Abstract
Sex differences exist in the regulation of energy homeostasis in response to calorie scarcity or excess. Brain-derived neurotrophic factor (BDNF) is one of the anorexigenic neuropeptides regulating energy homeostasis. Expression of Bdnf mRNA in the ventromedial nucleus of the hypothalamus (VMH) is closely associated with energy and reproductive status. We hypothesized that Bdnf expression in the VMH was differentially regulated by altered energy balance in male and female rats. Using dietary intervention, including fasting-induced negative energy status and high-fat diet (HFD) feeding-induced positive energy status, along with low-fat diet (LFD) feeding and HFD pair-feeding (HFD-PF), effects of diets and changes in energy status on VMH Bdnf expression were compared between male and female rats. Fasted males but not females had lower VMH Bdnf expression than their fed counterparts following 24-hour fasting, suggesting that fasted males reduced Bdnf expression to drive hyperphagia and body weight gain. Male HFD obese and HFD-PF non-obese rats had similarly reduced expression of Bdnf compared with LFD males, indicating that dampened Bdnf expression was associated with feeding a diet high in fat instead of increased adiposity. Decreased BDNF signaling during HFD feeding would increase a drive to eat and may contribute to diet-induced obesity in males. In contrast, VMH Bdnf expression was stably maintained in females when energy homeostasis was disturbed. These results suggest sex-distinct regulation of central Bdnf expression by diet and energy status.
Keywords: dietary intervention, glucose, leptin, estradiol, high-fat diet
1. Introduction
Energy homeostasis is regulated by a complex neuroendocrine system involving many central and peripheral signals. Neuronal signals in the hypothalamus and circulating hormones produced by peripheral endocrine and exocrine cells cooperatively control feeding and energy expenditure to ensure the presence of sufficient energy stores during periods of food scarcity and/or to avoid obesity during periods of food abundance [1]. Sex differences exist in the regulation of energy balance in response to calorie scarcity or excess, due to the different roles played by males and females in survival of the species. Specifically males are more responsible for hunting and gathering, while females are responsible for gestation, lactation and care-giving, and both must maintain energy homeostasis to support the survival and development of themselves as well as their offspring [2].
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family and plays an important role in the development, differentiation, growth, and maintenance of the nervous system. BDNF is also an anorexigenic signal. Bdnf mRNA is expressed at high levels in the ventromedial nucleus of the hypothalamus (VMH) [3, 4], an area associated with feeding and metabolism, and lesion of the VMH leads to hyperphagia and obesity in a variety of species, including humans [5]. The VMH is also a target for BDNF to regulate energy balance [6] and glucose homeostasis [7]. Central administration of BDNF attenuates hyperglycemia and improves glucose metabolism in an insulin-deficient diabetic model [7], and also potently decreases caloric intake [8–10], with estrous female rats responding to a lower dose of BDNF than males [8]. VMH Bdnf expression increases following the estradiol peak in female rats [8]. Bdnf deficient mice display hyperphagia, hyperglycemia, and obesity [11–14]. Bdnf expression in the VMH is regulated by alterations of energy status [15, 16] and nutrient-related signals, such as leptin [17] and glucose [18]. Leptin activates neurons within the VMH [19], and stimulates Bdnf expression and increases BDNF protein concentration in the VMH [17]. Systemic or central administration of glucose induces Bdnf mRNA expression only in the VMH [18]. Additionally, high blood glucose levels inhibit VMH Bdnf expression in rodents [16] and inhibit BDNF release into the circulation from the CNS in humans [20].
The mechanisms involved in obesity development have been investigated using a rodent model of high-fat diet (HFD)-induced obesity that produces hyperphagia and hyperleptinemia similar to human obesity. While previous studies have demonstrated that BDNF is involved in obesity development in genetic rodent models [11, 13, 15] and in chronic 14-week HFD feeding-induced obesity [16], no study has examined the role of BDNF at an early stage of obesity development using an acute HFD feeding model. As with many key regulators of energy balance, most previous rodent studies investigating hypothalamic Bdnf gene expression have been conducted exclusively in male rodents. Few studies have investigated sex differences in the regulation of hypothalamic Bdnf expression.
This study aimed to investigate whether any sex difference exists in the energy status-associated change of Bdnf expression. We hypothesized that VMH Bdnf expression was differentially regulated by altered energy status in male and female rats. Using quantitative PCR on microdissected VMH tissue, responses of VMH Bdnf mRNA levels to variations of caloric density or quantity in male and female rats were examined. Food deprivation for 24 hours produced an acute negative energy balance that corresponded to clinically relevant regimens as in glucose tolerance tests. HFD feeding for four days and four weeks produced an acute and a chronic positive energy status, respectively. Comparison between HFD feeding-induced acute and chronic positive energy status would determine if the difference in Bdnf expression is an early event that initiates long-term alteration in energy homeostasis leading to obesity development, i.e., whether the difference in Bdnf expression is a cause or consequence of HFD-induced obesity. Furthermore, an energy-restricted HFD pair-feeding (HFD-PF) method was used so that rats on a standard rodent diet with low-fat content (LFD) and rats on a HFD were fed identical amounts of calories. This technique was used to determine whether difference in Bdnf expression would remain once body weight and adiposity of HFD and LFD rats were normalized, and thus would allow us to dissociate the effects of eating a diet that is high in fat from the effects of resulting obesity. Circulating estradiol, leptin, and glucose concentrations were also measured to indicate any association between peripheral reproductive- and nutrient-related signals and central Bdnf expression.
2. Methods
2.1 Animals and diets
Seventy male and seventy female Long-Evans rats (Harlan, Indianapolis, IN; n=10 per group) were individually housed in separate rooms with controlled temperature (22–24 °C) and 12 h light-dark cycle (light on 0200 h – 1400 h). To compare sex differences in VMH Bdnf mRNA levels which are dependent on energy status and adiposity [15, 16], body fat-matched male rats at 8–10 weeks of age and female rats at 14–16 weeks of age were obtained. Ovarian cycles of female rats were determined by examining predominant cell types of vaginal cytology samples [8], and all female rats included were cycling normally throughout the current study. To be consistent, all female rats were analyzed in the estrus because estrogen levels of female rats fluctuate [21]. Rats were fed a LFD (Teklad, Madison, WI) and their body weights and caloric intake were monitored during acclimation. After acclimation, a HFD (D12451, Research Diets, Inc., New Brunswick, NJ) was provided to the HFD-fed groups. Each gram of LFD and HFD contain similar amounts of proteins (LFD: 0.243 g; HFD: 0.237 g) and carbohydrates (LFD: 0.402 g; HFD: 0.414 g), but different amounts of fat (LFD: 0.047 g; HFD: 0.236 g). Each gram of LFD provides 3.003 kcal, whereas each gram of HFD provides 4.728 kcal. All animal procedures were approved by the Institutional Animal Care and Use Committee of Miami University and were in accordance with the Guide for the Care and Use of Laboratory Animals.
2.2 Experimental design
2.2.1 Exp 1: 24-hour feeding or fasting
Twenty male and twenty female rats were assigned to either a feeding or a fasting group. Body weights and fat were measured when the feeding or fasting period was initiated and again 24 hours later before rats were sacrificed. Female rats started the feeding or fasting regimen in proestrus and were sacrificed 24 hours later during estrus.
2.2.2 Exp 2: 4-day LFD or HFD feeding
Twenty male and twenty female rats were assigned to either a LFD or a HFD group. Rats assigned to LFD groups remained on LFD, whereas rats assigned to HFD groups were placed on HFD for 4 days. Female rats started the HFD when in estrus and were sacrificed 4 days later when they were again in the estrous phase of the cycle.
2.2.3 Exp 3: 4-week LFD or HFD feeding
Thirty male and thirty female rats were assigned to one of the three groups, LFD, HFD, or HFD-PF. Rats in the LFD group remained on the LFD for the entire 4 weeks; rats assigned to the HFD groups had unlimited access to the HFD for 4 weeks; and rats assigned to HFD-PF groups were also placed on the HFD, but they were pair-fed so that they consumed the same number of calories as the same sex animals in the LFD group consumed. Female rats were sacrificed in the estrous phase after the 4-week feeding period. One study shows that mice exhibit anovulation 4 weeks after feeding a HFD containing 22% fat [22]. In another study, after feeding the same HFD as the current study for 6 weeks, 20% of diet-resistant rats and 80% of diet-induced obese rats fail to display regular estrous cycles [23]. Our unpublished observation indicates that female rats tend to have irregular ovarian cyclicity beginning the fifth week of HFD feeding. Thus, the current study was terminated after 4 weeks. HFD males and females gained more weight and fat than their same sex LFD groups, indicating that positive energy status was established 4 weeks after ad libitum HFD feeding.
2.3 Experimental procedures
2.3.1 Body weight, body composition, and caloric intake
Daily body weight and food intake were measured to the nearest 0.01 gram. Food intake was calculated by the difference of weights of food hoppers over 24 hours and corrected for spillage. Food intake data were then converted to calories to represent daily caloric intake. Cumulative caloric intake was calculated for the 4-day and 4-week feeding experiments. Weekly caloric totals were also determined for the 4-week regimen with intake determined 7 days/week for weeks 1–3 and for 6 days in the fourth week. An Echo MRI whole body composition analyzer (EchoMedical Systems, Houston, TX) was used to assess body fat mass in conscious rats before and after dietary intervention to provide longitudinal adiposity data for comparison.
2.3.2 Pair-feeding
In order to examine effects of HFD on Bdnf expression without a potential confound of different amounts of calories consumed by LFD and HFD groups or an increase in body weight due to HFD-induced obesity, HFD-PF groups were included in Exp 3. HFD-PF rats were provided HFD with the average amount of calories consumed by the same sex LFD group each day. HFD-PF rats were supplied their daily HFD allotment in two meals. One meal contained one third of the total calories and was fed to the HFD-PF rats toward the end of the light cycle, i.e. between 1100 h and 1200 h, and the other meal contained the remaining two thirds of the calories and was fed to the HFD-PF rats during the dark cycle between 1700 h and 1800 h. Our unpublished observation suggests that rats eat the majority of their food in large meals during their dark cycles. Separating total calories into two meals allows their food intake to be spread over a day with some food occasionally remaining when the next meal is offered, and produces similar body weights in the HFD-PF LFD rats [8].
2.3.3 Assays
Glucose concentration was measured using blood samples obtained from the tip of the tail vein using a glucose meter (US Diagnostics, New York, NY). Rats were sacrificed by decapitation between 1100 h and 1300 h. Plasma from the trunk blood samples was stored at −80 °C. 17β-estradiol was measured by double antibody 125I radioimmunoassay (MP Biomedicals LLC, Santa Ana, CA), with intra- and inter-assay coefficients of variation of 4.7–10.7% and 5.9–11.9% respectively, and sensitivity of 9 pg/ml. Leptin was measured by rat enzyme-linked immunosorbent assay (Crystal Chem Inc., Downers Grove, IL), with intra- and inter-assay coefficients of variation of ≤ 10%, and sensitivity of 0.2 ng/ml.
2.3.4 Quantitative real-time PCR for Bdnf mRNA level measurement
The brains were immediately removed after decapitation, frozen, and stored at −80 °C. Bilateral VMH was microdissected from frozen sections in 300 μm thickness between bregma −1.78 to −3.58 mm using hollow needles of 0.76 mm in diameter (Stoelting, Kiel, WI) according to anatomical landmarks [24]. Constitutively expressed gene ribosomal protein L32 was used as an endogenous control to normalize quantification of Bdnf levels. None of the feeding regimens changed the expression of L32 in the VMH. Accuracy of the VMH microdissection was verified by measuring proopiomelanocortin (Pomc) mRNA level that is abundant in the adjacent arcuate nucleus but not in the VMH. The primer sequences for rattus norvegicus L32 (NM_013226) were forward 5′-CAT CGT AGA AAG AGC AGC AC-3′ and reverse 5′-GCA CAC AAG CCA TCT ATT CAT-3′; for rattus norvegicus Bdnf (NM_012513) were forward 5′-GCG GCA GAT AAA AAG ACT GC-3′ and reverse 5′-GCA GCC TTC CTT CGT GTA AC-3′; and for rattus norvegicus Pomc (NM_139326) were forward 5′-TCC ATA GAC GTG TGG AGC TG -3′ and reverse 5′-ACT TCC GGG GAT TTT CAG TC -3′. Total RNA was isolated and cDNA was synthesized. Quantitative PCR reactions were performed using iQ SYBR Green Supermix and an iCycler (Bio-Rad, Hercules, CA) with 2-step amplification at 95 °C for 10 s and annealing temperature of 58°C for 30 s for 40 cycles. The identity of amplification products was confirmed by gel electrophoresis and melt curve analysis. CT values for L32 and Bdnf were between 19 and 21 and between 21 and 24, respectively; whereas CT values for Pomc were greater than 30, indicating very low Pomc expression and thus accurate VMH microdissection. Results were calculated by a 2−ΔΔCt method [25]. Data were presented using male fed or LFD group as 100%.
2.4 Statistical analysis
Data analysis was performed using Prism Statistical Software 5 (La Jolla, CA). Body weight, body fat, and daily caloric intake at different time points were analyzed by a two-factorial (diet × time) analysis of variance (ANOVA) for repeated measures. Measurements of change of body weight, body fat, cumulative caloric intake, glucose, leptin, estradiol concentrations, and VMH Bdnf expression were analyzed by a two-factorial (diet × sex) ANOVA. The Bonferroni’s corrected t-test was applied to calculate the statistical significance of mean differences. A P value < 0.05 was considered to be statistically significant.
3. Results
3.1 Exp 1: 24-hour feeding or fasting
3.1.1 Body weight and body fat
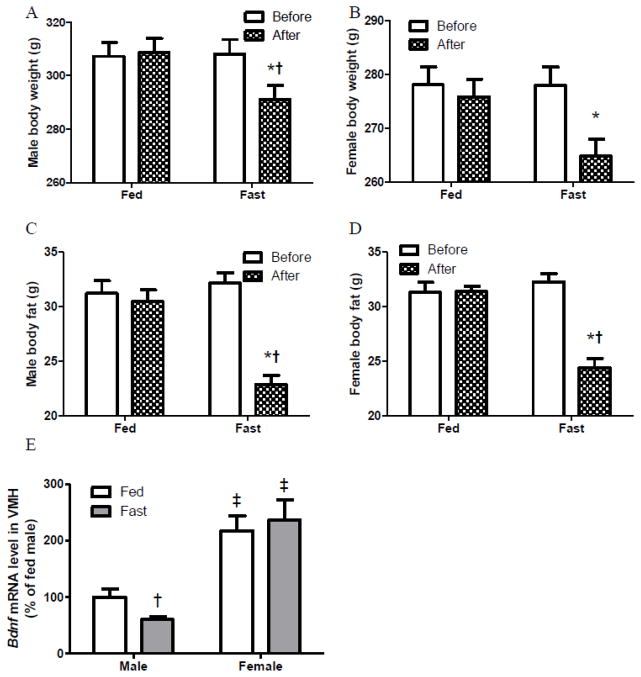
Body weight and fat were not changed in feeding groups, whereas they were decreased in 24-hour fasting groups (Fig 1A–D). Compared to their same sex fed counterparts, fasted males had lower body weights and fasted male and female groups had lower body fat (Fig 1A–D) and greater % decreases in their body weights and fat (Table 1), indicating a negative energy balance following 24-hour fasting. Additionally, fed females changed less % of body weight and fasted females lost less % of body weight than male counterparts (Table 1).
Fig. 1.
Body weight (A–B) and body fat (C–D) of male and female rats before and after 24 h feeding or fasting, and VMH Bdnf expression of male and female rats after 24 h feeding or fasting (E) (Exp 1).
*: Different between time points (before and after) within the same dietary treatment.
†: Different between dietary groups (feeding and fasting) within the same sex.
‡: Different between sexes (male and female) within the same dietary treatment.
Table 1.
Percentage changes of body weight and body fat, terminal measurements of blood glucose, plasma leptin and estradiol concentrations of male and female rats after 24-hour feeding or fasting. Data are means ± SEM.
| Exp 1: 24-hour feeding/fasting | Male | Female | ||
|---|---|---|---|---|
| Fed | Fast | Fed | Fast | |
| Body weight change % | 0.46±0.20 | −5.49±0.20† | −0.80±0.10 ‡ | −4.70±0.36†‡ |
| Body fat change % | −2.31±1.03 | −28.95±1.49† | 0.69±2.17 | −24.37±2.13† |
| Glucose (mg/dl) | 143.8±2.2 | 124.4±2.3† | 131.3±5.3‡ | 109.6±4.3†‡ |
| Leptin (ng/ml) | 5.94±0.44 | 4.02±0.50† | 3.85±0.28‡ | 3.31±0.26 |
| Estradiol (pg/ml) | 25.43±4.87 | 16.56±2.16 | 43.38±5.58‡ | 35.20±4.43‡ |
Different between dietary groups (feeding and fasting) within the same sex.
Different between sexes (male and female) within the same dietary treatment.
3.1.2 Circulating glucose, leptin, and estradiol concentrations
Blood glucose concentrations were lower in fasted rats than their fed counterparts, and females had lower blood glucose levels than males on the same dietary treatment (Table 1). Leptin levels were lower in fasted males than fed males, whereas they were similar between fed and fasted female rats. Additionally, plasma leptin concentrations were lower in female fed rats than male fed rats (Table 1), but were not significantly different in fasted males and females. Thus 24-hour fasting decreased leptin levels in males but not in females. Plasma estradiol concentrations were higher in females than males. Although there was a trend of lowering estradiol levels by fasting, two-factorial ANOVA indicated that diet did not significantly affect estradiol levels (P > 0.05), and Bonferroni’s corrected t-test indicated that estradiol levels were not significantly different between same sex fed and fasted groups (P > 0.05, Table 1).
3.1.3 VMH Bdnf expression
VMH Bdnf mRNA expression was higher in females than males regardless of dietary treatment (Fig 1E). This is consistent with previous findings that female rats had greater Bdnf expression in the VMH than male rats [8]. VMH Bdnf expression was lower in fasted male rats than their fed counterparts, whereas it was similar between fed and fasted females (Fig 1E).
3.2 Exp 2: 4-day LFD or HFD feeding
3.2.1 Body weight, body fat, and caloric intake
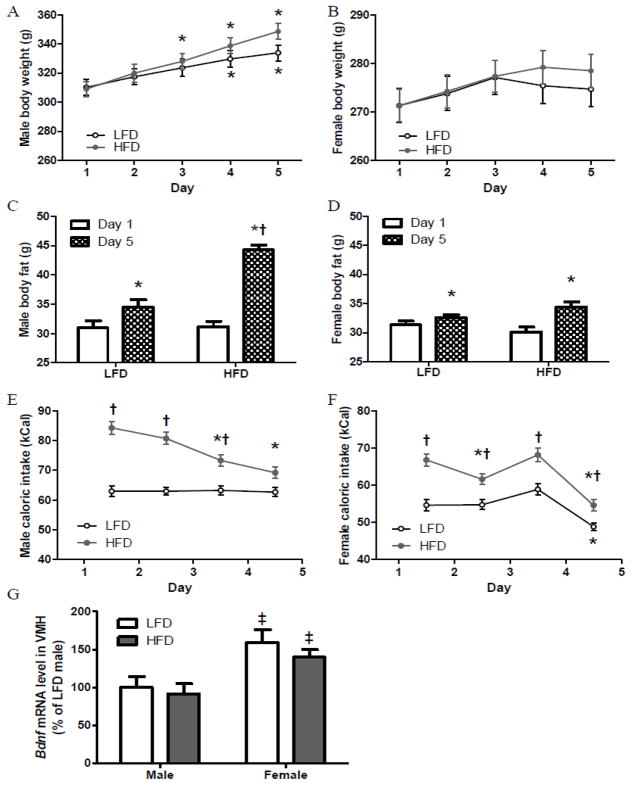
During 4-day ad libitum feeding, LFD and HFD males, but not females, increased their body weights (Fig 2A–B). Daily body weights of same sex LFD and HFD groups were similar. Males and females increased their body fat after the 4-day feeding regimen (Fig 2C–D). HFD males, but not females, had greater body fat than the same sex LFD group (Fig 2C–D). Additionally, the percentages change in body weight and fat were significantly greater in both male and female HFD groups compared to those fed a LFD (Table 2). Females gained less percentage of weight and fat than males with the same diet treatment (Table 2).
Fig. 2.
Daily body weight (A–B), body fat on Day 1 and Day 5 (C–D), and daily caloric intake (E–F) of male and female rats during 4-day LFD or HFD feeding, and VMH Bdnf expression of male and female rats after 4-day LFD or HFD feeding (G) (Exp 2).
*: Different compared with day 1 within the same dietary treatment.
†: Different between dietary groups (LFD and HFD) within the same sex.
‡: Different between sexes (male and female) within the same dietary treatment.
Table 2.
Percentage changes of body weight and body fat, cumulative caloric intake, terminal measurements of blood glucose, plasma leptin and estradiol concentrations of male and female rats after 4-day LFD or HFD feeding. Data are means ± SEM.
| Exp 2: 4-day LFD/HFD | Males | Females | ||
|---|---|---|---|---|
| LFD | HFD | LFD | HFD | |
| Body weight change % | 7.67±0.35 | 12.82±0.19† | 1.25±0.17‡ | 2.66±0.04†‡ |
| Body fat change % | 11.41±1.63 | 42.81±3.01† | 4.14±0.91‡ | 14.74 ±2.08†‡ |
| Caloric intake (kCal) | 251.89±3.90 | 307.62±6.79† | 217.13±5.27‡ | 251.22±6.29†‡ |
| Glucose (mg/dl) | 141.9±3.3 | 142.8±2.8 | 132.6±3.1 | 135.2±4.3 |
| Leptin (ng/ml) | 5.30±0.45 | 8.22±0.35† | 4.03±0.23‡ | 4.46±0.14‡ |
| Estradiol (pg/ml) | 20.20±1.28 | 25.26±3.29 | 44.36±4.74‡ | 48.61±4.08‡ |
Different between dietary groups (LFD and HFD) within the same sex.
Different compared with male rats within the same dietary treatment.
LFD: low-fat diet; HFD: high-fat diet.
Males fed a HFD consumed more calories than LFD males on the first 3 days of feeding. HFD males consumed fewer calories daily on days 3 and 4 than days 1 and 2, whereas LFD males consumed similar amounts of daily calories over 4 days (Fig 2E). Both female groups displayed changes in caloric intake related to the ovarian cycle, and consumed the most calories on day 3 between diestrus and proestrus and the least calories on day 4 between proestrus and metestrus (Fig 2F). HFD females consumed more calories on each of 4 days than LFD females. Both male and female HFD groups consumed more calories than their same sex LFD groups over the 4-day period. Additionally, within the same dietary treatment, female rats consumed fewer calories than males (Table 2).
3.2.2 Circulaing glucose, leptin, and estradiol concentrations
Blood glucose concentrations were similar between males and females within the same dietary treatment, and between LFD and HFD groups within the same sex (Table 2). Female rats had lower plasma leptin concentrations than males within the same dietary treatment. Plasma leptin concentrations were higher in HFD males than LFD males but were similar between female LFD and HFD groups (Table 2), consistent with greater adiposity of HFD males than LFD males and similar adiposity of between female groups. Plasma estradiol levels were higher in females than males and were similar between dietary groups within the same sex (Table 2).
3.2.3 VMH Bdnf expression
Female rats had higher Bdnf mRNA levels than males within LFD or HFD. Same sex LFD and HFD rats had similar Bdnf mRNA levels (Fig 2G).
3.3 Exp 3: 4-week LFD or HFD feeding
3.3.1 Body weight, body fat, and caloric intake
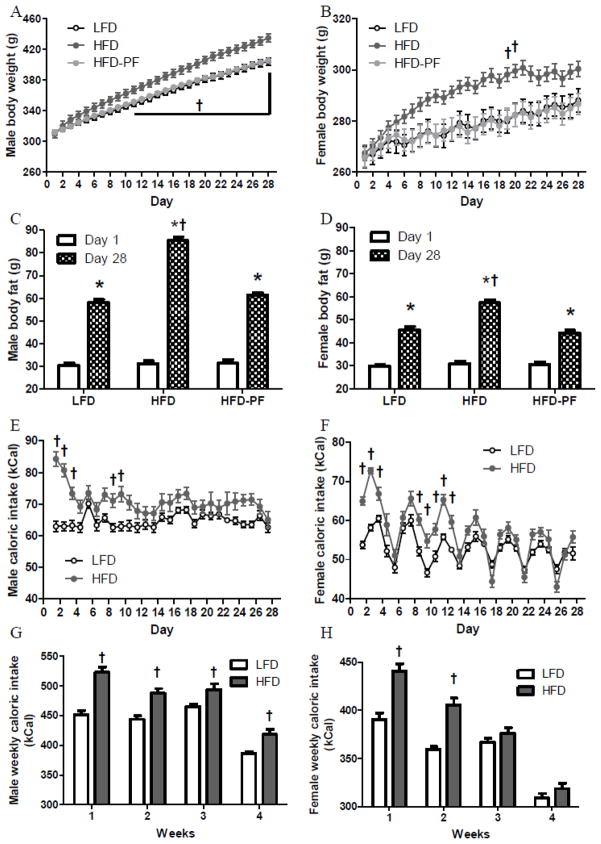
HFD males had greater body weights than LFD and HFD-PF males between day 11 and day 28. HFD-PF rats had similar body weights as LFD rats during the entire 4-week period (Fig 3A). Body weights of female rats fluctuated throughout 4 weeks. Different from the male groups, HFD females temporarily had greater body weights than LFD and HFD-PF females on days 19 and 20 and body weights of different groups were not significantly different on the rest of days (Fig 3B). Body fat levels of male and female groups were similar at the initiation of this 4-week feeding regimen and were increased after 4 weeks (Fig 3C–D). Male and female HFD groups had greater body fat than their LFD and HFD-PF counterparts, whereas LFD and HFD-PF groups had similar adiposity (Fig 3C–D). HFD males and females increased greater % of weight and fat than their LFD and HFD-PF counterparts. Female groups gained less % of weight and fat than the same diet male groups (Table 3).
Fig. 3.
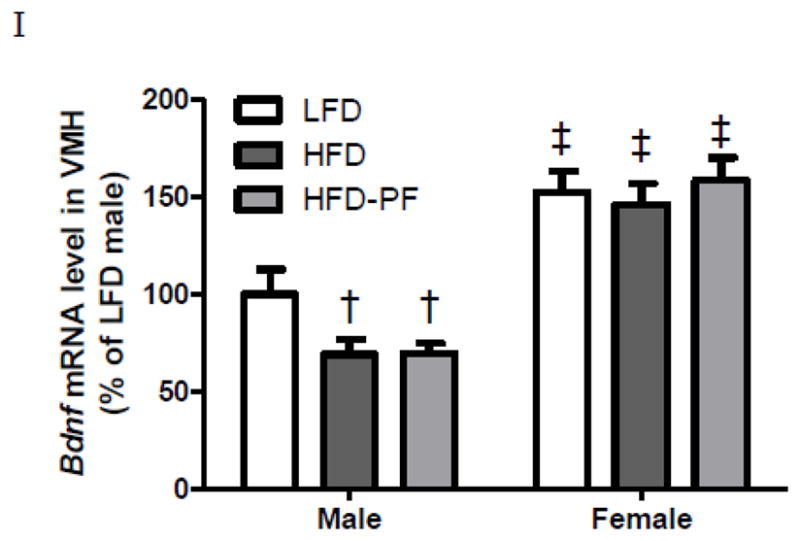
Daily body weight (A–B), body fat on Day 1 and Day 28 (C–D), daily caloric intake (E–F), weekly caloric intake (G–H) of male and female rats during 4-week LFD, HFD, or HFD-PF feeding, and VMH Bdnf expression of male and female rats after 4-week LFD, HFD, or HFD-PF feeding (I) (Exp 3). Figs 3A–B, E–F: only significant differences among dietary groups were noted. Figs 3G–H: weeks 1–3 included 7 days and week 4 included 6 days.
*: Different compared with day 1 within the same dietary treatment.
†: Different among dietary groups (LFD, HFD, and HFD-PF) within the same sex.
‡: Different between sexes (male and female) within the same dietary treatment.
Table 3.
Percentage changes of body weight and body fat, cumulative caloric intake, terminal measurements of blood glucose, plasma leptin and estradiol concentrations of male and female rats after 4-week LFD or HFD feeding. Data are means ± SEM.
| Exp 3: 4-week LFD/HFD | Males | Females | ||||
|---|---|---|---|---|---|---|
| LFD | HFD | HFD-PF | LFD | HFD | HFD-PF | |
| Body weight change % | 29.78±0.81 | 40.81±0.96† | 30.27±0.91 | 8.33±0.46‡ | 12.36 ±0.67†‡ | 7.91±0.44‡ |
| Body fat change % | 93.44±7.65 | 175.59±7.16† | 99.46±8.06 | 53.62±6.28‡ | 88.87±8.92†‡ | 46.03±5.99‡ |
| Caloric intake (kCal) | 1748.44 ±16.84 | 1921.64 ±28.45† | Same as LFD males | 1426.52 ±11.01‡ | 1541.43 ±14.03†‡ | Same as LFD females |
| Glucose (mg/dl) | 146.0 ±2.7 | 153.7±4.3 | 148.8±2.1 | 140.7 ±5.2 | 139.5±2.6‡ | 142.9±2.8 |
| Leptin (ng/ml) | 4.12 ±0.24 | 9.35±0.32† | 4.79±0.39 | 3.40±0.17 | 5.13±0.12†‡ | 3.77±0.12‡ |
| Estradiol (pg/ml) | 22.69±0.58 | 25.75±0.97 | 21.28±0.77 | 49.81±3.11‡ | 48.26±2.82‡ | 44.76±2.76‡ |
Different compared with LFD rats within the same sex.
Different compared with male rats within the same dietary treatment.
LFD: low-fat diet; HFD: high-fat diet; HFD-PF: high-fat diet pair-feeding.
Initially, there were significant, intermittent increases in caloric consumption in male and female rats fed a HFD which only lasted through the first two weeks of feeding (Fig 3E–F). Female rats displayed cyclic changes in caloric intake (Fig 3F). When weekly caloric intake was analyzed, male HFD rats consumed more calories than LFD males throughout 4 weeks, whereas female HFD rats consumed more calories than LFD females only during the first 2 weeks (Fig 3G–H). Female rats also consumed less amounts of cumulative calories than males within the same dietary treatment (Table 3). Additionally, HFD groups consumed greater amounts of calories compared with their same sex LFD counterparts over the 4-week period (Table 3).
3.3.2 Circulating glucose, leptin, and estradiol concentrations
Blood glucose concentrations were lower in HFD females than HFD males, while such sex difference did not exist in LFD or HFD-PF groups (Table 3). Plasma leptin concentrations were lower in HFD and HFD-PF females than males within the same dietary treatment, whereas they were similar between LFD males and females. Additionally, HFD groups had higher plasma leptin concentrations than same sex LFD and HFD-PF groups (Table 3), indicating increased circulating leptin levels accompanied with increased adiposity for both sexes. Female rats had higher estradiol levels than males within the same dietary treatment. Within the same sex, estradiol levels were similar among different dietary groups (Table 3).
3.3.3 VMH Bdnf expression
Regardless of the dietary treatment, female rats had significantly higher Bdnf mRNA levels than their male counterparts. HFD and HFD-PF males had lower VMH Bdnf expression compared to LFD males. In contrast all female groups had similar VMH Bdnf expression (Fig 3I).
4. Discussion
To elucidate how peripheral signals and central Bdnf expression were regulated in response to a HFD or perturbations of caloric intake-induced energy imbalance in male and female rats, caloric deficiency (fasting) and excess (HFD feeding) were used to assess whether both sexes responded in a similar manner. Our results identified both shared and sex-dependent responses. Male and female fasted rats had lower body fat and blood glucose than their fed counterparts (Exp 1), however they exhibited different responses in leptin and VMH Bdnf expression. Fasted males had lower circulating leptin levels and Bdnf expression than their fed counterparts; whereas the values of these parameters in fed and fasted females were comparable. These data suggested that a decrease in body fat or blood glucose per se was not associated with the reduction of leptin levels or VMH Bdnf expression in all rats, since such relationship was seen in males but not in females. To determine the role of central BDNF signaling in the development of HFD-induced obesity, Bdnf expression was assessed after acute (Exp 2) and chronic (Exp 3) HFD feeding. Although 4-day HFD males established positive energy balance with greater adiposity and elevated leptin levels compared with LFD males their Bdnf expression was similar, suggesting that an increase in body fat or circulating leptin levels did not lead to a change of Bdnf expression during the initial stage of obesity development. There are two possible explanations for such dissociation. One possibility is that the change of Bdnf expression might not be an early event or cause, but rather a consequence, for long-term HFD-induced obesity. The other possibility is that central Bdnf expression might be reversible during this early phase of disease development. Transcription of several noncoding exons and one coding exon of the rodent Bdnf gene and subsequent Bdnf expression in differential subcellular targets and specific regions in the CNS are regulated by distinct environmental cues [26], a process that might be initiated but not completed during early phase of HFD feeding. Thus a hyperphagia-obesity vicious cycle has not yet started. In response to chronic 4-week HFD feeding, ad libitum HFD males had greater adiposity and higher leptin levels than LFD males and HFD-PF males fed with the same amount of calories as their LFD counterparts. Interestingly, male HFD and HFD-PF rats had similarly decreased VMH Bdnf expression compared with LFD males in spite of differences in their energy status, suggesting that dietary high-fat content, instead of increases in body fat or leptin, was associated with the decrease in Bdnf expression in male rats. Increasing evidence supports the idea that dietary fat components and their metabolites could directly regulate gene transcription/post-transcription and second-messenger systems [27]. Fat metabolic enzymes may affect mRNA stability or translation efficiency and fat metabolites can be sensed via a regulatory segment of mRNA to change mRNA activity or even regulate RNA editing [27].
By controlling the amount of energy intake via a pair-feeding method, HFD-PF rats had similar body weight, adiposity, circulating glucose and leptin levels as their LFD counterparts. However, Bdnf expression was still lower in the VMH of HFD-PF males compared with LFD males. Lower Bdnf expressions in the VMH of HFD and HFD-PF male rats suggested an increased drive to eat due to HFD consumption in these rats, thus HFD-induced obesity would continue to develop after this 4-week period. This idea is consistent with a study showing that young Bdnf +/− mice with lower Bdnf expression exhibit increased caloric intake on a HFD even when they have similar body weight and fat as wild-type mice [28]. Our data were also consistent with two recent studies showing changes in Bdnf expression in rodents with similar adiposity as their controls. One study demonstrates that VMH Bdnf expression of male mice raised in an enriched environment significantly increases at an early time point of 2 weeks, well before changes of other metabolic markers including decreases in body weight and increases in hypothalamic gene expressions of neuropeptide Y (Npy) and agouti related peptide (Agrp) [29]. The other study reports that male mice with gut microbiota, an obesity-contributing environmental factor due to increased capacity to absorb energy, have reduced Bdnf expression, but similar Agrp, Npy, and Pomc content in the hypothalamus, compared to control mice with equal adiposity [30]. Therefore, unlike expressions of Agrp, Npy and Pomc, VMH Bdnf expression is not dependent on adiposity, but is based on the tendency of changes in adiposity. In other words, Bdnf expression decreases in animals with a tendency to gain weight/fat.
VMH Bdnf expression is also regulated by glucose [18], a short-term energy signal, and leptin [17], a long-term adiposity hormone. Suppressed VMH Bdnf expression accompanied with decreased blood glucose concentration in response to fasting was observed in Exp 1 and a previous study [18]. Under certain non-physiological conditions blood glucose affects VMH Bdnf expression, as a single intraperitoneal injection of 2 mg/kg glucose induces hyperglycemia and elevates VMH Bdnf expression in mice [18]. Leptin is secreted from adipose tissue proportional to adiposity and conveys the abundance of the body’s energy stores to the brain, where it acts to regulate feeding and energy expenditure [1]. A decline in leptin levels signals a state of negative energy balance, as was observed in 24-hour fasted male rats. The reduced VMH Bdnf expression would trigger robust counter-regulatory mechanisms to increase feeding. Interestingly, fasted female rats had similar plasma leptin levels as their fed counterparts. Plasma leptin levels were higher in male than female rats in current study and a few previous studies [32–35], a sex difference reversed from humans. The reason for this species difference with respect to the effects of sexual dimorphism of leptin levels is not known. Importantly, in this study leptin changes by alteration of energy status were quantitatively greater in males than females. Leptin levels were decreased in fasted males and increased in 4-day HFD males, but were comparable between fed and fasted or 4-day LFD and HFD females. Such sex-based differences in the change of leptin levels due to dietary disruption have also been reported previously from our group as well as others [32, 36, 37]. For examples, hyperleptinemia was observed in male but not female mice after 4-day HFD feeding [37]; in spite of the reduction of body fat in both sexes, 40% caloric restriction significantly reduced circulating leptin levels in male but not in female mice [36] and rats [32], with leptin levels being statistically correlated with body weight and fat in males but not in females [32]. The dynamic changes of leptin by dietary disruption in males, in contrast to the relatively stable levels of leptin in females, likely contributed to the robust alteration of VMH Bdnf expression in males but not females. It is noteworthy that an association between circulating leptin and VMH Bdnf expression is not yet completely understood. Expression of Bdnf mRNA is significantly greater in the VMH of ob/ob mice that lack leptin compared with wild-type controls [38], while expression of Bdnf in the VMH increases following intravenous administration of 10 μg/g of leptin [17]. Plasma leptin concentration was not measured in the latter study with intravenous administration of leptin at dose of 10 μg/g, but such procedure could increase leptin by 10-fold to a pharmacological level [39]. It is not clear whether physiologic hyperleptinemia, as seen in our 4-day and 4-week HFD rats, would change VMH Bdnf expression.
It is noteworthy that female 4-day and 4-week groups had lower Bdnf expression levels than 24-hour groups, which could not be explained by the differences in energy status or circulating signals measured in this study. Chronic stress over the 4-day or 4-week experimental period may affect hypothalamic Bdnf expression. BDNF signals colocalize with CRH signals in the paraventricular nucleus (PVN) of the hypothalamus [40], suggesting that BDNF signals could be regulated by HPA activity. Chronic stress decreases Bdnf mRNA levels in several regions of the brain, including in the VMH [41]. Additionally an up-regulation of hypothalamic levels of BDNF is associated with correction of impaired HPA activity in a mouse model with diminishing glucocorticoid receptor in the PVN [42].
Changes in energy state usually results in concomitant changes in gene expression of hypothalamic neuropeptides that regulate feeding behavior. BDNF is one of the anorexigenic neuropeptides. Two interesting points were noteworthy from the current study. First, consistent with previous reports [15, 16, 18] VMH Bdnf expression was down-regulated in males in response to acute energy restriction and chronic energy excess, indicating that Bdnf expression is decreased when energy balance is disturbed by either negative [15, 18] or positive [16] energy status. A pair-feeding paradigm was used to dissociate the effects of eating a HFD from the resulting obesity. VMH Bdnf expression was similarly down-regulated in HFD-PF and HFD rats compared with LFD rats, even though HFD-PF rats had a similar energy status as LFD rats. Thus, this finding extends our current understanding in the literature, demonstrating that lower Bdnf expression in the VMH was also a result of exposure to a HFD rather than the resulting fat gain. These results suggest that acute energy restriction and chronic HFD exposure may increase a drive to eat in males but not females. This opens up the possibility that decreased BDNF signaling may contribute to the weight gain associated with diet-induced obesity in males.
Second, we demonstrated for the first time that unlike males, no difference in VMH Bdnf expression was found in estrous females that underwent either fasting or HFD feeding. Female rats possessed a stable Bdnf expression in response to perturbation of their energy balance, indicating that female Bdnf expression is not solely regulated by their nutritional state or energy status, but is also regulated by estradiol across the ovarian cycle [8]. Suppression of VMH Bdnf expression during negative energy balance would lead to an active increase of caloric intake when food is available, ensuring rapid replenishment of energy stores and restoration of energy balance, as reported in males but not females following caloric restriction [36]. Suppression of VMH Bdnf expression during positive energy balance could lead to sustained hyperphagia in males but not females beyond the 4-week HFD feeding regimen, as reported that male rats continuously consume more calories from a HFD than from a LFD, whereas females consume similar amounts of total calories from both HFD and LFD’s over a 28-week period [35]. Reduced VMH Bdnf expression may indicate an intrinsic nature of susceptibility to diet-induced obesity. Female rodents are less susceptible to diet-induced obesity than males [35], which could be partially due to a stable level of VMH Bdnf expression. Further studies manipulating estrogen levels, including ovariectomy and hormone replacement, are needed to determine the involvement of female steroids in the regulation of hypothalamic BDNF signaling under differing energy states.
In conclusion, this is the first study that describes sex differences in VMH Bdnf expression in response to high-fat diet and changes of energy status. Our findings suggest that there are distinct sex differences in the central metabolic control system. The implication of these gender differences is profound in that the development of therapeutic approaches against obesity in men and women may involve very different strategies to achieve successful weight loss.
Highlights.
Sex differences existed in VMH Bdnf expression in response to a high-fat diet and changes of energy status.
VMH Bdnf expression was down-regulated by dietary intervention in male rats.
VMH Bdnf expression was stable in female rats under dietary disruption.
Acknowledgments
This work was supported by NIH R15 DK090823 and Madalene and George Shetler Diabetes Research Award to HS.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- HFD
high-fat diet
- HFD-PF
high-fat diet pair-feeding
- LFD
low-fat diet
- VMH
ventromedial nucleus of the hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 2.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamoto Y, Nakamura S, Nakano S, Oka N, Akiguchi I, Kimura J. Immunohistochemical localization of brain-derived neurotrophic factor in adult rat brain. Neuroscience. 1996;74:1209–26. doi: 10.1016/0306-4522(96)00245-x. [DOI] [PubMed] [Google Scholar]
- 5.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–44. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1053–R69. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meek TH, Wisse BE, Thaler JP, Guyenet SJ, Matsen ME, Fischer JD, et al. BDNF action in the brain attenuates diabetic hyperglycemia via insulin-independent inhibition of hepatic glucose production. Diabetes. 2013;62:1512–8. doi: 10.2337/db12-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Z, Liu X, Senthil Kumar SPD, Zhang J, Shi H. Central expression and anorectic effect of brain-derived neurotrophic factor are regulated by circulating estradiol levels. Horm Behav. 2013;63:533–42. doi: 10.1016/j.yhbeh.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1037–45. doi: 10.1152/ajpregu.00125.2007. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Bomberg E, Billington CJ, Levine AS, Kotz CM. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res. 2010;1336:66–77. doi: 10.1016/j.brainres.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–44. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446–53. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- 13.Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–57. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 14.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Wang Q, Huang XF. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase B mRNA expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience. 2009;160:295–306. doi: 10.1016/j.neuroscience.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 17.Komori T, Morikawa Y, Nanjo K, Senba E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience. 2006;139:1107–15. doi: 10.1016/j.neuroscience.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 18.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–74. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci U S A. 1998;95:741–6. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–8. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 21.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 22.Wu LLY, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, et al. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–45. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- 23.Balasubramanian P, Jagannathan L, Mahaley RE, Subramanian M, Gilbreath ET, MohanKumar PS, et al. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J Neuroendocrinol. 2012;24:748–55. doi: 10.1111/j.1365-2826.2011.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson LW. Brain maps: Structure of the rat brain. 2. Amsterdam; New York: Elsevier Science; 1999. [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–35. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clingman CC, Ryder SP. Metabolite sensing in eukaryotic mRNA biology. Wiley Interdiscip Rev RNA. 2013;4:387–96. doi: 10.1002/wrna.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox EA, Byerly MS. A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R994–R1004. doi: 10.1152/ajpregu.00727.2003. [DOI] [PubMed] [Google Scholar]
- 29.Cao L, Liu X, Lin EJD, Wang C, Choi EY, Riban V, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schéle E, Grahnemo L, Anesten F, Hallén A, Bäckhed F, Jansson JO. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (gcg) and brain-derived neurotrophic factor (BDNF) in the central nervous system. Endocrinology. 2013;154:3643–51. doi: 10.1210/en.2012-2151. [DOI] [PubMed] [Google Scholar]
- 31.Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22:583–93. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- 32.Guevara R, Valle A, Gianotti M, Roca P, Oliver J. Gender-dependent differences in serum profiles of insulin and leptin in caloric restricted rats. Horm Metab Res. 2008;40:38–43. doi: 10.1055/s-2007-1004525. [DOI] [PubMed] [Google Scholar]
- 33.Landt M, Gingerich RL, Havel PJ, Mueller WM, Schoner B, Hale JE, et al. Radioimmunoassay of rat leptin: sexual dimorphism reversed from humans. Clin Chem. 1998;44:565–70. [PubMed] [Google Scholar]
- 34.Mulet T, Picó C, Oliver P, Palou A. Blood leptin homeostasis: sex-associated differences in circulating leptin levels in rats are independent of tissue leptin expression. Int J Biochem Cell Biol. 2003;35:104–10. doi: 10.1016/s1357-2725(02)00092-4. [DOI] [PubMed] [Google Scholar]
- 35.Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–33. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H, Strader AD, Woods SC, Seeley RJ. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol Endocrinol Metab. 2007;293:E316–26. doi: 10.1152/ajpendo.00710.2006. [DOI] [PubMed] [Google Scholar]
- 37.Senthil Kumar SPD, Spicer EG, Goudjo-Ako AJ, Stumph JD, Zhang J, Shi H. Distinct metabolic effects following short-term exposure of different high-fat diets in male and female mice. Endocr J. 2014 doi: 10.1507/endocrj.ej13-0455. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komori T, Morikawa Y, Tamura S, Doi A, Nanjo K, Senba E. Subcellular localization of glucose transporter 4 in the hypothalamic arcuate nucleus of ob/ob mice under basal conditions. Brain Res. 2005;1049:34–42. doi: 10.1016/j.brainres.2005.04.079. [DOI] [PubMed] [Google Scholar]
- 39.Ahrén B, Baldwin RM, Havel PJ. Pharmacokinetics of human leptin in mice and rhesus monkeys. Int J Obes. 2000;24:1579–85. doi: 10.1038/sj.ijo.0801447. [DOI] [PubMed] [Google Scholar]
- 40.Givalois L, Naert G, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L. A single brain-derived neurotrophic factor injection modifies hypothalamo–pituitary–adrenocortical axis activity in adult male rats. Mol Cell Neurosci. 2004;27:280–95. doi: 10.1016/j.mcn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, et al. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 42.Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci U S A. 2012;109:1305–10. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]