Abstract
Background
Expanding access to effective treatments for heroin dependence is a global health priority that will also reduce HIV transmission. This study compares the efficacy for maintaining heroin abstinence, preventing relapse, and reducing HIV risk behaviors of three common treatments: detoxification followed by drug counseling only or drug counseling combined with opioid antagonist (naltrexone) or agonist (buprenorphine) maintenance treatment.
Methods
126 detoxified heroin dependent patients in Malaysia were randomly assigned to 24 weeks of medication maintenance with naltrexone, buprenorphine, or placebo, provided double-blind and double-dummy. All patients received manual-guided drug counseling. Primary outcomes, assessed by three times per week urine testing, were days to first heroin use, days to heroin relapse (3 consecutive opioid-positive urine tests), maximum consecutive days heroin abstinence, and, assessed by self-report at baseline, 3- and 6-months, reductions in HIV risk behaviors. The study was terminated after 22 months of enrolment, based on findings of superior buprenorphine efficacy in an interim safety analysis and the recommendation of the Data and Safety Monitoring Board. This study is registered with ClinicalTrials.gov, with the number NCT00383045.
Findings
We observed consistent, significant linear contrasts in days to first heroin use (p<0.001), days to heroin relapse (p<0.001), maximum consecutive days heroin abstinence (p<0.01), and retention (p<0.001), with all results best for buprenorphine, intermediate for naltrexone, and worst for placebo. Buprenorphine was associated with significantly greater time to first heroin use and retention compared to naltrexone (p<0.01 for both measures) or placebo (p<0.001 for both measures) and also significantly greater time to heroin relapse (p<0.01) and maximum consecutive weeks abstinent (p<0.01) compared to placebo. There were no significant differences between naltrexone and placebo on these measures. HIV risk behaviors were significantly reduced from baseline across all 3 treatments (p<0.001), but the reductions did not differ significantly among the 3 treatments.
Interpretation
The effectiveness of buprenorphine for maintaining prolonged periods of abstinence, delaying the time to resumption of heroin use or relapse, and retaining patients in treatment supports widespread dissemination of opioid agonist maintenance treatment.
Keywords: Heroin Dependence, Buprenorphine, Naltrexone, Randomized Clinical Trial, HIV/AIDS
Introduction
Heroin and injection drug use (IDU) are global problems and driving the AIDS epidemic in Malaysia, many other developing and transitional countries (including China, India, Indonesia, Iran, Pakistan, and Russia), and in the developed world (U.S., England, Europe, and Australia).1–7 Until recently, Malaysia along with many other developing countries implemented a largely punitive approach to drug problems, relying on imprisonment or long-term detention in “rehabilitation” programs and prohibiting medical treatments for heroin dependence.3,7 In Malaysia, the failure of this approach to stem drug problems and HIV transmission led to the introduction in 1996 of some medical treatments, including medically-supervised detoxification, drug counseling, and opioid antagonist maintenance treatment with naltrexone.7 Malaysia continued to prohibit opioid agonist maintenance treatment, however, until the start of the current study, which introduced buprenorphine maintenance treatment and led to the subsequent approval of methadone maintenance treatment. Concerns about the potential abuse liability of methadone, misconceptions about the therapeutic mechanisms of opioid agonist maintenance treatment (the belief that it simply substitutes one addiction for another), and lack of understanding of its effectiveness contributed to prohibition of this treatment approach. Notably, opioid agonist maintenance treatment remains prohibited in some countries, including Russia,3,8 and is provided only to an estimated 240,000 of 800,000 or more heroin addicts in the U.S.9 Dissemination of medical treatments will depend in part on evaluation of the comparative efficacy of the different treatments.
Heroin detoxification followed by drug counseling or referral to 12-step groups is a common treatment approach in the U.S., Malaysia and elsewhere, despite a lack of strong empirical evidence supporting this approach.10 Naltrexone, a relatively long-acting (24–72 hour following oral administration) opioid antagonist, was approved by the U.S. Food and Drug Administration for the treatment of opioid dependence in 1984, based on its safety and pharmacological efficacy for blocking opiate effects, and introduced in Malaysia in 1996. Several recent meta-analyses and reviews concluded, however, that there is insufficient evidence from placebo-controlled studies supporting the efficacy of naltrexone,11,12 although two recent placebo-controlled studies conducted in St. Petersburg, Russia, found significantly greater retention and reductions in relapse with naltrexone.8,13 Poor treatment retention and problems with patient acceptance have limited its effectiveness in clinical practice.11 Strong and consistent findings from randomized, placebo-controlled clinical trials, human laboratory, and observational and quasi-experimental studies support the effectiveness of maintenance treatment with the partial opioid agonist, buprenorphine, or the full agonist, methadone, for reducing illicit opioid use and HIV transmission risk.3,14–16 Studies directly comparing buprenorphine and methadone report comparable or greater efficacy of methadone.15,17 Advantages of buprenorphine, including decreased overdose risk, possibly lower abuse liability (especially when provided in a tablet formulation containing naloxone), and potential for three times per week dosing,18,19 facilitated its introduction in Malaysia when methadone was still prohibited. No studies have directly compared the efficacy of opioid agonist treatment (with either buprenorphine or methadone) and naltrexone maintenance treatment. The lack of direct comparisons may contribute to policy makers’ assumption that there are no advantages to introducing opioid agonist maintenance treatment (and continued prohibition of this treatment) if naltrexone maintenance treatment is available. Additionally, there are relatively few placebo-controlled clinical trials of either buprenorphine18,20–23 or naltrexone maintenance.8,11–13,24
Consequently, this double-blind, double-dummy, placebo-controlled randomized clinical trial compared the efficacy for maintaining heroin abstinence, preventing relapse, and reducing HIV risk behaviors following detoxification of 24 weeks of drug counseling alone (combined with placebo) or combined with maintenance with naltrexone or buprenorphine.
Methods
Patients
Patients met DSM IV TR criteria for current heroin dependence25 and had an opioid-positive urine toxicology. Patients were ineligible if alcohol, benzodiazepine or sedative dependent; had liver enzymes (alkaline phosphatase or alanine transaminase) greater than three times upper limit of normal; dangerous to themselves or others; psychotic or experiencing major depression; or experiencing life-threatening medical problems. Enrolment for the study began on 7/8/2003 and ended on 5/14/2005. During the 22-month enrolment period, 215 patients made at least one contact for initial screening; 18 made only an initial contact; 23 did not complete the evaluation; 12 were not eligible because of elevated liver enzymes; 19 completed the evaluation but did not enter the detoxification program; 17 did not complete detoxification phase; and 126 were randomized. Informed consent was obtained from all patients. The study was approved by the Human Investigation Committee, Yale University School of Medicine, and the Malaysian Ministry of Health Human Subjects Review Board.
Setting
The setting was an outpatient research clinic established for the study and a free-standing residential detoxification program.
Residential Detoxification
Prior to randomization, all patients completed a 14-day detoxification protocol26 in a residential setting, during which they were administered SL buprenorphine for the first three days (8 mg, 4 mg, and 2 mg) and oral naltrexone on days 4 (12.5 mg), 5 (25 mg) and 6 (50 mg). Withdrawal symptoms were treated, as needed, with oral clonidine, diazepam, naproxen, and metoclopromide.
Randomization
On day 14 in the residential detoxification center, patients were randomly assigned to drug counseling combined with maintenance treatment with placebo, naltrexone or buprenorphine. A simple, complete randomization sequence was generated by a computer program and maintained in the U.S. Two days before completion of detoxification, the treatment group assignment for each study participant was disclosed only to the study pharmacist in Malaysia to allow sufficient time for preparation of double-blind, double-dummy medications. All other study personnel were blind to treatment assignment throughout the entire study duration.
Drug Counseling
All patients were provided manual-guided weekly individual and group drug counseling in the outpatient research clinic. Group therapy, led by a psychiatrist with advanced training in addictions (MM), provided education about heroin addiction, HIV and AIDS, and the recovery process. Specific modules included relapse prevention and coping skills training, and 4–6 sessions specifically targeted reducing HIV risk associated with injection drug use, sharing of injection equipment and sexual activity. The treatment manual was adapted for use in Malaysia and took advantage of the importance of family in Malay and Chinese culture and the ethos of respect for parents (in particular for one’s mother). As part of the recovery process, the group therapy discussed the concept of “locked doors”—closing off the heart from recognizing the importance of others and accepting advice— and the importance of “turning over” to a higher power (e.g., God/Allah for Malay Muslims or one’s family), being humble, and seeing one’s parents (especially mother) or children as being more important than satisfying one’s own immediate needs or craving. Communication skills training components were modified to take into account that being outspoken is considered arrogant in Malaysian culture. Instead, patients were encouraged to cope with disagreement with others by lowering their expectations (recognizing that they can only change what is within their power to change (themselves) and that they can’t change someone else). Manual-guided individual drug counseling was provided by nurses and used a treatment manual adapted from the enhanced counseling provided to buprenorphine maintained patients in an earlier study conducted in a primary care clinic in the US.19 The nurses had no experience treating addictions prior to receiving 4 days of didactic training and then treating, under close supervision, at least 3 training cases during the pilot phase of the study. Individual counseling sessions lasted approximately 45 minutes; reviewed recent drug use or efforts at abstinence, urine test results, and self-help group attendance; and provided support and advice about preventing relapse. The nurses attempted to encourage patients who resumed heroin use or relapsed to remain in treatment and curtail heroin use and to reengage in treatment patients who missed appointments for counseling or medication.
Medications
Medications were prepared by a research pharmacist, who had no direct contact with participants. Buprenorphine mono tablets (containing only buprenorphine) and identical appearing placebo tablets were provided by the manufacturer. Naltrexone was purchased for the study; naltrexone tablets were crushed, and naltrexone or placebo was placed inside identical appearing capsules by the study pharmacist. All patients ingested oral capsules (containing either naltrexone or placebo) and dissolved sublingual tablets (containing either buprenorphine or placebo), under the direct observation of a study nurse who verified ingestion of the capsule and absorption of the tablet. To mask slight taste differences between active and placebo buprenorphine tablets, subjects first gargled with a mentholated, antiseptic mouthwash before administering the sublingual tablets. One 8-mg tablet of buprenorphine (or matching placebo) and one 50 mg tablet of naltrexone (or placebo) were administered daily during the first week of maintenance. Subsequently, patients received two 8-mg tablets of buprenorphine (or placebo) and two 50-mg tablets of naltrexone (or placebo) on Mondays and Wednesdays and three 8-mg tablets of buprenorphine (or placebo) and three 50-mg tablets of naltrexone (or placebo) on Fridays. Buprenorphine or matching placebo dose was increased to 24 mg (three 8-mg tablets) on Mondays and Wednesdays and to 36 mg (4 8-mg tablets and 2 2-mg tablets) on Fridays for patients who reported craving or withdrawal or had persistent heroin use. The success of the patient blind was assessed by asking patients at week 12 which medication they thought they were receiving: 30/32 (94%) of patients on buprenorphine, 16/21 (76%) naltrexone-treated patients and 4/20 (20%) of placebo-treated patients correctly identified their medication (Chi Square=31.8, df=2, p<0.001).
Outcomes
As specified in advance, the primary outcome measures were assessed for 24 weeks following randomization and included days to first heroin use (first opiate positive urine test after randomization); days to heroin relapse (3 consecutive opiate positive urine tests or opiate positive test followed by 2 consecutive positive or missing tests); the maximum consecutive days abstinent from heroin (the longest period of opioid-negative urine tests), and reductions in self-reported HIV risk behaviors over the past 3 months. Secondary outcomes included adverse events and treatment retention, which was defined as time to treatment completion or to last clinical contact, to protective transfer for unremitting heroin use (3 consecutive weeks of opioid positive tests), or to referral to an alternative treatment because of significant medical or psychiatric problem, as assessed by the study psychiatrist.
Outcome Assessments
Illicit drug use during treatment was measured by three times per week urine testing during treatment, using a semi-quantitative homogenous enzyme immunoassay for opioids (Microgenics Corp., Freemont, CA), with a cut-off set at 300 ng/ml. The AIDS Risk Inventory, assessing drug-related and sexual risk behaviors associated with HIV transmission,27 was administered prior to beginning detoxification and at 3- and 6-months following randomization by trained research assistants who were not involved in the treatment of patients. Liver enzymes were evaluated monthly. Serious adverse events (i.e., hospitalization or death) were recorded at the time of occurrence. Self-reported adverse effects were collected weekly using a structured questionnaire that assessed the severity of 32 symptoms (e.g., headache, constipation, drowsiness, etc.) on a 4-point scale (none, mild, moderate or severe).
Sample size
Based on findings from an unpublished pilot study of naltrexone maintenance in Malaysia that 72% of patients were abstinent at 6 months and data from a U.S. study that 42% of patients treated with buprenorphine at doses comparable to those planned for the study and 12–24% of patients treated with low doses (to estimate the effects of placebo) achieved sustained abstinence,28 we anticipated a medium effect size difference (equivalent to h=0.50) between naltrexone and buprenorphine (favoring naltrexone) in mean weeks abstinent and a larger effect size difference for the contrasts between drug counseling only (placebo) and either of the two maintenance treatments (favoring the two medications). The planned total sample size (N=180) would provide a power greater than 0.80, with a 2-sided Type I error of 0.05, to detect similar or larger effect size differences in mean maximum consecutive weeks abstinence among the 3 conditions. Data were not available for power calculations regarding the other primary outcome measures.
Statistical analysis
Characteristics at intake were compared among the 3 treatments using the chi-square and ANOVA as appropriate. Statistical analyses were planned in advance, and all randomized patients were included in the analyses.
Days to first heroin use, to heroin relapse, and remaining in treatment (all truncated at 168 days) were evaluated using the Life Table method and the Wilcoxon test. This approach is similar to the Kaplan-Meier Survival Analysis and Cox Regression procedures, and the same pattern of results was observed using all three approaches. Additionally, Cox Regression analysis was used to calculate proportional hazard ratios with their attendant confidence intervals regarding the pair-wise comparisons. A factorial analysis of variance procedure (ANOVA), including evaluation of polynomial contrasts and post-hoc, Scheffe adjusted pair-wise comparisons, was used to evaluate treatment group differences on the maximum consecutive weeks of abstinence. The mixed model procedure was used to assess treatment effects on the total score of the AIDS Risk Inventory and on the drug- and sexual-risk behaviors subscales. The differences in the frequency of adverse events across the three treatment groups were evaluated using omnibus Chi-Square tests. To facilitate comparisons with the results of other studies, treatment group differences regarding average number of days to first heroin use, to heroin relapse, or remaining in treatment (up to a maximum of 168) were evaluated using ANOVA. Consistent with the association between treatment discontinuation and relapse to illicit opioid use and as planned in advance, missing urine tests were coded as positive. Notably, 72 of the 84 patients who discontinued treatment prematurely for other than medical reasons had a morphine-positive urine test before leaving treatment. The completion rate of scheduled assessments was high: 87% (4301/4936) of the scheduled urine tests during the time patients remained in the study and 82% (311/378) of all scheduled ARI assessments were obtained.
Findings of an interim safety analysis conducted in June, 2005, on 103 patients who had completed treatment by the time of the interim analysis showed superior efficacy of one of the treatment conditions and were presented to the Data and Safety Monitoring Board. Based on the recommendations of the Board, the study was terminated on August 3, 2005.* All analyses used 2-tailed tests of significance and were performed using SPSS version 15.029 or, for the Cox Regression analysis, SAS version 9 (Cary, NC). To adjust for the interim analysis, p values <0.025 were considered statistically significant.
Role of the Funding Source
The study was funded by the National Institute on Drug Abuse and two of the investigators (Richard Schottenfeld and Marek Chawarski) also receive support from the State of Connecticut Department of Mental Health and Addiction Services. The funding agencies did not have any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of the patients enrolled are displayed in Table 1 and did not differ significantly among the 3 groups.
Table 1.
Baseline characteristics of randomized subjects.
| Buprenorphine n=44 | Naltrexone n=43 | Placebo n=39 | |
|---|---|---|---|
| Age - mean (SD) | 36.3 (9.3) | 38.2 (9.3) | 37.6 (8.2) |
| Malay Ethnicity | 31 (71%) | 28 (65%) | 27 (69.0%) |
| Single | 35 (79.5%) | 32 (74.4%) | 23 (59.0%) |
| HS Education or above | 2 (5%)) | 3 (7%) | 5 (13.0%) |
| Unemployed | 23 (52.3%) | 14 (32.6%) | 14 (35.9%) |
| Lifetime history of incarceration | 28 (63.6%) | 30 (69.8%) | 23 (59.0%) |
| History of previous drug treatment | 21 (47.7%) | 30 (69.8%) | 24 (61.5%) |
| Years of heroin use - mean (SD) | 14.5 (8.0) | 16.4 (9.0) | 14.8 (8.0) |
| Days of heroin use in the past 30 – mean (SD) | 27.0 (6.6) | 26.0 (8.3) | 28.3 (6.2) |
| Lifetime ATS abuse | 26 (59.1%) | 18 (41.9%) | 16 (41.0%) |
| Current ATS abuse | 20 (45.5%) | 17 (39.5%) | 13 (33.3%) |
| Lifetime Benzo abuse | 26 (59.1%) | 25 (58.1%) | 26 (66.7%) |
| Current Benzo abuse | 24 (54.5%) | 21 (48.4%) | 23 (59.0%) |
| Lifetime IDU | 33 (75.0%) | 36 (83.7%) | 31 (79.5%) |
| Current IDU | 20 (46%) | 16 (37%) | 16 (41%) |
| Lifetime needle sharing | 22 (50.0%) | 13 (30.2%) | 22 (56.4%) |
| Current needle sharing (past 30 days) | 14 (31.8%) | 8 (18.6%) | 12 (30.8%) |
| HIV seropositive* | 11 (25.6%) | 11 (26.2%) | 5 (13.2%) |
| Hepatitis C | 39 (88.6%) | 41 (95.3%) | 36 (92.3%) |
| Hepatitis B | 0 (0.0%) | 2 (4.7%) | 5 (12.8%) |
| Pulmonary TB | 5 (11.4%) | 10 (23.3%) | 6 (15.4%) |
| Consistent condom use | 3 (6.8%) | 4 (9.3%) | 2 (5.1%) |
| Multiple concurrent sex partners (lifetime) 17 (38.6%) | 15 (34.8%) | 9 (23.1%) | |
Retention
There were significant overall differences in retention (p<0.001), with retention highest for buprenorphine, intermediate for naltrexone, and lowest for placebo (Figure 1). In pairwise comparisons, retention was significantly higher for buprenorphine compared with naltrexone (p<0.01) or placebo (p<0.001), with hazard ratios (95% CI) of 1.55 (1.01 –2.37) and 2.15 (1.38–3.35), respectively. Retention did not differ significantly between naltrexone and placebo (p=0.59), with a hazard ratio (95% CI) of 1.32 (0.85–2.05). Treatment group differences in mean (95%CI) days remaining in treatment are provided in Table 2.
Figure 1. Retention.
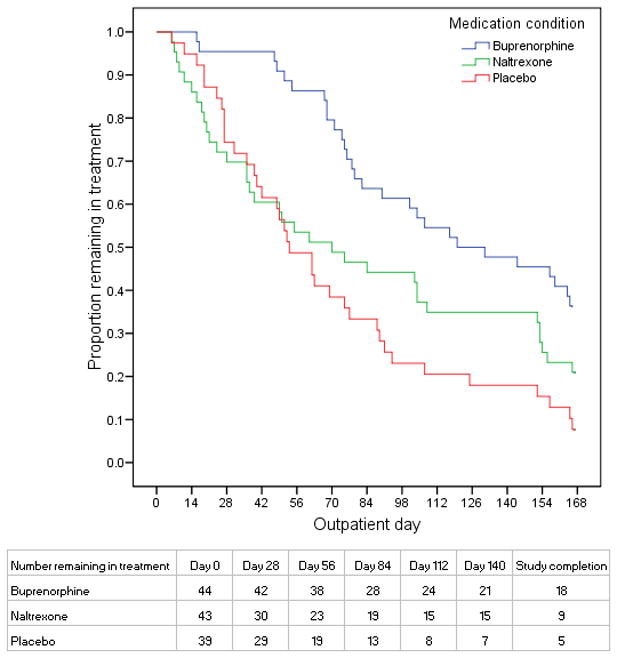
Figure 1 shows the proportion of enrolled patients in each treatment group on each study day following detoxification who were remaining in treatment
Table 2.
Effects of treatment group assignment on retention and heroin use outcomes (N=126)*
| Buprenorphine n=44 | Naltrexone n=43 | Placebo n=39 | Significance level of statistical comparisons | ||||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Linear contrast | Bup vs. Placebo | Bup vs. Ntx | Ntx vs. Placebo | |
| Days in treatment | 117 (102–132) | 84 (64–103) | 70 (54–87) | <0.001 | <0.001 | <0.025 | 0.516 |
| Days in treatment without heroin use | 51 (33–68) | 24 (11–37) | 18 (8–28) | <0.001 | <0.05 | <0.025 | 0.800 |
| Days in treatment without heroin relapse | 79 (61–98) | 64 (44–84) | 39 (25–53) | <0.01 | <0.01 | 0.422 | 0.121 |
| Maximum consecutive days abstinent | 59 (43–76) | 42 (28–57) | 24 (13–35) | <0.001 | <0.01 | 0.192 | 0.180 |
Heroin use and abstinence
There were significant overall treatment group differences for days to first heroin use (p<0.001) and days to heroin relapse (p<0.01) and a significant linear trend for the maximum consecutive days of heroin abstinence (p<0.001), with all results consistently best for buprenorphine, intermediate for naltexone, and worst for placebo (Figures 2 and 3; treatment group differences in days remaining in treatment without heroin use or without relapse and consecutive days abstinent are also provided in Table 2). In pairwise comparisons, days to first heroin use was significantly greater for buprenorphine compared to naltrexone (p<0.01) or placebo (p<0.001), with a hazard ratio (95% CI) of 1.87 (1.21–2.88) and 2.02 (1.29–3.16), respectively, and did not differ significantly between naltrexone and placebo (p=0.747), with a hazard ratio (95%CI) of 1.04 (0.67–1.61). Days to heroin relapse was also greater for buprenorphine compared to placebo (p<0.01) and naltrexone (p=0.052), with a hazard ratio (95% CI) of 2.17 (1.38–3.42) and 1.56 (1.01–2.41), respectively, and did not differ significantly between naltrexone and placebo (p=0.486), with a hazard ratio (95% CI) of 1.35 (0.86–2.11). Post-hoc comparisons indicated that buprenorphine-treated patients achieved longer maximum consecutive days of opiate abstinence compared to placebo-treated patients (p<0.01); the differences between buprenorphine and naltrexone (p=0.19) and between naltrexone and placebo (p=0.18) were not significant.
Figure 2. Time to first opiate use.
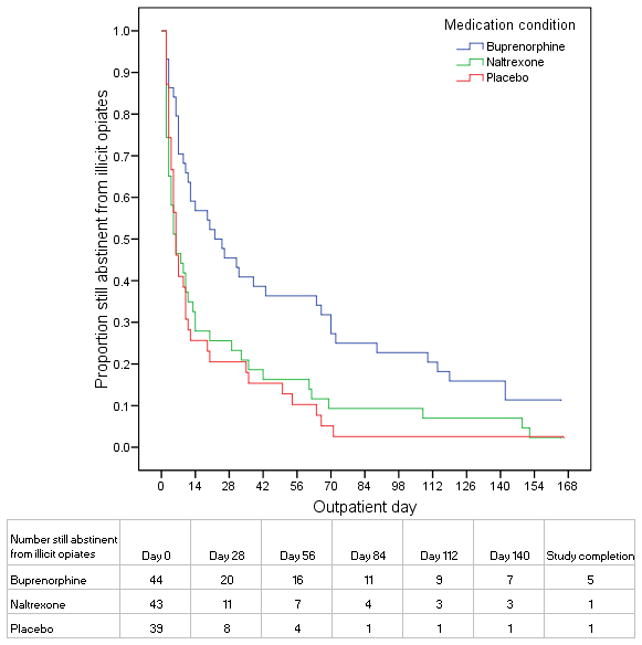
Figure 2 shows the proportion of enrolled patients in each treatment group on each study day following detoxification who were remaining in treatment and had not resumed illicit opiate use
Figure 3. Time to relapse.
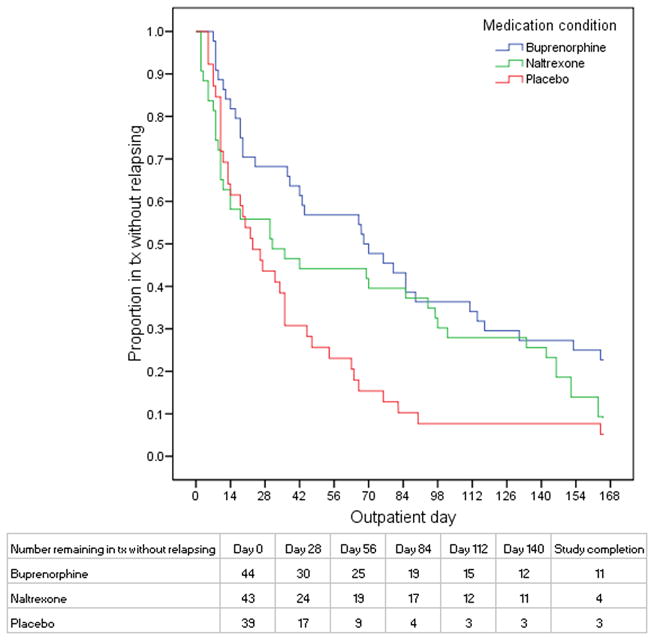
Figure 3 shows the proportion of enrolled patients in each treatment group on each study day following detoxification who were remaining in treatment and had not relapsed
AIDS Risk Behaviors
There were significant reductions from pre-treatment baseline to the three- and six-month assessments on the overall summary measure of the AIDS Risk Inventory (p<0.001) and the drug-risk subscale (p<0.01) but no significant differences in reductions associated with different treatment groups on either the summary scale (p=0.14) or the drug-risk subscale (p=0.13) and no significant reductions from baseline on the sex-risk subscale (p=0.14) (see Table 3). Review of ARI subscale scores and individual items indicated that the overall reductions were driven primarily by reductions in the recency and frequency of injection drug use; the proportion of patients reporting past month injection drug use in the buprenorphine, naltrexone and placebo groups respectively decreased substantially from baseline (49%, 38%, 41%) to 2%, 7% and 7% at 3 months and 14%, 7%, and 9% at 6 months.
Table 3.
Effects of treatment group assignment on AIDS Risk Behaviors (N=126)
| Buprenorphine | Naltrexone | Placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3-month | 6-month | Baseline | 3-month | 6-month | Baseline | 3-month | 6-month | Time effect | Medication effect | Interaction | |
| n=41 | n=42 | n=36 | n=42 | n=30 | n=29 | n=39 | n=29 | n=23 | p | p | p | |
| ARI total score Mean (95%CI) | 56.5(48.0–64.9) | 47.3(41.7–53.0) | 53.7(44.1–63.4) | 46.4(37.9–54.0) | 43.7(36. 7–50.6) | 43.1(33. 5–52.7) | 57.0(46.7–67.1) | 47.4(39.5–55.3) | 43.6(34.9–52.4) | <0.01 | 0.138 | 0.321 |
| ARI drug risks subscore Mean (95% CI) | 38.9(32.7–45.1) | 23.1(19.3–26.8) | 25.0(20.2–29.8) | 30.4(24.0–36.8) | 22.5(18.3–26.8) | 21.8(16.5–27.1) | 41.3(34.2–48.3) | 26.4(22.0–30.8) | 25.8(20.0–31.7) | <0.001 | 0.13 | 0.276 |
| ARI sex risks subscore Mean (95% CI) | 15.4(11.7–19.0) | 15.0(11.7–18.3) | 19.1(15.7–22.6) | 11.7(8.1–15.4) | 11.5(7.9–15.2) | 14.4(10.6–18.2) | 13.4(9.4–17.3) | 12.0(8.0–16.0) | 14.2(9.8–18.6) | 0.136 | 0.193 | 0.983 |
| IDU past 30 days n/N (%) | 20/41(49%) | 1/42(2.4%) | 5/36(14%) | 16/42(38%) | 2/30(6.7%) | 2/29(6.9%) | 16/39(41%) | 2/29(6.9%) | 2/23(8.7%) | <0.001 | 0.716 | 0.669 |
Adverse Effects
There were no deaths; two patients were hospitalized during the detoxification phase (1 for oversedation and 1 for pulmonary infection); and 12 patients were hospitalized during treatment, including 8 naltrexone-treated, 3 buprenorphine-treated, and 1 placebo-treated patient (p=0.035). Three of the naltrexone-treated patients were hospitalized for conditions considered possibly related to the study medication (severe insomnia and craving during the first week following randomization, consistent with protracted withdrawal symptoms; attempted suicide;30 and psychosis);31 all other hospitalizations were for conditions considered unrelated to the study medications (e.g., accidental injury, infections, asthma and chest pain). Three patients were withdrawn from the study for medical reasons (1 placebo-treated patient and 1 naltrexone-treated patient with psychosis and 1 buprenorphine-treated patient withdrawn from the study in advance of surgery for hernia repair). The number (proportion) of buprenorphine-, naltrexone- or placebo treated patients, respectively, experiencing any elevation of liver enzymes (13 (30%), 10 (23%), and 11 (28%); p=0.787) or elevations greater than 3 times the upper limit of normal (4 (9%), 3 (7%), and 5 (13%); p=0.662) did not differ significantly among treatment groups. During the course of treatment, a significantly higher proportion of buprenorphine-treated patients compared with naltrexone- or placebo-treated patients respectively made at least one report on a weekly assessment of severe constipation (51%, 23%, 22%; p<0.01), drowsiness (51%, 17%, 28%; p<0.025), urinary hesitancy (54%, 9%, 22%; p<0.001), or sweating (33%, 11%, 14%; p=0.036). These symptoms were usually transient and only reported in 1–2.5% of the total number of weekly assessments, and no patient discontinued treatment as a result of these symptoms.
Discussion
The results of this study provide strong support for the efficacy of buprenorphine maintenance treatment in sustaining abstinence, delaying time to resumption of heroin use and time to relapse, and retaining patients in treatment, as evidenced by the findings of consistent, significant linear contrasts on all of these measures, with best results for buprenorphine, intermediate results for naltrexone, and worst results for placebo. HIV risk behaviors decreased significantly from baseline for all 3 groups, primarily driven by substantial reductions in injection drug use, but did not differ significantly across treatments. While complete abstinence, elimination of HIV risk behaviors, and recovery of psychosocial functioning would be ideal outcomes, increasing the amount of time abstinent (i.e., consecutive weeks abstinent) and delaying the time to resumption of heroin use or relapse—positive outcomes found with buprenorphine maintenance in this study—are important treatment goals. By these measures, buprenorphine maintenance treatment is significantly more effective than placebo and significantly more effective on some measures than naltrexone. Since patients generally continue to improve the longer they remain in opioid agonist maintenance treatment,32 the significantly greater retention among patients receiving buprenorphine also supports the effectiveness of this medication.
To our knowledge, this is the first randomized clinical trial comparing opioid agonist maintenance treatment (with buprenorphine or methadone) and naltrexone and one of the few (and longest) placebo-controlled trials of buprenorphine or naltrexone. The findings in this study of significant superiority of buprenorphine compared to placebo on all of our primary drug use outcome measures and retention are consistent with the results of other placebo-controlled studies of buprenorphine in the U.S.,18,20,23 Norway22 and Sweden.21 The findings of significant superiority of buprenorphine compared to naltrexone for retention and time to resuming heroin use are unique. The results for buprenorphine treated patients in this study regarding retention and maximum duration of opioid abstinence are similar to previous studies of buprenorphine,19 and the results for naltrexone treated patients were comparable to the results of many studies,24,33,34 suggesting that the findings regarding the greater efficacy of buprenorphine compared to naltrexone or placebo may generalize to other populations and settings.
Drug use outcomes and retention for patients treated with naltrexone were consistently better but not significantly different than for placebo-treated patients in this study. The failure to detect significant differences is consistent with several other placebo-controlled studies of naltrexone and meta-analyses11,12 but contrast with the significant differences found in the St. Petersburg studies, where opioid agonist maintenance treatment is not available and there are strong family supports encouraging treatment participation.8,13 Notably, a recent meta-analytic review concluded that retention is the key variable explaining the discrepancies in findings regarding the efficacy of naltrexone in double-blind clinical trials and that significant differences in illicit opioid use are found between naltrexone- and placebo-treated patients in studies with higher retention,12 which may be achieved by enrolling more highly motivated patients or using contingency management, behavioral family therapy, or legal or other pressures to encourage medication adherence and continued treatment participation.35–37 The recent introduction of a long-acting, depot formulation of naltrexone may also improve treatment retention and the effectiveness of naltrexone in clinical practice.38
Despite the favorable findings with regard to buprenorphine, there is still room for improvement, since only 41% of patients remained in treatment and only 25% stayed in treatment and avoided relapse during the 24-week treatment period. Additionally, as found in other studies,3,39 HIV risk behaviors associated with injection drug use were reduced during treatment in all treatment groups, but sexual risk behaviors, including unprotected sex, did not decrease. Providing earlier dose increases, higher doses of buprenorphine, or take-home doses of buprenorphine might have improved retention and drug use outcomes in the buprenorphine group. Training available health care personnel, nurses, to provide the drug counseling is a strength of the study and improves the relevance of the study findings to Malaysia and other developing countries, where there are very few health professionals with advanced training or experience in addictions treatment, but developing and providing more effective counseling or other behavioral interventions might improve treatment outcomes. The added benefits of improved behavioral interventions would be expected in all treatment groups, however, and would not be expected to change the findings regarding the superiority of buprenorphine.
Limitations of this study include early termination of the study, due to the findings of significant superiority of buprenorphine on the primary drug use outcome measures, which resulted in a final sample size that was smaller than initially planned.40,41 The observed, non-significant differences in pair-wise comparisons on some drug use and HIV risk reduction measures and retention (e.g., between naltrexone- and placebo-treated patients on all of these measures or between buprenorphine- and naltrexone-treated patients on some of the measures) would not have been likely to reach the level of statistical significance with the originally planned sample size but could be large enough to be of potential clinical importance if found to differ significantly in a much larger study. Missing data, resulting mainly from the high attrition of patients treated with naltrexone or placebo and subsequent loss to follow-up of some non-completing patients, may have blunted the findings with regard to the efficacy of buprenorphine, since most patients who left treatment prematurely had resumed heroin use prior to leaving treatment, and premature discontinuation of treatment is usually followed by relapse. Drug use outcomes and retention were based on objective measures, but HIV risk behaviors were assessed by self-report and could not be validated by objective measures. Despite efforts to maintain the medication blind, differences in medication effects allowed most patients treated with buprenorphine or naltrexone to break the blind and identify correctly the medication they were receiving. Study eligibility criteria, the requirement that all patients complete a residential detoxification before randomization, and the use of a single study site and limited sample size limit the generalisability of the study findings to other patient populations or settings. Buprenorphine may have even greater effectiveness compared to detoxification followed by drug counseling alone or with naltrexone in “real world” settings where heroin-dependent patients are directly inducted on buprenorphine without first needing to undergo detoxification.
This study has important implications for clinical practice and public health policy. Although opioid agonist maintenance treatment remains unavailable in many areas, the findings of this study provide strong support for the efficacy of buprenorphine maintenance treatment for reducing illicit opioid use and retaining patients in treatment, compared to either naltrexone maintenance treatment or drug counseling only, and some support for the potential efficacy of all 3 treatments to reduce HIV transmission risk associated with injection drug use.3,14,15 The ease of induction onto buprenorphine in ambulatory settings, the low incidence of serious adverse events or medical symptoms leading to treatment discontinuation associated with buprenorphine in this study and previous studies,18,42 and the potential for providing more liberal take home doses than were provided in the clinical trial also facilitate dissemination of this treatment. These considerations support dissemination of opioid agonist maintenance treatment with buprenorphine or methadone (which has at least comparable efficacy, greater ease of induction, and generally lower medication cost compared to buprenorphine) as an important component of an effective public health approach for reducing problems associated with heroin dependence.
Acknowledgments
This work was supported by NIDA #’s K24 DA000445 (RSS), R01 DA14718.
The study was funded by the National Institute on Drug Abuse. Two of the investigators (Richard Schottenfeld and Marek Chawarski) also receive support from the State of Connecticut Department of Mental Health and Addiction Services. The funding agencies did not have any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Buprenorphine and buprenorphine placebo were provided at no cost by the manufacturer, Reckitt Benckiser. Reckitt Benckiser did not have any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Ten patients who had been randomized (4 treated with buprenorphine, 5 with naltrexone, and 1 with placebo) were still in the treatment phase at the time the study was terminated. Data from these ten patients are included in the analyses, consistent with an intention-to-treat analysis. The pattern of results does not change if data from these patients are excluded in the analyses. Inclusion of these patients in the analysis results in lower reported study completion, retention, and time to resumption of heroin use or relapse, however, than would be reported if these patients were excluded from the analyses, since data regarding these outcomes were truncated at the time of study termination.
Authors’ Contribution
I declare that I participated in the design, conduct and analysis of the study and that I have seen and approved the final version.
Conflict of Interest Statement
I have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aceijas C, Stimson GV, Hickman M, Rhodes T Countries UNRGoHAPaCaIiDaT. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS. 2004;18(17):2295–303. doi: 10.1097/00002030-200411190-00010. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS (Joint United Nations Programme on HIV/AIDS) UNAIDS 2006 report on the global AIDS epidemic: A UNAIDS 10th anniversary special edition. Geneva: 2006. [Google Scholar]
- 3.Institute of Medicine Committee on the Prevention of HIV Infection among Injecting Drug Users in High Risk Countries. Preventing HIV Infection among Injecting Drug Users in High Risk Countries. Washington, D.C: The National Academies Press; 2006. [Google Scholar]
- 4.Chawarski MC, Schottenfeld RS, Mazlan M. Heroin dependence and HIV infection in Malaysia. Drug Alcohol Depend. 2006;82(Suppl 1):S39–S42. doi: 10.1016/s0376-8716(06)80007-4. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan LE, Metzger DS, Fudala PJ, Fiellin DA. Decreasing international HIV transmission: the role of expanding access to opioid agonist therapies for injection drug users. Addiction. 2005;100(2):150–8. doi: 10.1111/j.1360-0443.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- 6.International Narcotics Control Board. Narcotic Drugs: Estimated World Requirements for 2006: Statistics for 2004. New York: United Nations; 2005. Part Four: Statistical Information on Narcotic Drugs. [Google Scholar]
- 7.Mazlan M, Schottenfeld RS, Chawarski MC. New challenges and opportunities in managing substance abuse in Malaysia. Drug Alcohol Rev. 2006;25(5):473–478. doi: 10.1080/09595230600883354. [DOI] [PubMed] [Google Scholar]
- 8.Krupitsky EM, Zvartau EE, Masalov DV, et al. Naltrexone with or without fluoxetine for preventing relapse to heroin addiction in St. Petersburg, Russia. J Subst Abuse Treat. 2006;31(4):319–328. doi: 10.1016/j.jsat.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Substance Abuse and Mental Health Services Administration - Office of Applied Studies. National Survey of Substance Abuse Treatment Servicies (N-SSATS): 2004 Data on Substance Abuse Treatment Facilities. Rockville, MD: DHHS Publication No. (SMA) 05–4112; 2005. [Google Scholar]
- 10.Mayet S, Farrell M, Ferri M, Amato L, Davoli M. Psychosocial treatment for opiate abuse and dependence. Cochrane Database Syst Rev. 2006;3:3. doi: 10.1002/14651858.CD004330.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence.[update of Cochrane Database Syst Rev. 2003;(2):CD001333; PMID: 12804405] Cochrane Database Syst Rev. 2006:1. doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101(4):491–503. doi: 10.1111/j.1360-0443.2006.01369.x. [DOI] [PubMed] [Google Scholar]
- 13.Krupitsky EM, Zvartau EE, Masalov DV, et al. Naltrexone for heroin dependence treatment in St. Petersburg, Russia. J Subst Abuse Treat. 2004;26(4):285–294. doi: 10.1016/j.jsat.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2003;(2):Art. No.: CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- 15.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2003;(2):Art. No.: CD002207. doi: 10.1002/14651858.CD002207.pub2:3. [DOI] [PubMed] [Google Scholar]
- 16.Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2004:4. doi: 10.1002/14651858.CD002025.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343(18):1290–7. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 18.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949–58. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 19.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 20.Ling W, Charuvastra C, Collins JF, et al. Buprenorphine maintenance treatment of opiate dependence: A multicenter, randomized clinical trial. Addiction. 1998;93:475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 21.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361:662–8. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 22.Krook AL, Brors O, Dahlberg J, et al. A placebo-controlled study of high dose buprenorphine in opiate dependents waiting for medication-assisted rehabilitation in Oslo, Norway. Addiction. 2002;97(5):533–42. doi: 10.1046/j.1360-0443.2002.00090.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 1995;40(1):17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- 24.San L, Pomarol G, Peri JM, Olle JM, Cami J. Follow-up after a six-month maintenance period on naltrexone versus placebo in heroin addicts. Br J Addict. 1991;86(8):983–90. doi: 10.1111/j.1360-0443.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- 26.O’Connor PG, Waugh ME, Weiss EC, et al. A comparison of clonidine, clonidine/naltrexone, and buprenorphine in primary care-based ambulatory opioid detoxification. Clinical Research. 1993;41:545A. [Google Scholar]
- 27.Chawarski MC, Schottenfeld RS, Pakes J, Avants K. Research Monograph. The College on Problems of Drug Dependence, Inc; 1996. AIDS Risk Inventory (ARI): a structured interview for assessing risk of HIV infection in apopulation of durg abusers; p. 174. [Google Scholar]
- 28.Schottenfeld RS, Pakes JR, Oliveto A, Zeidonis D, Kosten TR. Buprenorphine versus methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 29.SPSS for Windows, Release 13.0 [program] Chicago, IL: SPSS, Inc; [Google Scholar]
- 30.Miotto K, McCann MJ, Rawson RA, Frosch D, Ling W. Overdose, suicide attempts and death among a cohort of naltrexone-treated opioid addicts. Drug Alcohol Depend. 1997;45(1–2):131–4. doi: 10.1016/s0376-8716(97)01348-3. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan MA, Nunes EV. New-onset mania and psychosis following heroin detoxification and naltrexone maintenance. Am J Addict. 2005;14(5):486–487. doi: 10.1080/10550490500247230. [DOI] [PubMed] [Google Scholar]
- 32.Kakko J, Gronbladh L, Svanborg KD, et al. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164(5):797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- 33.National Research Council Committee on Clinical Evaluation of Narcotic Antagonists. Clincial evaluation of naltrexone treatment of opaiate-dependent individuals. Arch Gen Psych. 1978;35:335–340. [PubMed] [Google Scholar]
- 34.Curran S, Savage C. Patient response to naltrexone: issues of acceptance, treatment effects, and frequency of administration. NIDA Research Monograph. 1976;9:67–9. doi: 10.1037/e497452006-012. [DOI] [PubMed] [Google Scholar]
- 35.Fals-Stewart W, O’Farrell TJ. Behavioral family counseling and naltrexone for male opioid-dependent patients. [see comment] J Consult Clin Psychol. 2003;71(3):432–42. doi: 10.1037/0022-006x.71.3.432. [DOI] [PubMed] [Google Scholar]
- 36.Carroll KM, Ball SA, Nich C, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry. 2001;58(8):755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornish JW, Metzger D, Woody GE, et al. Naltrexone pharmacotherapy for opioid dependent federal probationers. J Subst Abuse Treat. 1997;14(6):529–34. doi: 10.1016/s0740-5472(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 38.Comer SD, Sullivan MA, Yu E, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63(2):210–8. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gowing L, Farrell M, Bornemann R, Ali R. Substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2004;(4):Art. No.: CD00415. doi: 10.1002/14651858.CD0045145.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Montori VM, Devereaux PJ, Adhikari NK, et al. Randomized trials stopped early for benefit: a systematic review. JAMA. 2005;294(17):2203–9. doi: 10.1001/jama.294.17.2203. [DOI] [PubMed] [Google Scholar]
- 41.Pocock SJ. When (not) to stop a clinical trial for benefit. JAMA. 2005;294(17):2228–30. doi: 10.1001/jama.294.17.2228. [DOI] [PubMed] [Google Scholar]
- 42.Lofwall MR, Stitzer ML, Bigelow GE, Strain EC. Comparative Safety and Side Effect Profiles of Buprenorphine and Methadone in the Outpatient Treatment of Opioid Dependence. Addictive Disorders & Their Treatment. 2005 Jun;4(2):49–64. [Google Scholar]


