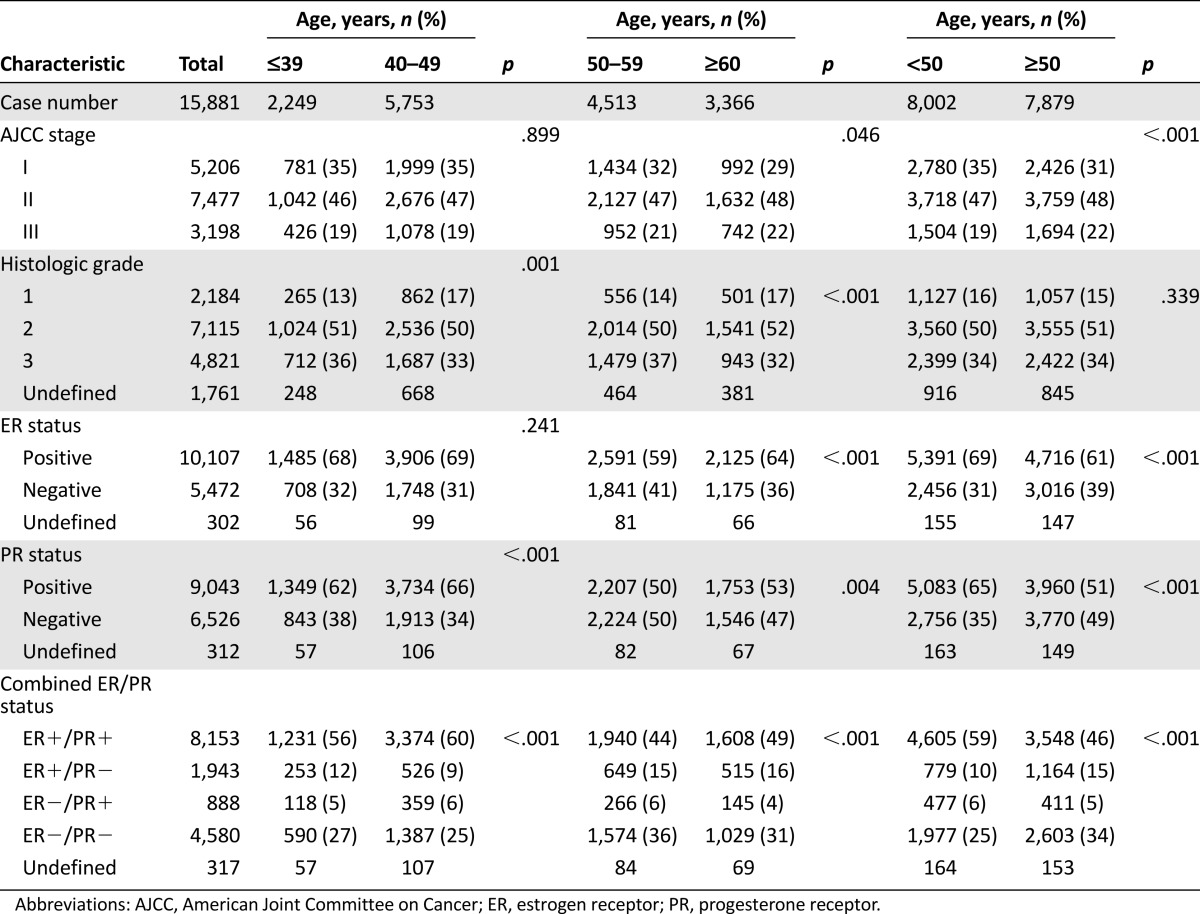
The demographic and clinical characteristics of the study population are shown in Table 1. The median age of the 15,881 patients was 49.4 years (range: 14.7–101.7 years), of whom 14.2% were aged ≤39 years and 50.4% were aged ≥49 years. Disease stages included 32.8% stage I disease, 47.1% stage II disease, and 20.1% stage III disease by AJCC staging. Histological grades included 15.5% grade 1, 50.4% grade 2, and 34.1% grade 3. Overall, 64.9% of the tumors were ER+ and 58.1% PR+. The clinicopathological features among the nine 5-year age groups are shown in Table 1. The comparisons of clinicopathological features among the four 10-year age groups (≤39, 40–49, 50–59, and ≥60 years) are shown in Table 2. Compared with the patients aged ≥50 years, the younger patients (<50 years) were significantly associated with higher frequencies of AJCC stage I (35% vs. 31%, p < .001), ER positivity (69% vs. 61%, p < .001), PR positivity (65% vs. 51%, p < .001), and ER+/PR+ disease (59% vs. 46%, p < .001) but not histological grade. Compared with the tumors in the patients aged ≤39 years, the tumors in the patients aged 40–49 years were associated with a higher frequency of histologic grade 1 and PR+ but not with AJCC stage or ER expression. Compared with the tumors in the patients aged 50–59 years, the tumors in the patients aged ≥60 years were significantly associated with a higher frequency of AJCC stage II/III, grade 1, ER positivity, and PR positivity (Table 2). Interestingly, histological grade 1 tumors were more common in the patients aged 40–49 years and ≥60 years and grade III tumors were more common in the patients aged ≤39 years and 50–59 years.
Table 1.
Clinicopathological characteristics of the breast cancer patients by age group at ascertainment
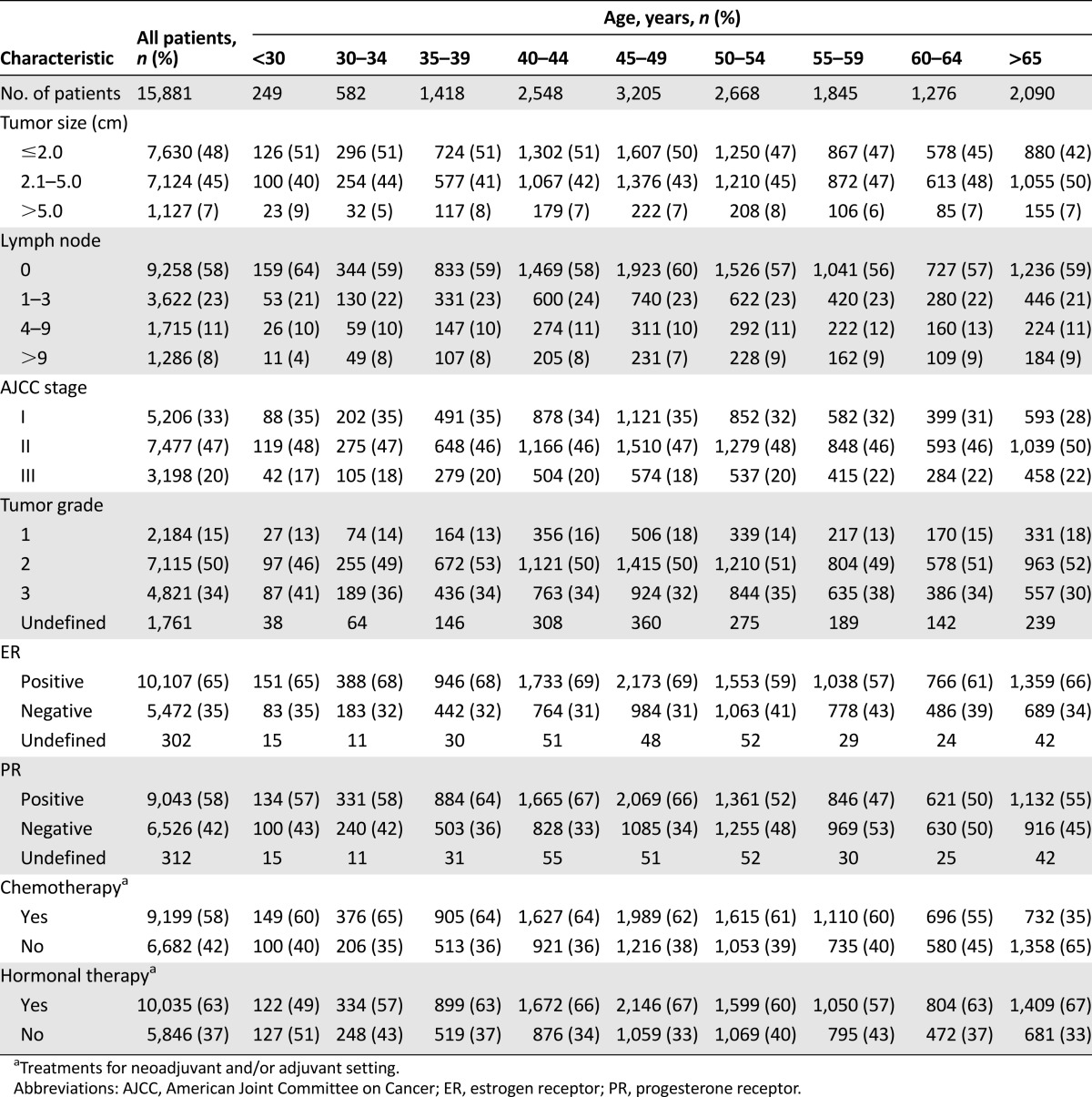
Table 2.
Comparison of clinicopathological features among the age groups
Analysis of Disease-Free Survival in the Whole Study Population
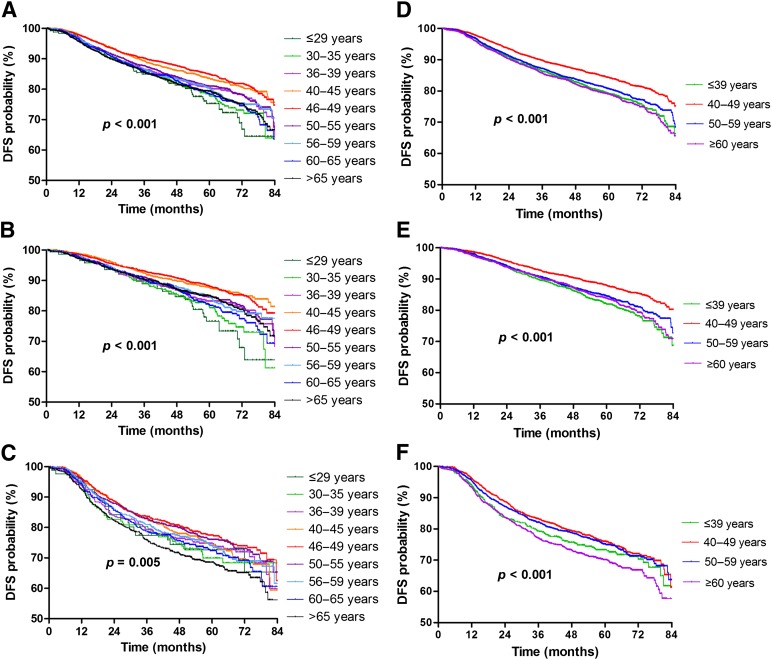
In univariate analysis of the whole population, larger tumor size, more positive axillary lymph nodes, and higher histological grade were significantly associated with shorter DFS. ER positivity, PR positivity, and the use of hormonal therapy were significantly associated with prolonged DFS. From the DFS curves of the whole population by the nine age groups (Fig. 2A), the patients aged 40–44 years and 45–49 years seemed to have a more favorable DFS than the other age groups. Consequently, we used the 40–44 age group as the reference control. Compared with the patients aged 40–44 years, univariate analysis showed that the patients aged ≤29, 30–34, 35–39, 50–54, 55–59, 60–64, and ≥65 years (crude HR, range: 1.17–1.54) were significantly associated with shorter DFS; however, the patients aged 45–59 years (crude HR: 0.94) did not have significantly different DFS (Table 3).
Figure 2.
DFS curves: whole population (A); patients with ER-positive tumors (B) and ER-negative tumors (C) by the nine age groups and in the whole population (D); patients with ER-positive tumors (E) and ER-negative tumors (F) by the four age groups (unadjusted analysis).
Abbreviation: DFS, disease-free survival.
Table 3.
Analysis of disease-free survival of the whole population
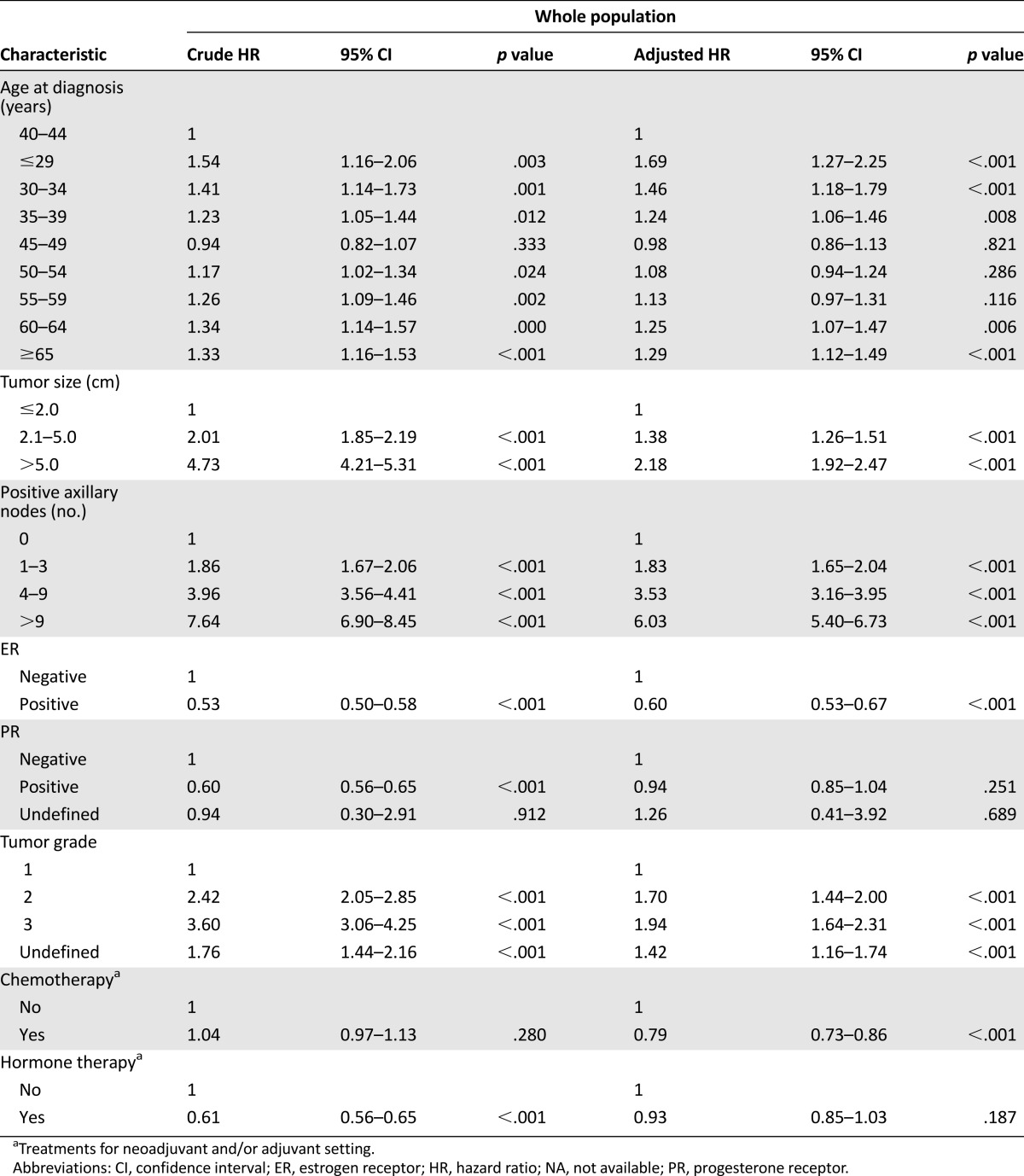
Multivariate analysis of the whole population showed that larger tumor size, more axillary lymph node involvement, higher histological grade, and ER negativity remained independent factors to predict unfavorable DFS. Compared with the patients aged 40–44 years, the patients aged ≤29, 30–34, 35–39, 60–64, and ≥65 years (adjusted HR, range: 1.24–1.69) were significantly associated with shorter DFS; however, there were no significant differences in DFS between the patients aged 45–59 years (adjusted HR: 0.98), 50–54 years (adjusted HR: 1.08), and 55–59 years (adjusted HR: 1.13) (Table 3).
Among the four 10-year age groups, compared with the patients aged 40–49 years, the patients aged ≤39, 50–59, and ≥60 years were significantly associated with shorter DFS in both univariate analysis (crude HR, range: 1.25–1.38) and multivariate analysis (adjusted HR, range: 1.11–1.36) (supplemental online Table 1; Fig. 2D).
Stratified Analysis of Disease-Free Survival by Estrogen Receptor Status
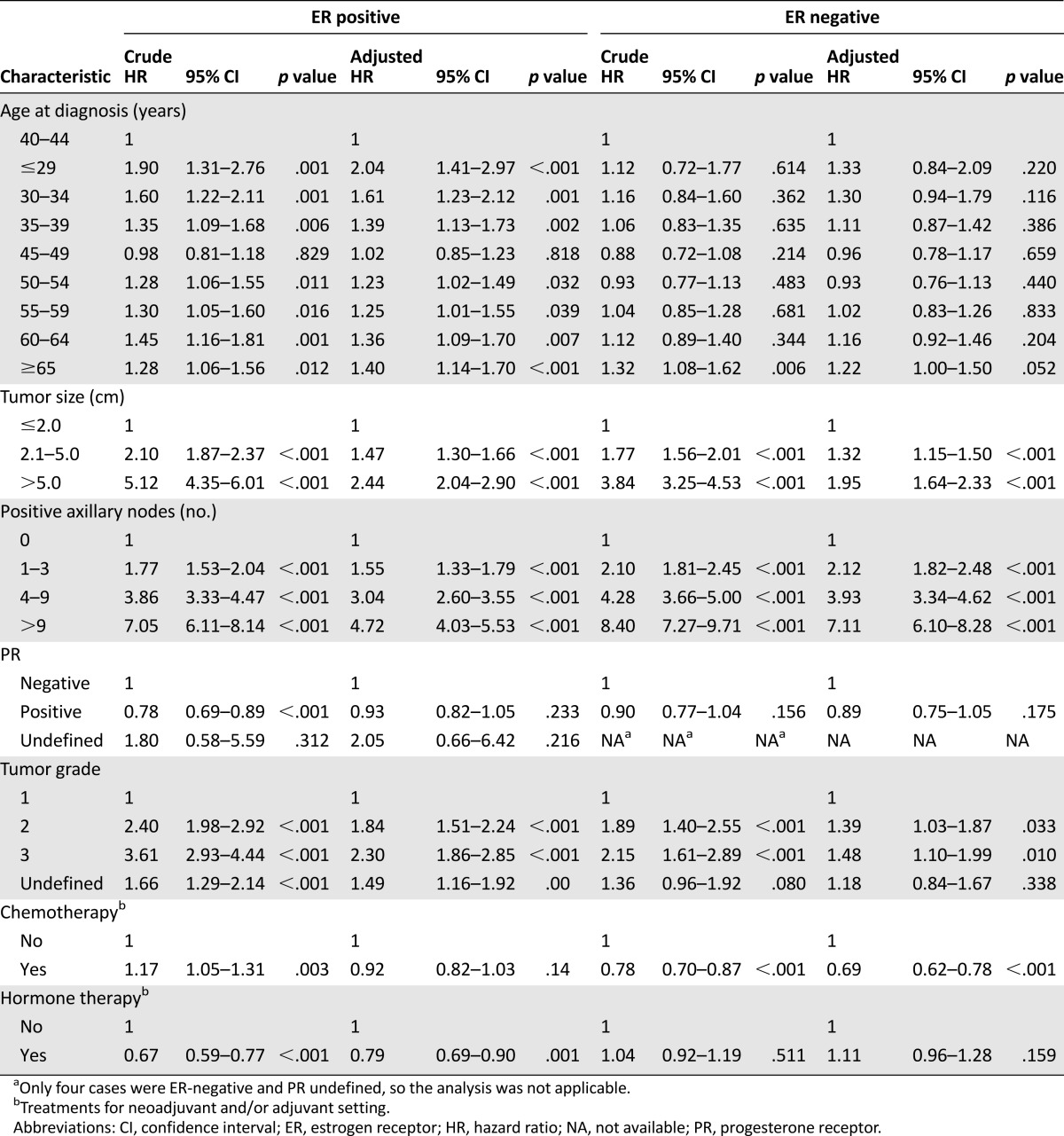
In the ER+ subgroup, compared with the patients aged 40–44 years, univariate analysis showed that the patients aged ≤29, 30–34, 35–39, 50–54, 55–59, 60–64, and ≥65 years (crude HR, range: 1.28–1.90) were significantly associated with shorter DFS; however, the patients aged 45–59 years did not have significantly different DFS (supplemental online Table 2, Fig. 2B). Multivariate analysis consistently showed that the patients aged ≤29, 30–34, 35–39, 50–54, 55–59, 60–64, and ≥65 years (adjusted HR, range: 1.23–2.04) were significantly associated with shorter DFS and that the patients aged 45–59 years did not have significantly different DFS (Table 3).
In the ER– subgroup, compared with the patients aged 40–44 years, univariate analysis showed that only the patients aged ≥65 years (crude HR: 1.32; p = .006) were significantly associated with shorter DFS and that the patients in the other age groups did not have significantly different DFS (supplemental online Table 2; Fig. 2C). Multivariate analysis consistently showed that only the patients aged ≥65 years (adjusted HR: 1.22; p = .052) had a trend toward DFS and that the patients in the other age groups did not have a significantly different DFS compared with the patients aged 40–44 years (Table 3).
Among the four 10-year age groups in the ER+ subgroup, compared with the patients aged 40–49 years, the patients aged ≤39, 50–59, and ≥60 years were significantly associated with shorter DFS in both univariate analysis (crude HR, range: 1.30–1.49) and multivariate analysis (adjusted HR, range: 1.22–1.50) (supplemental online Table 2; Fig. 2E). In the ER– subgroup, compared with the patients aged 40–49 years, only the patients aged ≥60 years (crude HR: 1.33) in univariate analysis and the patients aged ≤39 years (adjusted HR: 1.21) and ≥60 years (adjusted HR: 1.23) in multivariate analysis were significantly associated with shorter DFS (supplemental online Table 2; Fig. 2F).
Discussion
The unique association of YFBC in Taiwan with favorable pathological features and outcomes provides strong evidence that this population involves an emerging, distinct disease entity and should not be regarded as just a mirror image of its Western counterpart. The high hormonal receptor expression and the particularly favorable prognosis in the ER+ subgroup strongly imply that environmental changes related to endogenous estrogen synthesis or xenoestrogen exposure in women with certain genetic traits may contribute to YFBC carcinogenesis in Taiwan and in some other East Asian counties.
The higher frequencies of stage I, ER+, PR+, and ER+/PR+ tumors found in patients aged <50 years compared with patients ≥50 years have not been reported in other countries and are in contrast to previous reports from Western countries [10–12]. Data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) study in the U.S., for example, showed that the rates of ER and PR positivity increased with age among non-Hispanic whites [12]. Another recent study from the U.S. showed that tumors in young (age ≤45 years) breast cancer patients had significantly lower expressions of ER and PR mRNA and more high-grade histology than did tumors from older patients (aged ≥65 years) [7]. In our study, the tumors in patients aged 40–44 and 45–49 years had the highest frequencies of ER and PR positivity among the nine age groups examined and a high percentage of grade 1 histology. Even among very young patients, our study showed that patients aged <35 years had significantly higher frequencies of tumor size ≤2 cm (51% vs. 45%, p = .003), AJCC stage I disease (35% vs. 31%, p = .02), ER positivity (67% vs. 61%, p < .001), and PR positivity (58% vs. 51%, p < .001) and a marginally higher frequency of negative lymph nodes (61% vs. 57%, p = .09) (Table 1). These findings contrast with those of previous reports, which consistently describe breast tumors among younger women aged <30 or <35 years as exhibiting more aggressive features, including larger tumor sizes, positive lymph nodes, higher frequencies of poorly differentiated tumors, and the absence of hormone receptors [10, 11].
In terms of patient outcomes, the relapse risk among patients aged 40–49 years has not been reported to differ significantly from that in patients aged 50–59 years in Western countries [13–16]. However, in the current study, patients aged 40–44 and 45–49 years had significantly more favorable DFS in univariate analysis compared with patients in the other age groups. Although the difference compared with patients aged 50–54 and 55–59 years became insignificant after adjusting for other clinicopathological and treatment factors (Table 3), the multivariate stratified analysis of the ER+ subgroup consistently showed that patients aged 40–44 and 45–49 years had significantly more favorable DFS compared with patients in the other age groups. The difference in the ER– subgroup between patients aged 40–44 years and patients in the other age groups was not significant in multivariate analysis (Table 4, supplemental online Table 2). Because the emerging cases of YFBC in Taiwan are predominantly ER+, the unique association of favorable outcomes in patients aged 40–49 years with ER+ breast cancer provides further evidence that this emerging population may have a biology distinct from their Western counterparts.
Table 4.
Stratified analysis of disease-free survival by estrogen receptor status
The limitations of the present study include the lack of HER2 status and the relatively short follow-up duration. Although a prior study showed marginally higher HER2 overexpression by immunohistochemistry in patients aged ≤45 years than in patients aged ≥65 years [7], other studies have consistently shown no significant association of HER2 overexpression or amplification with patient age [17–19]. Our previous study, which analyzed the HER2 status of 1,028 breast cancer samples by immunohistochemistry and fluorescence in situ hybridization also did not show differences in HER2 overexpression or amplification based on patient age (<35 years vs. 35–50 years vs. >50 years, 20% vs. 20% vs. 21%; p = .848) [9]. Two phase III trials, published in 2005, showed that use of adjuvant trastuzumab significantly reduced disease recurrence [20, 21]. Because the present study enrolled patients diagnosed between 2002 and 2006, we conducted a sensitivity analysis to compare DFS between patients diagnosed in 2002–2004 and in 2005–2006. The analysis did not show a significant difference in DFS between these two periods for the whole population (crude HR: 0.99; p = .889), the ER+ subgroup (crude HR: 1.01; p = .898), or the ER– subgroup (crude HR: 1.04; p = .506) (data not shown). Consequently, the absence of HER2 status data is unlikely to affect our findings.
In the present study, the median follow-up period of 57.4 months was relatively short for survival analysis in breast cancer, thus we conservatively analyzed DFS instead of overall survival to avoid bias caused by an imbalanced performance status among the different age groups; there were a total of 2,739 events involving 17.6% of the study population. Furthermore, according to the survival curves (Fig. 2), the consistent separation of the curves between the 40–49 age group and the other age groups implied the robustness of our findings, although longer follow-up data are required to precisely predict the outcomes for Taiwanese patients and to make a direct comparison with the SEER database.
The high hormonal receptor expression and the particularly favorable prognosis in the ER+ subgroup of young patients imply that estrogen-related environmental changes play an important role in the carcinogenesis of YFBC in Taiwan. In addition to YFBC, we recently found a rapid increase in uterus and ovary endometrioid carcinomas among young women, and all of these tumor types showed a high prevalence of hormone receptor expression [22]. These findings suggest a dramatic increase in estrogen-related malignancies among young women in Taiwan. The discrepancy in the clinicopathological features and outcomes in YFBC patients between the East and West further suggests that unique environmental changes, beyond westernized lifestyle, are playing a major role in carcinogenesis. Regarding environmental factors, certain industrial environmental pollutants with estrogenic effects may be causative and deserve further investigation. The petrochemical industries, for example, have developed rapidly since the 1950s in some East Asian countries including Japan, Korea, and Taiwan [23]. Many compounds that have been widely used for the manufacture of plastic products are estrogenic, and people in these countries have been heavily exposed to a number of these chemicals. Previous studies revealed unexpectedly high exposures of Taiwanese people to phthalates and nonylphenol [24–26]. In addition, high concentrations of other estrogenic steroid pollutants in water and polycyclic aromatic hydrocarbons in air have been reported in Taiwan [27, 28].
Although environmental and lifestyle factors may play major roles, genetic factors with ethnic differences, particularly in genes involving estrogen synthesis or metabolism [29], may play a role in the carcinogenesis of YFBCs in East Asia; for example, genetic polymorphisms such as CYP1B1 [19] and COMT [30], which are related to estrogen metabolism, significantly differ between Asian and white people. Differences in these genetic traits may also explain the discrepancy of the clinicopathological features and outcomes in YFBC patients in the East and West; however, the interactions of environmental and genetic factors and their roles in carcinogenesis have not been systemically analyzed in this population. Determining the responsible gene-environment interactions is clearly warranted to stem the surge of these emerging malignancies in young women in East Asia.
Conclusion
The emerging YFBCs in Taiwan are uniquely associated with favorable pathological features. The high hormonal receptor expression and particularly favorable prognosis in the ER+ subgroup of young patients imply that estrogen–related environmental changes play an important role in the carcinogenesis of YFBC in Taiwan. The distinct pathological features and prognosis suggest that emerging YFBCs in Taiwan or other East Asian countries should not be regarded as the mirror image of its Western counterpart.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors thank the members of the Joint Breast Cancer Treatment Committee, Bureau of Health Promotion and Cancer Registry, and the Office of Taiwan Cancer Database for their support in the collection and assessment of the prospectively collected data. C.-H.L. and P.-Y.C. contributed equally to this work. S.-L.Y. and C.-H.T. contributed equally to this work. This study was supported by grants from the National Science Council, Taiwan (Grant NSC 99-2410-H-038-004), National Center of Excellence for Clinical Trial and Research, Taiwan (Grant DOH101-TD-B-001), and Bureau of Health Promotion, Department of Health, Executive Yuan, Taiwan.
Author Contributions
Conception/Design: Chao-Hsiun Tang, Ching-Hung Lin, Po-Ya Chuang, Yen-Shen Lu, Ann-Lii Cheng, Wen-Hun Kuo, Chiun-Sheng Huang, San-Lin You
Provision of study material or patients: Chao-Hsiun Tang, Ching-Hung Lin, Po-Ya Chuang, Chun-Ju Chiang, Mei-Shu Lai, San-Lin You
Collection and/or assembly of data: Chao-Hsiun Tang, Ching-Hung Lin, Po-Ya Chuang, Chun-Ju Chiang, Mei-Shu Lai, San-Lin You
Data analysis and interpretation: Chao-Hsiun Tang, Ching-Hung Lin, Po-Ya Chuang, Chun-Ju Chiang, Yen-Shen Lu, Ann-Lii Cheng, Wen-Hun Kuo, Chiun-Sheng Huang, Mei-Shu Lai, San-Lin You
Manuscript writing: Chao-Hsiun Tang, Ching-Hung Lin, Po-Ya Chuang, Chun-Ju Chiang, Yen-Shen Lu, Ann-Lii Cheng, Wen-Hun Kuo, Chiun-Sheng Huang, San-Lin You
Final approval of manuscript: Chao-Hsiun Tang, Ching-Hung Lin, Po-Ya Chuang, Chun-Ju Chiang, Yen-Shen Lu, Ann-Lii Cheng, Wen-Hun Kuo, Chiun-Sheng Huang, Mei-Shu Lai, San-Lin You
Disclosures
The authors indicated no financial relationships.
References
- 1.Sim X, Ali RA, Wedren S, et al. Ethnic differences in the time trend of female breast cancer incidence: Singapore, 1968-2002. BMC Cancer. 2006;6:261. doi: 10.1186/1471-2407-6-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo KY, Kim Y, Park SK, et al. Lifestyle, genetic susceptibility and future trends of breast cancer in Korea. Asian Pac J Cancer Prev. 2006;7:679–682. [PubMed] [Google Scholar]
- 3.Matsuno RK, Anderson WF, Yamamoto S, et al. Early- and late-onset breast cancer types among women in the United States and Japan. Cancer Epidemiol Biomarkers Prev. 2007;16:1437–1442. doi: 10.1158/1055-9965.EPI-07-0108. [DOI] [PubMed] [Google Scholar]
- 4.Shen YC, Chang CJ, Hsu C, et al. Significant difference in the trends of female breast cancer incidence between Taiwanese and Caucasian Americans: Implications from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1986–1990. doi: 10.1158/1055-9965.EPI-04-0932. [DOI] [PubMed] [Google Scholar]
- 5.Porter P. “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358:213–216. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 6.Chung M, Chang HR, Bland KI, et al. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996;77:97–103. doi: 10.1002/(SICI)1097-0142(19960101)77:1<97::AID-CNCR16>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 8.Azim HA, Jr, Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18:1341–1351. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 9.Lin CH, Liau JY, Lu YS, et al. Molecular subtypes of breast cancer emerging in young women in Taiwan: Evidence for more than just westernization as a reason for the disease in Asia. Cancer Epidemiol Biomarkers Prev. 2009;18:1807–1814. doi: 10.1158/1055-9965.EPI-09-0096. [DOI] [PubMed] [Google Scholar]
- 10.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 11.Walker RA, Lees E, Webb MB, et al. Breast carcinomas occurring in young women (< 35 years) are different. Br J Cancer. 1996;74:1796–1800. doi: 10.1038/bjc.1996.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson WF, Chu KC, Chatterjee N, et al. Tumor variants by hormone receptor expression in white patients with node-negative breast cancer from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2001;19:18–27. doi: 10.1200/JCO.2001.19.1.18. [DOI] [PubMed] [Google Scholar]
- 13.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 14.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande AD, Jeffe DB, Gnerlich J, et al. Racial disparities in breast cancer survival: An analysis by age and stage. J Surg Res. 2009;153:105–113. doi: 10.1016/j.jss.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg J, Chia YL, Plevritis S. The effect of age, race, tumor size, tumor grade, and disease stage on invasive ductal breast cancer survival in the U.S. SEER database. Breast Cancer Res Treat. 2005;89:47–54. doi: 10.1007/s10549-004-1470-1. [DOI] [PubMed] [Google Scholar]
- 17.Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: Features of disease at presentation. Ann Oncol. 2002;13:273–279. doi: 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- 18.Bertheau P, Steinberg SM, Merino MJ. C-erbB-2, p53, and nm23 gene product expression in breast cancer in young women: Immunohistochemical analysis and clinicopathologic correlation. Hum Pathol. 1998;29:323–329. doi: 10.1016/s0046-8177(98)90111-3. [DOI] [PubMed] [Google Scholar]
- 19.Caldarella A, Crocetti E, Bianchi S, et al. Female breast cancer status according to ER, PR and HER2 expression: A population based analysis. Pathol Oncol Res. 2011;17:753–758. doi: 10.1007/s12253-011-9381-z. [DOI] [PubMed] [Google Scholar]
- 20.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 21.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 22.Lin CH, Chen YC, Chiang CJ, et al. The emerging epidemic of estrogen-related cancers in young women in a developing Asian country. Int J Cancer. 2012;130:2629–2637. doi: 10.1002/ijc.26249. [DOI] [PubMed] [Google Scholar]
- 23.Chu WW. Import substitution and export-led growth: A study of Taiwan’s petrochemical industry. World Dev. 1994;22:781–794. [Google Scholar]
- 24.Lu YY, Chen ML, Sung FC, et al. Daily intake of 4-nonylphenol in Taiwanese. Environ Int. 2007;33:903–910. doi: 10.1016/j.envint.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Chen ML, Chen JS, Tang CL, et al. The internal exposure of Taiwanese to phthalate—an evidence of intensive use of plastic materials. Environ Int. 2008;34:79–85. doi: 10.1016/j.envint.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Chen ML, Lee HY, Chuang HY, et al. Association between nonylphenol exposure and development of secondary sexual characteristics. Chemosphere. 2009;76:927–931. doi: 10.1016/j.chemosphere.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 27.Chen CY, Wen TY, Wang GS, et al. Determining estrogenic steroids in Taipei waters and removal in drinking water treatment using high-flow solid-phase extraction and liquid chromatography/tandem mass spectrometry. Sci Total Environ. 2007;378:352–365. doi: 10.1016/j.scitotenv.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 28.Fang GC, Wu YS, Fu PP, et al. Polycyclic aromatic hydrocarbons in the ambient air of suburban and industrial regions of central Taiwan. Chemosphere. 2004;54:443–452. doi: 10.1016/S0045-6535(03)00706-9. [DOI] [PubMed] [Google Scholar]
- 29.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 30.Huang CS, Lin CH, Lu YS, et al. Unique features of breast cancer in Asian women—breast cancer in Taiwan as an example. J Steroid Biochem Mol Biol. 2010;118:300–303. doi: 10.1016/j.jsbmb.2009.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.