Abstract
Background
Pancreas cancer is highly lethal even at early stages. Adjuvant therapy with chemotherapy (CT) or chemoradiation (CRT) is standard following surgery to delay recurrence and improve survival. There is no consensus on the added value of radiotherapy (RT). We conducted a retrospective analysis of clinical outcomes in pancreas cancer patients treated with CT or CRT following surgery.
Methods
Patients with resected pancreas adenocarcinoma were identified in our institutional database. Relevant clinicopathologic and demographic data were collected. Patients were grouped according to adjuvant treatment: group A: no treatment; group B: CT; group C: CRT. The primary endpoint of overall survival was compared between groups B vs. C. Univariate and multivariate analyses of potential prognostic factors were conducted including all patients.
Results
A total of 146 evaluable patients were included (group A: n = 33; group B: n = 45; group C: n = 68). Demographics and pathologic characteristics were comparable. There was no significant survival benefit for CRT compared with CT (mOS 16.8 months vs. 21.5 months, respectively, p = 0.76). Local recurrence rates were similar in all three groups. Univariate analyses identified absence of lymph node involvement (hazards ratio [HR] 1.43, p = 0.0082) and administration of adjuvant therapy (HR 0.496, p = 0.0008) as significant predictors for improved survival. Multivariate analyses suggested that patients without nodal involvement derived the most benefit from adjuvant treatment.
Conclusions
The addition of RT to CT did not improve survival over CT. Lymph node involvement predicts inferior clinical outcome.
Pancreas cancer remains the fourth leading cause of cancer death in the United States.1 Prognosis continues to be dismal with a 5-year survival of <5 % across all stages and few advances made to improve outcomes in the past decade.2 Surgical resection offers the only chance for cure; however, only 10–15 % of pancreas cancers are classified as initially resectable with curative intent, and the majority of patients present with inoperable (locally advanced or metastatic) disease.3
Complete (R0) surgical resection offers the best chance for survival; however, the majority of these patients will have recurrent disease.4 Adjuvant therapy improves outcomes following resection, and administration of adjuvant treatment is considered the standard of care for patients who recover sufficiently within 4–8 weeks of surgery.5–8 Both chemotherapy (CT) alone and combined chemotherapy and radiation (chemoradiotherapy; CRT) have been studied in the adjuvant setting; however, there is no consensus about which approach is superior and both strategies are routinely recommended in clinical practice. Phase III trials demonstrate a clear benefit for the use of adjuvant chemotherapy with gemcitabine and suggest therapeutic equivalence between gemcitabine and fluoropyrimidines.5,6,9 Whereas studies investigating the use of combined chemotherapy and radiation (CRT) have been largely underpowered with flawed designs and mixed results, CRT remains a recommended treatment option, largely based on early phase III data.8,10,11
Limited phase III trials directly compare adjuvant chemotherapy to CRT in the treatment of resected pancreas cancer, and existing data are underpowered. In order to further investigate the comparative efficacy of these treatment modalities, we conducted a retrospective analysis of clinical outcomes in patients receiving adjuvant chemotherapy CT or CRT for resected pancreas cancer.
METHODS AND MATERIALS
Study Design, Inclusion, and Exclusion Criteria
This was a retrospective study of patients treated at the Ohio State University from 1991 to 2010. In order to be included in the study, patients were required to have undergone surgical resection of localized (stage I–II) pancreatic adenocarcinoma. Patients with locally advanced (stage III) tumors, neuroendocrine cancers, and ampullary carcinomas, as well as those with incomplete treatment data were excluded. Patients who received neoadjuvant therapy before surgery or intraoperative radiation therapy (IORT) were included in the data collection but ultimately were excluded from the final data analysis for other reasons. Data regarding patient demographics, clinicopathologic characteristics, treatment administration, and clinical outcomes were collected from a patient database.
To compare the efficacy of adjuvant therapies, patients were grouped according to type of adjuvant therapy received following surgery and clinical outcomes were compared between groups B and C: group A = no therapy; group B = chemotherapy only (CT); and group C = combined chemoradiation (CRT). In addition, to evaluate the effect of clinicopathologic factors on clinical outcome, univariate and multivariate analyses were planned for the entire study population, using the following prognostic factors: age (<70 vs. ≥70 years), tumor size (<3 cm vs. ≥3 cm), lymph node involvement (positive vs. negative); sex (female vs. male); surgical margins (microscopically positive [R1] vs. negative [R0]; tumor location (head vs. tail), tumor differentiation (poor vs. well/moderate); and treatment received (CT vs. CRT and any adjuvant therapy vs. no adjuvant therapy). Patients who received neoadjuvant therapy before surgery were excluded from the treatment analyses. This study was approved by the Ohio State University Institutional Review Board.
Statistical Analysis
The primary endpoint was overall survival, defined as the time from surgery until death from any cause. The initial objectives also included assessment of disease-free survival; however, insufficient data were available for accurate analyses. Survival was compared between group B(CT only) and group C (CRT). Due to the limited availability of patient data, recurrence patterns and time to recurrence were reported descriptively. Time to recurrence was defined as the time from date of surgery until documentation of recurrent disease. Time-to-event outcomes were estimated using the Kaplan–Meier method. Univariate and multivariate analyses were conducted including all patients, using the Cox proportional hazards model. The validity of proportional hazards assumption was assessed for each predictor.
RESULTS
Demographics
A total of 330 patients with pancreatic adenocarcinoma were identified in the patient database, and 146 of these patients had complete treatment information available and were included in analyses. Patient demographics are summarized in Table 1. Patient characteristics were well-balanced among the three groups with the exception of age, with untreated patients having the highest median age (p = 0.02). Numerically, there were higher proportions of patients with poorly differentiated tumors, R1 resections, and pancreatic tail tumors in the chemoradiation group, but none of these differences reached statistical significance. Adjuvant therapy was administered to 113 patients (chemotherapy only, 45; chemoradiation, 68) and 33 patients received no adjuvant treatment (reasons include patient/physician discretion (n = 17) or perioperative morbidity/mortality (n = 15). Most adjuvant chemotherapy was gemcitabine-based, whereas chemoradiation was most frequently administered using a fluoropyrimidine as the radiosensitizer. The majority of patients in all three groups had tumors <3 cm, positive lymph nodes, and negative (R0) resection margins. Tumors were most commonly located (85 %) in the pancreatic head and with well or moderate differentiation.
TABLE 1.
Patient characteristics (N = 146)
| Characteristic | N (% of patients with available data) |
|||
|---|---|---|---|---|
| Group A, no therapy (n = 33) |
Group B, CT (n = 45) |
Group C, CRT (n = 68) |
p value | |
| Age (years) | ||||
| <70 | 22 (69) | 22 (49) | 36 (53) | 0.12 |
| ≥70 | 10 (31) | 23 (51) | 32 (47) | |
| Median (range) | 70 (45–85) | 66 (37–85) | 64 (30–85) | 0.02 |
| Sex | ||||
| Male | 10 (30) | 22 (50) | 32 (47) | 0.09 |
| Female | 23 (70) | 22 (50) | 36 (53) | |
| Not available | 0 | 1 | 0 | |
| Adjuvant Chemotherapy | NA | |||
| Gemcitabine-based | 26 (87) | 10 (22) | NA | |
| Fluoropyrimidine | 4 (13) | 35 (78) | ||
| Not available | 15 (33) | 23 (34) | ||
| Tumor locationa | ||||
| Head | 29 (88) | 41 (91) | 54 (80) | 0.29 |
| Body/tail | 4 (12) | 4 (9) | 13 (20) | |
| Not available | 0 (0) | 0 | 1 (2) | |
| Tumor differentiation | ||||
| Well-moderate | 13 (59) | 39 (73) | 30 (56) | 0.25 |
| Poor | 9 (41) | 11 (27) | 24 (44) | |
| Not available | 11 | 5 | 14 | |
| Tumor size (cm) | ||||
| <3 | 22 (67) | 30 (67) | 49 (72) | 0.79 |
| ≥3 | 11 (33) | 15 (33) | 19 (28) | |
| Lymph nodes | ||||
| Positive (N1) | 19 (63) | 25 (61) | 36 (57) | 0.87 |
| Negative (N0) | 11 (37) | 16 (39) | 27 (43) | |
| Not available | 3 | 4 | 5 | |
| Resection margins | ||||
| R0 | 25 (78) | 36 (84) | 47 (71) | 0.32 |
| R1 | 7 (22) | 7 (16) | 19 (29) | |
| Not available | 1 | 2 | 2 | |
Bold value is statistically significant
Within the pancreas
R0 resection negative margins,
R1 resection microscopically positive
Clinical Outcomes
Overall survival was assessed according type of adjuvant treatment received (Fig. 1; Table 2). For patients receiving adjuvant therapy, median survival was 21.5 months (95 % confidence interval [CI] 13.4–24.6) for chemotherapy and 16.8 months (95 % CI 13.9–23.1) for CRT (p = 0.76). In patients treated with adjuvant therapy, median survival for node negative tumors was 24 months (95 % CI, 19.8–34.2) versus 13.8 months for node-positive tumors (95 % CI 10.9–15.2; p = 0.0003). There were no significant differences in survival according to surgical margin status, age, gender, tumor size, location, or level of tumor differentiation. Data regarding recurrence patterns and time to recurrence were available for the majority (54 %) of patients: 12 patients in group A, 26 patients in group B, and 41 patients in group C. Overall, 32 % of recurrences were purely local and 68 % were distant with 70 % of distant recurrences isolated to the liver. Local recurrence rates were 33 % in group A, 27 % in group B, and 34 % in group C. Median time to recurrence was 4.2 months in group A, 13.7 months in group B, and 12.9 months in group C.
FIG. 1.
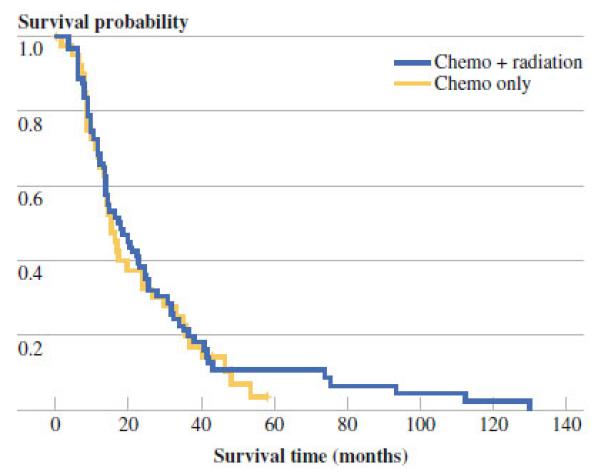
Kaplan Meier estimation of overall survival for patients receiving chemotherapy versus chemoradiation
TABLE 2.
Clinical outcomes according to treatment received
| Variable | mOS (m) | 95 % CI | p value |
|---|---|---|---|
| Any adjuvant therapy | 16.4 | 14–21 | 0.0007 |
| No adjuvant therapy | 6.3 | 1.5–10.1 | |
| Type of adjuvant therapy | |||
| CT | 21.5 | 13.4–24.6 | 0.76 |
| CRT | 16.8 | 13.9–23.1 | |
| Lymph node statusa | |||
| Negative | 24 | 19.8–34.2 | 0.0003 |
| Positive | 13.8 | 10.9–15.2 | |
| Resection margin status | |||
| Negative (R0) | 16.6 | 13.4–20.1 | 0.36 |
| Positive (R1)ab | 16.4 | 13.8–23.6 | |
| Tumor size (cm) | |||
| <3 | 16.4 | 12.1–21 | 0.0924 |
| ≥3 | 16.8 | 13.7–24 | |
| Tumor differentiation | |||
| Well-moderate | 15.2 | 8.7–25.6 | 0.54 |
| Poor | 16.4 | 13.9–23 | |
| Sex | |||
| Male | 17.1 | 13.9–24.3 | 0.55 |
| Female | 16.4 | 12.2–23.1 | |
| Age (years) | |||
| <70 | 20.4 | 13.7–29.9 | 0.0514 |
| ≥70 | 15.2 | 13.6–18.3 |
Bold values are statistically significant
In patients receiving adjuvant therapy
Microscopically positive
Univariate analyses (Fig. 2) identified lymph node involvement (hazards ratio [HR] 1.43, 95 % CI 1.1–1.86, p = 0.0082) as a predictor of inferior survival, whereas receipt of any adjuvant therapy was significantly associated with improved outcome (HR 0.496 in favor of adjuvant treatment, 95 % CI 0.328–0.749, p = 0.0008). Violation of proportional hazards was found for adjuvant therapy. To correct for this, a stratified Cox regression model was used for the multivariate survival analysis. The final model includes a stratification factor (adjuvant therapy) and one significant predictor (lymph node positivity). The model based HR of 1.77 (95 % CI 1.21–2.59) implied that patients with negative lymph nodes derive greater benefit from adjuvant therapy than those with any lymph node involvement.
FIG. 2.
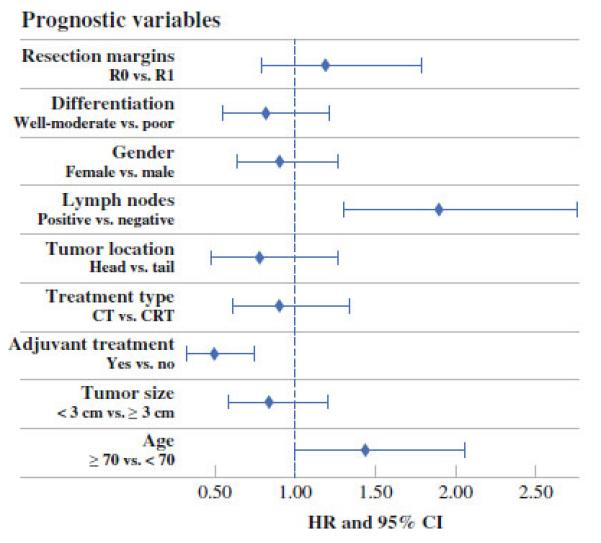
Univariate analysis of prognostic factors
DISCUSSION
Pancreas cancer remains a therapeutic challenge. Only a minority of patients present with resectable tumors, and following curative-intent resection, more than 90 % of these patients will have recurrent disease with a 5-year survival of <10 %.5,12 Adjuvant therapy improves clinical outcome; however, there is a lack of randomized data directly comparing chemotherapy versus chemoradiation in this setting. As a result, there is no universal agreement regarding the most effective treatment modality to represent a standard of care and on which to build future clinical trials.
Randomized trials of chemoradiation have generally been underpowered with design flaws; however, they do suggest lack of benefit and added toxicity versus chemotherapy or observation. Early results from the GITSG trial, which suggested a benefit for chemoradiation over observation, were not reproduced by the EORTC study.10,11 The ESPAC-1 trial, while criticized by some for the absence of a standard radiation protocol, suggested that chemoradiation resulted in inferior outcomes including inferior survival, higher recurrence rates, shorter recurrence-free survival, and increased toxicity versus chemotherapy.8 The existing data suggest that delay in the administration of systemic therapy along with increased toxicity may contribute to the inferior outcomes observed with chemoradiation.
In contrast, phase III trials of adjuvant chemotherapy alone demonstrate a significant survival advantage compared with observation.5,9 Preliminary results of the CONKO-001 trial of adjuvant gemcitabine versus observation showed a significant improvement in disease-free survival that was consistent across all subgroups, including node-positive disease and R1 resections.5 Updated results from this trial revealed a significant overall survival benefit for adjuvant gemcitabine.13 Results from the smaller III Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer trial 9 were similar to CONKO-001. The ESPAC-3 and RTOG 97-04 trials suggested overall therapeutic equivalence between adjuvant gemcitabine and bolus 5-fluorouracil, but increased toxicity associated with 5-FU, likely related to bolus administration.6,14 Table 3 includes the published results of the various large randomized adjuvant trials in pancreas cancer. Acknowledging the limitations of cross-study comparisons, the data consistently suggest that the addition of radiation in the adjuvant setting seems unlikely to enhance the benefit observed with adjuvant chemotherapy for all subset of patients.
TABLE 3.
Summary of postoperative adjuvant therapy trials in pancreas cancer
| Variable | Study |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ESPAC-18 |
CONKO-0015,13 |
ESPAC-36 |
RTOG 97-0414 |
Current study |
|||||||
| 5FU | CRT | GEM | Obs | 5FU | GEM | 5FU/CRT | GEM/CRT | Obs | CT | CRT | |
| % R1 | 19 | 19 | 19 | 15 | 35 | 35 | 33 | 35 | 22 | 16 | 29 |
| % LN positive | 53 | 50 | 71 | 73 | 70 | 73 | 65 | 68 | 63 | 61 | 57 |
| % LR | 35 | 34 | 41 | NR | NR | 28 | 23 | 33 | 27 | 34 | |
| mOS (m) | 20.1 | 15.9 | 22.5 | 20.2 | 23 | 23.6 | 16.9 | 20.6 | 6.3 | 21.5 | 16.8 |
| mDFS (m) | NR | NR | 13.4 | 6.9 | 14.1 | 14.3 | 11.1 | 11.2 | NA | NA | NA |
| % G3-4 toxicity | 4 | 6 | 5 | 1 | 14 | 7.5 | 62 | 79 | NA | NA | NA |
5FU 5-fluorouracil, CRT chemoradiation, GEM gemcitabine, Obs observation, R1 microscopically positive margins, LN lymph node, LR local recurrence, OS overall survival, DFS disease-free survival, CT chemotherapy, NA not available
We observed a nonstatistical but numerical advantage for adjuvant chemotherapy compared with chemoradiation that may have been limited by the small sample size. Importantly, our findings suggest no benefit derived from adjuvant chemoradiation compared with chemotherapy, and interestingly we observed survival outcomes remarkably similar to those published in the ESPAC-1 trial (Table 3).8 Our results are consistent with a recent meta-analysis of all randomized phase III trials of adjuvant therapy, which suggested that chemoradiation did not improve survival following surgery with a trend toward worse outcomes for these patients, while adjuvant chemotherapy reduced the risk of death by 25 % compared with observation.15 These results support the existing body of evidence, indicating that the addition of radiation to chemotherapy does not improve outcome over chemotherapy alone in the treatment of early stage pancreas cancer.
Our results are consistent with multiple studies of failure patterns following surgery for pancreas cancer, which have demonstrated that only approximately 15–30 % of patients have local recurrence only and the majority of recurrences are distant with a worse prognosis.12,16,17 In the subset of patients with available data on recurrence patterns, patients in all three groups had similar rates of local recurrence and, more importantly, chemoradiation did not seem to reduce the risk of local recurrence or delay recurrence compared with chemotherapy alone. This suggests that the potential locoregional control achieved with chemoradiation would likely not prevent the majority of recurrences. In the ESPAC-1 trial, only 18 % of patients had R1 resections, but nearly 50 % of patients experienced recurrent disease within 1 year, indicating that pancreas cancer should be treated as a systemic rather than a local disease. Interestingly, analysis from the ESPAC-1 study showed that patients with positive [R1] resection margins benefited from chemotherapy as much as or perhaps better than chemoradiation.18 Results from the CONKO-001 study also showed significant survival benefit for chemotherapy in R1 resections.5 These data support a treatment approach based on systemic chemotherapy only following surgery.
Consistent with previous data and randomized trials, our results suggest that lymph node involvement may be the most important predictor of inferior clinical outcomes, even with adjuvant treatment.5,7,15 Adjuvant treatment therefore is indicated strongly in this subgroup of patients, and positive lymph node status should be considered as a stratification factor in future clinical trials of adjuvant therapy for pancreas cancer.
Interpretation of our results is limited by the small sample size and the retrospective nature of the study, which introduces the potential for bias, most importantly in patient selection. Additionally, although not significant, there were numerically higher numbers of patients with microscopically positive margins, poorly differentiated tumors and tumors of the pancreatic tail in the group receiving chemoradiation versus chemotherapy. These factors have been associated with worse prognosis, may have affected results, and may have introduced a bias in our results in favor of chemotherapy alone. Our study population was treated for nearly two decades. Advances in treatment administration and supportive care over the years may have influenced treatment outcomes. Furthermore, we found that from 1991 to 1999, the majority (80 %) of patients receiving adjuvant treatment were treated with chemoradiation, whereas from 2000 to 2010, treatment was much more evenly distributed, with 54 % of patients receiving chemoradiation and 46 % of patients receiving chemotherapy. It is possible that evolution of our institutional treatment biases and differences in treatment quality, which we were not able to capture, may have contributed to the outcomes in patients receiving chemotherapy or chemoradiation. Lack of available data allowed for limited analyses of clinical outcome, and for this reason we were not able to accurately analyze disease-free survival or recurrence patterns.
CONCLUSIONS
Our results suggest no benefit from chemoradiation compared with chemotherapy for the adjuvant treatment of pancreas cancer. Based on the available data and, outside of a clinical trial, patients should be treated with chemotherapy alone following surgery. Additional well-designed, randomized trials are necessary to define the role of radiation therapy for resected pancreas cancer, including evaluation of new radiotherapy techniques with improved standardization of treatment protocols. Lymph node involvement predicts inferior clinical outcome following surgery and should serve as a stratifying factor in future clinical trials in this setting.
ACKNOWLEDGMENT
No funding source to disclose.
Footnotes
CONFLICT OF INTEREST The authors have no conflict of interest to disclose.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–99. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 3.Muller MW, Friess H, Koninger J, et al. Factors influencing survival after bypass procedures in patients with advanced pancreatic adenocarcinomas. Am J Surg. 2008;195(2):221–8. doi: 10.1016/j.amjsurg.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91(5):586–94. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 5.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemo-therapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–81. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 7.You DD, Lee HG, Heo JS, Choi SH, Choi DW. Prognostic factors and adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. J Gastrointest Surg. 2009;13(9):1699–706. doi: 10.1007/s11605-009-0969-5. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 9.Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101(6):908–15. doi: 10.1038/sj.bjc.6605256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 11.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230(6):776–82. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Frampton AE, Kyriakides C, et al. Loco-recurrence after resection for ductal adenocarcinoma of the pancreas: predictors and implications for adjuvant chemoradiotherapy. J Cancer Res Clin Oncol. 2012;138(6):1063–71. doi: 10.1007/s00432-012-1165-7. [DOI] [PubMed] [Google Scholar]
- 13.Nehaus P, Riess H, Post S, et al. CONKO-001: final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer (PC) (abstract) J Clin Oncol. 2008:26S. [Google Scholar]
- 14.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019–26. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 15.Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008;143(1):75–83. doi: 10.1001/archsurg.2007.17. discussion 83. [DOI] [PubMed] [Google Scholar]
- 16.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnerlich JL, Luka SR, Deshpande AD, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg. 2012;147(8):753–60. doi: 10.1001/archsurg.2012.1126. [DOI] [PubMed] [Google Scholar]
- 18.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234(6):758–68. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


