Abstract
OBJECTIVE
MRI abnormalities have been described in patients with increased intracranial pressure (ICP), including in those with idiopathic intracranial hypertension (IIH). Spontaneous cerebral spinal fluid (CSF)-filled outpouchings of the dura (meningoceles), and secondary CSF leaks can occur from elevated ICP in patients with IIH, however, few studies have evaluated these findings. Our objective was to evaluate the frequency of spontaneous intracranial meningoceles among IIH patients and determine their association with visual outcome.
SUBJECTS AND METHODS
We performed a retrospective case-control study of consecutive IIH patients between 2000 and 2011 who underwent MRI including T2-weighted imaging. Demographics, presenting symptoms, CSF opening pressure, and visual outcome were collected for the first and last evaluations. Controls included patients without headache or visual complaints with normal brain MRIs. Stratified analysis was used to control for potential confounding by age, gender, race, and body mass index.
RESULTS
We included 79 IIH patients and 76 controls. Meningoceles were found in 11% of IIH patients versus 0% of controls (p<0.003). Prominent Meckel’s caves without frank meningoceles were found in 9% of IIH patients versus 0% of controls (p<0.003). Among IIH patients, the presence of meningocele or prominent Meckel’s caves was not associated with demographics, symptoms, degree of papilledema, CSF opening pressure, visual acuity, or visual field defect severity.
CONCLUSION
Meningoceles are significantly more common in IIH patients than in controls, and can be considered an additional imaging sign for IIH. Meningoceles are not, however, associated with decreased CSF opening pressure or better visual outcome in IIH.
Introduction
Meningoceles are protrusions of the meninges through points of weakness, usually in the skull base, and are typically categorized into congenital, iatrogenic (following a craniotomy or sinus surgery), and spontaneous [1]. Congenital meningoceles are the most common, whereas spontaneous meningoceles are quite rare [2]. Meningoceles are most commonly asymptomatic, but depending on location may rupture and produce cerebral spinal fluid (CSF) leak. Meningoceles with secondary CSF leak can present as rhinorrhea, otorrhea, intracranial hypotension, and recurrent bacterial meningitis [3]. Spontaneous, post-traumatic, and post-surgical CSF leaks in the spine have also been described [4]. Spontaneous meningoceles and associated CSF leak may develop secondary to increased intracranial pressure (ICP) [5], usually in the setting of idiopathic intracranial hypertension (IIH) [3]. Those patients with spontaneous CSF leak and IIH often do not have signs of intracranial hypertension until after CSF leak repair [6], suggesting that the CSF leak may act to decompress the elevated ICP. Recent studies have emphasized MRI abnormalities in patients with increased ICP [7], especially those with IIH, but few have mentioned meningoceles in IIH patients and their clinical significance has not been determined. Our objective was to evaluate the frequency of meningoceles among IIH patients and to determine their association with IIH severity and outcome. Our hypothesis is that given a potential route for decompression of elevated ICP, visual outcome would be better in IIH patients with meningoceles than those without.
Subjects and Methods
Patients and Controls
This was a retrospective case-control study, approved by our Institutional Review Board. The study group included all consecutive IIH patients evaluated by the neuro-ophthalmology service in our tertiary medical center between 2000 and 2011 who had magnetic resonance imaging (MRI) with T2-weighted images performed at our institution. IIH was confirmed if patients met the modified Dandy criteria (symptoms and signs of increased ICP including papilledema, elevated CSF opening pressure equal or greater than 25 cm of water with normal CSF contents, brain imaging ruling-out an intracranial process and venous thrombosis) [8]. Demographic characteristics (age at diagnosis, gender, race, and body mass index {BMI}), presenting symptoms and signs, CSF opening pressure, as well as visual outcome parameters (papilledema grade, visual acuity, legal blindness, visual field severity score, Humphrey visual field mean deviation, and the overall clinical course) were collected for the first and last evaluations. Blindness was defined as best corrected visual acuity of ≥1 logMAR (20/200) or visual field of ≤20 degrees in the better-seeing eye. Visual field severity score was graded by the degree of Humphrey (based on the grey scale) or Goldmann visual field depression, using a 1–4 scale as previously described [9]: 1 = normal visual fields; 2 = enlarged blind spot; 3 = either nasal or temporal defect (but not both); 4 = diffuse constriction (in all 4 quadrants). Mean deviation was calculated as the mean of the right eye and left eye mean-deviations from Humphrey visual fields (where applicable) at first and last examinations. All patients were divided into “good” and “poor” overall clinical courses based on review of the entire clinical course. “Poor” clinical course included fulminant disease [10], progressive visual field defects, or need for surgical intervention for IIH management. Patients with a “good” clinical course had none of the “poor” clinical features.
The control group included patients without headache or visual complaints, who had an MRI of the brain at our institution (including similar axial and coronal T2-weighted images) that were interpreted as normal. Indications for having brain MRI included sensory disturbances, peripheral neuropathies, seizures, and metastatic screening.
MRI Technique and Image Analysis
Magnetic resonance imaging was performed at either 3.0-Tesla (Siemens Trio, Erlangen, Germany) or 1.5-Tesla (Siemens Avanto, Erlangen, Germany or GE Signa, Milwaukie, Wisconsin) using a standard head coil. All patients underwent standardized brain MRI protocol including unenhanced axial DWI, T1-weighted, T2*-weighted, T2-weighted, and sagittal T1-weighted images. T2-weighed images obtained were at 0.7 × 0.7-mm in plane resolution at a slice thickness of 5-mm.
All imaging studies were reviewed by an experienced neuroradiologist (AMS; 5 years experience) in addition to two trained investigators (OYB and MPR) in consensus for the presence or absence of meningoceles and enlargement of Meckel’s cave. Image review was performed blinded to the diagnosis of IIH. Axial T2-weighted images were reviewed for prominent or increased fluid signal expanding Meckel’s cave but not distorting the contours (considered prominent Meckel’s cave), or a frank meningocele extending into the petrous apex, anterior skull base, or mastoid temporal bone. If such signal was discovered, coronal T2-weighted images were reviewed for confirmation of the presence of a meningocele. A meningocele was defined as bulging of dura matter, arachnoid and CSF into Meckel’s cave, below the level of cranial base bony margin, or presence of meninges and CSF signal in the nasal cavity, paranasal sinuses, or mastoid air cells.
Statistical Analysis
Statistical analysis was performed using the software R: A language and environment for statistical computing (R Foundation for Statistical Computing, http://www.R-project.org) version 2.15.1. Fisher Exact Test was used for bivariate categorical comparisons, and an asymptotic general independence test was used when introducing a stratification variable. Wilcoxon Rank sum test was used to compare continuous measures between cases and controls and between IIH patients with and without meningocele. P<0.05 was considered significant.
Results
Of 297 consecutive patients with definite IIH evaluated in our institution between 2000 and 2011, 79 had the required imaging studies performed at our institution and available for review. We included 76 controls. The study group and controls demographics are presented in table 1.
Table 1.
Demographics of study group (IIH patients) and of controls
| Demographics | IIH | Controls | p-value |
|---|---|---|---|
| Number | 79 | 76 | |
| Age at diagnosis in years, median (IQR) | 28 (24–36) | 33.4a (30–40) | 0.005 |
| Women (%) | 77 (97%) | 62 (82%) | 0.001 |
| Black race (%) | 49 (62%) | 26/72b (36%) | 0.002 |
| BMI, median (IQR) | 40 kg/m2 (33–43) | 26 kg/m2 (22–30) | <0.001 |
| Meningoceles (%) | 9 (11%) | 0 (0%) | <0.003 |
| Meningocele or Meckel’s cave enlargement (%) | 16 (20%) | 0 (0%) | <0.003 |
For controls this is the median age at time of MRI examination
Race was available for 72/76 (95%) controls
IIH = Idiopathic intracranial hypertension. BMI= Body Mass Index. IQR = Interquartile range
MRI Findings
Meningoceles (of Meckel’s cave or the petrous apex) were found in 9/79 (11%) of IIH patients compared to none of the controls (p<0.003). Enlargement of Meckel’s cave was found in 7/79 (9%) of IIH patients but none of the controls (p<0.003) (table 1). The increased frequency of meningoceles (+/− enlargement of Meckel’s cave) seen among cases remained significant even after stratifying by age (at 30 years), BMI (at 25 kg/m2), gender, or race. Of note, 54% of the controls (n=41) had a BMI > 25. The range of BMI for controls was 18.9–52.7 and for cases was 22.7–55.5 kg/m2 (2/9 cases with cephaloceles had a BMI close to 25 [25.1 and 25.5]). Figure 1 provides representative examples of cephaloceles. No anterior cranial fossa meningoceles were found (Table 2).
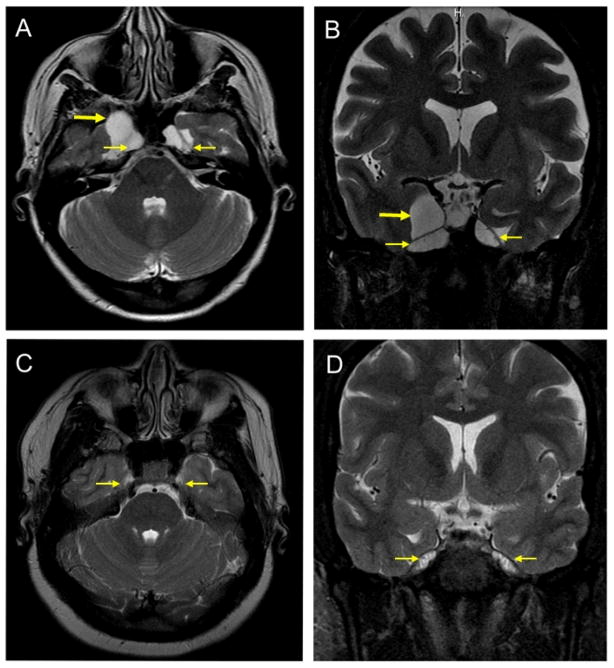
Fig. 1. Meningoceles on MRI.
A. Axial T2-weighted image in IIH case #9 (Table 2) demonstrating enlarged Meckel’s caves bilaterally (thin arrows)and a right petrous apex meningocele(thick arrrow).
B. Coronal T2-weighted image in IIH case #9 (Table 2) demonstrating enlarged Meckel’s caves bilaterally (thin arrows)and a right petrous apex meningocele(thick arrrow).
C. Axial T2-weighted image in a control showing normal appearance of the Meckel’s caves bilaterally (thin arrows)and absence of meningoceles.
D. Coronal T2-weighted image in a control showing normal appearance of the Meckel’s caves bilaterally (thin arrows)and absence of meningoceles.
Table 2.
Characteristics of patients with meningoceles or prominent Meckel’s caves
| # | Sex age |
Race | BMI, kg/m2 | CSF opening pressure (cm H2O) | Presenting symptoms | Overall clinical coursea | Meningocele | Enlargement of Meckel’s cave |
|---|---|---|---|---|---|---|---|---|
| 1 | W 27 |
Black | 50.8 | 31 | HA + TVO | Good | Left petrous apex | - |
| 2 | W 26 |
Black | 25.5 | 30 | HA | Good | Bilateral Meckel’s cave | NAb |
| 3 | W 28 |
Black | 35.7 | 32 | HA | Good | Right Meckel’s cave | NAb |
| 4 | W 25 |
White | 40.4 | 41 | HA | Good | Left petrous apex | - |
| 5 | W 53 |
White | 46.1 | 36 | HA + TVO | Poor | Bilateral Meckel’s cave | NAb |
| 6 | W 23 |
White | 34.4 | 38 | HA + TVO | Good | Bilateral Meckel’s cave | NAb |
| 7 | W 39 |
Black | 52.1 | High | None | Good | Right Meckel’s cave | NAb |
| 8 | W 42 |
Black | 30 | 27 | HA | Good | Bilateral petrous apex | - |
| 9 | W 54 |
White | 25.1 | 29 | None | Good | Bilateral Meckel’s cave + right petrous apex | NAb |
| 10 | W 27 |
White | 34 | 60 | HA+ Blurry vision | Good | - | Bilateral |
| 11 | W 31 |
Black | 36.6 | 28 | HA+ diplopia | Good | - | Right |
| 12 | W 30 |
Black | 32.6 | 34 | HA | Good | - | Bilateral |
| 13 | W 19 |
Black | 50.7 | 41 | HA | Good | - | Bilateral |
| 14 | W 18 |
Black | 31.8 | 50 | HA | Poor | - | Left |
| 15 | W 28 |
White | 43.9 | 31 | HA | Poor | - | Bilateral |
| 16 | W 35 |
Black | 41.2 | 31 | HA | Poor | - | Left |
Patients considered to have a “poor” clinical course had fulminant disease, progressive VF defects, or required a surgical procedure. Patients with a “good” clinical course had none of the “poor” clinical features.
Prominent Meckel’s cave cannot be determined in the presence of a Meckel’s cave meningocele.
BMI = body mass index, CSF = cerebral spinal fluid, W = woman, HA = headache, TVO = transient visual obscurations, NA= not applicable.
Clinical Characteristics and Visual Outcome
Among IIH patients, the presence of meningoceles or Meckel’s cave enlargement was not associated with their demographics, symptoms, degree of papilledema, or CSF opening pressure (Table 3). Median visual acuity of the study group at first examination (logMAR) was 0.31±0.95 (=20/40), and at last examination was 0.24±0.90 (=20/34). Among IIH patients, the presence of meningoceles or Meckel’s cave enlargement was not associated with visual acuity loss or visual field defect severity (table 3). Median visual acuity only in patients with meningoceles was 0.09 logMAR (=20/25) vs. 0 logMAR (=20/20) in patients without meningoceles (p=0.42). Median Humphrey visual field mean deviation was -3.6 in patients with meningoceles vs. -3.4 in patients without (p=0.94).
Table 3.
Comparison of IIH patients with and without meningoceles or prominent Meckel’s cave.
| Meningocephalocele or prominent Meckel’s cave Present (n=16) | Meningocephalocele or prominent Meckel’s cave Absent (n=63) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, years, median (IQR) | 28 (26–42) | 29 (25.7–36) | 0.78 |
| Sex (%) | 16 women (100%) | 61 women (97%) | 1.00 |
| Race (%) | 6 whites (37%) | 24 whites (38%) | 0.96 |
| BMI, kg/m2, median (IQR) | 36.6 (33.3–45) | 39.6 (33.3–42.8) | 0.96 |
| Presenting symptoms | |||
| Headache (%) | 16 (100%) | 55 (87%) | 0.19 |
| Diplopia (%) | 2 (12%) | 12 (19%) | 0.72 |
| Pulsatile tinnitus (%) | 7 (44%) | 25 (40%) | 0.76 |
| TVO (%) | 7 (44%) | 21 (33%) | 0.43 |
| Clinical characteristics | |||
| High grade papilledema (Frisén scale 4–5) (%) | 4 (25%) | 21 (33%) | 0.29 |
| Visual acuity (logMAR)b, median (IQR) | 0.07 (0.0–0.11) | 0.0 (0.0–0.09) | 0.5 |
| HVF mean deviationb, median (IQR) | 3.5 (1.8–6.3) | 3.4 (1.4–7.1) | 0.96 |
| VF severity scoreb, median (IQR) | 1.5 (1–3) | 1.5 (1–2) | 0.25 |
| VF severity score = 1–2b(%) | 8 (50%) | 37 (59%) | 0.25 |
| VF severity score = 2–3b (%) | 3 (19%) | 15 (24%) | 0.25 |
| VF severity score = 3b (%) | 5 (31%) | 6 (10%) | 0.25 |
| VF severity score = 4b (%) | 0 (0%) | 5 (8%) | 0.25 |
| Legally blind (both eyes) (%) | 1 (6%) | 7 (11%) | 1.00 |
| CSF opening pressure, cm H2O, median (IQR) | 32 (30.5–39.5) | 33.7 (29.4–40) | 0.98 |
| Good clinical coursed (%) | 12 (75%) | 45 (71%) | 0.48 |
| Required CSF-diverting Shunt or ONSF (%) | 4 (25%) | 17 (27%) | 1.0 |
Mean of both eyes
Either Humphrey or Goldmann visual fields
Patients considered to have a “poor” clinical course had fulminant disease, progressive visual field defects or required a surgical procedure. Patients with a “good” clinical course had none of the “poor” clinical features.
BMI = body mass index, TVO = transient visual obscurations, HVF = Humphrey visual fields, VF = visual fields, CSF = cerebral spinal fluid, ONSF = optic nerve sheath fenestration.
Overall good clinical outcome was present in all patients with meningoceles but one (89%) and in 12/16 patients (75%) with either meningocele or prominent Meckel’s cave, as compared to a slightly lower rate of good visual outcome in patients without meningocele (70%) (table 3). However, this difference was not statistically significant. The rate of surgical treatment was lower in the 9 patients with meningoceles versus those without meningoceles (11% vs. 21% for shunting and 0% vs. 10% for optic nerve sheath fenestration, respectively), however, the difference was not statistically significant (p=0.432) due to the small number of patients in each subgroup. When including patients with either meningocele or prominent Meckel’s cave, the rate of surgical treatment was similar to those without meningoceles (table 3).
Discussion
Meningoceles are most commonly congenital in origin with an estimated incidence of one in every 35,000 live births [11]. Spontaneous meningoceles or meningoencephaloceles account for only 8.6% of all meningoencephaloceles [2]. Meningoceles have been previously associated with CSF leaks and with intracranial hypertension [5,12]. According to one report, meningoceles were present in 50% of a subset of patients with CSF leaks and demographic similarities to patients with IIH [13]. Our results show that meningoceles and Meckel’s cave enlargement are significantly more common in patients with IIH than in controls.
The occurrence of meningoceles in our series (1 in 9 patients with IIH) was frequent enough in IIH patients to warrant consideration as a possible additional radiological sign for chronically elevated ICP. Several neuroimaging findings have been accepted as signs of intracranial hypertension or more specifically IIH. These include: empty sella, flattened posterior globe/sclera, enlarged perioptic nerve sheaths, increased optic nerve tortuosity, intraocular protrusion of the optic nerve head, slit-like ventricles, and transverse sinus stenosis (best seen on magnetic resonance venography [MRV]). The prevalence, sensitivity and specificity of these signs vary widely among previous publications [7]. Most radiological signs have high specificity (>90%), but lower sensitivities (ranging from 3.3% to 80%) [14]. Scleral flattening is the most specific sign, approaching specificity of 100% [15]. Brodsky et al [14] found that flattening of the posterior sclera and empty sella were the most prevalent radiological signs in patients with IIH (at 80% and 70% respectively, versus 5% of controls). Intraocular protrusion of the prelaminar optic nerves had the lowest occurrence at 30% of patients. Similarly, Agid et al [15] reported that optic nerve sheath distension was the most prevalent sign (at 66.7% in patients with IIH versus 17.9% in controls). Slit-like ventricles and optic nerve protrusion had the lowest occurrence (at 3.3% of IIH patients versus none of the controls).
The 20% prevalence of meningocele or prominent Meckel’s cave in our series of IIH patients is comparably less than that of several of these other radiological signs in previous IIH cohorts. However, it appears to be a very specific sign given that it was not present in any of our controls. The constellation of the traditional MRI signs may predict the presence of IIH in 90% of cases [14]; in fact, the incidental finding of traditional radiological signs of elevated ICP may prompt ophthalmological evaluation for possible papilledema. Hence, in this setting, the additional presence of meningocele should increase suspicion for elevated ICP and IIH.
All meningoceles in our study were located in the temporal bone, either petrous apex or Meckel’s cave. On MRI, a petrous apex meningocele appears as non-enhancing CSF isointense signal on both T1- and T2-weighted images. It shares radiological characteristics with an arachnoid cyst, but unlike a cyst, it is centered in the anterior petrous apex and often has connection to Meckel’s cave [16]. No anterior fossa meningoceles protruding into the nasal cavities or paranasal sinuses were found. The absence of meningoceles in other anatomical locations likely reflects their higher rate of symptomatic presentation [1,17]; meningoceles in other anatomical locations often produce symptomatic CSF leak or neurological deficits, leading to their initial presentation to an otolaryngologist or a neurosurgeon. The leak may decompress any underlying elevated ICP and preclude the development of symptoms or signs of elevated ICP.
The cause of spontaneous meningoceles is not well understood. Cerebrospinal fluid pressure and hydrostatic pulsatile forces may lead to the development of “pit-holes” in the cranial base [18], although herniation of the dura and arachnoid with or without brain tissue rarely occurs. Pathologic studies have reported relatively high rates of skull base defects but no meningoceles [19,20]. Recent publications in the otolaryngology literature consider increased ICP as the cause of meningoceles in a subset of patients [3,5]. It is possible that the formation of a meningocele may allow partial regulation of the ICP in various ways. A spontaneous meningocele is mechanically and conceptually similar to an empty sella: it increases the volume of the sub-arachnoid space [12], and thus may decrease elevated ICP. Additionally, meningoceles may intermittently and sub-clinically leak through micro-ruptures in the meninges [21], thus acting as a pressure release valve for elevated ICP.
No radiological signs have been shown so far to have prognostic implications for IIH patients [22,23]. In our study, visual outcomes were similar in patients with and without meningoceles, although there was a lower rate of surgical treatment among patients with meningoceles (excluding prominent Meckel’s cave). This may imply that meningocele formation does confer some protective effect, perhaps in the most severe cases of IIH. However, this finding did not reach statistical significance, likely because of the small number of patients with meningoceles in our study.
Our study has several limitations. First, it is a retrospective analysis and the relatively small sample size offers limited precision for the estimation of prevalence, sensitivity and specificity of meningoceles in IIH patients. Second, our study group and controls differed in their demographic characteristics and our control group may not represent the general adult population. However, meningoceles are unlikely to be related to age, race, gender, or BMI, as we found no evidence of confounding by these factors on stratified analysis. Indeed, after controlling for each of these factors, the presence of a meningocele remained significantly higher among IIH patients. The overall low occurrence of meningoceles within this cohort limits our ability to know if meningoceles provide any prognostic information. A study involving a larger cohort of IIH patients and controls could help further clarify the diagnostic and prognostic value of meningoceles in intracranial hypertension. Our study sample of patients with IIH also consisted of 97% women (only two men), and therefore any conclusions in men based on our sample size are limited. Finally, the study did not assess for the presence or absence of spinal meningoceles or any potential sources of CSF leak in the spine, since IIH patients generally do not have spinal MR imaging performed as part of their workup. It is possible that IIH patients may have an increased likelihood of developing spinal meningoceles and CSF leaks, and this should be explored further in subsequent studies.
In conclusion, our study shows that meningoceles are significantly more common in IIH patients than in controls, and can be considered an additional imaging sign for IIH. Meningoceles are not, however, associated with decreased CSF opening pressure or a better visual outcome in IIH.
Footnotes
The findings were presented as a poster at the 2013 annual meeting of the North American Neuro-Ophthalmology Society.
Disclosures:
This study was supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, and by the NIH/NEI core grant P30-EY06360 (Department of Ophthalmology).
Dr. Bialer reports no disclosures.
Dr. Perez-Rueda reports no disclosures.
Dr. Bruce receives research support from the NIH/NEI (K23-EY019341),
Dr. Newman received the Research to Prevent Blindness Lew R. Wasserman Merit Award.
Dr. Biousse reports no disclosures.
Dr. Saindane reports no disclosures
References
- 1.Schick B, Draf W, Kahle G, Weber R, Wallenfang T. Occult malformations of the skull base. Arch Otolaryngol Head Neck Surg. 1997;123:77–80. doi: 10.1001/archotol.1997.01900010087013. [DOI] [PubMed] [Google Scholar]
- 2.MacRae DL, Ruby RR. Recurrent meningitis secondary to perilymph fistula in young children. J otolaryngol. 1990;19:222–225. [PubMed] [Google Scholar]
- 3.Wise SK, Schlosser RJ. Evaluation of spontaneous nasal cerebrospinal fluid leaks. Curr Opin Otolaryngol Head Neck Surg. 2007;15:28–34. doi: 10.1097/MOO.0b013e328011bc76. [DOI] [PubMed] [Google Scholar]
- 4.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286–2296. doi: 10.1001/jama.295.19.2286. [DOI] [PubMed] [Google Scholar]
- 5.Jindal M, Hiam L, Raman A, Rejali D. Idiopathic intracranial hypertension in otolaryngology. Eur Arch Otorhinolaryngol. 2009;266:803–806. doi: 10.1007/s00405-009-0973-0. [DOI] [PubMed] [Google Scholar]
- 6.Wang EW, Vandergrift WA, Schlosser RJ. Spontaneous CSF Leaks. Otolaryngol Clin North Am. 2011;44:845–856. doi: 10.1016/j.otc.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Degnan AJ, Levy LM. Pseudotumorcerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32:1986–1993. doi: 10.3174/ajnr.A2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495. doi: 10.1212/01.wnl.0000029570.69134.1b. [DOI] [PubMed] [Google Scholar]
- 9.Bruce BB, Preechawat P, Newman NJ, Lynn MJ, Biousse V. Racial differences in idiopathic intracranial hypertension. Neurology. 2008;70:861–867. doi: 10.1212/01.wnl.0000304746.92913.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68:229–232. doi: 10.1212/01.wnl.0000251312.19452.ec. [DOI] [PubMed] [Google Scholar]
- 11.Matson D. Neurosurgery of infancy and childhood. 2. Springfield, IL: Thomas CC; 1969. p. 68. [Google Scholar]
- 12.Alorainy IA. Petrous apex cephalocele and empty sella: is there any relation? Eur J Radiol. 2007;62:378–384. doi: 10.1016/j.ejrad.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Silver RI, Moonis G, Schlosser RJ, Bolger WE, Loevner LA. Radiographic signs of elevated intracranial pressure in idiopathic cerebrospinal fluid leaks: a possible presentation of idiopathic intracranial hypertension. Am J Rhinol. 2007;21:257–261. doi: 10.2500/ajr.2007.21.3026. [DOI] [PubMed] [Google Scholar]
- 14.Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105:1686–1693. doi: 10.1016/S0161-6420(98)99039-X. [DOI] [PubMed] [Google Scholar]
- 15.Agid R, Farb RI, Willinsky RA, Mikulis DJ, Tomlinson G. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48:521–527. doi: 10.1007/s00234-006-0095-y. [DOI] [PubMed] [Google Scholar]
- 16.Razek AA, Huang BY. Lesions of the petrous apex: classification and findings at CT and MR imaging. Radiographics. 2012;32:151–173. doi: 10.1148/rg.321105758. [DOI] [PubMed] [Google Scholar]
- 17.Moore KR, Fischbein NJ, Harnsberger HR, et al. Petrous apex cephaloceles. AJNR Am J Neuroradiol. 2001;22:1867–1871. [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman B, Nulsen FE, Weiss MH, Brodkey JS, White RJ, Sykora GF. Acquired spontaneous, nontraumatic normal-pressure cerebrospinal fluid fistulas originating from the middle fossa. Radiology. 1977;122:379–387. doi: 10.1148/122.2.379. [DOI] [PubMed] [Google Scholar]
- 19.Kapur TR, Bangash W. Tegmental and petromastoid defects in the temporal bone. J Laryngol Otol. 1986;100:1129–1132. doi: 10.1017/s0022215100100702. [DOI] [PubMed] [Google Scholar]
- 20.Ahren C, Thulin CA. Fatal intracranial complications due to politerization of the outer ear canal in otitis therapy, caused by intracranial temporal bone defects. Sven Lakartidn. 1964;61:2421–2437. [PubMed] [Google Scholar]
- 21.DeBartolo HM, Vrabec D. Sphenoid encephalocele. Report of a case. Arch otolaryngol. 1977;103:172–174. doi: 10.1001/archotol.1977.00780200098012. [DOI] [PubMed] [Google Scholar]
- 22.Riggeal BD, Bruce BB, Saindane AM, et al. Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis. Neurology. 2013;80:289–295. doi: 10.1212/WNL.0b013e31827debd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saindane AM, Bruce BB, Riggeal BD, Newman NJ, Biousse V. Association of MRI findings and visual outcome in idiopathic intracranial hypertension. Am J Roentgol. doi: 10.2214/AJR.12.9638. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]