Abstract
Anxiety and depression are prevalent, impairing disorders. High comorbidity has raised questions about how to define and classify them. Structural models emphasise distinctions between “fear” and “distress” disorders while other initiatives propose they be defined by neurobiological indicators that cut across disorders. This study examined startle reflex (SR) modulation in adolescents with principal fear disorders (specific phobia; social phobia) (n = 20), distress disorders (unipolar depressive disorders, dysthymia, generalized anxiety disorder; post-traumatic stress disorder) (n = 9), and controls (n = 29) during (a) baseline conditions, (b) threat context conditions (presence of contraction pads over the biceps muscle), and (c) an explicit threat cue paradigm involving phases that signalled safety from aversive stimuli (early and late stages of safe phases; early stages of danger phases) and phases that signalled immediate danger of an aversive stimulus (late stages of danger phases). Adolescents with principal fear disorders showed larger SRs than other groups throughout safe phases and early stages of danger phases. SRs did not differ between groups during late danger phases. Adolescents with principal distress disorders showed attenuated SRs during baseline and context conditions compared to other groups. Preliminary findings support initiatives to redefine emotional disorders based on neurobiological functioning.
Keywords: Fear disorders, Distress disorders, Startle reflexes, Adolescents
1. Introduction
Anxiety and depression during adolescence are highly prevalent emotional disorders that cause significant concurrent and long-term impairment and economic burden (Bittner et al., 2007; Verduin and Kendall, 2007; Mathews et al., 2011). Furthermore, there is high comorbidity across the life span among anxiety disorders, and between anxiety and depressive disorders (e.g., Kashani and Orvaschel, 2000; for a review, see Craske and Waters, 2005). Such comorbidity has raised questions about how best to classify and define psychiatric disorders, including proposed revisions to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) and the International Classification of Diseases (ICD-10). While the DSM and ICD generally emphasise self-reported or observable behaviours and emotional features as criteria for individual disorders, the aim of the recent Research Domain Criteria (RDoC) initiative of the National Institute of Health is to characterize psychiatric disorders in terms of neurobiological indicators that cut across disorders as traditionally defined (Insel and Cuthbert, 2009; Craske, 2012).
Comorbidity between anxiety and depressive disorders has been explained in various models of psychiatric disorders as reflecting a broad “internalizing” factor (Kendler et al., 2003; Watson, 2005; Krueger and Markon, 2006; Lahey et al., 2008; Seeley et al., 2011) which subsumes two related sub-factors: “fear” and “distress” disorders (e.g., Clark and Watson, 2006; Prenoveau et al., 2010). Specific phobia, social phobia, separation anxiety disorder, panic disorder, and agoraphobia form the “fear” disorders, while generalised anxiety disorder (GAD), depression, post-traumatic stress disorder (PTSD), dysthymia, and possibly obsessive-compulsive disorder (OCD) form the “distress” disorders (Clark and Watson, 2006; Prenoveau et al., 2010). The major sources of evidence for the distinction between fear and distress disorders have come from self-report and genetic data (e.g., Kendler, 1996). However, more recent evidence emerging from cognitive science suggests that fear disorders may be characterised by attention biases away from threat (i.e., threat avoidance) while anxiety-related distress disorders (i.e., GAD) are characterised by attention biases towards threat (i.e., threat vigilance) (e.g., Salum et al., 2013; Waters et al., 2014b). Moreover, recent reviews of event-related negativity and eye blink startle reflex (SR) data as a function of anxiety and depressive disorders (see below) have revealed distinct neurophysiological indicators that show some consistency with structural models (e.g., Vaidyanathan et al., 2012).
The SR is a widely used psychophysiological index of human defensive responding. The SR involves the contraction of the orbicularis oculi muscle in response to a sudden, unexpected stimulus and is one of many obligatory somatic and visceral changes that comprise the human startle reaction (Graham, 1979). A variety of procedures have been used to examine SR modulation in threatening emotional states, including aversive picture-viewing and imagery experiments, as well as fear-potentiation paradigms when SR magnitudes have been assessed during explicit threat conditions (e.g., a cue signalling immediate threat of shock), when participants anticipate shocks, and during safe conditions that predict the absence of shocks. “Baseline” SR magnitudes have also been assessed either before and after fear-potentiation paradigms when threat of shock was explicitly absent or during non-cued phases throughout experiments, while context-potentiated SR refers to amplified SRs when a cue of an upcoming or unpredictable threat may be present.
Several studies have reported that individuals diagnosed with phobias (e.g., animal phobias; non-generalized social phobia), or with high levels of phobic symptoms or trait fearfulness, demonstrate greater SR magnitudes during aversive picture-viewing or imagery while showing relatively normal baseline SR magnitudes (de Jong et al., 1991; Vrana et al., 1992; Globisch et al., 1999; Cuthbert et al., 2003; Lang et al., 2007; McTeague et al. 2009; 2012; Vaidyanathan et al., 2009a). Inconsistent results have been found for panic disorder (Grillon et al., 1994; Vaidyanathan et al., 2009b; McTeague et al., 2011) and generalised social phobia (McTeague et al., 2009), suggesting they may be characterised by more generalised SR activation similar to the broader and more chronic anxiety disorders discussed below. In studies utilising fear-potentiation paradigms, SR may be larger overall in some fear disorders, such as panic disorder, throughout fear-potentiation paradigms (suggestive of anticipatory apprehension) but not during baseline phases before and after fear-potentiation paradigms when threat is explicitly absent (Grillon et al., 1994). Few studies of circumscribed fears, such as specific phobias and non-generalized social phobia have utilised fear-potentiation paradigms. However, the fear conditioning literature suggests that fear disorders, such as panic disorder and social phobia, are characterised by overgeneralized defensive responding to stimuli that are safe (i.e., CS−) but are associated with danger cues (i.e., CS+) (see Lissek et al., 2009; see Lissek, 2012, for review).
In contrast, distress-related anxiety disorders such as PTSD, OCD and GAD are more consistently associated with elevated baseline SR magnitudes and amplified context-potentiated SR relative to healthy controls (Morgan et al., 1995; Grillon et al., 1996; Kumari et al., 2001; Cuthbert et al. 2003; Grillon et al., 2008; Ray et al. 2009; Pole et al., 2009). Results regarding fear-potentiated SR have been more mixed (e.g., Kumari et al. 2001; Cuthbert et al., 2003; Kaviani et al., 2004; Lang et al., 2007). PTSD and GAD have also been linked to overgeneralized defensive responding to safe stimuli (i.e., CS−) in conditioning experiments (see Lissek, 2012; Lissek et al., 2013), and SR results for PTSD in particular have been considerably mixed compared with findings for other anxiety disorders (Grillon & Baas, 2003; Pole, 2007; Vaidyanathan et al., 2009b).
Other studies have shown that depression-related distress disorders have an attenuating effect upon affective and fear-potentiated SR relative to healthy controls (Allen et al., 1999; Kaviani et al., 2004; Forbes et al., 2005; McTeague et al., 2009) and anxious samples without depression (Melzig et al., 2007). Moreover, SR modulation during emotional picture viewing in anxiety disorders is blunted by comorbid depression (Taylor-Clift et al., 2011). Depression has also been linked to diminished physiological reactivity during the anticipation of threat in conditioning experiments in offspring of mothers with a principal depressive disorder relative to offspring of mothers with a principal anxiety disorder and low risk offspring (Waters et al., 2014a). These findings could suggest that depressive disorders are associated with blunted or context insensitive emotional responding (Rottenberg et al., 2005). However, other research has found that depressive disorders are characterised by elevated SR throughout fear-potentiation experiments, contextual anxiety (i.e., placement of shock electrodes) and when shock is predictable (Grillon et al., 2013), while other findings suggest that depressive disorders have no additional effect on the elevated SR magnitudes of adults with panic disorder relative to controls during fear-potentiation experiments (Shankman et al., 2013). Differences in findings may be due to variation in methodology. Blunting in depression has been obtained during viewing of pictures and films, or emotional imagery (Allen et al., 1999; Taylor-Clift et al., 2011; McTeague et al., 2012) or in anticipation of aversive tones (Waters et al., 2014a). Thus, threat is mild, imaginary, and/or may lack personal relevance (Grillon et al., 2013). Therefore, it may be adaptive for depressed individuals to disengage from these types of threats but not from more explicit danger such as a shock in fear-potentiation paradigms (Nesse, 2000; Grillon et al., 2013; Shankman et al., 2013).
As is clear, wide variation in methodology exists across studies. Furthermore, experimental protocols that separate baseline, context and explicit threat phases might help to clarify the conditions under which neurophysiological markers cut across versus differentiate fear-related and distress-related disorders (Watson, 2005; Insel and Cuthbert, 2009; Seeley et al., 2011; Craske, 2012). Therefore, the current study utilised a SR modulation protocol involving a baseline condition when threat of an aversive muscle contraction was explicitly absent, a context condition when a cue of later threat was present (i.e., presence of muscle contraction pads over the biceps muscle), and an explicit threat cue paradigm involving phases that signalled safety from aversive stimuli (i.e., early and late stages of safe phases; early stages of danger phases) and phases that signalled immediate danger of an aversive stimulus (i.e., late stages of danger phases).
If fear disorders are characterised by overgeneralized defensive responding to safe stimuli associated with threat (e.g., Grillon et al., 1994; Lissek et al., 2009), then adolescents with a principal fear disorder (i.e., either specific phobia or non-generalised social phobia) were expected to show amplified SRs during safe phases of the explicit threat cue paradigm compared to healthy controls and adolescents with principal distress disorders. However, groups were not expected to differ in SR magnitudes in response to immediate danger of an aversive stimulus (i.e., late stages of danger phases), reflecting a biologically imperative defence response to explicit threat (Lissek et al., 2005; Craske et al., 2009). In contrast, if distress disorders are associated with elevated SRs during baseline and context conditions (i.e., placement of shock electrodes) within experiments involving fear-potentiation paradigms (Grillon et al., 1996; Pole et al., 2009; Grillon et al., 2013), i.e., the explicit threat cue paradigm in the present study, then adolescents with a principal distress disorder (i.e., either GAD, PTSD or a depressive disorder) were expected to show larger SR magnitudes during the baseline and context conditions in comparison with controls and those with a principal fear disorder.
2. Methods
2.1 Participants
Participants were high school juniors from schools in suburban Chicago, Illinois, and suburban Los Angeles, California who participated in the Northwestern University-University of California, Los Angeles (NUCLA) Youth Emotion Project (YEP) (see Craske et al., 2009 and Zinbarg et al., 2010 for further details on recruitment and overall study design). Of 1269 students who completed an initial screening measure of neuroticism, 627 participants (69% female) had parental consent and gave youth assent to participate in the project. They all completed a baseline diagnostic assessment and 185 of these participants completed the startle modulation experiment. Of these, 132 did not meet criteria for any psychiatric disorder and the effects of neuroticism upon SR modulation are reported in Craske et al. (2009). Of the remaining 53 participants, 29 met criteria for one or more emotional disorders (19 girls; 10 boys). The remaining 24 participants met criteria for other psychiatric disorders, or did not have usable SR data, and are not included in this study.
Thus, the present study compared SR data from the 29 participants with emotional disorders with SR data from 29 healthy controls matched for gender and age (in years) selected from the 132 participants without a psychiatric disorder originally reported in Craske et al. (2009). There were no significant differences in any socio-demographic, self-report or SR measures between the 29 control children and the larger sample from which they were selected (i.e., Craske et al., 2009). Of the 29 participants with emotional disorders, 20 had a current principal (i.e., most severe) fear disorder of either specific phobia (n = 7) or social phobia (n = 13). The other nine of the 29 participants had a current principal (i.e., most severe) distress disorder; five had a principal unipolar depression-related distress disorder (MDD), dysthymia, minor depressive disorder) and four had a principal anxiety-related distress disorder (GAD, PTSD). The 29 participants with emotional disorders had an average of 1.69 diagnoses (SD = 0.85), all of which were emotional disorders. Thus, participants in the Distress and Fear Disorder groups did not meet criteria for psychiatric disorders other than anxiety and depressive disorders. None were on medication or receiving psychological treatment at the time of assessment. See Table 1 for patterns of comorbidity.
Table 1.
Summary of comorbid diagnoses as a function of diagnostic category
| Principal diagnostic category (number of participants) | Comorbid diagnostic category (number of participants) | Type of comorbid diagnoses |
|---|---|---|
| Fear disorder (20) | Distress disorder-Dep only (4) | MDD |
| Distress disorder-Anx only (3) | GAD, OCD | |
| Distress disorder-Dep & Anx (1) | GAD, MDD | |
| Distress disorder-Anx + Fear disorder (1) | PTSD, OCD, SP | |
| Distress disorder-Dep & Anx + Fear disorder (2) | PTSD, SP, Minor DD, GAD | |
| No comorbid diagnoses (9) | ||
| Distress disorder (9) | Distress disorder-Dep + Fear disorder (1) | Dysthymia, SoP |
| Fear disorder only (4) | SoP | |
| No comorbid diagnoses (4) |
Note: Dep = Depression, Anx = Anxiety; MDD = Major Depressive Disorder; GAD = Generalised Anxiety Disorder; OCD = Obsessive Compulsive Disorder; PTSD = Post Traumatic Stress Disorder; SP = Specific Phobia; Minor DD = Minor Depressive Disorder; SoP = Social Phobia
Age and ethnic composition of the total sample (n = 58) were similar to the larger sample (see Craske et al., 2009; 2012); 50% of the participants were Caucasian, 14% Hispanic/Latino American, 12% African American, 7% Asian American/Pacific Islander, and 17% “other” or multi-ethnic. Age ranged from 16 to 18 years (mean=17.0, SD=0.35). Participants received monetary compensation for their time and transportation costs.
2.2 Materials
2.2.1. Diagnosis
Lifetime and current Axis I psychopathology was assessed using the Structured Clinical Interview for DSM-IV (SCID-Non-Patient Version) (First et al., 2002). After completing interviews, interviewers rated the severity of each current diagnosis in the past month using the Di Nardo and Barlow (1988) 0 to 8 clinician-severity rating (CSR) scale, in which scores of 4 or above indicate clinically significant impairment or distress have been present for the past month. The 29 participants with emotional disorders all met diagnostic criteria for one or more current emotional disorders. Diagnostic reliability was adequate to good based on the larger YEP sample by having trained interviewers observe live SCIDs (n = 69) (κ = 0.65 – 0.83).
2.2.2 Symptoms
Anxiety and depression symptoms were assessed using the Multidimensional Anxiety and Mood Questionnaire which consists of five subscales: Mixed Symptoms, Anxiety Symptoms, Depressive Symptoms, Anxious Arousal, Anhedonic Depression (Watson et al., 1995a; 1995b). Each of the five scales had good internal consistency (α > 0.84) (see Sutton et al. 2011).
2.2.3 Electrophysiological equipment and data acquisition
The equipment and data acquisition were the same as in Craske et al. (2009). Auditory startle stimuli (105 dB, zero rise time, 50-ms white noise bursts) were presented binaurally through stereophonic headphones (Sony, Model MDRV700). The muscle contraction, delivered by a Digital 807 Electrical Muscle Stimulation Device (Everyway Medical Instruments Co.), was a 20.4 mA peak current (i.e., equating to 50 V peak) for 0.5 s. The intensity level was pre-set on the basis of pilot testing to represent an uncomfortable but not painful intensity but was similar to mean voltage levels of shock intensity in studies using shock work-up procedures (Neumann and Waters, 2006); individualized work-up procedures were not chosen, because pre-exposure to the muscle contraction might have decreased anticipatory anxiety during the explicit threat cue paradigm and/or weakened its aversiveness due to habituation (Baker et al., 1981). Startle reflex was measured by electromyogram (EMG) activity of the orbicularis oculi (see Craske et al., 2009, for further details).
2.2.4 Subjective Ratings
Anxiety was rated on a 10-point Likert scale (“calm and relaxed” to “really nervous or scared”) after each baseline and context condition and the explicit threat cue paradigm. After the experiment, participants rated the intensity and unpleasantness of the biceps contraction on 20-point scales (higher scores reflecting higher values).
2.3 Procedure
Data from 25 participants at UCLA and 33 participants at Northwestern University (NU) were used in this study. The two laboratories used identical hardware, software, manualized procedures, and technician training procedures.
Detailed information on the experimental procedure (including a Figure depicting the experimental conditions, the timing of startle probes and the muscle contraction) is reported in Craske et al. (2009). Briefly, after a 5-min resting period for adaptation, participants were presented with a single startle stimulus to reduce initial reactivity (discarded from analyses). During the first baseline condition, participants received 8 startle probes while focusing on a white fixation cross on the computer. For the first context condition, they were fitted with two contraction pads to the biceps muscle and told they would be informed when the contractions would happen later in the experiment. Eight more startle probes were presented while participants focused on the fixation cross. Before the explicit threat cue paradigm, participants were told there would be no muscle contractions delivered while the words ‘Safe: no contraction will be given’ were on the green screen, and they might receive a contraction when the words ‘Danger: contraction may be given’ were on the red screen. Participants were also told that for both phases, they would see a progressing bar showing the time from 0 to 55 s, and that if a biceps contraction occurred during a Danger phase, it would occur when the bar turned from pink to red in the last 15 s. They were then told they might receive a muscle contraction up to three times of increasing intensity each time. Participants received only one contraction in the final 15s of the fourth danger phase, half way through the paradigm. There were 8 safe and 8 danger phases in alternating order. A total of 32 startle probes were presented: 16 in the Danger and 16 in the Safe phases with two trials per phase at 5, 15, 35 or 45 s (the final startle probe presented during the final 15 s when threat of contraction was imminent in the Danger phase).
The muscle contraction pads were removed for the second baseline condition, and reattached for the second context condition. Following electrode removal, startle stimuli and muscle contractions were rated, hearing was tested (all participants passed), and participants were debriefed.
2.4 Response definitions and data analyses
Startle magnitudes were defined using conventional methods as described in Craske et al. (2009). In short, EMG magnitudes were expressed as the difference between the mean amplitude of the 200 ms of EMG preceding the startle stimulus and the peak response, in microvolts (μV). Analyses were performed on natural log (ln) transformed eye blink data as per Craske et al. (2009) using a linear mixed model for repeated measurements with Satterthwaite’s Approximation for degrees of freedom.
For baseline and context phases, the fixed effects were Group (Fear; Distress; CON), Block (Pre; Post Explicit Threat Cue Paradigm), Condition (Baseline; Context), and Trial Half (Trials 1 to 4; Trials 5 to 8). For the explicit threat cue paradigm, the fixed effects were Group, Block (Pre; Post Muscle Contraction), Phase (Safe; Danger), and Probe Time (Early; Late). Consistent with Craske et al. (2009), “Early” referred to 5-, 15-, and 35-s probe times and “Late” referred to the 45-s probe time, when the muscle contraction could occur within danger but not safe phases.
3. Results
3.1 Group comparisons
There were no significant group differences in age, F (2, 55) = 2.02, p = 0.14, nP2 = 0.07, ethnicity, χ2 (2, n = 58) = 0.15, p = 0.93, gender, χ 2 (2, n = 58) = 0.86, p = 0.65, or number of participants recruited from each site, χ 2 (2, n = 58) = 0.41, p = 0.81 There were no significant differences in the number of diagnoses, t(27) = 0.25, p = 0.85, or the severity of principal diagnoses between the clinical groups, t(27) = 0.22, p = 0.83. One-way analyses of variance (ANOVAs) of the MASQ subscales revealed significant group differences on the Mixed, F(2, 55) = 14.62, p < 0.001, nP2 = 0.34, Anxiety, F(2, 55) = 7.55, p = 0.001, nP2 = 0.22, Depressive, F(2, 55) = 20.7, p < 0.001, nP2 = 0.43, Anhedonic, F(2, 55) = 13.6, p < 0.001, nP2 = 0.33, and Anxious Arousal subscales, F(2, 55) = 5.90, p = 0.004, nP2 = 0.18 (see Table 2). The Fear and Distress Disorder groups had significantly higher scores than CONs on all subscales (all p < 0.048), but did not differ significantly from each other on any subscale (all p > 0.98) (see Table 2).
Table 2.
Descriptive information as a function of diagnostic category
| Measure | CON (n = 29) | Fear (n = 20) | Distress (n = 9) |
|---|---|---|---|
| Gender (male: female) | 10:19 | 7:13 | 3:6 |
| Age (years: months) | 17.0 (.2) | 16.9 (.4) | 17.2 (.4) |
| Principal diagnosis severity | 4.75 (1.02) | 4.67 (.70) | |
| Number of diagnoses | 1.85 (.99) | 1.66 (.70) | |
| Contraction | |||
| intensity | 12.4 (4.3) | 14.2 (3.9) | 15.1 (2.1) |
| Unpleasantness | 9.6 (4.3) | 9.4 (4.1) | 10.9 (3.3) |
| Startle stimulus | |||
| intensity | 10.6 (4.9) | 12.4 (4.0) | 12.2 (3.8) |
| Unpleasantness | 8.5 (3.7) | 9.1 (3.3) | 8.4 (3.9) |
| MASQ | |||
| Mixed | 28.1 (9.1) | 41.8 (11.3) | 42.6 (8.1) |
| Anxiety | 17.2 (6.5) | 25.6 (7.3) | 24.6 (8.9) |
| Depression | 20.1 (7.4) | 34.7 (11.7) | 37.4 (8.0) |
| Arousal | 23.2 (8.6) | 32.8 (12.8) | 32.7 (10.7) |
| Anhedonia | 41.8 (18.3) | 60.6 (18.1) | 77.1 (11.99) |
| Anxiety ratings | |||
| Baseline | 2.8 (2.2) | 4.1 (2.0) | 4.2 (2.2) |
| Context | 2.5 (2.1) | 3.3 (2.1) | 3.7 (1.9) |
| Explicit threat | 4.3 (2.6) | 6.4 (2.4) | 5.1 (2.2) |
| Baseline | 1.5 (1.6) | 2.0 (1.4) | 2.2 (1.9) |
| Context | 2.0 (2.1) | 3.0 (2.2) | 2.3 (1.7) |
3.2 Baseline and context conditions
Analysis revealed significant main effects for Block, F(1, 354) = 62.64, p <0.001, SRs were significantly smaller in the post- compared with the pre-explicit threat cue paradigm, Condition, F(1, 997) = 4.33, p = 0.038, SRs were significantly larger in the context compared with the baseline phases, Trial Half, F(1, 1734) = 16.73, p < 0.001, SRs were significantly larger in the first compared with the last half of trials, and Group, F(2, 211) = 10.00, p < 0.001, SRs in the Distress Disorder group were significantly smaller compared with the CON group (p < 0.001) and the Fear Disorder group (p < 0.001), which did not differ significantly from each other (p = 0.63) (see Fig. 1, upper panel). There was a significant Block × Condition interaction, F(1, 663) = 5.65, p = 0.018. The effect of Condition before the explicit threat cue paradigm was not significant, F(1, 699) = 2.34, p = 0.10. whereas SRs were significantly larger during the context compared with the baseline phase post-explicit threat cue paradigm, F(1, 431) = 13.07, p < 0.001.
Fig. 1.
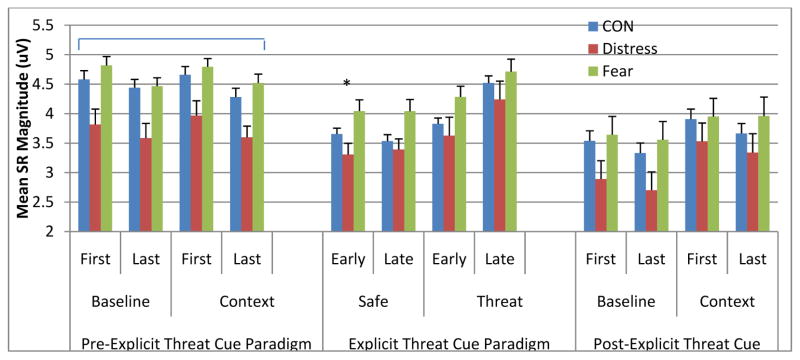
Startle reflex magnitude during baseline and context phases prior to (left panel) during (middle panel) and after (right panel) the explicit threat cue paradigm as a function of diagnostic category (^ = significant Group main effect (collapsed across Block and Condition); * = significant Group main effect at Early Probe Times (collapsed across Safe and Threat Phases); # = significant interaction at Late Probe Times due to significant Group differences during Late Probe Times within Safe Phases).
3.3 Explicit threat cue paradigm
Analysis revealed significant main effects of Phase, F(1, 1171) = 44.85, p < 0.001, Block, F(1, 466) = 8.47, p = 0.004, Probe Time, F(1, 1410) = 52.60, p < 0.001, and Group, F(2, 206) = 8.04, p < 0.001, which were subsumed by significant Phase × Probe Time, F(1, 1352) = 35.20, p < 0.001, Block × Phase × Probe Time, F(1, 1373) = 6.67, p = 0.01 and Phase × Probe Time × Group interactions, F(2, 1348) = 3.54, p = 0.029 (see Fig. 1, middle panel).
The three-way interaction between Phase, Probe Time and Group was driven by the simple 2-way Phase × Group interaction being larger for late probe times than for early probe times. The simple two-way interaction was not significant for the early probe times, F(1, 1220) = 1.95, p = 0.14 (however, significant main effects for Phase, F(1, 1217) = 11.92 = 0.001, and Group, F(2, 173) = 8.23, p < 0.001), whereas the simple two-way interaction was significant for the late probe times, F(2, 365) = 3.12, p = 0.045. As expected, the simple two-way interaction for the late probe times was driven by significantly larger group differences in the safe phases, F(2, 62) = 3.47, p = 0.037, than in the danger phases, F(2, 57) = 0.81, p = 0.45. At late probe times during safe phases, the Fear Disorder group had significantly larger SRs than the Distress Disorder and CON groups (both p < 0.036).
3.4. Subjective ratings
Subjective anxiety ratings1 differed significantly according to Condition, F(4, 49) = 29.88, p < 0.001, nP2 = 0.71, but not by Group, F(2, 52) = 2.66, p = 0.07, nP2 = 0.09, or in terms of a Group × Condition interaction, F(8, 44) = 1.14, p = 0.20, nP2 = 0.05. Anxiety was significantly higher during the explicit threat cue paradigm than all other conditions (all p < 0.001), and during the first baseline condition compared to the last baseline and context conditions (both p < 0.015) (see Table 2). There were no significant group differences in the ratings of the biceps contraction (intensity: F(2, 55) = 2.12, p = 0.13, nP2 = 0.07; unpleasantness: F(2, 55) = 0.40, p = 0.68, nP2 = 0.01) or the startle stimulus (intensity: F(2, 55) = 1.3, p = 0.29, nP2 = 0.04; unpleasantness: F(2, 55) = 0.19, p = 0.83, nP2 = 0.007) (see Table 2).
4. Discussion
Consistent with hypotheses, adolescents with a principal fear disorder showed significantly larger SRs during safe phases and early danger phases of the explicit threat cue paradigm compared to healthy controls and adolescents with principal distress disorders. As expected, groups did not differ significantly in SR magnitudes during late danger phases when threat of the aversive contraction was immediate and all participants would be expected to show neurobiologically imperative defensive responses (Lissek et al., 2006; Craske et al., 2009). Contrary to expectations, adolescents with a principal distress disorder displayed attenuated SRs compared to controls and the fear disorder group during baseline and context conditions before and after the explicit threat cue paradigm.
The finding of amplified defensive responding during safe stages of the explicit threat cue paradigm is broadly consistent with evidence from the few previous studies of fear-related disorders employing fear-potentiation protocols that have found larger SR during but not before and after the fear-potentiation protocol and overgeneralized threat responding to stimuli resembling threat (i.e., CS−) in conditioning experiments (Grillon et al., 1994; Lissek et al., 2009; Lissek, 2012). Moreover, the finding that elevation in SR magnitudes in the fear disorder group emerged only during the explicit threat cue paradigm and quickly dissipated by the final baseline and context conditions highlights that phasic defensive responding to safe cues that are associated with threat is a highly time-locked state in fear disorders, leading to rapid dissipation of fear when threat is not explicit (Davis et al., 1989; de Jongh et al., 2003).
On the other hand, distress-related anxiety disorders such as GAD, PTSD and affiliated traits in the domain of negative affectivity have been associated in past research with larger baseline and context-potentiated SR (e.g., Kumari et al. 2001; Cuthbert et al. 2003; Grillon et al. 2008; Ray et al. 2009). Moreover, they have been less reliably associated with elevated fear-potentiated SR (see Kumari et al. 2001; Kaviani et al. 2004; Lang et al. 2007). However, in contrast to expectations, we observed attenuated rather than amplified SR during baseline and context conditions both before and after the explicit threat cue paradigm in the distress disorder group. Consistent with some prior research (e.g., Allen et al., 1999; Kaviani et al., 2004; Forbes et al., 2005; Lang et al., 2007; Taylor-Clift et al., 2011; Waters et al., in press), this result could be due to the inhibiting effect of depressive disorders upon SR magnitudes; 5 of the 9 adolescents in this group had a principal diagnosis of a depressive disorder. However, Grillon et al. (2013) found enhanced rather than attenuated context-potentiated SR (i.e., placement of shock electrodes) in depression. As the aversive stimulus in the present study was an unpleasant “muscle contraction” rather than “shock”, threat following electrode placement may have been milder or more ambiguous than for shock, thereby attenuating SR due to disengagement/withdrawal (Grillon et al., 2013). The small sample of adolescents with a mix of anxiety and depressive disorders prevented potential distinctions in SR between anxiety-related and depression-related distress disorders from being examined (e.g., see Vaidyanathan et al., 2012). Nevertheless, SR in the distress disorder group did not differ significantly from controls during the explicit threat cue paradigm; thus, the blunting effect of distress disorders on SR appeared to diminish when threat was explicit2, consistent with the suggestion that emotional blunting in depression does not generalize to situations where strong defensive responses are evoked by actual threats (Grillon et al., 2013). In accord, we have shown previously that SR during safe phases of the explicit threat cue paradigm is a unique predictor of the onset of anxiety but not depressive disorders over an ensuing four year follow-up (Craske et al., 2012).
Unfortunately, due to the sample size in the present study, it was not possible to separate anxiety- from depression-related distress disorders to examine the effects of comorbid fear versus distress disorders or single diagnoses. Given that prior reviews of neurophysiological data suggest there may be some distinctions between (a) phobic and fear disorders, (b) non-phobic anxiety disorders and negative affect, and (c) depressive disorders (see Vaidyanathan et al., 2012), larger studies that can separate these diagnostic categories will be important for informing structural models of internalizing psychopathology (e.g., Watson and Clarke, 2006; Lahey et al., 2008; Seeley et al., 2011), as well as current initiatives, such as the RDoC, which aim to redefine the classification of emotional disorders along neurobiological lines (e.g., Insel and Cuthbert, 2009; Vaidyanathan et al., 2009; Craske, 2012). In contrast to studies of anxious adults (McTeague et al., 2012) but similar to studies with anxious children (Waters et al., 2014b), the present study did not find significant differences in the number of diagnoses or self-report symptom severity between the fear and distress disorder groups. These differences could reflect on developmental differences in the capacity to report on ones’ own problems as well as the actual severity and impairment associated with emotional disorders that increases with advancing age. Nevertheless, that distinct patterns of SR modulation were observed in adolescents with principal fear versus distress disorders that cut across anxiety and depressive disorders as traditionally defined and exist in the presence of high rates of comorbidity suggests further research as a function of diagnostic category is warranted.
In summary, the present study found that adolescents with principal fear disorders showed elevated SRs during safe phases of an explicit threat cue paradigm, possibly reflective of overgeneralized defensive responding to safe stimuli under explicit threat conditions, whereas adolescents with principal distress disorders showed attenuated SRs during baseline and context conditions, perhaps reflective of withdrawal from the environment when threat is mild or not explicit. These findings support current initiatives to redefine emotional disorders in terms of patterns of neurobiological functioning and encourage further research along these lines.
Acknowledgments
This work was supported by grants from the National Institutes of Health to Dr. Craske (MH065651) and Drs. Zinbarg and Mineka (MH065652) and from the Virginia Friedhofer Charitable Trust to Dr. Ornitz.
Footnotes
Subjective anxiety ratings were missing from one participant in the Fear Disorder Group and two participants in the CON group.
Supplementary analyses comparing the seven fear disorder participants with comorbid depression with the remaining 13 fear disorder participants without comorbid depressive disorders revealed no significant group differences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen NB, Trinder J, Brennan C. Affective startle modulation in clinical depression: preliminary findings. Biological Psychiatry. 1999;46:542–550. doi: 10.1016/s0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Bittner A, Egger HL, Erkanli A, Costello E, Foley DL, Angold A. What do childhood anxiety disorders predict? Journal of Child Psychology and Psychiatry. 2007;48:1174–1183. doi: 10.1111/j.1469-7610.2007.01812.x. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Distress and fear disorders: an alternative empirically based taxonomy of the ‘mood’ and ‘anxiety’ disorders. British Journal of Psychiatry. 2006;189:481–483. doi: 10.1192/bjp.bp.106.03825. [DOI] [PubMed] [Google Scholar]
- Craske MG. The R-DOC Initiative: science and practice. Depression & Anxiety. 2012;29:253–256. doi: 10.1002/da.21930. [DOI] [PubMed] [Google Scholar]
- Craske MG, Waters AM. Panic disorder, phobias, and generalized anxiety disorder. Annual Review of Clinical Psychology. 2005;1:197–225. doi: 10.1146/annurev.clinpsy.1.102803.143857. [DOI] [PubMed] [Google Scholar]
- Craske MG, Waters AM, Nazarian M, Mineka S, Zinbarg RE, Griffith JW, Ornitz EM. Does neuroticism in adolescents moderate contextual and explicit threat cue modulation of the startle reflex? Biological Psychiatry. 2009;65:220–226. doi: 10.1016/j.biopsych.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Wolitsky-Taylor K, Zinbarg R, Mineka S, Waters AM, Naliboff B, Ornitz E. Elevated responding to safety cues as a specific risk factor for anxiety versus depressive disorders: evidence from a longitudinal investigation. Journal of Abnormal Psychology. 2012;121:315–324. doi: 10.1037/a0025738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: fear memory imagery. Psychophysiology. 2003;40:407–422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- Davis M, Schlesinger LS, Sorenson CA. Temporal specificity of fear conditioning: effects of different conditioned stimulus-unconditioned stimulus intervals on the fear-potentiated startle effect. Journal of Experimental Psychology and Animal Behavior Processes. 1989;15:295–310. [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PJ, Merckelbach H, Arntz A. Eyeblink startle responses in spider phobics before and after treatment: a pilot study. Journal of Psychopathology and Behavioral Assessment. 1991;13:213–223. [Google Scholar]
- de Jongh R, Groenink L, van der Gugten J, Olivier B. Light-enhanced and fear-potentiated startle: temporal characteristics and effects of alpha-helical corticotropin-releasing hormone. Biological Psychiatry. 2003;54:1041–1048. doi: 10.1016/s0006-3223(03)00468-2. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Miller A, Cohn JF, Fox NA, Kovacs M. Affect-modulated startle in adults with childhood-onset depression: relations to bipolar course and number of lifetime depressive episodes. Psychiatry Research. 2005;134:11–25. doi: 10.1016/j.psychres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Globisch J, Hamm AO, Esteves F, Ohman A. Fear appears fast: Temporal course of startle reflex potentiation in animal fearful subjects. Psychophysiology. 1999;36:66–75. doi: 10.1017/s0048577299970634. [DOI] [PubMed] [Google Scholar]
- Graham FK. Distinguishing among orienting, defense, and startle reflexes. In: Kimmel HD, van Olst EH, Orlebeke JE, editors. The Orienting Reflex in Humans. Lawrence Erlbaum; Hillsdale, NJ: 1979. pp. 137–167. [Google Scholar]
- Grillon C, Ameli R, Goddard A, Woods SW, Davis M. Baseline and fear-potentiated startle in panic disorder patients. Biological Psychiatry. 1994;35:431–439. doi: 10.1016/0006-3223(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Molecular Psychiatry. 1996;1:278–297. [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Franco-Chaves JA, Mateus CF, Ionescu DF, Zarate CA. Major depression is not associated with blunting of aversive responses: evidence for enhanced anxious anticipation. PLoS One. 2013;8 (8):e70969. doi: 10.1371/journal.pone.0070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biological Psychiatry. 2009;66:988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Kashani JH, Orvaschel H. A community study of anxiety in children and adolescents. American Journal of Psychiatry. 1990;147:313–318. doi: 10.1176/ajp.147.3.313. [DOI] [PubMed] [Google Scholar]
- Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V. Affective modulation of the startle response in depression: Influence of the severity of depression, anhedonia, and anxiety. Journal of Affective Disorders. 2004;83:21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Major depression and generalised anxiety disorder: same genes, (partly) different environments—revisited. British Journal of Psychiatry. 1996;168(Suppl No. 30):68–75. [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Kaviani H, Raven PW, Gray JA, Checkley SA. Enhanced startle reactions to acoustic stimuli in patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2001;158:134–136. doi: 10.1176/appi.ajp.158.1.134. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Rathouz PJ, Van Hulle C, Urbano R, Krueger RF, Applegate B, Waldman ID. Testing structural models of DSM–IV symptoms of common forms of child and adolescent psychopathology. Journal of Abnormal Child Psychology. 2008;36:187–206. doi: 10.1007/s10802-007-9169-5. [DOI] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM, Cuthbert BN. Fear, anxiety, depression, and the anxiety disorder spectrum: A psychophysiological analysis. In: Treat TM, Bootzin RR, Baker TB, editors. Psychological Clinical Science: Papers in Honor of Richard M. McFall. Psychology Press; New York: 2007. pp. 167–195. [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Pine D, Grillon C. The strong situation: a potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology. 2006;72:265–270. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, Pine DS, Grillon C. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behaviour Research and Therapy. 2009;47:111–118. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Bradley MM. Aversive imagery in panic disorder: agoraphobia severity, comorbidity, and defensive physiology. Biological Psychiatry. 2011;70:415–424. doi: 10.1016/j.biopsych.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Wangelin BC, Laplante MC, Bradley MM. Defensive mobilization in specific phobia: fear specificity, negative affectivity, and diagnostic prominence. Biological Psychiatry. 2012;72:8–11. doi: 10.1016/j.biopsych.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews RRS, Hall WD, Vos T, Patton GC, Degenhardt L. What are the major drivers of prevalent disability burden in young Australians? The Medical Journal of Australia. 2011;194:232–235. doi: 10.5694/j.1326-5377.2011.tb02951.x. [DOI] [PubMed] [Google Scholar]
- Melzig CA, Weike AI, Zimmermann J, Hammn AO. Startle reflex modulation and autonomic responding during anxious apprehension in PD patients. Psychophysiology. 2007;44:846–854. doi: 10.1111/j.1469-8986.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Grillon C, Southwick SM, Davis M. Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry. 1995;36:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Is depression an adaptation? Archives of General Psychiatry. 2000;57:14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychological Bulletin. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective prediction of PTSD symptoms using fear-potentiated auditory startle responses. Biological Psychiatry. 2009;65:235–230. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenoveau JM, Zinbarg RE, Craske MG, Mineka S, Griffith JW. Testing a hierarchical model of anxiety and depression in adolescents: a tri-level model. Journal of Anxiety Disorders. 2010;24:334–344. doi: 10.1016/j.janxdis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Molnar C, Aikins D, Yamasaki A, Newman MG, Castonguay L, Borkovec TD. Startle response in generalized anxiety disorder. Depression and Anxiety. 2009;26:147–154. doi: 10.1002/da.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Salum GA, Mogg K, Bradley BP, Gadelha A, Pan P, Tamanaha AC, Pine DS. Threat bias in attention orienting: evidence of specificity in a large community-based study. Psychological Medicine. 2013;43:733–745. doi: 10.1017/S0033291712001651. [DOI] [PubMed] [Google Scholar]
- Seeley JR, Kosty DB, Farmer RF, Lewinsohn PM. The modeling of internalizing disorders on the basis of patterns of lifetime comorbidity. Journal of Abnormal Psychology. 2011;120:308–321. doi: 10.1037/a0022621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, McGowan SK, Katz AC, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122:322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton JM, Mineka S, Zinbarg RE, Craske MG, Griffith JW, Rose RD, Waters AM, Nazarian M, Mor N. The relationships of personality and cognitive styles with self-reported symptoms of depression and anxiety. Cognitive Therapy and Research. 2011;35:381–393. doi: 10.1007/s10608-010-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clift A, Morris BH, Rottenberg J, Kovacs M. Emotion-modulated startle in anxiety disorders is blunted by co-morbid depressive episodes. Psychological Medicine. 2011;41:129–139. doi: 10.1017/S003329171000036X. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat EM. Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology. 2009a;46:75–85. doi: 10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing psychopathology to neurobiological systems: affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychological Bulletin. 2009b;135:909–942. doi: 10.1037/a0017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Nelson LD, Patrick CJ. Clarifying domains of internalizing psychopathology using neurophysiology. Psychological Medicine. 2012;42:447–459. doi: 10.1017/S0033291711001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduin TL, Kendall P. Peer perceptions and liking of children with anxiety disorders. Journal of Abnormal Child Psychology. 2007;36:459–469. doi: 10.1007/s10802-007-9192-6. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Constantine JA, Westman JS. Startle reflex modification as an outcome measure in the treatment of phobia. Behavioral Assessment. 1992;14:279–291. [Google Scholar]
- Waters AM, Bradley BP, Mogg K. Biased attention to threat in paediatric anxiety disorders (generalized anxiety disorder, social phobia, specific phobia, separation anxiety disorder) as a function of ‘distress’ versus ‘fear’ diagnostic categorization. Psychological Medicine. 2014a;44 (3):607–616. doi: 10.1017/S0033291713000779. [DOI] [PubMed] [Google Scholar]
- Waters AM, Peters R, Forrest K, Zimmer-Gembeck M. Fear acquisition and extinction in offspring of anxious and depressed mothers. Developmental Cognitive Neuroscience. 2014b;7:30–42. doi: 10.1016/j.dcn.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Rethinking mood and anxiety disorders: a quantitative hierarchical model for DSM–V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995a;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptoms. Journal of Abnormal Psychology. 1995b;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, Mineka S, Craske MG, Griffith JW, Sutton J, Rose RD, Waters AM. The Northwestern-UCLA youth emotion project: associations of cognitive vulnerabilities, neuroticism and gender with past diagnoses of emotional disorders in adolescents. Behaviour Research and Therapy. 2010;48:347–358. doi: 10.1016/j.brat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


