Abstract
Background and objective
Cognitive impairment, including impairment of episodic memory, is frequently found in newly diagnosed Parkinson’s disease (PD). In this longitudinal observational study we investigated whether performance in memory encoding, retention, recognition and free recall is associated with reduced hippocampal radial distance.
Methods
We analysed baseline T1-weighted brain MRI data from 114 PD subjects without cognitive impairment, 29 PD subjects with mild cognitive impairment and 99 normal controls from the ParkWest study. Age- and education-predicted scores for the California Verbal Learning Test 2 (CVLT-2) and tests of executive function were regressed against hippocampal radial distance while adjusting for imaging centre.
Results
There was no association between encoding or performance on executive tests and hippocampal atrophy in the PD group. In the full PD sample we found bilaterally significant associations between lower delayed free recall scores and hippocampal atrophy in the CA1, CA3 and subiculum area (left, p=0.0013; right, p=0.0082). CVLT-2 short delay free recall scores were associated with bilateral hippocampal CA1 and subicular atrophy in the full PD sample (left, p=0.013; right, p=0.047). CVLT-2 recognition scores showed a significant association with right-sided subicular and CA1 atrophy in the full PD sample (p=0.043).
Conclusions
At the time of PD diagnosis, subjects’ verbal memory performance in recall and recognition are associated with atrophy of the hippocampus, while encoding is not associated with hippocampal radial distance. We postulate that impaired recall and recognition might reflect deficient memory consolidation at least partly due to structural hippocampal changes.
INTRODUCTION
Parkinson’s disease (PD) frequently involves cognitive decline early in the course of the disease, in the absence of dementia.1 Impaired cognition in a number of different cognitive domains, including executive and episodic memory impairments,2 can be found even at the time of initial diagnosis. Episodic memory is the memory of autobiographical events that can be explicitly stated, as opposed to implicit memory which is formed without conscious awareness. As the disease progresses, a further decline in memory has been reported.3 The early literature on cognitive deficits in PD explained most cognitive deficits, including memory, as being caused by impairment of executive function4 secondary to dysfunction in striato-prefrontal circuits.5
In the longitudinal observational ParkWest study, we recently observed deficits in episodic memory encoding in PD subjects, which we interpreted as being secondary to executive deficits. Yet, in the same study, deficits in composite memory measures such as short and long delay free recall could not be explained entirely by deficits of executive functions.6 Some of these memory deficits could be due to hippocampal pathology.
MRI studies in normal aging show that aging alone can result in a mild degree of hippocampal atrophy.7 Profound progressive short-term memory decline and hippocampal atrophy are hallmark features of Alzheimer’s disease (AD). Atrophy of the CA1 and subiculum areas of the hippocampus is associated with increased risk for conversion from mild cognitive impairment (MCI) to AD,8 and has been also found in cognitively normal individuals years prior to memory decline and progression to MCI and AD.9 MCI subjects with 1mm atrophy in the CA1 region are at twice greater risk for progression to AD.10 Increased mean diffusivity on diffusion tensor imaging (DTI) MRI was found in PD cognitive impairment (PDCN) versus controls, as a sign of microstructural changes of the hippocampus. This was associated with low memory scores even in the absence of volumetric hippocampal changes, showing that hippocampal changes exacerbate memory impairment in PD.11 Whether hippocampal atrophy is associated with increased risk of conversion to MCI or dementia in PD is still not known.
Hippocampal atrophy has been previously reported in imaging studies of PD. Some groups have even reported hippocampal atrophy in PD subjects without cognitive impairment,12 while others have not.13,14 Using ParkWest data we have recently demonstrated significantly smaller bilateral hippocampal radial distance in PDCN relative to normal controls (NC).15 Whether structural hippocampal changes relate to memory impairment in PD has so far not been studied in detail. In PD patients without dementia, California Verbal Learning Test 2 (CVLT-2) recall, but not recognition, correlated with reduced hippocampal volumes of the left hippocampal head.16 Both PD subjects with dementia (PDD) and PD patients with visual hallucinations (PD+VH) were reported to have significant hippocampal grey matter loss relative to controls, yet in the former the entire hippocampus was involved while in the latter the atrophy seemed confined to the hippocampal head.17 In the same study, memory impairment correlated with grey matter loss of the hippocampal head.17
Several factors can account for the previously published mixed results on hippocampal atrophy in PD, such as small sample size, study cohort differences between the groups (notably differences in disease duration13,14) or possible inclusion of PD subjects with mild cognitive impairment (PDMCI) in the PDCN cohorts12,13,18 as studies were published before MCI classification was done in PD. Imaging analysis techniques used in the past might have lacked sensitivity for detecting subtle hippocampal structural changes. The method that we use in this manuscript gives us significantly improved power for detecting subtle atrophy in the hippocampal structure relative to the region of interest (ROI) volumetric11,12 or the voxel based morphometry (VBM) approaches.17,19
In the present study we applied structural imaging techniques to examine the relationship between verbal memory performance (CVLT-2) and structural hippocampal changes in a large sample of PD patients. We were interested in studying if there were differences between the control subjects and the group including all PD subjects. We also wanted to study whether PDMCI and cognitively normal PD subjects (PDCN) had a different pattern of structural hippocampal changes. Our goal was to examine whether different aspects of verbal memory—encoding, retention, recognition and free recall—correlate with hippocampal atrophy in newly diagnosed PD subjects. We hypothesised that memory deficits in encoding, and recognition/recall will associate with hippocampal atrophy in PD.
METHODS
We analysed the baseline structural MRI data from the Norwegian ParkWest study.20 ParkWest is a population-based multicentre prospective longitudinal study of newly diagnosed drug-naïve PD subjects aiming to define the clinical progression of PD over 10 years and to identify promising biomarkers for PDMCI and PDD. The protocol was reviewed and approved by the Regional Committee for Medical Research Ethics, Western Norway.
ParkWest recruitment strategies and diagnostic procedures have previously been described in detail.20 Five neurology groups from southwestern Norway actively participated in recruitment and research evaluations of enrolled participants. Study recruitment materials were sent to all hospital departments and general practitioner offices in southwestern Norway. Reminders were sent twice during the study recruitment period. This comprehensive surveillance/referral mechanism resulted in 604 referrals of patients with possible PD between 1 November 2004 and 31 August 2006. All subjects were evaluated by one of the five participating neurology groups. Of the 604 PD referrals, 265 subjects were diagnosed with incident PD. A total of 207 drug-naïve incident PD subjects agreed to longitudinal participation. Normal control subjects were recruited from spouses, friends and relatives of patients with PD, and volunteers; 182 PD and 108 normal control (NC) subjects agreed to and received MRI. After visual inspection of the scans, subjects with strokes and/or structural lesions that could produce parkinsonian symptoms were excluded (N=7). Also excluded from the imaging analyses were subjects with baseline scan artefacts or scans of insufficient quality (n=12), and five subjects with baseline dementia. Of these, 258 ParkWest study participants (100 NC, 127 PDCN and 31 PDMCI) had useable imaging data. Of the resulting subjects, 143 patients and 99 controls had completed MRI scanning and had a complete CVLT-2, and were included in our study.
ParkWest subjects underwent standardised clinical, neuropsychiatric and neuropsychological examinations, brain MRI at study entry, and dopamine transporter imaging to improve diagnostic accuracy, when deemed necessary by the individual investigator (n=31). Bi-annual clinical assessments were done to evaluate drug response and to monitor disease progression. After a period from the screening visit, averaging 28 months, all participants of the study were given a final diagnosis. All available information was collected and evaluated by two neurologists who are specialised in movement disorders. Subjects who did not meet the PD criteria according to Gelb et al21 were not eligible for participation. Patients with atypical parkinsonism or dementia during the first year of motor onset were given a diagnosis of other parkinsonian syndromes according to published criteria22,23 or dementia with Lewy bodies (DLB) according to the revised DLB criteria.24 More details of the diagnostic ascertainment are reported in Alves et al.20
The severity of parkinsonian symptoms was assessed by the study neurologists with the Unified Parkinson’s Disease Rating Scale motor score (UPDRSm)25 and the modified Hoehn and Yahr (H&Y) staging.26
The ParkWest neuropsychological test battery27 consisted of the Mini-Mental State Examination (MMSE),28 verbal memory assessment with the CVLT-2,29 assessment of visuospatial abilities with the Silhouettes and Cube subtests from the Visual Object and Space Perception Battery (VOSP),30 and assessment of attention and executive functioning with semantic verbal fluency (animal fluency),31 the Stroop test.32 Depression was assessed using the Montgomery–Asberg depression rating scale (MADRS).33 Details of the diagnostics of MCI were described in detail in Aarsland et al.27
MRI acquisition and preprocessing
MRI was performed at four of the five study sites. Patients from the clinical site in Forde were scanned at the MRI centre in Bergen. Sagittal three-dimensional (3D) T1-weighted high-resolution images were acquired as previously described.15 The imaging protocol is uploaded as online supplementary material.
The images were subjected to intensity normalisation34 and spatial normalisation to the International Consortium for Brain Mapping (ICBM53) brain atlas using the Minctracc algorithm and nine-parameter (9P) transformation (three translations, three rotations, three scales).35 The aligned images were resampled in an isotropic space of 220 voxels along each axis (x, y and z), resulting in a final voxel size of 1 mm3.
Hippocampal segmentation
The hippocampal formations (including hippocampus proper, dentate gyrus and subiculum) of a randomly selected ParkWest training dataset were manually segmented on gapless coronal slices by one experienced rater (first author MKB) blinded to subjects’ age, sex and diagnosis following a detailed, well established protocol.15 The training dataset consisted of 29 subjects, with 12 subjects (4 NC, 4 PDCN and 4 PDMCI) from each of the two large imaging centres (Stavanger and Bergen), three subjects (one from each diagnostic group) from Arendal and two subjects from Haugesund (1 PDCN and 1 PDMCI). The training sample composition was proportionate to the ratio of subject enrolment across the four imaging centres, to prevent as far as possible any potential centre bias in the statistical sampling. The traces were closely inspected for accuracy by a second experienced hippocampal rater (last author LGA).
Next the hippocampi of the full dataset were segmented with AdaBoost—a validated automated machine-learning hippocampal segmentation algorithm, based on the adaptive boosting approach. Briefly, using the training dataset, AdaBoost develops statistical rules for hippocampal segmentation by selecting the optimally combination of features from a feature pool consisting of thousands of voxel-specific features, such as image gradients, local curvatures at image interfaces, grey or white matter classification, statistical information on the likely stereotaxic position of the hippocampus, etc. Following the algorithm labels each voxel in each new image as belonging to the hippocampus or not, based on the feature information contained in the positive and negative voxels of a training dataset. The AdaBoost algorithm has been extensively validated36 and utilised.37
Radial distance mapping
After modelling the segmented hippocampi as 3D parametric surface meshes, we computed the medial core (a medial curve threading down the centre of each structure) and the radial distance from the medial core to each surface point for each structure in each subject.38 Radial distance provides an intuitive measure of the thickness of the structure from its core to each point on its boundary.
Scanner reliability analyses
For scanner cross-validation, a human phantom study was performed. Three volunteers were scanned twice on each of the four scanners used in the ParkWest study. 3D T1-weighted and fluid attenuated inversion recovery (FLAIR) sequences were obtained. The hippocampi were manually traced and the volumes obtained as previously described.38 Whole brain volumes were obtained using SIENAX software.39 For inter-and intra-scanner reliability analyses on whole-brain and hippocampal volumes, we used PASW software (PASW for Windows V.18.0.1) reliability analyses to obtain Cronbach’s α coefficients.
Statistical analyses
Comparison of demographic variables was done using analysis of variance tests with post-hoc Scheffe tests for the normally distributed variables. Variables that were not normally distributed were analysed with the Kruskal–Wallis test corrected for multiple comparisons, with post-hoc Mann–Whitney U test. A χ2 test was used for analysis of sex differences between groups.
Education affects memory scores in our sample, hence we corrected the neuropsychological test performance scores for each individual (controls and patients) for the influence of age and education by a regression approach.27 Regression analyses were done in the healthy control group with age and education as predictors, and the CVLT-2 variables as dependent variable. The resulting regression equation was used to calculate expected CVLT-2-scores for all subjects individually. Finally, a difference score was calculated by subtracting the expected score from the observed score. To see if PD subjects with higher versus lower education had differences in delayed recall and recognition, we analysed the pooled sample of PD subjects in the highest quartile of education with the subjects within the lowest quartile of education comparing performances on CVLT-2 delayed recall and recognition scores.
Our main analyses were conducted with linear regression. The following CVLT-2 memory variables were analysed: list A sum trial 2–5 (composite encoding, consolidation and recall), short delay free recall (list A retest), long delay free recall, delayed recall list A, recognition score with D prime, retention (CVLT-2 short vs long delay free recall), and retrieval (CVLT-2 recall vs recognition). More details can be found in Bronnick et al.6 The image analyses were later rerun without two clinically depressed subjects, who had a MADRS score above 16.
Since we have previously shown that executive dysfunction can explain a substantial part of the memory deficits observed in PD,6 we also examined the associations between performance on the Stroop colour-word test and semantic verbal fluency (animal fluency) with hippocampal radial distance to control for a possible association between performances on attention and executive tests with hippocampal radial distance.
The difference scores between actual and predicted scores in the PD cohort were regressed against hippocampal radial distance while adjusting for imaging centre. Age and education correction had already been done for the neuropsychological tests. For 3D map-wise multiple comparisons correction, we ran 100 000 permutations thresholded at p<0.01.
To see if there is an interaction effect of (group×hippocampal volumes) on memory as measured with CVLT-2, we also analysed the memory variables that showed significant associations with hippocampal radial distance with linear regression.
RESULTS
The clinical and demographic between-group comparisons are summarised in table 1. Our PDMCI subjects were significantly older than the other two groups. Our normal controls were significantly more educated than PDCN subjects. Subjects with PDMCI had a significantly lower total MMSE score at baseline than subjects in the PDCN and NC groups. PD subjects in the highest versus the lowest quartile of education length did not perform significantly differently on tests of delayed recall or recognition. While the combined PD group had a significantly higher MADRS score relative to NC, only two PD subjects had a MADRS score >16, consistent with clinically significant depression.
Table 1.
Clinical and demographic between-group comparisons
| Variable | NC (N=99) | PDCN (N=114) | PDMCI (N=29) | p Value (all groups) | Groups compared | p Value |
|---|---|---|---|---|---|---|
| Age, years (SD) | 65.0 (9.5) | 65.8 (9.5) | 70.6 (8.3) | 0.017 | NC/PDMCI | 0.019 |
| PDCN/PDMCI | <0.05 | |||||
| Sex, M : F | 48 : 51 | 68 : 46 | 18 : 11 | 0.2 | ||
| Education, years (SD) | 12.7 (3.8) | 11.3 (3.3) | 11.4 (3.9) | 0.013 | NC/PDCN | 0.016 |
| H&Y (SD) | N/A | 1.8 (0.6) | 2.0 (0.5) | 0.1 | ||
| UPDRSm (SD) | N/A | 21.0 (10.1) | 24.2 (10.3) | 0.1 | ||
| MMSE (SD) | 28.8 (1.2) | 28.3 (1.6) | 26.9 (3.0) | 0.001 | PDCN/PDMCI | <0.019 |
| NC/PDMCI | 0.03 | |||||
| MADRS (SD) | 0.99 (2.1) | 4.1 (4.8) | 3.6 (4.2) | <0.001 | PDCN/NC | <0.001 |
| PDMCI/NC | <0.001 | |||||
| VOSP (SD) silhouettes | 19.8 (3.5) | 19.5 (3.8) | 15.9 (4.6) | <0.001 | PDMCI/NC | <0.001 |
| PDCN/PDMCI | <0.001 | |||||
| Stroop (SD) colour-word | 30.9 (8.3) | 29.3 (10.8) | 18.3 (9.3) | <0.001 | PDMCI/NC | <0.001 |
| PDCN/PDMCI | <0.001 |
Significant differences in bold.
Stroop colour-word; raw scores are shown.
F, female; M, male; MADRS, Montgomery–Aasberg Depression Rating Scale; MMSE, Mini-Mental State Examination; UPDRSm, Unified Parkinson’s Disease Rating Scale, motor subscale; VOSP silhouettes, Visual Object and Space Perception Battery, raw scores are shown.
Results from analyses of human phantom scans showed that variations in baseline whole brain volumes between centres were on average 2.95%. For whole brain volumes, inter-site Cronbach’s α was 0.96. Intra-site reliability, assessed using Cronbach’s α, for whole brain volumes were: Stavanger=0.97, Bergen=0.99, Arendal=0.90 and Haugesund=0.93.
For hippocampal volumes, inter-site Cronbach’s α was 0.97. Intra-site reliability statistics were similarly high: Stavanger=0.98, Bergen=0.97; Arendal=0.99 and Haugesund=0.96.
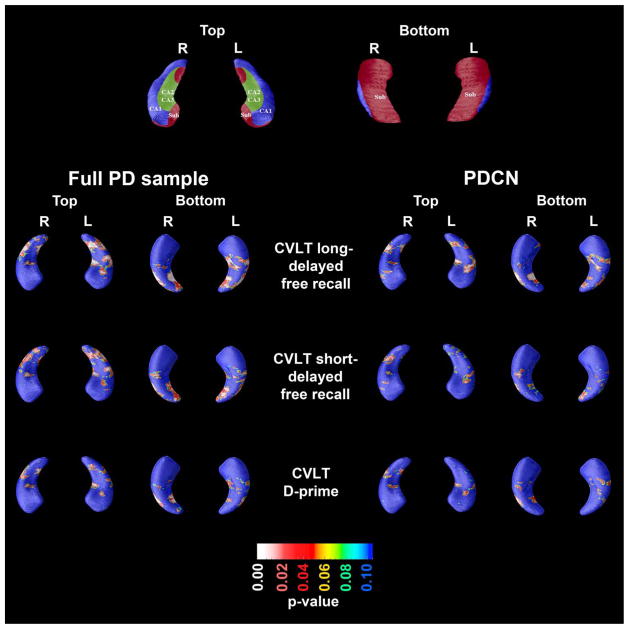
In the full PD sample consisting of both Parkinson’s disease non demented (PDND) and PDMCI (n=143), CVLT-2 delayed free recall scores showed a significant association with hippocampal radial distances in the CA1, CA3 and subiculum area (left, p=0.0013; right, p=0.0082). Analyses without two subjects with clinical depression (MADRS >16) did not affect the results. After excluding the PDMCI subjects (n=29) the association remained significant on the right (p=0.033) but became trend on the left (p=0.068). In the PDMCI sample (n=29) there was a trend level significant association between delayed free recall and hippocampal radial distance on the right side (p=0.055).
In the full PD sample we also found CVLT-2 short delay free recall scores to be associated with hippocampal radial distances in the CA1 and subiculum on the left (p=0.013) and on the right (p=0.047). After excluding the depressed subjects, the results became trend level on the right side (p=0.060) but remained significant on the left side (p=0.014).
CVLT-2 D-prime scores (recognition) showed a significant association with right-sided hippocampal radial distances of the subiculum and the CA1 area in the full PD sample (p=0.043) (figure 1). This result was unchanged after exclusion of the depressed subjects.
Figure 1.
Significance maps of the association between California Verbal Learning Test 2 (CVLT-2) long-delayed free recall score, short delay free recall score and recognition (D-prime) with hippocampal radial distance in the full Parkinson’s disease (PD) sample and the PD cognitive impairment (PDCN) group.
We found no significant relationship with encoding and consolidation (sum of list 2–5) and hippocampal radial distance.
We found no associations between performance on attention/executive tests and hippocampal radial distance in any of the groups.
No interaction effect of (group×hippocampal volumes) on memory as measured with CVLT-2 was found for any of the memory variables where we found associations with hippocampal radial distances (short delay free recall, long delay free recall and recognition with D-prime).
DISCUSSION
In this large group of drug-naïve newly diagnosed PD subjects, we studied the association between different components of verbal memory and executive function with hippocampal radial distance. As we hypothesised, a deficit in short and long delay free recall in the patient group was associated with significant hippocampal atrophy in PD subjects. After excluding the two PD subjects with clinically significant depression, the association of delayed free recall and recognition with hippocampal radial distance remained unchanged, while the association between short delay free recall was reduced to trend level on the right side but remained significant on the left side. These results add new insight into the aetiopathology of memory impairment in early PD, suggesting that component processes of memory are impaired partly due to structural changes in the hippocampi.
It has been long hypothesised that many of the cognitive deficits in PD result from disruption in the integrity of the frontal cortex and its connections.40 Here we report that encoding—the major component of memory impairment in our cohort—is not associated with structural hippocampal changes. This is further supported by the non-significant interaction analyses where we tested the effect of (group×hippocampal volumes) on short delay free recall, long delay free recall and recognition. The observed lack of association between encoding (ie, learning slope) and hippocampal radial distance agrees well with our recent hypothesis that the early memory deficits in PD are best explained by encoding deficits secondary to inefficient learning strategy following fronto-subcortical dysfunction.6 Indeed fMRI studies in healthy young individuals have demonstrated that successful activation of the medial temporal, fusiform and prefrontal brain regions during encoding predict subsequent successful recall performance.41 Thus we can speculate that changes in the prefrontal regions and perhaps also the postero-medial cortices are associated with encoding deficits in PD instead of the hippocampus.
Delayed recall and recognition were significantly associated with hippocampal radial distance, especially in the posterior portions of the hippocampus. Deficits in both short and long delay free recall were associated with areas of reduced hippocampal radial distances in PDCN subjects. Thus, the association between hippocampal volume and short and long delayed recall is probably related to deficient storage, or memory consolidation processes, not to learning strategy usage, which was a main source of encodig deficit in the previously reported memory study from our group.6
Our results show differences regarding the different components of CVLT-2 and the different subfields of the hippocampus. The CLVT-2 delayed free recall score showed a significant association with radial distance in the CA1, in keeping with the knowledge about this specific hippocampal region that is engaged in consolidation and late retrieval in aging and non-PD cognitive impairment.42 We also find an association with radial distance at CA3 that is reported to be associated with learning and early retrieval42 and delayed free recall. Our results differ from those of the above mentioned study, showing that more studies are needed to establish the relation between hippocampal subfields and neuropsychological deficits, although some areas seem to be common for different conditions.
Right- but not left-sided hippocampal radial distance changes in the CA1, CA3 and subiculum area were found to be associated with CVLT-2 long delay recall in PDMCI and PDCN. We did not expect significant laterality differences, and the observed findings might be due to greater radial distance variability of the left compared to the right hippocampus. Laterality changes were not reported in a study of CVLT-2 and hippocampal subfield changes in the elderly.42
Several strengths and limitations of the present study should be acknowledged. As far as we know this is the first study giving detailed results about the relation between hippocampal atrophy and aspects of verbal memory impairment in PD. Other major strengths include excellent recruitment and disease ascertainment strategies employed in this population-based multicentre prospective longitudinal cohort study of drug naïve new onset PD. Advanced imaging methodology was also used for these analyses. The ParkWest is a multicentre, multi-scanner study, which called for inter-scanner validation. Data from our human phantom validation study unequivocally show that the volumetric data are similar across manufacturers and models with high values for Cronbach’s α. Similarly, results from the ADNI study showed that the variance of the segmented volumes, particularly in the hippocampus, does not significantly change across platforms and field strengths.43
A limitation of the study is that one of the scanners we used is a 1T scanner while the others are 1.5 T, which may influence the image quality as it is found that higher field strength is associated with better segmentation results.44 Other authors who have performed multicentre studies have found that when applying automatic segmentation methods, the method must be flexible to cope with variation such as MR scanner types and field strengths.45 We preprocessed the images, including intensity normalisation and spatial normalisation, in a common pipeline. This can partly reduce the impact of different field strength,46 and is reflected in the excellent results from our validation study.
Another limitation is the use of only one test of memory, the CVLT-2, which is a test of verbal memory. As pointed out by Bronnick et al,6 applying both a visual and a verbal memory test, and also choosing a memory test less dependent on executive encoding strategies, could improve our understanding of the hippocampal involvement in PD memory impairment. Visual memory—in addition to verbal memory impairment—has previously been associated with hippocampal atrophy in a small convenience sample,47 and needs further study in a population based patient group.
We did not perform a power size calculation for this study, which is another limitation, but we have shown from previous studies of the same cohort that the sample size is sufficient to detect significant hippocampal changes in this cohort.15
In conclusion, we found that hippocampal atrophy relates to delayed recall and recognition but not to encoding, implying deficient storage or memory consolidation processes in newly diagnosed PD. Since this is, as far as we know, the first study of subfield changes in the hippocampus associated with memory impairment in early PD, more studies are needed to establish the generalisability of our findings.
Acknowledgments
Thanks are extended to Dr Robert Zivadinov, Buffalo Neuroimaging Analysis Center, University at Buffalo, Buffalo, NY, USA, for assistance in human phantom scanning whole brain analyses. Thanks are extended to Turi O Dalaker MD, PhD at the Norwegian Centre for Movement Disorders, Stavanger University Hospital, Stavanger, Norway and Ketil Oppedal, MSC, Centre for Age-related Medicine, Stavanger University Hospital, Stavanger, Norway for participation in, and planning of the human phantom scanning study.
Funding The ParkWest study in general is funded by the Research Council of Norway (grant number 177966). MKB is supported by the Western Norway Regional Health Authority (grant number 911218). This study was supported by a grant from the Norwegian Parkinson Research Foundation.
Data analyses were supported by the National Institute of Aging (AG16570), the Easton Consortium for Alzheimer’s Drug Discovery and Biomarker Development, the National Institute of Medical Imaging and Bioengineering (EB01651), the National Library of Medicine (LM05639) and the National Center for Research Resources (RR019771).
Footnotes
Additional data are published online only.
Contributors The authors all made substantial contributions to the contents of this paper. LGA, KSB, MKB, JPL, OBT: conception and design; LGA, KSB, MKB, KH, JS, PMT, NB: analysis and interpretation of data; all authors: drafting the article or revising it critically for important intellectual content; final approval of the version to be published: all authors. LGA and co-author KSB is a guarantor for the neuropsych parts of the paper.
Ethics approval Regional Committee for Medical Research Ethics, Western Norway.
Provenance and peer review Not commissioned; externally peer reviewed.
Competing interests JPL has served on scientific advisory boards for H Lundbeck A/S and GSK. KB holds stocks in Dual Attention and has received honoraria for presentations from H. Lundbeck A/S and Solvay Pharma. OBT has received honoraria for lectures and support to participate in scientific meetings from several companies engaged in Parkinson’s disease.
References
- 1.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–9. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone P, Aarsland D, Burn D, et al. Cognitive impairment in nondemented Parkinson’s disease. Mov Disord. 2011;26:2483–95. doi: 10.1002/mds.23919. [DOI] [PubMed] [Google Scholar]
- 3.Muslimovic D, Schmand B, Speelman JD, et al. Course of cognitive decline in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc. 2007;13:920–32. doi: 10.1017/S1355617707071160. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 5.Dujardin K, Defebvre L, Grunberg C, et al. Memory and executive function in sporadic and familial Parkinson’s disease. Brain. 2001;124:389–98. doi: 10.1093/brain/124.2.389. [DOI] [PubMed] [Google Scholar]
- 6.Bronnick K, Alves G, Aarsland D, et al. Verbal memory in drug-naive, newly diagnosed Parkinson’s disease. The retrieval deficit hypothesis revisited. Neuropsychology. 2011;25:114–24. doi: 10.1037/a0020857. [DOI] [PubMed] [Google Scholar]
- 7.Du AT, Schuff N, Chao LL, et al. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27:733–40. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63:693–9. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 9.Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077–88. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apostolova LG, Thompson PM, Green AE, et al. 3D comparison of low, intermediate, and advanced hippocampal atrophy in MCI. Hum Brain Mapp. 2010;31:786–97. doi: 10.1002/hbm.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlesimo GA, Piras F, Assogna F, et al. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology. 2012;78:1939–45. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- 12.Junque C, Ramirez-Ruiz B, Tolosa E, et al. Amygdalar and hippocampal MRI volumetric reductions in Parkinson’s disease with dementia. Mov Disord. 2005;20:540–4. doi: 10.1002/mds.20371. [DOI] [PubMed] [Google Scholar]
- 13.Burton EJ, McKeith IG, Burn DJ, et al. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 14.Beyer MK, Janvin CC, Larsen JP, et al. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:254–9. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostolova L, Alves G, Hwang KS, et al. Hippocampal and ventricular changes in Parkinson’s disease mild cognitive impairment. Neurobiol Aging. 2012;33:2113–24. doi: 10.1016/j.neurobiolaging.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouchard TP, Malykhin N, Martin WR, et al. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson’s disease. Neurobiol Aging. 2008;29:1027–39. doi: 10.1016/j.neurobiolaging.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Ibarretxe-Bilbao N, Ramirez-Ruiz B, Tolosa E, et al. Hippocampal head atrophy predominance in Parkinson’s disease with hallucinations and with dementia. J Neurol. 2008;255:1324–31. doi: 10.1007/s00415-008-0885-8. [DOI] [PubMed] [Google Scholar]
- 18.Laakso MP, Partanen K, Riekkinen P, et al. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology. 1996;46:678–81. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 19.Burton EJ, McKeith IG, Burn DJ, et al. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 20.Alves G, Muller B, Herlofson K, et al. Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2009;80:851–7. doi: 10.1136/jnnp.2008.168211. [DOI] [PubMed] [Google Scholar]
- 21.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 22.Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Auton Nerv Syst. 1998;74:189–92. [PubMed] [Google Scholar]
- 23.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 25.Fahn S, Elton R. Committee MotUD. Unified Parkinson’s disease Rating Scale. In: Fahn S, Marsden C, Calne D, et al., editors. Recent developments in Parkinson’s disease. Vol. 2. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–63. [Google Scholar]
- 26.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 27.Aarsland D, Bronnick K, Larsen JP, et al. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–6. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, Mc Hugh PR. Mini—mental State. A practical method for grading the mental state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Delis DC, Kramer JHEK, Ober BA. CVLT II. California verbal learning test. 2. San Antonio: The Psychological Corporation: Harcourt Assessment Inc; 2000. Adult version. [Google Scholar]
- 30.Warrington EK, James M. The visual object and space perception battery. Bury St Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- 31.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A clinical test. Arch Neurol. 1978;35:364–7. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- 32.Stroop JR. Studies in interference of serial verbal reactions. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- 33.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 34.Shattuck DW, Sandor-Leahy SR, Schaper KA, et al. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–76. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 35.Apostolova LG, Steiner CA, Akopyan GG, et al. Three-dimensional gray matter atrophy mapping in mild cognitive impairment and mild Alzheimer disease. Arch Neurol. 2007;64:1489–95. doi: 10.1001/archneur.64.10.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morra JH, Tu Z, Apostolova LG, et al. Validation of a fully automated 3D hippocampal segmentation method using subjects with Alzheimer’s disease mild cognitive impairment, and elderly controls. Neuroimage. 2008;43:59–68. doi: 10.1016/j.neuroimage.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apostolova LG, Morra JH, Green AE, et al. Automated 3D mapping of baseline and 12-month associations between three verbal memory measures and hippocampal atrophy in 490 ADNI subjects. Neuroimage. 2010;51:488–99. doi: 10.1016/j.neuroimage.2009.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–66. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 40.Taylor AE, Saint-Cyr JA, Lang AE. Memory and learning in early Parkinson’s disease: evidence for a “frontal lobe syndrome”. Brain Cogn. 1990;13:211–32. doi: 10.1016/0278-2626(90)90051-o. [DOI] [PubMed] [Google Scholar]
- 41.Dickerson BC, Miller SL, Greve DN, et al. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: an event-related functional-anatomic MRI study. Hippocampus. 2007;17:1060–70. doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller SG, Chao LL, Berman B, et al. Evidence for functional specialization of hippocampal subfields detected by MR subfield volumetry on high resolution images at 4 T. Neuroimage. 2011;56:851–7. doi: 10.1016/j.neuroimage.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jovicich J, Czanner S, Han X, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuro Image. 2009;46:177–92. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kruggel F, Turner J, Muftuler LT. Impact of scanner hardware and imaging protocol on image quality and compartment volume precision in the ADNI cohort. Neuroimage. 2010;49:2123–33. doi: 10.1016/j.neuroimage.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dyrby TB, Rostrup E, Baare WF, et al. Segmentation of age-related white matter changes in a clinical multi-center study. Neuro Image. 2008;41:335–45. doi: 10.1016/j.neuroimage.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 46.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–91. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 47.Jokinen P, Bruck A, Aalto S, et al. Impaired cognitive performance in Parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat Disord. 2009;15:88–93. doi: 10.1016/j.parkreldis.2008.03.005. [DOI] [PubMed] [Google Scholar]