Abstract
The fields of immunology and metabolism are rapidly converging on adipose tissue. During obesity, many immune cells infiltrate or populate in adipose tissue and promote a low-grade chronic inflammation. Studies to date have suggested that perturbation of inflammation is critically linked to nutrient metabolic pathways and to obesity-associated complications such as insulin resistance and type 2 diabetes. Despite these advances, however, many open questions remain including how inflammatory responses are initiated and maintained, how nutrients impact the function of various immune populations, and how inflamma-tory responses affect systemic insulin sensitivity. Here we review recent studies on the roles of various immune cells at different phases of obesity and discuss molecular mechanisms underlying obesity-associated inflammation. Better understanding of the events occurring in adipose tissue will provide insights into the pathophysiological role of inflammation in obesity and shed light on the pathogenesis of obesity-associated metabolic syndrome.
Keywords: immunity, inflammation, obesity, diabetes, lipids, cytokines, adipocytes
INTRODUCTION
A growing epidemic of obesity is threatening the health of millions of people around the world. Adipose tissue is the major site of triglyceride storage in the body and thus contributes significantly to nutrient metabolism in various tissues and influences plasma concentrations of free fatty acids and glucose. The molecular cloning of leptin (168) and adiponectin (128) in the mid-1990s has led to the notion of adipose tissue as a secretory organ that modulates the function of itself and peripheral tissues in an endocrine, paracrine, and autocrine fashion.
Recent studies have established that obesity is associated with systemic chronic inflammation and that this low-grade inflammation may play a causal role in obesity-associated insulin resistance, type 2 diabetes, and other complications (55, 56, 150). The elevated inflamma-tory status appears to originate from infiltrated macrophages or other emerging immune cells in adipose tissue (156, 162). Indeed, the role of the immune system in adipose tissues has become an exciting new area of research in the field of obesity and metabolic regulation (27, 107). Genetic ablation of various inflammatory genes or immune cells in hematopoietic or myeloid cell lineage has been shown to influence immune cell composition and inflammatory responses in adipose tissue as well as systemic glucose tolerance and insulin resistance (27, 107). Thus, the interplay between metabolic and immune systems may play a critical role in the pathogenesis of obesity and type 2 diabetes.
Given the complexity of the immune responses and the special microenvironment existing in adipose tissues during obesity, a complete understanding of the contributions of such diverse groups of immune cells to the inflammatory nature of obesity remains challenging but nonetheless critical. Despite recent advances, a comprehensive picture of inflammation and its initiation and resolution in adipose tissue in obesity has not yet emerged. Here we review recent reports on the roles of various immune cells and cytokines in the development of inflammatory responses in adipose tissues in the context of metabolic syndrome. We also discuss several current prevailing models of this emerging and exciting field.
OVERVIEW OF IMMUNITY
Inflammation is the process by which the body responds to injury or infection and as such represents the body's major initial defense mechanism to restore tissue homeostasis and function. In obesity, inflammatory responses are likely to be chronic and of much lower amplitude than the classical ones elicited by cancer, pathogen infection, or injury. The low-grade chronic inflammation associated with obesity may shift both immune and metabolic systems to a new homeostatic set point, which may lead to a gradual decline in systemic insulin sensitivity. In this section, basic concepts of immunity are briefly introduced, with a particular focus on two dichotomies: innate/adaptive immunity and the M1/M2 macrophage polarization.
Innate and Adaptive Immunity
The immune responses of vertebrates to pathogens are composed of the early reactions of innate immunity (also called natural immunity) followed by the later responses of adaptive immunity (also called acquired immunity) (59). Innate and adaptive immunity function cooperatively as a tightly linked and integrated host defense system: The innate response to microbes stimulates and controls the specificity and course of adaptive immunity, whereas the adaptive response further enhances the potency of the innate response.
Innate immunity provides a swift but nonspecific response against infectious agents prior to the initiation of adaptive immune responses. Innate immune cells sense and are activated by pathogens through pattern recognition receptors (PRRs). PRRs, including toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), C-type lectin receptors (CLRs), and retinoic acid–inducible gene (RIG)-I-like receptors (RLRs), recognize exogenous pathogen-associated molecular patterns (PAMPs) [e.g., lipopolysaccharides (LPS), flagellin, and pathogen nucleic acids] and endogenous damage-associated molecular patterns (DAMPs) from dead and dying cells [e.g., adenosine triphosphate (ATP), uric acid, heat-shock proteins, self nucleic acids], leading to the activation of innate immune cells and, as a consequence, adaptive immune cells (59, 98). Cells involved in innate immunity include macrophages, dendritic cells, mast cells, neutrophils, eosinophils, and natural killer (NK) cells.
Adaptive immunity represents a collection of highly evolved and sophisticated defense mechanisms mediated by B or T lymphocytes, including many subsets of CD4+, CD8+ T, and NK T (NKT) cells. These cells bear highly specific B and T cell receptors (BCR and TCR) that are generated by random gene rearrangements, known as V(D)J recombination, via the activity of recombination activating genes (Rag) and other mechanisms. This leads to an enormous number of antigen-specific receptors clonally distributed on T and B cells [except for invariant NKT cells (see below)] for a vast number of nonself antigens (59). Thus, unlike the innate immunity, adaptive immune responses require several days for clonal expansion, activation, and differentiation of effector lymphocytes. Two additional features of adaptive immunity are that it (a) is exquisitely specific for nonself antigens and (b) has memory so that a subsequent encounter with the same pathogen provokes a quicker and more robust response. Immune cell populations that have been identified in adipose tissue are illustrated in Figure 1 based on their pro- or anti-inflammatory nature.
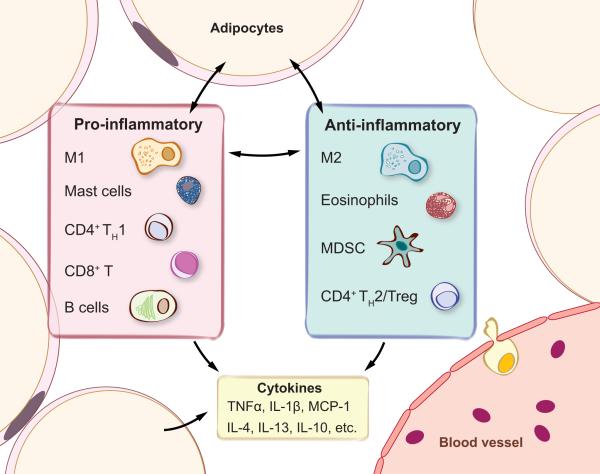
Figure 1.
Key immune populations and cytokines in white adipose tissue (WAT). Immune cells involved in both proand anti-inflammatory responses are present in adipose tissue. Cytokines allow immune cells and adipocytes to communicate with each other, maintain inflammatory homeostasis in WAT, and influence systemic insulin sensitivity. Abbreviations: IL, interleukin; M1, classically activated M1 macrophages; M2, alternatively activated M2 macrophages; MCP-1, monocyte chemotactic protein 1; MDSC, myeloid-derived suppressor cell; TH1/TH2, CD4+ type 1 or 2 helper T cells; TNFα, tumor necrosis factor alpha; Treg, regulatory T cells.
M1 and M2 Macrophage Polarization
Exposure of macrophages to either proor anti-inflammatory cytokines induces two distinct activation pathways leading to different polarization states (44), classically activated M1 or alternatively activated M2 macrophages. M1 macrophages, induced by interferon gamma (IFN-γ), tumor necrosis factor alpha (TNFα), and microbial products such as LPS, are key components of the polarized type 1 inflammation. They are generally characterized by interleukin (IL)-12hi, IL-23hi, and IL-10low and produce high levels of reactive oxygen and nitrogen intermediates and inflammatory cytokines. In contrast, M2 represents various forms of macrophage activation other than classical M1 macrophages. These cells are elicited by the stimulation of IL-4, IL-13, IL-10, and glucocorticoid hormones, among others. M2 cells are generally IL-12low, IL-23low, and IL-10high and have high levels of intracellular arginase 1 expression, which promotes cellular proliferation and growth.
Both types of macrophages are important components of inflammation. M1 macrophages tend to elicit chronic inflammation (i.e., are proinflammatory) and tissue injury, whereas M2 macrophages tend to resolve inflammation (i.e., are anti-inflammatory) and facilitate wound healing (44). Thus, cytokines and immune cells such as TH2 and regulatory T cells (Treg) that promote macrophages toward M2 polarization may be of therapeutic value in obesity-associated inflammation and type 2 diabetes.
It should be noted that although the classification is practically useful, it is a simplistic rigid view of macrophage functions. In vivo, there are probably no such clear-cut M1-versus-M2 states, but rather a dynamic continuum of the M1-M2 spectrum depending on the local cytokine microenvironment. M1 and M2 represent two extremes, and most macrophages fall somewhere between an M1 to M2 phenotype. This issue is further discussed in the Macrophages section below.
OVERVIEW OF INFLAMMATORY RESPONSES DURING THE PROGRESSION OF OBESITY
To date, much attention in the field has been devoted to the late stages of obesity, which are generally modeled by feeding animals a high-fat diet (HFD) for many weeks. The obese mice are compared with control mice fed carbohydrate-rich chow diet for the same amount of time, which is far from ideal because the content of chow is vastly different from that of semi-purified HFD. As a result, differences in phenotype may be unrelated to obesity but instead may be caused by differences in (micro)nutrient composition. For this reason, the use of a proper low-fat control group and alternative genetic obese mouse models such as leptin-deficient ob/ob or leptin-receptor-deficient db/db mice, as applied in a number of studies, should be advocated.
Studies in the past 15 years have led to the delineation of cellular events associated with well-established obesity, and they form the basis for the notion that obesity is associated with the activation of various immune cells in adi-pose tissue and, as a consequence, the development of inflammatory responses (27, 107). In contrast, what happens in the early stages of obesity or days after HFD feeding has remained unclear. Below we discuss the cellular events occurring in adipose tissue at these two stages.
At the Early Stages of Obesity
Several studies have suggested that changes in body weight, adiposity, and insulin resistance occur very early with short-term HFD feeding. HFD feeding, with 60% calories derived from fat, doubles adipose tissue weight, increases adipocyte cell size, quadruples triacylglycerol content in adipose tissue within a week (69, 81), and promotes hepatic insulin resistance even within three days (73, 126). Gene microarray analyses reveal that genes associated with inflammatory responses are altered in adipose tissue within three days of HFD feeding (69, 117), suggesting that short-term HFD may trigger an acute inflammatory response in adipose tissue. Lee et al. (79) recently showed that macrophage is not important for the development of insulin resistance with three days of feeding HFD because depletion of macrophages using liposome clodronate has no effect on insulin sensitivity. More studies are required to fully understand the events associated with the onset of obesity. Several key unsolved issues are how inflammation is initiated, how immune cells are activated, and what the roles of dietary lipids are in this process. Better understanding of the events associated with the onset of obesity may provide insights into the events associated with the later stage of obesity and help to develop intervention strategies or prevent irreversible changes at the later stages.
At the Late Stages of Obesity
Continued HFD feeding further increases body and adipose tissue weights with the development of hyperlipidemia and hyperinsulinemia. At this stage, animals or humans become mildly to severely glucose intolerant and insulin resistant in the liver, muscle, and adipose tissue. Increased adiposity is associated with elevated endoplasmic reticulum (ER) stress, cell death, and reduced secretion of adiponectin, an insulin-sensitizing cytokine secreted by adipocytes. Concomitantly, long-term HFD feeding or obesity affects the balance of pro- and anti-inflammatory cytokines in adipose tissue and, as a consequence, the M1/M2 polarization status of macrophages. Specifically, immune cells, most notably macrophages, CD8+ T, mast cells, and B cells, infiltrate into or accumulate in adipose tissues at later stages of obesity. By contrast, the levels of two immunosuppressive cells, Treg and myeloid-derived suppressor cells (MDSCs), decrease or increase, respectively, with adiposity. Collectively, these changes may progressively alter the status of inflammatory homeostasis in adipose tissue in obesity.
ADIPOSE-RESIDENT IMMUNE CELLS
Recruitment of immune cells from the circulation is a key feature of immune responses to tissue damage or infection. Below we discuss the recent findings on each cell type in adipose tissue classified into myeloid and lymphoid cells. The relative abundance of each immune cell type in adipose tissue and their dynamics under lean and obese states (Figure 2) are compiled based on recent literatures and our own unpublished data.
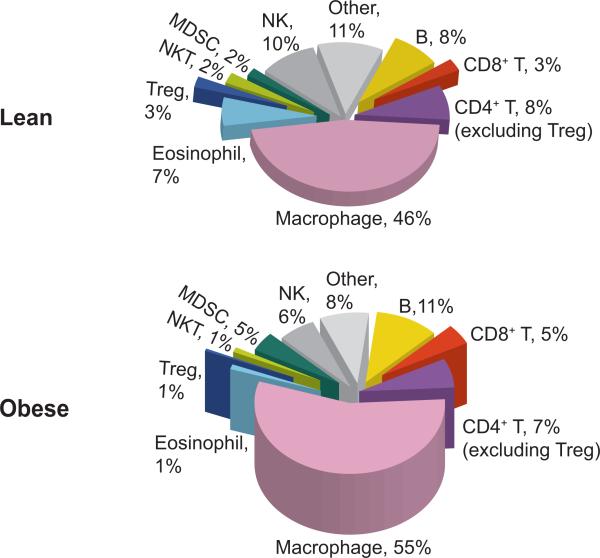
Figure 2.
Relative abundance of various immune cells in adipose tissue of lean and obese mice. Pie charts show the abundance of various immune cells in total CD45+ leukocytes present in stromal vascular fraction of epididymal adipose tissue of 14- to 20-week-old mice that have been on either LFD (lean) or HFD for 8 to 12 weeks (obese). The numbers shown in the charts indicate the percentage of each cell type in total CD45+ leukocytes. The height of the pies reflects the numbers of total CD45+ leukocytes in one epididymal fat pad, which are significantly increased in obese animals compared to age-matched lean mice. The relative abundances for B cells, Treg, eosinophils, MDSCs, and macrophages are from References 102 and 158–161; the others are from our unpublished data. These pie charts are our attempt to provide a glimpse of the relative abundance of each immune population and its dynamics in obesity. It is by no means precise, as data have been collected from different studies where different experimental methods and analysis tools have been employed. “Other” refers to mast cells, γδ T cells, and other unidentified immune cells. Abbreviations: HFD, high-fat diet; LFD, low-fat diet; MDSC, myeloid-derived suppressor cell; NK, natural killer cell; NKT, natural killer T cell; Treg, regulatory T cells.
Myeloid Cells
Macrophages
Macrophages, identified as F4/80+ CD11b+ cells, are important innate immune cells that not only phagocytose nonself antigens or cellular debris, but also act as professional antigen-presenting cells (APCs) (together with dendritic cells) to activate T lymphocytes of the adaptive immune system. They are produced by differentiation of monocytes in tissues in response to damaged tissues, damaged cells, pathogens, or cytokines, although the differentiation signals or cues for most tissue macrophages remain obscure. In some instances, it has been reported that macrophages can populate through proliferation induced by cytokines (60). Tissue-resident macrophages are believed to play a key role in steady-state homeostasis of the tissue via the clearance of dying cells or debris. As discussed above, macrophages are not a homogeneous population but rather consist of multiple macrophage phenotypes with different functions and divergent physiological effects, i.e., phenotypic plasticity (44). It is believed that macrophage activation dictates the quality, duration, magnitude, and specificity of most, if not all, inflammatory responses.
During obesity, macrophages infiltrate or undergo expansion in adipose tissues (156, 162). Changes in the number and function of these cells may have a significant impact on adi-pose tissue inflammation and systemic insulin sensitivity (16, 27, 107). Unlike proinflammatory M1 macrophages, M2 macrophages contribute to improved insulin sensitivity due to their ability to attenuate inflammation and promote tissue repair and angiogenesis (106). Moreover, depletion of macrophages using liposome clodronate significantly improves insulin resistance and glucose tolerance in HFD-fed and ob/ob mice (32, 79). However, other studies have reported no beneficial effect of liposome clodronate on systemic glucose homeostasis in obesity (20, 76, 139). One important question in the clodronate experiments is whether clodronate can specifically target and kill macrophages in adipose tissue with no or minimal off-target effects. The discrepancies among these studies may be derived from the differential depletion of macrophages, and they can be solved only by using alternative or more advanced techniques to target macrophages.
Another controversial issue is whether and how macrophages are recruited to adipose tissue during obesity. A recent study reported that macrophage accumulation in the pleural cavity after helminth infection with Litomosoides sigmodontis is a result of proliferation induced by IL-4 rather than recruitment (60). It will be interesting to determine whether this also holds true in adipose tissue during obesity. Nonetheless, it has been commonly assumed that macrophages populate in adipose tissue during obesity through recruitment or chemokine-mediated chemotaxis. Early studies have shown that chemokine receptor CCR2 or CCR2-ligand [also known as monocyte chemo-tactic protein 1 (MCP-1)] knockout (KO) mice have fewer macrophages in adipose tissue (63, 155), whereas overexpression of MCP-1 in adi-pose tissue increases macrophage infiltration and insulin resistance (62, 63). However, in other studies, MCP-1 KO mice exhibit no reduction in adipose-resident macrophages (17, 57). The discrepancies may be due to the mouse genetic background or the different techniques used to quantitate macrophages in adipose tissue. Although most studies used visual assessment of F4/80-positive cells or crown-like structures on adipose tissue sections, more accurate flow cytometric analysis of whole fat pad was used in one of the studies to calculate macrophage abundance (57).
Another highly debated issue is how obesity influences macrophage phenotypes in adipose tissue. It has been initially suggested that there is an M2-to-M1 phenotypic switch in adipose tissue during obesity (86): Obesity induced by a 20-week feeding of 45% HFD is associated with an increase of F4/80+ CD11b+ CD11c+ macrophages (or more precisely dendritic cells), elevated expression of proinflammatory markers such as inducible nitric oxide synthase and TNFα, and reduced expression of M2 markers such as arginase 1 in adipose tissue (86, 102). Ablation of CD11c+ macrophages using the toxin-based approach leads to a marked reduction of proinflammatory markers in adipose tissue and improvement of insulin sensitivity in mice following a 16-week feeding of 60% HFD (113). However, this binary switch model has been recently challenged by several studies in which mixed M1/M2 profiles are reported in obese adipose tissues of both human and mouse (12, 131, 167). Thus, further studies allowing the analysis of adipose-resident macrophages at both single-cell and population levels are required to delineate the heterogeneity of adipose-resident macrophages and to identify the macrophage state responsible for diabetic pathology.
Eosinophils
Eosinophils, comprising only 1% to 3% of circulating leukocytes, participate in engulfing and killing bacteria and other pathogens such as parasites (123). These cells are unique in that they possess large collections of granules, which contain cytotoxic cationic proteins and stain bright red with standard histology stains. The development of eosinophils is largely mediated by cytokine IL-5 and the transcriptional activity of the GATA-1 transcription factor (123). Overproduction of IL-5, as in IL-5-transgenic (IL-5tg) mice, leads to hypereosinophilia (78), and conversely, deletion of GATA1 expression in the ΔdblGATA mice blocks eosinophil lineage development (166). Using these animal models, a recent study investigated the role of these cells in obesity (160).
In a screen for IL-4-secreting cell types in adipose tissue of IL-4 reporter (4get) mice, Wu et al. (160) reported that nearly 90% of the IL-4-competent cells from perigonadal adipose tissue are eosinophils at 10 d following the infection with migratory helminth Nippostrongylus brasiliensis. Eosinophils, identified as CD11b+ F4/80+ Siglec-F+ cells, are made up of 30% of total CD11b+ F4/80+ cells in perigonadal adipose tissue and only 1.2% in subcutaneous adipose tissue. Eosinophil levels in adipose tissue are negatively correlated with adiposity and obesity in mice. Functionally, hypereosinophilia IL-5tg mice on a normal low-fat diet exhibit improved glucose tolerance. Using adoptive transfer of bone marrow cells from IL-5tg mice to eosinophil-deficient ΔdblGATA mice, the authors showed that eosinophils promote M2 polarization in adipose tissue via the secretion of IL-4 and IL-13 (160). Conversely, ΔdblGATA mice exhibit no change in adipose tissue weight but have elevated fasting glucose and impaired glucose tolerance upon 15 to 20 weeks of HFD feeding. Finally, infection of wild-type mice on HFD with N. brasiliensis increases levels of eosinophils in adipose tissue, improves glucose tolerance and insulin sensitivity, and, surprisingly, reduces the proportion of M1 macrophages in adipose tissue (160). How infection causes the loss of M1 macrophages remains unclear. Thus, eosinophils may play a role in the maintenance of M2 polarization in adipose tissue in obesity via the secretion of anti-inflammatory cytokines IL-4 and IL-13. The effect of IL-4 and IL-13 on metabolism is discussed in detail in the Cytokines section below.
Mast cells
Mast cells secrete a large number of proinflammatory and immunomodula-tory chemicals (e.g., histamine), cytokines, and chemokines and hence are important in allergic responses and tissue homeostasis and remodeling. Because the proliferation and survival of mast cells require the activity of cell-surface receptor kinase c-kit (39), the c-kit mutations in the KitW−sh/W−sh mice result in mast cell deficiency and profound reduction of tissue mast cells (45). Using this mouse model, a recent study showed the involvement of mast cells in the development of obesity and type 2 diabetes (84).
The abundance of mast cells is elevated in the adipose tissue of obese animals. Interestingly, genetic ablation of mast cells in KitW−sh/W−sh mice reduces body weight gain, improves glucose homeostasis, and increases energy expenditure after 12 weeks of HFD. Similarly, mice on 12 weeks of HFD exhibit reduced body and adipose weights and improved glucose tolerance and insulin sensitivity after treatment with the mast cell–stabilizing drug disodium cromoglycate (84), a drug that attenuates the release of mast cell granules (108). Interestingly, in both loss-of-function mouse models, fewer macrophages are observed in adipose tissue in comparison with wild-type control mice (84). Furthermore, adoptive transfer of bone marrow–derived mast cells into the KitW−sh/W−sh mice partially rescues the phenotypes. Interestingly, the effect of mast cells is mediated via IL-6 and IFN-γ but not via TNFα. Mechanistically, mast cell levels correlate with the level of cathepsins, enzymes involved in extracellular matrix proteolysis, and microvessel growth and angiogenesis (84). Thus, this study suggests that mast cells may link inflammation to tissue remodeling in adipose tissue in obesity.
Myeloid-derived suppressor cells
MDSCs are a heterogeneous immature myeloid cell population that is induced upon inflammation and subsequently causes immune suppression (38, 110). These immature myeloid cells, expressing both myeloid cell markers Gr-1 and CD11b in rodents, also accumulate in individuals experiencing traumatic stress and bacterial or parasitic infections (38, 110). Functionally, these cells suppress inflammation because they are able to attenuate CD8+ T cell activation, promote Treg cell development, block NK cell cytotoxicity, and skew M2 polarization (38, 110). Accumulating evidence suggests that MDSCs are precursors of mature monocytes (e.g., macrophages and dendritic cells) (41) but are distinct from mature macrophages in that the latter cell type is not normally associated with suppressive activities toward CD8+ T cells (38, 42, 110).
In obesity, Gr-1+ CD11b+ MDSCs are enriched in adipose tissue, where they attenuate inflammation and maintain inflammatory homeostasis in part via suppression of CD8+ T cells and skewing local macrophage polarization to the insulin-sensitizing M2 state (161). Indeed, downregulation of Gr-1+ CD11b+ MDSCs in obese animals using Gr-1 antibody leads to the deterioration of insulin sensitivity and glucose tolerance, whereas elevation of these cells in obese animals through adoptive transfer has the opposite effect (161). Hence, the accumulating MDSCs in obese animals may function as critical “homeostatic” regulators by countering proinflammatory cells such as CD8+ T cells and M1 macrophages. Because these cells are known to accumulate in response to inflammation (38, 110), chronic inflammation in obesity may be responsible for the induction of MDSCs. Thus, an autoregulatory feedback loop may be established during obesity in which MDSCs induced by chronic inflammation may function to prevent overt inflammatory responses and maintain inflammatory homeostasis.
Lymphoid Cells
In addition to myeloid cells, lymphocytes play important roles in the pathogenesis of obesity-associated insulin resistance, as demonstrated in lymphocyte-deficient Rag1-null mice (99, 134, 159). An intriguing issue is the identity of antigens that are responsible for the activation of T and B cells in adipose tissue in obesity. Here we introduce each lymphocyte population and detail its function in adipose inflammation and metabolic regulation.
CD4+ T cells
CD4+ T cells recognize polypeptides presented by class II major histocompatibility complex (MHC) molecules on the surface of APCs such as macrophages and dendritic cells. Activated or effector CD4+ T cells then release cytokines that recruit other immune cells to the area, resulting in inflammation. CD4+ effector T cells can be subdivided into at least three functionally distinct effector cells, T helper cells TH1, TH2, and recently discovered TH17 cells. They are defined by their signature cytokines, namely IFN-γ for TH1 cells, IL-4/IL-13 for TH2 cells, and IL-17 for TH17 cells. The balance between TH1 and TH2 cells is tuned by the crosstalk among various transcription factors. In particular, the signal transducer and activator of transcription 6 (STAT6) is a key positive regulator of TH2 cell differentiation and exerts inhibitory effects on TH1 cell differentiation. Thus, STAT6 deficiency in mice leads to TH2 deficiency.
The ratio of TH1 to TH2 cells is increased significantly during high-fat-diet-induced obesity as TH2 cells are progressively diluted by IFN-γ-producing TH1 cells (159). CD4+ T cells as a whole control the progression of obesity-associated inflammation via secretion of cytokines. Indeed, CD4+ T cell reconstitution into lymphocyte-deficient Rag1-null mice reduces body weight gain and adipocyte cell size and improves glucose tolerance and insulin signaling. However, the recipients of CD4+ T cells exhibit reduced body and adipose tissue weights and smaller adipocytes, which complicates the explanation for the improved metabolic parameters. In keeping with the notion that STAT6 is essential for TH2 cell differentiation and TH2 cells are key insulin-sensitizing cells, STAT6-deficient CD4+ T cells have no impact on glucose tolerance or on other metabolic parameters (159). Although the insulin-sensitizing effect of CD4+ T cells seems to depend on TH2 cells (159), the role of other emerging subsets of CD4+ T cells remains to be dissected.
Treg cells
CD4+ T cells can also differentiate into immunosuppressive CD4+ CD25+ Treg cells, an event regulated by the transcription factor forkhead box P3 (Foxp3) (85, 125). Notably, studies in both animals and humans have shown that depletion of Tregs not only elicits autoimmunity but also enhances immune response to nonself antigens. Most notably, Treg deficiency in humans with mutations in the FOXP3 gene leads to the immune dys-regulation, polyendocrinopathy, enteropathy, X-inked syndrome (IPEX) (4). These patients develop aggressive autoimmunity, including diabetes, thyroiditis, and eczema. Interestingly, a recent study has also implicated Treg cells in obesity-associated inflammation and metabolic disorders (34).
Obesity is associated with a reduction in the level of Treg cells in abdominal adipose tissue, but not subcutaneous adipose tissue, of various obese mouse models as well as obese human patients (34, 105, 159). Depletion of Treg in wild-type lean animals increases levels of insulin and proinflammatory cytokines in the circulation and adipose tissue (34). Conversely, upregulation of Treg cells via daily injection of IL-2 complexed with IL-2 antibody for six days in 15-week-HFD-fed obese animals increases levels of the anti-inflammatory cytokine IL-10 and improves insulin sensitivity and glucose tolerance (34). In keeping with the notion that Treg cells have insulin-sensitizing functions, HFD-fed mice injected daily with anti-CD3 antibody for five days have increased Treg cells in adipose tissue nine weeks later and exhibit improved insulin sensitivity and glucose homeostasis (159). These observations are in line with the known functions of Treg cells to suppress proinflammatory responses and promote M2 macrophage polarization via IL-4, IL-10, and IL-13 (147). With the discovery of different subsets of Tregs in basic immunology (e.g., natural and induced Treg) (85), their function in metabolic disorders will be topics of future studies.
CD8+ T cells
CD8+ T cells are important mediators of adaptive immunity against certain viral, protozoan, and bacterial pathogens. Naïve CD8+ T cells recognize and are activated by polypeptides presented by MHC class I molecules ubiquitously expressed on all nucleated cells. Activated CD8+ T cells produce large amounts of cytokines, chemokines, and microbicidal molecules to induce cytolysis of target cells. Unlike CD4+ T cells, CD8+ T cells respond to intracellular pathogens in most tissues and facilitate clearance of the infection due to the ubiquitous expression of MHC class I molecules on most nucleated cells. The most well-characterized CD8+ T cells are cytotoxic T lymphocytes.
A three- to fourfold increase in CD8+ T cells has been reported in adipose tissues in obese animals (118, 159) and humans (159). The increase of CD8+ T cells occurs following two weeks of 60% HFD feeding, hence preceding macrophage infiltration, which occurs after approximately eight to ten weeks (105). Upon infiltration, CD8+ T cells may not only increase the recruitment of macrophages to adipose tissues but also promote monocyte differentiation into M1 macrophages (105). Mice deficient in CD8+ T cells have fewer adipose macrophages with reduced levels of proinflammatory cytokines such as TNF-α and IL-6. Conversely, adoptive transfer of CD8+ T cells into obese animals further increases M1 macrophage infiltration and impairs glucose tolerance and insulin sensitivity (105). Interestingly, CD8+ T cell transfer into 12-week-old Rag1 KO mice on six-week HFD has only modest effect on glucose homeostasis and insulin sensitivity (159), suggesting that the metabolic effect of CD8+ T cells may require the presence of other lymphocytes. Thus, activated CD8+ T cells are important inflammatory cells in adipose tissue during obesity.
NKT cells
Unlike other conventional T cells, NKT cell development is dependent on the MHC class I homologue molecule CD1d, which binds and presents lipids (66). In mice, type I NKT cells, also known as invariant or iNKT cells, are the most prevalent and well-characterized NKT cells, with invariant Vα14-Jα18/Vβ8 TCR in mouse and Vα24 TCR in humans (43). Pointing to the importance of NKT cells in inflammatory responses, CD1d−/− mice with NKT cell deficiency have a reduced ability to clear bacterial infection (68, 94). In addition to glycosphingolipids such as the prototypical antigen α-galactosylceramide (66), NKT-specific lipid agonists include galactosyl diacylglycerols, phosphatidylinositol, and gangliosides (9, 74). Upon lipid activation, NKT cells secrete large amounts of both TH1 and TH2 cytokines, including IFN-γ and IL-4 (165). Numerous studies have shown that NKT cells may promote or suppress immune processes by skewing adaptive immune responses toward either a TH1 or TH2 response (9, 74).
NKT cells are quite abundant in lean adi-pose tissue (87), at the level of CD8+ and Treg cells in eight-week-old B6 mice (Figure 2) (Y. Ji, S. Sun, L. Yang, S. Xia, X. Li, S. Kersten, & L. Qi, unpublished findings). In humans, the abundance of NKT cells is reduced in obese omental tissue (87). Surprisingly, two recent studies using NKT-deficient mouse models demonstrated that loss of NKT cells has little, if any, effect on metabolic parameters following feeding of 45% HFD for over 26 weeks or 60% HFD for 12 weeks (72, 89). Most parameters, including food intake, weight gain, fat mass, metabolic rate, inflammation, and insulin resistance, are comparable between CD1d−/− and wild-type mice. Given their lipid-sensing nature, it will be interesting to see whether NKT cells are able to link dietary lipids to inflammation in response to acute HFD feeding.
B cells
B cells develop in the bone marrow and are an important component of the humoral immunity that produces immunoglobulins or antibodies to recognize the cognate antigen. This differs from the cell-mediated immunity where T cells recognize processed antigenic peptides presented by APCs. Upon binding to nonself antigens via BCR, B cells become activated and differentiate into plasma cells that secrete large amounts of antibodies with higher affinity. Antibodies bind to the antigens and hence trigger the activation of other immune cells such as T cells, leading to the further activation of plasma cells and the clearance of the nonself antigens. A recent study demonstrated the involvement of B cells in the development of obesity and type 2 diabetes (158).
B cells accumulate in adipose tissue early upon four weeks of 60% HFD feeding, which is associated with elevated concentrations of proinflammatory immunoglobulin G2c (IgG2c) in circulation and adipose tissue. B cells promote insulin resistance through the modulation of T cells and/or macrophage polarization via the production of pathogenic IgG antibodies. B cell–deficient animals are protected from insulin resistance following HFD feeding. Transfer of pathogenic IgG antibodies into HFD-feeding animals increases M1 polarization of adipose tissue and induces insulin resistance and glucose intolerance, which points to the significance of IgG antibodies. The IgGs may promote clearance of apoptotic and necrotic cell debris and enhance inflammatory responses. It will be interesting to determine whether pathogenic or proinflammatory IgGs have any impact on adipocyte function. Additionally, the role of B cells in other metabolic tissues merits further investigation.
MAJOR MEDIATORS OF INFLAMMATORY RESPONSES IN ADIPOSE TISSUE IN OBESITY
So far we have discussed the impact of individual immune cells in inflammation and metabolic regulation. Several questions arise. Importantly, what is the sequence of events leading to the activation of such a diverse group of immune cells? And does the activation of immune cells occur in adipose tissue or prior to their entry into adipose tissue, such as in lymph nodes or spleen? In other words, how is inflammation in obesity initiated? In this section we discuss studies that have provided some insights into these questions.
Besides being essential fuel molecules and components of cellular membranes, lipids are important signaling moieties for both immune responses and metabolic regulation. Pointing to the essential role of lipids in insulin signaling and metabolic regulation, lipid infusion in vivo attenuates the insulin effect on hepatic glucose production and glucose uptake by peripheral tissues (28) and enhances inflammatory gene expression in adipose tissue (132), both of which lead to systemic insulin resistance. Various mechanisms have been proposed to account for the effect of lipids on inflammation, including innate receptors, inflammasomes, nuclear receptors, cell death, ER stress, and possibly gut microbiota. All these possible mechanisms are illustrated in Figure 3 and are discussed in detail in this section. It is worth pointing out that this list is by no means comprehensive. For example, hypoxia, often associated with adipose expansion and inflammation, has been reviewed recently (163) and is not discussed here due to space limitations.
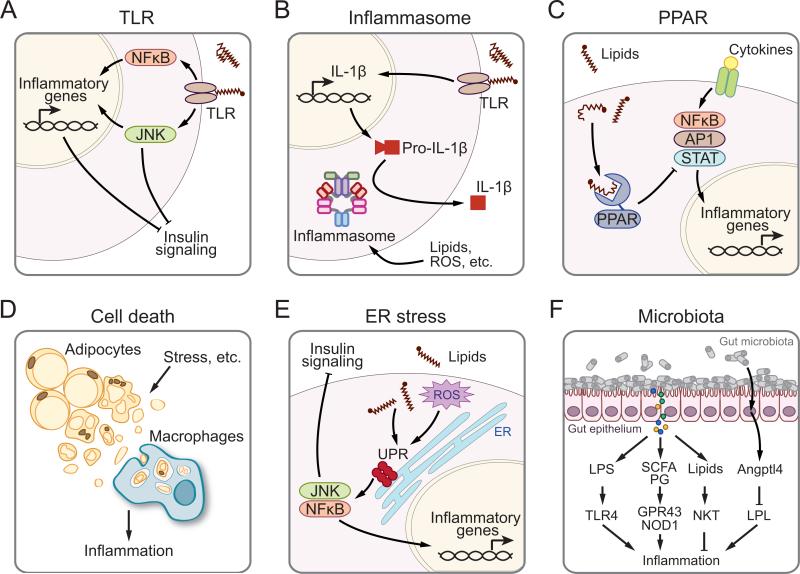
Figure 3.
Hypothetic models of how inflammation is initiated and developed in obesity. Stress signals such as lipids may signal through (a) TLRs or (b) inflammasomes to activate inflammatory pathways, which may be responsible for the downregulation of insulin signaling. (c) Lipids may downregulate inflammation via the activation of transcription factor PPAR. (d ) Stress signals induce adipocyte death, from which cell debris may be phagocytosed by macrophages and influence inflammation. (e) Lipids may promote ER stress and UPR, which may lead to the activation of JNK and NF-κB and consequently induce inflammation and attenuate insulin signaling. ( f ) Gut microbiota may influence inflammatory responses in the host by the release of LPS, SCFAs, and PG, which subsequently activate TLR4, GPR43, and NOD1 receptors, respectively. Lipid antigens derived from microbiota may also alter the activation status of NKT cells. Gut microbiota may influence the secretion of Angptl4 from enterocytes, which inhibits LPL activity and lipid uptake in macrophages. Abbreviations: Angptl4, angiopoietin-like 4; AP1, activator protein 1; ER, endoplasmic reticulum; GPR43, G protein–coupled receptor 43; JNK, c-Jun N-terminal kinases; LPL, lipoprotein lipase; LPS, lipopolysaccharide; NF-κB, nuclear factor-κB; NKT, natural killer T cells; NOD1, nucleotide-binding oligomerization domain-containing 1; PG, peptidoglycan; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; SCFAs, short-chain fatty acids; STAT, signal transducer and activator of transcription; TLR, Toll-like receptor; UPR, unfolded protein response.
Because the focus of this review is on the putative initiating mechanisms, we do not discuss the detailed intracellular signaling pathways, such as c-Jun N-terminal kinase ( JNK) and nuclear factor kappa-light-chain-enhancer of activated B cells [nuclear factor-κB (NF-κB)], leading to inflammation. Rather, in this paragraph we briefly introduce the role of the NF-κB pathway in inflammation and metabolic regulation (7). Mice with a deficiency in either NF-κB or upstream activating kinase IκB kinase β (IKKβ) in a global or tissue-specific (e.g., liver, hypothalamus, or myeloid cells) manner exhibit improved insulin sensitivity, whereas gain-of-function of IKKβ in the liver causes hepatic and systemic insulin resistance (7). However, two recent studies have shown that overexpression of either NF-κB or IKKβ under the control of the adipocyte/macrophage-specific aP2 promoter improves insulin sensitivity (61, 146) despite elevated systemic inflammation with over 20-fold increases of TNFα and IL-6 in the blood (146). These unexpected findings are likely due to decreased adiposity and increased energy expenditure, as the transgenic mice are protected from diet-induced obesity in both studies. Indeed, Tang et al. (146) showed that NF-κB inhibits the expression of a key adipogenic factor PPARγ in adipocytes. Thus, these studies suggest that inflammation not only influences insulin signaling and action but also regulates adipocyte biology and energy expenditure.
Toll-Like Receptors
TLR proteins, a family of conserved cell-surface and intracellular proteins, play an essential role in the induction of innate and adaptive immune responses against all known pathogens such as viruses, fungi, bacteria, and protozoa. It is well established that TLR signaling promotes T cell activation via the maturation and cytokine secretion of APCs. The discovery of TLR protein as a key defense gate against bacterial infection (80, 95, 115) has been awarded the 2011 Nobel Prize in Physiology and Medicine. Through a series of adaptor proteins recruited to TLRs such as myeloid differentiation factor 88, TLR signaling leads to the activation of various proinflammatory pathways, including extracellular signal-regulated kinase, mitogen-activated protein kinase, JNK, and NF-κB signaling pathways.
Among 10 human and 12 mouse TLRs, TLR2 and TLR4 are best known for their involvement in nutrient sensing and metabolic regulation. TLR4 is the best known and detects the lipopolysaccharides (LPS) found in most gram-negative bacteria, whereas TLR2 in conjunction with TLR1 or TLR6 recognizes lipopeptides and other components of gram-positive bacteria. Recent studies have suggested that TLR4 is essential for acute, lipid-induced insulin resistance (71, 132). Although results of TLR4-deficient mouse models are mixed, TLR4 seems to play essential roles in regulating adipose inflammation and systemic insulin sensitivity. Intriguingly, TLR2 deficiency has a similar effect (71). The phenotypes of TLR2- or TLR4-deficient animals from recent published studies (14, 21, 23, 30, 49, 67, 114, 116, 124, 132, 142, 149) are summarized in Table 1. Mechanistically, TLR2 and TLR4 may act as sensors for fatty acids (71) and then either initiate the classical inflammatory pathway or divert fatty acids into ceramides, both of which antagonize insulin action (50) (Figure 3a). Indeed, the detrimental effect of lipid infusion on insulin sensitivity is partially blunted in hematopoietic TLR4-deficient mice (124), suggesting that TLR4 in immune cells plays a role, but not exclusively, in mediating the lipid effect.
Table 1.
Effects of TLR2 or TLR4 deficiency on obesity-associated inflammation and insulin resistance
| Mouse models | Metabolic phenotypes | Inflammatory phenotypesb (in particular tissues) | Refs | ||||
|---|---|---|---|---|---|---|---|
| Mouse models (strain) | Length of HFD (% cal from fat) | Obesity/adiposity | Insulin sensitivitya | Hepatic steatosis | |||
| Global TLR4 deficiency | Tlr4−/− (C57BL/6) | 26 weeks (w) (58%) | ↑ (females) | ↑ | — c | ↓ (WAT, liver) | (132) |
| 8 w (60%) | =c | ↑ (thoracic aorta, liver) | — | ↓ (thoracic aorta) | (67) | ||
| Tlr4Lps–d (C3H/HeJ)d | 8 w (55%) | ↓ | ↑ | — | ↓ (WAT, liver, muscle) | (149) | |
| 16 w (60%) | = | — | — | ↓ (WAT) | (142) | ||
| 22 w (42%) | ↑ | ↑ (WAT only) | ↓ | ↓ (WAT) | (114) | ||
| Tlr4Lps–del (C57BL/10)d | 16 w (60%) | ↓ | ↑ | — | ↓ (WAT) | (22) | |
| 21 w (45%) | ↓ | = | ↓ | — | (116) | ||
| TLR4−/− BMT | BMT-Tlr4−/− (C57BL)e | 12 w (—) | = | ↑ | ↓ | ↓ (WAT, liver) | (124) |
| BMT-Tlr4−/− (C57BL/6)f | 12 w (41%) | = | = | — | ↓ (only in lean WAT) | (21) | |
| TLR2 deficiency | Tlr2−/− (C57BL/6) | 14 w (42%) | ↓ | ↑ | ↓ | ↓ (WAT) | (49) |
| Tlr2−/− (C57BL/6) | 20 w (58%) | ↓ | ↑ | ↓ | ↓ (WAT, liver, pancreas) | (30) | |
| Tlr2 ASONg (Swiss) | 8 w (55%) | — | ↑ | — | ↓ (WAT, muscle) | (14) | |
Insulin sensitivity as indicated by glucose tolerance test, insulin tolerance test, homeostasis model assessment analysis, insulin-induced insulin receptor, insulin receptor substrate-1 and AKT phosphorylation in specific tissues, or hyperinsulinemic euglycemic clamp studies.
Inflammatory phenotypes measured by inflammatory gene expression, circulation cytokine levels in serum, immunohistochemistry staining for macrophages in adipose tissues, or IκB phosphorylation and NF-κB activation in specific tissues.
—, no information is available; = , not altered in comparison with corresponding control cohort.
Tlr4Lps–d, C3H/HeJ mice with loss-of-function mutation of TLR4. Tlr4Lps–del, C57BL/10 mice with a deletion in the Tlr4 gene.
BMT of TLR4−/− C57BL/10 donor cells into irradiated wild-type C57BL/6 recipient mice.
BMT of TLR4−/− C57BL/6 donor cells into irradiated Ay/a; Ldlr−/− C57BL/6 recipient mice.
ASON, TLR2 antisense oligonucleotide intraperitoneal in eight-week HFD-fed mice for four days.
Abbreviations: BMT, bone marrow transplantation; HFD, high-fat diet; NF-κB, nuclear factor-κB; TLR, toll-like receptor; WAT, white adipose tissue.
However, our understanding of the function of TLR proteins in inflammation in the context of obesity and type 2 diabetes is incomplete. First, the relationship between TLR2 and TLR4 in vivo remains unclear. Mice with a deficiency of each protein exhibit strikingly similar phenotypes (Table 1), which begs an interesting question of whether they are sensing and activated by similar endogenous ligands in vivo. Second, the ability of saturated fatty acids to directly activate TLR2/4 was recently suggested because of LPS contamination (31). If so, how do saturated fatty acids activate inflammation? Presumably, saturated fatty acids may activate inflammation via ceramides (35) or indirectly via lipid rafts (51). A recent study showed that saturated fatty acids with an acyl chain of at least 16 carbons alter the membrane distribution and activation of membrane-anchored tyrosine kinase c-Src, leading to the activation of JNK (51). In contrast, unsaturated fatty acids prevent the redistribution of c-Src on the membrane and thus inhibit the activation of JNK (51). How TLR is involved in the saturated fatty acids-c-Src-JNK signaling pathway remains to be studied. Finally, in contrast to the phenotypes of TLR2/4-deficient animals, deletion of myeloid differentiation factor 88, an essential adaptor protein for most TLRs (except TLR3) and interleukin-1 receptor (IL-1R) signaling pathways, exacerbated insulin resistance (53), suggesting the presence of other insulin-sensitizing TLR proteins. Thus, the metabolic functions of TLR proteins, individually and collectively in a tissue-specific manner, deserve further investigations. If an individual TLR is proven to be involved in metabolic regulation, then it may serve as a safe and effective therapeutic target in type 2 diabetes (46).
Inflammasomes
Inflammasomes, high-molecular-weight multiprotein complexes in the cytosol, are responsible for the maturation of proinflamma-tory cytokines such as IL-1β and IL-18 upon activation by stress signals derived from infection and damaged tissue or cells (129). Since its first report in 2002 (91), four classes of inflammasomes, the NLR family, pyrin domain– containing 1 and 3 (NLRP1 and NLRP3), CARD domain–containing 4 (NLRC4, also known as IPAF), and absent in melanoma 2 (AIM2) inflammasomes, have been identified, among which the NLRP3 inflammasome is the best characterized. NLRP3 (also known as cyropyrin and NALP3) is a member of NLRs, and its activation leads to the recruitment of adaptor protein ASC (apoptosis-associated speck-like protein containing a carboxy-terminal CARD) and procaspase-1, forming an active inflammasome. The inflammasome subsequently releases bioactive capase-1, which in turn cleaves cytokine proforms, such as IL-1β, to mature IL-1β to be secreted extra-cellularly (22, 148). Such activity of the NLR inflammasome has been implicated in autoimmune diseases, cancer, inflammatory diseases, infection, and most recently in metabolic disorders.
Current understanding of NLRP3 inflammasome activation is illustrated in Figure 4. The expression of inflammasome components such as NLRP3 and caspase-1 as well as caspase-1 activity are elevated in the adipose tissue of diet-induced or genetically induced obese mouse models (6, 137, 140, 151). Conversely, calorie restriction reduces the NLRP3, ASC, and IL-1β transcript levels in adipose tissue (151). The NLRP3 inflammasome components including NLRP3, ASC, and caspase-1 are identified in both macrophages and adipocytes (70, 137, 151). Demonstrating the importance of inflammasome activation in the development of insulin sensitivity, mice with whole-body ablation of caspase-1, NLRP3, or ASC are protected from obesity and insulin resistance on long-term HFD (70, 137, 140, 151, 157, 169). These metabolic improvements are associated with reduced IL-1β in adipose tissue and IL-18 in the blood, as well as reduced effector T cells in the adipose tissue of KO mice (140, 151). Given that caspase-1, NLRP3, and ASC KO mice all exhibit increased energy expenditure and are resistant to diet-induced obesity and weight gain (137, 140), reduced inflammation and improved insulin sensitivity may be secondary to reduced adiposity and higher metabolic rates. Together, these reports point to a role of inflammasomes in adipose tissue in the development of obesity and insulin resistance.
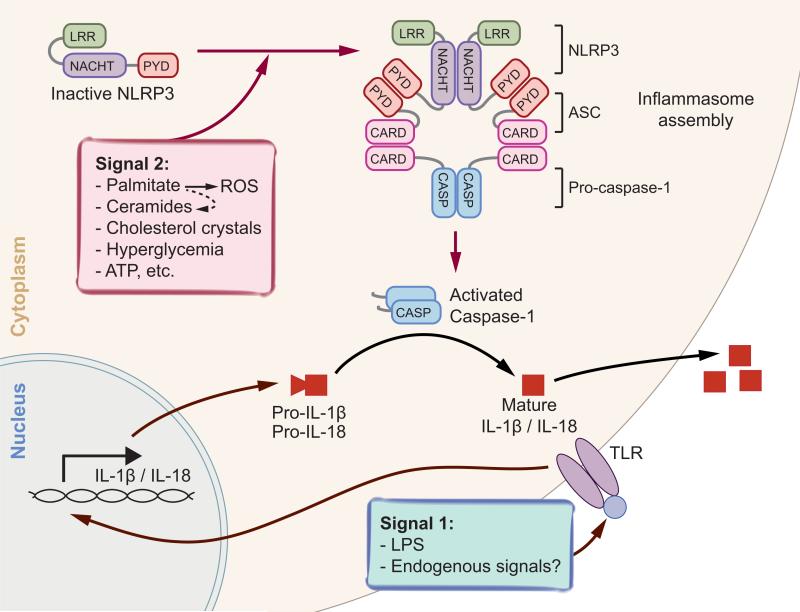
Figure 4.
Activation of NLRP3 inflammasomes. Two signals are required for the maturation of proinflammatory cytokines IL-1β and IL-18: transcriptional induction of pro-IL-1β and IL-18, and the activation of inflammasomes. Upon activation, NLRP3 oligomerizes and recruits PYD-containing adaptor ASC, whose CARD domain in turn recruits procaspase-1. Autocleavage of procaspase-1 leads to the formation of the active caspase-1 p10/p20 tetramers, which in turn splice inactive cytokines such as pro-IL-1β and pro-IL-18 to generate active molecules. Potential activating signals are indicated. Abbreviations: ASC, apoptosis-associated speck-like protein containing a carboxy-terminal CARD; ATP, adenosine triphosphate; IL, interleukin; LPS, lipopolysaccharide; LRR, leucine-rich repeat; NACHT, nucleotide-binding and oligomerization domain; NLRP3, NLR family, pyrin domain–containing 3; PYD, pyrin domain; ROS, reactive oxygen species; TLR, Toll-like receptor.
Studies in the immune system have revealed that the activation of inflammasomes, or synthesis, processing, and release of mature cytokines IL-1β and IL-18 by macrophages, ex vivo requires two stimuli: an inflammatory stimulus such as LPS to prime cells to transcribe and synthesize cytokine proforms and then a second stimulus such as ATP, nigericin, or bacterial toxins to activate inflammasome (129, 148). In the context of metabolic disorders and obesity, the nature of inflammasome-activating signals remains elusive. Several endogenous stress signals have been implicated in inflammasome activation, including glucose, palmitate, ceramides, cholesterol crystals, islet amyloid polypeptide, ATP, and reactive oxygen species (Figures 3b and 4). It will be of great interest to further investigate the physiological relevance of these putative activating signals in adipose tissue as well as other metabolic tissues, such as pancreas and the liver, in the context of obesity and type 2 diabetes.
Peroxisome Proliferator-Activated Receptors
The PPAR nuclear receptors, which include PPARα, PPARδ, and PPARγ, are transcription factors that mediate effects of fatty acids and fatty acid derivatives on gene expression. All three PPARs, but especially PPARδ and PPARγ, are well expressed in macrophages and adipose tissue and have been suggested as key modulators of macrophage polarization and adipose tissue inflammation. Pointing toward an anti-inflammatory effect of PPARα, ablation of PPARα leads to upregulation of various cytokines, chemokines, and macrophage markers in adipose tissue of mice fed HFD (138). Similarly, PPARβ/δ and PPARγ reduce adipose inflammation, promote M2 polarization of macrophages, and increase insulin sensitivity (48, 64, 90, 106, 144). Consistent with this notion, thiazolidinediones, which serve as PPARγ agonists and improve insulin sensitivity, have been shown to reduce adipose T-cell and macrophage infiltration and promote M2 polarization in obese mice (37, 136). Inasmuch as IL-4 augments PPARγ expression and activity via a mechanism involving STAT6 (144), the IL-4-induced shift toward M2 macrophages may be partly mediated by PPARγ (90, 106). Presently, conflicting data exist on whether the insulin-sensitizing effect of thiazolidinedione is mediated by PPARγ in macrophages or adipocytes (48, 90, 143). The reason for the discrepancy remains to be defined.
PPARs suppress inflammation via various mechanisms that are seemingly unrelated to the classical DNA response element–driven transcriptional upregulation of target genes. Both PPARα and PPARγ inhibit inflammatory gene expression in various cell types, including macrophages, by interfering with the activity of proinflammatory transcription factors NF-κB, AP-1, and STATs (24, 120) (Figure 3c). Additionally, it has been shown that ligands promote PPARγ SUMOylation, thereby targeting PPARγ to a corepressor complex on inflammatory gene promoters (112). This in turn impairs proteasome-dependent degradation of the corepressor complex, leading to sustained transcriptional repression (112). Although PPARδ is known to exert anti-inflammatory effects in many cell types, little is known about its underlying mechanism in macrophages (11). It has been suggested that PPARδ activation in macrophages disrupts the physical association between PPARδ and the anti-inflammatory transcriptional repressor BCL-6, thereby reducing levels of proinflammatory cytokines (8).
Cell Death
Cell death occurs through morphologically distinct cellular processes, mainly apoptosis and necrosis. How cells die dictates the outcome of inflammation (121). If cells are dying under pathological conditions (versus physiological cell death), they are potentially dangerous to the host through release of intracellular molecules known as damage-associated molecular patterns (DAMPs) (121). Concurrent with the release of DAMPs that are usually hidden in normal cells, innate and adaptive immune systems are alerted and activated. Although cell-death-induced inflammation may contribute to the pathogenesis of a number of diseases such as autoimmunity and diabetes, how the immune system identifies pathological cell death remains elusive.
Obesity is associated with the cell death of adipocytes via apoptosis (2) and/or necrosis (18). Adipocyte death is elevated at four to eight weeks 60% HFD feeding and peaks at 12 to 16 weeks, coinciding with the expression of inflammatory genes (100, 141). Dying adipocytes in obese adipose tissue may contribute to the recruitment of immune cells or release adipocyte-associated DAMPs to activate inflammasomes in situ (Figure 3d). Indeed, macrophages have been found to surround dying or dead adipocytes, forming the so-called crown-like structure (18, 100). Inhibition of cell death in mice deficient in the proapoptotic factor Bid reduces macrophages in adipose tissue as measured by Mac3+ cells in histology sections (2). This has been further confirmed in two independent lipodystrophic transgenic animal models where adipocyte cell death is associated with massive immune cell infiltration and inflammation (36, 47). Unexpectedly, inducing adipocyte apoptosis through the ligand-induced apoptotic model leads to infiltration of M2 but not M1 macrophages into adipose tissue, along with an upregulation of genes typically involved in tissue remodeling (36). Thus, how adipocyte death influences macrophage function as well as that of other immune cells remains an open question.
So what are the triggers for adipocyte death in obesity? Although a definitive answer remains elusive, a recent study excluded a possible involvement of macrophages or inflammation (33). It has been speculated that stress signals arising from nutrient overload following long-term HFD and obesity may be partly responsible. Below we discuss one of the possible stress signals derived from the ER.
Endoplasmic Reticulum Stress
ER is the site for the synthesis, folding, and secretion of membrane and secreted proteins. Many mechanisms are in place to safeguard the homeostasis of ER to ensure the fitness of the cells and organism. Certain conditions such as the acute demand for elevated protein synthesis and secretion or changes in ER microenvironment may cause sudden accumulation of misfolded proteins and disruption of ER homeostasis. The ensuing ER stress then initiates evolutionarily conserved signaling pathways, called the unfolded protein response (UPR), to maintain ER homeostasis (122).
Perturbation of ER homeostasis has been proposed to be a potential causal mechanism underlying many disease pathologies including obesity and type 2 diabetes (54, 111, 130). Obesity-associated factors such as nutrients and oxidative stress may induce the activation of ER stress sensor IRE1α or eIF2α kinase PERK in certain cell types, which in turn may affect inflammation and insulin sensitivity via the activation of inflammatory signaling cascades, such as the JNK and NF-κB pathways (54, 130) (Figure 3e). In addition, the activation of TLR2 and TLR4 engages the IRE1α-XBP1 branch of the UPR in macrophages, which is required for the optimal response to bacterial infections and sustained expression of a subset of inflamma-tory cytokines including IL-6 and TNFα (92). It should be noted, however, that the physiological relevance and importance of these ER-stress-initiated signaling cascades have not been well characterized in obesity. Indeed, a recent study showed that saturated fatty acids with an acyl chain of at least 16 carbons activate JNK and induce TNFα expression via altering the membrane distribution of c-Src protein kinase (51). Inhibition of c-Src activity has no effect on lipid-induced ER stress, whereas it attenuates JNK activation, suggesting that ER stress and JNK activation may be separate events induced by lipids. Thus, whether and how nutrient excess such as lipids and glucose perturbs ER homeostasis and activates ER stress in vivo requires further studies (130).
Gut Microbiota
Humans harbor ~1014 bacterial cells along the gastrointestinal tract, also known as commensal microbiota, with up to 1,000 species, including pathogenic and beneficial microbes. These microbiota are an integral part of human biology, shaped by coevolution with the host, and mostly exert beneficial effects in disease control and energy metabolism. The symbiotic relationship of the host and the vast numbers of both pathogenic and beneficial microbial species challenge the host immunity to maintain tissue homeostasis and to limit bacterial translocation across enterocytes (83).
Alterations of the commensal microbiota community can lead to peripheral inflammation via the following mechanisms (Figure 3f ). HFD feeding may increase the proportion of LPS-containing microbiota in the gut and somehow increase plasma LPS concentration or endotoxemia, which may contribute to inflammatory response in peripheral tissues through the activation of TLR4 protein (13). Although LPS injection in animals has produced mixed results (13, 153, 164), LPS injection in humans increases inflammatory gene expression in adipose tissue and induces insulin resistance (1, 3, 96). However, it should be noted that the diet used in the previous study (13) was 72% HFD with no carbohydrate, and when 40% HFD was used, no effect on metabolic endotoxemia was observed (13), suggesting that gut microbiota may have a limited contribution to endotoxemia under Western dietary conditions. Furthermore, gut microbiota may regulate the expression of angiopoietin-like 4 by enterocytes (5). Angiopoietin-like 4, an inhibitor of lipoprotein lipase, may subsequently affect lipoprotein metabolism and lipid storage in adipocytes as well as inflammatory responses in macrophages (82). Moreover, short-chain fatty acids and peptidoglycan released from gut microbiota may negatively or positively modulate inflammatory responses through G protein–coupled receptor 43 (93) and nucleotide-binding oligomerization domain-containing 1 receptors (19), respectively. The physiological relevance of short-chain fatty acids and peptidoglycan in adipose inflammation in the context of obesity requires further investigations. Finally, recent studies showed that gut microbiota may have a profound impact on the biology and function of NKT cells (154) and, conversely, NKT cells may influence the composition of intestinal microbiota (104). These studies point to an extensive crosstalk between NKT cells and gut microbiota, which may contribute to inflamma-tory responses in metabolic tissues. Together with a recent study showing that TLR5 KO mice have altered composition of the gut micro-biota (152), these studies have raised exciting possibilities that specific commensal bacteria or their products may have therapeutic benefit in metabolic disorders. The molecular mechanisms by which gut microbiota affect peripheral metabolism and inflammatory responses will no doubt be the subject of intense investigations.
CYTOKINES
Cytokines are the hormonal messengers responsible for most of the biological effects in cell-mediated immunity. Increasing evidence supports the functional involvement of multiple pro- and anti-inflammatory cytokines in adipose tissue in obesity, forming a circuitry controlling local and systemic glucose tolerance and insulin sensitivity. Although they are numerous, cytokines can be functionally divided into two groups: those that are anti-inflammatory, including IL-4, IL-13, and IL-1 receptor antagonist (IL-1Ra), and those that are proinflammatory, such as IL-1β and TNFα. The interplay among proand anti-inflammatory cytokines significantly influences the outcome of inflammatory responses and the function of immune cells in vivo (likely adipocytes as well). Here we discuss the functions of some key cytokines that have been implicated in inflammation and metabolic regulation. Because this review is focused on adipose inflammation, the effect of cytokines on the hypothalamus and central regulation of energy metabolism is not discussed here but can be found elsewhere (163).
Interleukin-4/Interleukin-13
The bias of immune responses to insulin-sensitizing TH2 responses and M2 polarization can be promoted by anti-inflammatory cytokines such as IL-4 and IL-13, two cytokines sharing the same receptor (10, 15). IL-4 and IL-13 are secreted by activated T lymphocytes (e.g., CD4+ T cells and NKT cells), eosinophils, and mast cells (44). They play an important role in modulating the balance of TH cell subsets, favoring expansion of the TH2 over TH1 lineage or skewing the polarization of M2 over M1 macrophages. The IL-4/IL-13 effect is largely mediated via the phosphorylation and activation of STAT6 (65, 133, 145). Following IL-4 stimulation, cytosolic STAT6 monomers are phosphorylated, leading to homodimerization and translocation to the nucleus. Imbalance of TH1 and TH2 lymphocyte subsets has been implicated in immunological diseases including allergy, inflammation, and autoimmune disease.
In adipose tissue, several studies have identified adipocytes (64) as potential sources of IL-13 and eosinophils (160) as potential sources of IL-4. In the absence of IL-4/IL-13, the number of adipose tissue macrophages remains similar, but the percentage of arginase-1-positive M2 macrophages of total F4/80+ CD11b+ macrophages is dramatically reduced in mice on normal chow diet (160). Furthermore, a recent study showed that intraperitoneal injection of IL-4 cytokine complexed with the IL-4 antibody (to increase the half-life of IL-4) improves glucose tolerance and insulin sensitivity in obese animals (119). Although the IL-4 treatment reduces inflammation in adipose tissue, the insulin-sensitizing effect of IL-4 treatment is largely due to the effect of IL-4 on liver. IL-4 treatment decreases fatty acid oxidation and increases glucose oxidation in a STAT6-dependent manner in hepatocytes. STAT6 interacts with PPARα and represses PPARα transcription activity on target genes involved in fatty acid oxidation. Thus, these data suggest that IL-4 signaling represses the catabolic activity of PPARα in the liver and attenuates inflammation in adipose tissue (119). Finally, a recent study showed that IL-4 and/or IL-13 may be involved in nonshivering thermogenesis (101). IL-4/IL-13 treatment increased the expression of catecholamines in alternatively activated macrophages, which may in turn promote thermogenesis in the brown adipose tissue and lipolysis in the white adipose tissue (101). Therefore, therapies targeting IL-4-secreting cells may have beneficial effects not only for obesity-associated inflammation but also for nutrient metabolism and energy expenditure.
Interleukin-1β/Interleukin-1Ra
IL-1β is a master regulator of the inflammatory response in various tissues. The processing of the immature form of IL-1β or pro-IL-1β to bioactive IL-1β cytokine requires two independent signals as described in the Inflammasome section (Figure 4). Upon signaling through its cognate receptor IL-1R, IL-1β stimulates the production of proinflammatory prostaglandins, nitric oxide, and chemokines in the target cells such as endothelial cells and smooth-muscle cells. IL-1β has been implicated in pancreatic β cell function in the context of type 1 diabetes as well as recently in inflammatory response in the context of obesity-associated insulin resistance and type 2 diabetes (26).
Interestingly, the immune system also produces IL-1Ra to counter the proinflammatory effect of IL-1β. IL-1Ra, also a naturally occurring cytokine, belongs to the IL-1 family and antagonizes the effects of IL-1β by competitive binding to IL-1R on target cells (25). Mice with IL-1Ra deficiency develop spontaneous pathogenic autoimmune inflammatory diseases with massive neutrophil infiltration at the joints or arteries (52, 103). The ratio of IL-1Ra to IL-1β seems important for the outcome of inflammation, as mice heterozygous for IL-Ra still develop arterial inflammation, albeit to a lesser extent (103). Thus, IL-1Ra protects the system from the deleterious effect of IL-1β.
The circulating level of IL-1β is found to be elevated with obesity, which in turn induces insulin resistance in insulin-sensitive cells and increases risk for type 2 diabetes (58, 75). Interestingly, IL-1Ra expression is induced by IL-1β, and serum levels of IL-1Ra are increased in obesity (97), suggesting the presence of a complex autoregulatory circuit in obesity. Indeed, studies have shown that IL-1β controls the circulating levels of many proinflammatory factors in patients with type 2 diabetes (77). In both humans and animal models, blockade of IL-1β activity with IL-1Ra or with an IL-1β neutralizing antibody improves insulin secretion and glycemia (29, 40, 77, 109, 127, 137). Indeed, the blockade of IL-1β signaling by IL-1Ra or IL-1β antibody is reported to help treat type 2 diabetes in recent clinical studies (77). These findings suggest that IL-1β is an important driver in the development and maintenance of insulin resistance. However, in other studies, IL-1β-deficient mice display glucose intolerance at three months of age (88), and mice with IL-1Ra ablation are resistant to obesity and have improved insulin sensitivity (135). Thus, more studies using tissue-specific KO animals are required to establish the physiological role of the IL-1 signaling pathway.
SUMMARY POINTS.
In addition to macrophages, many immune cells including lymphocytes and myeloid cells play important regulatory roles in adipose inflammation and hence systemic glucose tolerance and insulin sensitivity.
TLRs, inflammasomes, PPARs, cell death, ER stress, and gut microbiota may potentially link nutrient abundance with inflammation in obesity.
CD4+ TH2, Treg, eosinophils, and adipocytes can secrete TH2 cytokines and regulate inflammation in adipose tissue.
Whereas IL-4 and IL-13 augment TH2 responses in adipose tissue and attenuate inflammation, IL-1β promotes TH1 responses and augments inflammation.
Targeting the IL-1β proinflammatory signaling axis is a quite effective and promising therapeutic approach to reduce inflammation in human patients with type 2 diabetes.
FUTURE ISSUES.
Earlier studies have provided a framework for further dissecting the initiation, development, and resolution of inflammatory response in adipose tissue during obesity, including:
Delineating the mechanisms by which inflammation in adipose tissue affects systemic insulin sensitivity.
Delineating the interplay among various immune populations and adipocytes in adipose tissue and identifying the importance of other cell types such as TH17 cells in metabolic regulation.
Understanding the basis of inflammatory responses at different phases of obesity so as to predict the benefit from modulating the inflammatory responses.
Identifying the molecular mechanisms underlying the activation of immune cells in adi-pose tissue in obesity.
Identifying new and more effective therapeutic approaches based on the effect of obesity-associated inflammation.
Inflammation: a major defense mechanism to restore tissue homeostasis and function following pathogen infection or injury
Type 2 diabetes: adult-onset diabetes incapable of responding to insulin (i.e., insulin resistance) to maintain euglycemia, despite the presence of pancreatic islets
Macrophage polarization: a conceptual framework describing the activation status of macrophages
Pattern recognition receptors (PRRs): proteins expressed by innate immune cells that can recognize pathogen- or damage-associated molecular patterns
Toll-like receptors (TLRs): a type of PRRs that recognize extracellular PAMPs and may be activated by lipids such as saturated fatty acids
Pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs): molecules associated with pathogens or damaged tissue/cells capable of activating innate immunity
Antigens: nonself substances that stimulate adaptive immune responses
Classically activated M1 or alternatively activated M2 macrophages: a paradigm depicting the functional states of polarized macrophages mirroring the TH1/TH2 concept in CD4+ T cells
Immunosuppressive cells: cells that are able to suppress inflammatory responses, most notably regulatory T cells and myeloid-derived suppressor cells
Antigen-presenting cells (APCs): cells that engulf, process, and present antigens at the cell surface to activate T cells with B cells; macrophages and dendritic cells are professional APCs
Helminth: parasitic worms that live inside humans (such as in the digestive tract) and often protect themselves by stimulating host TH2 responses
T helper cells (TH1/TH2): a classification of CD4+ T cells based on the signature cytokines IFN-γ and IL-4, respectively
Inflammasomes: molecular platforms or complexes essential for the generation of bioactive proinflammatory caspases and IL-1β
Unfolded protein response (UPR): an adaptive cellular response to the disturbance or disruption of ER homeostasis or ER stress
Interleukin-1 receptor antagonist (IL-1Ra): a naturally occurring cytokine that antagonizes the effect of IL-1β by competitive binding to IL-1R
ACKNOWLEDGMENTS
The authors are grateful to Drs. Suzanne Ostrand-Rosenberg and Rinke Stienstra for their comments on the manuscript and apologize to those whose studies were omitted owing to space limitations. S.S. is an International Student Research Fellow of the Howard Hughes Medical Institute. The studies in the Kersten laboratory have been supported by grants from The Netherlands Organization for Scientific Research, the Dutch Diabetes Foundation, the Netherlands Heart Foundation, and the European Foundation for the Study of Diabetes. Studies in the Qi laboratory have been supported by grants from the American Federation for Aging Research (AFAR), the American Diabetes Association (ADA), and the National Institutes of Health (NIH; R01DK082582 and R21AA 020351).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. J. Clin. Endocrinol. Metab. 2000;85:3770–78. doi: 10.1210/jcem.85.10.6914. [DOI] [PubMed] [Google Scholar]
- 2.Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J. Biol. Chem. 2010;285:3428–38. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L, et al. Innate immunity modulates adipokines in humans. J. Clin. Endocrinol. Metab. 2007;92:2272–79. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 4.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J. Clin. Invest. 2006;116:1713–22. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 2007;104:979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai L, Sagiv Y, Liu Y, Freigang S, Yu KO, et al. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen alphaGalCer. Proc. Natl. Acad. Sci. USA. 2009;106:10254–59. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barish GD, Atkins AR, Downes M, Olson P, Chong LW, et al. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc. Natl. Acad. Sci. USA. 2008;105:4271–76. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 10.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–77. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 11.Bishop-Bailey D, Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol. Ther. 2009;124:141–50. doi: 10.1016/j.pharmthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–15. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 13.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 14.Caricilli AM, Nascimento PH, Pauli JR, Tsukumo DM, Velloso LA, et al. Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J. Endocrinol. 2008;199:399–406. doi: 10.1677/JOE-08-0354. [DOI] [PubMed] [Google Scholar]
- 15.Chawla A. Control of macrophage activation and function by PPARs. Circ. Res. 2010;106:1559–69. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011;11:738–49. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–80. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- 18.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid. Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010;16:228–31. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clementi AH, Gaudy AM, van Rooijen N, Pierce RH, Mooney RA. Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling. Biochim. Biophys. Acta. 2009;1792:1062–72. doi: 10.1016/j.bbadis.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52:318–28. doi: 10.1007/s00125-008-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011;29:707–35. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16:1248–55. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 24.Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J. Endocrinol. 2001;169:453–59. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N. Engl. J. Med. 2000;343:732–34. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 27.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 28.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 1999;103:253–59. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc. Natl. Acad. Sci. USA. 2009;106:13998–4003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53:1795–806. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- 31.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler. Thromb. Vasc. Biol. 2009;29:1944–49. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 32.Feng B, Jiao P, Nie Y, Kim T, Jun D, et al. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS ONE. 2011;6:e24358. doi: 10.1371/journal.pone.0024358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng D, Tang Y, Kwon H, Zong H, Hawkins M, et al. High-fat diet-induced adipocyte cell death occurs through a cyclophilin D intrinsic signaling pathway independent of adipose tissue inflammation. Diabetes. 2011;60:2134–43. doi: 10.2337/db10-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–39. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer H, Ellstrom P, Ekstrom K, Gustafsson L, Gustafsson M, Svanborg C. Ceramide as a TLR4 agonist; a putative signalling intermediate between sphingolipid receptors for microbial ligands and TLR4. Cell. Microbiol. 2007;9:1239–51. doi: 10.1111/j.1462-5822.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 36.Fischer-Posovszky P, Wang QA, Asterholm IW, Rutkowski JM, Scherer PE. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology. 2011;152:3074–81. doi: 10.1210/en.2011-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foryst-Ludwig A, Hartge M, Clemenz M, Sprang C, Hess K, et al. PPARgamma activation attenuates T-lymphocyte-dependent inflammation of adipose tissue and development of insulin resistance in obese mice. Cardiovasc. Diabetol. 2010;9:64. doi: 10.1186/1475-2840-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 40.Garcia MC, Wernstedt I, Berndtsson A, Enge M, Bell M, et al. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–13. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 41.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 42.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat. Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 44.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW−sh/W−sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 47.Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc. Natl. Acad. Sci. USA. 2010;107:240–45. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J. Clin. Invest. 2007;117:1658–69. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010;24:731–39. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 2011;121:1858–70. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holzer RG, Park EJ, Li N, Tran H, Chen M, et al. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147:173–84. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med. 2000;191:313–20. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88) deficiency increases risk of diabetes in mice. PLoS ONE. 2010;5:e1, 2537. doi: 10.1371/journal.pone.0012537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 57.Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–50. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 58.Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–51. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiao P, Feng B, Ma J, Nie Y, Paul E, et al. Constitutive activation of IKKbeta in adipose tissue prevents diet-induced obesity in mice. Endocrinology. 2012;153:154–65. doi: 10.1210/en.2011-1346. [DOI] [PubMed] [Google Scholar]
- 62.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, et al. Overexpression of monocyte chemoat-tractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006;281:26602–14. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 63.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–95. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–19. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 66.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, et al. CD1d-restricted and TCR-mediated activation of Valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–29. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 67.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res. 2007;100:1589–96. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 68.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–55. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 69.Kleemann R, van Erk M, Verschuren L, van den Hoek AM, Koek M, et al. Time-resolved and tissue-specific systems analysis of the pathogenesis of insulin resistance. PLoS ONE. 2010;5:e8817. doi: 10.1371/journal.pone.0008817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koenen TB, Stienstra R, van Tits LJ, de Graaf J, Stalenhoef AF, et al. Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1beta transcription in human adipose tissue. Diabetes. 2011;60:517–24. doi: 10.2337/db10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konner AC, Bruning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol. Metab. 2011;22:16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 72.Kotas ME, Lee HY, Gillum MP, Annicelli C, Guigni BA, et al. Impact of CD1d deficiency on metabolism. PLoS ONE. 2011;6:e25478. doi: 10.1371/journal.pone.0025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 74.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 75.Lagathu C, Yvan-Charvet L, Bastard JP, Maachi M, Quignard-Boulange A, et al. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 2006;49:2162–73. doi: 10.1007/s00125-006-0335-z. [DOI] [PubMed] [Google Scholar]
- 76.Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani PD, Leclercq IA. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G107–16. doi: 10.1152/ajpgi.00391.2009. [DOI] [PubMed] [Google Scholar]
- 77.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 78.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J. Immunol. 1997;158:1332–44. [PubMed] [Google Scholar]
- 79.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60:2474–83. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 81.Li J, Yu X, Pan W, Unger RH. Gene expression profile of rat adipose tissue at the onset of high-fat-diet obesity. Am. J. Physiol. Endocrinol. Metab. 2002;282:E1334–41. doi: 10.1152/ajpendo.00516.2001. [DOI] [PubMed] [Google Scholar]
- 82.Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 2010;12:580–92. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–23. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Divoux A, Sun J, Zhang J, Clément K, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009;15:940–45. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J. Immunol. 2011;187:2061–66. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lynch L, O'Shea D, Winter DC, Geoghegan J, Doherty DG, O'Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 2009;39:1893–901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 88.Maedler K, Schumann DM, Sauter N, Ellingsgaard H, Bosco D, et al. Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes. 2006;55:2713–22. doi: 10.2337/db05-1430. [DOI] [PubMed] [Google Scholar]
- 89.Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O'Doherty RM. Mice lacking NKT cells but with a complete complement of CD8 T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS ONE. 2011;6:e19831. doi: 10.1371/journal.pone.0019831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marathe C, Bradley MN, Hong C, Chao L, Wilpitz D, et al. Preserved glucose tolerance in high-fat-fed C57BL/6 mice transplanted with PPARγ−/−, PPARδ−/−, PPARγδ−/−, or LXRαβ−/− bone marrow. J. Lipid Res. 2009;50:214–24. doi: 10.1194/jlr.M800189-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 92.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010;11:411–18. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–86. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–29. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 95.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–97. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 96.Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–81. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J. Clin. Endocrinol. Metab. 2002;87:1184–88. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- 98.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 2011;11:807–22. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 99.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 100.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid. Res. 2008;49:1562–68. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–8. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007;282:35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 103.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J. Exp. Med. 2000;191:303–12. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J. Clin. Invest. 2009;119:1241–50. doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, et al. CD8 +effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 106.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 108.Orr TS, Cox JS. Disodium cromoglycate, an inhibitor of mast cell degranulation and histamine release induced by phospholipase A. Nature. 1969;223:197–98. doi: 10.1038/223197b0. [DOI] [PubMed] [Google Scholar]
- 109.Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. Treatment with an interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine. 2008;44:141–48. doi: 10.1016/j.cyto.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 112.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–63. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50:1267–76. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 115.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–88. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 116.Radin MS, Sinha S, Bhatt BA, Dedousis N, O'Doherty RM. Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia. 2008;51:336–46. doi: 10.1007/s00125-007-0861-3. [DOI] [PubMed] [Google Scholar]
- 117.Radonjic M, de Haan JR, van Erk MJ, van Dijk KW, van den Berg SA, et al. Genome-wide mRNA expression analysis of hepatic adaptation to high-fat diets reveals switch from an inflammatory to steatotic transcriptional program. PLoS ONE. 2009;4:e6646. doi: 10.1371/journal.pone.0006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lond.) 2008;32:451–63. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 119.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc. Natl. Acad. Sci. USA. 2010;107:22617–22. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 121.Rock KL, Kono H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 123.Rothenberg ME, Hogan SP. The eosinophil. Annu. Rev. Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 124.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–29. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 126.Samuel VT, Liu Z-X, Qu X, Elder BD, Bilz S, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004;279:32345–53. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 127.Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–18. doi: 10.1210/en.2007-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–49. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 129.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 130.Sha H, He Y, Yang L, Qi L. Stressed out about obesity: IRE1alpha-XBP1 in metabolic disorders. Trends Endocrinol. Metab. 2011;22:374–81. doi: 10.1016/j.tem.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet–induced obesity in mice. Diabetes. 2010;59:1171–81. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–33. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 134.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 135.Somm E, Cettour-Rose P, Asensio C, Charollais A, Klein M, et al. Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia. 2006;49:387–93. doi: 10.1007/s00125-005-0046-x. [DOI] [PubMed] [Google Scholar]
- 136.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J. Biol. Chem. 2008;283:22620–27. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 137.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stienstra R, Mandard S, Patsouris D, Maass C, Kersten S, Muller M. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148:2753–63. doi: 10.1210/en.2007-0014. [DOI] [PubMed] [Google Scholar]
- 139.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–22. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 140.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl. Acad. Sci. USA. 2011;108:15324–29. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFuria J, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–18. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 142.Suganami T, Yuan X, Shimoda Y, Uchio-Yamada K, Nakagawa N, et al. Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue. Circ. Res. 2009;105:25–32. doi: 10.1161/CIRCRESAHA.109.196261. [DOI] [PubMed] [Google Scholar]
- 143.Sugii S, Olson P, Sears DD, Saberi M, Atkins AR, et al. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. USA. 2009;106:22504–9. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–30. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 146.Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, et al. Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J. Biol. Chem. 2010;285:4637–44. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. USA. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010;10:210–15. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 149.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–98. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 150.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil G. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–14. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 151.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Virkamaki A, Yki-Jarvinen H. Mechanisms of insulin resistance during acute endotoxemia. Endocrinology. 1994;134:2072–78. doi: 10.1210/endo.134.5.8156907. [DOI] [PubMed] [Google Scholar]
- 154.Wei B, Wingender G, Fujiwara D, Chen DY, McPherson M, et al. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J. Immunol. 2010;184:1218–26. doi: 10.4049/jimmunol.0902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wen H, Gris D, Lei Y, Jha S, Zhang L, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12:408–15. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011;17:610–17. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Winer S, Chan Y, Paltser G, Truong D, Tsui H, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009;15:921–29. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–47. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b +myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J. Biol. Chem. 2011;286:23591–99. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu H, Barnes GT, Yang Q, Tan G, Yang D, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging. 2010;2:361–68. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yelich MR, Filkins JP. Insulin hypersecretion and potentiation of endotoxin shock in the rat. Circ. Shock. 1982;9:589–603. [PubMed] [Google Scholar]
- 165.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–47. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 166.Yu C, Cantor AB, Yang H, Browne C, Wells RA, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 2002;195:1387–95. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int. J. Obes. (Lond.) 2007;31:1420–28. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 169.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–40. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]