Abstract
Integration of retroviral elements into the host genome is a phenomena observed among many classes of retroviruses. Much information concerning integration of retroviral elements has been documented based on in vitro analysis or expression of selectable markers. To identify possible Tf1 integration events within silent regions of the S. pombe genome, we focused on performing an in vivo genome-wide analysis of Tf1 integration events from the nonselective phase of the retrotransposition assay. We analyzed 1000 individual colonies streaked from four independent Tf1 transposed patches under nonselection conditions. Our analysis detected a population of G418S/neo+ Tf1 integration events that would have been overlooked during the selective phase of the assay. Further RNA analysis from the G418S/neo+ clones revealed 50% of clones expressing the neo selectable marker. Our data reveals Tf1’s ability to insert within silent regions of S. pombe’s genome.
1. INTRODUCTION
Long terminal repeat (LTR) retrotransposons are retrovirus-like transposons that replicate by reverse transcription of an RNA intermediate producing cDNA. The life cycle is similar to some higher order retroviruses in that the final stage of the life cycle includes insertion of the cDNA –using a viral encoded integrase protein – into the host chromatin (Boeke and Devine, 1998); (Koonin et al., 2006). Because the eukaryotic cell chromatin is composed of euchromatic (regions of the chromatin that contains actively transcribing genes) and heterochromatic (regions of the chromatin referred to as “silent” for the transcription of genes) regions, careful target selection for integration is critical for propagation of the virus and survival of the host.
Integration of retroviral elements into the host genome is a phenomena observed among many classes of retroviruses. Much information concerning integration of retroviral elements has been documented based on in vitro analysis or expression of selectable markers (Asante-Appiah and Skalka, 1997). The epigenetic environment of the eukaryotic cell’s genome creates many challenges for target site selection. In order to fully analyze the cellular interactions of target site selection among retrotransposons it is critical to observe the impact of integration within the host environment.
Tf1 is an endogenous and active LTR retrotransposon of the fission yeast Schizosaccharomyces pombe. Tf1 contains a single open reading frame (ORF) that encodes 1,331 amino acids. The ORF is composed of capsid, protease, reverse transcriptase, and integrase proteins (Levin et al., 1993; Hizi and Levin, 2005). As a member of the Metavirus genus (formerly called Ty3/Gypsy family) (Malik and Eickbush, 1999; Jern et al., 2005) the integrase protein contains a conserved module called a chromodomain (CHD) located within the carboxyl terminal (C-terminal) domain of the protein. Chromodomains are found in several eukaryotic proteins. The regions are believed to be essential for methylation of histones as well as interaction with heterochromatic regions (Eissenberg, 2001). The presence of a CHD region within the C-terminal domain of Tf1 integrase suggests possible interaction with heterochromatic regions. Previous work has shown that mutations within the CHD of Tf1 affect transposition (Chatterjee et al., 2009).
Introduction of an in vivo assay system to monitor the life cycle of retroviral elements in prokaryotic (Naumann and Reznikoff, 2002) and eukaryotic (Boeke et al., 1985; Boeke and Corces, 1989; Sandmeyer et al., 1990; Curcio and Garfinkel, 1991; Jensen and Heidmann, 1991; Yang et al., 2007) cells has greatly advanced the field of retrovirology. Retrotransposition assays allow phenotypic monitoring of retroviral integrations de novo. The ability to isolate retroviral insertions is based on the cell’s ability to express a selectable marker within the genome. The presence of a selectable marker could create a selection bias based on integrations into genomic regions that could suppress transcription (Francis and Spiker, 2005). The retrotransposon assay used to monitor the life cycle of Tf1 consists mainly of two phases. Phase I is a nonselective phase that selects against the donor plasmid carrying a copy of Tf1 (Levin and Boeke, 1992). Phase II is the selective phase that selects for genomic integrated copies of Tf1 based entirely on expression of a selectable neomycin (neo) marker tagged to Tf1. Because Tf1 elements have never been observed to integrate into heterochromatic regions (Behrens et al., 2000; Singleton and Levin, 2002) it is unknown if a population of cells containing genomic Tf1 insertions exists within silent regions of the genome. Integration events within heterochromatic regions would go undetected in the selective phase of the retrotransposon assay. Previous reports have demonstrated this selection bias with T-DNA in the genome of Arabidopsis (Francis and Spiker, 2005; Kim et al., 2007; Gelvin, 2012).
Because Tf1 has been reported to harbor a chromodomain located within the C-terminal of the integrase protein, and because it is well documented that chromodomains are known to interact with heterochromatic regions of the genome, we anticipate possible Tf1 targeting to heterochromatic regions of S. pombe. Previous reports have isolated Tf1 insertions from the nonselective phase but bacteria were used to identify the insertion events (Singleton and Levin, 2002). In this study we report an in vivo genome-wide analysis of Tf1 transposition events. To identify possible Tf1 integration events within silent regions of the genome we focused on performing an in vivo analysis of Tf1 integration events from the nonselective phase of the retrotransposition assay. Our analysis detected a population of G418S/neo+ Tf1 integration events that would have been overlooked during the selective phase of the assay. Further RNA analysis from the G418S/neo+ clones revealed expression of the neo selectable maker. Because silencing in S. pombe has an essential role in the formation of heterochromatin at the centromeres, telomeres, the mating-type locus, and ribosomal DNA (rDNA) (Hansen et al., 2005), the use of this organism in understanding how cells may silence viral infections is critical in advancing the design of therapies used to treat viral infections and diseases in mammals and humans.
2. Materials and methods
2.1. Media
S. pombe liquid and agar plate media were composed according to Singleton and Levin, 2002. Plasmid propagation plates were composed of Edinburgh Minimal Medium (EMM) (Forsburg and Rhind, 2006) lacking uracil and supplemented with appropriate amino acid, and Vitamin B1 (Thiamine) to suppress the nmtl promoter. Nonselective plates were composed of EMM supplemented with 1mg/ml 5-Fluoroorotic acid (5-FOA) (Boeke et al., 1987) (Zymo Research) and uracil at a final concentration of 50ug/ml. Selective plates consisted of YES, supplemented with 500ug/ml Geneticin (G418) (Agilent) and 1mg/ml 5-FOA (YES/G418/5-FOA).
2.2. Construction of strains
The yeast strains and plasmids used in this study are described in Table 1. Yeast strain YHL6488 is a diploid S. pombe strain carrying the Tf1-ori/neo donor plasmid pTS1559-9 (Singleton and Levin, 2002). S. pombe strain YHL1282 is a haploid stain carrying the Tf1-neo donor plasmid pHL449-1 (Levin, 1995). S pombe strain YTS7078 is a haploid strain carrying the Tf1-ori/neo donor plasmid pTS1559-9.
TABLE 1.
Yeast strains and plasmids used in this study.
| Parental Yeast Strains | Genotype Parent/plasmid | Reference |
|---|---|---|
| 972 | Wild type | ATCCa |
| YHL912 | h− ura4-294 leu1-32 | Singleton and Levin, 2002 |
| YHL5661 | Stable diploid ura4-D18/ura4-D18 1eu1-32::nmt1-1acZ-1eu1-32::nmt-1acZ-1eu1 ade6-M210/ade6-M216 | Singleton and Levin, 2002 |
| Nontransposed Yeast Strains | ||
| YHL1282 | YHL912/pHL449-1 | ATCCa |
| YTS7078 | YHL912/pTS1559-9 | This study |
| YHL6488 | YHL5661/pTS1559-9 | This study |
| Transposed Yeast Strains | ||
| YTS6716† | YHL5661/pTS1559-9 | This study |
| YTS6932† | YHL912/pHL449-1 | This study |
| YTS7084‡ | YHL912/pTS1559-9 | This study |
| YTS7133† | YHL912/pTS1559-9 | This study |
| Plasmid | ||
| pTS1559-9 | Tf1-ori/neo | Singleton and Levin, 2002 |
| pHL449-1 | Tf1-neoAI | Levin, 1995 |
American Type Culture Collection
Transposition using solid media.
Transposition using liquid media.
2.3. Tf1 Transposition Assay
The Tf1 retrotransposition assay was performed as described previously (Singleton and Levin, 2002). Individual colonies were selected and used to grow patches on EMM lacking uracil supplemented with Vitamin B1 agar plates for 3 days at 30°C. Patches were replica-printed to EMM agar plates lacking uracil and Vitamin B1 to induce transcription of Tf1. Patches were grown for 4 days at 30°C then replica-printed to EMM agar plates supplemented with uracil and 5-FOA and allowed to grow for 3 days. This nonselective phase only allowed selection against cells containing the Tf1-neo and Tf1-ori/neo donor plasmids. Next, a selective phase consisted of replica-printing patches to YES/G418/5-FOA agar plates. Confluent cell growth was indicative of expression of the neo gene thereby creating G418-resistant (G418R) colonies. The lack of cells’ growth on YES-G418 is due to sensitivity to G418, and is G418-sensitive (G418S).
2.3.1 Phenotypic analysis of Tf1 transposed colonies
Tf1 transposed patches (YTS6716, YTS6932, YTS7084, and YTS7133) from the nonselective phase of the assay were streaked to obtain single colonies. Two hundred-fifty (250) individual colonies were patched on EMM agar plates supplemented with uracil, Vitamin B1, and 5-FOA. Patches were replica-printed to YES agar plates containing G418 to determine the number of G418S and G418R colonies in the population. G418S colonies were further analyzed by Southern blot hybridization.
2.4. Preparation of Digoxigenin (DIG)-labeled DNA probes for Southern and Northern analysis
All oligonucleotides (MWG Biotech) used for PCR are listed in Table 2. The primers selected for PCR are complementary to regions within the 5’ and 3’ genes adjacent to Tf1 targets. DIG-labeled DNA probes were amplified by incorporating a DIG-11-dUTP alkali-labile nucleotide (Roche Biochemicals) according to the manufacturer protocol. Long Amp enzyme (New England BioLabs) was used for all reactions. Cycle parameters for amplification: hot startdenaturation at 95°Cfor 5 min, followed by 30 cycles of amplification (95°C for 1 min; 60°C, 1 min; and 65°C, 3 min), and a final extension at 65°C for 10 min. The amplified DNA probes were analyzed on a 1.5% agarose gel in Tris-Borate-EDTA buffer. DIG-labeled probes ranged in size from 214–500bp.
Table 2.
Oligonucleotides
| Oligonucleotide | Sequence (5’ to 3’) | Use | Ref |
|---|---|---|---|
| TS217 | GTCAAGTCAGCGTAATGCTCT | 5’ oligo used to create neo gene probe | This study |
| TS218 | GGAAACGTCTTGCTCGAGGC | 3’ oligo used to create neo gene probe | This study |
| TS858 | GTACCACCAGACATAACAACGT | 5’ oligo for PCR probe to ORF Actin gene | This study |
| TS859 | ATGGCTCTGGTATGTGCAAAGC | 3’ oligo for PCR probe to ORF Actin gene | This study |
| TS586 | GGAGTACGGATAAAATGCTT | 5’ oligo for IPCR to neo upstream of HinPI | This study |
| TS587 | CGGTTTGGTTGATGCGAGTG | 3’ oligo for IPCR to neo upstream of HinPI | This study |
| TS760 | CCACTGCCAGTACTCTTGAGAAC | 5’ oligo for PCR probe to ORF SPCC1223.09 | This study |
| TS761 | ACCAAGACTCACACTGTCTACGA | 3’ oligo for PCR probe to ORF SPCC1223.09 | This study |
| TS764 | CGTCTCGTGGAATTAGTCGTGAC | 5’ oligo for PCR probe to ORF SPCC1223.10 | This study |
| TS765 | CCAGGAATGTGGACTGTGTACTA | 3’ oligo for PCR probe to ORF SPCC1223.10 | This study |
| TS851 | CTGATCCTCCTTATAGGCAGTATT | 5’ oligo for PCR probe to ORF SPCC1919.03 | This study |
| TS852 | CTCGAGGTGGAAGCTAATGAGA | 3’ oligo for PCR probe to ORF SPCC1919.03 | This study |
| TS855 | TGGACTGTAGTCAGAGCTGTAGT | 5’ oligo for PCR probe to ORF SPCC1919.04 | This study |
| TS856 | ATGAATCTTGCAGGCGCACGAA | 3’ oligo for PCR probe to ORF SPCC1919.04 | This study |
| TS833 | GAGTGCATCCACCTGGTCCTTT | 5’ oligo for PCR probe to ORF SPBC776.12 | This study |
| TS834 | GTTGACTTTGGATTAGCAGAGCG | 3’ oligo for PCR probe to ORF SPBC776.12 | This study |
| TS837 | CTGAGTATGCTGCTGATCTTGTT | 5’ oligo for PCR probe to ORF SPBC776.13 | This study |
| TS838 | TCTATGATAGCGCATCTCAGAG | 3’ oligo for PCR probe to ORF SPBC776.13 | This study |
| TS796 | GTGCTTCCTCGACATCAGCTG | 5’ oligo for PCR probe to ORF SPAC4A8.03 | This study |
| TS797 | CGTGGGTGGATACATGGCGG | 3’ oligo for PCR probe to ORF SPAC4A8.03 | This study |
| TS800 | TTCTACTGCCTGTGCTGCACCA | 5’ oligo for PCR probe to ORF SPAC4A8.04 | This study |
| TS801 | TGTTCGCTCAAATCAGGCTGCA | 3’ oligo for PCR probe to ORF SPAC4A8.04 | This study |
| TS813 | TAGACGATCTCTTCCAGCAGCTT | 5’ oligo for PCR probe to ORF SPAC57A7.05 | This study |
| TS814 | TCGAAGTCATAAGAGTCGTCTTC | 3’ oligo for PCR probe to ORF SPAC57A7.05 | This study |
| TS817 | GCATCATGTGGTCTCAACGTGACC | 5’ oligo for PCR probe to ORF SPAC57A7.04 | This study |
| TS818 | TAGCAGCATTGGCAGACTCGAC | 3’ oligo for PCR probe to ORF SPAC57A7.04 | This study |
| TS780 | CGATAGTGGCAGCAGAGGTGTGT | 5’ oligo for PCR probe to ORF SPAC13A11.02 | This study |
| TS781 | CGCTGACTTGTACCATGACCTTGA | 3’ oligo for PCR probe to ORF SPAC13A11.02 | This study |
| TS784 | TGGCACTAGCAATTACTACTGGTC | 5’ oligo for PCR probe to ORF SPAC13A11.03 | This study |
| TS785 | AGCTATCAAAGTATGGATTTGATCGGT | 3’ oligo for PCR probe to ORF SPAC13A11.03 | This study |
| TS786 | CCACACGCTTTGGACGAGTCTT | 5’ oligo for PCR probe to ORF SPAC3H5.04 | This study |
| TS787 | AGACACTTCAACGTATGTCAACGC | 3’ oligo for PCR probe to ORF SPAC3H5.04 | This study |
| TS792 | GCTTCTACTCATGAATCTAATTAC | 5’ oligo for PCR probe to ORF SPAC3H5.03 | This study |
| TS793 | TAGCTTGATCACTCTCGTAGGCT | 3’ oligo for PCR probe to ORF SPAC3H5.03 | This study |
| TS544 | CTGAGCTCCTGCACGTTGTAAAG | 5’ oligo for PCR probe to ORF SPCC74.03 | This study |
| TS545 | GGACCGCGTTGGACAGTCTCCG | 3’ oligo for PCR probe to ORF SPCC74.03 | This study |
| TS546 | GTACTGCAGCAATGCTTCTCGGC | 5’ oligo for PCR probe to ORF SPCC74.04 | This study |
| TS547 | ATTGAAGCCGTGATTCTTGCTAGAC | 3’ oligo for PCR probe to ORF SPCC74.04 | This study |
| TS845 | AGCTACCTGTTGGATGGGAATTC | 5’ oligo for PCR probe to ORF SPBC23G7.09 | This study |
| TS846 | CTCCACATCTCTCCAACCAGCT | 3’ oligo for PCR probe to ORF SPBC23G7.09 | This study |
| TS849 | ACCAGAGCTAACATCTATCAAGTC | 5’ oligo for PCR probe to ORF SPBC23G7.10 | This study |
| TS850 | GTCAACAGTTAGTCCTGCCACT | 3’ oligo for PCR probe to ORF SPBC23G7.10 | This study |
| TS839 | TTCACGTATCCAGACGGATCTG | 5’ oligo for PCR probe to ORF SPBC119.03 | This study |
| TS840 | TGACTATTTCATCCAGACCGGC | 3’ oligo for PCR probe to ORF SPBC119.03 | This study |
| TS843 | GCTGGATCGGTACATAGCGTTC | 5’ oligo for PCR probe to ORF SPBC119.04 | This study |
| TS844 | TCTCGTTAGGTCGAGGAACATGA | 3’ oligo for PCR probe to ORF SPBC119.04 | This study |
| TS827 | CGCATGGCGTTTCAGCTGCTT | 5’ oligo for PCR probe to ORF SPBPB2B2.09 | This study |
| TS828 | CAAGGAGTAATCTCACATGGGTG | 3’ oligo for PCR probe to ORF SPBPB2B2.09 | This study |
| TS831 | GGTGCTCTTTAGCTATCAGTAATG | 5’ oligo for PCR probe to ORF SPBPB2B2.10 | This study |
| TS832 | TATTCCTTCGGTTGTTGCTCAAG | 3’ oligo for PCR probe to ORF SPBPB2B2.10 | This study |
| TS612 | GATGATATATTTTTATCTTGTGCAATG | IPCR and sequencing primer | This study |
| TS614 | TATCGATTGTATGGGAAGCCCGA | IPCR and sequencing primer | This study |
| TS621 | CACAGAACATACCAAATATCCA | Sequencing primer for pTS1559-9 | This study |
2.5. Genomic DNA preparation and Southern Blot Hybridization
G418-sensitive cell cultures (10ml) were grown in YES with vigorous shaking overnight at 30°C. Cells were pelleted at 5,000 rpm for 10min and resuspended in 0.1M NaCl. After pelleting, cells were resuspended in Solution A (1M sorbitol, 50mM Na2PO4 [pH 7.5]). Betamercaptoethanol (Sigma Aldrich) and Zymolyase 100T (ICN) was added with an incubation of 37°C for 1 hr. Spheroplasts were collected and gently resuspended in Solution B (50mM Tris-HCl [pH 8.0], 50mM EDTA [pH8.0], 1% sarkosyl). RNase A (10mg/ml; 5ul) was added with an incubation of 37°C for 1 hr. Proteinase K (10mg/ml; 5ul) was added with an incubation of 65°C for 1 hr. Two chloroform:isoamyl alcohol (24:1) and one phenol:chloroform:isoamyl alcohol (25:24:1), and one final chloroform:isoamyl alcohol (24:1) extractions were performed. Genomic DNA was precipitated and resuspended in 1X TE (10mM Tris-HCl, 1mM EDTA) buffer.
Genomic DNA (10ug) was digested with SnaBI restriction enzyme, electrophoresed on a 0.7% agarose gel in 0.5X TBE, and subjected to capillary transfer to positively charged nylon membranes (Roche Biochemical) in Alkaline Transfer Buffer (0.4N NaOH, 1M NaCl). Nylon membranes were hybridized with an 851bp DIG-labeled neo probe in DIG Easy Hybridization Buffer (Roche Biochemical) at 42°C for 12–16 hrs. Chemiluminescent detection assay (Roche Biochemical) was performed according to the manufacturer. Membranes were exposed to x-ray film and analyzed using the ChemiDoc XRS Photodocumentation System (BioRAD).
2.6. Determination of Tf1-neo insertion sites by Inverse PCR
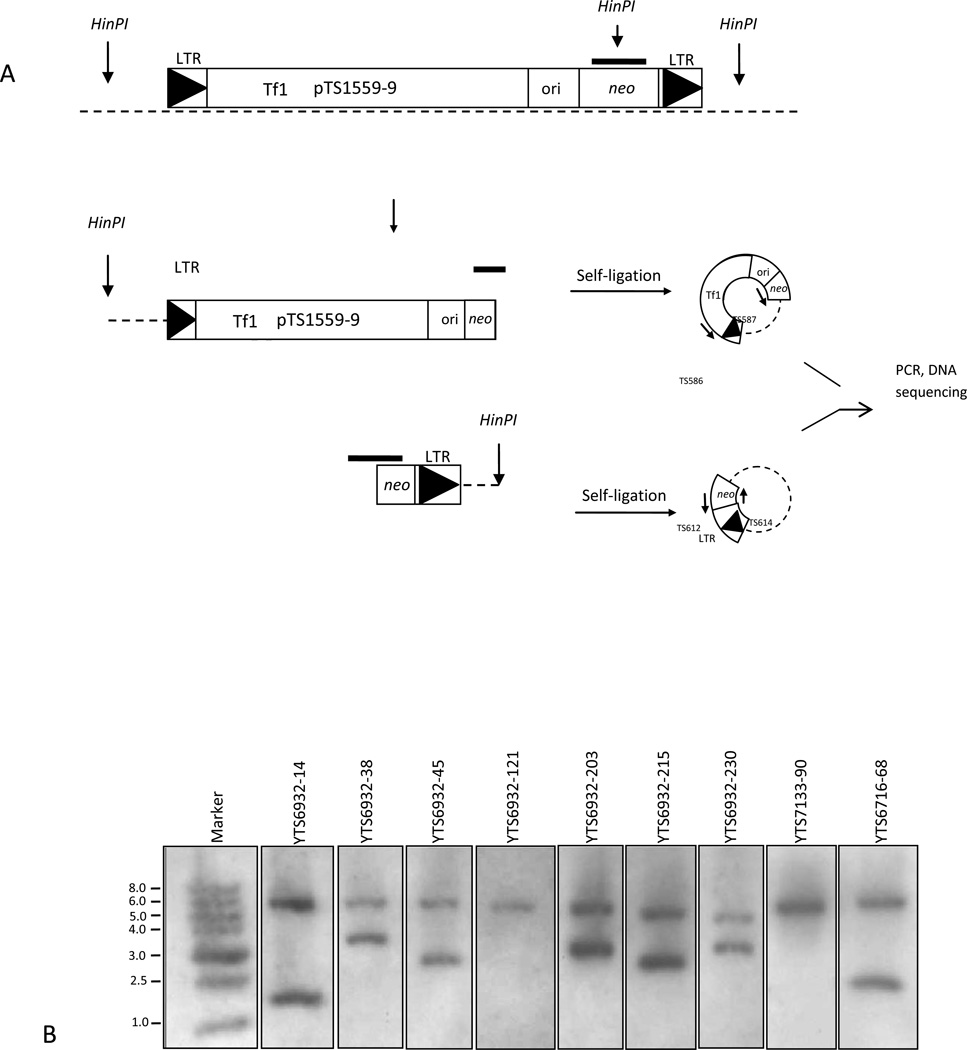
To determine the sequence of the genomic regions flanking the Tf1 integrations, 1ug of genomic DNA from all G418S/neo+ clones was digested with HinP1 restriction enzyme (Fig. 2A). Self-ligation reactions were performed using T4 DNA ligase (New England BioLabs) and incubated at 16°C for 16 hrs. After heat inactivation at 65°C for 10 min the HinP1 self-ligated DNA was subjected to Inverse PCR (iPCR) using the Long Amp Taq PCR system (New England BioLabs). Self-ligated constructs were amplified using primer set TS612/TS614 and primer set TS586/TS587 (Fig. 2A). Long Amp cycling conditions: 94°C for 30 seconds; 94°C for 30 seconds, 60°C for 1 min, and 65°C for 1 min (30 cycles); and a final extension cycle at 65°C for 10 min. Amplifications were analyzed on a 0.7% agarose gel. PCR products were purified (Qiagen PCR purification kit) and sequenced (Wake Forest DNA Sequencing Core, Winston-Salem NC) using primers TS612 and TS614. Tf1 genomic target site locations were determined using the S. pombe genome database of the Sanger Centre (htpp://www.sanger.ac.uk). Sequences were further analyzed using Lasergene software (DNAStar).
Figure 2. Determination of genomic Tf1-neo insertions in G418S/neo+ clones using Inverse PCR (iPCR).
(A). Genomic DNA from G418s/neo+ clones were digested with HinPI, blotted, and hybridized with an 851bp DIG-label neo probe. Genomic locations were identified using IPCR and the S. pombe sequencing database. (B). G418S/neo+ clones YTS6932-121 and YTS7133-90 hybridized with the 851bp DIG-labeled neo probed produced one single HinPI restriction fragment. DNA Marker is a 1kb DNA ladder. Dashed lines represent yeast genomic DNA. Solid bar represents the 851bp DIG-labeled neo probe. Arrows represent restriction sites and PCR primers. Large full triangles represent LTRs.
2.7. Total RNA preparation and Northern Blot Hybridization
Total cellular RNA was isolated from 1x109 G418S/neo+ cells using the Qiagen RNeasy Midi Kit (Qiagen) according to the manufacturer’s protocol. All solutions were treated with Diethylpyrocarbonate (DEPC) or prepared in DEPC treated water. For RNA blot analysis 20ug of total RNA from G418S/neo+ clones were resuspended in 2X loading buffer (50% formamide, 6% Formaldehyde, 1X MOPS, 10% glycerol, 0.05% bromphenol blue) and heated at 65°C for 10 min and cooled. Samples were electrophoresed on a 1% agarose-formaldehyde gel in 1X MOPS buffer for 8 hours under 50 volts. Total RNA was transferred to a positively charged nylon membrane (Roche Biochemical) by capillary transfer in DEPC-treated 20X SSC for 24 hrs. Hybridizations were performed in DIG Easy Hybridization solution with designated DIG-labeled probes. Membranes were analyzed using the DIG Detection system (Roche Biochemicals) and analyzed using the ChemiDoc XRS Photodocumentation System (BioRAD).
2.8. Trichostatin A (TSA) treatment
G418s/neo+ clones YTS6932-14, YTS6932-38, YTS6932-45, YTS6932-121, YTS6932-203, YTS6932-215, YTS6932-230, and YTS6716-68 were treated with the following concentrations of TSA (Sigma Aldrich): 0.6ng, 1.2ng, 2.0ng, 6.0ng, 91.0ng, 0.5ug, 1.0ug, 2.0ug, 3.0ug, 4.0ug, 5.0ug, 10.0ug, 35.0ug, 50.0ug, and 100.0ug/ml. Overnight cultures were diluted to an OD600 of 0.1 in YES broth and grown for 6 days at 30°C. Growth plots were taken at days 3 and 6. Cells were washed in 200ul YES and the whole culture was spotted onto YES-G418 agar plates. Plates were incubated for 3 days at 30°C. Control cultures (Wild type: 972; and G418s/neo+ clones) were grown in YES in the absence of TSA.
3. Results
3.1. In vivo Genome-wide analysis of Tf1 integration events under nonselective conditions
The retrotransposition assay developed to monitor and detect yeast genomic Tf1 integration is based on cell resistance to Geneticin (G418) due to expression of the neomycin (neo) selectable marker during the selective phase of the retrotransposition assay (Levin et al., 1993; Singleton and Levin, 2002). During the nonselective phase cells expressing the URA3 gene located on the donor plasmid are sensitive to 5-FOA present in the media and are therefore, eliminated from the cell population. The selective phase selects for Tf1 chromosomal integration events via expression of the neo selectable marker. To determine if Tf1 suppressed insertions can be identified, we chose to isolate and phenotypically analyze individual colonies isolated from Tf1 transposed patches growing under nonselective conditions. S. pombe strain YTS6932 was transposed with the Tf1-neo donor plasmid pHL449-1 (Levin et al., 1995). S. pombe strains YTS6716, YTS7133, and YTS7084 were transposed with the Tf1-ori/neo donor plasmid pTS1559-9 (Singleton and Levin, 2002). We isolated 250 individual colonies from individual Tf1 transposed patches YTS6932, YTS6716, YTS7084, and YTS7133 from the nonselective phase of the assay. All colonies were exposed to 5-FOA conferring 5-FOA-resistance (5-FOAR) and therefore, are void of the Tf1 donor plasmid. To determine resistance to G418, all individual colonies were replica-printed onto YES/G418 agar plates. We obtained 6 (2.4%) individual G418R colonies from patch YTS6932, 7 (2.8%) individual G418R colonies from patch YTS6716, 7 (2.8%) individual G418R colonies from patch YTS7133, and 4 (1.6%) individual G418R colonies from patch YTS7084 as shown in Table 3. Because Tf1-neo retrotransposition frequencies of 1.0 to 4.0% (Levin and Boeke, 1992) have been reported, our retrotransposition efficiency percentages reported in this study are well within range with patch YTS7084 more on the low end of the efficiency range. The remaining 244 (95% of the colony population), 243 (97.2% of the colony population), 243 (97% of the colony population), and 246 (98% of the colony population) G418S individual colonies derived from patches YTS6932, YTS6716, YTS7133, and YTS7084, respectively, represent cells possibly void of Tf1 insertions or Tf1 insertions suppressed by position effect variegation due to insertion within silent regions of the genome.
Table 3.
Phenotypic and Genotypic Analysis of Tf1 insertion events under nonselective conditions.
| Number of Colonies |
|||||||
|---|---|---|---|---|---|---|---|
| NSP | SP | NSP | NS* | ||||
| Transposed Patch | Tf1 Plasmid | 5-FOAR | G418R | G418S | G418S/neo+ | % Transposition† | Genotype |
| YTS6716 | pTS1559-9 | 250 | 7 | 243 | 2 | 2.8 | Diploid |
| YTS6932 | pHL449-1 | 250 | 6 | 244 | 7 | 2.4 | Haploid |
| YTS7084 | pTS1559-9 | 250 | 4 | 246 | 0 | 1.6 | Haploid |
| YTS7133 | pTS1559-9 | 250 | 7 | 243 | 1 | 2.8 | Haploid |
NSP = Nonselective Phase
SP = Selective Phase
NS* = neo+ by southern blot hybridization using an 851bp DIG-labeled neo probe.
= Transposition frequency of each patch was determined by calculating the total number of 5-FOAR colonies that were also G418R.
To investigate the possibility that the remaining 976 G418S clones could harbor silent Tf1 insertion events, we analyzed the genomes using Southern blotting (Fig. 1). The genomes of the 976 total G418S clones were screened by blot hybridization using an 851bp DIG-labeled neo probe generated by PCR. The probe is complementary to approximately 90% of the neo gene sequence. Surprisingly, blot analysis of all 976 G418S clones identified ten G418S clones signaling the presence of a neo gene as detectable using the 851bp DIG-labeled neo probe (Fig. 1B). We were not able to detect the presence of the neo gene in the genomic DNA of the 250 individual colonies patched from strain YTS7084. We also noticed that patch YTS7084 produced the lowest transposition frequency, which may explain our inability to detect any silent integration events. Our results revealed that approximately 1.0% of the total cell population in the nonselective phase of the transposition assay would have been overlooked under selective conditions. We clearly show the presence of a Tf1 insertion in S. pombe colonies that are 5-FOAR/G418S. The G418R clones YTS6716-2 and YTS7084-4 both produced strong positive signals for the presence of neo, as seen in Fig. 1B. Genomic DNA from wild type strain 972 gave no signal when hybridized with the DIG-labeled neo probe revealing the absence of any endogenous copies of Tf1-neo. The inability of the G418S/neo+ clones to phenotypically display G418R, hints to possible Tf1 insertion within silent regions of the genome.
Figure 1. G418S clones isolated under nonselective conditions contain Tf1 insertion events.
(A) Of the 976 G418S clones analyzed, ten G418S clones are represented here on YES media and replica-printed to YES plates containing G418. Lack of cell growth is due to non-expression of the Tf1-neo gene. Control strains YTS6716-2 and YTS7084-4 are G418R as seen on YES/G418 media plates due to expression of the neo gene. (B) Southern blot analysis of the ten G418S clones are represented. Genomic DNA was isolated from all G418S clones. The DNA was digested with BamHI and Bgl II (which does not restrict Tf1, ori, or neo sequences), separated by gel electrophoresis in a 0.7% agarose gel in 0.5X TBE. Genomic DNA was hybridized with an 851bp DIG-labeled neo probe. Yeast genomic DNA is represented by dashed lines. LTRs are represented by full dark triangles. Arrows depict BamHI and Bgl II restriction sites. The 851bp DIG-labeled neo probes are represented as a thick black horizontal bar above the neo gene.
3.2. Genomic locations of G418S/neo+ Tf1 insertion events
Identification of genomic Tf1 target sites allows analysis of transcriptional activity within the targeted region. To identify the genomic location of all ten G418S/neo+ clones, we used inverse PCR (iPCR) and the S. pombe sequence database. Previous methods used to identify Tf1 genomic insertions (Singleton and Levin, 2002) relied on genomic DNA restriction, recircularization of Tf1-ori/neo insertions with flanking genomic DNA, and bacterial transformations that could introduce selection biases. We employed the iPCR technique to identify the genomic location of all ten Tf1 insertion targets and to reduce any selection biases. Genomic DNA from the G418S/neo+ clones were restricted with HinP1 to produce two detectable fragments containing sections of the neo gene and flanking yeast genomic DNA, Fig. 2A. Because the 851bp DIG-labeled neo probe is complementary to 90% of the neo gene, two distinct HinP1 fragments were detectable in most of the clones. We observed that clones YTS7133-90 and YTS6932-121 both produced only one detectable HinP1 fragment, Fig. 2B. Oligonucleotides TS612 and TS614 and TS585/TS588 were used to sequence the self-ligated insertion events (Fig. 2A). Sequencing data concluded that Tf1 insertions occurred among the three chromosomes of S. pombe (Table 4). All insertions among the G418S/neo+ clones occurred within intergenic regions. Intergenic regions ranged in size from 871bp (YTS6932-230) to 4263bp (YTS6932-203). In Table 4 we report Tf1 insertion events as close as 114bp to the nearest ORF - observed in clone YTS6932-38 - or as far as 1375bp from the nearest ORF – observed in clone YTS6932-203. We were not able to obtain DNA sequence from clone YTS7133-90 possibly due to chromosomal recombination among existing solo LTRs. DNA sequencing of clones YTS6716-68 and YTS6716-102 revealed insertions that occurred at the same exact nucleotide. Interestingly clone YTS6716-68 was found to have inserted 443bp near the MAT1-MC gene, Table 4. The Mat1-MC gene is a known constricted silent region. We also noticed that majority of Tf1 insertions occurred between genes with divergent orientation, Table 4. Chromosomal plotting of all insertion events via contig location is represented in Fig. 3. Strains YTS6932-230, YTS6932-215, and YTS6716-68 contain Tf1 insertions near the centromere, (p7G5) on chromosome 1; the telomere (c212), on chromosome 1; and the matingtype locus (c23G7) on chromosome 2, respectively.
Table 4.
Analysis of Tf1 Intergenic Insertions.
| Strain | Chr | 5’ORF | IR (bp) | 3’ ORF | 5’ and 3’ ORF orientation |
Insertion distance from closest ORF (bp) |
Genotype |
|---|---|---|---|---|---|---|---|
| YTS6932-14 | 3 | SPCC1223.09 | 2533 | SPCC1223.10 (eaf1) | ←← | 866 | G418S |
| YTS6932-38 | 3 | SPCC1919.03 | 2103 | SPCC1919.04 | ← → | 114 | G418S |
| YTS6932-45 | 2 | SPBC776.12 (hsk1) | 1162 | SPBC776.13 (cnd1) | ← → | 174 | G418S |
| YTS6932-121 | 1 | SPAC4A8.03 (ptc4) | 2911 | SPAC4A8.04 (isp6) | ← → | 801 | G418S |
| YTS6932-203 | 1 | SPAC57A7.05 | 4263 | SPAC57A7.04 (pabp) | ← → | 1375 | G418S |
| YTS6932-215 | 1 | SPAC13A11.02 (erg11) | 1898 | SPAC13A11.03 (mcp7) | ← → | 153 | G418S |
| YTS6932-230 | 1 | SPAC3H5.04 (aar2) | 871 | SPAC3H5.03 (pkl1) | ← → | 159 | G418S |
| YTS6716-2 | 3 | SPCC74.03 (ssp2) | 4972 | SPCC74.04 | ← → | 215 | G418R |
| YTS6716-68 | 2 | SPBC23G7.09 (mat1-Mc) | 3239 | SPBC23G7.10 | → ← | 443 | G418S |
| YTS6716-102 | 2 | SPBC23G7.09 (mat1-Mc) | 3239 | SPBC23G7.10 | → ← | 443 | G418S |
| YTS7084-4 | 2 | SPBC119.03 | 1039 | SPBC119.04 (mei3) | → → | 580 | G418R |
| pTS1559-9 | 2 | SPBPB2B2.09 | 2406 | SPBPB2B2.10 | Donor Plasmid |
IR = intergenic region. Number represents distance in base pairs between the adjacent 5’ and 3’ open reading frame (ORF) from the target sequence; Chr = chromosome; bp = base pair
← → Divergent orientation
→ ← Convergent orientation
→ → Tandem orientation
← ← Tandem orientation
Figure 3. Chromosomal locations of all G418s/neo+ Tf1 integration events.
Integration events are bold and underlined. Contigs are represented in parenthesis. Telomere, centromere, mating-type locus, and ribosomal RNA regions are represented by black triangles, red circles, teal hexagons, and purple squares, respectively.
3.3. RNA analysis of G418S/neo+ clones reveals possible mechanisms for gene silencing
Because isolation of Tf1 insertions in this study was not based on expression of the neo gene, we asked whether the neo genes in G418S/neo+ clones are transcriptionally active. The inability of cells to establish resistance to G418 is due to the lack of neo gene expression or absence of the neo gene. To determine if the neo gene is transcriptionally active, we analyzed the eight G418S/neo+ clones by Northern blot hybridization. RNA blots of all eight G418S/neo+ insertion events were hybridized with the 851bp DIG-labeled neo probe. Surprisingly, the 851bp DIG-labeled neo probe was able to detect a 956bp signal in clones YTS6932-14, YTS6932-203, YTS6932-215, and YTS6932-230 representing expression of the neo gene transcripts Fig 4. The inability of the G418S/neo+ clones to grow in the presence of G418 hints to possible silencing of the neo gene. G418R clones YTS6716-2 (diploid) and YTS7084-4 (haploid) both produced strong 956bp signals when hybridized with the 851bp DIG-labeled neo probe, Fig. 4. We were not able to detect neo transcripts from clones YTS6932-38, YTS6932-45, YTS6932-121, and YTS6716-68 when hybridized with an 851bp DIG-labeled neo probe. To determine if the neo gene is active in clones YTS6932-38, YTS6932-45, YTS6932-121, and YTS6716-68, we chose to excise and self-ligate each insertion event and transform into bacteria. We were able to detect Kanamycin resistance in all G418s/neo+ stains. Our inability to detect in vivo transcripts in clones YTS6932-38, YTS6932-45, YTS6932-121, and YTS6716-68 hints to a possible different mechanism of silencing used by S. pombe.
Figure 4. Northern analysis of total RNA isolated from G418s/neo+ clones.
(A) Tf1 insertion clones were cultured in YES liquid and spotted onto YES agar plates. After 3 days the patches were replica-printed to YES agar plates supplemented with G418. All plates were incubated at 30°C. Colonies growing on YES/G418 agar plates represent G418R, and lack of growth represents G418S. (B) Re-hybridization of the RNA blot with an Actin DIG-labeled probe indicates approximately equal loading of RNA in each lane. Northern Blot analysis of G418S/neo+ clones utilized an 851bp DIG-labeled neo probe. Transcripts are detected in strains YTS6932-14, YTS6932-203, YTS6932-215, and YTS6932-230. No expression of neo was detected in strains YTS6932-38, YTS6932-45, YTS6932-121, and YTS6716-68. G418R strains YTS6716-2 and YTS7084-4 were grown in YES (Y), and YES supplemented with G418 (YG) prior to total RNA isolation. S pombe wild type strain 972 and a diploid wild type strain YHL5661 (Singleton and Levin, 2002) were grown in YES prior to total RNA isolation.
To observe transcriptional activity in genes surrounding Tf1’s target site, we analyzed expression of adjacent genes upstream and downstream from each target site using Northern blot hybridization (Table 5). Of the 22 genes analyzed using DIG-labeled PCR probes only three transcripts: SPCC1223.10 (eaf1), SPAC3H5.04 (aar2), and SPBC119.03 (conserved hypothetical protein) were detected (Table 5).
Table 5.
| Insertion Clone/Plasmid | Phenotype | Adjacent genes | Protein function |
|---|---|---|---|
| Gene expression (+/−) | |||
| YTS6932-14 | |||
| − | G418S | 5’-SPCC1223.09 | uricase* |
| + | 3’-SPCC1223.10 (eaf1) | RNA pol II transcription elongation factor | |
| YTS6932-38 | |||
| − | G418S | 5’-SPCC1919.03 (amk2) | AMP-activated protein kinase beta subunit* |
| − | 3’-SPCC1919.04 | Uncharacterized membrane protein | |
| YTS6932-45 | |||
| − | G418S | 5’-SPBC776.12 (hsk1) | Protein kinase |
| − | 3’-SPBC776.13 (cnd1) | Condensin complex subunit 1 | |
| YTS6932-121 | |||
| − | G418S | 5’-SPAC4A8.03 (ptc4) | protein phosphatase |
| − | 3’-SPAC4A8.04 (isp6) | vacuolar serine protease | |
| YTS6932-203 | |||
| − | G418S | 5’-SPAC57A7.05 | conserved hypothetical protein |
| − | 3’-SPAC57A7.04 (pabp) | mRNA export shuttling protein | |
| YTS6932-215 | |||
| − | G418S | 5’-SPAC13A11.02 (erg11) | Probable cytochrome P450 protein |
| − | 3’-SPAC13A11.03 (mcp7) | Required for meiotic recombination | |
| YTS6932-230 | |||
| + | G418S | 5’-SPAC3H5.04 (aar2) | U5 snRNP-associated protein |
| − | 3’-SPAC3H5.03 (pkl1) | kinesin-like protein | |
| YTS6716-2 | |||
| − | G418R | 5’-SPCC74.03 (ssp2) | serine/threonine protein kinase |
| − | 3’-SPCC74.04 | amino acid permease* | |
| YTS6716-68/102 | |||
| − | G418S | 5’-SPBC23G7.09 (mat1-Mc) | Mating-type M-specific polypeptide |
| − | 3’-SPBC23G7.10 | NADH-dependent flavin oxidoreductase* | |
| YTS7084-4 | |||
| + | G418R | 5’-SPBC119.03 | conserved hypothetical protein |
| − | 3’-SPBC119.04 (mei3) | meiosis inducing protein | |
| pTS1559-9 | |||
| − | Plasmid | 5’-SPBPB2B2.09 | 2-dehydropantoate 2-reductase |
| − | 3’-SPBPB2B2.10 | Galactose-1-phosphate uridylyltransferase | |
predicted protein function
3.4. TSA treatment restores G418-resistant phenotypes in G418s/neo+ clones
The loss of neo gene silencing, via the loss of G418-sensitivity as measured by the ability of cells to grow in the presence of G418, is related to possible alterations of histone modifications indicative of nonsilent chromatin. In order to study any relationship between genomic silencing of Tf1 integration events and possible histone deacetylation, G418s/neo+ clones YTS6932-14, YTS6932-45, YTS6932-121, YTS6932-203, YTS6932-215, YTS6932-230, and YTS6716-68 were grown in the presence of increasing concentrations of the histone deacelylase inhibitor Trichostatin A (TSA). All G418s/neo+ clones were treated with increasing concentrations of TSA (0.6, 1.2, 2.0, 6.0, 91.0ng/ml, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 10.0, 35.0, 50.0, and 100.0ug/ml). To determine the effect of TSA on the growth of G418s/neo+ clones, we chose to measure cell growth rate in liquid cultures prior to spotting the cultures onto YES/G418 agar plates. Figure 5B reveals the cellular growth curves of various concentrations of TSA and no TSA treated cultures. No significant defect in growth rates of cells treated with TSA as compared to growth rates without TSA treatment (Figure 5B) was observed. We did notice a higher cell count in cultures treated with 0.6ng and 1.2ng TSA compared to no TSA treatment. After TSA treatment whole culture suspensions (200ul) were spotted on YES-G418 agar plates. Surprisingly, G418-resistant colonies were observed among strains YTS6932-14, YTS6932-203, YTS6932-215, and YTS6932-230 Fig. 5A. Because undiluted whole cultures were spotted the population of colonies conferring G418-resistance could be observed. Strains YTS6932-14 resulted in more G418-resistant colonies at TSA concentration 3.0ug/ml. YTS6932-203 and YTS6932-215 produced more G418-resistant colonies within 1.0–5.0ug/ml TSA with 2.0ug/ml resulting in more colonies. Strain YTS6932-230 produced more G418-resistant colonies at the lower TSA concentrations of 91ng/ml, 0.5ug/ml, 1.0 and 3.0ug/ml. No colonies were observed at 100ug TSA concentration. Interestingly, we were not able to observe any G418-resistant colonies from TSA treated cultures: YTS6932-38 YTS6932-45, YTS6932-121, and YTS6716-68. Cultures YTS6932-14, YTS6932-203, YTS6932-215, and YTS6932-230 at TSA concentrations of 3.0ug/ml, 2.0ug/ml, 4.0ug/ml, and 0.5ug/ml, respectively, were spotted using a 5-fold dilution on YES/G418 agar, Fig. 6. Interestingly, we were able to draw a sharp correlation between G418s/neo+ clones void of an RNA transcript (Fig. 4B) and TSA treated G418s/neo+ clones (Fig 5A). It is clear that clones (YTS6932-38 YTS6932-45, YTS6932-121, and YTS6716-68) not expressing a neo transcript were also not able to produce G418-resistant clones after treatment with increasing concentrations of TSA,
Figure 5. TSA treatment of silent Tf1 insertions results in the loss of G418-sensitivity.
(A). Effects of TSA on the growth rates of G418s/neo+ clones. All cells were synchronized at an OD600 of 0.1. Growth rates at day 6 of TSA treated cells in increasing concentrations were plotted. The concentrations of TSA used in this study are presented. Wild-type (972) was also cultured in medium of increasing concentrations of TSA or lacking TSA. (B). Whole cultures (200ul TSA treated) were washed in YES media and spotted onto YES/G418 agar plates and incubated for 3 days at 30°C.
Figure 6. G418s/neo+ clones treated with Trichostatin A (TSA) results in a loss of neo silencing.
G418S/neo+ clones were cultured in medium containing TSA and allowed to grow for 6 days at 30°C. These colonies were obtained from the whole culture spots (Fig. 5B). Cell numbers were adjusted to OD600 of 1.0. Cells were washed in YES and serially diluted 1:5. In all cases, 3ul of each cell suspension was spotted onto a YES-G418 agar plate. Strain YTS6932-14, YTS6932-203, YTS6932-215, and YTS6932-230 were the only strains isolated from YES/G418 agar plates.
3.5. The 3’ flanking region of Tf1-neo donor plasmid reveals preexisting integration within a silent region of the genome
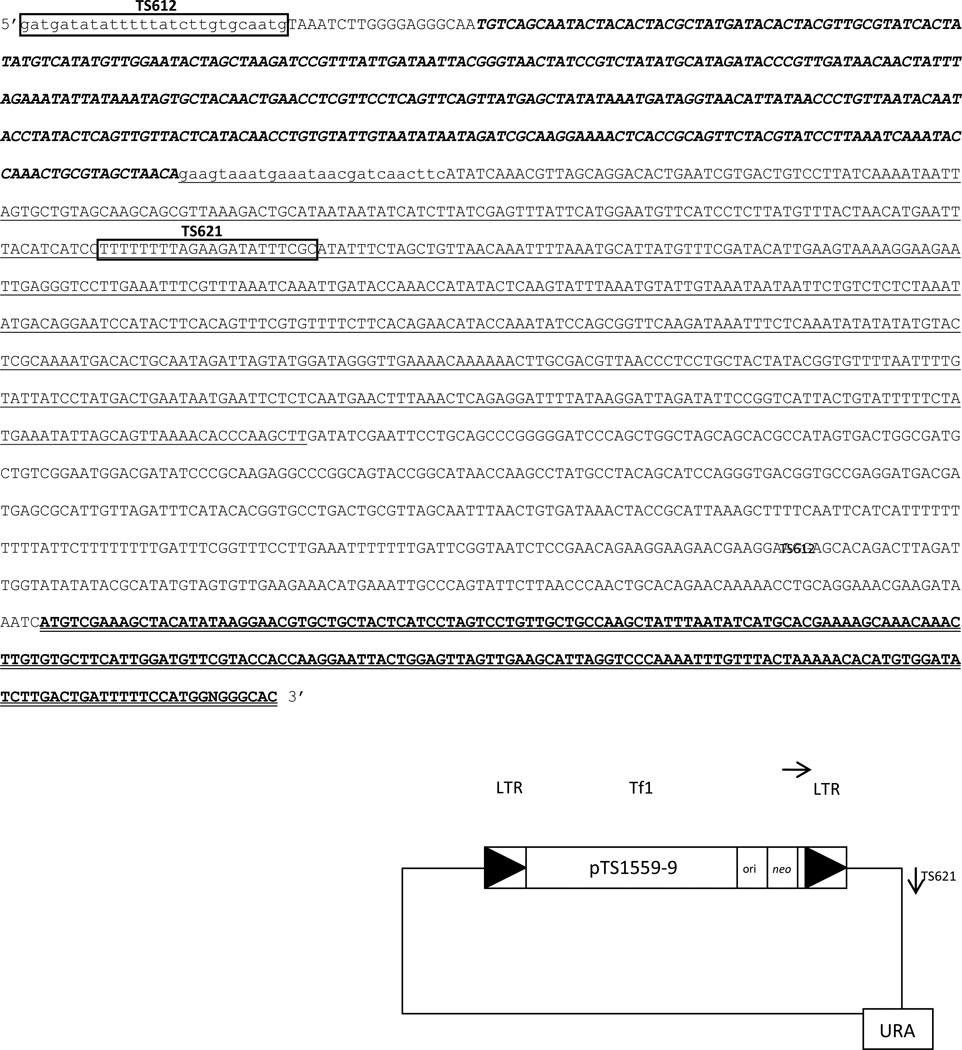
A genomic library of S. pombe NCYC132 was originally used to isolate Tf1-107 clone (Levin et al., 1990). The cloned XhoI-BamHI fragment contained flanking genomic DNA from the 3’ LTR. The flanking genomic DNA not only reveals the genomic location of the target site of Tf1-107 clone, but also revealed information about the genomic region chosen for integration. To identify the target site region chosen for this primitive Tf1 insertion, we chose to analyze genomic DNA sequence flanking the 3’LTR of the Tf1-ori/neo donor plasmid pTS1559-9. Plasmid pTS1559-9 contains the plasmid backbone for cloned Tf1-107 (Levin et al., 1990; Levin et al., 1993). Using sequencing primers TS612, and TS621, we were able to identify the genomic location chosen for Tf1-107 clone, Fig. 7. Surprisingly, Tf1-107 was found to have inserted 59kb upstream from the right end of chromosome 2. Insertion is within a 2.406kb intergenic region between genes SPBPB2B2.09 and SPBPB2B2.10. Tf1-107 inserted 2bp from the SPBPB2B2.09 ORF. More amazing is the presence of three solo LTRs downstream from the target site, indicating a favorable region for previous integrations. The most proximal anchored cosmid to the telomere region on chromosome 2 is pT2R1. We found that Tf1-107 inserted within cosmid pB2B2, which is located upstream from cosmid pT2R1 (ftp://ftp.ebi.ac.uk/pub/databases/pombase/pombe/Cosmid_assembly_data/SP_chr2_dump.text). Cosmid pT2R1 is defined as the telomeric cosmid for chromosome 2. Because telomeic regions are heterochromatic regions and defined as silent, we chose to analyze the genes adjacent to the Tf1-107 target site. We were not able to detect transcripts from genes SPBPB2B2.09 and SPBPB2B2.10. This further defines this area of chromosome 2 targeted for Tf1integration as silent.
Figure 7.
Bold italic sequence represents the 3’ LTR. Boxed sequences represent DNA primers. Single underlined sequence represents genomic flanking the 3’ LTR. Double-underlined region represents the Ura gene present on pTS1559-5.
4. Discussion
In this study we report an in vivo genome-wide analysis of Tf1 integration events from the nonselective phase of the retrotransposition assay. In this study we identified ten G418-sensitive clones harboring a genomic copy of Tf1-neo and Tf1-ori/neo. The ten G418S/neo+ clones were isolated from four independent transposed patches. Tf1 targets were sequenced by iPCR and found to span all three chromosomes of S. pombe. Analysis of each target site revealed a target preference for intergenic regions of the genome with insertion distances ranging from 100-400 nucleotides from the closest 5’ ORF as previously reported (Brehens et al., 2000; Singleton and Levin, 2002). The most surprising results came from the RNA analysis. In our experiments we were able to detect transcription activity from the neo gene in 50% of the G418S/neo+ clones (Fig. 3).
The techniques used in this study were designed to: (1) identify targets that may have occurred in silent regions of the genome that otherwise would have gone undetected, and (2) analyze the genome in vivo using the S. blot to eliminate any possible selection biases when transformed into bacteria. Because our previous report of Tf1 insertion events were based on self-ligation and selection using bacteria, (Behrens et al., 2000; Singleton and Levin, 2002) we were unable to analyze a cohort of Tf1 insertions in the parental cell. The techniques used in this study address the direct in vivo analysis of Tf1 insertion events thereby, allowing possible identification of insertions within silent regions of the genome.
The ability of cells to protect themselves upon viral infection is clearly demonstrated in bacteria via the restriction/modification system (Vasu and Nagaraja, 2013). Fission yeast cells have in place mechanisms such as DNA methylation, and histone modification, to control retroviral activity (Goto and Nakayama, 2011). There are increasing reports suggesting that RNAi may play a role in silencing virally infected eukaryotic cells (Haddad et al., 2011; Houzet and Jeang, 2011b; Vasu and Nagaraja, 2013). RNAi was also shown to be involved in the inhibition of viruses and silencing of viruses in plants, insects, fungi, and nematodes (Voinnet, 2001; Waterhouse et al., 2001; Wilkins et al., 2005; Wang et al., 2006; Segers et al., 2007). Our data suggests S. pombe is utilizing different silencing mechanisms that are observed in 50% of G418S/neo+ clones. Our inability to detect the neo transcript or produce G418-resistance in G418S/neo+ clones YTS6932-38, YTS6932-45, YTS6932-121, and YTS6716-68 (Fig. 3), suggest yet another silencing mechanism possibly not related to histone deacelylation. This mechanism could involve interactions of Tf1’s chromodomain and proteins involved in the establishment of heterochromatin. Future studies would clarify the mechanisms involved.
It is plausible that TSA-sensitive deacetylases in fission yeast are different based upon the area of chromatin chosen for silencing. Based on our findings, that G418s/neo+ clones are able to obtain G418-resistance at various TSA concentrations, indicates the existence of various TSA-sensitive histone deacetylase complexes. This explains the differences of G418-resistant clones obtained after various TSA concentration treatments.
Analysis of the Tf1 donor plasmid p1559-9 revealed essential information concerning integration of Tf1. Tf1-107 was originally isolated from S. pombe library NCYC132 (Levin et al., 1990). The flanking DNA was analyzed and found not to be tRNA-like sequences but location of the insertion was not determined at that time because sequencing was very limited. We chose to analyze the target site of Tf1-107 and, surprisingly, we identified the chromosomal location to be very close to the telomeric region on chromosome 2. Analysis of the available S. pombe genome database identified the target location on chromosome 2 within cosmid pB2B2. Amazingly, the insertion site is 59 kilobasepairs from the end of chromosome 2 and upstream from cosmid pT2R1 that contains the telomere. The presence of 3 solo-LTRs (one Tf2-type, and two Tf1 type) within the intergenic region suggests a favorable region for Tf1 insertion. The use of a library allowed isolation and cloning of Tf1-107. This insertion event would have gone undetectable under selectable conditions of the retrotransposition assay.
Research Highlights.
A genome-wide analysis of Tf1 integration events under nonselective conditions.
Southern blot analysis reveals the presence of Tf1 insertion events in G418s clones.
RNA analysis of G418-sensitive clones detects a neo transcript.
ACKNOWLEDGEMENTS
We sincerely thank Dr. Alisea Williams-McLeod for reading the manuscript. This work was funded by a grant from the National Institutes of Health, Grant No. 5P20MD002303-02 from the National Center on Minority Health and Health Disparities.
Abbreviations
- TSA
Trichostatin A
- HDAC
Histone deacetylase
- IPCR
Inverse Polymerase Chain Reaction
- G418
Geneticin
- Neo
neomycin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristina E. Cherry, Email: kriswilliams1234@gmail.com, Winston Salem State University.
Willis E. Hearn, Email: willishearn19@gmail.com, Winston Salem State University.
Osborne Y. Seshie, Email: oseshie111@rams.wssu.edu, Winston Salem State University.
Teresa L. Singleton, Winston Salem State University
Teresa L. Singleton, Associate Professor of Microbiology, Director, Undergraduate Biotechnology Degree Program Department of Life Science WBA Science Building, Room 406 Winston-Salem State University Winston-Salem, NC 27110 (336) 750-3238.
References
- Asante-Appiah E, Skalka AM. Molecular mechanisms in retrovirus DNA integration. Antiviral Res. 1997;36:139–156. doi: 10.1016/s0166-3542(97)00046-6. [DOI] [PubMed] [Google Scholar]
- Behrens R, Hayles J, Nurse P. Fission yeast retrotransposon Tf1 integration is targeted to 5' ends of open reading frames. Nucleic Acids Res. 2000;28:4709–4716. doi: 10.1093/nar/28.23.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke JD, Corces VG. Transcription and reverse transcription of retrotransposons. Annu Rev Microbiol. 1989;43:403–434. doi: 10.1146/annurev.mi.43.100189.002155. [DOI] [PubMed] [Google Scholar]
- Boeke JD, Devine SE. Yeast retrotransposons: finding a nice quiet neighborhood. Cell. 1998;93:1087–1089. doi: 10.1016/s0092-8674(00)81450-6. [DOI] [PubMed] [Google Scholar]
- Boeke JD, Garfinkel DJ, Styles CA, Fink GR. Ty elements transpose through an RNA intermediate. Cell. 1985;40:491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- Chatterjee AG, Leem YE, Kelly FD, Levin HL. The chromodomain of Tf1 integrase promotes binding to cDNA and mediates target site selection. J Virol. 2009;83:2675–2685. doi: 10.1128/JVI.01588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio MJ, Garfinkel DJ. Regulation of retrotransposition in Saccharomyces cerevisiae. Mol Microbiol. 1991;5:1823–1829. doi: 10.1111/j.1365-2958.1991.tb00806.x. [DOI] [PubMed] [Google Scholar]
- Eissenberg JC. Molecular biology of the chromo domain: an ancient chromatin module comes of age. Gene. 2001;275:19–29. doi: 10.1016/s0378-1119(01)00628-x. [DOI] [PubMed] [Google Scholar]
- Forsburg SL, Rhind N. Basic methods for fission yeast. Yeast. 2006;23:173–183. doi: 10.1002/yea.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis KE, Spiker S. Identification of Arabidopsis thaliana transformants without selection reveals a high occurrence of silenced T-DNA integrations. Plant J. 2005;41:464–477. doi: 10.1111/j.1365-313X.2004.02312.x. [DOI] [PubMed] [Google Scholar]
- Gelvin SB. Traversing the Cell: Agrobacterium T-DNA's Journey to the Host Genome. Front Plant Sci. 2012;3:52. doi: 10.3389/fpls.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto DB, Nakayama JI. RNA and epigenetic silencing: Insight from fission yeast. Dev Growth Differ. 2011 doi: 10.1111/j.1440-169X.2011.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Kashima S, Rodrigues ES, Azevedo R, Palma PV, de Magalhães DA, Zago MA, Covas DT. Silencing of HTLV-1 gag and env genes by small interfering RNAs in HEK 293 cells. J Virol Methods. 2011;173:92–98. doi: 10.1016/j.jviromet.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KR, Burns G, Mata J, Volpe TA, Martienssen RA, Bähler J, Thon G. Global effects on gene expression in fission yeast by silencing and RNA interference machineries. Mol Cell Biol. 2005;25:590–601. doi: 10.1128/MCB.25.2.590-601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizi A, Levin HL. The integrase of the long terminal repeat-retrotransposon tf1 has a chromodomain that modulates integrase activities. J Biol Chem. 2005;280:39086–39094. doi: 10.1074/jbc.M506363200. [DOI] [PubMed] [Google Scholar]
- Houzet L, Jeang KT. Genome-wide screening using RNA interference to study host factors in viral replication and pathogenesis. Exp Biol Med (Maywood) 2011a;236:962–967. doi: 10.1258/ebm.2010.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzet L, Jeang KT. MicroRNAs and human retroviruses. Biochim Biophys Acta. 2011b;1809:686–693. doi: 10.1016/j.bbagrm.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S, Heidmann T. An indicator gene for detection of germline retrotransposition in transgenic Drosophila demonstrates RNA-mediated transposition of the LINE I element. EMBO J. 1991;10:1927–1937. doi: 10.1002/j.1460-2075.1991.tb07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jern P, Sperber GO, Blomberg J. Use of endogenous retroviral sequences (ERVs) and structural markers for retroviral phylogenetic inference and taxonomy. Retrovirology. 2005;2:50. doi: 10.1186/1742-4690-2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SI, Veena and Gelvin SB. Genome-wide analysis of Agrobacterium T-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J. 2007;51:779–791. doi: 10.1111/j.1365-313X.2007.03183.x. [DOI] [PubMed] [Google Scholar]
- Koonin EV, Senkevich TG, Dolja VV. The ancient Virus World and evolution of cells. Biol Direct. 2006;1:29. doi: 10.1186/1745-6150-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HL. A novel mechanism of self-primed reverse transcription defines a new family of retroelements. Mol Cell Biol. 1995;15:3310–3317. doi: 10.1128/mcb.15.6.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HL, Boeke JD. Demonstration of retrotransposition of the Tf1 element in fission yeast. EMBO J. 1992;11:1145–1153. doi: 10.1002/j.1460-2075.1992.tb05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HL, Weaver DC, Boeke JD. Two related families of retrotransposons from Schizosaccharomyces pombe. Mol Cell Biol. 1990;10:6791–6798. doi: 10.1128/mcb.10.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HL, Weaver DC, Boeke JD. Novel gene expression mechanism in a fission yeast retroelement: Tf1 proteins are derived from a single primary translation product. EMBO J. 1993;12:4885–4895. doi: 10.1002/j.1460-2075.1993.tb06178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Chan CY, He ML. RNA interference and antiviral therapy. World J Gastroenterol. 2007;13:5169–5179. doi: 10.3748/wjg.v13.i39.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik HS, Eickbush TH. Modular evolution of the integrase domain in the Ty3/Gypsy class of LTR retrotransposons. J Virol. 1999;73:5186–5190. doi: 10.1128/jvi.73.6.5186-5190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann TA, Reznikoff WS. Tn5 transposase with an altered specificity for transposon ends. J Bacteriol. 2002;184:233–240. doi: 10.1128/JB.184.1.233-240.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer SB, Hansen LJ, Chalker DL. Integration specificity of retrotransposons and retroviruses. Annu Rev Genet. 1990;24:491–518. doi: 10.1146/annurev.ge.24.120190.002423. [DOI] [PubMed] [Google Scholar]
- Segers GC, Zhang X, Deng F, Sun Q, Nuss DL. Evidence that RNA silencing functions as an antiviral defense mechanism in fungi. Proc Natl Acad Sci U S A. 2007;104:12902–12906. doi: 10.1073/pnas.0702500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton TL, Levin HL. A long terminal repeat retrotransposon of fission yeast has strong preferences for specific sites of insertion. Eukaryot Cell. 2002;1:44–55. doi: 10.1128/EC.01.1.44-55.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasu K, Nagaraja V. Diverse functions of restriction-modification systems in addition to cellular defense. Microbiol Mol Biol Rev. 2013;77:53–72. doi: 10.1128/MMBR.00044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O. RNA silencing as a plant immune system against viruses. Trends Genet. 2001;17:449–459. doi: 10.1016/s0168-9525(01)02367-8. [DOI] [PubMed] [Google Scholar]
- Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse PM, Wang MB, Lough T. Gene silencing as an adaptive defence against viruses. Nature. 2001;411:834–842. doi: 10.1038/35081168. [DOI] [PubMed] [Google Scholar]
- Wilkins C, Dishongh R, Moore SC, Whitt MA, Chow M, Machaca K. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature. 2005;436:1044–1047. doi: 10.1038/nature03957. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhang F, Hancock CN, Wessler SR. Transposition of the rice miniature inverted repeat transposable element mPing in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U S A. 2007;104:10962–10967. doi: 10.1073/pnas.0702080104. [DOI] [PMC free article] [PubMed] [Google Scholar]