Abstract
Background
Despite regular surveillance colonoscopy, the metachronous colorectal cancer risk for mismatch repair (MMR) gene mutation carriers after segmental resection for colon cancer is high and total or subtotal colectomy is the preferred option. However, if the index cancer is in the rectum, management decisions are complicated by considerations of impaired bowel function. We aimed to estimate the risk of metachronous colon cancer for MMR gene mutation carriers who underwent a proctectomy for index rectal cancer.
Methods
This retrospective cohort study comprised 79 carriers of germline mutation in a MMR gene (18 MLH1, 55 MSH2, 4 MSH6, and 2 PMS2) from the Colon Cancer Family Registry who had had a proctectomy for index rectal cancer. Cumulative risks of metachronous colon cancer were calculated using the Kaplan–Meier method.
Results
During median 9 years (range 1–32 years) of observation since the first diagnosis of rectal cancer, 21 carriers (27 %) were diagnosed with metachronous colon cancer (incidence 24.25, 95 % confidence interval [CI] 15.81–37.19 per 1,000 person-years). Cumulative risk of metachronous colon cancer was 19 % (95 % CI 9–31 %) at 10 years, 47 (95 % CI 31–68 %) at 20 years, and 69 % (95 % CI 45–89 %) at 30 years after surgical resection. The frequency of surveillance colonoscopy was 1 colonoscopy per 1.16 years (95 % CI 1.01–1.31 years). The AJCC stages of the metachronous cancers, where available, were 72 % stage I, 22 % stage II, and 6 % stage III.
Conclusions
Given the high metachronous colon cancer risk for MMR gene mutation carriers diagnosed with an index rectal cancer, proctocolectomy may need to be considered.
Lynch syndrome is the most common hereditary cancer of the colon and rectum, resulting from germline mutations in one of the mismatch repair (MMR) genes, MLH1, MSH2, MSH6, and PMS2. Individuals with a MMR gene mutation(s) have an increased lifetime risk of colorectal cancer (CRC) with an estimated risk between 15 and 70 % by the age of 70 years.1–4 Lynch syndrome associated CRCs are diagnosed, on average, 1 or 2 decades earlier than sporadic CRCs. Carriers of a MMR germline mutation are advised to have regular colonoscopy. One study showed that, despite having colonoscopies with 1–2 year intervals, CRC risk is still 6 % over 10 years.5
Following their index cancer in the colon, MMR gene mutation carriers have a substantially higher risk of metachronous CRC after a segmental than a more extensive (total or subtotal colectomy) resection, which is the currently recommended option in these cases.6, 7 We previously reported cumulative risks of metachronous colorectal cancer to be 16, 41, and 62 % at 10, 20, and 30 years, respectively, after a segmental resection for colon cancer.6
Although proximal location is a hallmark of Lynch syndrome associated CRCs, about 15 %of MMR gene mutation carriers develop a rectal cancer and the surgical decision making for these patients is more complicated.7, 8 If the diagnosis of Lynch syndrome is known prior to the initial resection, the first question is whether to recommend a total proctocolectomy or a proctectomy. A total proctocolectomy with a permanent ileostomy or a restorative ileal pouch anal anastomosis (IPAA) will prevent the development of metachronous colon cancer but is associated with substantial issues related to postoperative morbidity and quality of life, including requirement of dietary restriction, incontinence or urgency, and decreased sexual function that would be expected to be similar to patients undergoing this operation for familial adenomatous polyposis (FAP).9–11 In contrast, a proctectomy [in the form of either an anterior resection (AR) with colorectal anastamosis or abdominoperineal resection (APR) with colostomy] may have technical and clinical advantages, but these operations are associated with the risk of metachronous colon cancer. Such a far-reaching decision on the extent of surgery (either proctocolectomy or proctectomy) is critically dependent on accurate estimates of the risk of metachronous colon cancer following a proctectomy for MMR gene mutation carriers.
There is little data about the risk of metachronous colon cancer following surgical resection for index rectal cancer in MMR gene mutation carriers, and whether ongoing colonoscopic surveillance prevents the risk of metachronous lesions. There are 3 series that reported 15–54 % of rectal cancer patients developed a metachronous colon cancer over a relatively short follow-up period.8, 12, 13 This study aimed to quantify the risk of metachronous colon cancer following surgery for index rectal cancer in a large cohort of MMR gene mutation carriers from a 4-nation collaborative group over a long study interval.
METHODS
Study Sample
The study sample comprised carriers of pathogenic germline mutations in 1 of the MMR genes (MLH1, MSH2, MSH6, or PMS2) who had had a surgical resection for index rectal cancer, identified through the Colon Cancer Family Registry. Details of recruitment methods can be found at http://epi.grants.cancer.gov/CFR/ and have been described previously.14 Families were ascertained via probands between 1997 and 2007. Population-based probands were recently diagnosed CRC cases from state or regional population cancer registries in the United States (Washington, California, Arizona, Minnesota, Colorado, New Hampshire, North Carolina, and Hawaii), Australia (Victoria), and Canada (Ontario). Clinic-based probands were enrolled from multiple-case families referred to family cancer genetics clinics in the United States (Mayo Clinic, Rochester, MN and Cleveland Clinic, Cleveland, OH), Australia (Melbourne, Adelaide, Perth, Brisbane, Sydney), and New Zealand (Auckland).
Permission was sought from the probands to contact their relatives to seek their participation in the study. For probands ascertained from population cancer registries, first-degree relatives were recruited at all centers, and recruitment extended to more distant relatives at some centers. For probands ascertained from family cancer clinics, prespecified rules governing which relatives were to be approached for recruitment were consistent across recruiting centers (for details, see Newcomb et al.14). Written informed consent was obtained from all study participants, and the study protocol was approved by the institutional research ethics review board at each center.
Data Collection
Information on demographics, personal characteristics, personal and family history of cancer, cancer screening history, history of polyps, polypectomy, and other surgeries were obtained by questionnaires at the time of enrollment into the registry. Participants were followed-up ~5 and 10 years after baseline to update this information. Baseline and follow-up questionnaires are available at: https://cfrisc.georgetown.edu/isc/dd.questionnaires.do. The present study was based on all available baseline and follow-up data. Reported cancer diagnoses and age at diagnosis were confirmed, where possible, using pathology reports, medical records, cancer registry reports, and/or death certificates. Blood samples and tumor tissues were collected for genetic testing from all probands and participating relatives.
For this study, details of index rectal cancer and metachronous colon cancer diagnoses and surgical resections were extracted from the pathology reports and/or medical records: date of surgery, type of surgery (anterior resection, abdominoperineal resection, polypectomy, proctocolectomy), length of bowel removed (centimeters), maximum dimension of tumor (millimeters), T- and N-stage, histological grade (well, moderately, poorly differentiated, other), and synchronous tumor (present, absent).
Mutation Screening and Testing
Screening for germline MLH1, MSH2, MSH6, and PMS2 mutations was performed for all population-based probands who had a colorectal tumor displaying evidence of impaired MMR function by either microsatellite instability (MSI) or by lack of MMR protein expression by immunohistochemistry, and for the youngest onset CRC case from each clinic-based family, regardless of MSI or MMR protein expression status. Mutation testing was performed by Sanger sequencing or denaturing high-pressure liquid chromatography (dHPLC), followed by confirmatory DNA sequencing. Large insertion and deletion mutations were detected by multiplex ligation dependent probe Amplification (MLPA) according to the manufacturer’s instructions (MRC Holland, Amsterdam, The Netherlands).3, 14, 15 All relatives of probands with a pathogenic mutation, who provided a blood sample, underwent genetic testing for the specific mutation identified in the proband. For this study, we have included both probands and their participating relatives with pathogenic MMR gene mutations who had a diagnosis of index rectal cancer.
Definitions
A metachronous colon cancer was defined as a primary colon cancer diagnosed more than 12 months after the diagnosis of a rectal cancer. T and N staging of the cancer was categorized according to the American Joint Committee on Cancer (AJCC) staging.16 A pathogenic mutation was defined as a variant that was predicted to result in a stop codon, a frameshift mutation, a large duplication or deletion, or a missense mutation in a coding region or splice site previously reported within the scientific literature and databases to be pathogenic.17
Statistical Analysis
Incidence rate of metachronous colon cancer per 1,000 person-years and corresponding 95 % confidence interval (95 % CI) was estimated for MMR gene mutation carriers who had had a proctectomy for index rectal cancer. The primary time scale for estimating risk started at the age of surgery for rectal cancer and ended at age at diagnosis ofmetachronous colon cancer (n = 21), last followup (n = 46), or death (n = 12), whichever occurred first.
Cox regression was performed to investigate the factors influencing the risk of metachronous colon cancer. We allowed for 2 time scales within the same model: age and time since surgery. We accommodated age in the model by splitting patient’s follow-up time into 5-year age brackets and including a separate risk parameter in the model for each age bracket. This is preferable to simply adjusting for age at surgery for index rectal cancer since the risk of metachronous cancer depends on age as well as time since surgery, and several patients contributed follow-up time to more than one 5-year age bracket. The model included sex, country of recruitment (Australia and New Zealand, Canada, the United States), cigarette smoking status (never, ever), the maximum dimension of tumor (per 1 centimeter), AJCC stage (stage I, II, and III), the histology grade (well, moderately, poorly, mucinous), synchronous tumor (absent, present), and height of individuals. We took account into the effect of clustering on family membership to allow for any correlation of risk between family members using Huber-White robust variance correction.18, 19
The Kaplan–Meier method was used to estimate the cumulative risk of metachronous colon cancer to 10, 20, and 30 years after surgical resection for rectal cancer.
Frequency of colonoscopy after surgery for rectal cancer, but before the diagnosis of metachronous colon cancer, was estimated from the self-reported questionnaire data using the same method done in a previous study.6 When assessing the number of colonoscopies for those with metachronous cancer, we excluded the most recent colonoscopy as we assumed it to be a diagnostic test. The frequency of colonoscopy was assumed to be distributed uniformly in the period between first and last age of colonoscopy. All statistical tests were 2-sided except in estimating incidence rates for MSH6 and PMS2 mutation carriers. P < 0.05 was assumed to be statistically significant. All statistical analyses were performed using Stata 11.0.20
RESULTS
Of the 109 MMR gene mutation carriers who had surgical resection for index rectal cancer, pathology reports were available for 88 (81 %). We excluded 9 carriers who had undergone total proctocolectomy as they had no risk of metachronous colon cancer. The remaining 79 carriers of germline mutation in MMR genes (18 MLH1, 55 MSH2, 4 MSH6, and 2 PMS2) from 72 families (18 MLH1, 48 MSH2, 4 MSH6, and 2 PMS2) were included in the analysis. Of all carriers, 38 (48 %) were recruited in Australia or New Zealand, 30 (38 %) in the United States, and 11 (14 %) in Canada. The baseline characteristics of carriers included in the study are summarized in Table 1. The mean age at diagnosis of index rectal cancer was 42.8 years (standard deviation [SD] 10.5 years), ranging from 17 to 70 years.
TABLE 1.
Baseline characteristics of mismatch repair gene mutation carriers included in the study
| All (n = 79) No. (%) |
Anterior resection (n = 50) |
Abdominoperineal resection (n = 29) |
|||
|---|---|---|---|---|---|
| No metachronous cancer (n = 37) No. (%) |
Metachronous cancer (n = 13) No. (%) |
No metachronous cancer (n = 21) No. (%) |
Metachronous cancer (n = 8) No. (%) |
||
| Sex, female | 44 (56) | 21 (57) | 8 (62) | 12 (57) | 3 (38) |
| Country | |||||
| Australia and New Zealand | 38 (48) | 22 (60) | 6 (46) | 7 (33) | 3 (37.5) |
| Canada | 11 (14) | 3 (8) | 2 (15) | 4 (19) | 2 (25) |
| United States | 30 (38) | 12 (32) | 5 (39) | 10 (48) | 3 (37.5) |
| MMR gene mutated | |||||
| MLH1 | 18 (23) | 8 (22) | 3 (23) | 5 (24) | 2 (25) |
| MSH2 | 55 (70) | 25 (67) | 10 (77) | 14 (66) | 6 (75) |
| MSH6 | 4 (5) | 3 (8) | 0 (0) | 1 (5) | 0 (0) |
| PMS2 | 2 (2) | 1 (3) | 0 (0) | 1 (5) | 0 (0) |
| Index rectal cancer | |||||
| Age at diagnosis (years), mean (SD) | 42.8 (10.5) | 43.3 (10.7) | 38.4 (12.5) | 44.3 (9.5) | 44.1 (8.8) |
| Length of bowel removed (cm), mean (SD) | 22.2 (8.0) | 20.8 (7.5) | 17.9 (6.8) | 26.9 (8.3) | 25.0 (6.2) |
| AJCC stage of tumora | |||||
| I | 28 (38) | 12 (35) | 4 (36) | 8 (38) | 4 (57) |
| II (A, B, C) | 33 (45) | 17 (50) | 3 (27) | 11 (52) | 2 (29) |
| III (A, B, C) | 12 (16) | 5 (15) | 4 (36) | 2 (10) | 1 (14) |
| Unknown | 6 | 3 | 2 | 0 | 1 |
| Histological grade | |||||
| Well | 6 (9) | 3 (9) | 1 (9) | 2 (11) | 0 (0) |
| Moderately | 44 (62) | 22 (65) | 5 (45) | 13 (68) | 4 (57) |
| Poorly | 18 (25) | 8 (23) | 3 (27) | 4 (21) | 3 (43) |
| Mucinous | 3 (4) | 1 (3) | 2 (18) | 0 (0) | 0 (0) |
| Unknown | 8 | 3 | 2 | 2 | 1 |
| Synchronous tumor | |||||
| Absent | 73 (96) | 35 (95) | 11 (100) | 21 (100) | 6 (86) |
| Present | 3 (4) | 2 (5) | 0 (0) | 0 (0) | 1 (14) |
| Unknown | 3 | 0 | 2 | 0 | 1 |
AJCC American Joint Committee on Cancer
Metastasis status was unavailable; and any of this stage could be stage IV
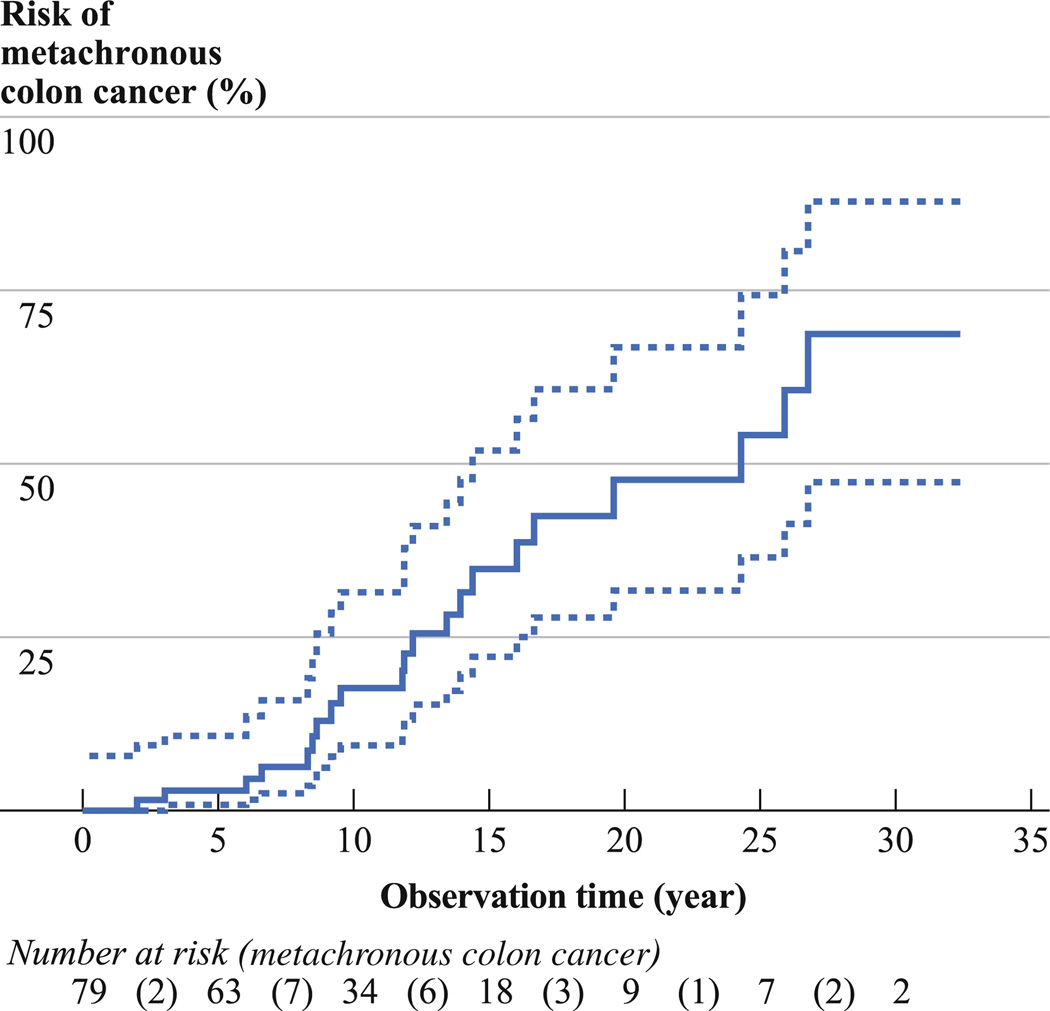
Of the total 79 carriers, 21 (27 %) developed a metachronous colon cancer over a mean follow-up 11 years (SD 8 years, median 9 years, range 1–32 years) with an incidence of 24.25 (95 % CI 15.81–37.19) per 1,000 person-years (Table 2). The cumulative risk of metachronous colon cancer was 19 % (95 % CI 9–31 %) at 10 years, 47 % (95 % CI 31–68 %) at 20 years, and 69 % (95 % CI 45–89 %) at 30 years after proctectomy (Fig. 1).
TABLE 2.
Incidence rates (per 1,000 person-years) of metachronous colon cancer following proctectomy for rectal cancer
| Total | Total years of observation |
Metachronous colon cancer No. (%) |
Rate per 1000 person-years (95% CI) |
|
|---|---|---|---|---|
| Combined | 79 | 866 | 21 (27) | 24.25 (15.81–37.19) |
| Sex | ||||
| Female | 44 | 458 | 11 (25) | 24.03 (13.31–43.39) |
| Male | 35 | 408 | 10 (29) | 24.49 (13.18–45.51) |
| Age at diagnosis (years) | ||||
| <40 | 30 | 379 | 11 (37) | 29.05 (16.09–52.45) |
| 40–49 | 30 | 346 | 6 (20) | 17.34 (7.79–38.59) |
| ≥50 | 19 | 141 | 4 (21) | 28.29 (10.62–75.38) |
| MMR gene mutated | ||||
| MLH1 | 18 | 174 | 5 (28) | 28.72 (11.96–69.01) |
| MSH2 | 55 | 628 | 16 (29) | 25.50 (15.62–41.62) |
| MSH6 | 4 | 43 | 0 (0) | 0 (0–67.30)a |
| PMS2 | 2 | 21 | 0 (0) | 0 (0–132.95)a |
| Country of recruitment | ||||
| Australia and New Zealand | 38 | 356 | 9 (24) | 25.30 (13.16–48.62) |
| Canada | 11 | 144 | 4 (36) | 27.78 (10.43–74.01) |
| United States | 30 | 366 | 8 (27) | 21.84 (10.92–43.67) |
| Type of surgery | ||||
| Anterior resection | 50 | 486 | 13 (26) | 26.75 (15.53–46.07) |
| Abdominoperineal resection | 29 | 380 | 8 (28) | 21.04 (10.52–42.08) |
One-sided 95 % confidence interval (CI)
FIG. 1.
Kaplan–Meier curve for the risk of metachronous colon cancer (bold line) and 95 % confidence intervals (dashed lines) following proctectomy for rectal cancer in mismatch repair gene mutation carriers
Of the 21 metachronous colon cancers, 16 (84 %) were in the right colon (5 cecum, 5 ascending colon, 2 hepatic flexure, and 4 transverse colon), 3 (16 %) were in the left colon (1 descending colon and 2 sigmoid colon), and the remaining 2 were not reported for the exact location in colon. The AJCC stage of the metachronous cancers was recorded for 18 of the 21 cancers: 13 (72 %) were stage I, 4 (22 %) were stage II, and 1 (6 %) was stage III. Stages of the remaining 3 cases could not be calculated because they were verified from biopsy or medical reports. Of all metachronous colon cancers, 12 (67 %) were moderately differentiated, 5 (28 %) were poorly differentiated, and 1 (6 %) was mucinous type. Details of MMR gene mutation carriers with a diagnosis of metachronous colon cancer and their surveillance colonoscopy status are shown in Supplementary Table 1.
Table 3 shows that risk of metachronous colon cancer was not associated with sex, age at diagnosis, country of recruitment, cigarette smoking status, maximum dimension, and histological grade of the rectal cancer. There was some evidence that risk of metachronous colon cancer was higher for carriers with index rectal cancer with a higher AJCC stage (hazard ratio [HR] 6.14, 95 % CI 1.21–13.14, P = 0.03) and those with synchronous tumor (HR 11.54, 95 % CI 1.06–125, P = 0.04).
TABLE 3.
Hazard ratios (HRs) and 95 % confidence intervals (95 % CIs) of metachronous colon cancer following proctectomy for rectal cancer in mismatch repair gene mutation carriers
| Univariable |
Multivariablea |
|||
|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Sex | ||||
| Female | 1.00 (referent) | 1.00 (referent) | ||
| Male | 0.96 (0.41–2.27) | 0.93 | 0.67 (0.12–3.84) | 0.65 |
| Age at diagnosis (years) | ||||
| <40 | 1.00 (referent) | 1.00 (referent) | ||
| 40–49 | 0.62 (0.23–1.68) | 0.97b | 0.52 (0.07–4.16) | 0.99b |
| ≥50 | 1.36 (0.42–4.34) | 1.84 (0.17–20.14) | ||
| Country of recruitment | ||||
| Australia and New Zealand | 1.00 (referent) | 1.00 (referent) | ||
| Canada | 0.82 (0.24–2.75) | 0.75 | 7.63 (0.25–232) | 0.24 |
| United States | 0.71 (0.27–1.86) | 0.48 | 0.84 (0.24–2.96) | 0.79 |
| Cigarette smoking | ||||
| Never user | 1.00 (referent) | 1.00 (referent) | ||
| Ever user | 1.06 (0.44–2.57) | .90 | 0.84 (0.17–4.18) | 0.83 |
| Synchronous tumor | ||||
| Absent | 1.00 (referent) | 1.00 (referent) | ||
| Present | 5.65 (0.67–47.68) | 0.11 | 11.54 (1.06–125) | 0.04 |
| Maximum dimension of tumor (per cm) | 1.04 (0.86–1.25) | 0.72 | 0.93 (0.65–1.32) | 0.68 |
| AJCC stage of tumor (per stage) | 1.68 (0.84–3.36) | 0.14 | 6.14 (1.21–13.14) | 0.03 |
| Histological grade (per grade) | 1.48 (0.85–2.57) | 0.17 | 2.00 (0.61–6.53) | 0.25 |
AJCC the American Joint Committee on Cancer
Adjusted for variables in the table and taken account into familial correlation
P trend: calculated from Cox regression models with ordinal variables as continuous measures
The frequency of surveillance colonoscopy after proctectomy was 1 colonoscopy per 1.16 years (95 % CI 1.01–1.31 years). There was no difference in the frequency of colonoscopy after surgical resection between carriers with and without metachronous colon cancer (Table 4). Of the 64 cases that we can determine the timing of colonoscopy with respect to the surgery for rectal cancer, 60 (94 %) reported a colonoscopy within 1 year of the age at surgery, and no synchronous CRCs were identified.
TABLE 4.
Frequency of surveillance colonoscopy following proctectomy for rectal cancer in mismatch repair gene mutation carriers
| Colonoscopy | All No. (%) | Metachronous cancer |
|
|---|---|---|---|
| Absent No. (%) | Present No. (%) | ||
| At least one | 62 (78) | 43 (90) | 19 (95) |
| Average frequency* | |||
| Every year | 46 (74) | 33 (76) | 13 (68) |
| Every 2 years | 8 (13) | 6 (14) | 2 (11) |
| Every 3 years | 2 (3) | 2 (5) | 0 (0) |
| Unknown frequency | 6 (10) | 2 (5) | 4 (21) |
| Mean interval (year) for 1 examination (95 % CI) | 1.16 (1.01–1.31) | 1.17 (0.97–1.36) | 1.13 (0.94–1.33) |
| None | 6 (8) | 5 (10) | 1 (5) |
| Missing | 11 (14) | 10 | 1 |
| Total | 79 | 58 | 21 |
Extracted from the self-reported questionnaire data. The frequency of colonoscopy was assumed to be distributed uniformly in the period between first and last age of colonoscopy
At 5 years after surgical resection, 47 (94 %) who had AR and 26 (90 %) who had APR were alive (Pearson χ2 P = 0.5). At 10 years, 44 (88 %) who had AR and 24 (83 %) who had APR were alive (Pearson χ2 P = 0.5).
DISCUSSION
In this relatively large observational study, MMR gene mutation carriers having a proctectomy (AR or APR) for rectal, as distinct from colon, cancer as their initial lesion had a high metachronous colon cancer risk. After treatment, almost a quarter developed a metachronous colon cancer by 10 years, nearly half by 20 years, and more than two-thirds by 30 years after the resection of their rectal cancer. These risks were evident despite an apparent 1–2 yearly colonoscopic surveillance interval after rectal surgery. No difference in the risk of metachronous colon cancer was found attributable to gender, age at diagnosis and cigarette smoking. The risk of metachronous cancer was greater in patients with a higher clinicopathological stage of their index rectal cancer or the presence of synchronous tumor, but not with the size and its histological characteristics of the tumor.
This metachronous cancer risk is similar to that we previously reported after segmental resection for index colon cancers in MMR gene mutation carriers.6 In that large observational study of 382 MMR gene mutation carriers followed-up for a mean of 9 years, we showed similar high cumulative risks of metachronous CRC for those treated by segmental resection contrasting with no occurrence in those undergoing extensive resection (total or subtotal colectomy). We argued that the overall high metachronous cancer risk in this series supported a strong case for more extensive surgical resection if the diagnosis of Lynch syndrome was known at the outset. Where the index cancer is in the colon, total colectomy with ileorectal anastomosis achieves this aim and avoids a permanent stoma, the risk of altered bowel function notwithstanding.
The clinical issues are more complex in the case of rectal cancer in Lynch syndrome since the options for a subtotal colectomy with ileorectal anastamosis are limited by the location of the primary cancer. The current study shows that the risk of metachronous colon cancer is substantial for Lynch syndrome patients following a proctectomy for index rectal cancer. This risk may be lethal if there is a rapid evolution of the tumor between surveillance colonoscopies, as is the behavior of cancer in Lynch syndrome. This weakens the argument for proctectomy despite patients having better quality of life compared with those who undergo total proctocolectomy, specifically better bowel function. If patients are young, healthy, and have good sphincter function, our data supports total proctocolectomy as a preferred option given they are likely to live long enough to get further metachronous cancers.
In contrast, our results could also be used to argue for a proctectomy. We observed that, overall, the patients with rectal cancer were young and had high survival rates. The metachronous cancers were, where stage was available, in early stages. The patients had undergone short interval surveillance colonoscopies during follow-up after proctectomy for rectal cancer. One could argue then, that proctectomy is appropriate as long as stringent follow-up is applied (assuming patients are willing to have such surveillance), as bowel function and quality of life can be maintained with little effect on survival.
We observed the majority of metachronous colon cancers were in the right colon in our cohort. This may be due to the commonly observed shift to the right colon cancers in Lynch syndrome and/or the relative difficulty to adequately complete colonoscopy in the right colon.21–24 It does raise the possibility of an alternative surgery comprising right hemicolectomy with proctectomy.
An important limitation of this study was the lack of information on the timing of the last surveillance colonoscopy, which meant we could not determine whether the metachronous cancer was likely to be an interval (missed) cancer or adenoma. We also cannot comment on the quality of colonoscopy performance. A further limitation of this study is the absence of detailed information on factors influencing choice of surgery, type of surgery (emergency or elective), and the status of adjuvant radiotherapy or chemotherapy and any complications. Finally, we also could not report the quality of life after surgery
Patients with Lynch syndrome who present with their index bowel cancer, regardless of whether it is in the colon or rectum, have a substantial high risk of metachronous cancer. Although an ileorectal anastomosis with ongoing endoscopic surveillance of the remaining rectum is an attractive straightforward option for those with index colon cancer, those presenting with index rectal cancer are disadvantaged by needing a more extensive restorative reconstruction at the outset. The final decision on extent of surgery when a Lynch syndrome patient presents with rectal cancer will be determined by age at presentation, factors that may increase the likelihood of a poor functional outcome, and patient choice informed by the high risk of metachronous colon cancers. If a proctectomy is chosen for index rectal cancer, it is critically important to ensure frequent surveillance of the remaining colonic mucosa.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all study participants of the Colon Cancer Family Registry and staff for their contributions to this project.
Funding This work was supported by the National Cancer Institute, National Institutes of Health under RFA #CA-95-011 and through cooperative agreements with members of the Colon Cancer Family Registry and Principal Investigators. Collaborating centers include Australasian Colorectal Cancer Family Registry (U01 CA097735), Familial Colorectal Neoplasia Collaborative Group (U01 CA074799) [USC], Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01 CA074800), Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783), Seattle Colorectal Cancer Family Registry (U01 CA074794), and University of Hawaii Colorectal Cancer Family Registry (U01 CA074806). AKW is supported by the Picchi Brothers Foundation Cancer Council Victoria Cancer Research Scholarship, Australia. MAJ is a Senior Research Fellow and JLH is an Australia Fellow of the National Health and Medical Research Council, Australia.
Footnotes
15th Annual Meeting of Collaborative Group of the Americas on Inherited Colorectal Cancer (CGA-ICC), Montreal, Canada, Oct 10–11, 2011. Metachronous colon cancer risk following surgery for first primary rectal cancer in Lynch syndrome.
Digestive Disease Week, San Diego, CA, May 19–22, 2012. Metachronous colon cancer risk following surgery for first rectal cancer in mismatch repair gene mutation carriers.
Electronic supplementary material The online version of this article (doi:10.1245/s10434-012-2858-5) contains supplementary material, which is available to authorized users.
DISCLOSURE The authors have no conflict of interest to declare with respect to this manuscript.
REFERENCES
- 1.Jenkins MA, Baglietto L, Dowty JG, Van Vliet CM, Smith L, Mead LJ, et al. Cancer risks for mismatch repair gene mutation carriers: a population-based early onset case-family study. Clin Gastroenterol Hepatol. 2006;4:489–498. doi: 10.1016/j.cgh.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Baglietto L, Lindor NM, Dowty JG, White DM, Wagner A, Gomez Garcia EB, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 2010;102:193–201. doi: 10.1093/jnci/djp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senter L, Clendenning M, Sotamaa K, Hampel H, Green J, Potter JD, et al. The clinical phenotype of Lynch syndrome due to germline PMS2 mutations. Gastroenterology. 2008;135:419–428. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, Guimbaud R, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 5.Vasen HFA, Abdirahman M, Brohet R, Langers AM, Kleibeuker JH, van Kouwen M, et al. One to 2-Year Surveillance Intervals Reduce Risk of Colorectal Cancer in Families With Lynch Syndrome. Gastroenterology. 2010;138:2300–2306. doi: 10.1053/j.gastro.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 6.Parry S, Win AK, Parry B, Macrae FA, Gurrin LC, Church JM, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut. 2011;60:950–957. doi: 10.1136/gut.2010.228056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalady MF. Surgical management of hereditary nonpolyposis colorectal cancer. Adv Surg. 2011;45:265–274. doi: 10.1016/j.yasu.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Kalady MF, Lipman J, McGannon E, Church JM. Risk of colonic neoplasia after proctectomy for rectal cancer in hereditary nonpolyposis colorectal cancer. Ann Surg. 2012;255:1121–1125. doi: 10.1097/SLA.0b013e3182565c0b. [DOI] [PubMed] [Google Scholar]
- 9.Coffey JC, Winter DC, Neary P, Murphy A, Redmond HP, Kirwan WO. Quality of life after ileal pouch-anal anastomosis: an evaluation of diet and other factors using the Cleveland Global Quality of Life instrument. Dis Colon Rectum. 2002;45:30–38. [PubMed] [Google Scholar]
- 10.Delaney CP, Fazio VW, Remzi FH, Hammel J, Church JM, Hull TL, et al. Prospective, age-related analysis of surgical results, functional outcome, and quality of life after ileal pouch-anal anastomosis. Ann Surg. 2003;238:221–228. doi: 10.1097/01.sla.0000080825.95166.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120–127. doi: 10.1097/00000658-199508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JS, Petrelli NJ, Rodriguez-Bigas MA. Rectal cancer in hereditary nonpolyposis colorectal cancer. Am J Surg. 2001;181:207–210. doi: 10.1016/s0002-9610(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 13.Moslein G, Nelson H, Thibodeau S, Dozois RR. [Rectal carcinomas in HNPCC] Langenbecks Arch Chir Suppl Kongressbd. 1998;115:1467–1469. [PubMed] [Google Scholar]
- 14.Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–2343. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 15.Southey MC, Jenkins MA, Mead L, Whitty J, Trivett M, Tesoriero AA, et al. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol. 2005;23:6524–6532. doi: 10.1200/JCO.2005.04.671. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 17.Win AK, Young JP, Lindor NM, Tucker KM, Ahnen DJ, Young GP, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30:958–964. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers WH. Regression standard errors in clustered samples. Stata Tech Bull. 1993;3:19–23. [Google Scholar]
- 19.Williams RL. A note on robust variance estimation for clustercorrelated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 20.Stata Statistical Software: Release 11. StataCorp LP: College Station; 2009. [computer program] [Google Scholar]
- 21.Young J, Simms LA, Biden KG, Wynter C, Whitehall V, Karamatic R, et al. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am J Pathol. 2001;159:2107–2116. doi: 10.1016/S0002-9440(10)63062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 23.Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858–864. doi: 10.1016/j.cgh.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Martinez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–841. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.