Abstract
The effectiveness of retinal prosthetics will depend on their ability to elicit patterns of neural activity that can be recognized by the visual cortex. While conventional short-duration pulses activate retinal neurons effectively, many nearby neurons are thought to respond similarly to a given pulse train – a situation that is non-physiological. Use of pulse trains delivered at rates > 1000 pulses per second (PPS) in cochlear prosthetics help to avoid phase-locked responses but have not been evaluated in the retina; here, we explored the response to trains of 2000 PPS. We found that ganglion cells respond robustly to these stimuli but that the properties of the response were highly sensitive to stimulus amplitude. At low amplitudes the response patterns were burst-like while at higher amplitudes elicited spikes had intervals that were more uniform. Because burst responses were insensitive to synaptic blockers, our results suggest that they arise from direct activation. This was surprising because previous studies indicated that burst responses arise only through indirect activation. Thus, our results suggest multiple mechanisms of burst creation may exist. Further, histograms of interspike intervals revealed that the response properties were different in different types of ganglion cells. While further testing is needed, the ability to create different patterns of activity in different types of ganglion cells raises the possibility that more natural spike patterns can be created.
I. Introduction
Retinal degenerative diseases such as macular degeneration and retinitis pigmentosa destroy the outer layers of the retina, primarily the photoreceptors, and result in blindness. Estimates suggest that these diseases affect 1 million people in the US alone [1]. Currently, there are no cures or treatments. Several groups are developing devices designed to electrically stimulate surviving retinal neurons thereby restoring vision [2–5]. The viability of this approach has been demonstrated in clinical trials during which the retinas of blind patients were electrically stimulated and light percepts, called phosphenes, were elicited. More recently, some subjects were able to read relatively simple words [6,7]. However, the ability to read was limited to only small subset of implanted patients and, on average, it took 1–2 minutes for a subject to identify a single letter. While all of the factors that contribute to high levels of elicited vision are not known, it is almost certain that better stimulation methods are needed. For example, although the location of individual phosphenes typically corresponds to the location of the activating electrode, simultaneous stimulation from multiple electrodes does not result in percepts that have predictable spatial properties [3,8]. Also, the appearance of even individual phosphenes can be highly variable, both within a single subject (stimulation from different electrodes within the MEA), as well as when comparing responses between different subjects. These factors severely limit the quality of spatial information that can reliably be conveyed with existing devices.
Pulses durations of ≥1 ms typically result in one or more bursts of action potentials from the ganglion cell [9–11]. These relatively long pulses have been shown to activate bipolar cells [10,12]; presumably the spike burst results from prolonged synaptic input from the bipolar to the ganglion cell. Individual short duration pulses elicit single action potentials that arise from direct activation of ganglion cells. This one spike per short pulse paradigm allows temporally precise trains of action potentials to be generated [10]. Unfortunately, methods to restrict activation to a single ganglion cell do not exist and it is likely that many ganglion cells respond simultaneously with identical response patterns – a situation that is quite different from the signaling schemes used physiologically.
To avoid ‘phase-locking’ of elicited spike patterns cochlear prosthetics deliver pulses at rates that are too fast for individual neurons to ‘follow.’ As a result, the spikes elicited from the population of activated cochlear neurons are not phase locked. While it is known that retinal ganglion cells can follow rates of up to several hundred pulses per second (PPS), the response to faster rates has not been explored.
Here we measured the response of retinal ganglion cells to pulse rates of 2,000 PPS. We found that at low stimulus amplitudes, the elicited spike trains were burst-like but at higher amplitudes the elicited spike trains consisted of individual spikes. Surprisingly, the properties of the responses were not affected by the application of synaptic blockers suggesting that all responses arise from direct activation of ganglion cells. Further, the response kinetics were different in different types of ganglion cells. Therefore, our results suggest that high rates of stimulation elicit different response properties in different types of ganglion cells.
II. Methods
A. Animal preparation and retina isolation
The care and use of animals followed all federal and institutional guidelines and all protocols were approved by the Institutional Animal Care and Use Committees of the Boston VA Healthcare System and/or the Subcommittee of Research Animal Care of the Massachusetts General Hospital. New Zealand white rabbits (~2.5 kg) were anesthetized with injections of xylazine/ketamine and subsequently euthanized with an intracardial injection of pentobarbital sodium. Immediately after death, the eyes were removed. All procedures following eye removal were performed under dim red illumination. The front of the eye was removed, the vitreous was eliminated, and the eye cup dissected so that the retina could be flattened. The retina was separated from the retinal pigment epithelium and mounted, photoreceptor side down, to a 10-mm square piece of Millipore filter paper (0.45 µm HA Membrane Filter) that was mounted with vacuum grease to the recording chamber (~1.0 ml volume). A 2 mm circle in the center of the Millipore paper allowed light from below to be projected on to the photoreceptors.
B. Light Responses and Electrophysiology
Patch pipettes were used to make small holes in the inner limiting membrane and ganglion cells were targeted under visual control. Spiking was recorded with a loose, cell-attached patch electrode (5–6 M´Ω), filled with Ames medium. Two silver-chloride coated silver wires served as the return; each was positioned approximately 8mm from the targeted cell and approximately 6 mm from each other.
The light stimulus and data acquisition software was controlled by custom software written in LabView (National Instruments and Matlab Mathworks) and written by G. Spor, T. Muench, and D. Balya. The electric stimulation software was written by D. Freeman. Light stimuli were projected onto the retina from below through a liquid crystal display projector (Dell) and focused onto the outer segments of the photoreceptors. A photopic background intensity was maintained throughout the experiment (~4 nW/m2) [13]. Light stimuli consisted of stationary flashed squares (size range: 100–1,000 µm), 1-s duration, centered at the soma as well as a series of moving bars (300 × 1,800 µm that moved at 600 µm/s in one of four orthogonal directions).
Cells were classified as directionally selective (DS) if their response to the flashed 200-µm square was ON–OFF and if their response to the moving bars was asymmetric, i.e., spiking levels were considerably higher in one direction than the opposite direction [14]. These cells are more accurately classified as ON–OFF DS cells to distinguish them from a different type of DS cell that generates responses only at light ON (ON DS cells). Furthermore, LED cells have been shown to generate ON and OFF responses to either light or dark stimuli and respond strongly to small squares (~100 µm) but poorly to larger squares [13,15]. Cells were classified as brisk transient/alpha cells (BT) if they responded with high frequency and transient bursts of spiking to stimuli centered in their receptive field. Consistent with previous reports, responses in these cells were largest for larger squares and were typically small or nonexistent for small squares (≤100 µm).
C. Electric Stimulation
Electric stimulation was delivered via a 10 kΩ Platinum-Iridium electrode (MicroProbes); the exposed area was conical with an approximate height of 125 µm and base diameter of 15 µm, giving a surface area of ~5,900 µm2, comparable to a 40 µm disk electrode. Two silver-chloride coated silver wires served as the return; each was positioned approximately 8mm from the targeted cell and approximately 12mm from each other. The height of the stimulating electrode remained fixed at 25 µm above the inner limiting membrane; the distance was calibrated by touching the surface of the inner limiting membrane with the tip of the electrode and then using the micromanipulator (Sutter, MP-385) to raise the height by 25 µm. Pulse stimuli were controlled by Multi-Channel Systems STG2004 hardware and software. The stimulating electrode was placed directly over the sodium-channel band on the proximal axon (see below).
D. Location of the Sodium Channel Band
In response to short-duration pulses, the location of the sodium-channel band has been shown to correspond to the center of the region with the lowest threshold and is generally centered between 20 and 60 µm from the soma along the proximal axon [16]. Using an iterative process, we were able to quickly find the center of the low-threshold region: movement of the stimulating electrode towards the center of the low-threshold region resulted in decreasing thresholds while movement away from the center resulted in increasing thresholds. We used this location as the approximate center of the sodium-channel band.
E. Rectangular Pulses
Pulsatile stimuli were biphasic pulses (equal and opposite rectangular phases, cathodal first) delivered at rates of 10 – 2000 pulses per second (PPS). Phase durations remained constant at 100 µs. For rates of ≥100 PPS, the interphase delay was equal to one-half of the period between consecutive pulses of the same phase, i.e. for 100 Hz, there was a 5 ms delay between the onset of consecutive cathodal and anodal pulses. While this approach introduced a variable delay between phases, it allowed the response to the cathodal phase to be studied in isolation – consistent with previous work [16,17]. For the 10 PPS rate, the interval between cathodal and anodal was 10 ms and the rate between anodal and cathodal determined by the pulse rate.
F. Stimulus Threshold and Statistical Tests
The threshold, T, was determined at 10 PPS prior to stimulation at the faster rates. The cells used in this study did not exhibit spontaneous firing and therefore all recorded spikes were assumed to be stimulus induced. Amplitudes at the faster rates were delivered in multiples of T.
III. Results
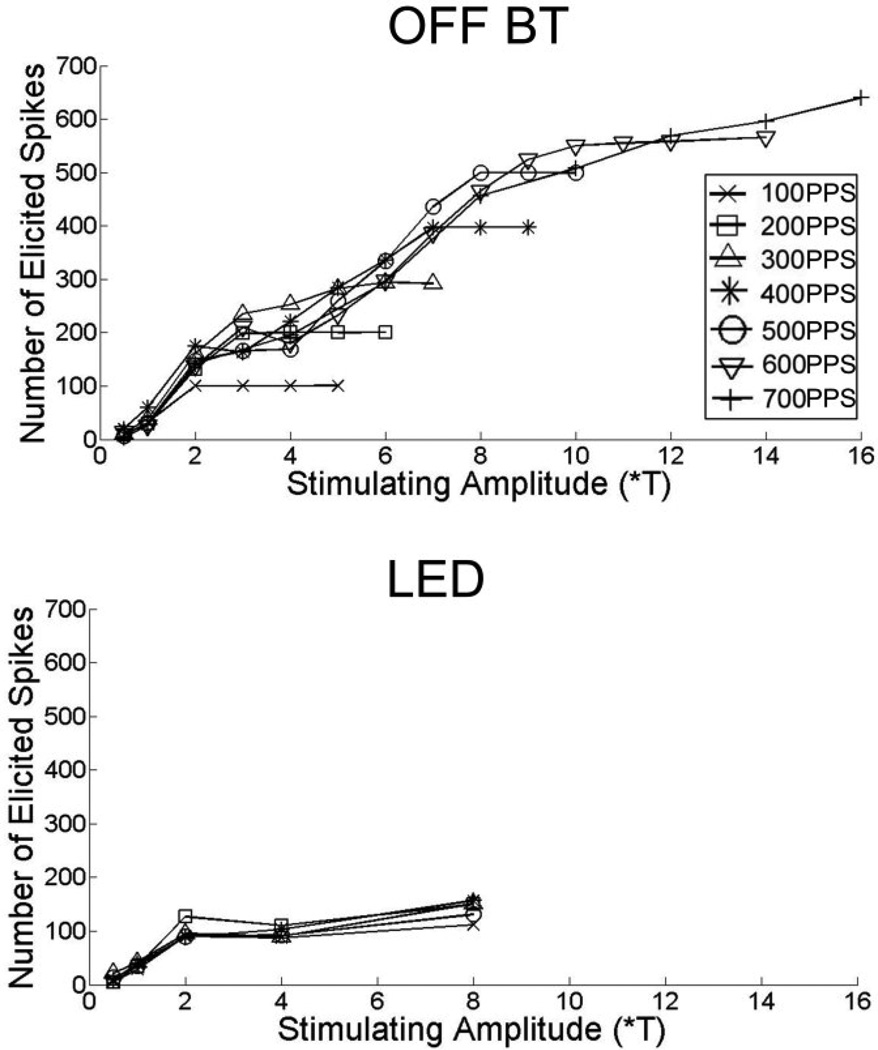
Previous studies have shown that different types of retinal ganglion cells respond differently to electric stimulation [ref]. For example, the ability of ganglion cells to ‘follow’ pulse trains consisting of short duration (~0.1 ms) pulses, i.e., generate one spike for each cathodal phase in the train, is variable (Fig. 1). At one extreme, the alpha OFF ganglion cell could reliably follow rates up to approximately 600 PPS (top) while at the other extreme the local edge detector (LED) type could not even follow rates of 200 PPS (bottom).
Fig. 1.
Spikes elicited as a function of amplitude. Each point represents the total number of spikes elicited in response to a 1-s train of pulses at pulse rates ranging from 100–700 PPS. (top) responses in a typical OFF-alpha cell. (bottom) Responses in a typical LED. Note the difference in vertical axes.
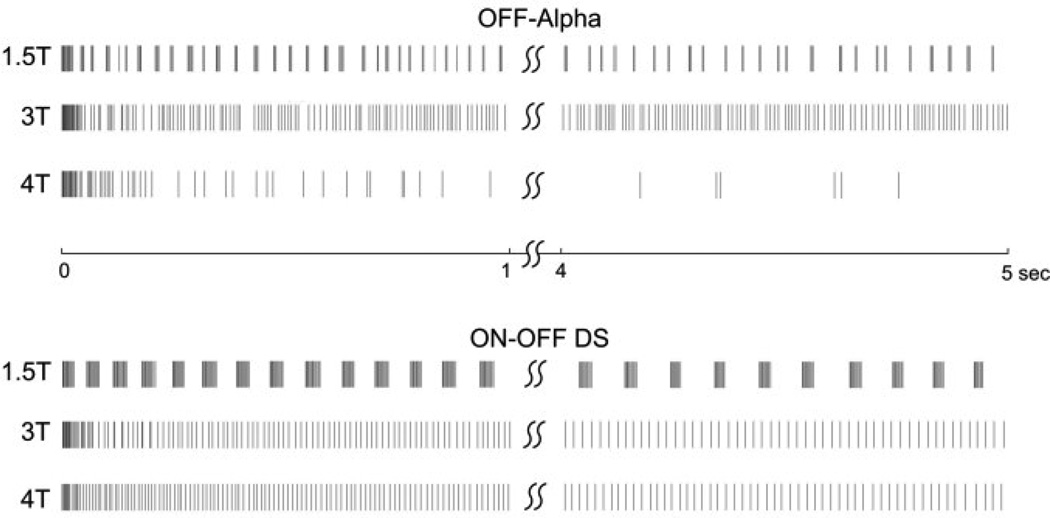
With these differences in mind, we explored the response of two different types of ganglion cells to stimulation at 2,000 PPS. Fig. 2 contains a portion of a typical response to a 5-s train delivered at 2,000 PPS. Responses to stimulation at three different amplitudes are shown: in preliminary experiments, we determined the amplitude at which 50% of the pulses in a 10 PPS train elicited spikes; we refer to that amplitude level as ‘T’. In all subsequent experiments, amplitudes are expressed in multiples of T. Responses from a typical OFF-alpha and from a typical ON-OFF DS cell are shown (top and bottom, respectively).
Fig. 2.
Responses to a 2,000 PPS pulse train. Each row represents the response to a 5-s duration train at the amplitude indicated to the left of the train. Each vertical line indicates an elicited spike. Trains were 5-s in duration; only the first and last second of the response are shown. Timing bar between the two plots refers to both cells. ‘T’ indicates the amplitude required to elicit spikes in 50% of the pulses in a 10 PPS train.
Consistent with much previous work, we found that the response to 2000 PPS pulse trains was highly dependent on pulse amplitude. At the lowest amplitudes we tested (1*T) (not shown), the pulse train elicited very few spikes. At an amplitude of 1.5*T, pulse trains elicited robust spiking; elicited spikes appeared burst-like, i.e. spikes were clustered together. This was consistent in both cell types we tested, although the properties of the bursts appeared somewhat different in the two types (compare the top rows from each cell type). Interestingly, as amplitude was increased the burst-like patterns disappeared. Also surprisingly, we found that at even higher amplitudes (4*T), the number of elicited spikes decreased in the OFF-alpha cell type but not the ON-OFF DS.
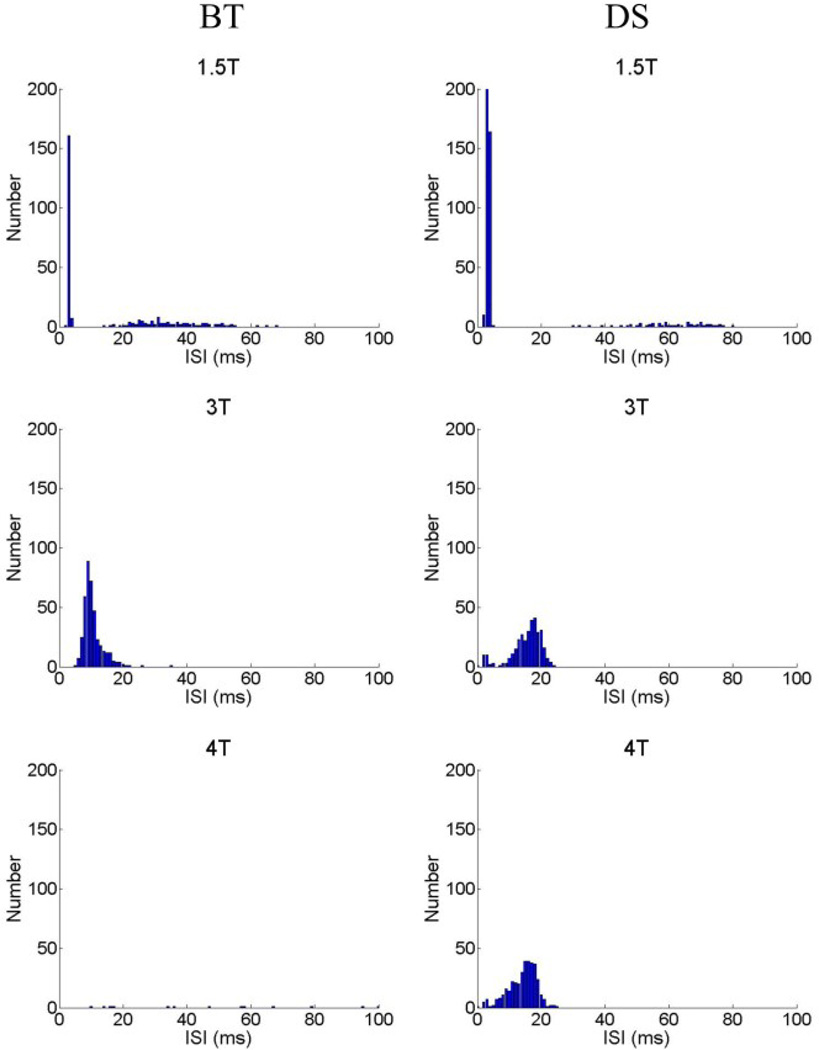
Histograms of the interspike intervals (ISIs) revealed peaks at two locations (Fig. 3). The first peak was centered at <10 ms and represents the short intervals between spikes within a burst; this peak was maximum for relatively low stimulus amplitudes. Consistent with the loss of burst patterns, the short ISI peak disappeared at higher amplitudes (bottom panels). The second peak occurred at 10–40 ms and was typically largest at amplitudes around 3*T. The center of the peak shifted as amplitude increased, i.e. 1.5*T to 3*T, and was also different for different cell types (compare the left and right middle panels). This peak corresponds to the non-burst spikes.
Fig. 3.
Interspike interval histograms in response to a 2,000 PPS pulse train. Interspike intervals were determined for the first second of the response. Histograms are presented for amplitudes of 1.5*T, 3*T and 4*T; left column is from a BT cell, right column from an ON-OFF DS cell.
Because much previous work suggests that burst spiking in response to electric stimulation arises from activation of presynaptic neurons, i.e. bipolar cells, we measured the response to high rate pulse trains in the presence of CNQX and AP-7, blockers of synaptic transmission between bipolar and ganglion cells. Surprisingly, neither the burst nor the non-burst spiking was sensitive to these agents (not shown). This suggests that all elicited responses to high rate pulse trains arise from direct activation of the ganglion cell.
IV. DISCUSSION
Our study is the first to measure the response of retinal ganglion cells to stimulation at rates in excess of 1,000 PPS. We found that although ganglion cells generally respond robustly to this type of stimulation, the response characteristics varied considerably as a function of pulse amplitude. At relatively low amplitudes the response patterns were burst-like while at increasing amplitudes the burst-like appearance is replaced with a pattern of more regular spiking. As amplitude increases further, the response in at least one ganglion cell type is greatly reduced.
The fact that pharmacological blockade of the synaptic inputs to ganglion cells did not affect the response to stimulation strongly suggests that responses to stimulation at 2000 PPS is mediated via direct activation of the ganglion cell. The fact that the burst-like responses observed at low stimulus amplitude levels originate from direct activation of the ganglion cell is surprising because much previous work suggests that burst-like responses originate only through indirect activation (i.e. via activation of bipolar cells). While our results do not shed light on the mechanism of activation, the appearance of burst and non-burst spiking raises the possibility that more than one activation mechanism may be utilized.
The fact that identical stimulation conditions elicited different response properties in different types of ganglion cells is intriguing because it brings into question whether this mode of activation is somehow utilizing an inherent property of the targeted ganglion cell. If so, the elicited responses may be more in line with the cell’s physiological signaling patterns and may therefore be useful for creating higher quality levels for the elicited percepts. Further testing is needed to evaluate these possibilities.
Acknowledgments
This work was supported in part by the U.S. Veterans Administration under Grants 1I01RX000350-01A1 and the Center for Innovative Visual Rehabilitation Grant, as well as by the NIH/NEI under grant 1 R01 EY019967-01.
Contributor Information
Shelley I. Fried, VA Boston Healthcare System and the Dept. of Neurosurgery, Mass. General Hospital/Harvard Medical School, Boston, MA 02114 USA (phone: 617-726-3888; fried.shelley@mgh.harvard.edu)
Changsi Cai, Department of Biomedical Engineering, School of Life Science, Shanghai Jiao-Tong University, Shanghai, China (ccs@sjtu.edu.cn).
Qiushi Ren, Department of Biomedical Engineering, Shanghai Jiao-Tong University, Shanghai, China and the Dept. of Biomedical Engineering, College of Engineering, Peking University, Beijing, China (renqsh@coe.pku.edu.cn).
References
- 1.Bunker CH, Berson EL, Bromley WC, Hayes RP, Roderick TH. Prevalence of retinitis pigmentosa in Maine. Am J Ophthalmol. 1984;97:357–365. doi: 10.1016/0002-9394(84)90636-6. [DOI] [PubMed] [Google Scholar]
- 2.Humayun MS, et al. Visual perception elicited by electrical stimulation of retina in blind humans. Arch Ophthalmol. 1996;114:40–46. doi: 10.1001/archopht.1996.01100130038006. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo JF, 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D. Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials. Invest Ophthalmol Vis Sci. 2003;44:5362–5369. doi: 10.1167/iovs.02-0817. [DOI] [PubMed] [Google Scholar]
- 4.Chow AY, et al. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol. 2004;122:460–469. doi: 10.1001/archopht.122.4.460. [DOI] [PubMed] [Google Scholar]
- 5.Zrenner E, et al. Subretinal microphotodiode array as replacement for degenerated photoreceptors? Ophthalmologe. 2001;98:357–363. doi: 10.1007/s003470170141. [DOI] [PubMed] [Google Scholar]
- 6.Zrenner E, et al. Blind Retinitis Pigmentosa Patients Can Read letters and Recognize the Direction of Fine Stripe Patterns With Subretinal Electronic Implants. Invest Ophthalmol Vis Sci. 2009;50:4581. Abstract #: [Google Scholar]
- 7.daCruz L, et al. Patients Blinded by Outer Retinal Dystrophies Are Able to Identify Letters Using the Argus II Retinal Prosthesis System. Invest. Ophthalmol. Vis. Sci. 2010;51:2023. Abstract #: [Google Scholar]
- 8.de Balthasar C, et al. Factors affecting perceptual thresholds in epiretinal prostheses. Invest Ophthalmol Vis Sci. 2008;49:2303–2314. doi: 10.1167/iovs.07-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stett A, Barth W, Weiss S, Haemmerle H, Zrenner E. Electrical multisite stimulation of the isolated chicken retina. Vision Res. 2000;40:1785–1795. doi: 10.1016/s0042-6989(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 10.Fried SI, Hsueh HA, Werblin FS. A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. J Neurophysiol. 2006;95:970–978. doi: 10.1152/jn.00849.2005. [DOI] [PubMed] [Google Scholar]
- 11.Jensen RJ, Ziv OR, Rizzo JF. Responses of rabbit retinal ganglion cells to electrical stimulation with an epiretinal electrode. J Neural Eng. 2005;2:S16–S21. doi: 10.1088/1741-2560/2/1/003. [DOI] [PubMed] [Google Scholar]
- 12.Margalit E, Thoreson WB. Inner retinal mechanisms engaged by retinal electrical stimulation. Invest Ophthalmol Vis Sci. 2006;47:2606–2612. doi: 10.1167/iovs.05-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- 14.Barlow HB, Levick WR. Mechanism of Directionally Selective Units in Rabbits Retina. J Physiol-London. 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Wyk M, Taylor WR, Vaney DI. Local edge detectors: a substrate for fine spatial vision at low temporal frequencies in rabbit retina. J Neurosci. 2006;26:13250–13263. doi: 10.1523/JNEUROSCI.1991-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried SI, Lasker AC, Desai NJ, Eddington DK, Rizzo JF., 3rd Axonal sodium-channel bands shape the response to electric stimulation in retinal ganglion cells. J Neurophysiol. 2009;101:1972–1987. doi: 10.1152/jn.91081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekirnjak C, et al. Electrical stimulation of mammalian retinal ganglion cells with multielectrode arrays. J Neurophysiol. 2006;95:3311–3327. doi: 10.1152/jn.01168.2005. [DOI] [PubMed] [Google Scholar]